Abstract
Milk thistle (Silybum marianum) extracts, one of the most widely used dietary supplements, contain a mixture of six major flavonolignans (silybin A, silybin B, isosilybin A, isosilybin B, silychristin, and silydianin) and other components. However, the pharmacokinetics of the free individual flavonolignans have been only partially investigated in humans. Furthermore, antioxidant effects of the extract, which may underlie the basis of many therapeutic effects, have not been thoroughly assessed. The present study evaluated the pharmacokinetics of the six major flavonolignans in healthy volunteers receiving single doses of either one (175 mg), two (350 mg), or three (525 mg) milk thistle capsule(s) on three separate study visits. Additionally, the steady-state pharmacokinetic parameters were determined after the subjects were administered one capsule three times daily for 28 consecutive days. Our results demonstrated that all six flavonolignans were rapidly absorbed and eliminated. In order of abundance, the exposure to free flavonolignans was greatest for silybin A followed by silybin B, isosilybin B, isosilybin A, silychristin, and silydianin. The systemic exposure to these compounds appeared linear and dose proportional. The disposition of flavonolignans was stereoselective, as evidenced by the apparent clearance of silybin B, which was significantly greater than silybin A, whereas the apparent clearance of isosilybin B was significantly lower than isosilybin A. The concentrations of urinary 8-epi-prostaglandin F2α, a commonly used biomarker of oxidative status in humans, were considerably decreased in study subjects after a 28-day exposure to the extract (1.3 ± 0.9 versus 0.8 ± 0.9 ng/mg creatinine) but failed to reach statistical significance (P = 0.076).
Introduction
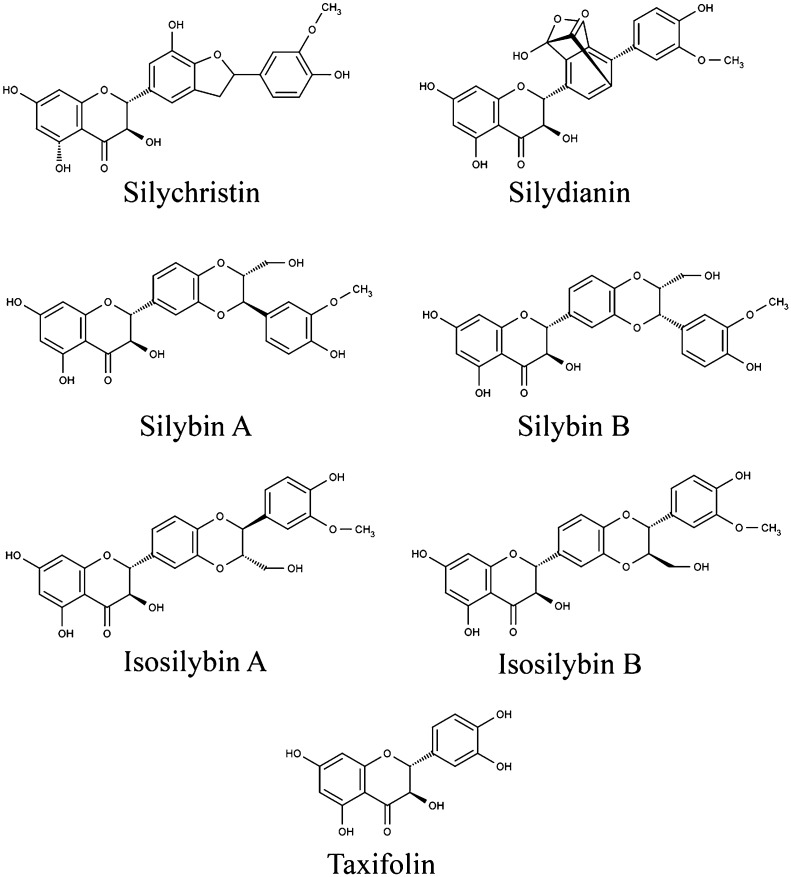
Milk thistle (Silybum marianum [L.] Gaertn. [Asteraceae]; synonym Carduus marianus L.) is an annual or biennial plant native to the Mediterranean and North African regions. Its achenes, the small, dry, indehiscent, one-seeded fruit, have long been sought after because of their many purported medicinal attributes (Kroll et al., 2007; Loguercio and Festi, 2011). The crude extract obtained from crushed achenes, termed silymarin, contains a complex mixture of constituents, typically including 65–80% total flavonolignans silybin A and silybin B, isosilybin A, isosilybin B, silychristin A, silychristin B, and silydianin (Fig. 1). Additionally, flavonoids (e.g., taxifolin and quercetin) are present. These constituents, both collectively and individually, are believed to confer a variety of pharmacological activities to the silymarin preparation (Kren and Walterova, 2005; Kroll et al., 2007). Beyond the flavonolignans and aforementioned flavonoids, the balance of the silymarin extract largely consists of various reduced and oxidized polyphenols as well as fatty acids (Javed et al., 2011).
Fig. 1.
Chemical structures of silybin A, silybin B, isosilybin A, isosilybin B, silychristin, silydianin, and taxifolin.
Silymarin exhibits potent antioxidant properties based on substantial in vitro, animal, and ex vivo literature. However, cautious interpretation must be taken in evaluating such studies given that the specific mixtures of constituents or isolated components/isomers of silymarin may differ from study to study and, perhaps more importantly, the natural product concentrations used may not be uniform (Ladas and Kelly, 2003; Kroll et al., 2007).
Oxidative stress/damage has been linked with a very wide range of chronic human diseases, including various cancers, cardiovascular disease, and hepatic disease (Jain et al., 2002). The potential hepatoprotective effects of silymarin and its purported utility in treating hepatitis, cirrhosis, and alcoholic liver disease and as an antidote to acute poisoning due to ingestion of the Amanita phalloides mushroom have been reported, although the mechanistic basis of the purported benefits is not fully understood (Ladas and Kelly, 2003; Kren and Walterova, 2005; Kroll et al., 2007; Javed et al., 2011; Loguercio and Festi, 2011). Furthermore, silymarin or one or more of its constituents is believed to produce anti-inflammatory, immunomodulatory, and favorable effects on lipid profiles and biliary function. There is also in vitro evidence of antiviral and antitumor therapeutic properties (Jain et al., 2002; Ladas and Kelly, 2003; Kren and Walterova, 2005; Kroll et al., 2007; Loguercio and Festi, 2011). Taxifolin, a flavanolol that is also present in silymarin extract, is thought to play a role in the antitumor activity of this mixture and has been reported to inhibit cancer cell growth (Kroll et al., 2007).
