Abstract
Waterfowl are the natural reservoir of all influenza A viruses, which are usually nonpathogenic in wild aquatic birds. However, in late 2002, outbreaks of highly pathogenic H5N1 influenza virus caused deaths among wild migratory birds and resident waterfowl, including ducks, in two Hong Kong parks. In February 2003, an avian H5N1 virus closely related to one of these viruses was isolated from two humans with acute respiratory distress, one of whom died. Antigenic analysis of the new avian isolates showed a reactivity pattern different from that of H5N1 viruses isolated in 1997 and 2001. This finding suggests that significant antigenic variation has recently occurred among H5N1 viruses. We inoculated mallards with antigenically different H5N1 influenza viruses isolated between 1997 and 2003. The new 2002 avian isolates caused systemic infection in the ducks, with high virus titers and pathology in multiple organs, particularly the brain. Ducks developed acute disease, including severe neurological dysfunction and death. Virus was also isolated at high titers from the birds' drinking water and from contact birds, demonstrating efficient transmission. In contrast, H5N1 isolates from 1997 and 2001 were not consistently transmitted efficiently among ducks and did not cause significant disease. Despite a high level of genomic homology, the human isolate showed striking biological differences from its avian homologue in a duck model. This is the first reported case of lethal influenza virus infection in wild aquatic birds since 1961.
Wild aquatic birds are the natural reservoir of influenza type A viruses and play an important role in the ecology and propagation of these viruses. Virus representatives of all 15 hemagglutinin (HA) and all 9 neuraminidase (NA) subtypes have been isolated from waterfowl (10, 27). Influenza viruses in wild aquatic birds have long been in a state of evolutionary equilibrium (evolutionary stasis), and infected hosts usually show no signs of disease (27). Most avian influenza viruses replicate preferentially in the gastrointestinal tract of wild ducks, are excreted at high levels in feces, and are transmitted through the fecal-oral route (10, 29). From this reservoir, influenza A viruses are occasionally transmitted to other avian and mammalian hosts, including humans, and can cause outbreaks of severe disease (27). In particular, viruses of the H5 and H7 subtypes can cause highly pathogenic avian influenza, a systemic disease of high morbidity and mortality in domestic poultry (23).
In 1997 in Hong Kong, a highly pathogenic H5N1 influenza virus caused serious outbreaks of influenza in chicken farms, with a mortality rate greater than 75%. In that same year, 18 Hong Kong residents were infected with H5N1 viruses, and 6 died. Chickens in the poultry markets were found to be the sources of these purely avian H5N1 viruses (22). This outbreak provided the first evidence that avian viruses could be transmitted directly to humans, without prior reassortment in a mammalian host or with a human virus (4, 25), and could cause severe disease (32). Fortunately, the H5N1 virus transmitted to humans in 1997 did not develop the capacity for human-to-human transmission. The outbreak was contained and the source of infection was eliminated by the decisive slaughter of more than 1.5 million birds in the Hong Kong poultry markets. However, H5N1 influenza viruses and their precursors still circulate among poultry and wild birds in Asia (3, 6-8, 28, 31), causing sporadic outbreaks and raising fear of the reappearance of H5N1 virus in humans. In 2001, H5N1 viruses originating from the aquatic bird reservoir (geese and ducks) reassorted with other aquatic avian viruses and reemerged among poultry in Hong Kong markets. These multiple genotypes of H5N1 viruses differed from the H5N1/97 viruses but were highly pathogenic to domestic poultry (6).
In late November and December 2002, new H5N1 outbreaks in two Hong Kong parks, Kowloon Park and Penfold Park, caused the deaths of many resident avian species, including waterfowl and greater flamingo. H5N1 viruses were also isolated from dead wild little egrets and grey herons and other wild migratory birds that overwinter in the New Territories of Hong Kong (4a). Concurrently with the Kowloon Park outbreak, H5N1 viruses were isolated from dead chickens in retail poultry markets and a local chicken farm. In February 2003, avian H5N1 influenza virus was isolated from two humans with acute respiratory distress, one of whom died (30).
Here we report the characterization of H5N1 influenza viruses that were isolated in wild and captive birds in Hong Kong in November and December 2002. These new viruses were compared to previously isolated H5N1 viruses by antigenic analysis and were assessed for their pathogenicity, replication, and transmission potential in ducks. Our findings show that the H5N1 viruses are evolving antigenically and biologically and that this evolution has a serious impact on the pathogenicity of the viruses in ducks. For the first time since 1961, influenza viruses have been reported to kill aquatic birds (2).
MATERIALS AND METHODS
Virus sampling in birds in Hong Kong parks.
Outbreaks of H5N1 avian influenza were detected in two waterfowl parks in Hong Kong, Penfold Park and Kowloon Park, in December 2002 (4a). The parks are located approximately 12 km apart on opposite sides of the Lion Rock Country Park and Kowloon Hills.
Penfold Park is a small nature park located in Shatin, New Territories, Hong Kong. It was populated with a variety of resident waterfowl including assorted geese (Anser spp.), assorted ducks (Anas spp.), and a pair of swans (Cygnus spp.). Penfold Park also had a variety of captive psittacine and passerine birds, free-ranging white pigeons, and a population of feral egrets (Egretta garzetta) that roosted on the park's trees.
Kowloon Park is located in a densely populated area of Tsim Sha Tsui on the Kowloon Peninsula of the city of Hong Kong. The popular park has extensive recreation and sports facilities, including an aviary and a bird lake that house many endangered and valuable birds. The avian population of the park included 26 species of captive pinioned waterfowl and flamingos, which lived around two open ponds, and 35 species of captive free-flying birds, which resided in a wire-meshed aviary complex physically separate from the waterfowl. Additionally, there were five species of feral birds that scavenged grain from the troughs where the waterfowl were fed and herons that regularly visited and searched for fish in the waterfowl ponds.
Staff of both Kowloon and Penfold parks submitted dead birds to Hong Kong's Agriculture, Fisheries and Conservation Department's (AFCD) Veterinary Laboratory for influenza virus testing as soon as unusual bird deaths were detected in early December 2002 (Penfold Park) and mid-December 2002 (Kowloon Park). Postmortem tissue samples from dead birds (spleen, pancreas, lung, trachea, brain, and cloaca), tracheal and cloacal swabs from sick and dead birds, and environmental swabs from the parks were submitted for virological and bacteriological examination. Influenza virus was detected by virus isolation in embryonated chicken eggs. If no virus was isolated on first passage in eggs, an additional passage was done before the sample was considered to be negative. Virus isolates were typed by the hemagglutination inhibition (HI) assay with reference antisera to all 15 avian influenza subtypes and to Newcastle disease virus. Rapid screening for H5 viruses was also performed by reverse transcription-PCR with primers specific for the H5 hemagglutinin. When the bird deaths became too numerous to deal with individually, pooled cloacal and tracheal swabs from each species of bird that died on a given day were screened for influenza virus as described above.
Viruses.
All influenza viruses used in this study were obtained from the AFCD of the Hong Kong SAR Government, the University of Hong Kong, or the St. Jude Children's Research Hospital Influenza Repository (Table 1). Stock viruses were grown in 10-day-old embryonated chicken eggs for 48 h at 35°C. The allantoic fluid was then harvested, and aliquots were stored at −80°C until use. The virus titer was determined by calculating the 50% egg infectious dose (EID50) per ml of virus stock, using the method of Reed and Muench (21). The lower limit of virus detection was 10 EID50 per ml. All experimental work with the H5N1 viruses, including animal studies, was performed in a biosafety level 3+ laboratory approved for use by the U.S. Department of Agriculture.
TABLE 1.
