Abstract
The ventrolateral periaqueductal gray (vlPAG) contributes to morphine antinociception and tolerance. Chronic inflammatory pain causes changes within the PAG that are expected to enhance morphine tolerance. This hypothesis was tested by assessing antinociception and tolerance following repeated microinjections of morphine into the vlPAG of rats with chronic inflammatory pain. Microinjection of morphine into the vlPAG reversed the allodynia caused by intraplantar administration of Complete Freund's Adjuvant (CFA), and produced antinociception on the hot plate test. Although there was a gradual decrease in morphine antinociception with repeated testing, there was no evidence of tolerance when morphine and saline treated rats with hind paw inflammation were tested with cumulative doses of morphine. In contrast, repeated morphine injections into the vlPAG caused a rightward shift in the morphine dose-response curve in rats without hind paw inflammation, as would be expected with the development of tolerance. The lack of tolerance in CFA treated rats was evident whether rats were exposed to repeated behavioral testing or not (Experiment 2) and whether they were treated with 4 or 8 prior microinjections of morphine into the vlPAG (Experiment 3). These data demonstrate that chronic inflammatory pain does not disrupt the antinociceptive effect of microinjecting morphine into the vlPAG, but it does disrupt the development of tolerance.
Keywords: opioid, analgesia, periaqueductal gray, inflammatory pain, morphine tolerance
Introduction
Opioids such as morphine are used clinically to manage chronic pain 8, 9. Unfortunately, the therapeutic effects are limited by unpleasant side effects and by the development of tolerance with repeated opioid administration 8. A number of studies report enhanced tolerance to systemically administered morphine in rodents with chronic inflammatory pain 10, 14, 26, 30, 31. This enhanced tolerance appears to be mediated by physiological changes in the central nervous system because tolerance does not occur when opioids are administered directly into inflamed tissue 51, 59.
The ventrolateral periaqueductal gray (vlPAG) is a midbrain structure known to contribute to opioid antinociception and tolerance. Microinjection of morphine into the vlPAG produces antinociception in both normal rats 4, 24, 42 and those with chronic inflammatory pain 34. Repeated opioid administration induces tolerance when nociception is assessed with acute pain tests such as the hot plate test in normal rats 3, 36, 39, 50, 52. Whether chronic inflammatory pain alters the development of tolerance to vlPAG morphine administration is not known. The induction of chronic inflammatory pain by intraplantar administration of Complete Freund's Adjuvant (CFA) induces a wide range of neural changes and activates neurons within the whole PAG, rather than only the vlPAG region 33. Some of the changes reported in these neurons include an increase in glutamate release, a decrease in GABA release, increased levels of neurotensin and brain-derived neurotrophic factor, and increased expression of serotonin and glutamate receptors 13, 18, 45, 47, 57, 58.
These changes in the PAG could contribute to the enhanced morphine tolerance in rats with chronic pain following systemic morphine administration 10, 14, 26, 30, 31. The objective of the present study was to test the hypothesis that the vlPAG contributes to enhanced morphine tolerance in rats with chronic inflammatory pain. This hypothesis was tested by assessing antinociception to repeated microinjections of morphine into the vlPAG of rats with chronic inflammatory pain induced by intraplantar administration of CFA.
Methods
Subjects
Adult (55-65 days old) male Sprague-Dawley rats (Harlan, Livermore, CA, USA) weighing 220-350 g were used. Rats were housed in a colony room on a reverse 12/12 hour light/dark cycle maintained at a temperature of 22 °C. Rats were provided with food and water ad libitum except during testing and surgery. Each rat was housed individually following surgery. All experiments were carried out according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee at Washington State University.
Surgery
Rats were anesthetized with pentobarbital (60 mg/kg i.p.; Sigma-Aldrich, St. Louis, MO, USA) and implanted with a 9 mm stainless steel guide cannula aimed 2 mm above the right vlPAG (AP: +1.7 mm, ML: ± 0.6 mm, DV: −4.6 mm from lambda). The guide cannula was attached to two screws in the skull with dental cement. A 9 mm stainless steel stylet was inserted to plug the guide cannula and prevent it from becoming clogged. Rats were allowed to recover under a heat lamp until awake and then moved to a clean home cage.
Behavioral Testing
Nociception was assessed using both thermal and mechanical tests. Thermal nociception was assessed using the Hot Plate Analgesia Meter (Columbus Instruments, Columbus, OH) set at a temperature of 52.5° C. The hot plate test assesses nociception by measuring the latency for the rat to lick a hind paw. The rat was removed from the hot plate if no response occurred within 50 s. Although the hot plate test typically is not used to assess nociception in rats with inflammatory pain, it was included so the present data could be compared to previous studies examining tolerance to vlPAG morphine administration using the hot plate test 3, 40.
Mechanical allodynia was assessed using the electronic von Frey test apparatus (IITC Life Science Inc, Woodland Hills, CA). The rat was placed in a Plexiglas square chamber (22 cm × 22 cm × 12.8 cm) on an elevated mesh surface to allow access to the plantar surface of the hind paw with the filament. All rats were allowed to habituate to the chamber for approximately 20 min prior to testing. A plastic semi-flexible von Frey filament was applied to the plantar surface of the CFA treated hind paw, and the amount of pressure exerted by the filament to induce paw withdrawal was recorded. The average of three measurements was used as the nociception score for each rat. The von Frey test was used because it allows assessment of allodynia resulting from chronic inflammatory pain 43. All behavioral testing was conducted in a dimly lit room by an experimenter blind to the drug administered on the preceding trials (i.e. saline or morphine).
Experiment 1: Four repeated morphine microinjections with repeated testing
Immediately following surgery to implant the guide cannula into the vlPAG, chronic inflammatory pain was induced by injecting CFA (Sigma, St. Louis, MO, USA) intradermally into the plantar surface of the right hind paw of half the rats 5. CFA (1.0 mg/ml), an emulsion of oil and water containing strains of heat-killed Mycobacterium, was injected in a volume of 0.1 ml using a 25-gauge needle. Control rats received a saline injection in the right hind paw in a volume of 0.1 ml. Nociception was assessed daily beginning 24 hrs after CFA or saline administration and continued for five consecutive days (Experimental days 2-6). Intraplantar CFA administration is a well-documented method for establishing long-term inflammation5, 27, 32. All of the rats injected with CFA, but not those injected with saline developed a red and swollen hind paw that was evident throughout the duration of the experiment.
