Abstract
Sulfotransferase (SULT) function has been well studied in healthy human subjects by quantifying mRNA and protein expression and determining enzyme activity with probe substrates. However, it is not well known if sulfotransferase activity changes in metabolic and liver disease, such as diabetes, steatosis, or cirrhosis. Sulfotransferases have significant roles in the regulation of hormones and excretion of xenobiotics. In the present study of normal subjects with nonfatty livers and patients with steatosis, diabetic cirrhosis, and alcoholic cirrhosis, we sought to determine SULT1A1, SULT2A1, SULT1E1, and SULT1A3 activity and mRNA and protein expression in human liver tissue. In general, sulfotransferase activity decreased significantly with severity of liver disease from steatosis to cirrhosis. Specifically, SULT1A1 and SULT1A3 activities were lower in disease states relative to nonfatty tissues. Alcoholic cirrhotic tissues further contained lower SULT1A1 and 1A3 activities than those affected by either of the two other disease states. SULT2A1, on the other hand, was only reduced in alcoholic cirrhotic tissues. SULT1E1 was reduced both in diabetic cirrhosis and in alcoholic cirrhosis tissues, relative to nonfatty liver tissues. In conclusion, the reduced levels of sulfotransferase expression and activity in diseased versus nondiseased liver tissue may alter the metabolism and disposition of xenobiotics and affect homeostasis of endobiotic sulfotransferase substrates.
Introduction
Sulfonation has a significant role in the biotransformation of numerous endogenous low-molecular weight compounds, including catecholamine neurotransmitters, steroids, and thyroid hormones (Negishi et al., 2001). Furthermore, it is an important pathway in the biotransformation of a number of xenobiotics, including drugs and chemicals (Negishi et al., 2001). Sulfonation reaction mechanism is based on the substitution of a hydrogen atom from the functional phenolic or alcoholic group of acceptor molecule with a sulfonyl group (–SO3−) from the universal donor molecule 3′-phosphoadenosine-5′-phosphosulfate (PAPS). Thus, the substrate of sulfonation is a neutral phenol or alcohol (R-OH), and the product of sulfonation is a highly polar sulfate (R-OSO3−) at physiologic pH (Klaassen and Boles, 1997).
One function of sulfonation is the defense mechanism against certain chemicals via elimination from the body. Sulfotransferase (SULT)1A1 is the isoform responsible for the metabolism and subsequent disposition of a number of exogenous substances possessing a small phenolic structure, such as acetaminophen (Reiter and Weinshilboum, 1982), 4-hydroxytamoxifen (Falany et al., 2006), and synthetic estrogens (bisphenol A, diethylstilbestrol) (Suiko et al., 2000). A second function of sulfonation is the inactivation of steroid hormones and neurotransmitters. The sulfated form of estradiol and testosterone are not receptor-active, and sulfonation by SULT2A1 and SULT1E1 serves to reduce concentration of active ligand (Falany, 1997). A third function of sulfonation involves biosynthesis of the androgen and estrogen hormones by enabling their precursor, dehydroepiandrosterone (DHEA), to be transported in plasma in the soluble form, DHEA-sulfate. DHEA-sulfate can be synthesized directly from cholesterol-sulfate or formed from DHEA by SULT2A1 (Falany, 1997).
The presence of sulfated metabolites of serotonin, norepinephrine, and pregnenolone in brain and plasma points out the role of sulfotransferases in neurotransmitter metabolism (Costa et al., 1983; Strobel et al., 1999; Schumacher et al., 2008). In addition, dopamine sulfate is detected in plasma at levels much higher than those of free dopamine. Free dopamine plasma concentration (<0.1 pmol/ml) is less than 1% of its sulfate-conjugated form concentration (Eisenhofer et al., 1999). SULT1A3 is responsible for dopamine sulfonation and is highly expressed in the gastrointestinal tract of humans, where the majority of dopamine sulfate (75%) is produced in the body. In animal models, dopamine sulfate regulates gut motility in mice (Haskel and Hanani, 1994), intestinal sodium absorption in weaning rats (Finkel et al., 1994), and gastroprotective effects in rats (Glavin, 1991). Its function in human is less well known.
Type 2 diabetes mellitus is defined as decreased insulin secretion or insulin sensitivity that results in elevated fasting blood glucose (Chiang et al., 2011), which presents a risk factor for nonalcoholic fatty liver disease (NAFLD) (Lattuada et al., 2011). NAFLD is the most common chronic liver disease in the industrialized countries. Current estimates of NAFLD range from 5% to 33% of United States population, but true prevalence is likely to be higher as many people remain undiagnosed in early stages due to the lack of inexpensive and noninvasive screening tests (Lazo and Clark, 2008; Moore, 2010). NAFLD is characterized as the accumulation of fat in liver in the absence of excessive alcohol intake. The main pathogenic mechanism of NAFLD is insulin resistance, which can be caused by genetic determinants, poor nutrition, and lifestyle (Pascale et al., 2010). NAFLD ranges from simple steatosis (fat without inflammation) to cirrhosis (McClain et al., 2004). Although development of cirrhosis subsequent to steatosis is becoming much more common, alcohol abuse and viral hepatitis currently remain the most common causes of cirrhosis (Starr and Raines, 2011). Approximately 90% of individuals who drink more than 60 g of alcohol daily develop steatosis (Crabb, 1999). This is a benign, asymptomatic condition, usually reversed within 4 to 6 weeks of abstaining from alcohol, with a low risk (5–15%) of developing fibrosis and cirrhosis (Leevy, 1962; Sorensen et al., 1984). However, the risk of progression into cirrhosis increases to 37% with continuous alcohol intake (Teli et al., 1995).
The aforementioned diseases have been found to alter the expression and activity of various enzymes important to drug disposition and homeostasis of endogenous molecules, such as steroid hormones, cholesterol, bile acids, and neurotransmitters (Buechler and Weiss, 2011; Merrell and Cherrington, 2011). Whereas sulfotransferase is an important regulator of the disposition of these endogenous and exogenous molecules, sulfotransferase activity has not been well characterized under disease conditions. The findings from this study are expected to clarify the liver tissue expression and activity levels of a major drug metabolism enzyme family at various liver disease states. Our results may help to determine therapeutic dosing while preventing the toxicity problems associated with drugs that are metabolized via sulfonation, and to better understand the effect of liver disease on homeostasis of endobiotic sulfotransferase substrates.
Materials and Methods
[35S]PAPS (1.5–2.54 Ci/mmol), [3H]17β-estradiol (110 Ci/mmol), and scintillation fluid (Optima Flo-M) were purchased from PerkinElmer Life and Analytical Sciences (Waltham, MA). Anti-SULT1A1 (ARP49134_P050) and anti-SULT1E1 (ARP48669_P050) were purchased from Aviva Systems Biology (San Diego, CA). Anti-SULT1A3 (ab92476) and anti-SULT2A1 (ab38416) were purchased from Abcam. Dopamine, p-nitrophenol (pNP), 2,6-dichlorophenolindophenol, dicumarol, and NADPH were purchased from Sigma-Aldrich (St. Louis, MO). DHEA was purchased from Steraloids Inc. (Newport, RI).
Cytosolic Fraction from Isolated Liver Tissue.
Human liver tissues were purchased from Liver Tissue Cell Distribution System, University of Minnesota, Minneapolis, MN. The details of the human liver donors are described (Supplemental Table 1), according to the data provided by the manufacturer: steatosis (n = 13 individuals), diabetes (n = 4), diabetic cirrhosis (n = 22), alcohol cirrhosis (n = 22) and nonfatty liver (n = 20). Liver samples were stored frozen at −80°C until the cytosolic fractions were prepared. Each liver tissue sample measured approximately 300 mg and was homogenized in buffer containing 0.25 M sucrose, 1 M Tris-HCl (pH 7.8), 0.01 M EDTA, 0.5 mM butylated hydroxytoluene, and 1 mM dithiothreitol with a PowerGen 125 tissue homogenizer three times for ∼10–20 seconds, keeping the tissue on ice between each interval. The homogenate was ultra-centrifuged at 100,000g for 1 hour, 4°C with Sorvall Discovery M120 SE (Hitachi Ltd., Tokyo, Japan). Aliquots of supernatant (cytosol) were stored at −80°C until assay. The protein concentration of cytosolic fractions was determined at 280 nm with Nano Drop ND1000 UV-Vis spectrophotometer (Thermo Fisher Scientific, Waltham, MA).