Viruses used in this study
| Virus | Subtype | Abbreviation | Source, isolation datea |
|---|---|---|---|
| A/Tern/South Africa/1961 | H5N3 | Tern/SA/61 | Feral birds, 1961 |
| A/Chicken/Pennsylvania/1370/1983 | H5N2 | Ck/PA/1370/83 | U.S. farm, 1983 |
| A/Hong Kong/156/1997 | H5N1 | HK/156/97 | Human patient, 05/97 |
| A/Chicken/Hong Kong/220/1997 | H5N1 | Ck/HK/220/97 | Poultry market, 03/97 |
| A/Chicken/Hong Kong/258/1997 | H5N1 | Ck/HK/258/97 | Poultry market, 03/97 |
| A/Goose/Hong Kong/437-4/1999 | H5N1 | Gs/HK/437-4/99 | Poultry market, 03/99 |
| A/Chicken/Hong Kong/FY150/2001 | H5N1 | Ck/HK/FY150/01 | Poultry market, 04/01 |
| A/Pheasant/Hong Kong/FY155/2001 | H5N1 | Ph/HK/FY155/01 | Poultry market, 04/01 |
| A/Chicken/Hong Kong/822.2/2001 | H5N1 | Ck/HK/822.2/01 | Poultry market, 05/01 |
| A/Chicken/Hong Kong/YU562/2001 | H5N1 | Ck/HK/YU562/01 | Poultry market, 04/01 |
| A/Chicken/Hong Kong/873.3/2001 | H5N1 | Ck/HK/873.3/01 | Poultry market, 05/01 |
| A/Chicken/Hong Kong/SV02-31.4/2002 | H5N1 | Ck/HK/31.4/02 | Poultry market, 01/02 |
| A/Chicken/Hong Kong/YU22/2002 | H5N1 | Ck/HK/YU22/02 | Poultry market, 01/02 |
| A/Chicken/Hong Kong/86.3/2002 | H5N1 | Ck/HK/86.3/02 | Poultry farm, 02/02 |
| A/Goose/Hong Kong/739.2/2002 | H5N1 | Gs/HK/739.2/02 | Penfold Park, 12/02 |
| A/Goose/Hong Kong/739.3/2002 | H5N1 | Gs/HK/739.3/02 | Penfold Park, 12/02 |
| A/Teal/Hong Kong/2978.1/2002 | H5N1 | Teal/HK/2978.1/02 | Hong Kong-Shenzhen border, 11/02 |
| A/Rosybilled Pochard/Hong Kong/821/2002 | H5N1 | RB Poch/HK/821/02 | Kowloon Park, 12/02 |
| A/Hong Kong/213/2003 | H5N1 | HK/213/03 | Human patient, 02/03 |
Apart from the first two entries, all dates are given as month/year.
Antigenic analysis.
The antigenic characteristics of the H5 influenza viruses were compared by HI tests with a panel of polyclonal antisera and monoclonal antibodies to the H5 hemagglutinin. Antisera were treated with receptor-destroying enzyme, and HI assays were performed with microtiter plates as previously described (17). The polyclonal monospecific hyperimmune goat serum was raised against purified HA from Tern/SA/61 (H5N3). This reference serum is part of a panel of goat antisera raised against the isolated HAs of reference strains of influenza A and B viruses produced by St. Jude Children's Research Hospital. Postinfection polyclonal antisera to Gs/HK/437-4/99 (H5N1) and Ck/HK/YU22/02 (H5N1) were prepared in chickens, and postinfection antiserum to Gs/HK/739.2/02 (H5N1) was prepared in ducks. Sheep antiserum raised against both of the baculovirus-expressed recombinant HA of human isolates HK/156/97 (H5N1) and HK/483/97 (H5N1) was kindly provided by the Food and Drug Administration. Monoclonal antibodies to the HA of A/Chicken/Pennsylvania/1370/83 (H5N2) and A/Chicken/Pennsylvania/8125/83 (H5N2) were used as described previously (22).
Pathogenicity screening of H5N1 viruses in mallards.
Two or three 4- to 6-week-old mallards (Anas platyrhynchos) were each inoculated with 1.0 ml of a 1:10 dilution of a given stock virus (for inoculation doses, see Table 3). A 0.5-ml volume of the inoculum was applied via the cloaca, 0.2 ml was applied via the trachea, and the remaining 0.3 ml was dripped into the mouth, nares, and eyes (0.1 ml in each). Two or three uninfected ducks were placed in the cage with the inoculated birds, sharing food and drinking water. All birds were weighed and observed daily for clinical signs of disease over a period of 7 days. Birds that exhibited severe disease signs were euthanized. Tracheal and cloacal swabs and drinking-water samples were collected 3 and 5 days after inoculation. Tracheal and cloacal swabs were collected in 0.5 and 1 ml of freezing medium, respectively. A 50-μl volume of antibiotic cocktail (200,000 U of penicillin per ml, 40,000 U of streptomycin per ml, 20,000 U of polymyxin B per ml, 5.0 mg of gentamicin per ml) and 50 μl of bovine serum albumin fraction V (Gibco BRL, Rockville, Md.) were added to 1.0 ml of the water sample before it was frozen. Influenza virus was detected in swabs and water samples by virus isolation in chicken embryos as previously described (8). Positive samples were subjected to titer determination for infectivity by determining the EID50.
TABLE 3.
Replication of H5N1/02-03 influenza viruses in mallards
| Virus | Inoculation dose (log10 EID50) | Detection of H5N1 virus at different times after infection
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 3 p.i.
|
Day 5 p.i.
|
||||||||||
| Inoculated ducksb
|
Contact ducksb
|
Drinking watera | Inoculated ducks
|
Contact ducks
|
Drinking water | ||||||
| Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | Trachea | Cloaca | ||||
| HK/156/97 | 5.75 | 2/2 (3.2) | 2/2 (2.4) | 1/2 (1) | 0/2 | − | 0/2 | 0/2 | 1/2 (1) | 1/2 (<1) | − |
| Ck/HK/220/97 | 8.5 | 2/2 (<1) | 1/2 (<1) | 1/2 (<1) | 0/2 | − | 0/2 | 0/2 | 0/2 | 2/2 (3.5) | − |
| Ck/HK/YU562/01 | 8.5 | 2/2 (4.5) | 1/2 (3.5) | 2/3 (<1) | 0/3 | + (1) | 0/2 | 0/2 | 2/3 (<1) | 3/3 (<1) | + (<1) |
| Ck/HK/FY150/01 | 8.25 | 2/2 (3.4) | 2/2 (3.0) | 1/2 (<1) | 0/2 | + (<1) | 1/2 (3.8) | 2/2 (2.2) | 2/2 (4.2) | 2/2 (2.5) | − |
| Ph/HK/FY155/01 | 8.25 | 2/2 (3.0) | 2/2 (4.4) | 1/3 (<1) | 3/3 (2.3) | + (<1) | 0/1d | 1/1d (<1) | 3/3 (5.0) | 3/3 (3.5) | + (2.5) |
| Ck/HK/822.2/01 | 8.25 | 2/2 (3.9) | 1/2 (<1) | 0/2 | 0/2 | + (<1) | 2/2 (2.5) | 0/2 | 0/2 | 0/2 | − |
| A/Ck/HK/873.3/01 | 7.5 | 2/2 (4.2) | 2/2 (<1) | 1/2 (3.5) | 2/2 (2.5) | + (<1) | 2/2 (3.7) | 1/2 (2.8) | 2/2 (4.5) | 2/2 (2.2) | + (<1) |
| Ck/HK/86.3/02 | 8.25 | 2/2 (4.7) | 1/2 (2.5) | NDc | ND | + (2.3) | 1/2 (<1) | 0/2 | ND | ND | − |
| Teal/HK/2978.1/02 | 7.75 | 2/2 (5.0) | 2/2 (<1) | 2/2 (5.0) | 2/2 (2.2) | + (3.5) | 2/2 (1) | 0/2 | 2/2 (3.4) | 1/2 (2.3) | + (3.5) |
| RB poch/HK/821/02 | 6.5 | 2/2 (4.3) | 0/2 | 2/2 (3.7) | 1/2 (<1) | + (3.3) | 0/0d | 0/0d | 2/2 (4.5) | 1/2 (2.3) | + (2.3) |
| Gs/HK/739.2/02 | 7.75 | 3/3 (5.7) | 3/3 (2.2) | 3/3 (5.6) | 2/3 (2.6) | + (3.5) | 1/1d (2.3) | 0/1d | 0/0d | 0/0d | + (<1) |
| HK/213/03 | 7.5 | 2/2 (5.6) | 2/2 (<1) | 1/2 (2.3) | 1/2 (<1) | + (<1) | 1/2 (<1) | 1/2 (2.5) | 0/2 | 1/2 (<1) | − |
Shared drinking water was tested for influenza virus (+, positive; −, negative). Positive samples were subsequently subjected to titer determination for infectious virus (virus titer [log10 EID50/milliliter]).
