Abstract
Despite increasing recognition of potential untoward interactions between herbal products and conventional medications, a standard system for prospective assessment of these interactions remains elusive. This information gap was addressed by evaluating the drug interaction liability of the model herbal product milk thistle (Silybum marianum) with the CYP3A probe substrate midazolam. The inhibitory effects of commercially available milk thistle extracts and isolated constituents on midazolam 1′-hydroxylation were screened using human liver and intestinal microsomes. Relative to vehicle, the extract silymarin and constituents silybin A, isosilybin A, isosilybin B, and silychristin at 100 μM demonstrated >50% inhibition of CYP3A activity with at least one microsomal preparation, prompting IC50 determination. The IC50s for isosilybin B and silychristin were ∼60 and 90 μM, respectively, whereas those for the remaining constituents were >100 μM. Extracts and constituents that contained the 1,4-dioxane moiety demonstrated a >1.5-fold shift in IC50 when tested as potential mechanism-based inhibitors. The semipurified extract, silibinin, and the two associated constituents (silybin A and silybin B) demonstrated mechanism-based inhibition of recombinant CYP3A4 (KI, ∼100 μM; kinact, ∼0.20 min−1) but not microsomal CYP3A activity. The maximum predicted increases in midazolam area under the curve using the static mechanistic equation and recombinant CYP3A4 data were 1.75-fold, which may necessitate clinical assessment. Evaluation of the interaction liability of single herbal product constituents, in addition to commercially available extracts, will enable elucidation of mechanisms underlying potential clinically significant herb-drug interactions. Application of this framework to other herbal products would permit predictions of herb-drug interactions and assist in prioritizing clinical evaluation.
Introduction
An estimated 20% of adults in the United States acknowledge taking herbal products (Bent, 2008), with nearly 70% failing to inform their health care provider (Gardiner et al., 2006; Kennedy et al., 2008). An herbal product that inhibits one or more drug-metabolizing enzymes can perpetrate untoward interactions with conventional medications (Hu et al., 2005; Izzo and Ernst, 2009; Gurley et al., 2012). Prominent among these enzymes are the cytochromes P450 (P450s). Inhibition of P450 activity by constituents of the herbal product can reduce “victim” drug clearance, leading to higher systemic drug concentrations and potential adverse effects and toxicity. Dietary substances, including herbal products, are not regulated in the same manner as drugs. Consequently, herb-drug interaction liability is not evaluated routinely prior to marketing. This information gap prevents both clinicians and consumers from making informed decisions about the risk of adding herbal products to pharmacotherapeutic regimens.
Despite increasing recognition of potential untoward herb-drug interactions, a standard system for prospective assessment of the drug interaction liability of herbal products remains elusive. In addition to inconsistent experimental methods, variability in phytochemical content further confounds interaction evaluations (Gurley, 2012). As such, knowledge of mechanisms underlying these interactions is scarce. One approach to developing a system to assess herb-drug interaction potential is to examine individual constituents from a well-characterized herbal product using traditional drug-drug interaction predictive tools. Milk thistle is a top-10-selling herbal product in the United States (Blumenthal et al., 2012) used predominately to self-treat hepatic disorders, particularly hepatitis C (Kroll et al., 2007; Post-White et al., 2007; Seeff et al., 2008). Milk thistle represents an ideal model herbal product due to the following key properties: high sales numbers ($12.8 million in 2011) indicate that a large number of consumers are at risk for milk thistle–drug interactions (Blumenthal et al., 2012); individual constituents have been identified, isolated, and scaled up to quantities sufficient for in vitro drug-drug interaction evaluation (Kim et al., 2003; Graf et al., 2007; Monti et al., 2010; Sy-Cordero et al., 2012); and in vitro–in vivo extrapolation has been inconsistent (Beckmann-Knopp et al., 2000; Zuber et al., 2002; Gurley et al., 2004, 2006; Han et al., 2009).
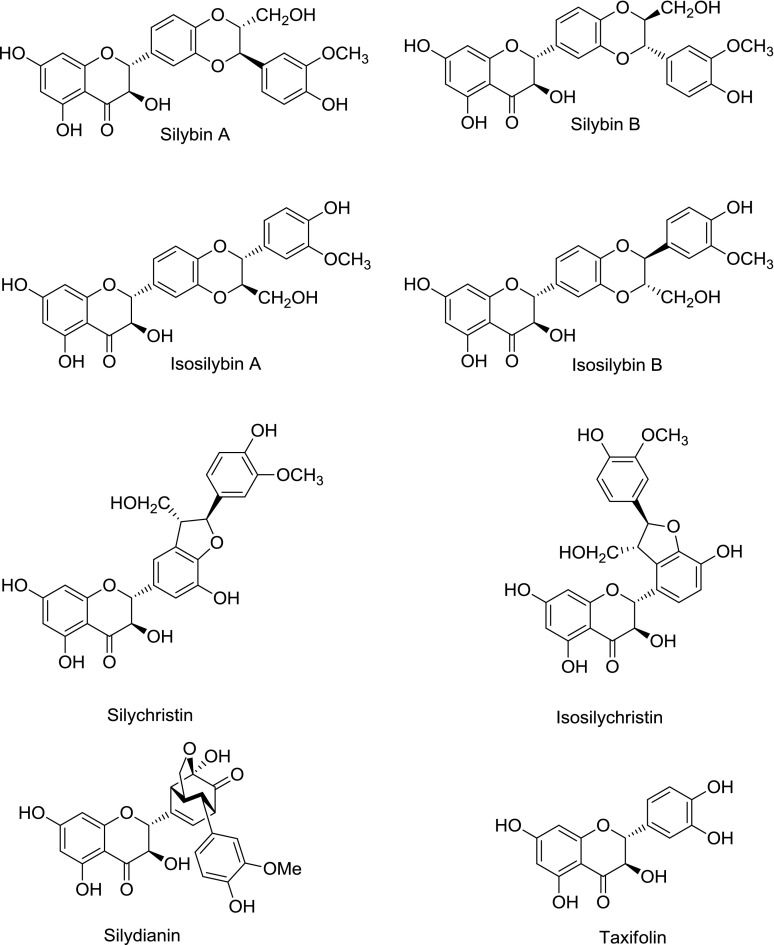
Commercial preparations of milk thistle [Silybum marianum (L.) Gaertn.] include the crude extract silymarin, consisting of at least seven flavonolignans (Fig. 1), the flavonoid taxifolin, and fatty acids; and the semipurified extract silibinin, consisting of the most prevalent flavonolignans (silybin A and silybin B). The relative composition of milk thistle constituents varies substantially between and among preparations (Davis-Searles et al., 2005; Wen et al., 2008). These extracts have been shown to inhibit the activity of several P450s in vitro, both reversibly (e.g., CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A) (Beckmann-Knopp et al., 2000; Zuber et al., 2002; Etheridge et al., 2007; Doehmer et al., 2008; Brantley et al., 2010) and irreversibly (e.g., CYP2C9 and CYP3A4) (Sridar et al., 2004). However, these in vitro observations generally have not manifested clinically. For example, two healthy volunteer studies demonstrated no interaction between the CYP3A probe substrate midazolam and one silymarin product (Gurley et al., 2004, 2006), whereas another study demonstrated a different silymarin product to significantly increase the systemic exposure to the CYP2C9/3A substrate losartan (Han et al., 2009). This discrepancy may be attributed to large variation in relative composition between herbal products. Rigorous characterization of P450 inhibition properties of individual constituents, as well as the commercial extracts, may assist explanation of these in vitro–in vivo disconnects (Paine and Oberlies, 2007; Won et al., 2012).
Fig. 1.
Structures of flavonolignans and flavonoid (taxifolin) from milk thistle.
CYP3A metabolizes >30% of clinically used drugs (Zanger and Schwab, 2013) and is expressed in both the intestine and liver (Thummel et al., 1996). Following oral administration of milk thistle, intestinal CYP3A likely will be exposed to higher concentrations of the constituents compared with hepatic CYP3A. This difference was demonstrated by the nearly 60-fold-higher mean (± S.D.) concentration in intestinal relative to hepatic tissue in cancer patients administered 1.4 g/day silibinin (140 ± 170 versus 2.5 ± 2.4 μM) (Hoh et al., 2006). Such high intestinal concentrations could markedly reduce the intestinal first-pass metabolism of susceptible CYP3A substrates, thereby increasing systemic drug exposure. Using milk thistle as a model herbal product, the objectives of this study were to 1) assess interaction liability of individual constituents toward CYP3A activity, 2) prioritize constituents for further evaluation, and 3) develop a framework to elucidate mechanisms underlying herb-drug interactions. Such mechanistic insight ultimately may assist clinicians and consumers to gauge the impact of adding herbal products to pharmacotherapeutic regimens.
