Abstract
Benzo[a]pyrene (BaP) is a prototypical polycyclic aromatic hydrocarbon (PAH); this ubiquitous environmental carcinogenic agent is found in tobacco smoke, charcoal-grilled foods, and PAH-contaminated surfaces of roofs, playgrounds, and highways. Cytochrome P450 1 wild-type, Cyp1a2(−/−), Cyp1b1(−/−), or Cyp1a2/1b1(−/−) knockouts, and mice with Cyp1a1 expression deleted in hepatocytes can ingest large oral BaP doses (125 mg/kg/d) without apparent toxicity. Cyp1a1(−/−) and Cyp1a1/1a2(−/−) knockouts and mice with Cyp1a1 expression deleted in gastrointestinal (GI) tract epithelial cells develop immunotoxicity and die within 32 days, indicating that GI tract inducible CYP1A1 is absolutely required for detoxication of oral BaP. Cyp1a1/1b1(−/−) and Cyp1a1/1a2/1b1(−/−) mice are rescued from immunosuppression and early death due to absent metabolic activation of BaP by CYP1B1 in immune cells. Ten-fold lower oral BaP doses result in adenocarcinoma of the proximal small intestine (PSI) in Cyp1a1(−/−) mice; Cyp1a1/1b1(−/−) double-knockout mice show no PSI cancer but develop squamous cell carcinoma of the preputial gland duct (PGD). BaP-metabolizing CYP1B1 in the PSI and CYP3A59 in the PGD are the most likely candidates to participate in tumor initiation in the epithelial cells of these two tissues; oncogenes and tumor-suppressor genes upregulated and downregulated during tumorigenesis are completely different between these tissues. This “oral BaP Cyp1” mouse paradigm represents a powerful teaching tool, showing that gene-environment interactions depend on route-of-administration: the same oral, but not intraperitoneal, BaP exposure leads to dramatic differences in target-organ toxicity and tumor type as a function of dose and Cyp1 genotype.
Introduction
Studies of dietary polycyclic aromatic hydrocarbons (PAHs) in laboratory animals have been underappreciated. It is well established that, in cigarette and cigar smokers, considerably greater amounts of PAHs are swallowed and enter the gastrointestinal (GI) tract compared with the amounts that enter the lung (Järup, 2003; Rozman and Klaassen, 2007). Furthermore, charcoal-grilled meat eaters, tar roofers, and those inhaling dust from heavily PAH-contaminated playgrounds and highways all ingest substantial amounts of PAHs.
In laboratory animals, PAH-induced lung tumor formation is common (Rubin, 2001), whereas PAH-caused GI tract cancer is rare. A common question over many decades has been, If more PAHs are swallowed than inhaled, why is GI tract cancer not more prevalent than lung cancer in smokers? One answer has been the rapid turnover of GI tract epithelial cells, which might prevent tumors from forming. However, head-and-neck epithelial cells as well as cells of the skin, lung, immune system, and bone marrow also turn over rapidly; PAH-induced malignancies commonly occur in these cell types, both in laboratory animals and in humans.
This minireview addresses this problem, focusing on benzo[a]pyrene (BaP) as a prototypic PAH. Over four decades, our laboratory has demonstrated that highly induced CYP1A1 in the GI tract is protective by way of oral BaP detoxication. This discovery seems to contradict the long-held belief that CYP1A1 should always be regarded as detrimental.
Results and Discussion
History.
After the landmark discovery in the 1950s (Conney et al., 1956, 1957) that rat liver drug- or xenobiotic-metabolizing enzyme (DME, XME) activities are inducible by PAHs, Wattenberg et al. (1962) proposed in 1962 that phase I oxidative enzymes are primarily essential for detoxifying PAHs such as oral BaP. Six years later, this concept was convincingly challenged when XME-mediated metabolism was shown unequivocally to generate reactive oxidative PAH intermediates capable of binding covalently to nucleic acids and proteins (Grover and Sims, 1968; Sims and Grover, 1968). Thus began more than 3 decades of a belief held by many that phase I XMEs and DMEs are fundamentally harmful because they can metabolically activate relatively inert xenobiotic substrates, including drugs, to reactive oxygenated intermediates—capable of initiating toxicity, birth defects, oxidative stress, mutations, and cancer (Phillips et al., 1978; Nebert, 1989; Wogan et al., 2004). In contrast, phase II DMEs are usually regarded as helpful because they conjugate reactive oxidative intermediates, thereby leading to excretion of detoxified, innocuous products (Talalay, 2000).
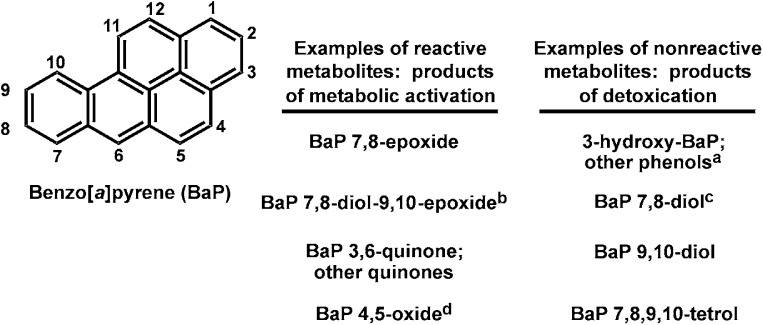
Figure 1 lists four (individual or classes of) BaP reactive oxygenated intermediates and four (individual or classes of) nonreactive products of detoxication. The concepts of “metabolic activation” versus “detoxication” of BaP, as well as any other PAH, are detailed further in Fig. 1.