Significant pharmacokinetic variability among the many commercially available milk thistle extracts has been documented, and the bioavailability of most oral formulations is generally regarded as poor and highly variable (Schrieber et al., 2008; Wen et al., 2008; Hawke et al., 2010; Schrieber et al., 2011). Major reasons cited for the low bioavailability of silymarin constituents include extensive presystemic conjugative metabolism, poor permeability across intestinal epithelial cells, and low aqueous solubility (Javed et al., 2011). Thus, the clinical pharmacokinetic assessments of silymarin extracts have not been well defined in view of the nonstandardization of extracts assessed, as well as differing study methodologies and analytical approach. The present study explores the pharmacokinetics of the major flavonolignans of a widely studied standardized silymarin extract at escalating dosages and under steady-state conditions.
Considerable research efforts have sought to identify reliable biomarkers of the oxidative/antioxidant status of the human body. These efforts have generally fallen in to one of two categories: measurement of oxidative damage and measurement of antioxidant protection (Pratico, 1999; Del Rio et al., 2002; Pratico et al., 2004). One such noninvasive measure of oxidative status is the measurement of urinary F2-isoprostane, 8-epi-prostaglandin F2α (8-epi-PGF2α), a marker of in vivo lipid peroxidation, which has recently been applied to assessing oxidative stress in chronic hepatitis C cirrhosis (Jain et al., 2002). A number of studies have shown the isoprostanes to be accurate markers of lipid peroxidation and have been shown to increase significantly during experimentally induced liver damage (Fam and Morrow, 2003). Because silymarin extracts have been shown to exert antioxidant activities in vitro and have purported hepatoprotective effects, a secondary goal of the present study includes a preliminary assessment of human subject urinary 8-epi-PGF2α at baseline relative to that at the end of a 28-day steady-state supplementation with milk thistle extract.
Materials and Methods
Chemicals and Reagents.
Milk thistle capsules (175 mg dried extract equivalent to 140 mg silymarin: Legalon 140) were generously donated by MADAUS GmbH (Cologne, Germany) and are described in further detail below. A certificate of analysis unique to the product lot number (#B0601214) used in the study was likewise provided by MADAUS GmbH. Authentic analytical reference standards of taxifolin, silychristin, and silydianin were obtained from ChromaDexTM (Santa Ana, CA), and silybin A, silybin B, isosilybin A, and isosilybin B were sourced from Phytolab GmbH & Co. (Vestenbergsgreuth, Germany). The internal standard (IS) naringenin was purchased from SAFC Supply Solutions (St. Louis, MO). 3,3,4,4-[2H]-8-epiprostaglandin F2α and the 8-epi-PGF2α immunoaffinity column were from Cayman Chemicals (Ann Arbor, MI). Liquid chromatography-mass spectrometry grade methanol, ammonium acetate, formic acid, and ethyl acetate were all purchased from Sigma-Aldrich (St. Louis, MO).
Choice and Characterization of Milk Thistle Preparation.
Historically, for clinical efficacy trials of botanical extracts, there has often been a lack of preclinical and preliminary clinical studies assessing tolerability, pharmacokinetics, and pharmacodynamics of a specific silymarin extract. In 2005, the U.S. National Center for Complementary and Alternative Medicine issued a Notice of Opportunity for Clinical Trial Collaboration to identify manufacturers of silymarin that would be interested in providing product for future clinical trials of silymarin extracts. After a review of responding manufacturers' submitted product information inclusive of extract chemistry, manufacturing and quality control data, and finally preclinical and clinical data, the proprietary brand of silymarin known as Legalon 140 (Madaus GmbH) was selected for use in both preclinical and clinical silymarin assessments (Reddy et al., 2012).
Legalon 140 is a milk thistle fruit extract [Silybum marianum (L) Gaertn. (Asteraceae)] standardized to 140 mg of silymarin per gelatin capsule (53% as total silybin, quantified photometrically). Standardized Legalon 140 capsules each contain 180 mg of dried extract of milk thistle achenes, or 140 mg of silymarin, which is the presumed active ingredient. We analyzed the capsules for the contents of the biologically active constituents utilizing a stereoselective high-performance liquid chromatographic–tandem mass spectrometric assay recently established in our laboratory (Brinda et al., 2012). The results showed that each Legalon 140 capsule contained the following major active components: silybin A (21.2 mg), silybin B (29.5 mg), isosilybin A (11.4 mg), isosilybin B (8.2 mg), silychristin (31.5 mg), silydianin (36.4 mg), and taxifolin (5.9 mg).
Human Subjects.
Fourteen healthy research volunteers provided written informed consent approved by the Medical University of South Carolina’s Office of Research Integrity (Charleston, SC). All were determined to be healthy by medical history and physical examination performed by the study physician. Furthermore, a satisfactory evaluation of baseline serum chemistries, complete blood counts, 12-lead electrocardiogram, and urinalysis were used to establish health status. Additionally, a urine drug screen, nicotine/cotinine screen, and urine pregnancy test (women) was obtained in each subject and preceded study participation. All participants were nonsmokers, not taking prescription or over-the-counter medications or botanical or dietary supplements (inclusive of vitamins). Additionally, participants were requested to abstain from grapefruit juice, caffeine-containing beverages, and ethanol use 2 weeks prior to and during the study period. Subjects were also asked to refrain from consumption of artichokes or artichoke-containing foods, as these foodstuffs are known to contain a constituent common to many silymarin extracts, taxifolin.
Study Design and Milk Thistle Dosing.
After an overnight fast, subjects arrived at the General Clinical Research Center (GCRC) of the Medical University of South Carolina at 7:00AM the morning of each of four separate blood drawing phases of the study. An indwelling venous catheter was placed in each subject’s arm to facilitate serial blood sampling. Just prior to 8:00AM, and after urinary void and collection of an aliquot for later creatinine analysis, subjects were provided either one (175 mg), two (350 mg), or three (525 mg) capsule(s), respectively, of silymarin extract (Legalon). This single-dose assessment of silymarin pharmacokinetics was an attempt to span typical ranges of recommended dosing and that often employed in formal clinical study. The capsules were administered with 240 ml of room temperature water, consumed in its entirety. Subjects remained in a fasted state for 4 hours after extract administration to minimize any influence of food on absorption. Standard meals were later provided by a registered dietician in the GCRC and did not include any sources of potentially interfering flavonoids. The composition and amounts of food eaten throughout the day were recorded. These pharmacokinetic assessments were performed on three distinct occasions separated by no less than a 7-day wash-out period. A total of 12 blood samples (10 ml each) were collected over the active study period at 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 10, 12, and 24 hours. The 24-hour blood samples were obtained via a separate venipuncture during a brief visit the following day. All samples were drawn in heparinized blood collection tubes (BD Vacutainer; Becton Dickinson, Franklin Lakes, NJ) and immediately placed on ice until centrifugation at 4°C within 15 minutes of sample collection. After centrifugation, plasma was transferred into plastic vials, acidified with 10 μl of 1 M acetic acid/ml plasma, and immediately stored at −70°C until analysis. After the last single-dose assessment and an ensuing 7-day (minimum) wash-out period, subjects initiated a 28-day exposure to the silymarin extract at the generally recommended and clinically studied dosage of one capsule three times daily (8:00AM, 1:00PM, and 8:00PM). After the 28 days of silymarin exposure, subjects returned to the GCRC for the determination of steady-state concentrations of silymarins in plasma. A blood sample was obtained immediately prior to dosing with the morning dose of extract (i.e., Ctrough), and 0.5, 1.0, 2.0, 3.0, 4.0, and 8.0 hour time points after silymarin dosing. A second silymarin dose, corresponding with the three times daily dosing regimen each subject was asked to maintain the previous 28 days, was administered at 1:00PM. A 4-week exposure was selected to assure the presumed steady state was reached and to significantly extend the length of exposure over most existing pharmacokinetic assessments of silymarin extracts in healthy human subjects, which generally have been only brief in duration. In addition, the extended duration of exposure permitted the gathering of pilot data assessing the antioxidant as measured via the measurement of urinary isoprostanes and creatinine pre- and post-silymarin exposure.