Sulfotransferase Activity.
Sulfotransferase enzymes present in the cytosolic fraction of liver tissue were incubated with radiolabeled sulfonyl donor 35S-PAPS and a prototype substrate in 20 mM potassium phosphate (pH 7.0). For these assays PAPS was used at a concentration of 3–6 μM, which is near its Km value. The reaction mixture was incubated for a defined time at 37°C, stopped by heating in boiling water for 30 seconds, and centrifuged at 14,000g for 1 minute to pellet the protein. For separation of sulfated products of pNP, estradiol, and DHEA, the resulting supernate was injected onto Synergi Polar-RP column (50 × 2.00 mm, 4 μm) (Phenomenex, Torrance, CA). An appropriate gradient of 20 mM potassium phosphate (pH 2.7) and acetonitrile was used as mobile phase to separate excess 35S-PAPS from 35S-product. For dopamine-sulfate separation, a Hypersil Duet C18/SAX column (150 × 4.6 mm, 5 μm; Thermo Fisher Scientific) with a mobile phase of ammonium bicarbonate (pH 8.0) and acetonitrile was used. Radiolabeling was detected and quantified on a flow scintillation analyzer (500 TR series; Packard Bioscience, Meriden, CT) with PerkinElmer Ultima Flo-M scintillation cocktail. The 35S-PAPS was eluted with the solvent front, pNP sulfate at 1.5 minutes, DHEA sulfate at 5 minutes, estradiol sulfate at 4.5 minutes, and dopamine sulfate at 3.5 minutes (Supplemental Fig. 1). The dopamine sulfate produced was eluted as a single peak and was not further analyzed for regioisomeric composition.
Immunoblot Analysis.
Protein expression of four major hepatic sulfotransferases was quantified by immunoblotting. To reduce experimental variability, 38 samples were loaded on a single gel (20 cm × 20 cm) so that all samples were contained in two large gels. This two-gel set was subsequently transblotted, and antibody incubations were conducted such that all 76 samples were treated simultaneously. The two-gel set was conducted a total of four times for incubation with each of the four anti-SULT isoform antibodies. Cytosolic protein (40 μg protein/well) was resolved by SDS-polyacrylamide gel electrophoresis (12% resolving and 8% stacking gel). Proteins were transblotted onto polyvinylidene difluoride membrane (Millipore Corporation, Billerica, MA) at 100 V for 40 minutes. After washing the membrane with Tris-buffered saline for 2 hours, it was incubated with primary antibody (anti-SULT1A1, anti-SULT1E1, or anti-SULT1A3) diluted in Tris-buffered saline with Tween 20 overnight at 4°C. For SULT2A1 detection, the membrane was incubated for 1 hour with anti-SULT2A1 antibody. Each membrane was then washed and incubated with infrared dye-labeled secondary antibody (Li-Cor, Lincoln, NE) for 1 hour at room temperature in the dark. Infrared signal of Western blot bands were detected and quantified using an Odyssey Infrared Imaging System (Li-Cor). Antibody selectivity was tested as shown in Supplemental Fig. 2.
RNA Extraction.
Total RNA from human livers (280–320 mg of each sample) were isolated by phenol-chloroform extraction using RNA Bee reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer's protocol. Total RNA quantification was made by measuring the absorbance at 260 nm in a UV-visible spectrophotometer (NanoDrop ND 1000; Thermo Fisher Scientific). RNA integrity was verified by formaldehyde-agarose gel electrophoresis.
Branched DNA Signal Amplification Assay for mRNA Quantification.
Assay was similar to previously published (Hardwick et al., 2013). All reagents for analysis, including oligonucleotide primers designed specifically for each sulfotransferase, lysis buffer, amplifier/label probe diluent, and substrate solution, were supplied in the QuantiGene HV signal amplification kit (Panomics, Fremont, CA). Primers designed specifically for each sulfotransferase were diluted in lysis buffer. On the first day, RNA samples diluted to 1 μg/μl were added to each of the 96-well plates containing 50 μl of capture hybridization buffer and 100 μl of diluted probe set. The RNA was allowed to hybridize overnight with a probe set at 53°C. On the second day of the assay, subsequent hybridization steps were followed as mentioned in the manufacturer's protocol, and luminescence was measured with a Quantiplex 320 branched DNA luminometer interfaced with Quantiplex Data Management Software (version 5.02; Bayer Corp., Tarrytown, NY). The luminescence for each well was reported as relative light units per 10 μg of total RNA.
Quantitative Real-Time Polymerase Chain Reaction for mRNA Quantification of Human SULT1A3.
Quantitative real-time polymerase chain reaction was used to measure mRNA expression of SULT1A3, which was not available in the QuantiGene Plex used. Total RNA was converted to cDNA, then mRNA levels were quantified by quantitative real-time polymerase chain reaction using a Roche LightCycler 480 System (Roche Applied Science, Mannheim, Germany). SYBR green chemistry was used, and relative target gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase. The SULT1A3 primers used were published by Dooley et al. (2000).
Statistical Analysis.
Raw data from mRNA quantification were normalized to housekeeping gene 60S ribosomal protein L13a (RPL13A). Sulfotransferase activity and normalized mRNA data were log transformed and then plotted as quantile-quantile plots (QQplot) and box plots. The linearity of the points from QQplot suggests that the data were normally distributed. The differences in mRNA quantification and sulfotransferase activity quantification between groups and genders were determined by multivariate analysis of variance using a significance level of 0.05. Data from SULT protein quantification were normalized to anti–glyceraldehyde-3-phosphate dehydrogenase quantification and was analyzed by SPSS version 19.0 software (IBM, Armonk, NY) using analysis of variance and considering the difference of P ≤ 0.05 as statistically significant. The diabetes group, containing only four individuals, was too small for group statistical comparisons, and the data were used for qualitative comparison only. The remaining groups had sufficient sample size, and the log transformation of raw data demonstrated a normal distribution.
Results
Liver Disease Affects Sulfotransferase Activity.
SULT1A1 (using 4 µM pNP), SULT2A1 (10 µM DHEA), SULT1E1 (20 nM estradiol), and SULT1A3 (10 µM dopamine) activities were determined at concentrations that were selective in human liver cytosol for each isoform (Falany et al., 1995; Tabrett and Coughtrie, 2003; Yasuda et al., 2007; Huang et al., 2010). Incubation time and amount of cytosolic protein to achieve appropriate linear kinetic conditions for each assay were empirically determined. Activity assays for each isoform in each tissue sample were performed in duplicate except for SULT1A3, which was taken as a single measurement per tissue sample. Sulfonation was quantified in liver samples from humans diagnosed with steatosis (n = 13 individuals), diabetes (n = 4), diabetic cirrhosis (n = 22), alcohol cirrhosis (n = 22) and nonfatty liver (n = 20) (Figs. 1A, 2A, 3A, and 4A). The diabetes group, containing only four individuals, was too small for group statistical comparisons and the data are shown for qualitative comparison only.
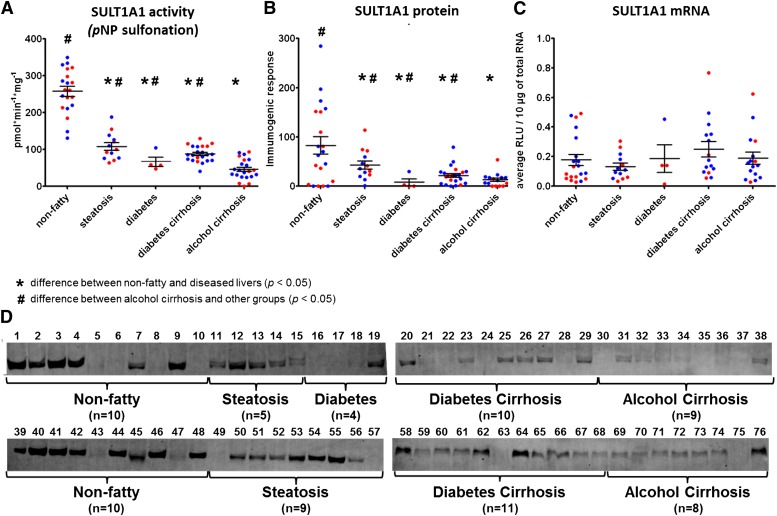
Fig. 1.