Fig. 1.
Molecular structure of the prototypical polycyclic aromatic hydrocarbon, benzo[a]pyrene (BaP), with its standardized numbering system. Among 709 possible oxygenated BaP metabolites, which includes all syn- and anti-isomers, four products (or classes of products) of metabolic activation that are highly reactive intermediates are listed in the left column; four products (or classes of products) of detoxication that are negligibly reactive are listed in the right column. Electrophilic oxides and epoxides can react with nucleophilic groups of cellular macromolecules, can rearrange to become hydroxyl products, or can become conjugated with moieties such as glucuronide, glutathione, or sulfate. Phenols and the tetrol are generally nontoxic, and conjugation renders them even more hydrophilic and easy to excrete from the cell. Whereas the epoxide oxygen is derived from diatomic oxygen, the addition of a second oxygen atom across the same C—C bond to form a diol comes from cellular water. aBaP phenols are major nonreactive metabolites and therefore represent important detoxication pathways; all BaP phenols have been tested for carcinogenicity and are inactive except for 2-hydroxyBaP, which is active but not a known biologic metabolite. bOnly one of the four possible optically active diol-epoxide metabolites is carcinogenic. cAlthough the 7,8-diol is not “reactive,” it is highly carcinogenic because of its further metabolism. dWhereas BaP 4,5-oxide is chemically reactive and also mutagenic, it is not carcinogenic. In summary, some chemically reactive metabolites are not carcinogenic but can be toxic and/or mutagenic, whereas some nonreactive metabolites can be carcinogenic, toxic, and/or mutagenic due to further metabolism (reviewed in Conney et al., 1994).
In parallel with these events, striking genetic differences in PAH-inducible aryl hydrocarbon hydroxylase (AHH) activity were described among inbred strains of mice (Nebert and Gelboin, 1969). AHH inducibility was subsequently found to be inherited principally as a Mendelian trait—C57BL/6N (B6) expressing dominance over DBA/2N (D2) mice, which showed lack of PAH-inducible AHH activity as an autosomal recessive trait (Nebert et al., 1971; Gielen et al., 1972). An independent study reported this same mode of inheritance (Thomas et al., 1972).
Early work in rat liver had shown that PAH treatment caused a shift in the carbon monoxide (CO)-reduced cytochrome P450 (P450) spectrum from 450 to 448 nm (Alvares et al., 1967; Kuntzman et al., 1968). Subsequently, AHH induction in the Ah-responsive but not Ah-nonresponsive mouse liver was demonstrated to be associated with a hypsochromic spectral shift in CO-reduced cytochrome P450, indicating the formation of a new form of induced P450 protein (Nebert, 1970; Gielen et al., 1972).
Next, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, “dioxin”) as an inducer of AHH activity in chick embryo was established to be >30,000 times more potent than any PAH (Poland and Glover, 1974). Because of its potency, TCDD as an AHH inducer was then explored in rodents. After TCDD treatment, hepatic AHH activity in Ah-nonresponsive D2 was inducible to levels as high as those in Ah-responsive B6 mice; however, the D2 dose-response curve was shifted ∼20-fold to the right compared with that of B6 mice (Poland et al., 1974). The shape of the dose-response curve and its shift were predicted (Poland et al., 1974) to represent differences not in AHH but rather a regulatory protein [later determined to be aryl hydrocarbon receptor (AHR)]; 2 decades later, specific amino-acid changes in B6 versus D2 AHR responsible for differences in receptor affinity were demonstrated (Poland et al., 1994).
Thus began the research field of “genetic differences in DME- and/or XME-mediated toxicity, carcinogenesis, mutagenesis, and teratogenesis” (reviewed in Nebert, 1989). Oxidative phase I metabolism, especially via P450s, was generally believed to be detrimental in any cell type of every vertebrate, including microsomal studies in vitro and studies in cell culture (Conney et al., 1994; Nebert et al., 2004). In contrast, conjugative phase II metabolism was regarded as almost always beneficial (Nebert et al., 2000; Talalay, 2000).
Target Organ Toxicity of BaP as a Function of Route of Administration.
For studies of toxicity or cancer, xenobiotics can be administered intraperitoneally, topically, subcutaneously, intramuscularly, intravenously, orally in food or drinking water, or by injection into the stomach, trachea, eye, or rectum. Largely for convenience, intraperitoneal and topical administration have been the most common means of treating laboratory animals with environmental test compounds, including drugs.
It seems likely, however, that the presentation, dose, and rate of administration of any test chemical orally in the diet or drinking water or as a stomach gavage might lead to different pharmacokinetic responses than intraperitoneal or topical administration. This minireview highlights one such difference in pharmacokinetics (absorption, distribution, metabolism, excretion); moreover, if the test compound is also an inducer of its own metabolism in proximal tissues, such as AHH activity in the GI tract induced by oral BaP (Wattenberg et al., 1962) or AHH activity in the skin induced by topical PAH (Schlede and Conney, 1970), this will lead to even more dramatic changes in the pharmacokinetics of the test compound. Chronic administration of a chemical such as BaP, which can cause both tumor initiation and promotion, will cause differences in phenotype compared with a xenobiotic not having these properties.