Materials and Methods
Chemicals and Reagents
Human liver (pooled from 50 donors, mixed gender) and intestinal (pooled from 18 donors, mixed gender) microsomes (HLMs and HIMs, respectively) were purchased from Xenotech, LLC (Lenexa, KS). Baculovirus insect cell–expressed CYP3A4 (rCYP3A4), supplemented with cDNA-expressed reductase and cytochrome b5, was purchased from BD Biosciences (San Jose, CA). Midazolam, 1′-hydroxymidazolam, alprazolam, silibinin, ketoconazole, NADPH, glutathione, superoxide dismutase, catalase, 5,5-dimethyl-1-pyrroline N-oxide, acetonitrile, dimethylsulfoxide (DMSO), high-performance liquid chromatography (HPLC)-grade water, methanol, ammonium acetate, and 1-propanol were purchased from Sigma-Aldrich (St. Louis, MO). 6′,7′-Dihydroxybergamottin (DHB) was purchased from Cayman Chemical (Ann Arbor, MI). Silymarin was obtained from Euromed S.A. (Barcelona, Spain). This product has been shown to consist of 16% silybin A, 24% silybin B, 6.4% isosilybin A, 4.4% isosilybin B, 17% silydianin, 12% silychristin, 2.2% isosilychristin, and 1.6% taxifolin; the remainder consists of uncharacterized polyphenols and aliphatic fatty acids (Davis-Searles et al., 2005). Silybin A, silybin B, isosilybin A, isosilybin B, silychristin, isosilychristin, silydianin, and taxifolin were purified as described previously (Graf et al., 2007); all milk thistle constituents were >97% pure as determined by HPLC.
Evaluation of the Stability of Individual Milk Thistle Constituents
Stability in Potassium Phosphate Buffer.
Each constituent was dissolved in DMSO to yield a 100 mM solution. Each solution was diluted in potassium phosphate buffer (0.1 M, pH 7.4), supplemented with MgCl2 (3.3 mM) to yield a final concentration of 100 μM. Solutions were placed immediately into a heated (37°C) autosampler, and aliquots (0.3 μl) were collected serially from 0–1440 minutes and analyzed via UPLC-UV (described below).
Metabolic Lability of Selected Constituents.
Silybin A, silybin B, isosilybin A, and isosilybin B were added to potassium phosphate buffer as described above with the addition of HLMs (0.05 mg/ml). Incubation mixtures were placed on a dry heat block and equilibrated for 5 minutes. Reactions were initiated with the addition of NADPH to yield a final concentration of 1 mM. Aliquots (100 μl) were removed from 0–60 minutes, and reactions were terminated with 2 volumes of ice-cold acetonitrile. Each constituent was quantified by UPLC-UV (described below).
Analysis of Incubations for Milk Thistle Constituents
Milk thistle constituents were quantified with an Acquity UPLC system (Empower 2 software; Waters Corp., Milford, MA) at a flow rate of 0.80 (stability study) or 0.75 (lability study) ml/min using an Acquity binary solvent manager and an Acquity HSS-T3 column (1.8 μm, 2.1 × 50 mm; Waters Corp.). The elution gradient for the stability study ranged from 10:90 to 40:60 methanol/water over 0.45 minutes, then from 40:60 to 55:45 over 1.5 minutes. The elution gradient for the lability study ranged from 40:60 to 55:45 methanol/water over 2 minutes. Signals were monitored at UV 288 nm using an Acquity PDA detector, and the volume injected was 0.3 (stability study) or 7.5 (lability study) μl by an Acquity sample manager.
Evaluation of Milk Thistle Constituents as Inhibitors of CYP3A Activity
The inhibitory effects of silymarin, silibinin, and individual constituents on midazolam 1′-hydroxylation activity were evaluated using HLMs, HIMs, and rCYP3A4. Midazolam and ketoconazole were dissolved in methanol to yield 10 mM and 1 mM solutions, respectively. Milk thistle constituents were dissolved in DMSO to yield a 200 mM solution. NADPH was prepared fresh in potassium phosphate buffer to yield a 4 mM solution. Under all experimental conditions, the amount of 1′-hydroxymidazolam formed was linear with respect to incubation time and concentration of microsomal or rCYP3A4 protein (data not shown).
Initial Testing.
Incubation mixtures consisted of HLMs or HIMs (0.05 mg/ml protein), midazolam (4 μM), milk thistle constituent/extract (1, 10, or 100 μM), and potassium phosphate buffer. Control mixtures contained 0.1% (v/v) DMSO in place of milk thistle constituent/extract. As a positive control for CYP3A inhibition, mixtures contained ketoconazole (1 μM) in place of milk thistle constituent/extract. The mixtures were equilibrated in a dry heat block at 37°C for 5 minutes before initiating the reactions with NADPH (1 mM final concentration) to yield a final volume of 200 μl. After 2 (HLMs) or 4 (HIMs) minutes, reactions were terminated with 2 volumes of ice-cold acetonitrile containing 20 μg/ml alprazolam as the internal standard. After centrifugation (1350g for 10 minutes at 4°C), supernatants were analyzed for 1′-hydroxymidazolam by HPLC-MS/MS as described previously (Wang et al., 2007; Ngo et al., 2009). Individual constituents demonstrating ≥50% inhibition in either microsomal preparation were selected for further evaluation.
Screening of Constituents as Reversible Inhibitors of CYP3A Activity in HLMs and HIMs.
Incubation mixtures were prepared as described above, only milk thistle constituent/extract concentrations ranged from 0–200 μM. Reaction mixtures were processed and analyzed for 1′-hydroxymidazolam as described above.
Screening of Constituents as Metabolism-Dependent Inhibitors of CYP3A Activity in HLMs, HIMs, and rCYP3A4.
IC50 shift experiments (Obach et al., 2007) were used to screen milk thistle constituents and extracts as potential mechanism-based inhibitors of CYP3A activity. Primary incubation mixtures consisting of HLMs, HIMs (0.25 mg/ml), or rCYP3A4 (20 pmol/ml), milk thistle constituent/extract (0–1000 μM), and potassium phosphate buffer were equilibrated at 37°C for 5 minutes before initiating reactions with NADPH (1 mM). Control reactions were identical except NADPH was replaced with potassium phosphate buffer. After 15 minutes, an aliquot (40 μl) was removed and diluted 5-fold into a secondary incubation mixture containing midazolam (4 μM) and NADPH (1 mM). After 2 (HLMs) or 4 (HIMs and rCYP3A4) minutes, the secondary reactions were terminated and processed as described above. Individual constituents demonstrating a >1.5-fold shift in IC50 were selected for further evaluation.
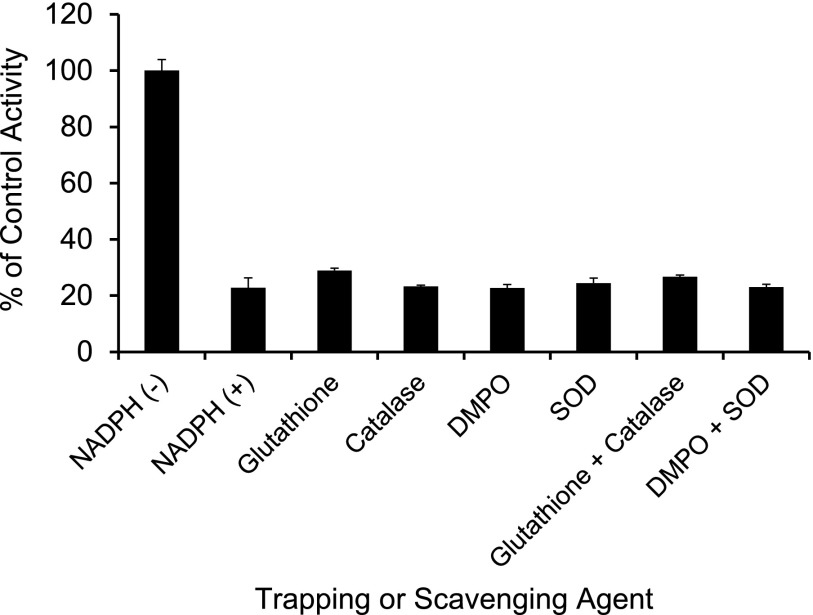
Effect of Nucleophilic Trapping Agents and Reactive Oxygen Species Scavengers on CYP3A Inactivation in HLMs.