Expression and activity of SULT1A1 in nonfatty (control) and diseased human livers. Each data point represents a single tissue (average of determinations) categorized by gender and disease type (n = 20 for nonfatty; n = 13–14 for steatosis; n = 4 for diabetes; n = 21–22 diabetic cirrhosis; and n = 17–22 for alcohol cirrhosis). Tissue from female patients is displayed in red; from male patients, in blue. *Statistically significant differences (P < 0.05) between nonfatty and diseased livers. #Statistically significant differences (P < 0.05) between alcohol cirrhosis and other groups. (A) Enzyme activity was determined by incubating 4 μM pNP with human liver cytosols for 30 minutes in the presence of the 35S-labeled cofactor PAPS. (B) Protein expression of SULT1A1 was quantified by Western blotting analysis in human livers. (C) Messenger RNA expression of SULT1A1 was quantified by branched DNA signal amplification assay (Affymetrix Inc., Santa Clara, CA). (D) Membranes loaded with 40 μg protein and probed with anti-SULT1A1 antibody. Lane numbers refer to sample ID as noted in Supplemental Table 1.
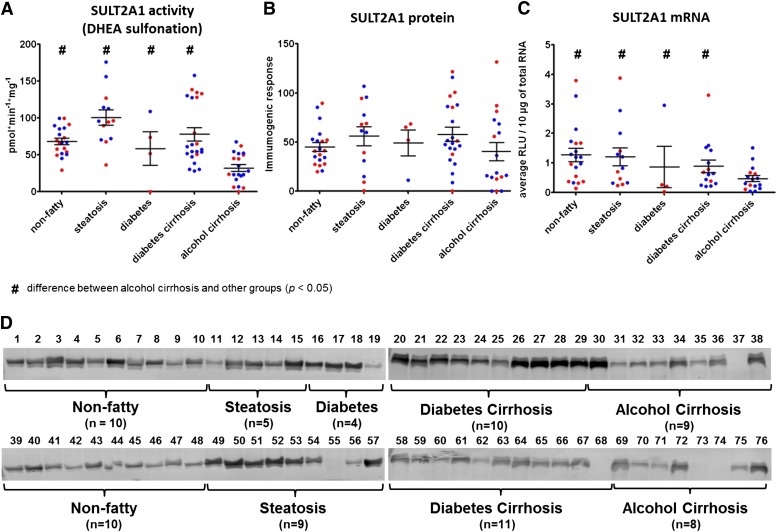
Fig. 2.
Expression and activity of SULT2A1 in nonfatty (control) and diseased human livers. Each data point represents a single tissue (average of determinations) categorized by gender and disease type (n = 20 for nonfatty; n = 13–14 for steatosis; n = 4 for diabetes; n = 21–22 diabetic cirrhosis; and n = 17–22 for alcohol cirrhosis). Tissue from female patients is displayed in red; from male patients, in blue. #Statistically significant differences (P < 0.05) between alcohol cirrhosis and other groups. (A) Enzyme activity was determined by incubating 10 μM DHEA with human liver cytosols for 30 minutes in the presence of the 35S-labeled cofactor PAPS. (B) Protein expression of SULT2A1 was quantified by Western blotting analysis in human livers. (C) Messenger RNA expression of SULT2A1 was quantified by branched DNA signal amplification assay (Affymetrix Inc.). (D) Membranes loaded with 40 μg protein and probed with anti-SULT2A1 antibody. Lane numbers refer to sample ID as noted in Supplemental Table 1.
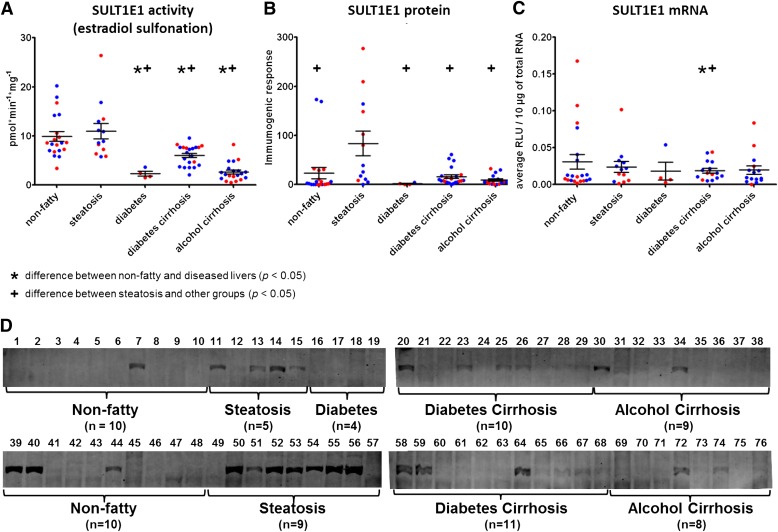
Fig. 3.
Expression and activity of SULT1E1 in nonfatty (control) and diseased human livers. Each data point represents a single tissue (average of determinations) categorized by gender and disease type (n = 20 for nonfatty; n = 13–14 for steatosis; n = 4 for diabetes; n = 21–22 diabetic cirrhosis; and n = 17–22 for alcohol cirrhosis). Tissue from female patients is displayed in red; from male patients, in blue. *Statistically significant differences (P < 0.05) between nonfatty and diseased liver groups. +Statistically significant differences (P < 0.05) between steatosis and other groups. (A) Enzyme activity was determined by incubating 20 nM 3H-labeled estradiol with human liver cytosols for 30 minutes in the presence of the 35S-labeled cofactor PAPS. (B) Protein expression of SULT1E1 was quantified by Western blotting analysis in human livers. (C) Messenger RNA expression of SULT1E1 was quantified by branched DNA signal amplification assay (Affymetrix Inc.). (D) Membranes loaded with 40 μg protein and probed with anti-SULT1E1 antibody. Lane numbers refer to sample ID as noted in Supplemental Table 1.
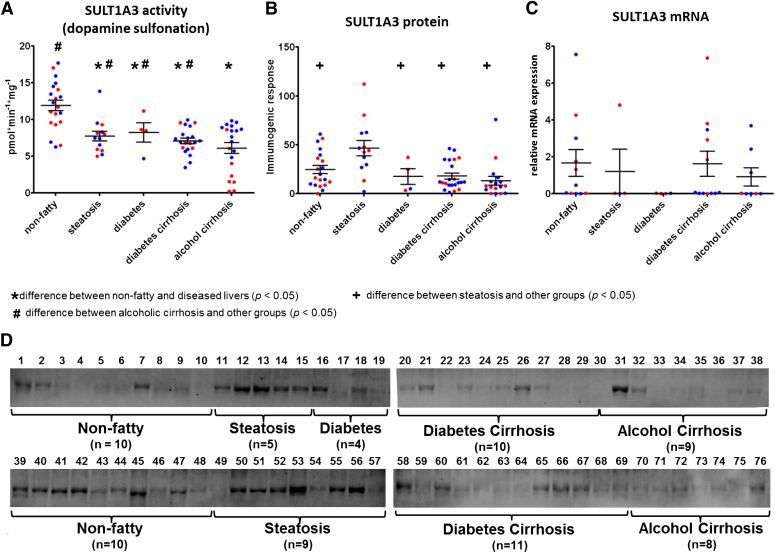
Fig. 4.
