Summary
Marked familial aggregation of chronic kidney disease suggests that inherited factors play a major role in nephropathy susceptibility. Molecular genetics analyses have identified a number of genes reproducibly associated with a broad range of renal phenotypes. Most associations show polygenic inheritance patterns with limited effect size. In contrast, genetic association between the apolipoprotein L1 (APOL1) gene and several severe nondiabetic forms of kidney disease in African Americans approach Mendelian inheritance patterns and account for a large proportion of glomerulosclerosis in populations of African ancestry. Emerging data support an important role for APOL1 in the progression of diverse etiologies of kidney disease, in concert with requisite environmental (gene*environment) and inherited (gene*gene) interactions. This article reviews the current status of APOL1-associated nephropathy and discusses research questions under active investigation in the search for a cure for these severe and often progressive kidney diseases.
Keywords: African American, APOL1, FSGS, HIV, kidney disease, progression
Identification of the impressive apolipoprotein L1 (APOL1) association with several previously unrelated forms of nondiabetic kidney disease was a significant scientific breakthrough, one likely to advance our understanding of the pathogenesis of glomerulosclerosis with interstitial fibrosis and renal microvascular disease.1–3 The APOL1 G1 and G2 coding nephropathy risk variants appear to have been selected in sub-Saharan Africa within the past 10,000 years. This is based on the protection that the presence of one of these variants affords from parasitic infection with Trypanosoma brucei rhodesiense, a cause of the fatal disease African sleeping sickness. G1 and G2 risk variants are virtually absent in populations of European and Asian ancestry, showing their relatively recent origin, well after modern human beings departed the African continent.4,5
Strong nephropathy association initially was detected between polymorphisms on chromosome 22q in the nonmuscle myosin heavy chain 9 (MYH9) and adjacent APOL1 genes in African Americans with biopsy-proven forms of focal segmental glomerulosclerosis (FSGS) using admixture mapping.6–8 Odds ratios [OR] for APOL1 association with FSGS are 17, for human immunodeficiency virus (HIV)-associated collapsing glomerulopathy (herein referred to as HIV-associated nephropathy [HIVAN]) are 29, and for nondescript forms of end-stage kidney disease (ESKD) that historically were ascribed to high blood pressure (putative hypertension-attributed or arteriolar nephrosclerosis) are 7.3.1,9 Table 1 contains representative ORs for APOL1 association. A somewhat weaker association was observed in Hispanic Americans of Puerto Rican, Dominican, and other Caribbean ancestries residing in New York City.2 These population groups had approximately 30% African ancestry, far higher than typically seen in Mexican Americans and other Hispanic Americans residing outside of the northeastern United States.
Table 1.
Reported Odds Ratio (Recessive Model) for APOL1-Associated Forms of Nephropathy
| Kidney Disease | Clinical Features | Odds Ratio (95% CI) |
|---|---|---|
| HIVAN9 | Collapsing glomerulopathy | 29.2 (13.1-68.5) |
| Idiopathic FSGS9 | — | 16.9 (11.0-26.5) |
| FGGS with interstitial and vascular alterations17 |
All participants in AASK with putative hypertension-attributed nephropathy |
2.6 (1.9-3.6) |
| FGGS with interstitial and vascular alterations17 |
AASK participants with eventual increase in serum creatinine level > 3 mg/dL |
4.6 (3.1-6.8) |
| FGGS with interstitial and vascular alterations17 |
AASK participants with baseline urine protein: creatinine ratio > 0.6 g/g |
6.3 (3.9-10.1) |
| Nondiabetic ESKD in African Americans1 | Putative hypertension-attributed ESKD (African ancestry) | 7.3 (5.6-9.5) |
| Sickle call nephropathy31 | Proteinuria | 3.4 (not reported) |
| Lupus nephritis–associated ESKD35 | African ancestry | 2.4 (1.8-3.3) |
Abbreviation: CI, confidence interval.
The initial MYH9 extended-1–risk haplotype association in African ancestry populations likely reflected strong linkage disequilibrium with APOL1 because 89% of those with APOL1 G1 and 76% with APOL1 G2 have MYH9 extended-1–risk haplotypes.1 However, residual (albeit weaker) MYH9 association with nephropathy in European and Asian populations suggests that additional susceptibility alleles exist in this region because the G1 and G2 APOL1 risk variants are virtually absent in these groups.4,5,10,11 Whether additional risk variants reside in MYH9 or reflect linkage disequilibrium with the neighboring APOL1 to APOL6 gene region remains unknown.