Intraperitoneal PAHs and dioxin at sufficiently high concentrations result in toxicity of the liver, immune system, bone marrow and white cells, adipose tissue, spleen, thymus, and arterial cell wall (Nebert, 1989; Collins et al., 1991; Miller and Ramos, 2001; Bock and Kohle, 2009); it has been proposed that future studies of the lymphatic system and on the participation of chylomicron particle release of PAHs and dioxin will provide valuable insight into understanding toxicity in these tissues (Nebert and Dalton, 2006). On the other hand, oral BaP at sufficiently high doses most dramatically causes acute immunotoxicity, whereas acute toxicity of liver, adipose tissue, and the arterial cell wall appears to be negligible (Uno et al., 2001, 2004, 2006, 2008).
Apparent Paradox with Oral BaP in B6 versus D2 Mice.
Dietary BaP was first studied in mice 4 decades ago (Robinson et al., 1975). Curiously, at high BaP doses (125 mg/kg/d), Ah-nonresponsive D2 mice died by 28–32 days with striking immunosuppression and toxic chemical depression of bone marrow, whereas Ah-responsive B6 and B6D2F1 mice remained healthy. In fact, on a high-BaP chronic diet, Ah-responsive mice reproduced normally and even lived longer than Ah-responsive mice eating normal laboratory chow without BaP (Robinson et al., 1975)!
At lower oral BaP doses (12 and 6 mg/kg/d), D2 mice did not die quickly; instead, they exhibited higher rates of leukemia and thymoma than B6 or B6D2F1 mice. Administration of 120 mg/kg/d of dietary α-naphthoflavone, a competitive inhibitor of AHH activity but not of other P450 enzymes, lowered substantially this risk of malignancy (Nebert and Jensen, 1979). In mice receiving topical 3-methylcholanthrene, D2 showed more leukemia than B6 mice (Duran-Reynals et al., 1978). Therefore, we might consider that oral and topical PAH administration representing GI tract and skin are proximal tissues, whereas bone marrow and thymus might be considered as distal tissues.
After 15 years, a paradigm became apparent (Table 1). PAH administration to Ah-responsive mice causes in proximal target tissues AHH induction, increased cancer, mutagenesis, DNA-adduct formation, various forms of toxicity, oxidative stress, and birth defects. Consequently, lower levels of PAHs reach the distal tissues, where one sees negligible AHH induction and lower rates of cancer, mutagenesis, DNA-adduct formation, toxicity, oxidative stress, and birth defects (reviewed in Nebert, 1989). A single oral dose of BaP to Ah-responsive rats was found to result in elevated blood and tissue BaP levels, whereas giving the same BaP dose for several days (or inducing with a different PAH) leads to markedly decreased blood and tissue BaP levels (Schlede et al., 1970); these results represent the same phenomenon observed with Ah-responsive mice receiving oral BaP (Robinson et al., 1975).
TABLE 1.
Association of oral PAH-inducible AHH activity with various cellular effects in proximal versus distal tissues or organs (reviewed in detail in Nebert, 1989).
| Ahr Genotypea | PAH-Induced Effect | Proximal Tissues/Organsb | Distal Tissues/Organsc |
|---|---|---|---|
| B6 and B6D2F1 (Ahrb1/b1, Ahrb1/d) | AHH induction | Increased | Decreased |
| Neoplasia; mutagenesis | Increased risk | Decreased risk | |
| DNA-adduct formation | Increased | Decreased | |
| Toxicity; oxidative stress | Increased | Decreased | |
| Teratogenesis | Increased risk | Decreased risk | |
| Detoxication | Increased | Decreased | |
| D2 (Ahrd/d) | AHH induction | Decreased | Increased |
| Neoplasia; mutagenesis | Decreased risk | Increased risk | |
| DNA-adduct formation | Decreased | Increased | |
| Toxicity; oxidative stress | Decreased | Increased | |
| Teratogenesis | Decreased risk | Increased risk | |
| Detoxication | Decreased | Increased |
Standardized mouse genetic nomenclature states that the Ahr allele for B6 is b1, for D2 is d (Poland et al., 1994).
“Proximal” in this context denotes tissues or organs in contact with, or in close proximity to, the incoming PAH. Interestingly, this includes in utero fetuses when a PAH is administered intraperitoneally (Nebert, 1989).
“Distal” in this context denotes tissues or organs not in contact with, or in close proximity to, the incoming PAH. This includes in utero fetuses when the PAH is administered orally (Nebert, 1989).
In contrast, administering the same PAH dose to Ah-nonresponsive mice resulted in negligible AHH induction in proximal tissues and hence decreased risk of cancer, mutagenesis, DNA-adduct formation, toxicity, oxidative stress, and teratogenesis in these tissues. This resulted in more PAHs reaching distal target tissues, where we found increased risk of cancer, mutagenesis, DNA-adduct formation, toxicity, oxidative stress, and birth defects (Nebert, 1989) (summarized in Table 1).