Side Effect Monitoring.
A modified treatment emergent side effect scale (TESS) (NIH, 1985) was administered by the research nurse after placement of the indwelling venous catheter but prior to administration of silymarin extracts. A second TESS was administered to all subjects 2 hours postdosing, a time expected to approximate the maximum blood concentration (Cmax) of silymarin per existing literature values, and ostensibly, a greater likelihood of any associated side effects. Subjects in the 28-day exposure phase of the study reported to the outpatient GCRC on four separate occasions to pick up a 7-day supply of silymarin extracts, at which time an additional TESS was administered.
Analysis of Silymarin Flavonolignan.
A novel high-performance liquid chromatographic-tandem mass spectrometric assay was recently developed in our laboratory for the simultaneous analysis of the free (nonconjugated) flavonolignans silybin A, silybin B, isosilybin A, isosilybin B, silychristin, silydianin, and the flavonoid taxifolin in human plasma (Brinda et al., 2012). In brief, to 1 ml of plasma, 20 ng/ml of the IS naringenin and 2 ml of ethyl acetate containing 0.1% formic acid were added. The samples were then shaken at 200 cycles/min for 10 minutes and centrifuged at 3000 rpm for 10 minutes at room temperature. The organic phase was then transferred to clean glass tubes. This extraction was then repeated and produced approximately 4 ml of organic solvent per sample. This sample volume was evaporated to dryness under a stream of nitrogen at 40°C, and the remaining residue was reconstituted with 100 µl of mobile phase, 40 µl of which were injected for analysis.
Measurement of individual silymarin constituents was achieved by running samples through a C18 guard column (4 mm × 20 mm, SecurityGuard, Torrance, CA) before separation on a Phenomenex Luna 5u C18 column (100 A 250 × 2 mm, 5 µm, Torrance, CA). The mobile phase consisted of 51% methanol and 49% water containing 0.1% formic acid and 10 mM ammonium acetate, and was delivered at a low rate of 0.25 ml/min. The MS was operated in negative ion mode using turbo electrospray ionization. The MS tuning parameters were optimized for each analyte by infusing 0.1 µg/ml of each compound dissolved in mobile phase at a low rate of 20 µl/min. The following parameters were used for the MS analysis: curtain gas, 8 psi; nebulizer gas (gas 1), 12 psi; CAD gas, 6 psi; TurboIonSpray voltage, −4500 V; entrance potential, −10 V; collision cell exit potential, −7 V; declustering potential, −71 V; collision energy, 40 eV for m/z: 481 > 125, 26 eV for m/z: 271 > 151, 30 eV for m/z: 303 > 125; source temperature, 400 ◦C; and dwell time, 250 ms. The following transitions were monitored in the Multiple Reaction Monitoring mode: taxifolin, m/z 303 > 125; silychristin, silydianin, silybin A, silybin B, isosilybin A, and isosilybin B, m/z 481 > 125; naringenin (IS), m/z 271 > 151. Data were acquired and analyzed by AB Sciex Analyst software, version 1.4.2 (AB Sciex, Toronto, Canada). The lower limit of quantification was 2 ng/ml for each constituent. Calibration curves were linear over the range of 2 to 100 ng/ml for all analytes (r2 > 0.99). The intra- and interday accuracies were 91.0–106.5% and 95.1–111.9%, respectively. The intra- and interday precision was within 10.5%.
Urinary 8-Epi-PGF2α Analysis Using Immunoaffinity Extraction-Gas Chromatography-Negative Ion Chemical Ionization-Mass Spectrometry.
A modification of the immunoaffinity extraction-gas chromatography-negative ion chemical ionization-mass spectrometry method of Tsikas and associates (2003) was used. Urine (1 ml) was fortified with 3,3,4,4-[2H4]-8-epi- prostaglandin F2α (1 ng in 10 µl methyl acetate), centrifuged (5 minutes at 500g), then applied to a commercial 8-epi-PGF2α immunoaffinity column. After being washed with eicosanoid affinity column buffer, followed by water (2 ml), the 8-epi-PGF2α isomer was eluted with 95% ethanol (2 ml). The sample was dried under nitrogen, and the analyte was transferred to a silanized microvial insert in 50 µl ethanol. After drying under nitrogen, the analyte was esterified using 10% pentafluorobenzylbromide in acetonitrile (40 µl) and 10% diisopropylethylamine in acetonitrile (20 µl). The sample was subject to heating (40°C for 20 minutes) and then evaporated under a stream of nitrogen. Silyl ethers were formed by treating the residue with bis(trimethylsilyl)trifluoroacetamide (50 µl) at 60°C for 15 minutes. The derivatized sample was then analyzed by GC-NICI-MS using an Agilent model 5973N instrument. GC separations were achieved using a 30 m × 0.25 PM film 5% phenylmethylpolysiloxane column (DB-5MS; J&W Scientific, Folsom, CA) with a helium linear velocity of 50 cm/min). The column was held at 190°C for 2 minutes after injection (2 µl; pulsed splitless), then ramped to 300°C at 20°C min−1 and held for 6 minutes. Methane was used as the reagent gas. With selected ion monitoring, the debenzylated fragment ions m/z 569 for the analyte and m/z 573 for the tetradeuterated internal standard were detected at 8.10 and 8.02 minutes, respectively, after injection. Urinary 8-epi-PGF2α concentrations were standardized to milligrams of urinary creatinine. Urine creatinine concentrations were determined by colorimetric assay utilizing a creatinine (urinary) assay kit (Cayman Chemical Co., Ann Arbor, MI).
Pharmacokinetic Analysis.