CLASSIFYING KIDNEY DISEASE IN POPULATIONS OF AFRICAN ANCESTRY: HYPERTENSION-ATTRIBUTED NEPHROPATHY RESIDES IN THE SPECTRUM OF FSGS
High blood pressure is reported by nephrologists as the inciting cause of ESKD in approximately 35% of African Americans initiating renal replacement therapy in the United States12; however, this clinical diagnosis frequently is incorrect.13,14 APOL1-associated forms of glomerulosclerosis are present in the majority of African Americans labeled with hypertension-attributed ESKD and hypertension-attributed nephropathy.15,16 Recent genetic analyses in African American Study of Kidney Disease and Hypertension (AASK) trial participants clearly show that this is the case.17
Nephropathy progression rates among AASK participants with putative “hypertensive nephropathy” were not appreciably slowed by intensive blood pressure lowering or with particular classes of antihypertensive agents including angiotensin-converting enzyme inhibitors (ACEi).18 Although a slight benefit of ACEi and intensive blood pressure control was seen in the early years of the AASK trial,19 using these two measures in all subjects in the AASK cohort phase saw nearly 60% of participants reach a primary study end point (death, dialysis, or doubling of serum creatinine concentration) after 10 years.20 Clearly, blood pressure control did not slow nephropathy progression in AASK participants, suggesting that an alternative initiating cause of kidney disease was present.21
APOL1 showed impressive genetic association with kidney disease in all 663 AASK participants with available DNA samples (OR, 2.57; P = 1.39E−8); association was strengthened in the subset whose nephropathy progressed to a serum creatinine concentration greater than 3 mg/dL or ESKD (OR, 4.61; P = 5.60E−15) or with a baseline urine protein:creatinine ratio greater than 0.6 g/g (OR, 6.29; P = 2.62E−14).17
Kidney biopsy specimens in a small number of AASK participants showed histologic changes that initially were interpreted as consistent with hypertension, focal global glomerulosclerosis (FGGS) and occasionally FSGS, with interstitial fibrosis and vascular changes.22 In retrospect, these biopsy changes relate to APOL1-associated disease and not high blood pressure. This observation likely explains why blood pressure lowering and ACEi were poorly effective. AASK subjects have a kidney disease that lies in the spectrum of FSGS with low relatively level proteinuria.23 In addition, an ACEi can best slow nephropathy progression in the setting of heavy proteinuria.24
HIV-ASSOCIATED COLLAPSING GLOMERULOPATHY
Of all common kidney diseases, HIVAN shows the most marked African American to European American disparity in incidence rates.25 In the early days of the acquired immune deficiency syndrome (AIDS) epidemic, AIDS was reported as a cause of FSGS in African Americans residing in the northeastern and southeastern United States.26 Subsequent reports from the West Coast failed to detect this association, prompting many to attribute FSGS on the East Coast to the effects of intravenous drug use, not HIV infection. These geographic differences are related to the presence of APOL1 risk variants in East Coast HIV populations with African ancestry, relative to the initial West Coast AIDS epidemic that more often impacted European Americans.27
In a similar vein, it is fascinating to note the relative lack of APOL1 nephropathy risk variants in Africans from Ethiopia (whether they reside in Ethiopia or emigrated to Israel), along with their associated low frequency of HIVAN.28 Ethiopians have approximately 50% Middle Eastern and 50% African ancestry; however, demarcation is observed in allele frequencies between Western and Eastern Africa. Consistent associations between APOL1 risk variants and kidney disease in those with West African ancestry, and lower nephropathy risk in Ethiopians with HIV infection who lack APOL1 risk variants, show the powerful role of variation in this one gene on nondiabetic kidney disease.29
Fine et al30 evaluated renal pathology in 98 African American patients with HIV infection and nephropathy, based on APOL1 genotypes. The frequency of FSGS was significantly higher among those with two APOL1 risk variants, whereas immune complex–mediated glomerular diseases were more common in those without risk variants. Progression to ESKD was also significantly more common in those with two risk variants, versus zero or one.
SICKLE CELL DISEASE–ASSOCIATED NEPHROPATHY, PROGRESSIVE IGA NEPHROPATHY, AND LUPUS NEPHRITIS
Variations in APOL1 (and MYH9) recently was shown to underlie risk for sickle cell disease (hemoglobin SS)-associated nephropathy in African Americans.31 In addition, individuals with sickle cell trait (hemoglobin AS) do not face an increased risk of nephropathy, before or after adjustment for APOL1.32 A report in Han Chinese with progressive IgA nephropathy also detected MYH9 association.11
Our initial report in small numbers of African Americans with lupus nephritis (LN)-associated ESKD suggested that there was an MYH9/APOL1 association; however, we were unable to replicate this observation when additional patients with mild forms of LN were included.33 Lin et al34 subsequently reported MYH9 association with LN in EAs (and less strongly in the Gullah population), but no evidence of APOL1 or MYH9 association in African Americans, Asians, Amerindians, or Hispanics with non-ESKD LN.
To address the issue of severity of LN on genetic risk, a national sample of 668 African Americans with LN with ESKD was tested for chromosome 22q nephropathy risk variant associations. This study detected an OR of 2.35 (95% confidence interval 1.77-3.3; P = 4.25E−9) for APOL1 association with LN ESKD.35 Significant differences were not observed in the OR for association between the 456 African American cases with kidney biopsy-proven LN and the 212 African American cases without a kidney biopsy (whose physician attributed ESKD to LN). This suggests that severe LN with ESKD is associated with APOL1; leading to the evolving concept that APOL1 risk variants underlie progression of several forms of nephropathy to end stage, but may not necessarily initiate kidney disease. Table 2 includes a list of kidney diseases associated with variation in the chromosome 22q gene region.
Table 2.