Following testing on Experimental day 6, rats received a sham microinjection in which an 11 mm long stainless steel injector was inserted through the guide cannula into the vlPAG. The purpose of this procedure was to habituate the rats to the microinjection procedure and to reduce the possibility of confounds from mechanical activation of neurons on the test day. Rats received morning and afternoon microinjections of either morphine sulfate (0.5 μg/0.4 μl; n = 8) or saline (0.4 μl; n = 8) at a rate of 0.1 μl/10 s into the vlPAG twice daily for two consecutive days (Experimental days 7 and 8). The injections were administered 6 hours apart and were given directly into the vlPAG via a 31-gauge injection cannula (0.25 mm OD and 0.127 mm ID) that extended 2 mm beyond the tip of the guide cannula. The injector remained in place for 20 s after the injection to reduce back flow of drug up the cannula track. Antinociception was assessed 15 min after the injection using the von Frey and hot plate tests, respectively. The morphine dose and injection regimen were chosen based on previous experiments showing that microinjections of morphine given twice daily for two consecutive days into the vlPAG produce tolerance in non-inflamed rats3, 4, 6, 11, 20, 21, 32, 35, 36, 38, 39. Non-CFA treated rats were tested in the same manner as the CFA treated rats (n = 7-9/group). Given the consistency of tolerance to vlPAG morphine microinjections in non-CFA treated rats, additional non-CFA treated rats were not included in Experiments 2 and 3.
On Experimental day 9, morphine potency was assessed in rats previously treated with morphine or saline by microinjecting cumulative doses of morphine into the vlPAG resulting in third log steps (1.0, 2.2, 4.6, 10, and 22 μg/0.4 μl) 39. Microinjections were administered every 20 min, and the von Frey test was conducted 15 min after each injection followed by the hot plate test approximately 2 min later. Tolerance was defined as a significant rightward shift in the morphine dose-response curve.
Experiment 2: Four repeated morphine microinjections without repeated testing
Given that behavioral tolerance is known to develop with repeated nociceptive testing 15, 28, 37, a second experiment was conducted in which the same surgical and microinjection procedures were performed as described above, except that no behavioral testing was conducted until the dose response procedure on the final day of the experiment. After surgery and administration of CFA into the right hind paw, rats were handled daily until the tolerance induction procedure on Experimental days 7 and 8. Rats were injected twice a day for two days with morphine (n = 11) or saline (n = 8) as described above. Cumulative doses of morphine were microinjected into the vlPAG on Experimental day 9 and rats were tested on the von Frey and hot plate tests to assess tolerance.
Experiment 3: Eight repeated morphine microinjections without repeated testing
Although previous studies with normal, non-injured rats showed that four microinjections of morphine was sufficient to produce tolerance 3, 39, morphine tolerance may take longer to develop during chronic pain conditions. Therefore, a third experiment was conducted in which rats underwent the same procedures as in Experiment 2 except that morphine (n = 8) and saline (n = 8) were injected twice a day for four instead of two consecutive days. No nociceptive testing was conducted except for assessment of tolerance on the final day.
Histology
Immediately following testing, each rat was given an overdose of Halothane (Sigma, St. Louis, MO, USA) and decapitated. The brain was extracted and placed in a 10% formalin solution. At least 48 hours later, the brain was sliced coronally (100 μm) with a vibratome (Intracel Vibratome 1500 sectioning system), and the location of the injection site in the vlPAG was determined 44. Only injections located in the vlPAG were included in data analysis. Microinjections in the lateral and dorsal PAG (n = 14) were not included because tolerance does not occur with repeated microinjections in these regions 3, 23, 39, 50, 53. Cannulae terminating in the cerebral aqueduct (n = 11) or passing all the way through the vlPAG (n = 8) were not included because the site of morphine action is not known.
Statistical Analysis
Dose response curves for morphine antinociception were plotted and the half maximal antinociceptive effect (D50) was generated using non-linear regression (GraphPad Prism 5 Software, San Diego, CA, USA). The upper and lower limits for the dose response curves were set at the mean response latency produced by the highest dose of morphine (22 μg/0.4 μl) and the mean baseline latency, respectively. Differences in D50 between the control and experimental groups were analyzed using ANOVA. Baseline nociception was analyzed using a one-way repeated-measures ANOVA, and the effects of morphine treatment were analyzed using a twoway repeated-measures ANOVA.
Results
Microinjections were within the boundaries of the caudal vlPAG in 67 rats44. The locations of the vlPAG microinjections were similar for both morphine and saline treated rats as well as for CFA and non-CFA treated rats. The injection sites were in the same region of the vlPAG in all three experiments. The locations of these injections for Experiment 1 are displayed in Figure 1.
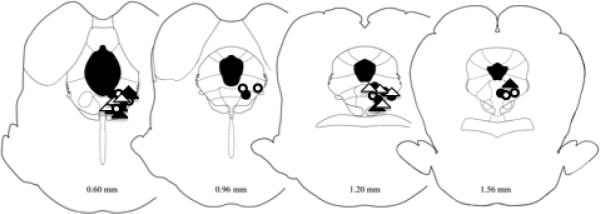
Figure 1.
Location of microinjection sites from Experiment 1. Only injection sites within the vlPAG were included in data analysis. There was no difference in location between CFA treated rats (triangles) and non-CFA rats (circles) nor in rats treated with saline (open shapes) and morphine (closed shapes). The locations of the coronal section relative to the interaural line are indicated at the bottom of each section [36]. Microinjection sites for Experiments 2 and 3 were similar.
Experiment 1: Four repeated morphine microinjections with repeated testing
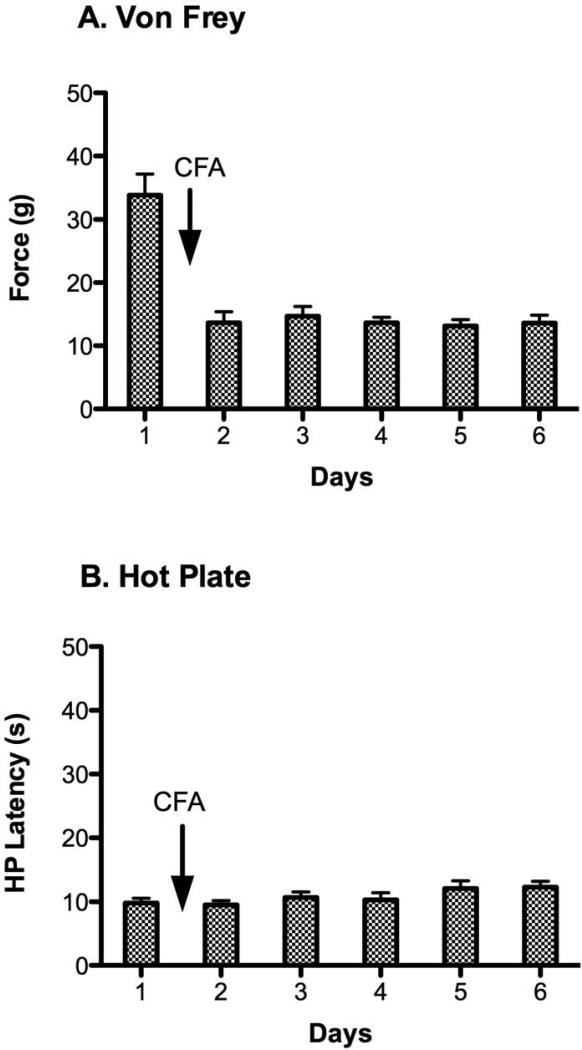
Administration of CFA caused a significant decrease from baseline in the force needed to evoke hind paw withdrawal on the von Frey test [Figure 2A; F (5, 75) = 26.05, p < 0.05]. Allodynia was evident 24 hrs after the CFA injection and persisted across the four subsequent days of testing. In contrast, CFA induced inflammation had no effect on hot plate latency or the choice of paw licked. Hot plate latency was consistent from the baseline test to the 5 days following administration of CFA [Figure 2B; F (5, 75) = 22.03, p > 0.05]. Although only the right paw was inflamed, rats were just as likely to lick the inflamed paw (47%) as the non-inflamed paw (53%) to terminate the test.