Plasma samples were obtained from 13 healthy volunteers participating in a dose-escalation study after receiving single oral doses of 175, 350, and 525 mg of standardized milk thistle extract, respectively, on three separate occasions. Silymarin determinations were made via LC-MS/MS assay as described above. Additionally, determinations of steady-state concentrations of flavonolignans after 28 days of exposure to 175 mg three times daily were assessed. Pharmacokinetic parameters of individual silymarin flavonolignans were estimated by a noncompartmental analysis using WinNonlin 5.3 (Pharsight, Mountain View, CA). The maximum plasma concentration (Cmax) and time to maximum plasma concentration (Tmax) were obtained directly from the plasma concentration-time data. The terminal elimination rate constant (λz) was estimated by linear least-squares regression of the terminal portion of the plasma concentration-time curve, and the corresponding elimination half-live (t1/2) was then calculated using the formula t1/2 = 0.693/λz. The area under the plasma concentration-time curve from time 0 to infinity (AUC0→∞) for single-dose assessments and AUC 0→8h for steady-state evaluations were calculated according to the linear trapezoidal rule. The apparent clearance (CL/F) was calculated using the formula dose/AUC0→∞. The apparent volume distribution (V/F) was estimated by dividing CL/F by λz.
Statistical Analysis.
Results are presented as median (range) for Tmax and mean ± S.D. for other data. The concentrations of urinary 8-epi-PGF2a after a 28-day exposure to silymarin extract were compared with the baseline concentrations using the paired t test. The differences are considered statistically significant when the P values are less than 0.05.
Results
Safety Assessments.
Thirteen research volunteers (8 men and 5 women) aged 23 to 44 years (mean ± S.D., 29 ± 5.5 years; weight, 71.6 ± 18.3 kg) completed the study. One subject was discontinued from the study because of a protocol violation. No unexpected adverse events occurred that were attributable to exposure to the milk thistle extract or any study procedure. Specifically, there were no indicators on the modified TESS forms of side effects or adverse reactions clearly associated with exposure to the silymarin extract at any dosage level in the single dose assessments or during the 28-day exposure.
Pharmacokinetic Parameters of Silymarin Flavonolignans in Human Plasma.
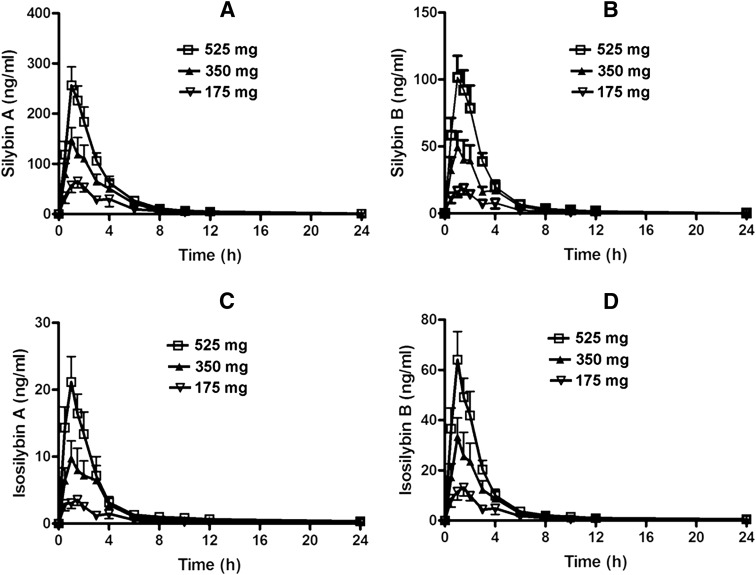
Pharmacokinetic analysis indicated that after oral administration of the standardized milk thistle extract Legalon, flavonolignans were rapidly absorbed and eliminated. Pharmacokinetic parameters for the single-dose escalation assessment are provided in Table 1, whereas results from the 28-day steady-state assessment are provided in Table 2. In order of abundance, exposure to silymarin flavonolignans was greatest for silybin A followed by silybin B, isosilybin B, isosilybin A, silychristin, and silydianin. For the single-dose assessment at escalating doses of 175, 350, and 525 mg, there was evidence of dose proportionality as assessed by the observed mean Cmax and AUC0-24 in the circulating concentrations of the major flavonolignans as dosages were increased (Fig. 2). No significant differences of apparent clearance (CL/F) across the three dosage groups were observed for these flavonolignans. Thus, the systemic exposure to these compounds occurred in a linear and dose-proportional fashion after single oral doses of silymarin extract 175 to 525 mg. The mean ± S.D. Cmax values of free (unconjugated) silybin A were 106.9 ± 49.2, 200.5 ± 98., and 299.3 ± 101.7 ng/ml, respectively, approximately two to three times higher than those observed for silybin B, which were 30.5 ± 16.3, 74.5 ± 45.7, and 121.0 ± 52.2 ng/ml, respectively. These observations as well as the much greater AUC0–∞ and lower CL/F values for silybin A are consistent with the known stereoselective glucuronidation of silybin B (Jančová et al., 2011). With regard to the free concentrations of diastereomers isosilybin A and isosilybin B in the dose escalation assessment, the mean Cmax of isosilybin A was 6.1 ± 2.9, 18.2 ± 13.5, and 24.7 ± 11.8 ng/ml, respectively. For isosilybin B, observed concentrations were 22.0 ± 10.7, 46.4 ± 31, and 75.8 ± 32.3 ng/ml, respectively (Table 1; Fig. 2, C and D). Silychristin concentrations were below the limit of detection in individuals dosed with a single 175 mg capsule. However, with the two capsule and three capsule dosing levels, the Cmax were determined to be 4.6 ± 1.1 and 8.5 ± 3.4 ng/ml, respectively (Table 1). With regard to silydianin and the flavonol taxifolin, measurable Cmax were only detectable under the three capsule dosing condition and were 6.5 ± 3.8 and 5.1 ± 2.7 ng/ml, respectively (Table 1), despite the fact that silydianin was the flavonolignan found in greatest abundance in the administered extract (i.e., 36.4 mg/capsule). The median Tmax values of all flavonolignans were between 1.0 and 1.5 hours at all dosage levels in the single-dose escalation assessment.
TABLE 1.
Pharmacokinetic parameters of silybin A, silybin B, isosilybin A, isosilybin B, silychristin, silydianin, and taxifolin after single oral doses of milk thistle capsules (Legalon)
The Tmax values are expressed as median (range) and others are the mean ± S.D. (n = 13).