Furthermore, bone marrow of Ah-nonresponsive mice was replaced with immune-compatible marrow from Ah-responsive mice; these animals were compared with Ah-responsive mice carrying bone marrow from Ah-nonresponsive mice (Legraverend et al., 1983). High daily oral BaP doses produced marrow toxicity in Ah-nonresponsive mice, regardless of the source of marrow. The conclusion was that—due to detoxication of oral BaP by induced AHH activity in GI tract and/or liver of Ah-responsive mice—bone marrow is protected; in contrast, with negligible detoxication of oral BaP in Ah-nonresponsive mice, much larger amounts of BaP reach the marrow, causing toxicity (Legraverend et al., 1983; Nebert, 1989).
Re-Examination of the Paradigm in Cyp1a1(−/−) Knockout Mice.
Is the observed oral BaP detoxication in B6 mice due to PAH-inducible CYP1A1, CYP1A2, or CYP1B1, or any two, or all three CYP1 enzymes? Or is another PAH-inducible PAH-metabolizing P450 involved? Also, is the inducible enzyme(s) primarily located in the liver or GI tract? These questions could only be answered by studying conventional, as well as conditional, knockout mouse lines.
Oral versus intraperitoneal BaP was first assessed in wild-type versus Cyp1a1(−/−) knockout mice; striking differences between mice with different genotypes occurred via oral, but not intraperitoneal, route of administration (Uno et al., 2001). At high oral BaP doses (125 mg/kg/d), Cyp1a1(−/−) mice of >99.8% B6 genetic background died at 28–32 days (Uno et al., 2001), which was virtually identical to the results found previously in D2 mice (Robinson et al., 1975). Significant anemia, methemoglobinemia, and elevated plasma enzymes indicative of damage of various tissues occurred in Cyp1a1(−/−) mice at 125 and 12.5 mg/kg/d doses (Uno et al., 2004). After oral BaP gavage, Cyp1(+/+) wild-type mice were shown to clear BaP from blood at least 4 times more rapidly than Cyp1a1(−/−) mice (Uno et al., 2004). Moreover, BaP-DNA adduct levels in the liver were 4-fold greater in Cyp1a1(−/−) than Cyp1(+/+) mice (Uno et al., 2001, 2004). In fact, at BaP doses of 12.5 mg/kg/d, elevated BaP-DNA adducts were observed 18 days later in Cyp1a1(−/−) liver, GI tract, spleen, and bone marrow. Even at BaP doses of 1.25 mg/kg/d, significantly elevated BaP-DNA adducts were detectable in Cyp1a1(−/−) spleen (Uno et al., 2004).
Studies of All Three CYP1 Enzymes.
In the epithelial cells of duodenum, jejunum, ileum, and colon, we determined that maximally inducible CYP1A1 mRNA and protein levels were ∼3–10 times greater than CYP1B1, which in turn were ∼3–10 times greater than CYP1A2 (Uno et al., 2008). Whereas ablation of Cyp1a2 or Cyp1b1 gene expression made little difference in CYP1 mRNA or protein levels, when the Cyp1a1 gene was deleted, the CYP1B1 mRNA and protein levels in liver, but especially the proximal small intestine (PSI; the first 5 cm from the pyloric valve, including the duodenum and proximal jejunum) were strikingly increased compared with those of wild-type animals (Uno et al., 2006); this effect in the GI tract is believed to be a compensatory response to the absence of CYP1A1.
To address this point, we studied Cyp1a1(−/−) (Dalton et al., 2000), Cyp1a2(−/−) (Liang et al., 1996), and Cyp1b1(−/−) (Buters et al., 1999) single-knockout lines and Cyp1a1/1b1(−/−) and Cyp1a2/1b1(−/−) double-knockout mouse lines (Uno et al., 2006). Generation of Cyp1a1/1a2(−/−) knockout mice was problematic because the two genes were separated by only 13,954 base pairs. More than 1,100 pups from the Cyp1a1(−/−) × Cyp1a2(−/−) genetic cross were genotyped but failed to achieve any double-knockout crossover pups; subsequently, a Cre recombinase-mediated interstrand excision via two loxP sites located 26,173 bp apart was successful (Dragin et al., 2007). This made it possible to include oral BaP studies on Cyp1a1/1a2(−/−) double-knockout as well as Cyp1a1/1a2/1b1(−/−) triple-knockout lines (Dragin et al., 2008).
Table 2 summarizes the data for the highest dose (125 mg/kg/d) of oral BaP. The Cyp1a2(−/−) and Cyp1b1(−/−) single-knockout and Cyp1a2/1b1(−/−) double-knockout mice behave similarly to the wild-type mice. Severe effects of oral BaP occur in both Cyp1a1(−/−) and Cyp1a1/1a2(−/−) mice; those life-threatening effects were largely alleviated in Cyp1a1/1b1(−/−) double-knockout as well as in Cyp1a1/1a2/1b1(−/−) triple-knockout mice. Hence, all data implicate CYP1A1 as the enzyme that, when missing, leads to oral BaP-induced immunosuppression and early death. Moreover, our data show that when CYP1B1 is also missing, severe effects of oral BaP on the immune system are mollified.
TABLE 2.