Expression and activity of SULT1A3 in nonfatty (control) and diseased human livers. Each data point represents a single tissue (average of determinations) categorized by gender and disease type (n = 20 for nonfatty; n = 13–14 for steatosis; n = 4 for diabetes; n = 21–22 diabetic cirrhosis; and n = 17–22 for alcohol cirrhosis). Tissue from female patients is displayed in red; from male patients, in blue. *Statistically significant differences (P < 0.05) between nonfatty and diseased livers. +Statistically significant differences (P < 0.05) between steatosis and other groups. #Statistically significant differences (P < 0.05) between alcoholic cirrhosis and other groups. (A) Enzyme activity was determined by incubating 10 μM dopamine with human liver cytosols for 30 minutes in the presence of the 35S-labeled cofactor PAPS. (B) Protein expression of SULT1A3 was quantified by Western blotting analysis in human livers. (C) Messenger RNA expression of SULT1A3 was quantified by quantitative real-time polymerase chain reaction using a Roche LightCycler 480 System (Roche Applied Science, Mannheim, Germany). (D) Membranes loaded with 40 μg protein and probed with anti-SULT1A3 antibody. Lane numbers refer to sample ID as noted in Supplemental Table 1.
Mean SULT1A1 activity was found to be decreased substantially in steatosis, diabetic cirrhosis, and alcoholic cirrhosis versus nonfatty controls, with no difference in men versus women (Fig. 1A; Table 1). For example, steatosis samples contained 42% of nonfatty control activity, diabetic cirrhosis samples contained 34% of nonfatty control activity, and alcoholic cirrhosis samples contained 18% of nonfatty control activity (P ≤ 0.0001). It is especially noteworthy that the SULT1A1 activity in alcoholic cirrhosis tissues was so low that it was also significantly decreased (P ≤ 0.02) from other disease states: a further 42% reduction from steatotic samples and a 52% reduction from diabetic cirrhotic samples. This indicates that tissues from individuals with cirrhosis caused by alcohol exposure have even lower SULT1A1 activity than tissues from individuals with diabetic cirrhosis (i.e., nonalcoholic cirrhosis).
TABLE 1.
Summary of changes in sulfonation activity of the four major sulfotransferases in diseased human livers versus nonfatty tissue
Left-right arrow (↔) indicates no change from the nonfatty, nondiseased liver. A single arrow (↓) indicates 59–65% of activity in nonfatty, nondiseased liver. A double arrow (↓↓) indicates 42–51% of activity in nonfatty, nondiseased liver. A triple arrow (↓↓↓) indicates 27–34% of activity in nonfatty, nondiseased liver. Four arrows (↓↓↓↓) indicates 18% of activity in nonfatty, nondiseased liver.
| Disease State | SULT1A1 | SULT1A3 | SULT1E1 | SULT2A1 |
|---|---|---|---|---|
| Steatosis | ↓↓ | ↓ | ↔ | ↔ |
| Diabetes cirrhosis | ↓↓↓ | ↓ | ↓ | ↔ |
| Alcohol cirrhosis | ↓↓↓↓ | ↓↓ | ↓↓↓ | ↓↓ |
In contrast to SULT1A1, neither steatotic nor diabetic cirrhotic tissues differed in their SULT2A1 activity versus nonfatty tissues (Fig. 2A; Table 1). However, mean SULT2A1 activity was found to be decreased substantially in the alcohol cirrhosis group. For example, alcohol cirrhosis samples contained 47% of nonfatty control activity, 32% of steatosis activity, and 41% of diabetic cirrhosis activity (P ≤ 0.0001). Gender difference was observed only in diabetic cirrhosis samples, with women possessing somewhat higher DHEA-sulfonation than men (P ≤ 0.03).
SULT1E1 activity in steatotic samples was not significantly reduced versus the nonfatty control samples (Fig. 3A; Table 1). However, similar to SULT1A1 and SULT2A1, SULT1E1 activity in the other disease states was significantly reduced versus nonfatty controls: 61% in diabetic cirrhosis and 27% in alcohol cirrhosis samples. Gender difference was observed in alcohol cirrhosis samples, with women possessing somewhat lower estradiol-sulfonation activity than males (P ≤ 0.04).
Similar to SULT1A1 activity, SULT1A3 activity was found to be decreased substantially in steatosis, diabetic cirrhosis, and alcoholic cirrhosis samples compared with nonfatty controls (Fig. 4A; Table 1). For example, steatosis samples contained 65% of nonfatty control activity, diabetic cirrhosis samples contained 59%, and alcoholic cirrhosis samples contained 51% (P ≤ 0.015). Of note, the SULT1A3 activity in alcoholic cirrhosis tissues was also significantly less than that seen in other disease states: 74 and 86% of activity measured in diabetic cirrhotic samples, respectively (P ≤ 0.02). Gender difference was observed in female alcohol cirrhosis samples, which contained substantially lower SULT1A3 activity than those from men (P ≤ 0.0001). Because the dopamine sulfate formed was eluted as a single 35S-labeled peak, it was not further analyzed for 3-O- or 4-O-sulfate regioisomeric position.
Table 1 summarizes the aforementioned effects of liver disease on human sulfotransferase activity. SULT1A1 and SULT1A3 activities were lower in disease states relative to nonfatty tissues. Alcoholic cirrhotic tissues further contained lower SULT1A1 and 1A3 activities than either of the two other disease states. SULT2A1, on the other hand, was reduced only in alcoholic cirrhotic tissues. SULT1E1 was reduced in both diabetic cirrhosis and alcoholic cirrhosis tissues, relative to nonfatty liver tissues.
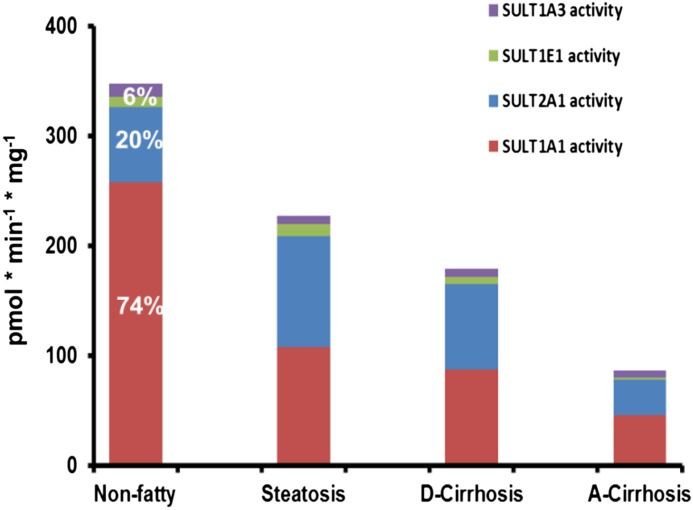
Figure 5 illustrates total and relative sulfonation capacity measured in nonfatty and diseased liver tissues. The relative contribution of each isoform to the total sulfotransferase activity demonstrates that SULT1A1 and SULT2A1 carried out more sulfonation than SULT1E1 and SULT1A3 in nonfatty and all disease types. In nonfatty livers, SULT1A1 and SULT2A1 were responsible for 74 and 20% of total sulfonation, whereas SULT1E1 and SULT1A3 together were responsible for only 6% of total sulfonation in nonfatty liver. This is consistent with results published previously (Riches et al., 2009) for nondiseased liver tissue. However, we found that sulfotransferase activity decreased with increased severity of liver disease from steatotic liver to cirrhosis. Compared with sulfotransferase activity in nonfatty livers, total sulfonation in steatosis samples was only 65%, sulfonation in diabetic cirrhosis samples was 51%, and sulfonation in alcohol cirrhosis samples was only 25% of total sulfonation in the nonfatty control group.
Fig. 5.
Total and relative sulfonation of SULT1A1, SULT2A1, SULT1E1, and SULT1A3 in non-fatty (control) and diseased human livers. Total sulfotransferase activity is indicated by colored bars in each tissue type. The percentage of sulfotransferase activities of four major isoforms were calculated for nonfatty (control) group.
Effects of Liver Disease on Sulfotransferase Protein Expression.
