Abstract
Bupropion is widely used for treatment of depression and as a smoking-cessation drug. Despite more than 20 years of therapeutic use, its metabolism is not fully understood. While CYP2B6 is known to form hydroxybupropion, the enzyme(s) generating erythro- and threohydrobupropion have long remained unclear. Previous experiments using microsomal preparations and the nonspecific inhibitor glycyrrhetinic acid suggested a role for 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) in the formation of both erythro- and threohydrobupropion. 11β-HSD1 catalyzes the conversion of inactive glucocorticoids (cortisone, prednisone) to their active forms (cortisol, prednisolone). Moreover, it accepts several other substrates. Here, we used for the first time recombinant 11β-HSD1 to assess its role in the carbonyl reduction of bupropion. Furthermore, we applied human, rat, and mouse liver microsomes and a selective inhibitor to characterize species-specific differences and to estimate the relative contribution of 11β-HSD1 to bupropion metabolism. The results revealed 11β-HSD1 as the major enzyme responsible for threohydrobupropion formation. The reaction was stereoselective and no erythrohydrobupropion was formed. Human liver microsomes showed 10 and 80 times higher activity than rat and mouse liver microsomes, respectively. The formation of erythrohydrobupropion was not altered in experiments with microsomes from 11β-HSD1-deficient mice or upon incubation with 11β-HSD1 inhibitor, indicating the existence of another carbonyl reductase that generates erythrohydrobupropion. Molecular docking supported the experimental findings and suggested that 11β-HSD1 selectively converts R-bupropion to threohydrobupropion. Enzyme inhibition experiments suggested that exposure to bupropion is not likely to impair 11β-HSD1-dependent glucocorticoid activation but that pharmacological administration of cortisone or prednisone may inhibit 11β-HSD1-dependent bupropion metabolism.
Introduction
Bupropion ([(±)-1-(3-chlorophenyl)-2-[(1,1-dimethylethyl) amino]-1-propanone]; Wellbutrin; GlaxoSmithKline, Research Triangle Park, NC) has been used for the treatment of depression for more than 20 years (Holm and Spencer, 2000). It is also administered as a smoking-cessation drug (Zyban). Furthermore, bupropion has recently been proposed for the treatment of attention-deficit/hyperactivity disorders (Jafarinia et al., 2012). According to a recent review, approximately 40 million patients worldwide have been treated with bupropion (Fava et al., 2005). Despite its frequent use, the mechanisms of bupropion metabolism are not fully understood. The identification and characterization of the enzymes involved may help to optimize the therapeutic use of bupropion and to avoid potential drug-drug interactions.
Therapeutically, bupropion is used as a racemic mixture of R- and S-bupropion and acts as a dopamine and norepinephrine reuptake inhibitor. The first studies with bupropion in humans in the 1980s led to the identification of the three major metabolites: hydroxybupropion, erythrohydrobupropion, and threohydrobupropion (Schroeder, 1983; Laizure et al., 1985; Martin et al., 1990; Wang et al., 2010); however, the enzymes responsible for its metabolism remained unknown. A decade later, CYP2B6 was identified as the enzyme responsible for the formation of hydroxybupropion (Faucette et al., 2000; Hesse et al., 2000). Another ten years later, experiments with human and baboon placental and liver microsomes and the nonspecific 11β-hydroxysteroid dehydrogenase (11β-HSD) inhibitor 18β-glycyrrhetinic acid (GA) suggested that bupropion is metabolized by one of the 11β-HSDs to erythrohydrobupropion and threohydrobupropion (Wang et al., 2010, 2011; Molnari and Myers, 2012). Incubation with the nonspecific inhibitor GA yielded lower amounts of both threohydrobupropion and erythrohydrobupropion, suggesting the involvement of 11β-HSD1 in the carbonyl reduction of bupropion.
Two distinct 11β-HSD enzymes are known: 11β-HSD1 is responsible for conversion of the inactive 11-ketoglucocorticoids cortisone (humans) and 11-dehydrocorticosterone (rodents) to the active 11β-hydroxyglucocorticoids cortisol (humans) and corticosterone (rodents), whereas 11β-HSD2 catalyzes the reverse reaction (White et al., 1997). 11β-HSD2 plays a crucial role in protecting mineralocorticoid receptors from activation by glucocorticoids (Odermatt and Kratschmar, 2012). Although 11β-HSD2 is able to act as a reversible enzyme for some substrates, such as dexamethasone/11-ketodexamethasone, under in vitro conditions (Rebuffat et al., 2004), it functions exclusively as a dehydrogenase in vivo, and a role in the reduction of bupropion can be excluded.
11β-HSD1 is expressed in many metabolically active tissues, such as liver, adipose, and skeletal muscle (Atanasov and Odermatt, 2007). In addition to the reduction of cortisone, 11β-HSD1 essentially converts the prodrug prednisone to its active form prednisolone (Hult et al., 1998), thereby enabling activation of the glucocorticoid receptor and regulating glucocorticoid-receptor-dependent target genes. Due to the adverse metabolic effects of prolonged periods of exposure to excessive glucocorticoid levels and the observed metabolic disturbances in transgenic mice overexpressing 11β-HSD1 in adipose tissue (Masuzaki and Flier, 2003), there are considerable efforts to develop inhibitors for the treatment of metabolic syndrome, with ongoing phase II trials (Hughes et al., 2008; An et al., 2013; Anagnostis et al., 2013; Gathercole et al., 2013; Venier et al., 2013). In addition, 11β-HSD1 inhibitors are currently under investigation for the treatment of several other diseases, including osteoporosis, glaucoma, age-associated impaired cognitive function, aging skin, and wound healing (Gathercole et al., 2013; Luo et al., 2013; Tiganescu et al., 2013).
Nevertheless, 11β-HSD1 is a multifunctional carbonyl reductase with broad substrate specificity (Odermatt and Nashev, 2010). It is able to reduce endogenous sterols such as 7-ketocholesterol (Hult et al., 2004; Schweizer et al., 2004), the secondary bile acid 7-oxolithocholic acid (Odermatt et al., 2011), 7-ketodehydroepiandrosterone (Nashev et al., 2007), and several xenobiotics, including triadimefon (Meyer et al., 2013), 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK; nicotine-derived nitrosamine ketone) (Maser et al., 2003), oracin (Wsól et al., 2003), metyrapone (Maser and Bannenberg, 1994), and ketoprofen (Hult et al., 2001).
The evidence from earlier studies using microsomes and the nonspecific inhibitor GA suggested a role for 11β-HSD1 in the formation of the metabolites erythrohydrobupropion and threohydrobupropion. Since it still remained unclear whether indeed 11β-HSD1 is responsible for the generation of these two metabolites, and whether it plays a major or minor role, we used hepatic microsomes, a selective 11β-HSD1 inhibitor, and a recombinant enzyme to assess the role of 11β-HSD1 in bupropion metabolism. Furthermore, we investigated species-specific differences in the carbonyl reduction of bupropion by human, rat, and mouse liver microsomes. The contribution of 11β-HSD1 was further assessed using microsomes from liver-specific 11β-HSD1 knockout mice. Finally, the putative binding of bupropion to 11β-HSD1 was investigated by molecular modeling, suggesting that 11β-HSD1 selectively generates threohydrobupropion from R-bupropion.
Materials and Methods
Chemicals and Reagents.
Microsomes from the liver of a 77-year-old male Caucasian were purchased from Celsis In Vitro Inc. (Baltimore, MD). Human embryonic kidney (HEK)-293 cells from ATCC (No. CRL-1573) were purchased from LGC Standards SARL (Molsheim Cedex, France). Cell culture medium was purchased from Invitrogen (Carlsbad, CA), tricyclo[3.3.1.13,7]dec-1-yl-6,7,8,9-tetrahydro-5H-1,2,4-triazolo[4,3-a]azepine (T0504) from Enamine (Kiev, Ukraine), and steroids from Steraloids (Newport, RI). The metabolites hydroxybupropion, erythrohydrobupropion, and threohydrobupropion were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada), and bupropion and all other chemicals from Sigma-Aldrich Chemie GmbH (Buchs, Switzerland). The solvents were of analytical and high-performance liquid chromatography grade and reagents were of the highest grade available.