Number shedding/number sampled (virus titer [log10 EID50/milliliter]). The virus titer is the mean of positive samples.
ND, not determined.
Ducks died.
Duck infection and transmission studies.
Three 4-week-old mallards were inoculated as described above with 2 × 107.75 EID50 of virus. Three uninfected ducks were placed in direct contact with the inoculated birds. All birds were weighed and observed daily for clinical signs of disease. Tracheal and cloacal swabs and drinking-water samples were collected daily for 10 days after inoculation and tested for the presence of influenza virus. Positive samples were titrated for infectivity by determining the EID50.
Histopathology studies and determination of the virus titer in organs.
Seven adult ducks were inoculated with 2 × 107.75 EID50 of virus as described above. Starting 1 day after inoculation, one bird was killed daily and its organs were studied histopathologically. Briefly, tissues of the brain, trachea, lungs, liver, pancreas, spleen, kidneys, intestine, and bursa were collected. Tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin; 5-μm sections were stained with hematoxylin and eosin and studied by light microscopy. Tissue samples from an uninfected control bird were collected and prepared in parallel. Samples of brain, lung, liver, pancreas, spleen, kidneys, and bursa were also subjected to virus titer determination. The samples were weighed and homogenized (approximately 1 g/ml) in sterile phosphate-buffered saline with antibiotics. To detect virus and determine the EID50, tissue homogenates were injected into the allantoic cavities of 10-day-old embryonated chicken eggs. The lower limit of virus detection was 10 EID50 per ml of tissue homogenate.
RESULTS
Influenza outbreak in Hong Kong waterfowl parks in December 2002.
At Penfold Park, the unusual deaths of waterfowl were first noted by staff at the beginning of December 2002 and the first postmortem specimens were submitted to the AFCD veterinary laboratory on 4 December 2002. A total of 31 waterfowl (23 geese, 6 ducks, and 2 swans) died before depopulation of the remaining birds on 10 December 2002. Clinical signs included lack of appetite, dullness, weakness, reluctance to move, and poor balance. Birds that showed signs of disease were usually dead within a day. H5N1 viruses were isolated from eight dead waterfowl submitted for investigation to AFCD. A total of 62 clinically healthy waterfowl (34 ducks and 28 geese) were depopulated, and pooled cloacal and tracheal swabs from 5 of these birds tested positive for H5N1 viruses.
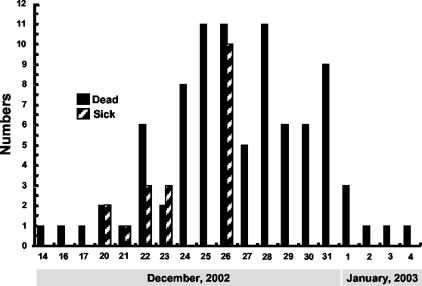
The first unusual deaths among resident birds at Kowloon Park were recorded on 14 to 17 December 2002 (Fig. 1). The first confirmed case of H5N1 avian influenza occurred in a Rosybill Pochard duck on 17 December 2002. On 20 December, more deaths were recorded and two ducks showed signs of central nervous system (CNS) dysfunction. Once H5N1 avian influenza was identified, the two park ponds were cordoned off, drained, and thoroughly disinfected and all of the resident waterfowl were isolated and quarantined. Many birds died rapidly without clinical signs of disease. Some showed slight inactivity, lack of appetite, and ruffled feathers before dying. A minority of birds showed a clear watery nasal discharge, lacrimation, or slight diarrhea before death. About 40% of the birds showed CNS involvement with signs such as depression, paralysis with or without tremors, and an intermittent headshake or an unusual position of the head. At least one-quarter of the ducks showing CNS dysfunction recovered over time. A minority of ducks had an occasional necrotic ulcer or scab on the beak or webbed feet during the outbreak. A total of 105 birds from the open ponds, including 80 (43.7%) of 183 ducks, 9 (37.5%) of 24 geese and swans, and 16 (11.1%) of 144 flamingos, died during the outbreak. Avian influenza of the H5N1 subtype was confirmed by virus isolation in 95 (90.5%) of these cases. There was no major mortality among terrestrial and feral birds. In fact, only one pigeon from the aviary section died during the outbreak (of salpingitis/peritonitis); the bird tested negative for avian influenza. The last H5N1 virus was isolated from birds in this outbreak on 3 January 2003. However, occasional deaths attributed to postinfection complications continued to occur among waterfowl in the month following the outbreak.
FIG. 1.
H5N1 avian influenza virus outbreak in Kowloon Park, Hong Kong. Shown are the numbers of birds identified as sick or dead on each calendar day. H5N1 influenza virus infection was confirmed by virus isolation from the affected birds.
Antigenic analysis of the 2002/2003 H5N1 viruses.
We used HI testing to compare the antigenic characteristics of the 2002/2003 Hong Kong H5N1 viruses with those of H5N1 viruses previously isolated in the region. The H5N1 virus isolates Gs/HK/739.2/02, Dk/HK/739.3/02, Teal/HK/2978.1/02, and RB Poch/HK/821/02 were compared with H5N1 viruses isolated in 1997 from humans (HK/156/97) and poultry markets (Ck/HK/258/97), with representative viruses of the different H5N1 genotypes identified in Hong Kong in 2001 (Ck/HK/FY150/01, Ph/HK/FY155/01, Ck/HK/822.2/01, Ck/HK/YU562/01, and Ck/HK/873.3/01) and early 2002 (Ck/HK/31.4/02, Ck/HK/YU22/02, and Ck/HK/86.3/02), and with reference H5 viruses homologous to the antisera and monoclonal antibodies used in the assay (Tern/SA/61, Ck/PA/1370/83, and Gs/HK/437-4/99) (Table 2). All post-2001 viruses except CK/HK/86.3/02 cross-reacted poorly with the reference monospecific goat antiserum raised against the HA of Tern/SA/61 (H5N3). In fact, the postinfection polyclonal antisera raised against viruses Gs/HK/437-4/99 and Ck/HK/YU22/02 cross-reacted to higher titers with the 2001 and 2002 H5N1 viruses than did the reference antisera. Interestingly, these chicken antisera cross-reacted to high titers with the human isolate HK/156/97 in particular, as did the reference antiserum. The postinfection duck antiserum against Gs/HK/739.2/02 reacted with its homologous virus, as well as with three other 2002 viruses, but with none of the 2001 viruses. Interestingly, it cross-reacted to highest titers with the human isolate HK/156/97.
TABLE 2.