In addition to sulfotransferase activity, sulfotransferase protein expression was determined by Western blot. We have used commercially available anti-SULT1A1, anti-SULT2A1, anti-SULT1E1, and anti-SULT1A3 antibodies to detect the protein expression in the same human liver cytosols that we used to measure the sulfotransferase activity. Similar to activity, there was considerable individual variation in protein expression within the disease groups. The expression of four SULT proteins, SULT1A1, SULT2A1, SULT1E1, and SULT1A3, was detected at varying levels in human liver tissues affected by steatosis (n = 14 individuals), diabetes (n = 4), diabetic cirrhosis (n = 21), alcohol cirrhosis (n = 17), and in nonfatty livers (n = 20) (Figs. 1, B and D, 2, B and D, 3, B and D, and 4, B and D). The discrepancy in number of tissues in each group versus activity data was due to limited sample volumes. In contrast to activity and mRNA measurements, many of the immunogenic protein values were below the level of detection. This is not unexpected, because the sensitivities of the antibodies are limited.
In accordance with enzyme activity data, protein expression of SULT1A1 was found to be expressed at substantially higher amounts in nonfatty livers versus diseased livers. The expression was significantly reduced in diseased livers, including steatosis, diabetes cirrhosis, and alcohol cirrhosis (P ≤ 0.0001) (Fig. 1B). SULT2A1 protein expression was not different between groups, although the range of highest to lowest protein was larger in the diseased versus nonfatty tissues (Fig. 2B). SULT1E1 and SULT1A3 protein expressions were higher in steatosis samples compared with nonfatty, diabetes cirrhosis, and alcohol cirrhosis livers (P ≤ 0.05) (Figs. 3B and 4B).
Effects of Liver Disease on Sulfotransferase mRNA Expression.
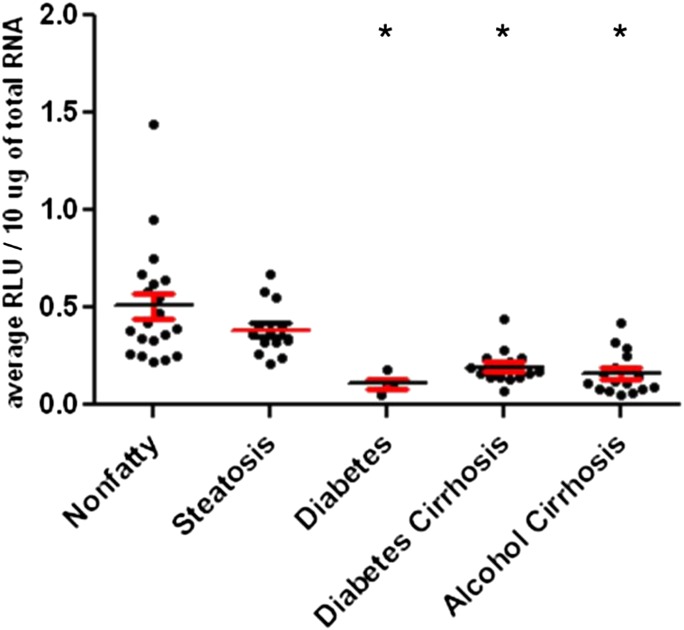
Along with sulfotransferase activity and protein expression, we have quantified mRNA expression of SULT1A1, SULT2A1, SULT1E1, SULT1A3, and PAPSs2 (PAPS synthase 2) in the same human liver tissues (Figs. 1C, 2C, 3C, 4C, and 6). Similar to the activity variations we observed, substantial interindividual variation was noted within disease groups. Specifically, SULT1A1 mRNA expression was detected at unexpectedly low levels in all liver samples, including nonfatty and disease groups, and there was no statistically significant difference in SULT1A1 mRNA detected between any groups (Fig. 1C). SULT2A1 mRNA expression was substantially lower in the alcohol cirrhosis group than in the nonfatty group and the other disease groups (P ≤ 0.05) (Fig. 2C). Gender difference was observed in SULT2A1 mRNA expression, which was found to be less abundant in female than male subjects (P ≤ 0.04). SULT1E1 mRNA expression in the steatosis group was similar to that in the nonfatty control group. SULT1E1 expression was significantly decreased in diabetes cirrhosis but not in alcohol cirrhosis group or the nonfatty and steatosis groups (P ≤ 0.05) (Fig. 3C). SULT1A3 mRNA expression did not show any significant difference between groups. PAPSs2 mRNA expression in the steatosis group trended lower than expression in the nonfatty control group, but the difference was not statistically significant (Fig. 6). However, PAPSs2 expression in diabetic and alcoholic cirrhosis groups was significantly reduced versus nonfatty controls and versus steatosis groups (P ≤ 0.0002). A gender difference was observed only in the alcohol cirrhosis group, with women having more PAPSs2 mRNA expression than men (P ≤ 0.0001) (Fig. 6).
Fig. 6.
PAPSs2 mRNA expression in humans in nonfatty (control) and diseased human livers. PAPSs2 mRNA expression was quantified by branched DNA signal amplification assay (Affymetrix Inc.). *Statistically significant differences (P < 0.05) between nonfatty and diseased livers.
Discussion
Phase II biotransformation enzymes have an essential role in termination of pharmacologic activity, detoxification, and elimination of xenobiotics from the body by conjugating small molecules to xenobiotics and thus altering their pharmacokinetic characteristics. To understand the contribution of these enzymes to the xenobiotic and endobiotic metabolism, we must elucidate the enzymes’ characteristics, including their tissue expression, activity, and regulation. Human sulfotransferases have been well characterized in healthy liver tissues (Riches et al., 2009), but little has been reported in diseased liver tissue (Elekima et al., 2000; Yeo et al., 2010; Hardwick et al., 2013). In this paper, we report sulfotransferase activity and expression in healthy liver tissue versus tissue of individuals diagnosed with steatosis, diabetes, diabetic cirrhosis, and alcohol cirrhosis.
One challenge in profiling these sulfotransferases is whether to focus on functional (activity) measurements or expression measurements at the translational (protein) or transcriptional (mRNA) level. This challenge is especially relevant in the sulfotransferase field because several studies have found weak correlations of activity to immunogenic protein to message (e.g., Duanmu et al., 2006; Riches et al., 2009; Hardwick et al., 2013). In our hands, we are most confident in the sulfotransferase activity measures and have emphasized these results throughout the Discussion section.
Our results show that, with expected interindividual differences, hepatic expression and activity of sulfotransferases varied between diseased and nonfatty livers. As required for its role in drug metabolism and disposition in liver, SULT1A1 catalyzed the majority of hepatic sulfonation. However, we found that the capacity of SULT1A1 diminished significantly in intact livers with steatosis and in more seriously diseased liver tissue. Furthermore, SULT1A1 protein expression was previously reported to be lost in the liver tissues of patients with hepatocellular carcinogenesis (Yeo et al., 2010). This reduction in SULT1A1 sulfonation may result in accumulation of xenobiotics eliminated through sulfate conjugation and, subsequently, the onset of adverse effects. Safe dosages and exposures may require re-evaluation for individuals with fatty liver disease.
SULT2A1 activity (DHEA sulfonation) was found to be decreased significantly only in the alcohol cirrhosis group but not in steatotic or diabetic cirrhotic livers compared with nonfatty controls. Steatotic livers resemble the early stage of NAFLD in which fat accumulation occurs without inflammation, fibrosis, or hepatocyte changes (Hashimoto and Tokushige, 2011). Interestingly, DHEA sulfonation of diabetic cirrhosis samples resulted in two subgroups—one resembling the high sulfonation seen in nonfatty livers and the other resembling the diminished sulfonation seen in livers with alcohol cirrhosis. A plausible explanation is that our diabetic cirrhosis group contained tissue samples reflecting a wide range of disease states, including livers with the onset of mild inflammation and others with severe inflammation in which the liver cells have been replaced by scar tissue. This observation will need further study.