Cell Culture and Transfection.
HEK-293 cells were grown at 37°C in Dulbecco’s modified Eagle’s medium, containing 4.5 g/l glucose, 10% fetal bovine serum, 100 U/ml penicillin, 0.1 mg/ml streptomycin, 1 × minimum essential medium, nonessential amino acids, and 10 mM HEPES buffer, pH 7.4. For the experiments with recombinant 11β-HSD1, HEK-293 cells were transiently transfected by the calcium phosphate transfection method as described earlier (Meyer et al., 2013) with plasmids for human, rat, or mouse 11β-HSD1 (Arampatzis et al., 2005). Cells were harvested 48 hours post-transfection, centrifuged at 900g for 4 minutes, and cell pellets were immediately shock-frozen and stored at −80°C until further use. Protein concentration was determined using the Pierce BCA protein assay kit (Thermo Fisher Scientific Inc., Rockford, IL).
Preparation of Liver Microsomes.
Microsomes were prepared as described earlier (Meyer et al., 2013). Livers were taken from adult male Sprague Dawley rats, C57BL/6J mice, and liver-specific HSD11B1 knockout mice generated by crossing albumin-Cre transgenic mice on a C57BL/6J background with floxed homozygous HSD11B1 mice on a mixed C57BL/6J/129SvJ background (Lavery et al., 2012). Liver tissue was homogenized, and microsomes were obtained after differential centrifugation as described (Meyer et al., 2013). Microsomes were finally resuspended in a buffer containing 0.15 M potassium chloride, 0.25 M sucrose, and 10 mM Tris-maleate, pH 7.0. Aliquots were stored at −80°C until further use. The microsomal protein concentration was measured using the Pierce BCA protein assay kit (Thermo Fisher Scientific Inc.). The quality of the microsomal preparations was analyzed using the cytochrome C reductase assay kit (Sigma-Aldrich Chemie GmbH) and by assessing the latent activity of the 11β-HSD1-dependent oxoreduction of cortisone in the presence of glucose-6-phosphate (G6P).
Enzyme Activity Measurements Using Liver Microsomes.
The oxoreduction of cortisone by liver microsomes was measured as reported earlier (Meyer et al., 2013). The metabolism of bupropion was determined at 37°C (1-hour incubation) in a final reaction volume of 22 µl of TS2 buffer (100 mM NaCl, 1 mM EGTA, 1 mM EDTA, 1 mM MgCl2, 250 mM sucrose, 20 mM Tris–HCl, pH 7.4) containing 1 µM of bupropion and either human liver microsomes [final concentration (f.c.) of 0.4 mg/ml], or rat, mouse, or liver-specific HSD11B1 knockout mouse–liver microsomes (all at an f.c. of 1 mg/ml), supplemented with either 1 mM G6P or 1 mM NADPH in the presence or absence of 20 µM of the selective 11β-HSD1 inhibitor T0504. Reactions were stopped by adding 200 µl 0.3 M zinc sulfate in a 1:1 (v/v) mixture of water and methanol. Atrazine was added as an internal standard at an f.c. of 50 nM, followed by vortexing for 10 seconds and centrifugation for 10 minutes at 12,000g on a table-top centrifuge. Samples were further purified by an ethyl acetate extraction. Supernatants (180 µl) were added to 600 µl ethyl acetate and incubated for 10 minutes on a thermomixer at 700 rpm. Following centrifugation for 10 minutes at 12,000g, supernatants (550 µl) were evaporated to dryness, reconstituted in 100 µl methanol and stored at −20°C until analysis by liquid chromatography–tandem mass spectrometry (see below).
Enzyme Activity Measurements Using Lysates of Transfected HEK-293 Cells.
Frozen pellets of HEK-293 cells transiently expressing human, rat or mouse 11β-HSD1 were resuspended in TS2 buffer and sonicated. Lysates were then incubated for 1 hour at 37°C in the presence of 1 mM NADPH and different concentrations of bupropion (8, 4, 2, and 1 µM, and 500, 250, and 125 nM) in a final volume of 22 µl to estimate apparent Km and apparent Vmax values. Substrate conversion was kept below 25% in all experiments. Reactions were stopped and processed as described above.
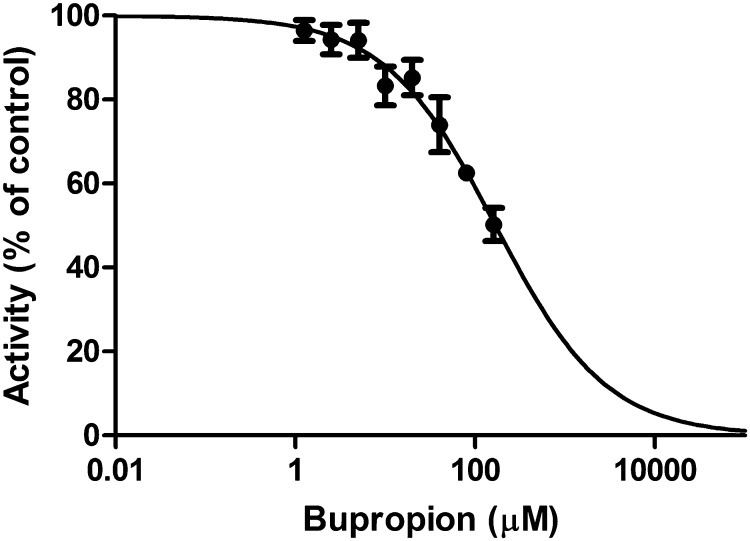
For measuring the reductase activity of 11β-HSD1, cell lysates were incubated in the presence of 1 µM cortisone or 1 µM bupropion as substrate and various concentrations of either bupropion or cortisone and prednisone as the respective inhibitor. IC50 values were calculated by non-linear regression using four parametric logistic curve fitting (GraphPad Prism; GraphPad Software, Inc., La Jolla, CA).
Liquid Chromatography–Tandem Mass Spectrometry Measurements.
An Acquity ultra-performance liquid chromatography (UPLC) BEH C18 column (1.7-µm particle size, 130-Å pore diameter, 2.1-mm internal diameter × 150-mm column length, ID; Waters, Milford, MA) and an Agilent 1290 Infinity Series chromatograph (Agilent Technologies, Basel, Switzerland) were used for chromatographic separations.
The mobile phase consisted of solvent A (H2O/acetonitrile, 95:5 (v/v), containing 0.1% formic acid), and solvent B (H2O/acetonitrile, 5:95 (v/v), containing 0.1% formic acid), at a flow rate of 0.5 ml/minute. Bupropion, hydroxybupropion, threohydrobupropion, and erythrohydrobupropion were separated using 15% solvent B for 6 minutes, followed by a linear gradient from 6 to 10 minutes to reach 100% solvent B, and then 100% solvent B for 3 minutes. The column was then re-equilibrated with 15% solvent B. Cortisone and cortisol were resolved as described earlier (Meyer et al., 2013).
The UPLC was interfaced to an Agilent 6490 triple quadropole tandem mass spectrometer. The entire UPLC–tandem mass spectrometer system was controlled by MassHunter Workstation software, version B.01.05 (Agilent Technologies). The injection volume of each sample was 5 µl. The mass spectrometer was operated in electrospray ionization positive ionization mode, a source temperature of 350°C, a nebulizer pressure of 20 psi, and a capillary voltage of 4000 V.