| One Capsule |
Two Capsules |
Three Capsules |
|
|---|---|---|---|
| Silybin A (21.2 mg/capsule) | |||
| Tmax (h) | 1.5 (0.5–8.0) | 1.0 (0.5–4) | 1.0 (0.5–4) |
| Cmax (ng/ml) | 106.9 ± 49.2 | 200.5 ± 98.3 | 299.3 ± 101.7 |
| t1/2 (h) | 1.6 ± 0.5 | 1.8 ± 0.5 | 1.9 ± 0.5 |
| AUC0–24h (ng×h/ml) | 231.2 ± 91.6 | 462.3 ± 222.2 | 737.3 ± 270.0 |
| AUC0–∞ (ng×h/ml) | 233.5 ± 92.1 | 466.1 ± 222.4 | 744.3 ± 269.7 |
| CL/F (l/h) | 108.9 ± 53.5 | 109.9 ± 45.2 | 96.9 ± 37.3 |
| V/F (l) | 244.5 ± 108.2 | 281.8 ± 130.9 | 259.4 ± 127.0 |
| Silybin B (29.5 mg/capsule) | |||
| Tmax (h) | 1.5 (0.5–6.0) | 1.0 (0.5–4) | 1.0 (0.5–4) |
| Cmax (ng/ml) | 30.5 ± 16.3 | 74.5 ± 45.7 | 121.0 ± 52.2 |
| t1/2 (h) | 2.4 ± 0.7 | 2.6 ± 0.8 | 2.2 ± 0.8 |
| AUC0–24h (ng×h/ml) | 63.6 ± 27.3 | 150.4 ± 94.0 | 279.1 ± 129.7 |
| AUC0–∞ (ng×h/ml) | 65.6 ± 27.1 | 155.2 ± 96.2 | 285.8 ± 130.8 |
| CL/F (l–/h) | 554.6 ± 298.5 | 514.8 ± 252.5 | 377.6 ± 130.8 |
| V/F (l) | 1944.0 ± 1307.7 | 1945.4 ± 1171.3 | 1190.8 ± 676.4 |
| Isosilybin A (11.4 mg/capsule) | |||
| Tmax (h) | 1.0 (0.5–6.0) | 1.0 (0.5–3.0) | 1.0 (0.5–3.0) |
| Cmax (ng/ml) | 6.1 ± 2.9 | 18.2 ± 13.5 | 24.7 ± 11.8 |
| t1/2 (h) | ND | 3.2 ± 1.5 | 2.8 ± 1.3 |
| AUC0–24h (ng×h/ml) | ND | 33.6 ± 21.0 | 57.2 ± 28.7 |
| AUC0–∞ (ng×h/ml) | ND | 36.4 ± 21.6 | 61.1 ± 28.6 |
| CL/F (l/h) | ND | 899.8 ± 560.1 | 675.6 ± 297.6 |
| V/F (l) | ND | 3781.1 ± 1961.4 | 2883.4 ± 2281.9 |
| Isosilybin B (8.2 mg/capsule) | |||
| Tmax (h) | 1.5 (0.5–8.0) | 1.0 (0.5–4.0) | 1.0 (0.5–4.0) |
| Cmax (ng/ml) | 22.0 ± 10.7 | 46.4 ± 31.0 | 75.8 ± 32.3 |
| t1/2 (h) | 1.9 ± 0.6 | 2.0 ± 0.7 | 1.9 ± 0.6 |
| AUC0–24h (ng×h/ml) | 40.4 ± 17.7 | 90.1 ± 53.6 | 158.6 ± 72.1 |
| AUC0–∞ (ng×h/ml) | 42.8 ± 17.6 | 91.9 ± 54.0 | 162.1 ± 72.5 |
| CL/F (l/h) | 237.9 ± 138.7 | 236.3 ± 119.3 | 183.5 ± 85.6 |
| V/F (l) | 662.0 ± 430.5 | 677.2 ± 353.9 | 506.4 ± 251.4 |
| Silychristin (31.5 mg/capsule) | |||
| Tmax (h) | ND | 1.5 (0.5–4.0) | 1.5 (0.5–4.0) |
| Cmax (ng/ml) | ND | 4.6 ± 1.1 | 8.5 ± 3.4 |
| t1/2 (h) | ND | ND | 2.7 ± 1.5 |
| AUC0–24h (ng×h/ml) | ND | ND | 32.9 ± 14.3 |
| AUC0–∞ (ng×h/ml) | ND | ND | 36.6 ± 14.6 |
| CL/F (l/h) | ND | ND | 2987.6 ± 1158.0 |
| V/F (l) | ND | ND | 11,079.9 ± 5506.1 |
| Silydianin (36.4 mg/capsule) | |||
| Tmax (h) | ND | ND | 1.5 (0.5–9.0) |
| Cmax (ng/ml) | ND | ND | 6.4 ± 3.8 |
| t1/2 (h) | ND | ND | ND |
| AUC0–24h (ng×h/ml) | ND | ND | ND |
| AUC0–∞ (ng×h/ml) | ND | ND | ND |
| CL/F (l/h) | ND | ND | ND |
| V/F (l) | ND | ND | ND |
| Taxifolin (5.9 mg/capsule) | |||
| Tmax (h) | ND | ND | 1.0 (0.5–3.0) |
| Cmax (ng/ml) | ND | ND | 5.1 ± 2.7 |
| t1/2 (h) | ND | ND | ND |
| AUC0–24h (ng×h/ml) | ND | ND | ND |
| AUC0–∞ (ng×h/ml) | ND | ND | ND |
| CL/F (l/h) | ND | ND | ND |
| V/F (l) | ND | ND | ND |
ND, not determined due to the plasma concentrations were below the low limits of quantification.
TABLE 2.
The steady-state pharmacokinetic parameters of silybin A, silybin B, and isosilybin B in healthy volunteers orally administered with one capsule of silymarin extract (Legalon, 175 mg) three times daily for 28 days
The Tmax values are expressed as median (range) and other parameters are the mean ± S.D. (n = 13).
| Silybin A | Silybin B | Isosilybin B | |
|---|---|---|---|
| Tmax (h) | 2.0 (0.5–3.0) | 1.0 (0.5–3.0) | 2.0 (0.5–3.0) |
| Ctrough (ng/ml) | 5.9 ± 7.9 | 2.2 ± 2.5 | 0.9 ± 1.2 |
| Cmax (ng/ml) | 134.7 ± 72.0 | 42.1 ± 27.3 | 26.8 ± 19.9 |
| AUC0–8h(ng×h/ml) | 375.6 ± 200.8 | 99.7 ± 61.8 | 66.8 ± 48.4 |
Fig. 2.
Plasma concentrations of silybin A (A), silybin B (B), isosilybin A (C), isosilybin B (D) versus time profiles after oral administration of single doses of one (175 mg), two (350 mg), and three (525 mg) Legalon capsules in healthy volunteers. Data are the means from 13 subjects with error bars presenting S.D.
In the steady-state assessment in which major pharmacokinetic parameters (Cmax, Ctrough, Tmax, and AUC0–8h) were determined after 28 days of exposure to milk thistle extracts (one single 175-mg capsule taken three times daily), results were available for the major flavonolignans silybin A, silybin B, and isosilybin B, although concentrations were not sufficient to provide these parameters for isosilybin A (Table 2). The Cmax was 134.7 ± 72.0, 42.1 ± 27.3, and 26.8 ± 19.9 ng/ml, respectively, whereas Ctrough was 5.9 ± 7.9, 2.2 ± 2.5, and 0.9 ± 1.2 ng/ml. The median Tmax values were 2, 1, and 1 hours, respectively.