Summary of the response to oral BaP (125 mg/kg/d) by all possible Cyp1 genotypes
CYP1A1 and CYP1B1 metabolize PAHs, whereas CYP1A2 metabolism of PAHs is low but detectable; CYP1A2 metabolizes N-arylamines most efficiently (Nebert et al., 2004). Subtle differences in BaP metabolite profiles generated by CYP1A1 versus CYP1B1 versus CYP1A2 are well known (Guengerich and Shimada, 1998) and are probably tissue- and cell-type-specific.
| Genotypesa | Cyp1(+/+), Cyp1a2(−/−), Cyp1b1(−/−), Cyp1a2/1b1(−/−) | Cyp1a1(−/−), Cyp1a1/1a2(−/−) | Cyp1a1/1b1(−/−), Cyp1a1/1a2/1b1(−/−) |
|---|---|---|---|
| Clinical outcome | Healthy for lifetime | Dies 28–32 d | Reverts almost completely to wild-type phenotype |
| Blood BaP levels (ng/ml)b | ∼2.0 | ∼50 | ∼150 |
| ALT, AST,c hemoglobin, hematocrit, methemoglobin levels | Normal | Abnormal | Near normal |
| Bone marrow, spleen | Normal | Severe hypocellularity | Slight hypocellularity |
| Liver, thymus | Normal | AHR activationd | AHR activationd |
The knockout genotypes in all lines were backcrossed into C57BL/6J (B6) mice at least 8 times, rendering all animals with >99.8% B6 genetic background; hence, B6 inbred mice were used as Cyp1(+/+) controls. These data are summarized from Uno et al. (2004, 2006) and Dragin et al. (2007).
Measured after 125 mg/kg/d of oral BaP for 5 days. All other parameters were described after oral BaP at this dose was given for 18 days.
Plasma alanine aminotransferase (ALT) levels are to assess hepatocellular injury; plasma aspartate aminotransferase (AST) levels also are to assess liver injury but can also be a sign of cardiac and skeletal muscle damage.
Activation reflects the fact that daily BaP treatment causes chronic AHR activation, which in turn leads to proliferation of the endoplasmic reticulum and increased liver weight and thymus weight; these effects are AHR-dependent but are independent of CYP1 metabolism (Uno et al., 2006).
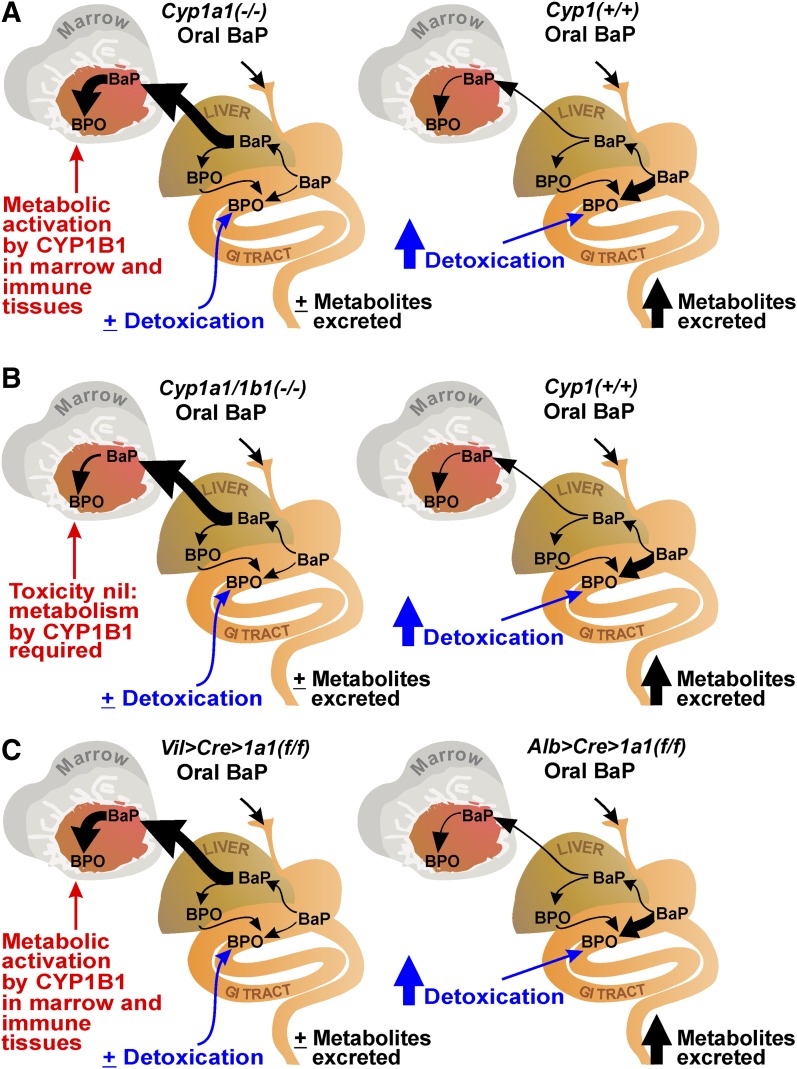
Figure 2 illustrates the paradigm that explains our data. In wild-type mice as well as in those missing a functional Cyp1a2 or Cyp1b1 gene, oral BaP-treated CYP1A1 quickly becomes massively induced in liver and/or GI tract, thereby leading to enhanced detoxication and excretion of BaP metabolites; the result is rapid clearance of blood BaP, with only small amounts of BaP reaching distal tissues such as bone marrow and immune tissue.
Fig. 2.