The compounds were analyzed using multiple-reaction monitoring and identified by comparing their retention time and mass-to-charge ratios (m/z) with those of authentic standards. The transitions, collision energy, and retention time were m/z 240.1/184.1, 19 V, and 4.9 minutes for bupropion; m/z 242/168, 20 V, and 5.4 minutes for threohydrobupropion, m/z 242/168, 20 V, and 4.8 minutes for erythrohydrobupropion; m/z 256/238.1, 17 V, and 3.0 minutes for hydroxybupropion, and m/z 216/174, 16 V, and 5 minutes for the internal standard atrazine.
The UPLC–tandem mass spectrometer method was validated for accuracy, precision, sensitivity, recovery, and calibration range. Acceptable interday assay precision (≤ 6.2%) and accuracy (94.1–105.0%) were achieved over a linear range of 50–5000 nM for bupropion, hydroxybupropion, threohydrobupropion, and erythrohydrobupropion. Recovery of bupropion, hydroxybupropion, threohydrobupropion, and erythrohydrobupropion were 96, 80, 79, and 82%, respectively, in all extractions. For each experiment a new calibration curve was determined.
Molecular Modeling.
The 2D structures of R- and S-bupropion were generated using ChemBioDraw Ultra 12.0 (1986-2010 CambridgeSoft; PerkinElmer, Waltham, MA). The 2D structures were converted into 3D structures using ChemBio3D Ultra 12.0 (1986–2010 CambridgeSoft). The docking studies were performed using GOLD (Cambridge Crystallographic Data Centre, Cambridge, UK) (Jones et al., 1997; Verdonk et al., 2003), which uses a genetic algorithm to produce low-energy binding solutions for small molecules in the ligand-binding pocket. The X-ray crystal structure of 11β-HSD1 was obtained from the Protein Data Bank (www.pdb.org) (Berman et al., 2000). Both stereoisomers of bupropion were docked into the ligand-binding site of 11β-HSD1 (PDB ID 2BEL, Chain A) (X. Wu et al., submitted manuscript). The binding site was defined as a 10-Å sphere, centered on the hydroxyl-oxygen of Ser170 (x: 3.84, y: 22.49, and z: 13.34). The protein side chains were handled as rigid and the ligand conformations as flexible during the docking run. The program was set to define the atom types of the ligands and the protein automatically. GoldScore was selected as a scoring function. The program was allowed to terminate the docking run in cases where three best-ranked solutions were within an root-mean-square deviation of 1.0 Å from each other. Using these settings, the program successfully reproduced the binding mode of the cocrystallized ligand carbenoxolone, thus validating the docking settings.
Results
Carbonyl Reduction of Bupropion by Human, Rat, and Mouse Liver Microsomes.
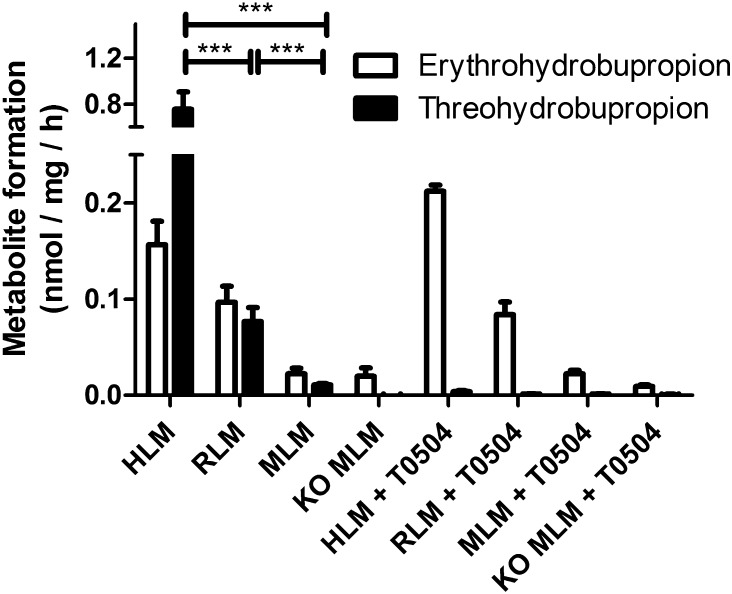
Earlier studies using the nonspecific 11β-HSD inhibitor GA and microsomes prepared from human placenta (Wang et al., 2010) and liver (Molnari and Myers, 2012) or from baboon liver (Wang et al., 2011) suggested a role for 11β-HSD enzymes in the metabolism of bupropion. To test our assumption that 11β-HSD1 catalyzes the carbonyl reduction of bupropion, we first measured the metabolism of bupropion in human liver microsomes that were incubated in the presence of G6P. In intact liver microsomes the lumen of the endoplasmic reticulum (ER) is protected by the microsomal membrane; these microsomes contain an endogenous NADPH-regenerating system consisting of hexose-6-phosphate dehydrogenase (Meyer et al., 2013). Therefore, 11β-HSD1 reductase activity can be measured by simultaneous incubation of microsomes with G6P and the substrate. Upon incubation with G6P and bupropion, human liver microsomes efficiently formed threohydrobupropion and to a lesser extent (4–5-fold) erythrohydrobupropion (Fig. 1). Surprisingly, the selective 11β-HSD1 inhibitor T0504 completely blocked the formation of threohydrobupropion but had no effect on the formation of erythrohydrobupropion.
Fig. 1.
Carbonyl reduction of bupropion by human, rat, and mouse liver microsomes. HLMs (f.c. 0.4 mg/ml), rat liver microsomes (RLM; f.c. 1 mg/ml), mouse liver microsomes (MLM; f.c. 1 mg/ml), and microsomes from livers of liver-specific 11β-HSD1-deficient mice (f.c. 1 mg/ml) were incubated for 1 hour at 37°C with 1 µM bupropion and 1 mM glucose-6-phosphate, in the absence or presence of 20 µM of the 11β-HSD1 inhibitor T0504. Data (mean ± S.D.) were obtained from at least three independent experiments using pooled microsomes. ***P < 0.001, multiple measures analysis of variance found significant species differences in bupropion reduction, posthoc analysis by Tukey test was used for multiple comparisons. KO, knockout.
To assess possible species-specific differences, we compared the activities of human, rat, and mouse liver microsomes. The rat and mouse liver microsomes were 10- and 80-fold less active than human liver microsomes in generating threohydrobupropion. It is important to note that under the same conditions rat liver microsomes showed a 2-fold higher capacity to reduce the substrate cortisone than human or mouse liver microsomes, which had comparable activities (Meyer et al., 2013). Rat liver microsomes formed equal amounts of threohydrobupropion and erythrohydrobupropion, whereas mouse liver microsomes formed about 2-fold more erythrohydrobupropion than threohydrobupropion. As with the human liver microsomes, the 11β-HSD1 inhibitor T0504 selectively blocked threohydrobupropion, suggesting that 11β-HSD1 stereoselectively reduces bupropion to threohydrobupropion. To further support a role for 11β-HSD1 in bupropion metabolism, we used liver microsomes from liver-specific 11β-HSD1 knockout mice. Threohydrobupropion formation was completely abolished, while erythrohydrobupropion formation was unaffected and comparable to that in wild-type mice, suggesting that another enzyme is responsible for the formation of erythrohydrobupropion.
Impact of Cofactor on Bupropion Metabolism.