Primary mixtures consisted of HLMs (0.25 mg/ml), silybin A (0, 30, or 100 μM), potassium phosphate buffer, and trapping agent [5,5-dimethyl-1-pyrroline N-oxide (1 mM), superoxide dismutase (1,000 U/ml), glutathione (2 mM), or catalase (5,000 U/ml)]. The mixtures were equilibrated at 37°C for 5 minutes before initiating reactions with NADPH (1 mM). Control reactions were identical except NADPH was replaced with potassium phosphate buffer. Reactions proceeded as described for the IC50 shift experiment.
Determination of Mechanism-Based Inhibition Kinetics.
Time- and concentration-dependent inhibition of CYP3A4 was assessed as described previously (Paine et al., 2004). Briefly, primary incubation mixtures consisting of HLMs, HIMs (2.5 mg/ml), or rCYP3A4 (0.2 nmol/ml), milk thistle constituent/extract (0–200 μM), and potassium phosphate buffer were equilibrated at 37°C for 5 minutes before initiating the primary reactions with NADPH. As a positive control for mechanism-based inhibition (MBI), incubation mixtures contained DHB (5 μM) in place of milk thistle constituent/extract (Paine et al., 2004). At designated times (0–15 minutes), an aliquot (4 μl) was removed and diluted 50-fold into a secondary reaction mixture containing midazolam (8 μM) and NADPH (1 mM). The secondary reactions were terminated and processed as described for the reversible inhibition experiments.
Data Analysis
Determination of Apparent IC50.
The apparent IC50 of milk thistle constituent/extract was recovered according to previously published methods (Brantley et al., 2010). Initial estimates of IC50s were determined by visual inspection of the velocity of 1′-hydroxymidazolam formation versus the natural logarithm of milk thistle constituent/extract concentration data. Final parameter estimates were recovered by fitting eq. 1 or 2 to untransformed data using WinNonlin (version 5.3; Pharsight, Mountain View, CA):
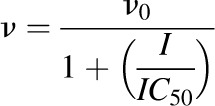 |
(1) |
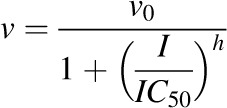 |
(2) |
where v denotes the velocity of 1′-hydroxymidazolam formation, v0 denotes the initial velocity of 1′-hydroxymidazolam formation, I denotes the concentration of milk thistle constituent/extract, and h denotes the Hill coefficient. The best-fit equation was assessed from visual inspection of the observed versus predicted data, randomness of the residuals, Akaike information criteria, and S.E.'s of the parameter estimates.
Determination of KI and kinact.
MBI parameters were recovered according to previously published methods (Paine et al., 2004; Obach et al., 2007). Briefly, the natural logarithm of the percentage of CYP3A activity remaining was plotted as a function of primary reaction time. The apparent inactivation rate constant (kinact,app) associated with each inhibitor concentration was determined from the slope of the initial mono-exponential decline in activity. Initial estimates of KI and kinact were obtained from a Kitz-Wilson plot. Final parameter estimates were obtained by nonlinear least-squares regression using eq. 3:
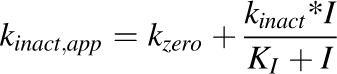 |
(3) |
where kzero denotes the rate of CYP3A inactivation in the absence of inhibitor. The efficiency of inactivation was calculated as the ratio of kinact to KI.
Milk Thistle–Midazolam Drug Interaction Prediction.
The interaction between milk thistle constituents and midazolam was predicted using the intestinal portion of the static mechanistic MBI equation detailed previously (Obach et al., 2007; US FDA, 2012):
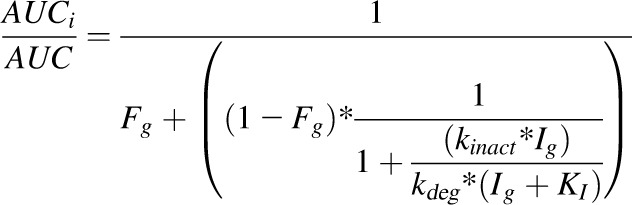 |
(4) |
where AUCi/AUC denotes the predicted ratio of in vivo exposure of a victim drug, Ig denotes the inhibitor concentration in the enterocyte (140 μM) after a silibinin dose of 1.4 g (Hoh et al., 2006), kdeg denotes the rate of intestinal CYP3A4 degradation (0.00481 min−1) (Obach et al., 2007), and Fg denotes the fraction of the dose of midazolam escaping first-pass extraction in the intestine (0.57) (Paine et al., 1996).
Statistical Analysis
Data are presented as means ± S.D.'s of triplicate determinations unless indicated otherwise. Enzyme kinetic parameters are presented as the estimates ± S.E.'s. All statistical comparisons were made according to previously published methods (Brantley et al., 2010). In brief, concentration-dependent inhibition of single constituents/extracts was evaluated by a one-way analysis of variance; post hoc comparisons were made using Tukey’s test when an overall significance resulted. Statistical differences between calculated IC50 values were evaluated by a Student’s t test of two independent samples. A P < 0.05 was considered significant for all statistical tests.
Results
Selected Milk Thistle Constituents Are Metabolically Stable in HLMs for up to 15 Minutes
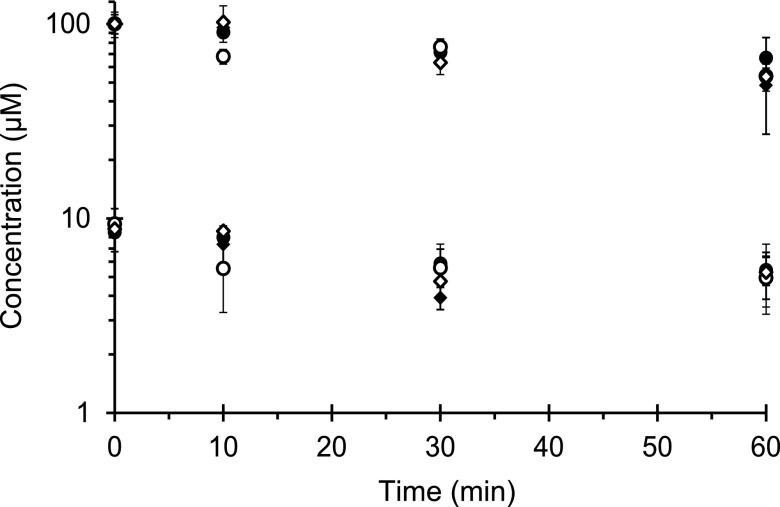
All tested constituents were stable (>95% remaining) for 1 hour at 37°C in potassium phosphate buffer (data not shown). Metabolic stability experiments determined the optimal primary incubation time such that inhibitor depletion would be ≤20%. All constituents at 10 μM demonstrated ≥50% oxidative depletion by 60 minutes (Fig. 2). Isosilybin A was the least stable, showing 40% depletion by 10 minutes, whereas the remaining constituents were depleted by <10%. At 100 μM, silybin A was slightly more stable than the other constituents (∼40% versus 50% depletion by 60 minutes). Isosilybin A at 100 μM was again the least stable, showing 30% depletion by 10 minutes, whereas the remaining constituents were depleted by <10%. Based on these observations, the primary incubation time of the IC50 shift and MBI experiments involving selected constituents, with the exception of isosilybin A, was limited to 15 minutes.
Fig. 2.
Metabolic lability of selected milk thistle constituents in HLMs. Microsomes (0.05 mg/ml) were incubated with silybin A (solid circles), silybin B (solid diamonds), isosilybin A (open circles), or isosilybin B (open diamonds) at 10 or 100 μM in potassium phosphate buffer. Incubations were initiated with NADPH (1 mM) and quenched at designated times. Symbols and error bars denote means and S.D.'s, respectively, of triplicate incubations.
Milk Thistle Constituents Differentially Inhibit CYP3A-Mediated Midazolam 1′-Hydroxylation
Initial Testing.