Kidney Diseases Associated With APOL1 (and MYH9)
| Histopathology | Clinical Diagnosis | Population Group (s) | Comments |
|---|---|---|---|
| FSGS | Idiopathic FSGS | African and European | 1,2,9 |
| HIV-associated collapsing glomerulopathy |
HIV-associated nephropathy | African | 9 |
| Nondiabetic ESKD (likely FGGS and FSGS) |
Hypertension-attributed ESKD | African and Hispanic | Hispanics from New York City had significant African admixture1,2 |
| FGGS with arteriolar changes and interstitial fibrosis |
Hypertension-attributed nephropathy |
African | 1,2,17 |
| Sickle cell nephropathy | Sickle cell nephropathy | African | 31 |
| Lupus nephritis-associated ESKD |
Advanced lupus nephritis with ESKD |
African | 35 |
| Progressive IgA nephropathy | Advanced IgA nephropathy | Chinese | MYH9 association11 |
| Diabetic and nondiabetic CKD | Nondiabetic CKD (nonspecific glomerulosclerosis) |
European | MYH9 association4,5,10 |
Abbreviation: CKD, chronic kidney disease.
APOL1 ASSOCIATIONS WITH MILD NEPHROPATHY
Although African Americans have higher rates of severe kidney disease relative to European-derived populations,12 they do not appear to have excess rates of early nephropathy.36 This fact, along with the markedly weaker APOL1 association with milder forms of nephropathy, strengthens the hypothesis that APOL1 is a risk factor for nephropathy progression.
Friedman et al37 evaluated African Americans from the large population-based Dallas Heart Study with a mean age of 44.8 years (SD, 10.3 y). Although microalbuminuria and/or MDRD GFR less than 60 mL/min per 1.73 m2 were observed in 19.2% of nondiabetic subjects with two APOL1 risk alleles, relative to 6.7% of those with fewer than two risk variants, it is important to note that the mean GFR and urine albumin to creatinine ratio (UACR) were similar and within the normal range in participants from both genotype groups. Shriner et al38 also reported a weak association of the APOL1 G2 risk variant with eGFR in a population-based sample of African Americans residing in the Washington, DC, area; evidence of G1 association was not detected in these individuals lacking advanced nephropathy.
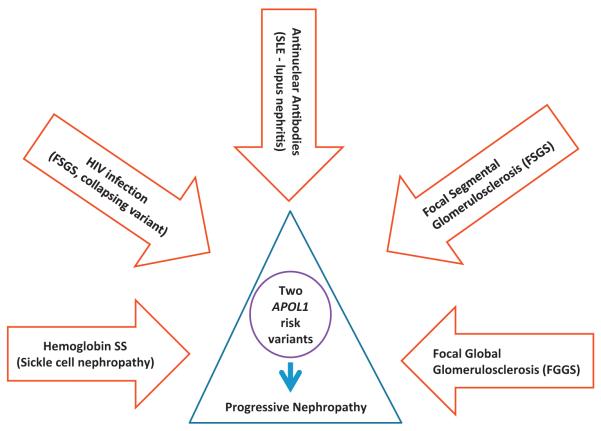
As in the Dallas Heart Study, we observed weaker APOL1 association with mild nephropathy in first-degree relatives of African Americans with nondiabetic forms of ESKD.8 Although high-risk relatives were enriched for APOL1 risk variants (23% had two risk variants and 46% had one risk variant, compared with 12% and 39% of the general African American population), MDRD GFR less than 60 mL/min per 1.73 m2 and/or UACR greater than 30 mg/g were present in 22.9% of those with two risk variants and in 21.1% of those with fewer than two risk variants. A major difference between our family based results and those in the Dallas Heart Study was seen in those with fewer than two APOL1 risk variants; lower frequencies of kidney disease were detected in the Dallas Heart Study compared with high-risk first-degree relatives of index cases with ESKD. Based on the robust evidence of APOL1 association with severe forms of nephropathy and weaker association with mild disease, we conclude that APOL1 likely contributes to more rapid progression from early stage kidney disease to ESKD (Fig. 1).
Figure 1.
The spectrum of APOL1-associated progressive nephropathy. SLE, systemic lupus erythematosus.
GENETIC PREDICTION OF OUTCOMES AFTER KIDNEY TRANSPLANTATION
APOL1 variation appears to underlie the poorer allograft survival rates in kidney transplants from deceased African American donors, relative to European Americans.39 As for the caveolin 1 and the drug transporter P-glycoprotein genes (encoded by the adenosine triphosphate-binding cassette, subfamily B, member 1 gene) on kidney allograft survival transplant outcomes, effects are associated with donor genotypes, not those of recipients.40,41
APOL1 risk variants were genotyped in 106 African American deceased kidney donors at one center and graft survival was assessed in 136 resulting transplants.39 Cox proportional hazard models were used to test for association between time to graft failure and donor genotypes. Sixteen percent of transplanted kidneys (22 of 136) were from high-risk donors with two APOL1 nephropathy risk variants. Overall, 25 grafts failed during follow-up evaluation, 32% (8 of 22) of them had two APOL1 risk variants. In a multivariate model accounting for donor APOL1 genotypes, genome-wide African ancestry, expanded criteria versus standard criteria donation, recipient age and sex, HLA mismatch, cold ischemia time (CIT), and panel reactive antibody titer, graft survival was significantly shorter in donor kidneys with two APOL1 risk variants (hazard ratio, 3.84; P = .008) and higher HLA mismatch (hazard ratio, 1.52; P = .03), but not overall African ancestry excluding APOL1. Kidneys from African American deceased donors that harbored two APOL1 risk variants failed far more rapidly after transplantation than those with zero or one risk variant.