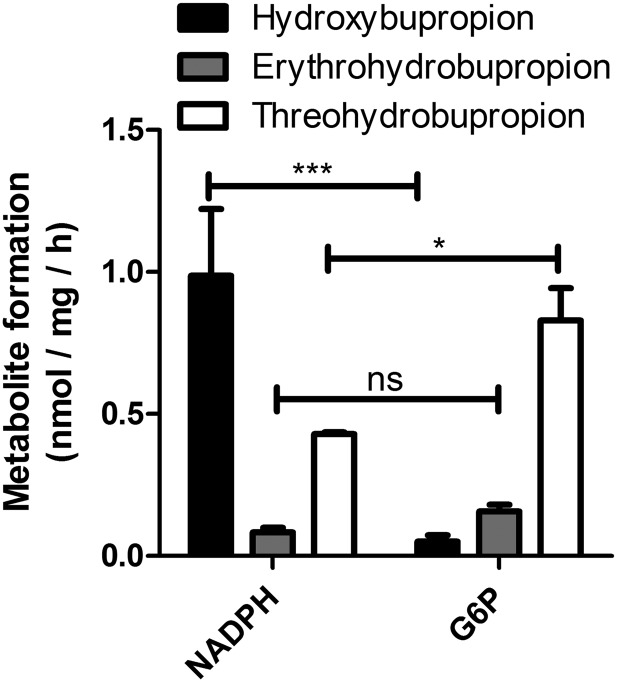
As reported recently, the preparation of rodent microsomes used in this study yields intact vesicles with approximately 90% showing an orientation such that the luminal compartment is protected by the vesicular membrane and the cytoplasmic side is facing the solution. Thus, these microsomal preparations show about 90% latent activities of luminal enzymes. The commercially available human liver microsomes showed about 75% latency (Meyer et al., 2013). Nevertheless, incubation of human liver microsomes with G6P yielded approximately 8-fold higher amounts of threohydrobupropion than erythrohydrobupropion, but only minor amounts of hydroxybupropion (Fig. 2). As expected, incubation of microsomes with NADPH mainly led to the cytochrome P450-dependent formation of hydroxybupropion. The formation of threohydrobupropion is probably due to the microsomal fraction with reverse orientation, because its formation could be completely blocked by the 11β-HSD1 inhibitor T0504. Similar observations were made with mouse and rat liver microsomes, and even higher differences between NADPH- and G6P-dependent formation of hydroxybupropion versus erythro and threohydrobupropion, respectively, were measured (data not shown).
Fig. 2.
Impact of cofactor on the metabolism of bupropion by human liver microsomes. HLMs (f.c. 0.4 mg/ml) were incubated for 1 hour at 37°C in the presence of 1 µM bupropion and either 1 mM NADPH or 1 mM G6P. Data represent mean ± S.D. from at least three independent experiments using pooled microsomes. Ns, not significant; *P < 0.05; ***P < 0.001. Multiple measures analysis of variance found significant differences in the groups, posthoc analysis by Tukey test was used for multiple comparisons.
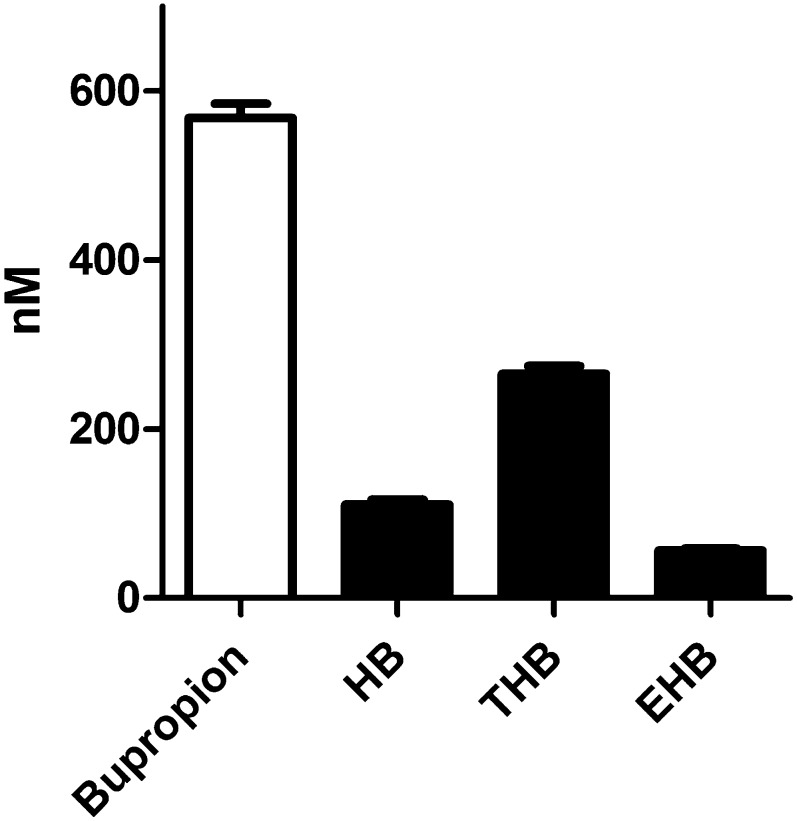
To roughly estimate the relative activities of cytochrome P450–dependent hydroxylation and 11β-HSD1-dependent carbonyl reduction in vitro, human liver microsomes were incubated in the presence of both NADPH and G6P (Fig. 3). Threohydrobupropion was the major product formed, followed by hydroxybupropion and erythrohydrobupropion, suggesting that 11β-HSD1-dependent threohydrobupropion formation is a major route of bupropion metabolism in humans.
Fig. 3.
Bupropion and its major metabolites after incubation of human liver microsomes with NADPH and G6P. HLMs(f.c. 0.2 mg/ml) were incubated for 1 hour at 37°C in the presence of 1 µM bupropion, 1 mM NADPH, and 1 mM G6P. Data represent mean ± S.D. from at least three independent experiments with pooled microsomes. EHB, erythrohydrobupropion; HB, hydroxybupropion; THB, threohydrobupropion.
Carbonyl Reduction of Bupropion by Recombinant Human 11β-HSD1 Measured in Cell Lysates.
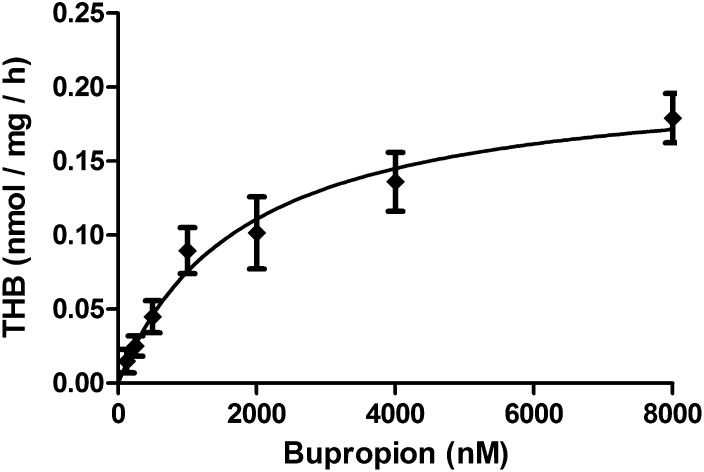
The lysates of HEK-293 cells transiently transfected with human 11β-HSD1 efficiently converted bupropion to threohydrobupropion (Fig. 4). Importantly, no other metabolites were detected, and lysates of untransfected HEK-293 cells did not metabolize bupropion. These incubations were performed in the presence of NADPH, because the cells were lysed by sonication to obtain multilamellar vesicles and vesicles with mixed orientation, therefore allowing direct access of NADPH to 11β-HSD1. An apparent Km of 2.1 ± 0.9 µM and Vmax of 0.22 ± 0.03 nmol/mg per hour for the carbonyl reduction of bupropion was obtained for human 11β-HSD1, suggesting that bupropion is less efficiently reduced by 11β-HSD1 than cortisone (Km of 0.34 ± 0.04 µM and Vmax of 1.88 ± 0.23 nmol/mg per hour) (Frick et al., 2004).
Fig. 4.
Concentration-dependent reduction of bupropion to threohydrobupropion (THB). HEK-293 cells transiently transfected with plasmid for human 11β-HSD1 were sonicated to obtain mixed vesicles, followed by incubation for 1 hour at 37°C in the presence of 1 mM NADPH and different concentrations of bupropion as given in Materials and Methods. Apparent Km (2.1 µM ± 0.9 µM) and apparent Vmax (0.22 ± 0.03 nmol/mg per hour) values were calculated. Data represent mean ± S.D. from at least three independent experiments.