Figure 2.
Daily assessment of nociception using the von Frey and hot plate tests before and after intraplantar administration of CFA. A) CFA administration caused a pronounced and prolonged decrease in von Frey pressure thresholds. This decrease from baseline von Frey threshold was significantly different from the threshold on the subsequent 5 days. B) There was no significant difference in mean hot plate latency before and after CFA administration.
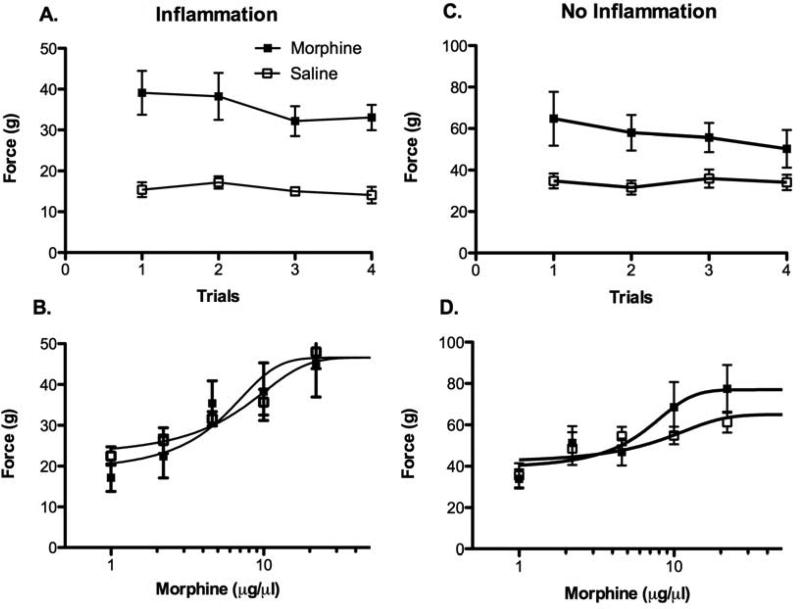
Microinjection of morphine into the vlPAG of CFA treated rats caused an increase in mean von Frey threshold compared to rats microinjected with saline [Figure 3A; F (1, 14) = 32.0, p < 0.05]. This antinociception was relatively stable across the four microinjections as evident by a lack of group by trial interaction [F (3, 42) = 0.533, p > 0.05]. Microinjection of cumulative doses of morphine one day after Trial 4 produced a dose dependent increase in thresholds (Figure 3B). This antinociception was relatively mild and did not differ between rats treated with morphine or saline on Trials 1 – 4. That is, there was no evidence of tolerance in rats with inflammation as indicated by no significant difference in morphine antinociceptive potency between morphine and saline treated rats [F (1, 76) = 0.00, p > 0.05]. This lack of tolerance to repeated morphine microinjections into the vlPAG could be caused by chronic inflammatory pain or because nociception was assessed with the von Frey test. Given that tolerance to morphine administered in the vlPAG has been shown using the hot plate test in non-inflamed rats4, 39, rats with chronic inflammatory pain also were tested on the hot plate.
Figure 3.
Assessment of morphine antinociception and tolerance in CFA-treated rats using the von Frey test. A) Microinjection of morphine produced a significant increase in pressure thresholds that was consistent across the four trials. B) Morphine produced a dose dependent increase in von Frey threshold, but there was no difference in morphine potency on Trial 5 between rats treated with morphine or saline on Trials 1 - 4. The same result was evident in non-inflamed rats except that the range of scores was different because of the lack of allodynia (note the difference in range on the y-axis between Figures A & B vs. C & D). C) Microinjection of morphine caused a significant increase in von Frey threshold that persisted for all four days. D) There was no difference in morphine potency on Trial 5 in non-inflamed rats treated with morphine or saline on Trials 1 – 4.
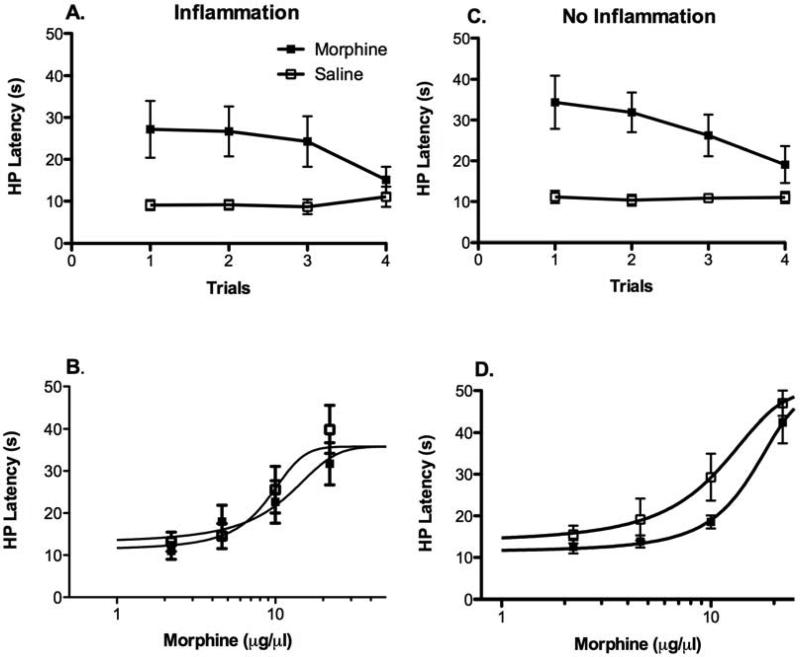
For CFA treated rats, microinjection of morphine into the vlPAG caused a significant increase in hot plate latency compared to control rats receiving microinjection of saline [Figure 4A; F (1, 14) = 7.102, p < 0.05]. The magnitude of this antinociception decreased with each subsequent morphine microinjection [F (3, 42) = 4.10, p < 0.05]. Despite this decrease in antinociception across trials, cumulative microinjections of morphine into the vlPAG on the day following the last morphine injection caused a similar dose dependent increase in hot plate latency in CFA treated rats given morphine or saline on Trials 1 - 4 (Figure 4B). That is, there was no significant difference in morphine antinociceptive potency between groups [F (1, 76) = 0.52, p > 0.05]. The difference between the decrease in antinociception across trials and the lack of a difference in morphine potency following the Trials 1 – 4 highlights the difference between behavioral and pharmacological tolerance. Behavioral tolerance is a reduction in antinociception with repeated testing 12, 28, 37 whereas pharmacological tolerance is caused by changes in morphine potency with repeated exposure to morphine. The present data show that pharmacological tolerance to morphine does not occur despite behavioral tolerance caused by repeated testing.