Antigenic analysis of H5N1 influenza viruses from Hong Kong by the HI assay
| Virus | Polyclonal antisera HI titers to:
|
Monoclonal antibody HI titers to:
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anti-A/HK/156 (483)/97 (sheep) | Anti-A/Gs/HK/ 437-4/99 (chicken) | Anti-A/Tern/ SA/61 (goat) | Anti-A/Ck/HK/ YU22/02 (chicken) | Anti-A/Gs/ HK/739.2/02 (duck) | A/Ck/PA/1370/83
|
A/Ck/PA/8125/83
|
|||||
| CP24 | CP25 | CP46 | CP58 | 176/26 | 406/07 | ||||||
| HK/156/97 | 1,280b | 5,120 | 10,240 | 1,280 | 320 | 1,600 | 3,200 | 6,400 | 6,400 | 12,800 | 12,800 |
| Ck/HK/258/97 | 1,280 | 2,560 | 160 | 160 | —a | 1,600 | 1,600 | —a | 6,400 | 12,800 | 400 |
| Ck/HK/FY150/01 | 320 | 1,280 | 40 | 160 | — | 400 | 400 | 1,600 | 6,400 | 6,400 | 3,200 |
| Ph/HK/FY155/01 | 160 | 1,280 | — | 80 | — | 800 | 800 | 800 | 6,400 | 3,200 | 1,600 |
| Ck/HK/822.2/01 | 1,280 | 2,560 | — | 160 | — | 1,600 | 200 | — | 6,400 | 200 | — |
| Ck/HK/YU562/01 | 320 | 1,280 | — | 160 | — | 800 | 800 | 800 | 1,600 | 100 | 100 |
| Ck/HK/873.3/01 | 160 | 1,280 | — | 160 | — | 1,600 | 800 | 200 | 800 | 800 | — |
| Ck/HK/31.4/02 | 640 | 1,280 | — | 160 | — | 100 | 100 | — | 200 | 100 | — |
| Ck/HK/YU22/02 | — | 1,280 | — | 320 | — | 100 | 100 | — | — | 100 | — |
| Ck/HK/86.3/02 | 40 | 2,560 | 160 | 640 | 80 | — | — | — | — | 100 | — |
| Teal/HK/2978.1/02 | 320 | 160 | 40 | 80 | — | 800 | 400 | 400 | 800 | 100 | — |
| RB poch/HK/821/02 | — | 320 | — | 40 | 40 | — | — | — | — | — | — |
| Gs/HK/739.3/02 | — | 640 | — | 160 | 40 | 100 | — | — | — | — | — |
| Gs/HK/739.2/02 | — | 1,280 | — | 320 | 80 | 400 | — | — | — | — | — |
| Reference strains | |||||||||||
| Tern/SA/61 | 40 | 1,280 | 160 | 40 | — | — | — | 1,600 | 200 | 800 | — |
| Ck/PA/1370/83 | NDc | ND | ND | ND | ND | 12,800 | 12,800 | 6,400 | 3,200 | 6,400 | 6,400 |
| Gs/HK/437-4/99 | ND | 5,120 | ND | ND | ND | ND | ND | ND | ND | ND | ND |
— titer, <1:40 for antisera and <1:100 for monoclonal antibodies.
Underlined values are titers of homologous virus.
ND, not determined.
The viruses tested formed two distinguishable groups based on their HI reactivity pattern with the antiserum against HK/156(483)/97. Most of the viruses isolated in 2002 failed to react with this antiserum. A similar pattern emerged with the panel of six monoclonal antibodies against the HA of Ck/PA/1370/83 (H5N2) and Ck/PA/8125/83 (H5N2). All of the 2001 viruses reacted to high titers with monoclonal antibodies CP24, CP25, CP58, and 176/26. Of the 2002 viruses, only Teal/HK/2978.1/02 reacted strongly with these monoclonal antibodies, and its reactivity pattern with all six monoclonal antibodies and most antisera was reminiscent of that of the 2001 viruses.
Lack of reactivity of H5 viruses with monoclonal antibody CP46 is correlated with the presence of a glycosylated carbohydrate at residue 158 of the HA (22). None of the 2002 viruses except Teal/HK/2978.1/02 were reactive with CP46. In contrast, most of the 2001 viruses and the human isolate HK/156/97 reacted with this discriminating monoclonal antibody.
The differences in reactivity patterns indicate that the majority of the 2002 viruses are antigenically distinguishable from the viruses isolated in 1997 and 2001. This fact is evidence of considerable antigenic drift in the HA of recent H5N1 viruses.
Pathogenicity of H5N1 viruses in mallards.
In light of the antigenic drift that has occurred among H5N1 viruses isolated in Hong Kong and the recent observation of an H5N1 outbreak with significant morbidity and mortality among waterfowl, we compared the pathogenicity to mallards of various H5N1 viruses isolated in Hong Kong since 1997. All viruses replicated in the inoculated birds, and almost all were transmitted to the contact ducks within 5 days postinfection (p.i.) (Table 3). The only virus that was not transmitted to the contact birds was A/Ck/HK/822.2/01. The majority of screened viruses had replicated to high titers by 3 days p.i. in the inoculated ducks, and titers were generally higher in the tracheal swabs. Interestingly, at 3 days p.i., the 2002 virus isolates had replicated to higher levels than the earlier virus isolates in the tracheas of contact ducks (>102.3 EID50/ml versus <101 EID50/ml). The only exception to this pattern was the virus A/Ck/HK/873.3/01, which also replicated to high levels in the tracheas of contact ducks (Table 3). Both human isolates HK/156/97 and HK/213/03 replicated in the inoculated ducks and were each transmitted to one contact duck by 3 days p.i. The human isolates replicated only to low titers in the contact ducks, and virus was mostly undetectable in the drinking water. In contrast, higher virus titers were detected in the drinking water after exposure to the 2002 H5N1 viruses than after exposure to earlier virus isolates. This finding reinforces the observation that more virus appears to be shed from the upper respiratory tract than from the intestinal tract of ducks infected with the late 2002 avian H5N1 isolates.
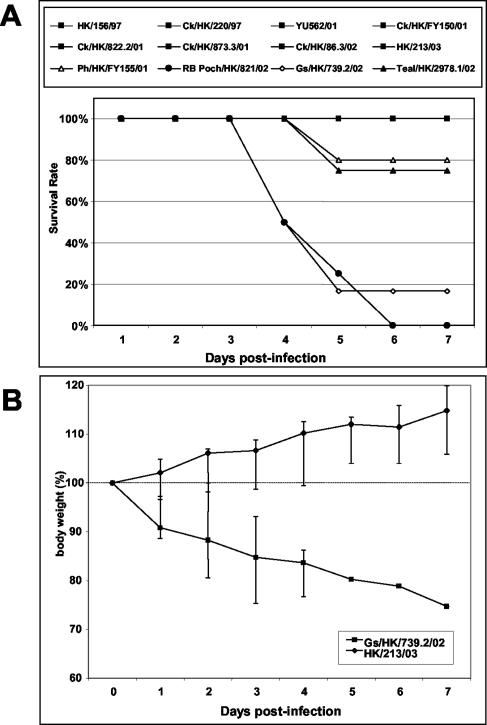
Ducks were observed for a week to compare the morbidity and mortality caused by the different viruses. Infection with viruses isolated before early 2002 did not elicit any overt signs. The ducks were very active, eating and drinking normally and gaining weight. One of four ducks infected with Ph/HK/FY155/01 died at 5 days p.i. without having shown any sign of disease, not even progressive weight loss. All other ducks infected with this virus remained healthy. In contrast, infection with the late-2002 H5N1 isolates (Teal/HK/2978.1/02, RB Pochard/HK/821/02, and Gs/HK/739.2/02) caused significant morbidity and mortality (Fig. 2). By 3 days p.i., the birds showed signs of lethargy and ataxia and had lost considerable weight. By 4 days p.i., one of four ducks infected with RB Pochard/HK/821/02 and three of six ducks infected with Gs/HK/739.2/02 were dead. Infection with any of the three viruses induced severe CNS dysfunction (violent tremors, uncontrollable shaking, marked loss of balance, and lack of coordination) in the surviving birds. All three ducks infected with RB Pochard/HK/821/02 and one duck infected with teal/HK/2978.1/02 were euthanized on days 4 to 6 because of the severity of their neurological signs. Infection with the human isolate HK/213/03 caused no clinical signs of disease in mallards, which remained healthy for the duration of the study and gained weight (Fig. 2). This finding contrasts sharply with the clinical picture observed after infection with Gs/HK/739.2/02 although these two viruses are from the same genotype and have a high degree of homology (>99.0%) in all genes (8a).
FIG. 2.
Survival (A) and weight loss (B) of mallards infected with different H5N1 influenza virus isolates. Ducks were observed for 7 days after inoculation with 1.0 ml of a 1:10 dilution of stock virus. Ducks with severe neurological signs were euthanized and were categorized as having died of infection. (A) Solid squares represent 8 of the 12 viruses tested, which did not cause mortality and whose lines overlap at the 100% survival rate. (B) Data points represent median values, and error bars represent the data range at each time point.
These results indicate that the H5N1 avian viruses recently isolated in Hong Kong are highly pathogenic to ducks. To characterize this new phenomenon in more detail, we undertook a more thorough analysis of the pathogenicity and transmission of virus Gs/HK/739.2/02 in ducks. This virus was selected because it had caused the most rapid mortality in ducks and was the virus most closely related at a genomic level to the human isolate HK/213/03.