Furthermore, we assessed whether 11β-HSD1 catalyzes the reverse reaction by incubating cell lysates with threohydrobupropion and NADP+. No bupropion could be detected under the conditions applied, indicating that 11β-HSD1 exclusively catalyzes the reduction reaction under physiologic conditions (data not shown).
Inhibition of 11β-HSD1-Dependent Cortisone Reduction by Bupropion and Vice Versa.
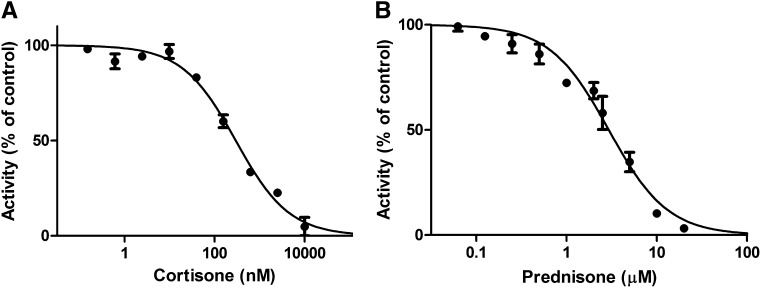
To test whether the substrates influence each other, we first assessed the effect of bupropion on glucocorticoid activation. The reduction of cortisone was inhibited with an IC50 value of 165 ± 51 µM (Fig. 5). Next, we tested the impact of cortisone and the widely used synthetic glucocorticoid prednisone on the carbonyl reduction of bupropion. The conversion of bupropion to threohydrobupropion was inhibited by cortisone and prednisone with IC50 of 193 ± 40 nM (Fig. 6A) and 2.9 ± 0.3 µM, respectively (Fig. 6B).
Fig. 5.
Inhibition of 11β-HSD1-dependent reduction of cortisone by bupropion. Lysates of HEK-293 cells transiently transfected with human 11β-HSD1 were incubated with 1 µM cortisone, 1 mM NADPH, and different concentrations of bupropion for 15 minutes at 37°C. Data were normalized to vehicle control (0.05% dimethyl sulfide) and represent mean ± S.D. from three independent experiments.
Fig. 6.
Inhibition of 11β-HSD1-dependent threohydrobupropion reduction by cortisone and prednisone. Lysates of HEK-293 cells transiently transfected with human 11β-HSD1 were incubated with 1 µM bupropion, 1 mM NADPH, and different concentrations of cortisone (A) or prednisone (B) for 60 minutes at 37°C. Data were normalized to activity of vehicle control (0.05% dimethyl sulfide) and represent mean ± S.D. from three independent experiments.
Binding Mode Prediction of Bupropion to 11β-HSD1 by Molecular Docking.
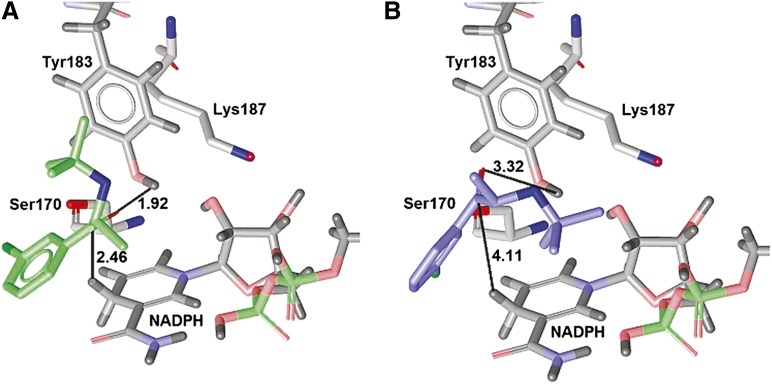
Both enantiomers of bupropion geometrically fit the binding site of 11β-HSD1, and both are predicted to bind next to the catalytic triad Ser170-Tyr183-Lys187 and the cofactor NADPH. However, the stereochemistry of these two enantiomers allows only one of them, R-bupropion, to be metabolized by 11β-HSD1. Since the hydrogens in the reduction reaction are transferred to the substrate via the cofactor and Tyr183 (Oppermann et al., 1997; Kavanagh et al., 2008), it is essential that the carbonyl-oxygen of bupropion is located next to these residues. This is the case for R-bupropion (Fig. 7A): the carbonyl oxygen points toward the hydroxyl of Tyr183 with a distance of 1.92 Å, and the cofactor is at 2.46 Å distance from the carbonyl-carbon. In contrast, S-bupropion is located in the same place, but because of the different stereochemistry, the tert-butyl-group points toward the cofactor, thus pushing the carbonyl group further away from the hydroxyl of Tyr183 (3.32 Å) and the cofactor (4.11 Å), respectively (Fig. 7B). Thus, the S-bupropion carbonyl group is more distant from the catalytic H-donors and has a nonfavorable interaction angle with the Tyr183 hydroxyl group. These docking results support our biologic findings that 11β-HSD1 exclusively forms threohydrobupropion. Erythrohydrobupropion is not formed because of steric hindrance coming from the stereochemistry of S-bupropion.
Fig. 7.
Proposed binding modes of R-bupropion and S-bupropion in the ligand-binding pocket of human 11β-HSD1. R-bupropion (A) is colored green and S-bupropion (B) is blue. The catalytic triad and the cofactor are gray. The distances between the substrate and the protein are given in Ångstroms.
Discussion
Based on earlier studies using microsomes from human and baboon liver and placenta, together with the nonspecific inhibitor GA, it was suggested that 11β-HSD enzymes are involved in the formation of both erythrohydrobupropion and threohydrobupropion (Wang et al., 2010, 2011; Molnari and Myers, 2012). However, since GA might inhibit other enzymes, the relative contribution of 11β-HSD enzymes remained unclear. In the present study, we used liver microsomes and the highly selective 11β-HSD1 inhibitor T0504, also known as Merck-544 (Arampatzis et al., 2005; Hermanowski-Vosatka et al., 2005), as well as recombinant 11β-HSD1 to characterize the carbonyl reduction of bupropion.
The comparison of human, rat, and mouse liver microsomes revealed clearly that human liver microsomes are more active than either rat or mouse liver microsomes in catalyzing the carbonyl reduction of bupropion, and threohydrobupropion was the preferred metabolite formed (Fig. 1). These findings provide an explanation for the observations by Welch et al., who found low levels of these metabolites in plasma of mice and rats (Welch et al., 1987). Furthermore, these authors reported that hydroxybupropion was a major urinary metabolite in human, mouse, and dog, whereas rats predominantly excreted side-chain cleavage products of bupropion such as m-chlorobenzoic acid. It was proposed that the distinct metabolism of bupropion might account for the species-specific pharmacological response of bupropion. Thus, our findings further support earlier studies indicating that rodents are not adequate models for the prediction of bupropion metabolism in humans.
The specific 11β-HSD1 inhibitor completely abolished the formation of threohydrobupropion from the racemic mixture of bupropion by liver microsomes from all three species, without affecting the formation of erythrohydrobupropion. Importantly, microsomes from liver-specific knockout mice were unable to generate threohydrobupropion, but the formation of erythrohydrobupropion was comparable to that by wild-type mouse liver microsomes. These results indicate that 11β-HSD1 is the major if not the only enzyme responsible for the formation of threohydrobupropion and emphasize the existence of another carbonyl reductase responsible for the formation of erythrohydrobupropion. The fact that erythrohydrobupropion is generated upon incubation of microsomes with G6P indicates that the unknown enzyme is localized within the ER, as is 11β-HSD1, and is dependent on hexose-6-phosphate dehydrogenase activity. We speculate that the unknown NADPH-dependent oxoreductase enzyme belongs to the short-chain dehydrogenase/reductase family. Currently, 11β-HSD1 is the only NADPH-dependent enzyme of this family that has been demonstrated to face the ER lumen; however, the function and intracellular localization of 30–40% of the members of this family (currently 72 members are known in the human genome) still remain unknown.