This effect held true only for the genotypes of kidney donors. In a subsequent study, long-term effects on graft survival were not observed based on APOL1 genotypes of kidney transplant recipients.42 Although this result makes it tempting to speculate that an important pathophysiologic role exists for APOL1 gene expression in kidney cells (possibly in podocytes) leading to glomerulosclerosis, and that resulting kidney disease or allograft failure is less likely related to aberrant circulating ApoL1 protein or high-density lipoprotein (HDL) cholesterol, we do not believe that firm conclusions should be drawn from the kidney transplant model. Kidney transplants are impacted by the effects of prolonged CIT and nephrotoxin exposure including calcineurin inhibition. As such, subclinical nephropathy that may have been present at the time of organ harvesting may be accelerated to graft failure by CIT and nephrotoxin exposure.
It remains difficult to determine exactly what processes lead to subclinical kidney disease in donors; this remains an area of intensive investigation. It is not difficult to appreciate that some deceased individuals screened for kidney disease before organ donation could have undetected mild nephropathy. APOL1 association with nonproteinuric mild nephropathy in AASK participants and relatives of cases with non-diabetic ESKD show this phenomenon.8,17
As a result of this work, debate has arisen over whether to screen potential African American living kidney donors for APOL1. Cohen et al43 argued persuasively that this is the most ethical approach because kidney donors may face higher risk for subsequent ESKD if they donate half of their renal mass. In addition, recipients of these kidneys may not fare as well. It is true that African American live kidney donors have somewhat higher rates of ESKD than European ancestry donors.44 However, overall rates of ESKD postdonation remain low.45 This suggests that the current evaluation process works fairly well; but APOL1 genotyping may further inform patients, family members, and transplant physicians. We await replication of our initial finding in the deceased kidney donor population. If this finding is replicated, we would encourage APOL1 genotyping in potential African American living kidney donors, as well as in deceased African American kidney donors if it can be performed rapidly. As such, APOL1 genotypic data ultimately may transform the kidney donor evaluation process.
SECOND HITS AND MODIFYING FACTORS
All individuals inheriting two APOL1 risk variants will not develop nephropathy. For example, HIVAN develops in approximately 50% of untreated African Americans with HIV infection.9 A smaller percentage of non–HIV-infected individuals with two APOL1 risk variants are expected to develop kidney disease. Therefore, modifying factors such as environmental exposures or second gene interactions likely are involved in APOL1-associated nephropathy.46–48 We believe that different modifiers likely lead to the observed histologic findings in this disorder, from collapsing glomerulosclerosis seen with HIV infection to idiopathic forms of FSGS and FGGS, likely related to other modifying factors.14
Variants in the glomerulosclerosis-associated podocin gene (NPHS2), and other genes, could induce nondiabetic ESRD in concert with APOL1.49 Although such gene*gene interactions are likely, environmental exposures that interact with APOL1 potentially may be amenable to treatment and lead to novel prevention strategies.
We screened for the presence of viral infections with characteristics similar to HIV (eg, lymphotropism and persistent urinary tract/renal reservoirs of infection) that could interact with APOL1, as in HIVAN.50 Evidence of active infection with two lymphotropic viruses (Human Herpes Virus 6 and cytomegalovirus) and two viruses with urinary tract reservoirs (JC polyoma virus [JCV] and BK polyoma virus) were assessed in 300 first-degree relatives of African American index cases with nondiabetic ESKD, a population enriched for APOL1 risk variants. Although bloodstream infection with Human Herpes Virus 6 and cytomegalovirus were extremely rare, 30% and 9.7% of relatives, respectively, had detectable JCV and BK polyoma virus in the urine. Adjusting for familial age at nephropathy, sex, and ancestry, JCV genomic DNA in the urine and APOL1 risk alleles were associated negatively with renal phenotypes, including increased cystatin C (P = .006), UACR greater than 30 mg/g (P = .0002), and kidney disease defined as an eGFR less than 60 mL/min per 1.73 m2 and/or UACR greater than 30 mg/g (P = .000017) in an additive model. BK viruria was not associated with kidney disease. Hence, African Americans with two APOL1 risk variants and JC viruria had a lower prevalence of kidney disease, suggesting that JCV interacts with APOL1. Potential mechanisms might include inhibition of urinary tract replication by other more nephropathic viruses or impact on gene expression profiles that may alter susceptibility to APOL1-associated nephropathy. This observation requires replication; however, it provides hope that modifiable or preventable viral infections may one day reduce the rates of nondiabetic nephropathy in individuals with two APOL1 risk variants.
DISEASE PATHOGENESIS
Mechanisms underlying the development of progressive nephropathy in individuals with two APOL1 risk variants remain unknown (Table 3). There appears to be a consensus developing that APOL1 is expressed in podocytes and ApoL1 protein is present in these cells.51–53 It is less clear whether the gene is expressed in renal tubular cells, glomerular endothelial cells, and intimal and medial cells of small intrarenal blood vessels. Only a few small studies have been performed to date and results are equivocal. Differences in gene expression based on APOL1 genotypes have not been observed. Several cell lines have been transfected with wild type, G1, and G2 APOL1 transcripts. In general, cells transfected with nephropathy risk variants appeared to have higher rates of autophagic cell death and shorter survival,54,55 as initially postulated in the landmark study by Genovese et al.1
Table 3.