Transmission and shedding of Gs/HK/739.2/02 in infected ducks.
To determine the susceptibility and transmissibility of virus Gs/HK/739.2/02, we inoculated three juvenile ducks and housed three contact ducks in the same cage. The contact ducks were highly susceptible to infection with naturally transmitted Gs/HK/739.2/02 (Table 4). All three inoculated ducks showed efficient replication of the virus in both the trachea and cloaca for at least 3 days. Additionally, virus Gs/HK/739.2/02 was transmitted efficiently from inoculated ducks to contact ducks. One day after inoculation, all of the contact ducks had detectable virus in their tracheas and one had detectable virus in the cloaca as well.
TABLE 4.
Transmission and clinical signs of infection with virus Gs/HK/739.2/02 in ducks
| Time (days) p.i. | Inoculated ducks
|
Contact ducks
|
||||
|---|---|---|---|---|---|---|
| No. positivea in:
|
Signs | No. positiveb in:
|
Signs | |||
| Trachea | Cloaca | Trachea | Cloaca | |||
| 1 | 3/3 | 3/3 | None | 3/3 | 1/3 | None |
| 2 | 3/3 | 3/3 | None | 3/3 | 2/3 | None |
| 3 | 3/3 | 3/3 | Lethargy, diarrhea, mucus, labored breathing, cloudy eyes | 3/3 | 2/3 | Lethargy |
| 4 | 1/1 | 1/1 | Death (2/3) | 2/2 | 2/2 | Lethargy, violent tremors, death (1/3) |
| 5 | 1/1 | 0/1 | Cloudy eyes, lethargy, mucus, ruffled feathers | —d | — | — |
| 6 | 0/1 | 0/1 | Cloudy eyes, ataxia | — | — | — |
| 7 | 1/1 | 0/1 | Cloudy eyes, ataxia, labored breathing | — | — | — |
| 8 | 1/1 | 0/1 | Cloudy eyes, ataxia, weight lossc | — | — | — |
| 9 | 1/1 | 0/1 | Cloudy eyes, ataxia | — | — | — |
| 10 | 1/1 | 0/1 | Cloudy eyes, ataxia, depression | — | — | — |
| 11 | 0/1 | 0/1 | Cloudy eyes, ataxia, depression | — | — | — |
Number of positive ducks/number of live inoculated ducks.
Number of positive ducks/number of live contact ducks.
Bird lost 29.5% of body weight over 8 days.
—, all ducks dead.
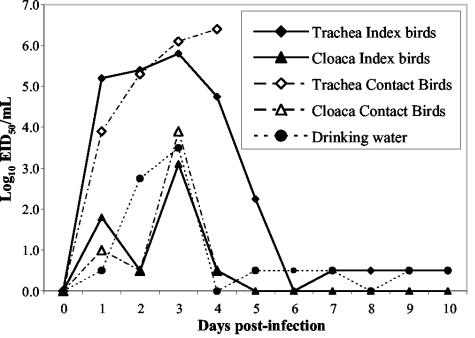
In the wild, influenza viruses are usually spread among waterbirds via the oral-fecal route. Because Gs/HK/739.2/02 infection in ducks did not appear to be restricted to the intestinal tract, virus shedding over the course of infection was examined to determine whether the oral-fecal route should be considered the main mode of transmission of this novel virus. Virus shedding peaked at 3 to 4 days p.i. for both experimentally infected and contact ducks (Fig. 3). Virus shedding from the trachea was more persistent, and titers were consistently more than 100 times higher than titers in the cloaca in both inoculated and contact ducks. Although inoculated ducks shed more virus than did contact birds on day 1 p.i., the levels of shedding were very similar in the two groups from day 2 onward. Most of the ducks died at 3 to 4 days p.i., and therefore the titers of shed virus could be quantified only for one infected duck at 5 days p.i. The surviving duck continued to shed virus from the trachea at a low level for 10 days. However, no cloacal shedding was observed after 4 days p.i. Low levels of virus could also be detected in drinking water samples for 10 days.
FIG. 3.
Virus titers in ducks experimentally infected with Gs/HK/739.2/02, in contact ducks, and in the drinking water provided for the ducks. Values plotted are the mean virus titers of all live birds on the indicated day (see Table 4). For calculation of the mean, influenza virus-positive samples with a virus titer of <1 log10 EID50/ml were assigned a value of 0.5 log10 EID50/ml. Index ducks (n = 3) were inoculated with 2 × 107.75 EID50 of virus, and uninoculated contact ducks (n = 3) shared their cage, food, and drinking water.
These results show that the virus Gs/HK/739.2/02 replicates efficiently in ducks and is easily transmitted to susceptible hosts by direct contact. Aerosol transmission and oral-oral contamination via drinking water may be more important than oral-fecal transmission because of the higher levels of virus shed from the trachea and because of the long-lasting residual shedding.
Clinical signs of Gs/HK/739.2 infection in ducks.
Starting on day 3, both experimentally infected ducks and contact birds showed obvious signs of disease and had lost over 20% of their body weight (Table 4). Two of the inoculated ducks and one of the contact ducks were dead by 4 days p.i. Later on day 4, another contact duck died and the remaining contact duck was euthanized because of severe neurological signs. One experimentally infected duck survived for 12 days, although it showed persistent signs of disease (Table 4). Considering its overall condition, it probably would not have survived in the wild.
Pathology of Gs/HK/739.2/02 infection in ducks.
The epidemiological data from the Hong Kong park outbreaks clearly show the high rate at which H5N1 influenza virus was isolated from dead birds, including a wide variety of ducks and other waterfowl. Influenza infection in waterfowl is usually restricted primarily to the intestinal tract and does not kill its host. However, the mortality and severe neurological disorders observed among ailing birds in two Hong Kong parks and in our own experiments suggest that the new H5N1 viruses replicate in other organs. To assess the systemic dissemination and the pathology of virus Gs/HK/739.2/02, we inoculated adult ducks and collected tissues sequentially for virus titer determination and histopathological analysis. Table 5 shows the results of virus titer determination for the organs. On day 1 p.i., high virus titers were detected in the lungs, spleen, liver, and bursa. The virus titers remained elevated in the lungs for approximately 3 days and then dropped sharply, so that by 5 days p.i., no virus was detectable. By 2 days p.i., high titers of virus were detected in all organs except the brain. Titers remained high in the spleen and kidneys but dropped sharply by 4 days p.i. Interestingly, high virus titers (104 to 106.5 EID50/ml) were detected in the brain later in the course of infection (no virus was detected before 3 days p.i.). Virus was detected at high levels in the bursa starting at 1 day p.i., with the highest titers observed on day 2. Titers of virus dropped after 3 days, but virus remained detectable for up to 6 days p.i.
TABLE 5.
Gs/HK/739.2/02 virus titers in duck organs
| Time (days) p.i. | Virus titera in:
|
||||||
|---|---|---|---|---|---|---|---|
| Brain | Bursa | Kidneys | Liver | Lungs | Pancreas | Spleen | |
| 1 | —b | 103.25 | <101 | 102.5 | 104.75 | <101 | 103.25 |
| 2 | — | 104.5 | 105.75 | 103.75 | 105.75 | 103.25 | >106.5 |
| 3 | 104 | 103.5 | 104.75 | <101 | 105 | <101 | 105.25 |
| 4 | 106.5 | 101 | <101 | <101 | 103.25 | — | 101 |
| 5 | — | <101 | — | — | — | — | — |
| 6 | — | 101 | — | — | — | — | — |
| 7 | — | — | — | — | — | — | — |
Virus titers are expressed as EID50 per milliliter.
—, no detectable virus.
Samples of brain and bursa were collected postmortem for virus titer determination from all juvenile ducks used in the infection and transmission study. All of the ducks had high titers of virus in the brain (105 to 106.5 EID50 units/ml) and bursa (105.4 to 106.5 EID50 units/ml), confirming the systemic dissemination of the virus in ducks.