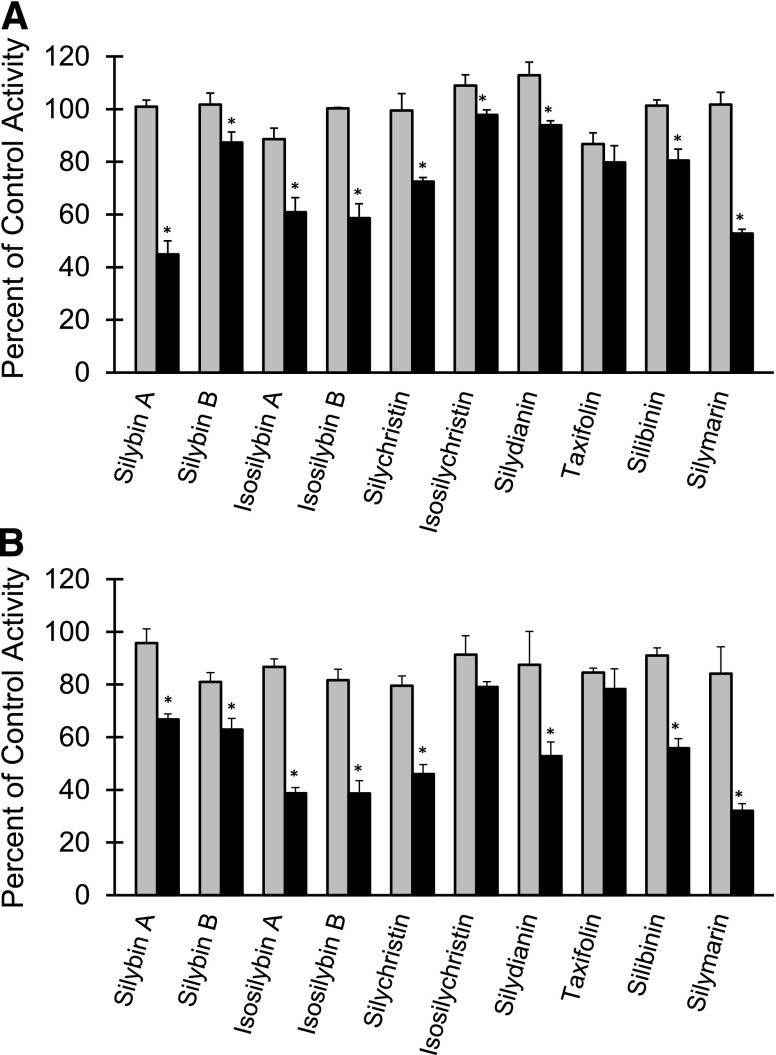
All flavonolignans and the two extracts (silibinin and silymarin) inhibited midazolam 1′-hydroxylation in a concentration-dependent manner (10 versus 100 μM) in HLMs (Fig. 3A) and, with the exception of isosilychristin, in HIMs (Fig. 3B). The sole flavonoid, taxifolin, showed no concentration-dependent inhibition with either preparation. Only silybin B (HLMs), silychristin (HIMs), and silydianin (HIMs) showed concentration-dependent inhibition from 1 to 10 μM (1 μM data not shown). With HLMs, silybin A at 100 μM was the most potent, followed by silymarin, isosilybin B, and isosilybin A (Fig. 3A); with HIMs, silymarin at 100 μM was the most potent, followed by isosilybin A, isosilybin B, and silychristin (Fig. 3B). Ketoconazole inhibited CYP3A activity in both microsomal preparations by at least 95% (data not shown). Based on >50% inhibition at 100 μM in at least one microsomal preparation, silybin A, isosilybin A, isosilybin B, and silychristin were selected for IC50 determination. Additionally, based on the high concentrations (>140 μM) observed in enterocytes following silibinin administration to human subjects (Hoh et al., 2006), silybin B was selected for further evaluation.
Fig. 3.
Inhibitory effects of flavonolignans on midazolam 1′-hydroxylation activity in HLMs (A) and HIMs (B). Microsomes (0.05 mg/ml) were incubated with midazolam (4 μM) and flavonolignan (10 or 100 μM; gray and black bars, respectively) for 2 (HLMs) or 4 (HIMs) minutes. Silymarin and silibinin “concentrations” were calculated by assuming a consistent molecular weight of 482 g/mol (Davis-Searles et al., 2005). Reactions were initiated with NADPH (1 mM). Midazolam 1′-hydroxylation activity in the presence of vehicle control [0.1% (v/v) DMSO] was 800 ± 40 pmol/min/mg or 280 ± 16 pmol/min/mg microsomal protein for HLMs and HIMs, respectively. Bars and error bars denote means and S.D.'s, respectively, of triplicate incubations. *P < 0.05 versus 10 μM (paired Student's t-test).
Reversible Inhibition.
With both HLMs and HIMs, isosilybin B was the most potent constituent, followed by silychristin, silybin B, and silybin A (Table 1). Because isosilybin A was not soluble at concentrations of >100 μM, a complete IC50 curve could not be recovered.
TABLE 1.
Comparison of IC50s for milk thistle constituents under reversible inhibition experimental design
Values represent the estimate ± S.E. from nonlinear regression using WinNonlin (version 5.3).
| Enzyme Source | IC50 of: | ||||
|---|---|---|---|---|---|
| Silybin A | Silybin B | Isosilybin A | Isosilybin B | Silychristin | |
| μM | |||||
| HLMs | 180 ± 16 | 150 ± 7.6 | N.D. | 56 ± 4.4 | 90 ± 8.8 |
| HIMs | 130 ± 12 | 90 ± 10 | N.D. | 60 ± 4.8 | 93 ± 8.7 |
N.D., not determined due to insolubility at concentrations of >100 μM.
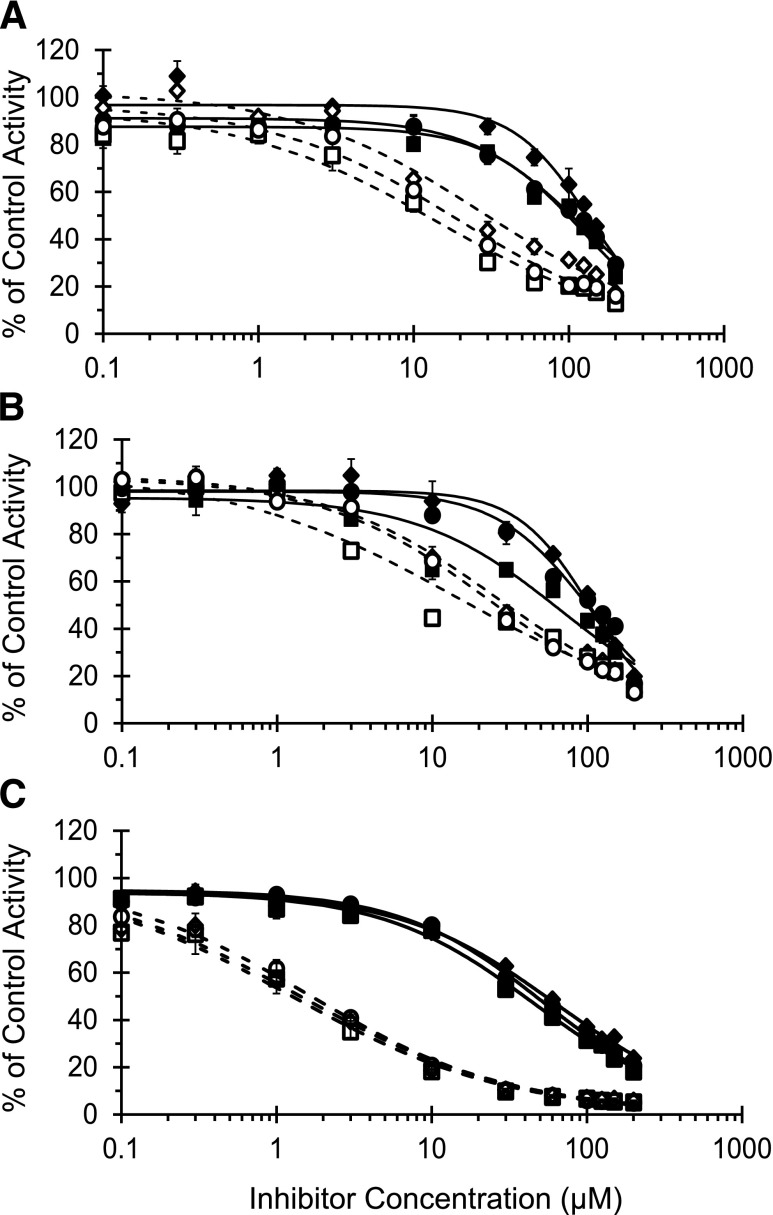
IC50 Shift.