Using the recombinant enzyme, and under the conditions applied, we observed that human 11β-HSD1 exclusively catalyzes the carbonyl reduction of bupropion to threohydrobupropion. Analysis of the binding of bupropion and its metabolites to 11β-HSD1 by molecular modeling indicates that R-bupropion adopts a favorable binding position in the substrate pocket of 11β-HSD1, allowing electron transfer from the cofactor to form threohydrobupropion. In contrast, steric hindrance prevents optimal binding of S-bupropion and erythrohydrobupropion, suggesting that electron transfer is unlikely to occur. Unfortunately, pure S- and R-bupropion are currently not commercially available and will need to be tested in a future study to verify the prediction by molecular modeling.
In a study on exercise performance and neuroendocrine responses to exercise, the effect of bupropion on plasma adrenocorticotropic hormone and cortisol was measured in eight healthy well-trained male cyclists (Piacentini et al., 2004). While bupropion did not affect performance, it did slightly enhance ACTH and cortisol at the end of exercise, suggesting a central noradrenergic effect on the hormonal response to exercise. In another study, the response to a single dose of sustained-release bupropion on nocturnal urinary free cortisol was determined in 20 patients with unipolar major depressive disorder (Rao et al., 2005). Interestingly, bupropion significantly increased nocturnal urinary free cortisol in individuals not responding to the antidepressant effect, whereas no such change could be detected in responders. The nocturnal urinary free cortisol positively correlated with the severity of depression symptoms at the end of the treatment. The authors suggested that a differential sensitivity of the noradrenergic and/or dopaminergic system might be responsible for the observed effects. These findings suggest an effect of bupropion on hypothalamic-pituitary-adrenal activity. To start to understand whether administration of bupropion might interfere with intracellular 11β-HSD1-dependent glucocorticoid activation, we determined IC50 for cortisone reduction. Given the rapid metabolism of bupropion in vivo (Welch et al., 1987) and the high IC50 of 165 ± 51 µM of bupropion to inhibit cortisone reduction, it is unlikely that exposure to bupropion will significantly inhibit the 11β-HSD1-dependent conversion of endogenous cortisone to cortisol.
On the other hand, cortisone efficiently inhibited the carbonyl reduction of bupropion. The low IC50 value of cortisone to inhibit bupropion reduction suggests that pharmacological use as well as elevated endogenous cortisone levels during stress may abolish the concomitant carbonyl reduction of bupropion. The pharmacological use of prednisone is also likely to inhibit the 11β-HSD1-dependent carbonyl reduction of bupropion. An oral administration of a dose of 100-mg prednisone results in Cmax values of about 600 nM (Czock et al., 2005). Intrahepatic drug concentrations after first pass through the liver can be several-fold higher than circulating concentrations, suggesting that prednisone concentrations equal to or higher than the IC50 of 2.9 ± 0.3 µM obtained in the in vitro assay to inhibit bupropion carbonyl reduction may be reached.
Bupropion and its metabolites show different potency regarding the inhibition of biogenic amine uptake; they also differ in half-life and area under the curve (Laizure et al., 1985; Golden et al., 1988; Martin et al., 1990; Hsyu et al., 1997; Horst and Preskorn, 1998; Jefferson et al., 2005). It has been described earlier that hydroxybupropion, the metabolite generated by CYP2B6, has the highest potency (Schroeder, 1983; Martin et al., 1990). Pharmacological administration of cortisone and prednisone, high endogenous cortisone during stress, or the use of 11β-HSD1 inhibitors (currently in development to treat metabolic syndrome and other diseases (An et al., 2013; Anagnostis et al., 2013; Gathercole et al., 2013; Luo et al., 2013; Tiganescu et al., 2013; Venier et al., 2013) are likely to result in higher hydroxybupropion levels, which will necessitate a readjustment of the therapeutic dose of bupropion. Subjects receiving hormone replacement therapy, which leads to inhibition of CYP2B6, had diminished hydroxybupropion levels and increased erythro- and threohydrobupropion levels (Palovaara et al., 2003).
It has been shown that the glucuronides of erythro- and threohydrobupropion account for 13% of the urinary excretion of bupropion in man after a single 200-mg dose of bupropion (Welch et al., 1987). The localization of both 11β-HSD1 and the UDP-glucuronosyltransferase enzymes on the luminal side of the ER membrane facilitates glucuronidation of the newly formed threohydrobupropion. Similarly, our results suggest a luminal orientation of the unknown enzyme responsible for the formation of erythrohydrobupropion, which would facilitate subsequent glucuronidation. An impaired carbonyl reduction of bupropion by the unknown enzyme and by 11β-HSD1 is expected to result in delayed excretion, which may enhance the pharmacological effects of bupropion and hydroxybupropion.
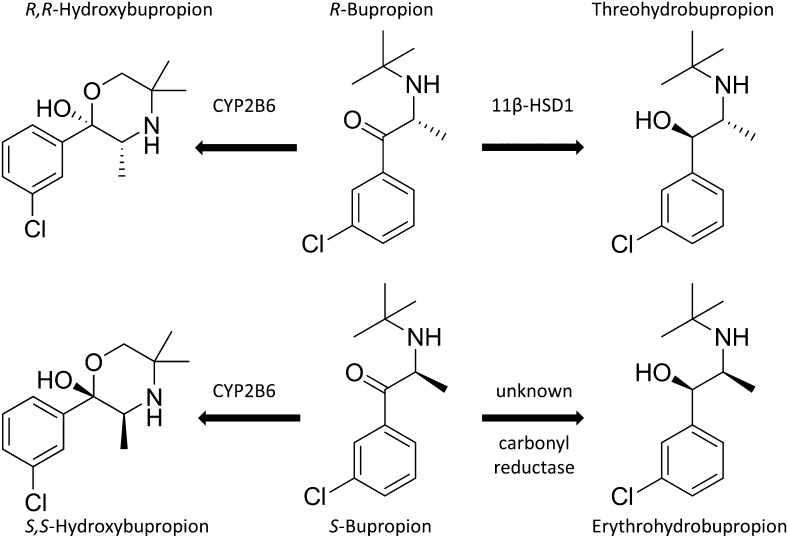
In conclusion, our results indicate that 11β-HSD1 exclusively catalyzes the carbonyl reduction of R-bupropion to threohydrobupropion and suggest that another ER luminal enzyme is responsible for the formation of erythrohydrobupropion (Fig. 8). 11β-HSD1-dependent carbonyl reduction of bupropion is about 10 and 80 times more efficient with human enzymes than with rat or mouse enzymes, while cortisone reduction is less than 2-fold different between these three species. Whereas bupropion is unlikely to impair 11β-HSD1-dependent glucocorticoid activation, the metabolism of bupropion is expected to be inhibited by high endogenous cortisone or pharmacological cortisone or prednisone, and dose adjustments of bupropion might be necessary to achieve optimal therapeutic effects. Further studies are needed to identify the ER luminal enzyme responsible for erythrohydrobupropion formation and to examine the consequences of 11β-HSD1 inhibition on bupropion metabolism in humans.
Fig. 8.
Schematic model of bupropion metabolism.
Acknowledgments
The authors thank Thierry Da Cunha for technical assistance with UPLC–tandem mass spectrometer.
Abbreviations
- 11β-HSD
11β-hydroxysteroid dehydrogenase
- ER
endoplasmic reticulum
- f.c.
final concentration
- G6P
glucose-6-phosphate
- GA
18β-glycyrrhetinic acid
- HEK
human embryonic kidney
- m/z
mass-to-charge ratio
- NNK/nicotine-derived nitrosamine ketone
4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
- T0504
tricyclo[3.3.1.13,7]dec-1-yl-6,7,8,9-tetrahydro-5H-1,2,4-triazolo[4,3-a]azepine
- UPLC
ultra-performance liquid chromatography
- UPLC-MS/MS
ultra-performance liquid chromatography–tandem mass spectrometry
Authorship Contributions
Participated in research design: Meyer, Vuorinen, Schuster, Lavery, Odermatt.