Comparisons of the response of oral BaP (125 mg/kg/d) for 18 days in mice of different genotypes. BPO denotes the first step in oxygenated BaP; BPO can be the reactive intermediate as well as undergo all the detoxication possibilities, as detailed in Fig. 1. The ratio of reactive-intermediates-to-detoxified-products is most likely tissue or cell-type specific, depends on how “tightly coupled” phase II conjugation systems might be to the membrane-bound P450 enzymes (Nebert and Dalton, 2006), and is also likely to depend on the degree of CYP1 induction. The relative amounts of CYP1A1, CYP1A2, and CYP1B1 protein in each tissue or cell type and the route and rate of administration as well as the function of time during induction by BaP will also affect this ratio. (A) Cyp1(+/+) wild-type (right) versus Cyp1a1(−/−) single-knockout mice (left). Oral BaP in Cyp1(+/+) mice rapidly induces intestinal CYP1A1, leading to detoxication and rapid excretion of BaP metabolites. Absence of CYP1A1 in the GI tract of Cyp1a1(−/−) mice (left) results in negligible detoxication and therefore a 25-fold greater blood BaP level than in the wild-type mice; larger amounts of BaP reaching nonhepatic tissues then result in BaP-induced CYP1B1-mediated immunosuppression, wasting, and death. (B) Cyp1(+/+) (right) versus Cyp1a1/1b1(−/−) double-knockout mice. Lack of CYP1B1 in marrow and immune tissues (left) results in negligible metabolic activation of BaP and thus markedly diminished immunosuppression, toxicity, and prevention of early death—despite a 3-fold more BaP body burden than in the Cyp1a1(−/−) mice seen in panel A. (C) Alb>Cre>Cyp1a1(f/f) versus Vil>Cre>Cyp1a1(f/f) conditional knockout mice. Alb>Cre>Cyp1a1(f/f) mice (right) respond to oral BaP much like wild-type mice, whereas Vil>Cre>Cyp1a1(f/f) mice (left) respond much like the Cyp1a1(−/−) mice; these experiments demonstrate unequivocally that it is the CYP1A1 in the GI tract, not the liver, that is most important in oral BaP detoxication and hence protection from immunosuppression, immunotoxicity, and early death.
In oral BaP-treated Cyp1a1(−/−) knockout mice (Fig. 2A, left), with or without functional Cyp1a2, absence of CYP1A1 in liver and/or GI tract results in negligible detoxication; this leads to ∼25-fold higher blood BaP levels than those in wild-type, with much more BaP reaching distal tissues such as bone marrow and immune tissue, causing marrow toxicity, immunosuppression, and early death.
In Cyp1a1/1b1(−/−) mice (Fig. 2B, left), with or without a functional Cyp1a2 gene, absence of CYP1A1 in liver and/or GI tract results in negligible detoxication; however, the additional absence of CYP1B1 in marrow and immune cells leads not only to 75-fold higher blood BaP levels than those in wild-type and greater amounts of BaP reaching distal tissues (Fig. 2B, left), but lack of CYP1B1—which is known to participate in PAH metabolism in bone marrow and immune tissue (Galván et al., 2005, 2006; N’jai et al., 2010)—results in diminished metabolic activation of BaP by CYP1B1. Therefore, the ultimate outcome is a substantial reversion to wild-type phenotype—that is, relatively healthy mice, despite even 3-fold more BaP body burden than that of immunosuppressed Cyp1a1(−/−) mice. This is a pharmacologic example in which total body burden, or rate of clearance, of a foreign chemical is not necessarily correlated with specific target-organ toxicity or clinical outcome.
Studies with Cyp1a1(−/−) Conditional Knockout Mice.
The liver is regarded as the principal organ of metabolic activation as well as detoxication. Is CYP1A1 located in the liver or CYP1A1 located in the GI tract more important in detoxication of oral BaP? Or are hepatic and GI tract hepatic CYP1A1 equally important? Answers to these questions can only be resolved by using conditional Cyp1a1(−/−) knockout lines. The Alb>Cre>Cyp1a1(f/f) line was thus created in which CYP1A1 is specifically ablated from albumin-expressing hepatocytes, whereas CYP1A1 is present and inducible in the rest of the animal, including the GI tract (Shi et al., 2010a). The Vil>Cre>Cyp1a1(f/f) line was also developed in which CYP1A1 function is deleted from villin-expressing epithelial cells of the GI tract (also renal epithelial cells, which are of no consequence in these experiments), meaning that CYP1A1 is present and inducible in the rest of the animal including the hepatocytes (Shi et al., 2010a).
Figure 2C summarizes the data with these two Cyp1a1 conditional knockout mice. The response of Alb>Cre>Cyp1a1(f/f) mice to daily oral BaP was similar to that of wild-type Cyp1(+/+) mice. Although CYP1A1 expression is absent in liver, CYP1A1 becomes massively induced by BaP in the GI tract (Fig. 2C, right), which in turn results in enhanced detoxication and excretion of BaP metabolites, rapid clearance of blood BaP levels, and no detectable immunotoxicity because only small amounts of BaP reach distal tissues.
On the other hand, the response of Vil>Cre>Cyp1a1(f/f) mice to daily oral BaP was similar to that of Cyp1a1(−/−) mice. Absence of CYP1A1 in the GI tract, rather than hepatocytes, results in negligible detoxication (Fig. 2C, left); this leads to severely impaired clearance of BaP total body burden, large amounts of BaP reaching distal tissues such as bone marrow and immune tissue, and resultant CYP1B1-mediated bone marrow toxicity, immunosuppression, and death. These two conditional knockout mouse models were supported with further pharmacokinetics parameters, blood and plasma enzyme measurements, and histology (Shi et al., 2010a). It can be unequivocally concluded that GI tract–inducible CYP1A1, but not CYP1B1 or CYP1A2, can detoxify enormous amounts of oral BaP.