Figure 4.
Comparison of morphine antinociception and tolerance in CFA and non-CFA treated rats on the hot plate test. A) CFA treated rats showed a significant increase in hot plate latency following microinjection of morphine that decreased with each subsequent injection [F (3, 42) = 4.10, p < 0.05]. B) Morphine produced a dose dependent increase in hot plate latency for CFA-treated rats when tested 18 hours after the pretrial 4 treatment, but there was no significant difference in morphine potency on Trial 5 between rats treated with morphine and saline on Trials 1 - 4. C) Non-CFA treated rats showed a similar increase in hot plate latency following microinjection of morphine F (1, 14) = 19.5, p < 0.05]. Although this antinociception decreased with each subsequent injection, there was no significant group by trial interaction [F (3, 42) = 1.52, p > 0.05]. D) Repeated morphine injections into the vlPAG of control rats not treated with CFA caused a significant rightward shift in the morphine dose response curve compared to saline treated rats [D50M = 15.7 vs. 10.4 μg, respectively; F (1, 60) = 7.56, p < 0.05] as would be expected with the development of tolerance.
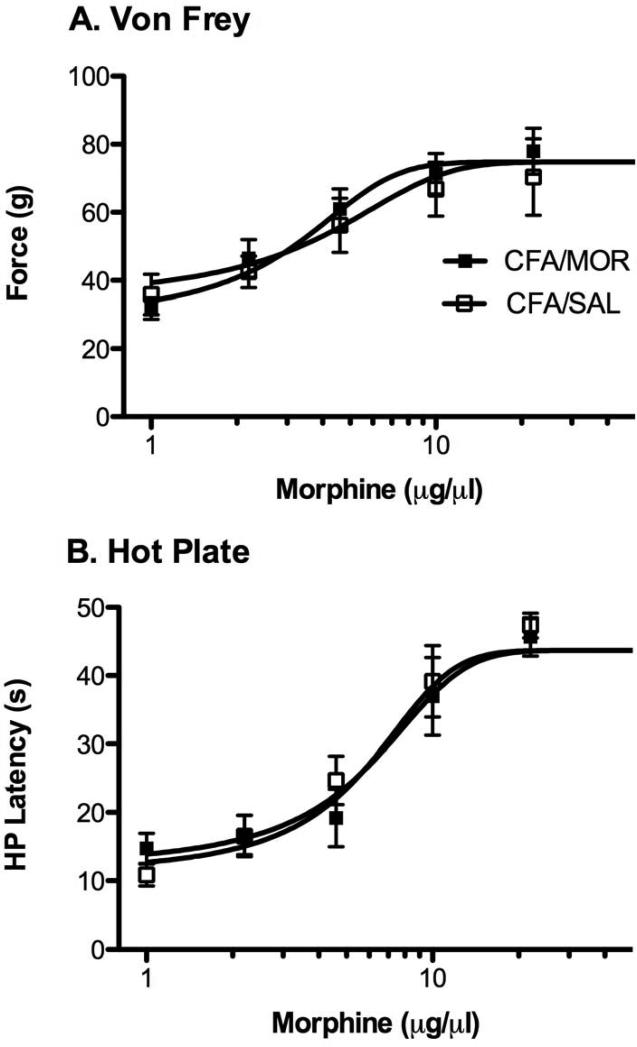
Non-CFA treated rats also were assessed for antinociception and tolerance using the hot plate and von Frey tests. Morphine produced a mild increase in von Frey threshold in non-CFA treated rats compared to rats injected with saline (86% increase vs. 154% increase in CFA rats injected with morphine; Figure 3C). This change is not surprising given that the von Frey test is used to assess allodynia and these rats did not have inflammation48, 56. Likewise, there was no difference in morphine potency between groups on the test day following the last morphine and saline treatment [F (1, 74) = 1.32, p > 0.05]. Von Frey threshold increased from a baseline of 31.1 g to 60.8 g following the highest dose of morphine (22 μg) in rats treated with saline on Trials 1 - 4 and from a baseline of 33.9 g to 77.4 g in morphine treated rats (Figure 3D).
When these non-inflamed rats were tested on the hot plate, microinjections of morphine into the vlPAG caused a significant increase in antinociception [Figure 4C; F (1, 14) = 19.5, p < 0.05]. This increase gradually declined with each subsequent injection, although there was no significant group by trial interaction [F (3, 42) = 1.52, p > 0.05]. Non-CFA treated rats given morphine on Trials 1 – 4 displayed a significant decrease in morphine potency (15.7 μg) on Trial 5 on the hot plate test compared to saline treated controls (10.4 μg) [Figure 4D; F (1, 60) = 7.56, p < 0.05]. These data demonstrate that morphine tolerance was evident in normal, but not in CFA treated rats. D50 values for morphine antinociception on the von Frey and hot plate tests in rats with hind paw inflammation are shown in Table 1.
Table 1.
Comparison of D50 values ± 95% confidence intervals (μg) for rats tested repeatedly or only on the final test day.
| Hot Plate D50 | Von Frey D50 | |||
|---|---|---|---|---|
| Repeat test | Single test | Repeat test | Single test | |
| Saline | 8.9 ± 5.8 | 5.1 ± 2.8 | 4.9 ± 4.1 | 3.4 ± 4.2 |
| Morphine | 11.1 ± 9.9 | 6.1 ± 3.8 | 4.5 ± 5.6 | 2.7 ± 1.9 |
There are no significant differences in D50 values between morphine and saline treated rats when tested repeatedly or with a single test.
Experiment 2: Four repeated morphine microinjections without repeated testing
The lack of tolerance found in CFA rats in Experiment 1 could be caused by a dampening of antinociception in both groups as a result of repeated testing 9, 12, 28, 37. Thus, CFA treated rats in the present experiment were subjected to the same procedures as in Experiment 1 except they were tested with the von Frey and hot plate tests only during the cumulative dose response procedure on the last day of the experiment. Given that morphine tolerance was evident in non-inflamed rats in Experiment 1, only CFA treated rats were used in this experiment.