Circulating levels of DHEA sulfate in serum have been reported in patients diagnosed with NAFLD in the absence of inflammation and with advanced NAFLD. Plasma DHEA sulfate levels were found to be increased with elevated alanine transaminase levels in NAFLD patients (Völzke et al., 2010; Koga et al., 2011). This may indicate a compensatory increase of adrenal secretion and sulfonation of DHEA to protect the liver. Indeed, DHEA was able to inhibit inflammatory cytokines and hepatocyte apoptosis and decrease serum alanine transaminase levels in a mouse model of hepatitis (Yoneda et al., 2004). However, with advanced NAFLD, where nonalcoholic steatohepatitis is present, DHEA sulfate levels have been found to be decreased (Charlton et al., 2008). This might be due to decreased hepatic sulfonation or reduced efflux of DHEA as a result of impaired activity of enzymes and transporters, respectively.
Administration of DHEA to rodents at physiologic concentrations and DHEA sulfate at much lower concentrations increased the size and number of peroxisomes present in the liver (Peters et al., 1996). To determine whether the nuclear receptor PPAR regulated the peroxisomal gene induction by DHEA sulfate, mice lacking a functional PPARα gene were administered DHEA sulfate and clofibrate, a known PPAR agonist. Both treatments induced peroxisome proliferator response in wild-type mice but not in PPARα-deficient mice (Peters et al., 1996). In addition, clofibrate treatment of primary cultured human hepatocytes increased human SULT2A1 mRNA, protein, and enzymatic activity (Fang et al., 2005). Therefore, PPAR is thought to contribute to DHEA sulfate–stimulated hepatic peroxisomal gene induction. Thus, the decreased SULT2A1 expression in alcohol cirrhosis livers could be associated with PPARα expression, which might be a potential target to maintain hormonal homeostasis in diseased liver tissue.
In addition to DHEA, SULT2A1 catalyzes metabolism of testosterone, whereas SULT1E1 catalyzes elimination of estrogens (Luu-The et al., 1996). Thus, both sulfotransferases can regulate the concentration of biologically active sex steroids in the liver and systemic circulation. Our results indicate decreased activity of SULT1E1 in livers with cirrhosis (both alcoholic and diabetic) versus nonfatty and steatotic livers. This may contribute to elevated levels of estrogens (estradiol and estrone) and continuous activation of estrogen receptor in cirrhotic livers. Elevated levels of systemic estrogen have been reported in patients with liver cirrhosis (Becker et al., 1991; Nakamuta et al., 1994; Montalto et al., 1997). In addition, gynecomastia has been commonly observed in male patients with cirrhosis, likely due to impaired estrogen metabolism (Narasaka et al., 2000). However, the biologic role of liver SULT1E1 is unclear at this time.
In contrast to the excess estrogen associated with cirrhosis, low estrogen conditions may play a role in development of NAFLD. NAFLD has been found to be more prevalent in men, postmenopausal women, and women with polycystic ovary syndrome than in premenopausal women, suggesting the hepatoprotective roles of estrogens in the healthy liver (Shimizu and Ito, 2007; Gutierrez-Grobe et al., 2010). Hepatic steatosis became evident in the aromatase-deficient mouse, which cannot produce estrogen, and steatosis was attenuated after estradiol treatment (Nemoto, et al., 2000). In contrast, nonobese, nondiabetic patients treated with tamoxifen were reported to develop progressive steatosis, which occasionally induced NASH and liver cirrhosis (Akhondi-Meybodi et al., 2011).
Previous studies could not detect immunogenic SULT1A3 protein in human liver cytosols (Teubner et al., 2007; Riches et al., 2009); however, we were able to detect protein expression of SULT1A3 in nonfatty and diseased human livers. The lack of previous detection of hepatic SULT1A3 protein expression could be related to binding affinity of the antibodies used in previous studies. By using a newly available commercial antibody that was highly specific to SULT1A3 protein and did not recognize recombinant-expressed SULT1A1, we were able to detect bands corresponding to the predicted molecular weight for SULT1A3 protein (Supplemental Fig. 2; Supplemental Table 2). In addition to expression, we were able to detect dopamine sulfonation in nonfatty and diseased livers. Our results indicate decreased activity of SULT1A3 in steatotic and cirrhotic (both diabetic and alcoholic) liver tissues compared with nonfatty livers. This may result in reduced sulfonation of catecholamines in liver disease; however, little is understood about the role of phenylethylamine or catecholamine action in hepatobiliary-intestinal function.
The universal sulfate donor PAPS is synthesized by PAPS synthase (PAPSs), a bifunctional protein including ATP-sulfurylase and APS-kinase activities (Venkatachalam, 2003). mRNA expression of PAPSs in diabetic and alcoholic cirrhosis samples was significantly reduced versus nonfatty controls and versus steatosis samples, indicating that cofactor deficiency may be an additional factor contributing to low sulfonation capacity in individuals with liver cirrhosis.
The diminished sulfotransferase expression and activity in liver disease could be related to inflammation (Bryan et al., 2013). Previous studies have shown the induction of proinflammatory cytokines with diet-induced obesity, which reduces levels of drug metabolism enzymes, including sulfotransferases (Ghose et al., 2011). Rodent models revealed lipopolysaccharide-induced inflammation caused suppression of Sult1a1 and Sult2a1 gene expression (Shimada et al., 1999; Kim et al., 2004). Accordingly, severe inflammation in alcohol-induced cirrhosis may contribute to the dramatically low levels of sulfotransferases found in cirrhotic human livers.
Use of human donor tissue often carries the concern of tissue integrity. Because our data showed decreased SULT activity in diseased liver tissue, we chose to measure an additional parameter known to be upregulated by liver disease, NADPH:quinone oxidoreductase 1 (NQO1) (Hardwick et al., 2010). NQO1 is a cytosolic protein that catalyzes the two-electron reduction of reactive quinones, which are capable of producing reactive oxygen species (Jaiswal, 2000). Our results showed an increasing trend in NQO1 activity with the progression of NAFLD (Supplemental Fig. 3), in agreement with Hardwick et al. (2010), indicating the potential to measure increased enzyme activity in the samples analyzed.
In summary, in this study we characterized the alteration of sulfotransferases at transcriptional and translational levels in human livers diagnosed with steatosis, diabetes, and cirrhosis. Both alcohol- and diabetes-induced cirrhosis showed profound reduction of major hepatic sulfotransferase isoforms (SULT1A1, SULT1E1, and SULT1A3), whereas the milder steatosis reduced only SULT1A1 and SULT1A3. The current study cannot confirm causation versus association but may lead to improved understanding of such important consequences as xenobiotic accumulation and toxicity due to diminished SULT1A1, alteration in androgen synthesis and metabolism due to modified SULT2A1, alteration in estrogen metabolism due to modified SULT1E1, or accumulation of catecholamines due to diminished SULT1A3 levels in diseased liver tissue.
Supplementary Material
Abbreviations
- DHEA
dehydroepiandrosterone
- NQO1
NADPH:quinone oxidoreductase 1
- NAFLD
nonalcoholic fatty liver disease
- pNP
para-nitrophenol
- PAPS
3′-phosphoadenosine-5′-phosphosulfate
- SULT
sulfotransferase
Authorship Contributions
Participated in research design: Yalcin, King.
Conducted experiments: Yalcin, More, Neira.
Contributed new reagents or analytic tools: Slitt.
Performed data analysis: Yalcin, Lu.
Wrote or contributed to the writing of the manuscript: Yalcin, King, Slitt, Cherrington.
Footnotes
This work was supported in part by the University of Rhode Island (URI) Foundation (R.S.K.); the URI Council for Research (R.S.K.); a URI Graduate Fellowship (E.B.Y.); and a Summer Undergraduate Research Fellowship from RI-INBRE (K.L.N.).
This work also was supported in part by the National Institutes of Health [Grants 5R-01ES-016042-03, 3R-01ES-016042-2S2, and 5K-22ES-013782-03] (to A.L.S.); and by the Rhode Island IDeA Network of Biomedical Research Excellence [P20RR016457-10] from the National Institutes of Health National Center for Research Resources (to R.S.K. and A.L.S.).