These results indicate that Gs/HK/739.2/02 replicated efficiently in various organs of infected ducks. The rate of replication seemed to be at its highest at 2 to 3 days p.i. in organs other than the brain, in which the highest virus titers were detected at 4 days p.i. Most virus had been cleared in all organs by 5 days p.i.
Histopathological analysis.
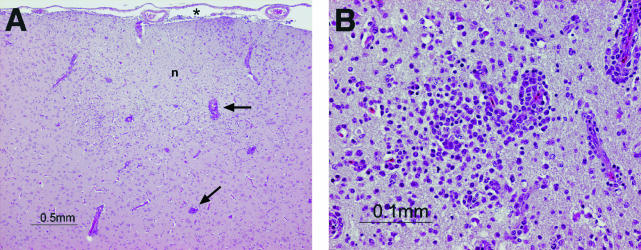
The different organ tissues collected from 1 to 7 days p.i. were histologically analyzed and compared to tissues from an uninfected duck. Although there was a noticeable focal splenic vasculitis at 5 days p.i., the brain and bursa were the only organs consistently affected at multiple time points. Microscopic lesions were observed in brains collected at 3, 4, and 6 days p.i. only. At 3 days p.i, there were signs of a moderate multifocal encephalitis with heterophil infiltrates in the neuropil and mononuclear cell infiltrates in the perivascular spaces. The encephalitis at 4 and 6 days p.i. was more severe than that seen on day 3, and the inflammatory infiltrates in both the neuropil and perivascular space were predominantly mononuclear cells on days 4 and 6 (Fig. 4). Additionally, the inflammatory cell infiltrates observed in the neuropil and perivascular spaces at 4 and 6 days p.i. were more intense and more extensive, with meningeal involvement. The inflammatory infiltrate of the neuropil at 4 and 6 days p.i. was also associated with edema, and there were clear foci of neuronal degeneration and necrosis.
FIG. 4.
Hematoxylin- and eosin-stained sections of the brain of a duck infected with Gs/HK/739.2/02. The brain was collected 6 days after inoculation with 2 × 107.75 EID50 of virus. (A) A prominent inflammatory cell infiltrate is apparent in the meninges (*), neuropil (n), and perivascular spaces (arrows); the pallor of the neuropil indicates edema. (B) The inflammatory infiltrate in the neuropil and perivascular spaces comprises predominantly mononuclear cells. Magnifications: ×4 (A) and ×20 (B).
Various changes were observed in the bursa over time. At 3 days p.i. the bursa was infiltrated with heterophils, while at 4 days p.i. there was a moderate depletion of the lymphoid tissue. At 5, 6, and 7 days p.i., the bursa showed signs of lymphoid hyperplasia. All of these histological changes occurred after viremia in the bursa had reached its peak (2 days p.i.). Despite the presence of virus at high titers in other organs, such as the kidneys, liver, pancreas, and lungs, no histopathological signs were observed.
These results confirm that the systemic spread of virus Gs/HK/739.2/02 in ducks seriously affects organs such as the brain and the bursa and is not limited to the intestinal tract or the respiratory tract.
DISCUSSION
Highly pathogenic H5N1 influenza viruses continue to cause sporadic outbreaks in poultry in Hong Kong. However, the outbreaks in December 2002 caused significant mortality among waterfowl and wild birds in two Hong Kong parks. In the present study, we have shown that the H5N1 avian influenza viruses isolated in late 2002, which caused the first cases of lethal influenza virus infection reported in wild aquatic birds since 1961, were antigenically distinct from previously described H5N1 viruses and were neurotropic and pathogenic in ducks.
Most of the outbreaks of pathogenic H5N1 infection have occurred in domestic chickens. There are few reported cases of avian influenza that is pathogenic in wild aquatic birds (2), and none of the highly pathogenic H5N1 viruses had been shown to be pathogenic in ducks (1, 19, 28). H5N1 virus isolates from 1997 did not replicate efficiently in ducks (22) and induced only mild tissue lesions, if any (19). A pathogenic H5N1 outbreak among waterfowl and wild birds is therefore novel and has serious implications. These new viruses caused disease and a high level of mortality in many different species of water birds and in some wild migratory birds such as egrets and grey herons (4a). Hence, this outbreak was not an isolated event caused by an unusual virus. Acute pathogenicity was not restricted to a single virus genotype or to a single host population but instead appears to be a new characteristic of the late 2002 and 2003 H5N1 viruses in Hong Kong. Our findings showed that three different H5N1 viruses isolated in late 2002 induced severe disease and death in mallards. Preliminary data from our present studies show that other H5N1 viruses isolated in 2003 have the same pathogenicity in mallards.
Previous phylogenetic studies had shown low evolutionary rates of avian influenza viruses in waterfowl. Therefore, it was generally accepted that influenza viruses were in evolutionary stasis in wild aquatic birds, with no evidence of clear evolution over the past 60 years (5). The data presented in this paper raise the possibility that this balance may be changing in ducks or that it has been disrupted by the introduction of novel viruses to ducks from some other avian source. These “imported” viruses may contain some new elements that prove to be highly pathogenic in ducks.
The H5N1/02 influenza viruses isolated in Hong Kong have a different antigenic reactivity pattern from that of previously isolated H5N1 viruses. Most of the 2001 and 2002 Hong Kong H5N1 isolates did not react with the reference H5-monospecific goat antisera (anti-Tern/SA/61), which had previously been used to type H5 isolates. Further, the 2002 H5N1 isolates reacted differently from 1997 and 2001 H5N1 viruses with a panel of monoclonal antibodies. Previous studies had reported limited antigenic variation among H5 viruses isolated between 1979 and 1997 in Hong Kong (22). However, H5N1 viruses that arose among poultry in 2001 differed antigenically from previously identified viruses (6), and our results have confirmed considerable antigenic variation in the H5 surface antigen over the last 2 to 3 years. Antigenic variation in influenza virus is not a novel occurrence. However, it is unclear why antigenic variation is suddenly occurring among H5N1 viruses.
Transfer of viruses between species results in increased antigenic variation, particularly in the surface glycoproteins, due to strong immune selective pressure (15, 16). Interspecies transmission of avian influenza has previously been considered to flow from waterfowl to terrestrial birds, since many viruses isolated from domestic poultry contain genes of aquatic avian origin. However, recent phylogenetic analysis of emerging H9N2 viruses in southern China showed that virus lineages established in terrestrial poultry have now been transmitted back to ducks. Hence, there appears to be a two-way flow of influenza viruses between aquatic and domestic birds in southern China and possibly Hong Kong (12). Most H5 and H7 viruses that are highly pathogenic to terrestrial domestic poultry have multiple basic amino acids at the HA cleavage site. In contrast, most H5 and H7 viruses previously isolated from waterfowl and wild birds (with the exception of Tern/SA/61) have not had this signature motif (24), although it was present in the H5N1/02 viruses isolated from waterfowl and characterized in this study (8a). Therefore, it is reasonable to postulate that the emerging H5N1 viruses in Hong Kong may have been reintroduced into waterfowl from domestic poultry, as has been the case with other subtypes of influenza virus (12). Antigenic variation like that characterized in this study could be explained by the constant transmission of H5N1 viruses to new hosts and the resulting increased probability of changes on surface proteins such as HA. When H5N1 appeared in domestic poultry in 1997 in southern China and Hong Kong, it may have found an ecological niche between the large poultry population and the resident wild aquatic birds. A detailed phylogenetic analysis of newly emerging H5N1 viruses among waterfowl in Hong Kong should help to answer this question.
Another possibility that could explain the recent increased antigenic variation among H5 viruses isolated in Hong Kong is strong immune pressure caused by the use of a vaccine. Antibody-mediated immunity is directed primarily against surface antigens such as HA. Wide use of a vaccine against H5N1 viruses could have created a survival advantage for H5 viruses that undergo antigenic variation. Considering the economic loss that can be caused by highly pathogenic H5N1 in poultry flocks, it is possible that some unapproved use of unregistered H5 vaccine in farms not under regular inspection for poultry trade purposes may have occurred in the larger region.