Cumulative microinjections of morphine on the last day caused a dose dependent increase in von Frey thresholds for both morphine and saline treated rats. As in Experiment 1, there was no difference in morphine potency between groups [Figure 5A; F (1, 91) = 0.83, p > 0.05]. The D50 values for the morphine (2.7 μg) and saline (3.4 μg) groups were 30 – 40% lower than the values reported for rats tested repeatedly in Experiment 1 (Table 1) as would be expected to occur with repeated testing.
Figure 5.
Lack of morphine tolerance in CFA-treated rats not exposed to repeated behavioral testing. A) Morphine produced a dose dependent increase in von Frey threshold with no difference in potency between rats treated with morphine and saline on Trials 1 - 4. B) Hot plate latency also increased in a dose dependent manner to morphine microinjections with no difference in potency between morphine and saline treated groups.
Morphine and saline treated rats also showed a dose dependent increase in hot plate latency when injected with cumulative doses of morphine into the vlPAG on Trial 5 (Figure 5B). No significant difference in morphine potency measured with the hot plate test was evident between groups [F (1, 91) = 0.75, p > 0.05]. Although these rats had much lower D50 values than the rats undergoing repeated testing in Experiment 1 (Table 1), there was no evidence of tolerance to morphine.
Experiment 3: Eight repeated morphine microinjections without repeated testing
The lack of tolerance in Experiments 1 and 2 could indicate that rats with CFA need greater morphine exposure to induce tolerance. This hypothesis was tested by microinjecting morphine or saline into the vlPAG eight times over 4 consecutive days as opposed to four times over 2 days as in Experiments 1 and 2. Microinjection of morphine following 4 days of treatment produced similar dose dependent antinociception on the von Frey test for rats treated with morphine (D50 3.7 μg) and saline (D50 4.2 μg). There was no significant difference in morphine potency between the groups [F (1, 84) = 0.017, p > 0.05]. Only the highest doses of morphine (10 and 22 μg/0.4 μl) produced antinociception on the hot plate test in the morphine and saline treated groups, and there was no significant difference in morphine potency between these groups (D50 = 17.4 and 22.4 μg for morphine and saline treated groups, respectively) [F (1, 86) = 2.18 p > 0.05]. These results indicate that morphine tolerance does not occur in rats with chronic inflammation even after eight microinjections of morphine into the vlPAG. However, the decrease in morphine potency in rats receiving eight instead of four microinjections suggests that tissue damage from repeated microinjections or repeated handling may reduce morphine potency.
Discussion
The present data indicate that the antinociceptive effect of microinjecting morphine into the vlPAG is maintained in rats with chronic inflammatory pain. Contrary to our hypothesis, tolerance to repeated microinjections of morphine into the vlPAG was not evident in rats with chronic inflammatory pain compared to normal rats without inflammation. This lack of tolerance was consistent whether rats were treated with four or eight morphine microinjections, exposed to repeated nociceptive testing, or tested with the von Frey or hot plate test. These data differ from the rapid development of tolerance to morphine microinjections into the vlPAG of rats without chronic pain 3, 23, 39, 50, 53.
Tolerance to the antinociceptive effect of morphine has been shown to occur with as little as a single injection of morphine into the vlPAG 3 and to persist for over a week with repeated administration 41. Both behavioral studies 28 and in vitro slice recordings 2, 20, 22 indicate that changes in vlPAG neurons are sufficient to cause tolerance. The only difference between vlPAG studies showing tolerance and the present study in which tolerance did not occur is the presence of hind paw inflammation. The present study used the same strain of rat, morphine dose, microinjection procedure, and nociceptive test (i.e., hot plate test) as previous studies in non-inflamed rats 3, 39. Additionally, the non-inflamed rats in the current study displayed morphine tolerance.
Studies examining unilateral hind paw inflammation have not used the hot plate test to assess nociception because rats can terminate the test by licking either the inflamed or non-inflamed paw. We used the hot plate test in the present study to link our data to previous studies showing morphine tolerance on the hot plate test in rats without inflammation. Surprisingly, we found no decrease in paw lick latency with the induction of inflammation. This lack of thermal hyperalgesia may have been caused by the lack of specificity of the stimulus to the inflamed paw. The hot plate test allows rats to compensate for pain in the inflamed paw by distributing more weight among the other three paws, whereas the von Frey test applies the stimulus directly to the inflamed paw. Thus, the von Frey test showed a clear and consistent decrease in withdrawal threshold on all of the test days following CFA administration, as has been reported in previous studies 43, 49. Despite this difference, no tolerance to morphine antinociception was evident in CFA treated rats whether nociception was assessed with the hot plate or von Frey test.
Morphine tolerance has been repeatedly reported in healthy rats without a chronic pain condition. This tolerance is evident whether rats were given morphine via repeated microinjections 3, 23, 39, 50, 53 or continuously into the vlPAG 29. The present study showed that this tolerance was only evident using the hot plate test in non-inflamed rats, but that is because morphine had minimal effect on the von Frey threshold in these rats. The von Frey test measures allodynia, and thus, is a test of limited use in the absence of chronic pain. Nonetheless, it was necessary to apply the von Frey test to non-CFA treated rats so that both inflamed and non-inflamed rats underwent identical testing procedures. In contrast to the von Frey test, the hot plate test assesses nociception whether rats are in chronic pain or not. The present data show a clear rightward shift in the morphine dose response curve in non-CFA rats treated with morphine compared to saline when assessed on the hot plate test. This tolerance to repeated microinjections of morphine into the vlPAG is consistent with numerous previous studies in normal rats 3, 6, 10, 19, 20, 23, 31, 32, 35, 37, 39, 50, 52, 53.
Similar to healthy rats, tolerance to morphine antinociception has been reported following systemic administration in rats with chronic inflammatory pain, 10, 14, 26, 30, 31, 54 but these reports are countered by other studies showing a lack of morphine tolerance in animals in chronic pain 1, 7, 19, 25, 55. This discrepancy suggests that methodological differences may determine whether tolerance occurs or not. For example, many of the studies reporting a lack of tolerance used intraplantar injections of formalin to induce chronic pain 1, 25, 55 whereas many of the studies reporting tolerance induced chronic pain with administration of CFA into the hind paw, although tolerance does not always occur7. Additionally, many studies reporting tolerance have administered morphine centrally, whereas studies showing a lack of tolerance have administered morphine directly into the inflamed tissue. These studies indicate that the mechanisms underlying morphine tolerance are mediated by the central nervous system. The current data suggest that susceptibility to morphine tolerance also may vary depending on the central site of action. For example, repeated morphine administration causes activation of astrocytes and microglia in the PAG, and this activation and the development of morphine tolerance is blocked in rats with CFA-induced inflammation7.