A portion of this work was previously presented: Emine Bihter Yalçin, Karissa Neira, Renae Gupta, Angela L. Slitt and Roberta S. King (2011) Sulfotransferase expression and activity is altered by human metabolic disease. 17th North America Regional International Society for the Study of Xenobiotics (ISSX) Meeting; 2011 Oct 16–20; Atlanta, GA. P78. Drug Metab Rev 42(S2).
 This article has supplemental material available at dmd.aspetjournals.org.
This article has supplemental material available at dmd.aspetjournals.org.
References
- Akhondi-Meybodi M, Mortazavy-Zadah MR, Hashemian Z, Moaiedi M. (2011) Incidence and risk factors for non-alcoholic steatohepatitis in females treated with tamoxifen for breast cancer. Arab J Gastroenterol 12:34–36 DOI:10.1016/j.ajg.2011.01.003. [DOI] [PubMed] [Google Scholar]
- Becker U, Gluud C, Farholt S, Bennett P, Micic S, Svenstrup B, Hardt F. (1991) Menopausal age and sex hormones in postmenopausal women with alcoholic and non-alcoholic liver disease. J Hepatol 13:25–32 [DOI] [PubMed] [Google Scholar]
- Bryan S, Baregzay B, Spicer D, Singal PK, Khaper N. (2013) Redox-inflammatory synergy in the metabolic syndrome. Can J Physiol Pharmacol 91:22–30 DOI:10.1139/cjpp-2012-0295. [DOI] [PubMed] [Google Scholar]
- Buechler C, Weiss TS. (2011) Does hepatic steatosis affect drug metabolizing enzymes in the liver? Curr Drug Metab 12:24–34 [DOI] [PubMed] [Google Scholar]
- Charlton M, Angulo P, Chalasani N, Merriman R, Viker K, Charatcharoenwitthaya P, Sanderson S, Gawrieh S, Krishnan A, Lindor K. (2008) Low circulating levels of dehydroepiandrosterone in histologically advanced nonalcoholic fatty liver disease. Hepatology 47:484–492 DOI:10.1002/hep.22063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang DJ, Pritchard MT, Nagy LE. (2011) Obesity, diabetes mellitus, and liver fibrosis. Am J Physiol Gastrointest Liver Physiol 300:G697–G702 DOI:10.1152/ajpgi.00426.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa JL, Launay JM, Kirk KL. (1983) Exploration of the role of phenolsulfotransferase in the disposition of serotonin in human platelets: implications for a novel therapeutic strategy against depression. Med Hypotheses 10:231–246 [DOI] [PubMed] [Google Scholar]
- Crabb DW. (1999) Pathogenesis of alcoholic liver disease: newer mechanisms of injury. Keio J Med 48:184–188 [DOI] [PubMed] [Google Scholar]
- Dooley TP, Haldeman-Cahill R, Joiner J, Wilborn TW. (2000) Expression profiling of human sulfotransferase and sulfatase gene superfamilies in epithelial tissues and cultured cells. Biochem Biophys Res Commun 277:236–245 [DOI] [PubMed] [Google Scholar]
- Duanmu Z, Weckle A, Koukouritaki SB, Hines RN, Falany JL, Falany CN, Kocarek TA, Runge-Morris M. (2006) Developmental expression of aryl, estrogen, and hydroxysteroid sulfotransferases in pre- and postnatal human liver. J Pharmacol Exp Ther 316:1310–1317 [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Coughtrie MW, Goldstein DS. (1999) Dopamine sulphate: an enigma resolved. Clin Exp Pharmacol Physiol Suppl 26:S41–S53 [PubMed] [Google Scholar]
- Elekima OT, Mills CO, Ahmad A, Skinner GR, Ramsden DB, Bown J, Young TW, Elias E. (2000) Reduced hepatic content of dehydroepiandrosterone sulphotransferase in chronic liver diseases. Liver 20:45–50 [DOI] [PubMed] [Google Scholar]
- Falany CN. (1997) Enzymology of human cytosolic sulfotransferases. FASEB J 11:206–216 [DOI] [PubMed] [Google Scholar]
- Falany CN, Krasnykh V, Falany JL. (1995) Bacterial expression and characterization of a cDNA for human liver estrogen sulfotransferase. J Steroid Biochem Mol Biol 52:529–539 [DOI] [PubMed] [Google Scholar]
- Falany JL, Pilloff DE, Leyh TS, Falany CN. (2006) Sulfation of raloxifene and 4-hydroxytamoxifen by human cytosolic sulfotransferases. Drug Metab Dispos 34:361–368 DOI:10.1124/dmd.105.006551. [DOI] [PubMed] [Google Scholar]
- Fang HL, Strom SC, Cai H, Falany CN, Kocarek TA, Runge-Morris M. (2005) Regulation of human hepatic hydroxysteroid sulfotransferase gene expression by the peroxisome proliferator-activated receptor alpha transcription factor. Mol Pharmacol 67:1257–1267 DOI:10.1124/mol.104.005389. [DOI] [PubMed] [Google Scholar]
- Finkel Y, Eklöf AC, Granquist L, Soares-da-Silva P, Bertorello AM. (1994) Endogenous dopamine modulates jejunal sodium absorption during high-salt diet in young but not in adult rats. Gastroenterology 107:675–679 [DOI] [PubMed] [Google Scholar]
- Ghose R, Omoluabi O, Gandhi A, Shah P, Strohacker K, Carpenter KC, McFarlin B, Guo T. (2011) Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters. Life Sci 89:57–64 DOI:10.1016/j.lfs.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glavin GB. (1991) Dopamine and gastroprotection. The brain-gut axis. Dig Dis Sci 36:1670–1672 [DOI] [PubMed] [Google Scholar]
- Gutierrez-Grobe Y, Ponciano-Rodríguez G, Ramos MH, Uribe M, Méndez-Sánchez N. (2010) Prevalence of non alcoholic fatty liver disease in premenopausal, posmenopausal and polycystic ovary syndrome women. The role of estrogens. Ann Hepatol 9:402–409 [PubMed] [Google Scholar]
- Hardwick RN, Ferreira DW, More VR, Lake AD, Lu Z, Manautou JE, Slitt AL, Cherrington N. (2013) Altered UDP-glucuronosyltransferase (UGT) and sulfotransferase (SULT) expression and function during progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 41:554-561 DOI:10.1124/dmd.112.048439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. (2010) Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos 38:2293–2301 DOI:10.1124/dmd.110.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto E, Tokushige K. (2011) Prevalence, gender, ethnic variations, and prognosis of NASH. J Gastroenterol 46 (Suppl 1):63–69 DOI:10.1007/s00535-010-0311-8. [DOI] [PubMed] [Google Scholar]
- Haskel Y, Hanani M. (1994) Inhibition of gastrointestinal motility by MPTP via adrenergic and dopaminergic mechanisms. Dig Dis Sci 39:2364–2367 [DOI] [PubMed] [Google Scholar]
- Huang J, Bathena SP, Tong J, Roth M, Hagenbuch B, Alnouti Y. (2010) Kinetic analysis of bile acid sulfation by stably expressed human sulfotransferase 2A1 (SULT2A1). Xenobiotica 40:184–194 DOI:10.3109/00498250903514607. [DOI] [PubMed] [Google Scholar]
- Jaiswal AK. (2000) Regulation of genes encoding NAD(P)H:quinone oxidoreductases. Free Radic Biol Med 29:254–262 [DOI] [PubMed] [Google Scholar]
- Kim MS, Shigenaga J, Moser A, Grunfeld C, Feingold KR. (2004) Suppression of DHEA sulfotransferase (Sult2A1) during the acute-phase response. Am J Physiol Endocrinol Metab 287:E731–E738 DOI:10.1152/ajpendo.00130.2004. [DOI] [PubMed] [Google Scholar]
- Klaassen CD, Boles JW. (1997) Sulfation and sulfotransferases 5: the importance of 3′-phosphoadenosine 5′-phosphosulfate (PAPS) in the regulation of sulfation. FASEB J 11:404–418 [DOI] [PubMed] [Google Scholar]