Studies with a 10-Fold Lower Dose of Oral BaP.
We had reported previously that lower daily oral BaP doses given to D2 mice resulted in higher rates of leukemia and thymoma compared with no malignancies in B6 or B6D2F1 mice (Nebert and Jensen, 1979). In other words, if mice do not die quickly after high toxic doses of daily oral BaP, they can live sufficiently long to develop cancer.
Therefore, we decreased the daily oral BaP dose of 125 mg/kg/d to 12.5 mg/kg/d; this dose allowed Cyp1a1(−/−) mice to live beyond 20 weeks of age instead of dying at 28–32 days when given the higher BaP dose (Shi et al., 2010b). Adenocarcinoma of the PSI developed in Cyp1a1(−/−) mice between 8 and 12 weeks of oral BaP (12.5 mg/kg/d). Interestingly, in Cyp1a1/1b1(−/−) double-knockout mice between 8 and 12 weeks on this same BaP oral dose, no GI tract cancer occurred; however, squamous cell carcinoma (SCC) of the preputial gland duct (PGD) appeared (Shi et al., 2010b) (Table 3).
TABLE 3.
Summary of the response to oral BaP (12.5 mg/kg/d) by four genotypes
| Genotypesa | Cyp1(+/+), Cyp1b1(−/−) | Cyp1a1(−/−) | Cyp1a1/1b1(−/−) |
|---|---|---|---|
| Clinical outcome | Healthy for lifetime | Adenocarcinoma of PSI (at 8-12 wk) | Squamous cell carcinoma of preputial gland duct (at 8–12 wk) |
| Blood BaP levels (ng/ml)b | ∼0.1 | ∼4 | ∼13 |
| ALT, AST, hemoglobin, hematocrit, methemoglobin levels | Normalb | Borderline abnormalb | Normalb |
| Bone marrow, spleenb | Normal | Slight hypocellularity | Normal |
| Liver, thymusb | Normal | AHR activationc | AHR activationc |
Knockout genotype in all lines was backcrossed into C57BL/6J (B6) mice at least 8 times, rendering all animals with >99.8% B6 genetic background; hence, B6 inbred mice were used as Cyp1(+/+) controls. These data are summarized from Shi et al. (2010b) and Gálvez-Peralta et al. (2013).
Measured after 12.5 mg/kg/d oral BaP for 4 weeks. Abbreviations are the same as in Table 2.
Activation reflects the fact that daily BaP treatment causes chronic AHR activation, which in turn leads to proliferation of the endoplasmic reticulum and increased liver weight and thymus weight; these effects are AHR-dependent but are independent of CYP1 metabolism (Uno et al., 2006).
In addition to characterizing the tumors histologically, we performed cDNA microarray analyses of the PSI and PGD during formation of the malignancies (Shi et al., 2010b; Gálvez-Peralta et al., 2013). Zero versus 4-week-interval time points of oral BaP (12.5 mg/kg/d) were compared between relevant genotypes. Greatest attention was given to XME-related genes and cancer-related genes that were most highly upregulated and downregulated as the cancer process began (4 and 8 weeks of oral BaP) and then progressed (8 and 12 weeks and beyond).
Adenocarcinoma of the PSI.
Many of the top-ranked upregulated and downregulated genes during PSI adenocarcinoma formation are discussed in detail by Shi et al. (2010b). Genes involved in inflammation and acute phase response were among those strikingly upregulated by daily oral BaP after 4 weeks. Although PSI adenocarcinomas showed no immunohistochemical evidence of being lymphatic in origin, paradoxical overexpression of a large number of immunoglobulin kappa and heavy chain variable genes was observed (Shi et al., 2010b); this has previously been reported, with studies showing Igk and Igh gene expression paradoxically expressed in various malignancies of epithelial origin (Hu et al., 2008).
It is noteworthy that oral BaP-treated Cyp1a1(−/−) mice exhibit markedly increased CYP1B1 mRNA levels in the GI tract (Uno et al., 2006; Shi et al., 2010a,b). Association of elevated CYP1B1 in the same epithelial cells (Uno et al., 2008) that develop the adenocarcinoma strongly suggests that metabolic activation of oral BaP by CYP1B1 might be pivotal during the process of tumor initiation. Hence, PSI adenocarcinoma occurs in oral BaP-treated Cyp1a1(−/−) mice, which have highly induced CYP1B1 present in GI tract, but no GI tract cancer arises in oral BaP-treated Cyp1a1/1b1(−/−) mice, which have CYP1B1 deleted. This is additional evidence that CYP1B1-mediated metabolic activation of BaP is likely an important factor in GI tract tumorigenesis.
The putative oncogenes and tumor-suppressor genes in the PSI that are most strikingly upregulated and downregulated as a function of time of oral BaP exposure and adenocarcinoma formation (especially at the 4- and 8-week time points) include upregulation of the Xist gene, suggesting epigenetic silencing; upregulation of the Rab30 oncogene; and downregulation of the Nr0b2 tumor-suppressor gene during adenocarcinoma development (Shi et al., 2010b). This mouse model may be relevant to human PAH-induced or inflammation-induced epithelial cancers of the GI tract.