The consequences of antigenic variation are multiple. Antigenic variation could directly affect the pathogenicity of H5N1 viruses via any biological mechanism that involves interaction with the globular head of the HA protein, such as immune evasion, tissue tropism, and cell and/or host range. H5 antigenic variation can also limit the value of reference reagents used in surveillance. Reference antisera are broadly cross-reactive to detect as many variants as possible within a subtype. Our results showed that most of the 2001 and 2002 H5N1 viruses arising in Hong Kong are not detected by antisera against Tern/SA/61, the current H5 reference reagent. Similarly, the monoclonal antibodies used in this study, considered to be useful diagnostic reagents for H5 viruses after the 1997 outbreak (22), did not react with the novel viruses in an HI test. Considering the repeated reemergence of H5N1 viruses recently in Asia, it may be necessary to complement the current reference reagents to ensure the accurate typing of these new viruses.
Earlier studies showed that highly pathogenic avian influenza viruses that were lethal in domestic poultry could replicate in the internal organs of ducks but caused no disease signs (11). Previously described H5N1 infection in ducks tended to be pneumotropic, with mild lesions localized to the respiratory tract and some virus detected in the spleen and bursa (19). However, in our studies, infection of mallard ducks with Gs/HK/739.2/02 resulted in severe disease, and significant titers of virus were isolated from all organs collected. Although inoculation procedures differed among studies, these results imply that this new H5N1 virus can infect a broader range of cell types than previous H5N1 viruses do. A broader tissue tropism could play a role in the increased pathogenicity of Gs/HK/739.2/02 virus in ducks. H5N1 viruses have been reported to infect the CNS and cause histopathological changes in the brain in different species of domestic poultry and birds, as well as in mammalian models (6, 13, 14, 18-20, 22, 33). However, to our knowledge, this is the first description of an influenza infection affecting the CNS in ducks. Many of the waterfowl affected by the recent Hong Kong outbreaks showed signs of neurological disorders. This observation was confirmed by our experimental infections. Several of the newly emerging H5N1 viruses induced severe neurological signs, and lesions were found in the brains of affected ducks. Ducks that survived the infection had persistent neurological sequelae and never fully recuperated. These results show that the newly emerging H5N1 viruses have acquired neurotropic characteristics in waterfowl, as they had previously done in other avian species. We were unable to determine the specific cause of death of the ducks infected with the late 2002 H5N1 viruses. However, because previous H5N1 viruses infected multiple organs without causing significant disease and the new H5N1 viruses are neurotropic, it is reasonable to postulate that this new ability to infect the CNS is central to the pathogenicity of these viruses. Future studies are needed to confirm this hypothesis.
Other investigators have suggested that the observed tropism of a virus may depend on its site of entry and its pathway of spread in the infected host. There have been multiple reports on the impact of the site of inoculation on the subsequent pattern of viral distribution (26). Our inoculation of ducks via various routes with a large quantity of virus may have affected the spread of the virus in their organs. However, the clinical signs observed in the contact birds confirmed that the naturally transmitted virus spreads systemically and induces severe neurological signs and death. The high titers of virus isolated postmortem from the brain and bursa of contact birds were consistent with the titers observed in the organs of the experimentally infected animals.
Influenza viruses reportedly replicate preferentially in the intestinal tracts of wild ducks, are excreted at high titers in feces (29), and are thought to spread to other wild birds and domestic poultry via contamination of water. However, a recent report described ducks as shedding Ck/HK/220/97 primarily from the upper respiratory tract (19). Our findings indicate that the new H5N1 viruses are causing systemic and respiratory infection in ducks, not just intestinal infection. Elevated virus titers were observed in drinking water in the cages, indicating oropharyngeal shedding. The cages used in this study did not allow the ducks to swim in the water pans or contaminate them with feces. The preferential replication of avian influenza viruses in the digestive tracts of waterfowl and their spread through contaminated water have up to now been thought to minimize the probability of avian-to-human transmission. However, this assumption should be reevaluated in light of our findings. Although our study did not allow us to discriminate between aerosol transmission and drinking-water contamination as the route of infection, it is clear that viruses such as Gs/HK/739.2/02 are no longer restricted to the duck intestinal tract (18, 29) and are now likely to be transmissible via aerosol.
In addition to the possibility of increased aerosol shedding of H5N1 viruses by ducks, there is some reason for concern about the observed duration of shedding. Although most of the infected ducks died rapidly, the surviving duck shed virus for up to 10 days after infection. If this observation is a realistic representation of natural events, it should be of concern in view of the migratory habits of some of the waterfowl affected in Hong Kong. Prolonged shedding would allow these new viruses to be spread over a large geographical area, thereby increasing the exposure of humans and domestic poultry.
Because H5N1 viruses have proven to be highly pathogenic to poultry and to humans, there is great interest in understanding the molecular basis of their high virulence (9). One particularly intriguing result of this study was the distinctively different outcome of duck infection with viruses HK/213/03 and Gs/HK/739.2/02. As mentioned above, these two viruses have a high degree of genomic homology (8a). However, HK/213/03 was poorly transmitted and nonpathogenic in ducks whereas Gs/HK/739.2/02 was efficiently transmitted, neurotropic, and highly pathogenic in ducks. More detailed molecular studies are needed to fully elucidate these striking biological differences between two highly homologous viruses.
Our results clearly show that ducks can be infected with human-adapted H5N1 viruses such as HK/156/97 and HK/213/03 without showing signs of disease. This fact may be of great public health concern. Because they are not pathogenic in ducks, these viruses could become widespread in this host population without raising alarm. If the viruses retain certain characteristics, such as cell receptor-binding profiles or specific gene segments that enable them to be transmitted to humans and cause disease, we would face the possibility that viruses with pandemic potential are silently carried by ducks over large geographical areas.
There is currently great concern about the spread of H5N1 viruses in Asia and possibly further. These viruses have had tremendous economic impact in Hong Kong, requiring the complete or partial slaughter of the poultry population of Hong Kong three times in the last 5 years to prevent the spread of the viruses. Their transmission to humans in Hong Kong in 1997 and in southern China in 2003 emphasizes the risk that this avian influenza subtype represents for human health and the importance of continued characterization of the virus subtypes in poultry and other fowl that live in close proximity to humans. Even if these new viruses do not appear to be transmitted efficiently from human to human, the appearance of new strains brings novel avian influenza viruses into contact with human influenza viruses, with the distinct possibility of reassortment and acquisition of the genes necessary for efficient human transmission. Such an event could result in the emergence of new pathogenic human influenza viruses. It is therefore crucial to gain a good understanding of the natural history and pathogenesis of avian influenza A viruses, particularly highly pathogenic viruses such as the H5N1 subtype. Future studies using reverse genetics will focus on understanding the molecular basis of the pathogenicity of the Gs/HK/739.2/02 virus in ducks. These studies may help us better understand what makes this novel virus neurotropic, lethal, and potentially able to infect humans.
Acknowledgments
These studies were supported by Public Health research grant AI95357 from the National Institute of Allergy and Infectious Diseases and by the American Lebanese Syrian Associated Charities (ALSAC).
We thank Scott Krauss, Patrick Seiler, and Jennifer Humberd for excellent technical assistance and Sharon Naron for editorial assistance.