The IC50 for silybin A, silybin B, and silibinin was 73%–98% lower in the presence compared with the absence of NADPH in the primary incubation (Table 2). rCYP3A4 was more sensitive to inhibition than HLMs and HIMs (Fig. 4; Table 2). The IC50 for silychristin did not decrease with either HLMs or HIMs (Table 2), precluding further evaluation of this constituent. Reactive species scavengers did not ameliorate the NADPH-dependent increase in potency of silybin A toward CYP3A activity (Fig. 5).
TABLE 2.
Comparison of IC50s for milk thistle constituents and extracts under IC50 shift experimental design
Values represent the estimate ± S.E. from nonlinear regression using WinNonlin (version 5.3).
| Constituent/Extract | Enzyme Source | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HLMs | HIMs | rCYP3A4 | |||||||
| IC50 | Fold Change | IC50 | Fold Change | IC50 | Fold Change | ||||
| − NADPHa | + NADPHb | − NADPH | + NADPH | − NADPH | + NADPH | ||||
| μM | μM | μM | |||||||
| Silybin A | 120 ± 4.8 | 28 ± 3.1 | 4.3 | 100 ± 6.9 | 23 ± 1.7 | 4.3 | 53 ± 2.0 | 1.6 ± 0.2 | 33 |
| Silybin B | 140 ± 5.7 | 19 ± 2.1 | 7.4 | 100 ± 3.8 | 27 ± 1.9 | 3.7 | 63 ± 3.2 | 1.4 ± 0.2 | 45 |
| Silibinin | 120 ± 5.7 | 14 ± 2.2 | 8.6 | 67 ± 7.9 | 15 ± 3.0 | 4.5 | 45 ± 2.8 | 1.2 ± 0.2 | 38 |
| Silychristin | 26 ± 3.3 | 52 ± 5.2 | 0.5 | 25 ± 4.2 | 72 ± 7.7 | 0.3 | N.D. | N.D. | N.D. |
| Isosilybin B | 78 ± 6.2 | 30 ± 3.3 | 2.6 | 54 ± 3.3 | 23 ± 2.3 | 2.3 | N.D. | N.D. | N.D. |
| Silymarin | 95 ± 12 | 28 ± 4.5 | 3.4 | 29 ± 2.7 | 7.0 ± 0.8 | 4.1 | N.D. | N.D. | N.D. |
N.D., not determined.
NADPH was absent during the primary incubation.
NADPH was present during the primary incubation.
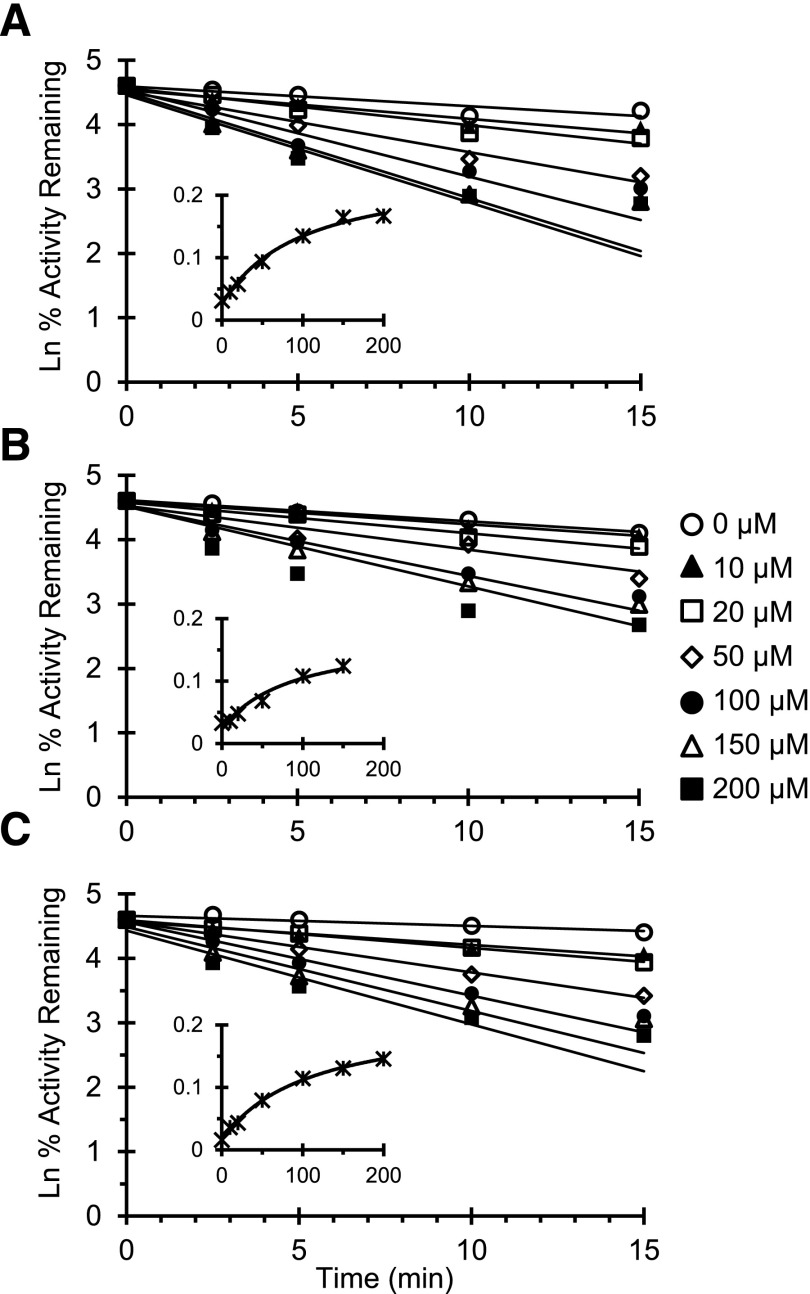
Fig. 4.
IC50 shift plot for silybin A, silybin B, and silibinin. HLMs (A), HIMs (B), and rCYP3A4 (C) were incubated with silybin A (circles), silybin B (diamonds), or silibinin (squares) (0.1–200 μM) in the presence (open symbols) or absence (solid symbols) of NADPH (1 mM). The primary reaction mixture was diluted 5-fold to initiate the secondary reaction, which contained NADPH (1 mM) and midazolam (4 μM). Midazolam 1′-hydroxylation activity in the presence of vehicle control [0.1% (v/v) DMSO] was 2000 ± 90 pmol/min/mg (HLMs), 350 ± 10 pmol/min/mg (HIMs), and 7.9 ± 1.0 pmol/min/pmol (rCYP3A4). Symbols and error bars denote means and S.D.'s, respectively, of triplicate incubations. Open symbols denote observed data when NADPH was present in the primary incubation; solid symbols denote observed data when NADPH was absent in the primary incubation. Curves denote nonlinear least-squares regression of observed data using WinNonlin (version 5.3).
Fig. 5.
Effect of traditional reactive species scavengers. HLMs were incubated for 15 minutes with silybin A (100 μM) and traditional reactive species scavengers in the presence or absence of NADPH. The primary reaction mixture was diluted 5-fold to initiate the secondary reaction, which contained NADPH (1 mM) and midazolam (4 μM). Midazolam 1′-hydroxylation activity in the presence of vehicle control [0.1% (v/v) DMSO] was 1700 ± 46 pmol/min/mg microsomal protein. With the exception of glutathione and catalase (duplicate incubations), bars and error bars denote means and S.D.'s, respectively, of triplicate incubations.
Silybin A, Silybin B, and Silibinin Demonstrate MBI of rCYP3A4 But Not Microsomal CYP3A Activity
MBI of CYP3A activity by silybin A, silybin B, and silibinin was evaluated to derive relevant kinetic parameters. No tested constituent or extract demonstrated time- and concentration-dependent inhibition of CYP3A activity with either HLMs or HIMs (data not shown). Unlike with microsomes, rCYP3A4 demonstrated both time- and concentration-dependent inhibition (Fig. 6). Because the rate of rCYP3A4 inactivation for silybin B at 200 μM deviated from linearity (Fig. 6B), these data were excluded from further analysis. The kinetics were similar between silybin A and silybin B; the kinetics of the 1:1 mixture of silybin A and silybin B (silibinin) were similar to those recovered for the single constituents (Table 3). Using the rCYP3A4 inactivation parameters recovered for silibinin and assuming the enterocyte concentration of silibinin approximates that measured in intestinal tissue (Hoh et al., 2006), a 1.75-fold increase in midazolam area under the curve was predicted by the static mechanistic equation (Obach et al., 2007; US FDA, 2012).