Measurement of Oxidative Status via Urinary F2-isoprostane.
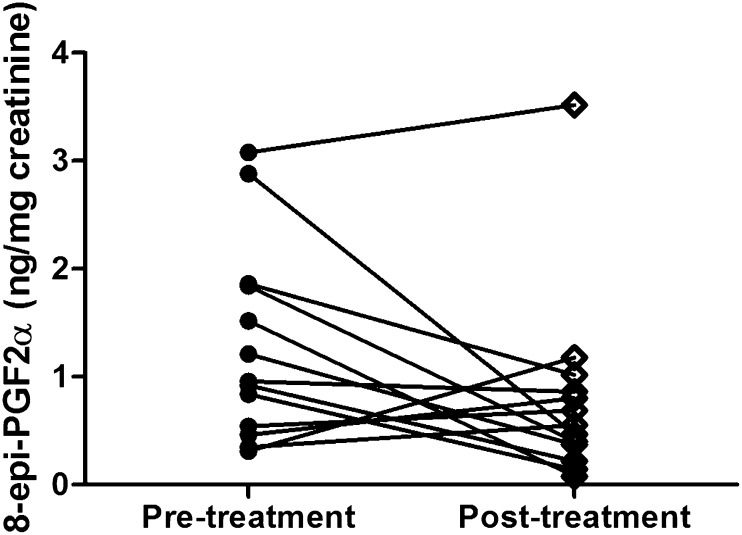
The exposure to 175-mg silymarin extract capsules three times daily for 28 days resulted in considerable decreases of 8-epi-PGF2α concentrations in urine (1.3 ± 0.9 versus 0.8 ± 0.9 ng/mg creatinine) in healthy volunteers (Fig. 3). However, the differences between the pre- and postexposure did not reach statistical significance (P = 0.076), mainly due to marked interindividual variability in response.
Fig. 3.
Urinary concentrations of 8-epi-PGF2α in 13 healthy volunteers pre- and postexposure to 175-mg silymarin extract capsules (Legalon) three times daily for 28 days.
Discussion
Pharmacokinetic analysis indicated that, in general, silymarin flavonolignans were rapidly absorbed and eliminated with short t1/2 after oral administration of a standardized extract. The present findings are in general agreement with previous studies of the plasma pharmacokinetics and metabolism of silybin A and silybin B in humans (Schrieber et al., 2008; Wen et al., 2008; Hawke et al., 2010; Schrieber et al., 2011). For the single-dose assessment at escalating doses of 175, 350, and 525 mg, there was evidence of dose proportionality as assessed by the mean Cmax and AUC0–∞ in the circulating concentrations as well as CL/F of the major flavonolignans as dosages were increased.
Although the content of silybin B was greater than the content of silybin A in the milk thistle extract capsules used in the present study (i.e., 29.5 versus 21.2 mg), a substantially greater systemic exposure to free concentrations of silybin A compared with silybin B was observed. Likewise, the isosilybin A content in the capsules was higher than isosilybin B (11.4 versus 8.2 mg/capsule), and systemic exposure to isosilybin B was greater than that observed for isosilybin A. Both of these observations indicate the apparent clearance of silybin and isosilybin diastereomers proceeds in a stereoselective manner. The calculated CL/F values of silybin B and isosilybin A were significantly higher than their respective isomers (Table 1).
Numerous studies report potentially beneficial pharmacological effects for one or more components of milk thistle extracts that may have therapeutic implications. However, the overwhelming number of these reports used in vitro cellular models for their assessments. For instance, the in vitro chemotherapeutic effects of silymarin have been well documented in various studies, but the in vivo effects in humans have generally not been demonstrated (Jain et al., 2002; Kren and Walterova, 2005; Loguercio and Festi, 2011). Additionally, silymarin components have been reported to inhibit numerous metabolic enzymes such as UGT1A1 and CYP2C9 and drug transporters such as P-glycoprotein and multiple OATP isoforms (Sridar et al., 2004; Brantley et al., 2010; Lee and Choi, 2010; Kock et al., 2013). Previous studies examining the pharmacokinetic properties of silymarin have demonstrated erratic and generally poor bioavailability and intestinal absorption, which are thought to occur because of the relative insolubility of silybin in aqueous media. The variability in silymarin formulations coupled with the ambiguous nomenclature of milk thistle extract can make the interpretation of current literature challenging. One goal of the present study was to determine if the concentrations of silymarin constituents used in in vitro studies are comparable with the concentrations observed in our in vivo pharmacokinetic study or even likely to be achievable with higher dosing.
Silymarin extracts undergo extensive metabolism, the majority of which are subject to phase II metabolic processes. Mono-, di-, and sulpho-glucuronides are known to be formed, and as many as 31 metabolites have been identified (Calani et al., 2012). Silybin diastereomers are extensively conjugated with glucuronic acid and sulfate. Most studies have measured only the total amount of silybin after glucuronidase/sulfatase hydrolysis. Thus, the amounts of silybin A and silybin B that exist in the native or free form over time is not generally presented in earlier studies.
Of the total concentrations of silybin in the plasma after the administration of silymarin extracts, it appears that only 10–30% is circulating as the free dihydroxy derivatives of silybin. In vitro assessments of oxidative biotransformation have found a significant role only for cytochrome P450 CYP2C8, with a minor contribution from CYP3A4 (Jančová et al., 2007). With regard to the purported drug interaction potential with milk thistle extracts a number of reports are available assessing both cytochrome isozymes and various drug transporters. For example, Brantley and associates (2010) reported that certain constituents of the silymarin mixture had inhibitory activity on cytochrome P450 isoform 2C9, the second most heavily expressed hepatic P450 isozyme. A series of experiments were carried out using human liver microsomes and recombinant CYP2C9 enzymes. The investigators used (S)-warfarin as a probe to measure the influence on inhibition of 1 μM (500 ng/ml), 10 μM (5,000 ng/ml), and 100 μM (50,000 ng/ml) of silybin A, silybin B, isosilybin A, and isosilybin B, respectively, on warfarin metabolism. The authors indicated that silybin A, silybin B, isosilybin A, and isosilybin B all inhibited the activity of CYP2C9 with reported IC50 values of 8.2 μM (4,100 ng/ml), 18 μM (9,000 ng/ml), 74 μM (37,000 ng/ml), and >100 μM (50,000 ng/ml), respectively (Brantley et al., 2010). Furthermore, silymarin extracts have been implicated as an inhibitor of CYP3A4 in several in vitro studies (Sridar et al., 2004; Doehmer et al., 2008; Lee and Choi, 2010; Doehmer et al., 2011). However, these in vitro findings of drug-drug interactions have never been demonstrated in focused clinical studies. This may be explained by the incorporation of silymarin constituent concentrations into in vitro model systems that are far in excess of that is achievable clinically (Markowitz and Zhu, 2012). A more recent study examined the inhibitory effects of the various silymarin flavonolignans on multiple OATP isoforms. The group used overexpressing cell lines to examine inhibition of OATP1B1- and OATP1B3-mediated estradiol-17β-glucuronide uptake and OATP2B1-mediated estrone-3-sulfate uptake by silybin A, silybin B, silychristin, and the silymarin mixture as a whole. The investigators reported significant inhibition of OATP1B1-, OATP1B3-, and OATP2B1-mediated substrate uptake by silybin A [IC50 values of 9.7 µM (4,850 ng/ml), 2.7 µM (1,350 ng/ml), and 4.5 µM (2,250 ng/ml), respectively], silybin B [IC50 values of 8.5 µM (4,250 ng/ml), 5 µM (2,500 ng/ml), 0.8 µM (400 ng/ml), respectively], and silychristin [IC50 values of 9.0 µM (4,500 ng/ml), 36.4 µM (18,200 ng/ml), and 3.6 µM (1,800 ng/ml), respectively] (Kock et al., 2013).