REFERENCES
- 1.Alexander, D. J., G. Parsons, and R. J. Manvell. 1986. Experimental assessment of the pathogenicity of eight avian influenza A viruses of H5 subtype for chickens, turkeys, ducks and quail. Avian Pathol. 15:647-662. [DOI] [PubMed] [Google Scholar]
- 2.Becker, W. B. 1966. The isolation and classification of Tern virus: influenza A-Tern South Africa—1961. J. Hyg. (London) 64:309-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cauthen, A. N., D. E. Swayne, S. Schultz-Cherry, M. L. Perdue, and D. L. Suarez. 2000. Continued circulation in China of highly pathogenic avian influenza viruses encoding the hemagglutinin gene associated with the 1997 H5N1 outbreak in poultry and humans. J. Virol. 74:6592-6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claas, E. C., A. D. Osterhaus, R. van Beek, J. C. de Jong, G. F. Rimmelzwaan, D. A. Senne, S. Krauss, K. F. Shortridge, and R. G. Webster. 1998. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 351:472-477. [DOI] [PubMed] [Google Scholar]
- 4a.Ellis, T. M., R. B. Bousfield, L. Bissett, K. C. Dyrting, G. S. M. Luk, Y. Guan, S. T. Tsim, K. Sturm-Ramirez, R. G. Webster, and J. S. M. Peiris. Investigation of outbreaks of highly pathogenic H5N1 avian influenza in waterfowl and wild birds in Hong Kong in late 2002. Avian Pathol., in press. [DOI] [PubMed]
- 5.Gorman, O. T., W. J. Bean, and R. G. Webster. 1992. Evolutionary processes in influenza viruses: divergence, rapid evolution, and stasis. Curr. Top. Microbiol. Immunol. 176:75-97. [DOI] [PubMed] [Google Scholar]
- 6.Guan, Y., J. S. Peiris, A. S. Lipatov, T. M. Ellis, K. C. Dyrting, S. Krauss, L. J. Zhang, R. G. Webster, and K. F. Shortridge. 2002. Emergence of multiple genotypes of H5N1 avian influenza viruses in Hong Kong SAR. Proc. Natl. Acad. Sci. USA 99:8950-8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan, Y., K. F. Shortridge, S. Krauss, P. S. Chin, K. C. Dyrting, T. M. Ellis, R. G. Webster, and M. Peiris. 2000. H9N2 influenza viruses possessing H5N1-like internal genomes continue to circulate in poultry in southeastern China. J. Virol. 74:9372-9380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan, Y., K. F. Shortridge, S. Krauss, and R. G. Webster. 1999. Molecular characterization of H9N2 influenza viruses; were they the donors of the “internal” genes of H5N1 viruses in Hong Kong? Proc. Natl. Acad. Sci. USA 96:9363-9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8a.Guan, Y., L. L. M. Poon, C. Y. Cheung, T. M. Ellis, W. Lim, A. S. Lipatov, K. H. Chan, K. M. Sturm-Ramirez, C. L. Cheung, Y. H. C. Leung, K. Y. Yuen, R. G. Webster, and J. S. M. Peiris. H5N1 influenza: a protean pandemic threat. Proc. Natl. Acad. Sci. USA, in press. [DOI] [PMC free article] [PubMed]
- 9.Hatta, M., P. Gao, P. Halfmann, and Y. Kawaoka. 2001. Molecular basis for high virulence of Hong Kong H5N1 influenza A viruses. Science 293:1840-1842. [DOI] [PubMed] [Google Scholar]
- 10.Hinshaw, V. S., R. G. Webster, and B. Turner. 1980. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can. J. Microbiol. 26:622-629. [DOI] [PubMed] [Google Scholar]
- 11.Kawaoka, Y., A. Nestorowicz, D. J. Alexander, and R. G. Webster. 1987. Molecular analyses of the hemagglutinin genes of H5 influenza viruses: origin of a virulent turkey strain. Virology 158:218-227. [DOI] [PubMed] [Google Scholar]
- 12.Li, K. S., K. M. Xu, J. S. Peiris, L. L. Poon, K. Z. Yu, K. Y. Yuen, K. F. Shortridge, R. G. Webster, and Y. Guan. 2003. Characterization of H9 subtype influenza viruses from the ducks of southern China: a candidate for the next influenza pandemic in humans? J. Virol. 77:6988-6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lipatov, A. S., S. Krauss, Y. Guan, M. Peiris, J. E. Rehg, D. R. Perez, and R. G. Webster. 2003. Neurovirulence in mice of H5N1 influenza virus genotypes isolated from Hong Kong poultry in 2001. J. Virol. 77:3816-3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu, X., T. M. Tumpey, T. Morken, S. R. Zaki, N. J. Cox, and J. M. Katz. 1999. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J. Virol. 73:5903-5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ludwig, S., L. Stitz, O. Planz, H. Van, W. M. Fitch, and C. Scholtissek. 1995. European swine virus as a possible source for the next influenza pandemic? Virology 212:555-561. [DOI] [PubMed] [Google Scholar]
- 16.Matrosovich, M., N. Zhou, Y. Kawaoka, and R. Webster. 1999. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J. Virol. 73:1146-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palmer, D. F., M. T. Coleman, W. R. Dowdle, and G. C. Schild. 1975. Advanced laboratory techniques for influenza diagnosis, p. 51-52. Immunology Series no. 6. U.S. Department of Health, Education and Welfare, Washington, D.C.
- 18.Perkins, L. E., and D. E. Swayne. 2001. Pathobiology of A/chicken/Hong Kong/220/97 (H5N1) avian influenza virus in seven gallinaceous species. Vet. Pathol. 38:149-164. [DOI] [PubMed] [Google Scholar]
- 19.Perkins, L. E., and D. E. Swayne. 2002. Pathogenicity of a Hong Kong-origin H5N1 highly pathogenic avian influenza virus for emus, geese, ducks, and pigeons. Avian Dis. 46:53-63. [DOI] [PubMed] [Google Scholar]
- 20.Perkins, L. E., and D. E. Swayne. 2003. Varied pathogenicity of a Hong Kong-origin H5N1 avian influenza virus in four passerine species and budgerigars. Vet. Pathol. 40:14-24. [DOI] [PubMed] [Google Scholar]
- 21.Reed, L. J., and H. Muench. 1938. A simple method for estimating fifty percent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 22.Shortridge, K. F., N. N. Zhou, Y. Guan, P. Gao, T. Ito, Y. Kawaoka, S. Kodihalli, S. Krauss, D. Markwell, K. G. Murti, M. Norwood, D. Senne, L. Sims, A. Takada, and R. G. Webster. 1998. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology 252:331-342. [DOI] [PubMed] [Google Scholar]
- 23.Suarez, D. L. 2000. Evolution of avian influenza viruses. Vet. Microbiol. 74:15-27. [DOI] [PubMed] [Google Scholar]
- 24.Suarez, D. L., M. L. Perdue, N. Cox, T. Rowe, C. Bender, J. Huang, and D. E. Swayne. 1998. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J. Virol. 72:6678-6688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Subbarao, K., A. Klimov, J. Katz, H. Regnery, W. Lim, H. Hall, M. Perdue, D. Swayne, C. Bender, J. Huang, M. Hemphill, T. Rowe, M. Shaw, X. Xu, K. Fukuda, and N. Cox. 1998. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science 279:393-396. [DOI] [PubMed] [Google Scholar]
- 26.Tyler, K. L., and B. N. Fields. 1996. Pathogenesis of viral infections, p. 161-206. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fundamental virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 27.Webster, R. G., W. J. Bean, O. T. Gorman, T. M. Chambers, and Y. Kawaoka. 1992. Evolution and ecology of influenza A viruses. Microbiol. Rev. 56:152-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster, R. G., Y. Guan, M. Peiris, D. Walker, S. Krauss, N. N. Zhou, E. A. Govorkova, T. M. Ellis, K. C. Dyrting, T. Sit, D. R. Perez, and K. F. Shortridge. 2002. Characterization of H5N1 influenza viruses that continue to circulate in geese in southeastern China. J. Virol. 76:118-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Webster, R. G., M. Yakhno, V. S. Hinshaw, W. J. Bean, and K. G. Murti. 1978. Intestinal influenza: replication and characterization of influenza viruses in ducks. Virology 84:268-278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wuethrich, B. 2003. Infectious disease. An avian flu jumps to people. Science 299:1504. [DOI] [PubMed] [Google Scholar]
- 31.Xu, X., Subbarao, N. J. Cox, and Y. Guo. 1999. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 261:15-19. [DOI] [PubMed] [Google Scholar]
- 32.Yuen, K. Y., P. K. Chan, M. Peiris, D. N. Tsang, T. L. Que, K. F. Shortridge, P. T. Cheung, W. K. To, E. T. Ho, R. Sung, and A. F. Cheng. 1998. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351:467-471. [DOI] [PubMed] [Google Scholar]
- 33.Zitzow, L. A., T. Rowe, T. Morken, W. J. Shieh, S. Zaki, and J. M. Katz. 2002. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 76:4420-4429. [DOI] [PMC free article] [PubMed] [Google Scholar]