Tolerance is often defined as a decrease in morphine antinociception over time 14, 16, 17. However, a decrease in antinociception with repeated testing is called behavioral tolerance and has been reported following repeated morphine administration systemically 12, 37 or into the vlPAG 28. Behavioral tolerance is distinct from pharmacological tolerance, which is caused by neural adaptations as a result of drug exposure. The present data provide a good example of this difference. A decrease in morphine antinociception was evident with each subsequent microinjection of morphine on Trials 1 - 4 (Figure 4), but the dose response analysis on Trial 5 revealed no difference in morphine potency between CFA rats treated with morphine or saline. These results show that the decrease in morphine antinociception with repeated hot plate testing was a result of behavioral tolerance rather than pharmacological tolerance. Although repeated behavioral testing weakened the potency of morphine antinociception, the dose response analysis revealed a further rightward shift in the dose response curve in morphine compared to saline treated rats lacking inflammation. This additional decrease in morphine potency is distinct from behavioral tolerance. Additionally, microinjecting morphine in the absence of repeated hot plate testing, as was done in Experiment 2, enhanced morphine potency compared to rats in Experiment 1 that underwent repeated testing (see Table 1). However, there was no evidence of pharmacological tolerance to morphine in either experiment as demonstrated by the lack of a difference in morphine potency on the dose response analysis in CFA rats treated with morphine or saline.
Likewise, there was no evidence of tolerance in CFA treated rats even when rats were treated with eight morphine microinjections over 4 days. These rats also showed very little antinociception. Whether this is caused by the magnitude of behavioral tolerance, mechanical damage to PAG neurons from repeated microinjections, or some other cause is not known. Whatever the cause, if tolerance to morphine were present, then a further decrease in potency would have been evident in morphine treated rats, and yet this was not the case. These data do not demonstrate that tolerance to vlPAG morphine does not occur, just that tolerance to repeated morphine microinjections does not occur in rats with inflammation of the hind paw.
The induction of chronic pain causes many changes in the nervous system. Within the PAG, CFA administration into the hind paw has been shown to increase glutamate and decrease GABA release, increase levels of neurotensin and brain-derived neurotrophic factor, and increase expression of serotonin and glutamate receptors 13, 18, 46, 47, 57, 58. In addition, the PAG projects to the RVM, and changes in the RVM or spinal cord caused by inflammation could counteract tolerance produced by the PAG. Which of these, if any, contribute to the loss of morphine tolerance to vlPAG morphine administration in CFA treated rats is unknown, but recent evidence suggests that CFA-induced inhibition of PAG glia plays a role7.
In conclusion, the current study demonstrates that repeated microinjections of morphine into the vlPAG produces antinociception against mechanical allodynia and thermal nociception in rats with chronic inflammatory pain, but repeated morphine microinjections into the vlPAG failed to produce tolerance. These results suggest that the presence of chronic inflammatory pain may cause physiological changes within the vlPAG that counteract the development of morphine tolerance. Therefore, the vlPAG may serve as a primary location for further identification of tolerance mechanisms.
Perspective.
The present data show that induction of chronic inflammatory pain does not disrupt the antinociceptive effect of microinjecting morphine into the vlPAG, but it does attenuate the development of tolerance. This finding indicates that tolerance to opioids in rats with inflammatory pain is mediated by structures other than the vlPAG.
Acknowledgements
The assistance of Steve Kallio, Katie Suchland, Rachel Reid, and Michelle Cyr is greatly appreciated.
Funding was provided by NIH grant R01 DA027625.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts of interests related to this work.
Reference List
- 1.Abbott FV, Franklin KB, Ludwick RJ, Melzack R. Apparent lack of tolerance in the formalin test suggests different mechanisms for morphine analgesia in different types of pain. Pharmacol Biochem Behav. 1981;15:637–640. doi: 10.1016/0091-3057(81)90222-7. [DOI] [PubMed] [Google Scholar]
- 2.Bagley EE, Chieng BC, Christie MJ, Connor M. Opioid tolerance in periaqueductal gray neurons isolated from mice chronically treated with morphine. Br J Pharmacol. 2005;146:68–76. doi: 10.1038/sj.bjp.0706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bobeck EN, Haseman RA, Hong D, Ingram SL, Morgan MM. Differential development of antinociceptive tolerance to morphine and fentanyl is not linked to efficacy in the ventrolateral periaqueductal gray of the rat. J Pain. 2012;13:799–807. doi: 10.1016/j.jpain.2012.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bobeck EN, McNeal AL, Morgan MM. Drug dependent sex-differences in periaqueducatal gray mediated antinociception in the rat. Pain. 2009;147:210–216. doi: 10.1016/j.pain.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calvino B, Crepon-Bernard MO, Le Bars D. Parallel clinical and behavioural studies of adjuvant-induced arthritis in the rat: possible relationship with ‘chronic pain’. Behav Brain Res. 1987;24:11–29. doi: 10.1016/0166-4328(87)90032-5. [DOI] [PubMed] [Google Scholar]
- 6.Cyr MC, Morgan MM. Early methylphenidate exposure enhances morphine antinociception and tolerance in adult rats. Neuropharmacology. 2009;57:673–677. doi: 10.1016/j.neuropharm.2009.07.030. [DOI] [PubMed] [Google Scholar]
- 7.Eidson LN, Murphy AZ. Persistent peripheral inflammation attenuates morphine-induced periaqueductal gray glial cell activation and analgesic tolerance in the male rat. J Pain. 2013;14:393–404. doi: 10.1016/j.jpain.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenberg E, McNicol ED, Carr DB. Efficacy of mu-opioid agonists in the treatment of evoked neuropathic pain: Systematic review of randomized controlled trials. Eur J Pain. 2006;10:667–676. doi: 10.1016/j.ejpain.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 9.Farrar JT, Messina J, Xie F, Portenoy RK. A novel 12-week study, with three randomized, double-blind placebo-controlled periods to evaluate fentanyl buccal tablets for the relief of breakthrough pain in opioid-tolerant patients with noncancer-related chronic pain. Pain Med. 2010;11:1313–1327. doi: 10.1111/j.1526-4637.2010.00939.x. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Duenas V, Pol O, Garcia-Nogales P, Hernandez L, Planas E, Puig MM. Tolerance to the antinociceptive and antiexudative effects of morphine in a murine model of peripheral inflammation. J Pharmacol Exp Ther. 2007;322:360–368. doi: 10.1124/jpet.106.118901. [DOI] [PubMed] [Google Scholar]