Conducted experiments: Meyer, Vuorinen, Zielinska, Strajhar.
Contributed new reagents or analytic tools: Schuster, Lavery, Odermatt.
Performed data analysis: Meyer, Vuorinen, Schuster, Odermatt.
Wrote or contributed to the writing of the manuscript: Meyer, Vuorinen, Lavery, Schuster, Odermatt.
Footnotes
This work was supported by the Swiss National Science Foundation [Grant PDFMP3-127330] (to A.O.); and a BBSRC David Philips fellowship [Grant BB/G023468/1] (to G.L.).
A.O. has a Chair for Molecular and Systems Toxicology by the Novartis Research Foundation. A.V. is supported by a Ph.D. grant from the Austrian Academy of Sciences and has received financial support from the University of Innsbruck’s Young Talents Grants (Nachwuchsförderung). D.S. is financed by the Erika Cremer Habilitation Program of the University of Innsbruck.
References
- An G, Liu W, Katz DA, Marek G, Awni W, Dutta S. (2013) Effect of ketoconazole on the pharmacokinetics of the 11β-hydroxysteroid dehydrogenase type 1 inhibitor ABT-384 and its two active metabolites in healthy volunteers: population analysis of data from a drug-drug interaction study. Drug Metab Dispos 41:1035–1045 [DOI] [PubMed] [Google Scholar]
- Anagnostis P, Katsiki N, Adamidou F, Athyros VG, Karagiannis A, Kita M, Mikhailidis DP. (2013) 11β-Hydroxysteroid dehydrogenase type 1 inhibitors: novel agents for the treatment of metabolic syndrome and obesity-related disorders? Metabolism 62:21–33 [DOI] [PubMed] [Google Scholar]
- Arampatzis S, Kadereit B, Schuster D, Balazs Z, Schweizer RA, Frey FJ, Langer T, Odermatt A. (2005) Comparative enzymology of 11β-hydroxysteroid dehydrogenase type 1 from six species. J Mol Endocrinol 35:89–101 [DOI] [PubMed] [Google Scholar]
- Atanasov AG, Odermatt A. (2007) Readjusting the glucocorticoid balance: an opportunity for modulators of 11β-hydroxysteroid dehydrogenase type 1 activity? Endocr Metab Immune Disord Drug Targets 7:125–140 [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. (2000) The Protein Data Bank. Nucleic Acids Res 28:235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czock D, Keller F, Rasche FM, Häussler U. (2005) Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 44:61–98 [DOI] [PubMed] [Google Scholar]
- Faucette SR, Hawke RL, Lecluyse EL, Shord SS, Yan B, Laethem RM, Lindley CM. (2000) Validation of bupropion hydroxylation as a selective marker of human cytochrome P450 2B6 catalytic activity. Drug Metab Dispos 28:1222–1230 [PubMed] [Google Scholar]
- Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, Johnston JA. (2005) 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry 7:106–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick C, Atanasov AG, Arnold P, Ozols J, Odermatt A. (2004) Appropriate function of 11β-hydroxysteroid dehydrogenase type 1 in the endoplasmic reticulum lumen is dependent on its N-terminal region sharing similar topological determinants with 50-kDa esterase. J Biol Chem 279:31131–31138 [DOI] [PubMed] [Google Scholar]
- Gathercole LL, Lavery GG, Morgan SA, Cooper MS, Sinclair AJ, Tomlinson JW, Stewart PM. (2013) 11β-Hydroxysteroid Dehydrogenase 1: Translational and Therapeutic Aspects. Endocr Rev DOI: 10.1210/er.2012-1050 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Golden RN, De Vane CL, Laizure SC, Rudorfer MV, Sherer MA, Potter WZ. (1988) Bupropion in depression. II. The role of metabolites in clinical outcome. Arch Gen Psychiatry 45:145–149 [DOI] [PubMed] [Google Scholar]
- Hermanowski-Vosatka A, Balkovec JM, Cheng K, Chen HY, Hernandez M, Koo GC, Le Grand CB, Li Z, Metzger JM, Mundt SS, et al. (2005) 11β-HSD1 inhibition ameliorates metabolic syndrome and prevents progression of atherosclerosis in mice. J Exp Med 202:517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse LM, Venkatakrishnan K, Court MH, von Moltke LL, Duan SX, Shader RI, Greenblatt DJ. (2000) CYP2B6 mediates the in vitro hydroxylation of bupropion: potential drug interactions with other antidepressants. Drug Metab Dispos 28:1176–1183 [PubMed] [Google Scholar]
- Holm KJ, Spencer CM. (2000) Bupropion: a review of its use in the management of smoking cessation. Drugs 59:1007–1024 [DOI] [PubMed] [Google Scholar]
- Horst WD, Preskorn SH. (1998) Mechanisms of action and clinical characteristics of three atypical antidepressants: venlafaxine, nefazodone, bupropion. J Affect Disord 51:237–254 [DOI] [PubMed] [Google Scholar]
- Hsyu P-H, Singh A, Giargiari TD, Dunn JA, Ascher JA, Johnston JA. (1997) Pharmacokinetics of bupropion and its metabolites in cigarette smokers versus nonsmokers. J Clin Pharmacol 37:737–743 [DOI] [PubMed] [Google Scholar]
- Hughes KA, Webster SP, Walker BR. (2008) 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitors in type 2 diabetes mellitus and obesity. Expert Opin Investig Drugs 17:481–496 [DOI] [PubMed] [Google Scholar]
- Hult M, Elleby B, Shafqat N, Svensson S, Rane A, Jörnvall H, Abrahmsen L, Oppermann U. (2004) Human and rodent type 1 11beta-hydroxysteroid dehydrogenases are 7beta-hydroxycholesterol dehydrogenases involved in oxysterol metabolism. Cell Mol Life Sci 61:992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hult M, Jörnvall H, Oppermann UC. (1998) Selective inhibition of human type 1 11β-hydroxysteroid dehydrogenase by synthetic steroids and xenobiotics. FEBS Lett 441:25–28 [DOI] [PubMed] [Google Scholar]
- Hult M, Nobel CS, Abrahmsen L, Nicoll-Griffith DA, Jörnvall H, Oppermann UC. (2001) Novel enzymological profiles of human 11β-hydroxysteroid dehydrogenase type 1. Chem Biol Interact 130-132:805–814 [DOI] [PubMed] [Google Scholar]
- Jafarinia M, Mohammadi M-R, Modabbernia A, Ashrafi M, Khajavi D, Tabrizi M, Yadegari N, Akhondzadeh S. (2012) Bupropion versus methylphenidate in the treatment of children with attention-deficit/hyperactivity disorder: randomized double-blind study. Hum Psychopharmacol 27:411–418 [DOI] [PubMed] [Google Scholar]
- Jefferson JW, Pradko JF, Muir KT. (2005) Bupropion for major depressive disorder: Pharmacokinetic and formulation considerations. Clin Ther 27:1685–1695 [DOI] [PubMed] [Google Scholar]
- Jones G, Willett P, Glen RC, Leach AR, Taylor R. (1997) Development and validation of a genetic algorithm for flexible docking. J Mol Biol 267:727–748 [DOI] [PubMed] [Google Scholar]
- Kavanagh KL, Jörnvall H, Persson B, Oppermann U. (2008) Medium- and short-chain dehydrogenase/reductase gene and protein families : the SDR superfamily: functional and structural diversity within a family of metabolic and regulatory enzymes. Cell Mol Life Sci 65:3895–3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laizure SC, DeVane CL, Stewart JT, Dommisse CS, Lai AA. (1985) Pharmacokinetics of bupropion and its major basic metabolites in normal subjects after a single dose. Clin Pharmacol Ther 38:586–589 [DOI] [PubMed] [Google Scholar]