However, in interpreting all of these in vitro results it is essential to recognize that the concentrations used in these experiments that were sufficient to produce metabolic inhibition surpassed even the highest systemic concentrations attained by any milk thistle constituent we were able to measure at even the highest dosage level (i.e., 3 capsules) by more than one order of magnitude. However, it should be noted that flavonolignans undergo rapid and extensive phase II metabolism in the liver, forming sulfate and glucuronide conjugates. Very low bioavailability of flavonolignans has been observed in both humans and rats due to an extensive first-pass effect. As a result, the concentrations of flavonolignans in the gut lumen and the portal circulation postingestion can be significantly higher than that in plasma. Thus, it remains an open question whether the concentrations of flavonolignans in the gut lumen and the liver can in fact reach the requisite concentrations to exert significant inhibitory effects on drug metabolizing enzymes and transporters. Nevertheless, to our knowledge, no in vivo clinical assessment of silymarin extracts in humans has ever demonstrated clinically significant inhibition of CYP3A4/5.
Isoprostane 8-epi-PGF2α in both the plasma and urine is among the most commonly used biomarkers of oxidative stress in vivo. Elevated plasma and/or urinary concentrations of 8-epi-GPF2α have been observed in patients with Alzheimer’s disease, stroke, myocardial infarction, hepatitis, and numerous other diseases (Comporti et al., 2009; Stephens et al., 2009; Pratico, 2010; Davies and Roberts, 2011; Il'yasova et al., 2012; Lee et al., 2012; Zhang, 2012). Silymarin exhibits potent antioxidant and anti-inflammatory properties. At least one previously published rodent study demonstrated that silybin significantly decreased the concentrations of isoprostanes in the liver and heart from mice fed a methionine-choline-deficient diet (Salamone et al., 2012). However, the potential antioxidant effects of orally administered milk thistle extracts in human subjects have not been defined to date. In the present study, a trend toward decreased urinary 8-epi-PGF2α concentrations was observed in healthy volunteers dosed with 175 mg milk thistle three times daily for 28 days (1.3 ± 0.9 versus 0.8 ± 0.9 ng/mg creatinine, P = 0.076). We speculate that this antioxidant effect (i.e., as indicated by decreased urinary 8-epi-PGF2α) may be more easily observed and/or of greater magnitude in patients with certain chronic diseases associated with elevated oxidative stress, such as chronic liver diseases, as opposed to the healthy volunteer subjects in the present study. Furthermore, the milk thistle extract dosage of 175 mg three times daily may not be the optimal dosing regimen to achieve maximal antioxidant effects.
In conclusion, after the oral administration of the standardized milk thistle extract Legalon, flavonolignans were rapidly absorbed and eliminated. Escalating single-dose assessments suggested dose proportionality. In order of abundance, the exposure to free (i.e., unconjugated) silymarin flavonolignans was greatest for silybin A followed by silybin B, isosilybin B, isosilybin A, silychristin, and silydianin. Stereoselective metabolism was also in evidence. Plasma concentrations of the flavonolignans were generally lower than those used in in vitro studies assessing various pharmacodynamic effects, although some such as silybin A, silybin B, and isosilybin B reached systemic concentrations in the free form that could produce clinical effects. Further investigation is warranted to study antioxidant effects of silymarin extract in patients exhibiting elevated levels of oxidative stress.
Acknowledgments
Madaus (Cologne, Germany) provided Legalon milk thistle extract for the study.
Abbreviations
- 8-epi-PGF2α
8-epi- prostaglandin F2α
- AUC
area under the plasma concentration-time curve
- CL/F
apparent clearance
- Cmax
maximum plasma concentration
- Ctrough
concentration immediately prior to dosing during the multiple-dose regimen
- GCRC
General Clinical Research Center
- IS
internal standard
- λz
terminal elimination rate constant
- t1/2
elimination half-life
- TESS
treatment emergent side effect scale
- Tmax
time to maximum plasma concentration
- V/F
apparent volume distribution
Authorship Contributions
Participated in research design: Markowitz.
Conducted experiments: Zhu, Brinda, Chavin, Bernstein, Markowitz.
Performed data analysis: Zhu.
Wrote or contributed to the writing of the manuscript: Zhu, Brinda, Patrick, Markowitz.
Footnotes
This work was made possible by a grant from the National Institutes of Health National Center for Complementary and Alternative Medicine [Grant R21AT02817]. We also acknowledge the National Institutes of Health National Center for Research Resources [Grant M01 RR01070-18] for the funding of the clinical study at the Medical University of South Carolina GCRC.