- 11.Fyfe LW, Cleary DR, Macey TA, Morgan MM, Ingram SL. Tolerance to the antinociceptive effect of morphine in the absence of short-term presynaptic desensitization in rat periaqueductal gray neurons. J Pharmacol Exp Ther. 2010;335:674–680. doi: 10.1124/jpet.110.172643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunn A, Bobeck EN, Weber C, Morgan MM. The influence of non-nociceptive factors on hot-plate latency in rats. J Pain. 2011;12:222–227. doi: 10.1016/j.jpain.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo W, Robbins MT, Wei F, Zou S, Dubner R, Ren K. Supraspinal brain-derived neurotrophic factor signaling: a novel mechanism for descending pain facilitation. J Neurosci. 2006;26:126–137. doi: 10.1523/JNEUROSCI.3686-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gutstein HB, Trujillo KA, Akil H. Does chronic nociceptive stimulation alter the development of morphine tolerance? Brain Res. 1995;680:173–179. doi: 10.1016/0006-8993(95)00259-s. [DOI] [PubMed] [Google Scholar]
- 15.Hayes RL, Mayer DJ. Morphine tolerance: is there evidence for a conditioning model? Science. 1978;200:343–345. doi: 10.1126/science.635595. [DOI] [PubMed] [Google Scholar]
- 16.He L, Fong J, von Zastrow M, Whistler JL. Regulation of opioid receptor trafficking and morphine tolerance by receptor oligomerization. Cell. 2002;108:271–282. doi: 10.1016/s0092-8674(02)00613-x. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez-Delgadillo GP, Cruz SL. Dipyrone potentiates morphine-induced antinociception in dipyrone-treated and morphine-tolerant rats. Eur J Pharmacol. 2004;502:67–73. doi: 10.1016/j.ejphar.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 18.Hu J, Wang Z, Guo YY, Zhang XN, Xu ZH, Liu SB, Guo HJ, Yang Q, Zhang FX, Sun XL, Zhao MG. A role of periaqueductal grey NR2B-containing NMDA receptor in mediating persistent inflammatory pain. Mol Pain. 2009;5:71. doi: 10.1186/1744-8069-5-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imai S, Narita M, Hashimoto S, Hashimoto S, Nakamura A, Miyoshi K, Nozaki H, Hareyama N, Takagi T, Suzuki M, Narita M, Suzuki T. Differences in tolerance to anti-hyperalgesic effects between chronic treatment with morphine and fentanyl under a state of pain. Nihon Shinkei Seishin Yakurigaku Zasshi. 2006;26:183–192. [PubMed] [Google Scholar]
- 20.Ingram SL, Fossum EN, Morgan MM. Behavioral and electrophysiological evidence for opioid tolerance in adolescent rats. Neuropsychopharmacology. 2007;32:600–606. doi: 10.1038/sj.npp.1301139. [DOI] [PubMed] [Google Scholar]
- 21.Ingram SL, Macey TA, Fossum EN, Morgan MM. Tolerance to repeated morphine administration is associated with increased potency of opioid agonists. Neuropsychopharmacology. 2008;33:2494–2504. doi: 10.1038/sj.npp.1301634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ingram SL, Vaughan CW, Bagley EE, Connor M, Christie MJ. Enhanced opioid efficacy in opioid dependence is caused by an altered signal transduction pathway. J Neurosci. 1998;18:10269–10276. doi: 10.1523/JNEUROSCI.18-24-10269.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jacquet YF, Carol M, Russell IS. Morphine-induced rotation in naive, nonlesioned rats. Science. 1976;192:261–263. doi: 10.1126/science.1257766. [DOI] [PubMed] [Google Scholar]
- 24.Jacquet YF, Lajtha A. Paradoxical effects after microinjection of morphine in the periaqueductal gray matter in the rat. Science. 1974;185:1055–1057. doi: 10.1126/science.185.4156.1055. [DOI] [PubMed] [Google Scholar]
- 25.Javan M, Ahmadiani A, Motamadi F, Kazemi B. Changes in G proteins genes expression in rat lumbar spinal cord support the inhibitory effect of chronic pain on the development of tolerance to morphine analgesia. Neurosci Res. 2005;53:250–256. doi: 10.1016/j.neures.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Kayser V, Neil A, Guilbaud G. Repeated low doses of morphine induce a rapid tolerance in arthritic rats but a potentiation of opiate analgesia in normal animals. Brain Res. 1986;383:392–396. doi: 10.1016/0006-8993(86)90047-8. [DOI] [PubMed] [Google Scholar]
- 27.Kramer PR, Kerins CA, Schneiderman E, Bellinger LL. Measuring persistent temporomandibular joint nociception in rats and two mice strains. Physiol Behav. 2010;99:669–678. doi: 10.1016/j.physbeh.2010.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lane DA, Morgan MM. Antinociceptive tolerance to morphine from repeated nociceptive testing in the rat. Brain Res. 2005;1047:65–71. doi: 10.1016/j.brainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 29.Lane DA, Tortorici V, Morgan MM. Behavioral and electrophysiological evidence for tolerance to continuous morphine administration into the ventrolateral periaqueductal gray. Neuroscience. 2004;125:63–69. doi: 10.1016/j.neuroscience.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 30.Li JY, Wong CH, Huang KS, Liang KW, Lin MY, Tan PP, Chen JC. Morphine tolerance in arthritic rats and serotonergic system. Life Sci. 1999;64:PL111–116. doi: 10.1016/s0024-3205(99)00008-9. [DOI] [PubMed] [Google Scholar]
- 31.Liang DY, Guo T, Liao G, Kingery WS, Peltz G, Clark JD. Chronic pain and genetic background interact and influence opioid analgesia, tolerance, and physical dependence. Pain. 2006;121:232–240. doi: 10.1016/j.pain.2005.12.026. [DOI] [PubMed] [Google Scholar]
- 32.Loyd DR, Morgan MM, Murphy AZ. Sexually dimorphic activation of the periaqueductal gray-rostral ventromedial medullary circuit during the development of tolerance to morphine in the rat. Eur J Neurosci. 2008;27:1517–1524. doi: 10.1111/j.1460-9568.2008.06100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loyd DR, Murphy AZ. Sex differences in the anatomical and functional organization of the periaqueductal gray-rostral ventromedial medullary pathway in the rat: a potential circuit mediating the sexually dimorphic actions of morphine. J Comp Neurol. 2006;496:723–738. doi: 10.1002/cne.20962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loyd DR, Wang X, Murphy AZ. Sex differences in micro-opioid receptor expression in the rat midbrain periaqueductal gray are essential for eliciting sex differences in morphine analgesia. J Neurosci. 2008;28:14007–14017. doi: 10.1523/JNEUROSCI.4123-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macey TA, Bobeck EN, Hegarty DM, Aicher SA, Ingram SL, Morgan MM. Extracellular signal-regulated kinase 1/2 activation counteracts morphine tolerance in the periaqueductal gray of the rat. J Pharmacol Exp Ther. 2009;331:412–418. doi: 10.1124/jpet.109.152157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer PJ, Fossum EN, Ingram SL, Morgan MM. Analgesic tolerance to microinjection of the micro-opioid agonist DAMGO into the ventrolateral periaqueductal gray. Neuropharmacology. 2007;52:1580–1585. doi: 10.1016/j.neuropharm.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milne RJ, Gamble GD, Holford NH. Behavioural tolerance to morphine analgesia is supraspinally mediated: a quantitative analysis of dose-response relationships. Brain Res. 1989;491:316–327. doi: 10.1016/0006-8993(89)90066-8. [DOI] [PubMed] [Google Scholar]