Fig. 6.
Time- and concentration-dependent plot of CYP3A activity. Recombinant CYP3A4 was incubated with silybin A (A), silybin B (B), and silibinin (C) (0–200 μM). The primary reaction mixture was diluted 50-fold to initiate the secondary reaction, which contained NADPH (1 mM) and midazolam (8 μM), at designated times. Symbols denote means of duplicate incubations; all replicates deviated by <30%. Lines denote linear regression of the initial mono-exponential decline in activity. The inset depicts the rate of CYP3A4 inactivation as a function of inhibitor concentration. Symbols denote observed inactivation rates at each inhibitor concentration. Curves denote nonlinear least-squares regression of observed values using WinNonlin (version 5.3).
TABLE 3.
Inactivation kinetics of selected milk thistle constituents and extract
Values represent the estimate ± S.E. from nonlinear regression using WinNonlin (version 5.3).
| Constituent/Extract | KI | kinact | kinact/KI | t1/2 | kzero |
|---|---|---|---|---|---|
| μM | min−1 | μl/min/pmol | min | min−1 | |
| Silybin A | 100 ± 27 | 0.22 ± 0.02 | 2.2 | 3.2 | 0.025 ± 0.005 |
| Silybin B | 89 ± 60 | 0.15 ± 0.04 | 1.7 | 4.5 | 0.023 ± 0.008 |
| Silibinin | 110 ± 15 | 0.20 ± 0.01 | 1.8 | 3.5 | 0.016 ± 0.002 |
t1/2, half-life.
Discussion
Herb-drug interactions are a growing concern in clinical practice as consumers turn increasingly to herbal products as a means to self-treat various conditions. Despite increasing recognition of these potential untoward interactions, a standard system for prospective assessment of the drug interaction liability of herbal products remains elusive. Milk thistle was selected as a model herbal product based on market share, well-characterized nature, and clinical relevance. Milk thistle extracts have been reported to inhibit P450 activity in vitro (Beckmann-Knopp et al., 2000; Zuber et al., 2002; Doehmer et al., 2008); however, clinical interaction liability has been inconsistent, particularly with CYP3A-mediated interactions (Gurley et al., 2004, 2006; Han et al., 2009). Taken together, inhibition of CYP3A by single constituents was evaluated to address these in vitro–in vivo disconnects, as well as to develop a framework to elucidate potential mechanisms underlying herb-drug interactions.
As demonstrated previously with CYP2C9 activity [i.e., (S)-warfarin 7-hydroxylation] (Brantley et al., 2010), milk thistle constituents differentially inhibited CYP3A-mediated midazolam 1′-hydroxylation (Fig. 3). The crude extract silymarin was consistently one of the most potent inhibitors of CYP3A activity in microsomal preparations. Among single constituents, the relatively less abundant isosilybin A and isosilybin B (Davis-Searles et al., 2005; Wen et al., 2008) were two of the more potent inhibitors in both HLMs and HIMs (Fig. 3). Inhibitory kinetic parameters were recovered for isosilybin B, whereas metabolic lability (Fig. 2) and insolubility above 100 μM precluded recovery of these parameters for isosilybin A. Inhibition of midazolam 1′-hydroxylation in HLMs by silymarin at 100 μM (47%) was comparable to that by a different silymarin product at approximately 50 μM (43%) in a previous study (Etheridge et al., 2007). With microsomal preparations, silibinin was a slightly more potent inhibitor of both nifedipine dehydrogenation (IC50, 27–60 μM) (Zuber et al., 2002) and testosterone 6β-hydroxylation (IC50, 50 μM) (Jancova et al., 2007) compared with midazolam 1′-hydroxylation (IC50, 67–120 μM). Although these comparisons of IC50 values have inherent limitations, the apparent substrate-dependent inhibition is consistent with multiple CYP3A4 substrate-binding domains (Schrag and Wienkers, 2001; Galetin et al., 2003). Because midazolam was the only substrate tested in the current work, the reversible inhibition kinetics of individual constituents may not correlate with other CYP3A subclasses. In addition, the extent of inhibition by silymarin at 100 μM was greater than expected assuming additive inhibition by flavonolignans in aggregate (Fig. 3), suggesting the potential for synergistic inhibition of CYP3A by milk thistle flavonolignans. Further evaluation is needed to confirm this putative synergism.
Except for silychristin, all tested constituents and both extracts demonstrated potential MBI of CYP3A activity with HLMs, HIMs, and rCYP3A4 (Table 2), as evidenced by a >1.5-fold shift in IC50 (Berry and Zhao, 2008). However, the kinetic parameters associated with MBI could only be recovered using rCYP3A4. The KI for silibinin in the current work (110 μM) was within, whereas the kinact (0.20 min−1) was roughly 3 times faster than, corresponding parameters reported using the CYP3A substrates 7-benzyloxy-4-(trifluoromethyl) coumarin (32 μM and 0.06 min−1) and testosterone (132 μM and 0.08 min−1) (Sridar et al., 2004). The difference in kinact may reflect different enzyme sources (recombinant versus reconstituted CYP3A4). Loss of rCYP3A4 activity was rapid, occurring within the 2- to 4-minute incubation times for the reversible inhibition experiments. As such, some MBIs would be expected under these conditions; however, the presence of midazolam should mitigate enzyme inactivation through substrate protection (Silverman, 1995).
Unlike with rCYP3A4 (current work) and reconstituted CYP3A4 (Sridar et al., 2004), MBI was not evident with either microsomal preparation. P450-mediated metabolism can generate superoxide and/or hydrogen peroxide, potentially leading to enzyme autoinactivation (Kalgutkar et al., 2007). Isolated enzyme systems are believed to be more sensitive to reactive oxygen species, manifesting as MBI-like behavior; however, inclusion of reactive species scavengers would abrogate this process (Kalgutkar et al., 2007). The likely presence of CYP3A5 in the microsomal preparations also may have compensated for the loss of CYP3A4 activity. Unlike the IC50 shift experiment, saturating substrate concentrations were used for the MBI experiment, allowing potential compensation by CYP3A5 if this enzyme is less susceptible to MBI (McConn et al., 2004).
The major oxidative metabolite of silibinin is reported to be an O-demethylated product produced by CYP2C8 and CYP3A4 (Jancova et al., 2007). Further oxidation of the resulting catechol could create a reactive 1,2-benzoquinone moiety. However, MBI by this mode was unlikely, as MBI was not observed with compounds containing either the catechol (taxifolin) (data not shown) or the 2-methoxyphenol (silychristin) moiety (Fig. 1; Table 2). The only constituents that tested positive for MBI contained a 1,4-dioxane moiety (Fig. 1). Oxidation of this region may create reactive oxygen species capable of inactivating CYP3A4. Since activity loss was not abrogated by the trapping agents, the inactivating species exerted their effect before leaving the CYP3A4 active site. If this proposed mode of MBI is verified, this chemical moiety may be considered an addition to the list of structural alerts (Kalgutkar et al., 2007).
Following typical “doses” (<600 mg/day) of milk thistle preparations, individual constituents achieve relatively low peak systemic concentrations (Cmax, <1 μM) and are cleared rapidly from the systemic circulation (half-life, <4 hours) (Wen et al., 2008). Accordingly, the interaction potential of milk thistle due to reversible inhibition likely is limited to inhibition of first-pass clearance of sensitive substrates. The clinical interaction potential for mechanism-based inhibitors is higher than that for reversible inhibitors, because restoration of P450 activity is dependent on de novo protein synthesis rather than removal of the perpetrator compound(s) (Watanabe et al., 2007). For example, MBI has been hypothesized as the only means by which fruit juices can elicit clinically significant interactions with CYP3A substrates (Hanley et al., 2012). Whereas the maximum rates of rCYP3A4 inactivation by silybin A and silybin B were roughly half those reported for a major mechanism-based inhibitor in grapefruit juice, DHB (∼0.20 versus 0.41 min−1) (Paine et al., 2004), the KIs for silybin A and silybin B were ∼100 times greater than those for DHB (∼100 versus 1.1 μM). Thus, at equivalent exposures, DHB will inactivate CYP3A4 much more efficiently than the milk thistle constituents (370 versus ∼2 μl/min/pmol).