- Lavery GG, Zielinska AE, Gathercole LL, Hughes B, Semjonous N, Guest P, Saqib K, Sherlock M, Reynolds G, Morgan SA, et al. (2012) Lack of significant metabolic abnormalities in mice with liver-specific disruption of 11β-hydroxysteroid dehydrogenase type 1. Endocrinology 153:3236–3248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo MJ, Thieringer R, Springer MS, Wright SD, Hermanowski-Vosatka A, Plump A, Balkovec JM, Cheng K, Ding GJ, Kawka DW, et al. (2013) 11β-HSD1 inhibition reduces atherosclerosis in mice by altering proinflammatory gene expression in the vasculature. Physiol Genomics 45:47–57 [DOI] [PubMed] [Google Scholar]
- Martin P, Massol J, Colin JN, Lacomblez L, Puech AJ. (1990) Antidepressant profile of bupropion and three metabolites in mice. Pharmacopsychiatry 23:187–194 [DOI] [PubMed] [Google Scholar]
- Maser E, Bannenberg G. (1994) 11 β-hydroxysteroid dehydrogenase mediates reductive metabolism of xenobiotic carbonyl compounds. Biochem Pharmacol 47:1805–1812 [DOI] [PubMed] [Google Scholar]
- Maser E, Friebertshäuser J, Völker B. (2003) Purification, characterization and NNK carbonyl reductase activities of 11β-hydroxysteroid dehydrogenase type 1 from human liver: enzyme cooperativity and significance in the detoxification of a tobacco-derived carcinogen. Chem Biol Interact 143-144:435–448 [DOI] [PubMed] [Google Scholar]
- Masuzaki H, Flier JS. (2003) Tissue-specific glucocorticoid reactivating enzyme, 11 β-hydroxysteroid dehydrogenase type 1 (11 β-HSD1)—a promising drug target for the treatment of metabolic syndrome. Curr Drug Targets Immune Endocr Metabol Disord 3:255–262 [DOI] [PubMed] [Google Scholar]
- Meyer A, Vuorinen A, Zielinska AE, Da Cunha T, Strajhar P, Lavery GG, Schuster D, Odermatt A. (2013) Carbonyl reduction of triadimefon by human and rodent 11β-hydroxysteroid dehydrogenase 1. Biochem Pharmacol 85:1370–1378 [DOI] [PubMed] [Google Scholar]
- Molnari JC, Myers AL. (2012) Carbonyl reduction of bupropion in human liver. Xenobiotica 42:550–561 [DOI] [PubMed] [Google Scholar]
- Nashev LG, Chandsawangbhuwana C, Balazs Z, Atanasov AG, Dick B, Frey FJ, Baker ME, Odermatt A. (2007) Hexose-6-phosphate dehydrogenase modulates 11β-hydroxysteroid dehydrogenase type 1-dependent metabolism of 7-keto- and 7β-hydroxy-neurosteroids. PLoS ONE 2:e561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odermatt A, Da Cunha T, Penno CA, Chandsawangbhuwana C, Reichert C, Wolf A, Dong M, Baker ME. (2011) Hepatic reduction of the secondary bile acid 7-oxolithocholic acid is mediated by 11β-hydroxysteroid dehydrogenase 1. Biochem J 436:621–629 [DOI] [PubMed] [Google Scholar]
- Odermatt A, Kratschmar DV. (2012) Tissue-specific modulation of mineralocorticoid receptor function by 11β-hydroxysteroid dehydrogenases: an overview. Mol Cell Endocrinol 350:168–186 [DOI] [PubMed] [Google Scholar]
- Odermatt A, Nashev LG. (2010) The glucocorticoid-activating enzyme 11β-hydroxysteroid dehydrogenase type 1 has broad substrate specificity: Physiological and toxicological considerations. J Steroid Biochem Mol Biol 119:1–13 [DOI] [PubMed] [Google Scholar]
- Oppermann UC, Filling C, Berndt KD, Persson B, Benach J, Ladenstein R, Jörnvall H. (1997) Active site directed mutagenesis of 3 β/17 β-hydroxysteroid dehydrogenase establishes differential effects on short-chain dehydrogenase/reductase reactions. Biochemistry 36:34–40 [DOI] [PubMed] [Google Scholar]
- Palovaara S, Pelkonen O, Uusitalo J, Lundgren S, Laine K. (2003) Inhibition of cytochrome P450 2B6 activity by hormone replacement therapy and oral contraceptive as measured by bupropion hydroxylation. Clin Pharmacol Ther 74:326–333 [DOI] [PubMed] [Google Scholar]
- Piacentini MF, Meeusen R, Buyse L, De Schutter G, De Meirleir K. (2004) Hormonal responses during prolonged exercise are influenced by a selective DA/NA reuptake inhibitor. Br J Sports Med 38:129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao U, Ott GE, Lin KM, Gertsik L, Poland RE. (2005) Effect of bupropion on nocturnal urinary free cortisol and its association with antidepressant response. J Psychiatr Res 39:183–190 [DOI] [PubMed] [Google Scholar]
- Rebuffat AG, Tam S, Nawrocki AR, Baker ME, Frey BM, Frey FJ, Odermatt A. (2004) The 11-ketosteroid 11-ketodexamethasone is a glucocorticoid receptor agonist. Mol Cell Endocrinol 214:27–37 [DOI] [PubMed] [Google Scholar]
- Schroeder DH. (1983) Metabolism and kinetics of bupropion. J Clin Psychiatry 44:79–81 [PubMed] [Google Scholar]
- Schweizer RA, Zürcher M, Balazs Z, Dick B, Odermatt A. (2004) Rapid hepatic metabolism of 7-ketocholesterol by 11β-hydroxysteroid dehydrogenase type 1: species-specific differences between the rat, human, and hamster enzyme. J Biol Chem 279:18415–18424 [DOI] [PubMed] [Google Scholar]
- Tiganescu A, Tahrani AA, Morgan SA, Otranto M, Desmoulière A, Abrahams L, Hassan-Smith Z, Walker EA, Rabbitt EH, Cooper MS, et al. (2013) 11β-hydroxysteroid dehydrogenase blockade prevents age-induced skin structure and function defects. J Clin Invest 10.1172/JCI64162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venier O, Pascal C, Braun A, Namane C, Mougenot P, Crespin O, Pacquet F, Mougenot C, Monseau C, Onofri B, et al. (2013) Discovery of SAR184841, a potent and long-lasting inhibitor of 11β-hydroxysteroid dehydrogenase type 1, active in a physiopathological animal model of T2D. Bioorg Med Chem Lett 23:2414–2421 [DOI] [PubMed] [Google Scholar]
- Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD. (2003) Improved protein-ligand docking using GOLD. Proteins 52:609–623 [DOI] [PubMed] [Google Scholar]
- Wang X, Abdelrahman DR, Fokina VM, Hankins GD, Ahmed MS, Nanovskaya TN. (2011) Metabolism of bupropion by baboon hepatic and placental microsomes. Biochem Pharmacol 82:295–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Abdelrahman DR, Zharikova OL, Patrikeeva SL, Hankins GDV, Ahmed MS, Nanovskaya TN. (2010) Bupropion metabolism by human placenta. Biochem Pharmacol 79:1684–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RM, Lai AA, Schroeder DH. (1987) Pharmacological significance of the species differences in bupropion metabolism. Xenobiotica 17:287–298 [DOI] [PubMed] [Google Scholar]
- White PC, Mune T, Agarwal AK. (1997) 11 β-Hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev 18:135–156 [DOI] [PubMed] [Google Scholar]
- Wsól V, Szotáková B, Skálová L, Maser E. (2003) Stereochemical aspects of carbonyl reduction of the original anticancer drug oracin by mouse liver microsomes and purified 11β-hydroxysteroid dehydrogenase type 1. Chem Biol Interact 143-144:459–468 [DOI] [PubMed] [Google Scholar]