- 38.Morgan MM, Bobeck EN, Ingram SL. Glutamate modulation of antinociception, but not tolerance, produced by morphine microinjection into the periaqueductal gray of the rat. Brain Res. 2009;1295:59–66. doi: 10.1016/j.brainres.2009.07.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan MM, Fossum EN, Levine CS, Ingram SL. Antinociceptive tolerance revealed by cumulative intracranial microinjections of morphine into the periaqueductal gray in the rat. Pharmacol Biochem Behav. 2006;85:214–219. doi: 10.1016/j.pbb.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Morgan MM, Fossum EN, Stalding BM, King MM. Morphine antinociceptive potency on chemical, mechanical, and thermal nociceptive tests in the rat. J Pain. 2006;7:358–366. doi: 10.1016/j.jpain.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Morgan MM, Tierney BW, Ingram SL. Intermittent dosing prolongs tolerance to the antinociceptive effect of morphine microinjection into the periaqueductal gray. Brain Res. 2005;1059:173–178. doi: 10.1016/j.brainres.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 42.Morgan MM, Whitney PK, Gold MS. Immobility and flight associated with antinociception produced by activation of the ventral and lateral/dorsal regions of the rat periaqueductal gray. Brain Res. 1998;804:159–166. doi: 10.1016/s0006-8993(98)00669-6. [DOI] [PubMed] [Google Scholar]
- 43.Nagakura Y, Okada M, Kohara A, Kiso T, Toya T, Iwai A, Wanibuchi F, Yamaguchi T. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J Pharmacol Exp Ther. 2003;306:490–497. doi: 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- 44.Paxinos G, Watson SJ. The rat brain, in stereotaxic coordinates. 2nd edition Academic Press; Sydney: 2005. [Google Scholar]
- 45.Renno WM. Microdialysis of excitatory amino acids in the periaqueductal gray of the rat after unilateral peripheral inflammation. Amino Acids. 1998;14:319–331. doi: 10.1007/BF01318851. [DOI] [PubMed] [Google Scholar]
- 46.Renno WM. Prolonged noxious stimulation increases periaqueductal gray NMDA mRNA expression: a hybridization study using two different rat models for nociception. Ir J Med Sci. 1998;167:181–192. doi: 10.1007/BF02937933. [DOI] [PubMed] [Google Scholar]
- 47.Renno WM, Beitz AJ. Peripheral inflammation is associated with decreased veratridine-induced release of GABA in the rat ventrocaudal periaqueductal gray: microdialysis study. J Neurol Sci. 1999;163:105–110. doi: 10.1016/s0022-510x(98)00327-x. [DOI] [PubMed] [Google Scholar]
- 48.Saghaei E, Moini Zanjani T, Sabetkasaei M, Naseri K. Enhancement of Antinociception by Co-administrations of Nefopam, Morphine, and Nimesulide in a Rat Model of Neuropathic Pain. Korean J Pain. 2012;25:7–15. doi: 10.3344/kjp.2012.25.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scoto GM, Arico G, Iemolo A, Ronsisvalle S, Parenti C. Involvement of the Nociceptin/Orphanin FQ-NOP receptor system in the ventrolateral periaqueductal gray following mechanical allodynia in chronic pain. Life Sci. 2009;85:206–210. doi: 10.1016/j.lfs.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 50.Siuciak JA, Advokat C. Tolerance to morphine microinjections in the periaqueductal gray (PAG) induces tolerance to systemic, but not intrathecal morphine. Brain Res. 1987;424:311–319. doi: 10.1016/0006-8993(87)91476-4. [DOI] [PubMed] [Google Scholar]
- 51.Stein C, Pfluger M, Yassouridis A, Hoelzl J, Lehrberger K, Welte C, Hassan AH. No tolerance to peripheral morphine analgesia in presence of opioid expression in inflamed synovia. J Clin Invest. 1996;98:793–799. doi: 10.1172/JCI118852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tortorici V, Nogueira L, Salas R, Vanegas H. Involvement of local cholecystokinin in the tolerance induced by morphine microinjections into the periaqueductal gray of rats. Pain. 2003;102:9–16. doi: 10.1016/s0304-3959(02)00153-7. [DOI] [PubMed] [Google Scholar]
- 53.Tortorici V, Robbins CS, Morgan MM. Tolerance to the antinociceptive effect of morphine microinjections into the ventral but not lateral-dorsal periaqueductal gray of the rat. Behav Neurosci. 1999;113:833–839. doi: 10.1037//0735-7044.113.4.833. [DOI] [PubMed] [Google Scholar]
- 54.Uhelski ML, Boyette-Davis JA, Fuchs PN. Chronic inflammatory pain does not attenuate the development of tolerance to chronic morphine in adult male rats. Pharmacol Biochem Behav. 2011;98:325–330. doi: 10.1016/j.pbb.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 55.Vaccarino AL, Marek P, Kest B, Ben-Eliyahu S, Couret LC, Kao B, Liebeskind JC. Morphine fails to produce tolerance when administered in the presence of formalin pain in rats. Brain Res. 1993;627:287–290. doi: 10.1016/0006-8993(93)90332-h. [DOI] [PubMed] [Google Scholar]
- 56.Wegert S, Ossipov MH, Nichols ML, Bian D, Vanderah TW, Malan TP, Porreca F. Differential activities of intrathecal MK-801 or morphine to alter responses to thermal and mechanical stimuli in normal or nerve-injured rats. Pain. 1997;71:57–64. doi: 10.1016/s0304-3959(97)03337-x. [DOI] [PubMed] [Google Scholar]
- 57.Williams FG, Beitz AJ. Chronic pain increases brainstem proneurotensin/neuromedin-N mRNA expression: a hybridization-histochemical and immunohistochemical study using three different rat models for chronic nociception. Brain Res. 1993;611:87–102. doi: 10.1016/s0006-8993(93)90001-4. [DOI] [PubMed] [Google Scholar]
- 58.Xie H, Ma F, Zhang YQ, Gao X, Wu GC. Expression of 5-HT(2A) receptor mRNA in some nuclei of brain stem enhanced in monoarthritic rats. Brain Res. 2002;954:94–99. doi: 10.1016/s0006-8993(02)03347-4. [DOI] [PubMed] [Google Scholar]
- 59.Zollner C, Mousa SA, Fischer O, Rittner HL, Shaqura M, Brack A, Shakibaei M, Binder W, Urban F, Stein C, Schafer M. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest. 2008;118:1065–1073. doi: 10.1172/JCI25911. [DOI] [PMC free article] [PubMed] [Google Scholar]