The high KI and low systemic exposure of silybin A and silybin B following milk thistle administration indicate a low risk for an interaction between milk thistle at typical doses and midazolam. The much higher colorectal tissue concentrations of silibinin compared with peripheral blood concentrations (140 ± 170 versus 3.0 ± 2.3 μM) following administration of 1.4 g/day to cancer patients (Hoh et al., 2006) further indicate that any clinically relevant MBI of CYP3A likely will be limited to the intestine. Enterocyte concentrations of silibinin this high would be expected to produce a 1.75-fold increase in systemic midazolam exposure based on the mechanistic static MBI equation (Obach et al., 2007; US FDA, 2012). Although the extent of MBI was not consistent between different enzyme preparations, predictions using parameters recovered from recombinant enzymes will provide more conservative estimates of interaction liability.
The large milk thistle doses required to achieve a relatively low risk of interaction with midazolam suggest that the clinical interaction liability of milk thistle may be limited to substrates sensitive to extensive intestinal first-pass extraction (e.g., simvastatin, felodipine) and/or to patient populations at risk for elevated milk thistle exposure (e.g., impaired hepatic function). Self-administration of milk thistle products is particularly popular among hepatitis C patients, in which an estimated 33% have used milk thistle as part of their therapeutic regimen (Seeff et al., 2008). Compared with healthy volunteers, patients with impaired hepatic function, including those with hepatitis C, demonstrated increased plasma exposure to milk thistle flavonolignans (Schrieber et al., 2008). Whereas the disease-related decrease in hepatic function alone could place these patients at increased risk for metabolic drug-drug interactions, intake of milk thistle products may provide an additional insult, increasing the risk for adverse events.
In summary, herb-drug interaction predictions are challenging due to the multitude of bioactive constituents typically composing herbal products. As such, identification of individual perpetrator compound(s) is necessitated. The current work outlines a framework to facilitate prospective evaluation of herb-drug interaction potential using milk thistle as a model herbal product. Of the eight constituents tested, this approach identified two constituents, silybin A and silybin B, which may perpetrate interactions via MBI of intestinal CYP3A activity. Products enriched with these constituents, such as silibinin, may have increased herb-drug interaction liability compared with other milk thistle products. Intestinal concentrations of these constituents could achieve those near the KI (∼100 μM), particularly with gram doses of silibinin that have been tested in patient populations (Flaig et al., 2007). Modeling and simulation incorporating these higher doses would help assess interaction liability of milk thistle products with CYP3A substrates sensitive to first-pass elimination. Refinement and application of this framework to other herbal products ultimately may assist clinicians and consumers to make informed decisions about the consequences of adding an herbal product to conventional pharmacotherapeutic regimens.
Acknowledgments
The authors thank Dr. Dhiren Thakker for his insightful discussions during the planning of experiments. M.F.P. dedicates this article to Dr. David P. Paine.
Abbreviations
- DHB
6′,7′-dihydroxybergamottin
- DMSO
dimethylsulfoxide
- HIMs
human intestinal microsomes
- HLMs
human liver microsomes
- HPLC
high-performance liquid chromatography
- MBI
mechanism-based inhibition
- P450
cytochrome P450
- rCYP3A4
recombinant CYP3A4
Authorship Contributions
Participated in research design: Brantley, Graf, Oberlies, Paine.
Conducted experiments: Brantley, Graf.
Contributed new reagents or analytic tools: Graf, Oberlies.
Performed data analysis: Brantley, Graf, Oberlies, Paine.
Wrote or contributed to the writing of the manuscript: Brantley, Graf, Oberlies, Paine.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01-GM077482] and National Center for Research Resources and National Center for Advancing Translational Sciences [Grant UL1TR000083]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. WinNonlin software was generously provided to the Division of Pharmacotherapy and Experimental Therapeutics, UNC Eshelman School of Pharmacy, by Certara as a member of the Pharsight Academic Center of Excellence Program.
This work was previously presented in part as a poster presentation: Brantley SJ, Graf TN, Oberlies NH, and Paine MF (2011) Identification of a single compound from the herbal supplement milk thistle as a candidate mechanism-based inhibitor of CYP3A activity. American Association of Pharmaceutical Scientists Annual Meeting and Exposition; 2011 Oct 23-27; Washington, DC.
References
- Beckmann-Knopp S, Rietbrock S, Weyhenmeyer R, Böcker RH, Beckurts KT, Lang W, Hunz M, Fuhr U. (2000) Inhibitory effects of silibinin on cytochrome P-450 enzymes in human liver microsomes. Pharmacol Toxicol 86:250–256 [DOI] [PubMed] [Google Scholar]
- Bent S. (2008) Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. J Gen Intern Med 23:854–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry LM, Zhao Z. (2008) An examination of IC50 and IC50-shift experiments in assessing time-dependent inhibition of CYP3A4, CYP2D6 and CYP2C9 in human liver microsomes. Drug Metab Lett 2:51–59 [DOI] [PubMed] [Google Scholar]
- Blumenthal M, Lindstrom A, Ooyen C, Lynch ME. (2012) Herb supplement sales increase 4.5% in 2011. HerbalGram 95:60–64 [Google Scholar]
- Brantley SJ, Oberlies NH, Kroll DJ, Paine MF. (2010) Two flavonolignans from milk thistle (Silybum marianum) inhibit CYP2C9-mediated warfarin metabolism at clinically achievable concentrations. J Pharmacol Exp Ther 332:1081–1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis-Searles PR, Nakanishi Y, Kim NC, Graf TN, Oberlies NH, Wani MC, Wall ME, Agarwal R, Kroll DJ. (2005) Milk thistle and prostate cancer: differential effects of pure flavonolignans from Silybum marianum on antiproliferative end points in human prostate carcinoma cells. Cancer Res 65:4448–4457 [DOI] [PubMed] [Google Scholar]
- Doehmer J, Tewes B, Klein KU, Gritzko K, Muschick H, Mengs U. (2008) Assessment of drug-drug interaction for silymarin. Toxicol In Vitro 22:610–617 [DOI] [PubMed] [Google Scholar]
- Etheridge AS, Black SR, Patel PR, So J, Mathews JM. (2007) An in vitro evaluation of cytochrome P450 inhibition and P-glycoprotein interaction with goldenseal, Ginkgo biloba, grape seed, milk thistle, and ginseng extracts and their constituents. Planta Med 73:731–741 [DOI] [PubMed] [Google Scholar]
- Flaig TW, Gustafson DL, Su LJ, Zirrolli JA, Crighton F, Harrison GS, Pierson AS, Agarwal R, Glodé LM. (2007) A phase I and pharmacokinetic study of silybin-phytosome in prostate cancer patients. Invest New Drugs 25:139–146 [DOI] [PubMed] [Google Scholar]
- Galetin A, Clarke SE, Houston JB. (2003) Multisite kinetic analysis of interactions between prototypical CYP3A4 subgroup substrates: midazolam, testosterone, and nifedipine. Drug Metab Dispos 31:1108–1116 [DOI] [PubMed] [Google Scholar]
- Gardiner P, Graham RE, Legedza AT, Eisenberg DM, Phillips RS. (2006) Factors associated with dietary supplement use among prescription medication users. Arch Intern Med 166:1968–1974 [DOI] [PubMed] [Google Scholar]
- Graf TN, Wani MC, Agarwal R, Kroll DJ, Oberlies NH. (2007) Gram-scale purification of flavonolignan diastereoisomers from Silybum marianum (Milk Thistle) extract in support of preclinical in vivo studies for prostate cancer chemoprevention. Planta Med 73:1495–1501 [DOI] [PubMed] [Google Scholar]