Potential Mechanisms Underlying APOL1-Associated Nephropathy
| Potential Mechanism | Primary Site (Effect) | Additional Sites |
|---|---|---|
| Expression in the kidney | Podocyte (? autophagy) | Glomerular endothelium and tubules |
| Expression in small arterioles | Media | Intima |
| Altered circulating ApoL1 protein |
Glomerular filtration, subsequent proximal tubule reabsorption |
Direct effects on glomerular endothelium |
| Altered HDL cholesterol | Effects on glomerular and vascular endothelium | — |
Another potentially important observation is that ApoL1 protein is detectable in renal tubular and vascular cells, although it may not be expressed in those cells.52 If the protein is not produced within these cells, circulating and/or filtered ApoL1 protein may be taken up by these cells with potentially toxic consequences. Again, it will be important to detect genotype-specific effects.
ApoL1 protein travels in the circulation in association with HDL cholesterol. Nondiabetic nephropathies include arteriolar changes that are consistent with vascular damage. Hence, it is conceivable that altered properties of HDL cholesterol based on ApoL1 content could lead to small-vessel and glomerular endothelial cell injury. APOL1 genotype impacts HDL particle concentration; as a result, HDL could play a role in glomerulosclerosis and renal microvascular injury.23 APOL1 genotypes also were associated with race-specific relationships between HDL cholesterol concentration and kidney function.56 Higher HDL level was associated with higher eGFR in Han Chinese and European American populations, whereas an inverse association between HDL and eGFR was seen in African Americans and West Africans. The effect was significant only in African Americans who had APOL1 risk genotypes (not in West Africans). The authors concluded that the disease mechanisms underlying APOL1-associated nephropathy could involve HDL cholesterol.
RESPONSE TO TREATMENT
APOL1-associated nephropathies are often severe, lead to rapid progression of chronic kidney disease, and present with earlier ages at onset of ESRD.57,58 Although little is known about response rates to standard therapies for FSGS, Kopp et al9 reported that after 8 or more weeks of steroid therapy, African American patients with FSGS and two APOL1 risk variants had a 29% (12 of 42) response rate, relative to 33% (5 of 15) of patients with 0/1 risk variants (P > .5). FSGS associated with other Mendelian genetic mutations, including the recessively inherited podocin gene (NPHS2), typically are resistant to glucocorticoid therapy.59
The FSGS Controlled Trial randomized children and young adults with glucocorticoid-resistant FSGS to either 1 year of cyclosporine plus low-dose daily steroids or mycophenolate pulse oral dexamethasone and low-dose daily steroids.60 Only 32 study subjects were African American (of those, 23 [72%] had two APOL1 risk alleles); an additional four study subjects had two APOL1 risk alleles (two were Hispanic). Subjects with two APOL1 risk alleles had a lower eGFR, a higher frequency of severe lesions (collapsing FSGS, cortical atrophy/fibrosis, arteriosclerosis), and similar levels of proteinuria relative to those with fewer than two risk alleles. Although remission rates to the cyclosporine and mycophenolate-based regimens were not significantly different based on genotypes in this small series, the risk for progression to ESKD during follow-up evaluation was significantly higher (by three-fold) in those with two APOL1 risk variants versus those with fewer than two risk variants.60 Additional trials evaluating responses to existing and novel treatments for APOL1-associated nephropathy are required.
CONCLUSIONS
Much of the existing epidemiology regarding non-diabetic ESKD in African Americans was based on the assumption that mild to moderate essential hypertension commonly initiated nephropathy. Instead, it is now clear that a spectrum of APOL1-associated proteinuric and nonproteinuric disorders manifesting as FSGS and FGGS with interstitial and vascular changes exists in those previously labeled with hypertension-attributed nephropathy. These disorders relate to selection for trypanolytic gene variants protective of African sleeping sickness. Risk variants appear to predispose to severe kidney disease from a variety of nondiabetic systemic and renal-limited diseases. As such, it appears likely that they promote progression of nondiabetic forms of nephropathy to ESKD. The mechanisms whereby APOL1 accelerates nephropathy progression remain to be determined; however, modifying genetic and environmental triggers appear necessary and different modifying factors likely determine the final histopathology (eg, collapsing FSGS, nonspecific FSGS, or FGGS). This molecular genetics breakthrough is likely to lead to novel treatments for FSGS and related nondiabetic renal disorders in those with African ancestry. Novel therapies will be critical for the nephrology community because nondiabetic nephropathies in African Americans previously have proven relatively refractory to existing treatments.
Footnotes
Financial disclosure and conflict of interest statements: none.