References
- Brantley SJ, Oberlies NH, Kroll DJ, Paine MF. (2010) Two flavonolignans from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations. J Pharmacol Exp Ther 332:1081–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinda BJ, Zhu HJ, Markowitz JS. (2012) A sensitive LC-MS/MS assay for the simultaneous analysis of the major active components of silymarin in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci 902:1–9 [DOI] [PubMed] [Google Scholar]
- Calani L, Brighenti F, Bruni R, Del Rio D. (2012) Absorption and metabolism of milk thistle flavanolignans in humans. Phytomedicine 20:40–46 [DOI] [PubMed] [Google Scholar]
- Comporti M, Arezzini B, Signorini C, Vecchio D, Gardi C. (2009) Oxidative stress, isoprostanes and hepatic fibrosis. Histol Histopathol 24:893–900 [DOI] [PubMed] [Google Scholar]
- Davies SS, Roberts LJ., 2nd (2011) F2-isoprostanes as an indicator and risk factor for coronary heart disease. Free Radic Biol Med 50:559–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Rio D, Serafini M, Pellegrini N. (2002) Selected methodologies to assess oxidative/antioxidant status in vivo: a critical review. Nutr Metab Cardiovasc Dis 12:343–351 [PubMed] [Google Scholar]
- Doehmer J, Tewes B, Klein KU, Gritzko K, Muschick H, Mengs U. (2008) Assessment of drug-drug interaction for silymarin. Toxicol In Vitro 22:610–617 [DOI] [PubMed] [Google Scholar]
- Doehmer J, Weiss G, McGregor GP, Appel K. (2011) Assessment of a dry extract from milk thistle (Silybum marianum) for interference with human liver cytochrome-P450 activities. Toxicol In Vitro 25:21–27 [DOI] [PubMed] [Google Scholar]
- Fam SS, Morrow JD. (2003) The isoprostanes: unique products of arachidonic acid oxidation-a review. Curr Med Chem 10:1723–1740 [DOI] [PubMed] [Google Scholar]
- Hawke RL, Schrieber SJ, Soule TA, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, et al. SyNCH Trial Group (2010) Silymarin ascending multiple oral dosing phase I study in noncirrhotic patients with chronic hepatitis C. J Clin Pharmacol 50:434–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Il’yasova D, Scarbrough P, Spasojevic I. (2012) Urinary biomarkers of oxidative status. Clin Chim Acta 413:1446–1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain SK, Pemberton PW, Smith A, McMahon RF, Burrows PC, Aboutwerat A, Warnes TW. (2002) Oxidative stress in chronic hepatitis C: not just a feature of late stage disease. J Hepatol 36:805–811 [DOI] [PubMed] [Google Scholar]
- Jančová P, Anzenbacherová E, Papousková B, Lemr K, Luzná P, Veinlichová A, Anzenbacher P, Simánek V. (2007) Silybin is metabolized by cytochrome P450 2C8 in vitro. Drug Metab Dispos 35:2035–2039 [DOI] [PubMed] [Google Scholar]
- Jančová P, Siller M, Anzenbacherová E, Křen V, Anzenbacher P, Simánek V. (2011) Evidence for differences in regioselective and stereoselective glucuronidation of silybin diastereomers from milk thistle (Silybum marianum) by human UDP-glucuronosyltransferases. Xenobiotica 41:743–751 [DOI] [PubMed] [Google Scholar]
- Javed S, Kohli K, Ali M. (2011) Reassessing bioavailability of silymarin. Altern Med Rev 16:239–249 [PubMed] [Google Scholar]
- Köck K, Xie Y, Hawke RL, Oberlies NH, Brouwer KL. (2013) Interaction of silymarin flavonolignans with organic anion-transporting polypeptides. Drug Metab Dispos 41:958–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kren V, Walterová D. (2005) Silybin and silymarin—new effects and applications. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 149:29–41 [DOI] [PubMed] [Google Scholar]
- Kroll DJ, Shaw HS, Oberlies NH. (2007) Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther 6:110–119 [DOI] [PubMed] [Google Scholar]
- Ladas EJ, Kelly KM. (2003) Milk thistle: is there a role for its use as an adjunct therapy in patients with cancer? J Altern Complement Med 9:411–416 [DOI] [PubMed] [Google Scholar]
- Lee CK, Choi JS. (2010) Effects of silibinin, inhibitor of CYP3A4 and P-glycoprotein in vitro, on the pharmacokinetics of paclitaxel after oral and intravenous administration in rats. Pharmacology 85:350–356 [DOI] [PubMed] [Google Scholar]
- Lee R, Margaritis M, Channon KM, Antoniades C. (2012) Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr Med Chem 19:2504–2520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loguercio C, Festi D. (2011) Silybin and the liver: from basic research to clinical practice. World J Gastroenterol 17:2288–2301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JS, Zhu HJ. (2012) Limitations of in vitro assessments of the drug interaction potential of botanical supplements. Planta Med 78:1421–1427 [DOI] [PubMed] [Google Scholar]
- NIMH (1985) TESS (treatment emergent symptom scale-write-in). Psychopharmacol Bull 21:1069–1072 [Google Scholar]
- Praticò D. (1999) F(2)-isoprostanes: sensitive and specific non-invasive indices of lipid peroxidation in vivo. Atherosclerosis 147:1–10 [DOI] [PubMed] [Google Scholar]
- Praticò D. (2010) The neurobiology of isoprostanes and Alzheimer’s disease. Biochim Biophys Acta 1801:930–933 [DOI] [PubMed] [Google Scholar]
- Praticò D, Rokach J, Lawson J, FitzGerald GA. (2004) F2-isoprostanes as indices of lipid peroxidation in inflammatory diseases. Chem Phys Lipids 128:165–171 [DOI] [PubMed] [Google Scholar]
- Reddy KR, Belle SH, Fried MW, Afdhal N, Navarro VJ, Hawke RL, Wahed AS, Doo E, Meyers CM, SyNCH Study Group (2012) Rationale, challenges, and participants in a Phase II trial of a botanical product for chronic hepatitis C. Clin Trials 9:102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone F, Galvano F, Marino Gammazza A, Paternostro C, Tibullo D, Bucchieri F, Mangiameli A, Parola M, Bugianesi E, Li Volti G. (2012) Silibinin improves hepatic and myocardial injury in mice with nonalcoholic steatohepatitis. Dig Liver Dis 44:334–342 [DOI] [PubMed] [Google Scholar]
- Schrieber SJ, Hawke RL, Wen Z, Smith PC, Reddy KR, Wahed AS, Belle SH, Afdhal NH, Navarro VJ, Meyers CM, et al. (2011) Differences in the disposition of silymarin between patients with nonalcoholic fatty liver disease and chronic hepatitis C. Drug Metab Dispos 39:2182–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrieber SJ, Wen Z, Vourvahis M, Smith PC, Fried MW, Kashuba AD, Hawke RL. (2008) The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic Fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos 36:1909–1916 [DOI] [PubMed] [Google Scholar]
- Sridar C, Goosen TC, Kent UM, Williams JA, Hollenberg PF. (2004) Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug Metab Dispos 32:587–594 [DOI] [PubMed] [Google Scholar]
- Stephens JW, Khanolkar MP, Bain SC. (2009) The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis 202:321–329 [DOI] [PubMed] [Google Scholar]
- Tsikas D, Schwedhelm E, Suchy MT, Niemann J, Gutzki FM, Erpenbeck VJ, Hohlfeld JM, Surdacki A, Frölich JC. (2003) Divergence in urinary 8-iso-PGF(2alpha) (iPF(2alpha)-III, 15-F(2t)-IsoP) levels from gas chromatography-tandem mass spectrometry quantification after thin-layer chromatography and immunoaffinity column chromatography reveals heterogeneity of 8-iso-PGF(2alpha). Possible methodological, mechanistic and clinical implications. J Chromatogr B Analyt Technol Biomed Life Sci 794:237–255 [DOI] [PubMed] [Google Scholar]
- Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. (2008) Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos 36:65–72 [DOI] [PubMed] [Google Scholar]
- Zhang ZJ. (2012) Systematic review on the association between F2-isoprostanes and cardiovascular disease. Ann Clin Biochem 50:108–114 [DOI] [PubMed] [Google Scholar]