- Gurley BJ. (2012) Pharmacokinetic herb-drug interactions (part 1): origins, mechanisms, and the impact of botanical dietary supplements. Planta Med 78:1478–1489 [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Barone GW, Williams DK, Carrier J, Breen P, Yates CR, Song PF, Hubbard MA, Tong Y, Cheboyina S. (2006) Effect of milk thistle (Silybum marianum) and black cohosh (Cimicifuga racemosa) supplementation on digoxin pharmacokinetics in humans. Drug Metab Dispos 34:69–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurley BJ, Fifer EK, Gardner Z. (2012) Pharmacokinetic herb-drug interactions (part 2): drug interactions involving popular botanical dietary supplements and their clinical relevance. Planta Med 78:1490–1514 [DOI] [PubMed] [Google Scholar]
- Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Carrier J, Khan IA, Edwards DJ, Shah A. (2004) In vivo assessment of botanical supplementation on human cytochrome P450 phenotypes: Citrus aurantium, Echinacea purpurea, milk thistle, and saw palmetto. Clin Pharmacol Ther 76:428–440 [DOI] [PubMed] [Google Scholar]
- Han Y, Guo D, Chen Y, Chen Y, Tan ZR, Zhou HH. (2009) Effect of silymarin on the pharmacokinetics of losartan and its active metabolite E-3174 in healthy Chinese volunteers. Eur J Clin Pharmacol 65:585–591 [DOI] [PubMed] [Google Scholar]
- Hanley MJ, Masse G, Harmatz JS, Court MH, Greenblatt DJ. (2012) Pomegranate juice and pomegranate extract do not impair oral clearance of flurbiprofen in human volunteers: divergence from in vitro results. Clin Pharmacol Ther 92:651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoh C, Boocock D, Marczylo T, Singh R, Berry DP, Dennison AR, Hemingway D, Miller A, West K, Euden S, et al. (2006) Pilot study of oral silibinin, a putative chemopreventive agent, in colorectal cancer patients: silibinin levels in plasma, colorectum, and liver and their pharmacodynamic consequences. Clin Cancer Res 12:2944–2950 [DOI] [PubMed] [Google Scholar]
- Hu Z, Yang X, Ho PC, Chan SY, Heng PW, Chan E, Duan W, Koh HL, Zhou S. (2005) Herb-drug interactions: a literature review. Drugs 65:1239–1282 [DOI] [PubMed] [Google Scholar]
- Izzo AA, Ernst E. (2009) Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 69:1777–1798 [DOI] [PubMed] [Google Scholar]
- Jancová P, Anzenbacherová E, Papousková B, Lemr K, Luzná P, Veinlichová A, Anzenbacher P, Simánek V. (2007) Silybin is metabolized by cytochrome P450 2C8 in vitro. Drug Metab Dispos 35:2035–2039 [DOI] [PubMed] [Google Scholar]
- Kalgutkar AS, Obach RS, Maurer TS. (2007) Mechanism-based inactivation of cytochrome P450 enzymes: chemical mechanisms, structure-activity relationships and relationship to clinical drug-drug interactions and idiosyncratic adverse drug reactions. Curr Drug Metab 8:407–447 [DOI] [PubMed] [Google Scholar]
- Kennedy J, Wang CC, Wu CH. (2008) Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med 5:451–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim NC, Graf TN, Sparacino CM, Wani MC, Wall ME. (2003) Complete isolation and characterization of silybins and isosilybins from milk thistle (Silybum marianum). Org Biomol Chem 1:1684–1689 [DOI] [PubMed] [Google Scholar]
- Kroll DJ, Shaw HS, Oberlies NH. (2007) Milk thistle nomenclature: why it matters in cancer research and pharmacokinetic studies. Integr Cancer Ther 6:110–119 [DOI] [PubMed] [Google Scholar]
- McConn DJ, 2nd, Lin YS, Allen K, Kunze KL, Thummel KE. (2004) Differences in the inhibition of cytochromes P450 3A4 and 3A5 by metabolite-inhibitor complex-forming drugs. Drug Metab Dispos 32:1083–1091 [DOI] [PubMed] [Google Scholar]
- Monti D, Gazák R, Marhol P, Biedermann D, Purchartová K, Fedrigo M, Riva S, Kren V. (2010) Enzymatic kinetic resolution of silybin diastereoisomers. J Nat Prod 73:613–619 [DOI] [PubMed] [Google Scholar]
- Ngo N, Yan Z, Graf TN, Carrizosa DR, Kashuba AD, Dees EC, Oberlies NH, Paine MF. (2009) Identification of a cranberry juice product that inhibits enteric CYP3A-mediated first-pass metabolism in humans. Drug Metab Dispos 37:514–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obach RS, Walsky RL, Venkatakrishnan K. (2007) Mechanism-based inactivation of human cytochrome p450 enzymes and the prediction of drug-drug interactions. Drug Metab Dispos 35:246–255 [DOI] [PubMed] [Google Scholar]
- Paine MF, Criss AB, Watkins PB. (2004) Two major grapefruit juice components differ in intestinal CYP3A4 inhibition kinetic and binding properties. Drug Metab Dispos 32:1146–1153 [DOI] [PubMed] [Google Scholar]
- Paine MF, Oberlies NH. (2007) Clinical relevance of the small intestine as an organ of drug elimination: drug-fruit juice interactions. Expert Opin Drug Metab Toxicol 3:67–80 [DOI] [PubMed] [Google Scholar]
- Paine MF, Shen DD, Kunze KL, Perkins JD, Marsh CL, McVicar JP, Barr DM, Gillies BS, Thummel KE. (1996) First-pass metabolism of midazolam by the human intestine. Clin Pharmacol Ther 60:14–24 [DOI] [PubMed] [Google Scholar]
- Post-White J, Ladas EJ, Kelly KM. (2007) Advances in the use of milk thistle (Silybum marianum). Integr Cancer Ther 6:104–109 [DOI] [PubMed] [Google Scholar]
- Schrag ML, Wienkers LC. (2001) Covalent alteration of the CYP3A4 active site: evidence for multiple substrate binding domains. Arch Biochem Biophys 391:49–55 [DOI] [PubMed] [Google Scholar]
- Schrieber SJ, Wen Z, Vourvahis M, Smith PC, Fried MW, Kashuba AD, Hawke RL. (2008) The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic Fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos 36:1909–1916 [DOI] [PubMed] [Google Scholar]
- Seeff LB, Curto TM, Szabo G, Everson GT, Bonkovsky HL, Dienstag JL, Shiffman ML, Lindsay KL, Lok AS, Di Bisceglie AM, et al. HALT-C Trial Group (2008) Herbal product use by persons enrolled in the hepatitis C Antiviral Long-Term Treatment Against Cirrhosis (HALT-C) Trial. Hepatology 47:605–612 [DOI] [PubMed] [Google Scholar]
- Silverman RB. (1995) Mechanism-based enzyme inactivators. Methods Enzymol 249:240–283 [DOI] [PubMed] [Google Scholar]
- Sridar C, Goosen TC, Kent UM, Williams JA, Hollenberg PF. (2004) Silybin inactivates cytochromes P450 3A4 and 2C9 and inhibits major hepatic glucuronosyltransferases. Drug Metab Dispos 32:587–594 [DOI] [PubMed] [Google Scholar]
- Sy-Cordero AA, Day CS and Oberlies NH (2012) Absolute configuration of isosilybin A by X-ray crystallography of the heavy atom analogue 7-(4-bromobenzoyl)isosilybin A. J Nat Prod 75:1879–1881. [DOI] [PMC free article] [PubMed]
- Thummel KE, O’Shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, Wilkinson GR. (1996) Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther 59:491–502 [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration (2012) Draft Guidance: Drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendations, US Food and Drug Administration, Rockville, MD. [Google Scholar]
- Wang MZ, Wu JQ, Bridges AS, Zeldin DC, Kornbluth S, Tidwell RR, Hall JE, Paine MF. (2007) Human enteric microsomal CYP4F enzymes O-demethylate the antiparasitic prodrug pafuramidine. Drug Metab Dispos 35:2067–2075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe A, Nakamura K, Okudaira N, Okazaki O, Sudo K. (2007) Risk assessment for drug-drug interaction caused by metabolism-based inhibition of CYP3A using automated in vitro assay systems and its application in the early drug discovery process. Drug Metab Dispos 35:1232–1238 [DOI] [PubMed] [Google Scholar]
- Wen Z, Dumas TE, Schrieber SJ, Hawke RL, Fried MW, Smith PC. (2008) Pharmacokinetics and metabolic profile of free, conjugated, and total silymarin flavonolignans in human plasma after oral administration of milk thistle extract. Drug Metab Dispos 36:65–72 [DOI] [PubMed] [Google Scholar]
- Won CS, Oberlies NH, Paine MF. (2012) Mechanisms underlying food-drug interactions: inhibition of intestinal metabolism and transport. Pharmacol Ther 136:186–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanger UM, Schwab M. (2013) Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138:103–141 [DOI] [PubMed] [Google Scholar]
- Zuber R, Modrianský M, Dvorák Z, Rohovský P, Ulrichová J, Simánek V, Anzenbacher P. (2002) Effect of silybin and its congeners on human liver microsomal cytochrome P450 activities. Phytother Res 16:632–638 [DOI] [PubMed] [Google Scholar]