REFERENCES
- 1.Genovese G, Friedman DJ, Ross MD, Lecordier L, Uzureau P, Freedman BI, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329:841–5. doi: 10.1126/science.1193032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tzur S, Rosset S, Shemer R, Yudkovsky G, Selig S, Tarekegn A, et al. Missense mutations in the APOL1 gene are highly associated with end stage kidney disease risk previously attributed to the MYH9 gene. Hum Genet. 2010;128:345–50. doi: 10.1007/s00439-010-0861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman BI, Kopp JB, Langefeld CD, Genovese G, Friedman DJ, Nelson GW, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21:1422–6. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Seaghdha CM, Parekh RS, Hwang SJ, Li M, Kottgen A, Coresh J, et al. The MYH9/APOL1 region and chronic kidney disease in European-Americans. Hum Mol Genet. 2011;20:2450–6. doi: 10.1093/hmg/ddr118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooke JN, Bostrom MA, Hicks PJ, Ng MC, Hellwege JN, Comeau ME, et al. Polymorphisms in MYH9 are associated with diabetic nephropathy in European Americans. Nephrol Dial Transplant. 2012;27:1505–11. doi: 10.1093/ndt/gfr522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopp JB, Smith MW, Nelson GW, Johnson RC, Freedman BI, Bowden DW, et al. MYH9 is a major-effect risk gene for focal segmental glomerulosclerosis. Nat Genet. 2008;40:1175–84. doi: 10.1038/ng.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kao WH, Klag MJ, Meoni LA, Reich D, Berthier-Schaad Y, Li M, et al. MYH9 is associated with nondiabetic end-stage renal disease in African Americans. Nat Genet. 2008;40:1185–92. doi: 10.1038/ng.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Freedman BI, Langefeld CD, Turner J, Nunez M, High KP, Spainhour M, et al. Association of APOL1 variants with mild kidney disease in the first-degree relatives of African American patients with non-diabetic end-stage renal disease. Kidney Int. 2012;82:805–11. doi: 10.1038/ki.2012.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kopp JB, Nelson GW, Sampath K, Johnson RC, Genovese G, An P, et al. APOL1 genetic variants in focal segmental glomerulosclerosis and HIV-associated nephropathy. J Am Soc Nephrol. 2011;22:2129–37. doi: 10.1681/ASN.2011040388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pattaro C, Aulchenko YS, Isaacs A, Vitart V, Hayward C, Franklin CS, et al. Genome-wide linkage analysis of serum creatinine in three isolated European populations. Kidney Int. 2009;76:297–306. doi: 10.1038/ki.2009.135. [DOI] [PubMed] [Google Scholar]
- 11.Cheng W, Zhou X, Zhu L, Shi S, Lv J, Liu L, et al. Polymorphisms in the nonmuscle myosin heavy chain 9 gene (MYH9) are associated with the progression of IgA nephropathy in Chinese. Nephrol Dial Transplant. 2011;26:2544–9. doi: 10.1093/ndt/gfq768. [DOI] [PubMed] [Google Scholar]
- 12.US Renal Data System . US Renal Data System, USRDS 2011 annual data report: atlas of end-stage renal disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2011. [Google Scholar]
- 13.Schelling JR, Zarif L, Sehgal A, Iyengar S, Sedor JR. Genetic susceptibility to end-stage renal disease. Curr Opin Nephrol Hypertens. 1999;8:465–72. doi: 10.1097/00041552-199907000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Freedman BI, Bowden DW, Rich SS. Genetic basis of kidney disease. In: Taal MW, editor. Brenner & Rector’s the kidney. 9th ed Elsevier Saunders; Philadelphia: 2012. pp. 1554–69. [Google Scholar]
- 15.Freedman BI, Hicks PJ, Bostrom MA, Cunningham ME, Liu Y, Divers J, et al. Polymorphisms in the non-muscle myosin heavy chain 9 gene (MYH9) are strongly associated with end-stage renal disease historically attributed to hypertension in African Americans. Kidney Int. 2009;75:736–45. doi: 10.1038/ki.2008.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freedman BI, Langefeld CD. The new era of APOL1-associated glomerulosclerosis. Nephrol Dial Transplant. 2012;27:1288–91. doi: 10.1093/ndt/gfr812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipkowitz MS, Freedman BI, Langefeld CD, Comeau ME, Bowden DW, Kao WH, et al. Apolipoprotein L1 gene variants associate with hypertension-attributed nephropathy and the rate of kidney function decline in African Americans. Kidney Int. 2013;83:114–20. doi: 10.1038/ki.2012.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appel LJ, Wright JT, Jr, Greene T, Kusek JW, Lewis JB, Wang X, et al. for the African American Study of Kidney Disease and Hypertension Collaborative Research Group Long-term effects of renin-angiotensin system-blocking therapy and a low blood pressure goal on progression of hypertensive chronic kidney disease in African Americans. Arch Intern Med. 2008;168:832–9. doi: 10.1001/archinte.168.8.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright JT, Jr, Bakris G, Greene T, Agodoa LY, Appel LJ, Charleston J, et al. Effect of blood pressure lowering and antihypertensive drug class on progression of hypertensive kidney disease: results from the AASK trial. JAMA. 2002;288:2421–31. doi: 10.1001/jama.288.19.2421. [DOI] [PubMed] [Google Scholar]