- Koga M, Saito H, Mukai M, Saibara T, Kasayama S. (2011) Serum dehydroepiandrosterone sulphate levels in patients with non-alcoholic fatty liver disease. Intern Med 50:1657–1661 10.2169/internalmedicine.50.4682. [DOI] [PubMed] [Google Scholar]
- Lattuada G, Ragogna F, Perseghin G. (2011) Why does NAFLD predict type 2 diabetes? Curr Diab Rep 11:167–172 10.1007/s11892-011-0190-2. [DOI] [PubMed] [Google Scholar]
- Lazo M, Clark JM. (2008) The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis 28:339–350 10.1055/s-0028-1091978. [DOI] [PubMed] [Google Scholar]
- Leevy CM. (1962) Fatty liver: a study of 270 patients with biopsy proven fatty liver and review of the literature. Medicine (Baltimore) 41:249–276 [DOI] [PubMed] [Google Scholar]
- Luu-The V, Bernier F, Dufort I. (1996) Steroid sulfotransferases. J Endocrinol 150 (Suppl):S87–S97 [PubMed] [Google Scholar]
- McClain CJ, Mokshagundam SP, Barve SS, Song Z, Hill DB, Chen T, Deaciuc I. (2004) Mechanisms of non-alcoholic steatohepatitis. Alcohol 34:67–79 [DOI] [PubMed] [Google Scholar]
- Merrell MD, Cherrington NJ. (2011) Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metab Rev 43:317–334 10.3109/03602532.2011.577781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalto G, Miceli M, Soresi M, Amodio R, Carroccio A, Cartabellotta A, Castagnetta L. (1997) Sex hormones in patients with liver cirrhosis and hepatocellular carcinoma. Oncol Rep 4:173–176 [PubMed] [Google Scholar]
- Moore JB. (2010) Non-alcoholic fatty liver disease: the hepatic consequence of obesity and the metabolic syndrome. Proc Nutr Soc 69:211–220 10.1017/S0029665110000030. [DOI] [PubMed] [Google Scholar]
- Nakamuta M, Ohashi M, Goto K, Tanabe Y, Hiroshige K, Nawata H. (1994) The re-evaluation of sex steroids metabolism in patients with non-alcoholic liver cirrhosis. Fukuoka Igaku Zasshi 85:187–194 [PubMed] [Google Scholar]
- Narasaka T, Moriya T, Endoh M, Suzuki T, Shizawa S, Mizokami Y, Matsuoka T, Sasano H. (2000) 17Beta-hydroxysteroid dehydrogenase type 2 and dehydroepiandrosterone sulfotransferase in the human liver. Endocr J 47:697–705 [DOI] [PubMed] [Google Scholar]
- Negishi M, Pedersen LG, Petrotchenko E, Shevtsov S, Gorokhov A, Kakuta Y, Pedersen LC. (2001) Structure and function of sulfotransferases. Arch Biochem Biophys 390:149–157 [DOI] [PubMed] [Google Scholar]
- Nemoto Y, Toda K, Ono M, Fujikawa-Adachi K, Saibara T, Onishi S, Enzan H, Okada T, Shizuta Y. (2000) Altered expression of fatty acid-metabolizing enzymes in aromatase-deficient mice. J Clin Invest 105:1819–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascale A, Pais R, Ratziu V. (2010) An overview of nonalcoholic steatohepatitis: past, present and future directions. J Gastrointestin Liver Dis 19:415–423 [PubMed] [Google Scholar]
- Peters JM, Zhou YC, Ram PA, Lee SS, Gonzalez FJ, Waxman DJ. (1996) Peroxisome proliferator-activated receptor alpha required for gene induction by dehydroepiandrosterone-3 beta-sulfate. Mol Pharmacol 50:67–74 [PubMed] [Google Scholar]
- Reiter C, Weinshilboum RM. (1982) Acetaminophen and phenol: substrates for both a thermostable and a thermolabile form of human platelet phenol sulfotransferase. J Pharmacol Exp Ther 221:43–51 [PubMed] [Google Scholar]
- Riches Z, Stanley EL, Bloomer JC, Coughtrie MW. (2009) Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: the SULT “pie”. Drug Metab Dispos 37:2255–2261 DOI:10.1124/dmd.109.028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher M, Liere P, Akwa Y, Rajkowski K, Griffiths W, Bodin K, Sjövall J, Baulieu EE. (2008) Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int 52:522–540 DOI:10.1016/j.neuint.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Shimada M, Watanabe E, Iida Y, Nagata K, Yamazoe Y. (1999) Alteration of hepatic sulfation by endotoxin. Jpn J Pharmacol 80:371–373 [DOI] [PubMed] [Google Scholar]
- Shimizu I, Ito S. (2007) Protection of estrogens against the progression of chronic liver disease. Hepatol Res 37:239–247 [DOI] [PubMed] [Google Scholar]
- Sørensen TI, Orholm M, Bentsen KD, Høybye G, Eghøje K, Christoffersen P. (1984) Prospective evaluation of alcohol abuse and alcoholic liver injury in men as predictors of development of cirrhosis. Lancet 2:241–244 [DOI] [PubMed] [Google Scholar]
- Starr SP, Raines D. (2011) Cirrhosis: diagnosis, management, and prevention. Am Fam Physician 84:1353–1359 [PubMed] [Google Scholar]
- Strobel G, Friedmann B, Siebold R, Bärtsch P. (1999) Effect of severe exercise on plasma catecholamines in differently trained athletes. Med Sci Sports Exerc 31:560–565 [DOI] [PubMed] [Google Scholar]
- Suiko M, Sakakibara Y, Liu MC. (2000) Sulfation of environmental estrogen-like chemicals by human cytosolic sulfotransferases. Biochem Biophys Res Commun 267:80–84 [DOI] [PubMed] [Google Scholar]
- Tabrett CA, Coughtrie MW. (2003) Phenol sulfotransferase 1A1 activity in human liver: kinetic properties, interindividual variation and re-evaluation of the suitability of 4-nitrophenol as a probe substrate. Biochem Pharmacol 66:2089–2097 [DOI] [PubMed] [Google Scholar]
- Teli MR, Day CP, Burt AD, Bennett MK, James OF. (1995) Determinants of progression to cirrhosis or fibrosis in pure alcoholic fatty liver. Lancet 346:987–990 [DOI] [PubMed] [Google Scholar]
- Teubner W, Meinl W, Florian S, Kretzschmar M, Glatt H. (2007) Identification and localization of soluble sulfotransferases in the human gastrointestinal tract. Biochem J 404:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam KV. (2003) Human 3′-phosphoadenosine 5′-phosphosulfate (PAPS) synthase: biochemistry, molecular biology and genetic deficiency. IUBMB Life 55:1–11 [DOI] [PubMed] [Google Scholar]
- Völzke H, Aumann N, Krebs A, Nauck M, Steveling A, Lerch MM, Rosskopf D, Wallaschofski H. (2010) Hepatic steatosis is associated with low serum testosterone and high serum DHEAS levels in men. Int J Androl 33:45–53 DOI:10.1111/j.1365-2605.2009.00953.x. [DOI] [PubMed] [Google Scholar]
- Yasuda S, Liu MY, Suiko M, Sakakibara Y, Liu MC. (2007) Hydroxylated serotonin and dopamine as substrates and inhibitors for human cytosolic SULT1A3. J Neurochem 103:2679–2689 DOI:10.1111/j.1471-4159.2007.04948.x. [DOI] [PubMed] [Google Scholar]
- Yeo M, Na YM, Kim DK, Kim YB, Wang HJ, Lee JA, Cheong JY, Lee KJ, Paik YK, Cho SW. (2010) The loss of phenol sulfotransferase 1 in hepatocellular carcinogenesis. Proteomics 10:266–276 DOI:10.1002/pmic.200900721. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Wada K, Katayama K, Nakajima N, Iwasaki T, Osawa E, Mukasa K, Yamada Y, Blumberg RS, Sekihara H, et al. (2004) A novel therapy for acute hepatitis utilizing dehydroepiandrosterone in the murine model of hepatitis. Biochem Pharmacol 68:2283–2289 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.