- 20.Appel LJ, Anderson CA. Compelling evidence for public health action to reduce salt intake. N Engl J Med. 2010;362:650–2. doi: 10.1056/NEJMe0910352. [DOI] [PubMed] [Google Scholar]
- 21.Freedman BI, Murea M. Target organ damage in African American hypertension: role of APOL1. Curr Hypertens Rep. 2012;14:21–8. doi: 10.1007/s11906-011-0237-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogo A, Breyer JA, Smith MC, Cleveland WH, Agodoa L, Kirk KA, et al. Accuracy of the diagnosis of hypertensive nephrosclerosis in African Americans: a report from the African American Study of Kidney Disease (AASK) trial. AASK Pilot Study Investigators. Kidney Int. 1997;51:244–52. doi: 10.1038/ki.1997.29. [DOI] [PubMed] [Google Scholar]
- 23.Freedman BI, Langefeld CD, Murea M, Ma L, Otvos JD, Turner J, et al. Apolipoprotein L1 nephropathy risk variants associate with HDL subfraction concentration in African Americans. Nephrol Dial Transplant. 2011;26:3805–10. doi: 10.1093/ndt/gfr542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cravedi P, Ruggenenti P, Remuzzi G. Proteinuria should be used as a surrogate in CKD. Nat Rev Nephrol. 2012;8:301–6. doi: 10.1038/nrneph.2012.42. [DOI] [PubMed] [Google Scholar]
- 25.Abbott KC, Hypolite I, Welch PG, Agodoa LY. Human immunodeficiency virus/acquired immunodeficiency syndrome-associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival in the pre highly active antiretroviral therapy era. J Nephrol. 2001;14:377–83. [PubMed] [Google Scholar]
- 26.Rao TK, Filippone EJ, Nicastri AD, Landesman SH, Frank E, Chen CK, et al. Associated focal and segmental glomerulosclerosis in the acquired immunodeficiency syndrome. N Engl J Med. 1984;310:669–73. doi: 10.1056/NEJM198403153101101. [DOI] [PubMed] [Google Scholar]
- 27.Bourgoignie JJ. Acquired immunodeficiency syndrome (AIDS)–related renal disease. Klin Wochenschr. 1989;67:889–94. doi: 10.1007/BF01717345. [DOI] [PubMed] [Google Scholar]
- 28.Behar DM, Shlush LI, Maor C, Lorber M, Skorecki K. Absence of HIV-associated nephropathy in Ethiopians. Am J Kidney Dis. 2006;47:88–94. doi: 10.1053/j.ajkd.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Behar DM, Kedem E, Rosset S, Haileselassie Y, Tzur S, Kra-Oz Z, et al. Absence of APOL1 risk variants protects against HIV-associated nephropathy in the Ethiopian population. Am J Nephrol. 2011;34:452–9. doi: 10.1159/000332378. [DOI] [PubMed] [Google Scholar]
- 30.Fine DM, Wasser WG, Estrella MM, Atta MG, Kuperman M, Shemer R, et al. APOL1 risk variants predict histopathology and progression to ESRD in HIV-related kidney disease. J Am Soc Nephrol. 2012;23:343–50. doi: 10.1681/ASN.2011060562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashley-Koch AE, Okocha EC, Garrett ME, Soldano K, De Castro LM, Jonassaint JC, et al. MYH9 and APOL1 are both associated with sickle cell disease nephropathy. Br J Haematol. 2011;155:386–94. doi: 10.1111/j.1365-2141.2011.08832.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hicks PJ, Langefeld CD, Lu L, Bleyer AJ, Divers J, Nachman PH, et al. Sickle cell trait is not independently associated with susceptibility to end-stage renal disease in African Americans. Kidney Int. 2011;80:1339–43. doi: 10.1038/ki.2011.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freedman BI, Edberg JC, Comeau ME, Murea M, Bowden DW, Divers J, et al. The non-muscle myosin heavy chain 9 gene (MYH9) is not associated with lupus nephritis in African Americans. Am J Nephrol. 2010;32:66–72. doi: 10.1159/000314688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin CP, Adrianto I, Lessard CJ, Kelly JA, Kaufman KM, Guthridge JM, et al. Role of MYH9 and APOL1 in African and non-African populations with lupus nephritis. Genes Immun. 2012;13:232–8. doi: 10.1038/gene.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freedman BI, Langefeld CD, Comeau ME, Hebert L, Segal MS, Edberg JC, et al. Apolipoprotein L1 risk variants associate with lupus nephritis-induced end-stage renal disease in African Americans [abstract] J Am Soc Nephrol. 2012;23:248A. [Google Scholar]
- 36.McClellan W, Warnock DG, McClure L, Campbell RC, Newsome BB, Howard V, et al. Racial differences in the prevalence of chronic kidney disease among participants in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Cohort Study. J Am Soc Nephrol. 2006;17:1710–5. doi: 10.1681/ASN.2005111200. [DOI] [PubMed] [Google Scholar]
- 37.Friedman DJ, Kozlitina J, Genovese G, Jog P, Pollak MR. Population-based risk assessment of APOL1 on renal disease. J Am Soc Nephrol. 2011;22:2098–105. doi: 10.1681/ASN.2011050519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shriner D, Herbert A, Doumatey AP, Zhou J, Huang H, Erdos MR, et al. Multiple loci associated with renal function in African Americans. PLoS One. 2012;7:e45112. doi: 10.1371/journal.pone.0045112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reeves-Daniel AM, Depalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. The APOL1 gene and allograft survival after kidney transplantation. Am J Transplant. 2011;11:1025–30. doi: 10.1111/j.1600-6143.2011.03513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore J, McKnight AJ, Simmonds MJ, Courtney AE, Hanvesakul R, Brand OJ, et al. Association of caveolin-1 gene polymorphism with kidney transplant fibrosis and allograft failure. JAMA. 2010;303:1282–7. doi: 10.1001/jama.2010.356. [DOI] [PubMed] [Google Scholar]
- 41.Moore J, McKnight AJ, Dohler B, Simmonds MJ, Courtney AE, Brand OJ, et al. Donor ABCB1 variant associates with increased risk for kidney allograft failure. J Am Soc Nephrol. 2012;23:1891–9. doi: 10.1681/ASN.2012030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee BT, Kumar V, Williams TA, Abdi R, Bernhardy A, Dyer C, et al. The APOL1 genotype of African American kidney transplant recipients does not impact 5-year allograft survival. Am J Transplant. 2012;12:1924–8. doi: 10.1111/j.1600-6143.2012.04033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen DM, Mittalhenkle A, Scott DL, Young CJ, Norman DJ. African American living-kidney donors should be screened for APOL1 risk alleles. Transplantation. 2011;92:722–5. doi: 10.1097/TP.0b013e31822eec39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lentine KL, Schnitzler MA, Xiao H, Saab G, Salvalaggio PR, Axelrod D, et al. Racial variation in medical outcomes among living kidney donors. N Engl J Med. 2010;363:724–32. doi: 10.1056/NEJMoa1000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaston RS, Young CJ. Living donor nephrectomy: understanding long-term risk in minority populations. Am J Transplant. 2010;10:2574–6. doi: 10.1111/j.1600-6143.2010.03324.x. [DOI] [PubMed] [Google Scholar]
- 46.Bostrom MA, Freedman BI. The spectrum of MYH9-associated nephropathy. Clin J Am Soc Nephrol. 2010;5:1107–13. doi: 10.2215/CJN.08721209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Atta MG, Estrella MM, Kuperman M, Foy MC, Fine DM, Racusen LC, et al. HIV-associated nephropathy patients with and without apolipoprotein L1 gene variants have similar clinical and pathological characteristics. Kidney Int. 2012;82:338–43. doi: 10.1038/ki.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hays T, Wyatt CM. APOL1 variants in HIV-associated nephropathy: just one piece of the puzzle. Kidney Int. 2012;82:259–60. doi: 10.1038/ki.2012.129. [DOI] [PubMed] [Google Scholar]
- 49.Bostrom MA, Kao WH, Li M, Abboud HE, Adler SG, Iyengar SK, et al. Genetic association and gene-gene interaction analyses in African American dialysis patients with nondiabetic nephropathy. Am J Kidney Dis. 2012;59:210–21. doi: 10.1053/j.ajkd.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freedman BI, Murea M, Rocco MV, Ma L, Bowden DW, Ornelles DA, et al. JC polyoma virus interacts with apolipoprotein L1 genetic risk in African Americans with non-diabetic nephropathy [abstract] J Am Soc Nephrol. 2012;23:178A. [Google Scholar]
- 51.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR. APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol. 2011;22:2119–28. doi: 10.1681/ASN.2011010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O’Toole JF, Madhavan SM, Konieczkowski M, Ganesan S, Barisoni L, Thomas DB, et al. apol1 expression and localization pattern in kidney diseases with and without association to risk genotype [abstract] J Am Soc Nephrol. 2012;23:323A. [Google Scholar]
- 53.Wakashin H, Kopp JB. ApolipoproteinL1-B splice variant is expressed by podocytes in vitro and in vivo and shows both nuclear and cytoplasmic distribution [abstract] J Am Soc Nephrol. 2012;23:819A. [Google Scholar]
- 54.Chen Z, Freedman BI, Graham WD, Ross NA, Pollak MR, Ross MD, et al. Dose-dependent expression of apol1 protein sensitizes cell death pathways [abstract] J Am Soc Nephrol. 2012;23:136A. [Google Scholar]
- 55.Dummer PD, Hickman A, Espositio D, Winkler CA, Pisitkun T, Kopp JB. Apolipoprotein-L1 kidney risk variants demonstrate differential intracellular retention and altered cell biological pathways [abstract] J Am Soc Nephrol. 2012;23:249A. [Google Scholar]
- 56.Bentley AR, Doumatey AP, Chen G, Huang H, Zhou J, Shriner D, et al. Variation in APOL1 contributes to ancestry-level differences in HDLc-kidney function association. Int J Nephrol. 2012;2012:748984. doi: 10.1155/2012/748984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kanji Z, Powe CE, Wenger JB, Huang C, Ankers E, Sullivan DA, et al. Genetic variation in APOL1 associates with younger age at hemodialysis initiation. J Am Soc Nephrol. 2011;22:2091–7. doi: 10.1681/ASN.2010121234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzur S, Rosset S, Skorecki K, Wasser WG. APOL1 allelic variants are associated with lower age of dialysis initiation and thereby increased dialysis vintage in African and Hispanic Americans with non-diabetic end-stage kidney disease. Nephrol Dial Transplant. 2012;27:1498–505. doi: 10.1093/ndt/gfr796. [DOI] [PubMed] [Google Scholar]
- 59.Ruf RG, Lichtenberger A, Karle SM, Haas JP, Anacleto FE, Schultheiss M, et al. Patients with mutations in NPHS2 (podocin) do not respond to standard steroid treatment of nephrotic syndrome. J Am Soc Nephrol. 2004;15:722–32. doi: 10.1097/01.asn.0000113552.59155.72. [DOI] [PubMed] [Google Scholar]
- 60.Kopp JB, Zhao X, Winkler CA, Woroniecki RP, Radeva M, Gassman J, et al. APOL1 variant-associated FSGS is more aggressive but manifests similar response to cyclosporine and mycophenolate compared to other forms of primary FSGS [abstract] J Am Soc Nephrol. 2012;23:41A. [Google Scholar]