SCC of the PGD.
Intriguingly, between 8 and 12 weeks, SCC formation occurred in the PGD of Cyp1a1/1b1(−/−) double-knockout mice receiving daily oral BaP (12.5 mg/kg/d). One might ask, Of all tissues, why should we see cancer in preputial gland duct? Actually, PGD tumors are well known to occur commonly in male rodents during toxicity and cancer testing with many different environmental chemicals (Mitsumori and Elwell, 1988). PAHs commonly cause hyperkeratosis of PGD epithelium; indeed, among the best examples of an AHR ligand causing hyperkeratosis is dioxin-induced chloracne in humans (reviewed in Bock and Kohle, 2006). It seems likely that BaP-induced keratin-plugging of secretions from the PGD could lead to pruritus; the mouse’s response by scratching might set up secondary infections. BaP-induced hyperkeratinization and BaP as a well-known tumor promoter and cause of inflammation (Bock and Kohle, 2006) might therefore combine to cause both initiation and promotion of SCC in the PGD.
Many of the most dramatically upregulated and downregulated genes during SCC formation in the PGD are described in detail by Gálvez-Peralta et al. (2013). If both CYP1A1 and CYP1B1 are absent, and these two enzymes are well-known metabolic activators of BaP, what enzyme might compensate and take their place when both CYP1 genes are missing? Various CYP2C (Meehan et al., 1988; Yun et al., 1992; Bauer et al., 1995) and CYP3A (Yun et al., 1992; Bauer et al., 1995; Sun et al., 1995; Koley et al., 1997; Fukuhara et al., 1999; James et al., 2005) enzymes, some of which are PAH-inducible, have been shown in various vertebrates to metabolize PAHs such as BaP. Interestingly, microarray data revealed by far the most striking increases in CYP3A59 mRNA upregulation, which peaked at 8 weeks of oral BaP; these data strongly suggest—but do not prove—that CYP3A59 is the most likely BaP-inducible candidate responsible for initiation of BaP-induced SCC (Gálvez-Peralta et al., 2013).
Future studies might include Western immunoblot data to confirm that CYP3A59 protein is indeed highly induced in the PGD of oral BaP-treated Cyp1a1/1b1(−/−) mice, if an antibody specific for mouse CYP3A59 can be made. CYP3A59 cDNA expression studies in cell culture or bacteria would also be informative to confirm that CYP3A59 does indeed specifically metabolize BaP and what the metabolite profile might be. Specifically knocking out the Cyp3a59 gene and finding no SCC in the PGD of oral BaP-treated Cyp1a1/1b1/3a59(−/−) mice would provide the ultimate means to prove CYP3A59 is responsible for SCC initiation. [And it is also possible in an oral BaP-treated Cyp1a1/1b1/3a59(−/−) triple-knockout that a new P450 might arise to take the place of CYP3A59 in the PGD epithelium!]
It is worth noting that oral BaP-treated Cyp1a1/1b1(−/−) double-knockout, but not oral BaP-treated Cyp1a1(−/−) single-knockout, mice develop SCC of the PGD. This suggests that CYP1B1 in the PGD of oral BaP-treated Cyp1a1(−/−) mice might function in the role of BaP detoxication; in other words, when CYP1B1 and CYP1A1 are absent, only then does BaP result in SCC formation. And with both CYP1B1 and CYP1A1 missing, this would lead to a higher accumulation of BaP in the PGD; consequently, the compensatory response of CYP3A59 induction and then presumably CYP3A59-mediated metabolic activation of BaP become crucial in initiating SCC tumorigenesis.
Twenty-six cancer-related genes plus eight Serpin genes were upregulated and downregulated as a function of time during oral BaP exposure and development of SCC (especially at the 4- and 8-week time points). Among these 26 genes, eight were rat sarcoma (RAS)-related oncogenes—which have often been associated with PAH-induced cancers (Gálvez-Peralta et al., 2013).
Inflammation-associated genes were also highly upregulated at the 4- and 8-week time points; thus, specific mechanisms by which cancer-related genes are responsible for SCC tumor progression in the PGD remain to be elucidated. This mouse model of SCC in the PGD may be relevant to human PAH-induced as well as inflammation-mediated epithelial cell cancers.
Conclusions
This oral BaP mouse paradigm represents an exciting example of gene-environment interactions in which the same chronic exposure of a PAH carcinogen results in dramatic differences in target-organ toxicity and tumor type, as a function of zero versus one Cyp1 gene missing versus two Cyp1 genes missing. With an intact genome in Cyp1(+/+) wild-type mice, enormous amounts of BaP can be consumed without apparent toxicity to the animal because CYP1A1 in GI tract epithelial cells quickly becomes highly induced and functions to detoxify oral BaP very efficiently.
Removal of just one gene—Cyp1a1 globally or from only the GI tract epithelium—results in immunosuppression and death within 28–32 days at high daily oral BaP doses; immunotoxicity is mediated by CYP1B1 metabolic activation of BaP in marrow and other immune tissues. It should be emphasized that the striking difference between wild-type and Cyp1a1(−/−) mice happens with oral but not intraperitoneal route of administration (Uno et al., 2001). At 10-fold lower daily oral BaP doses, absence of the Cyp1a1 gene leads to adenocarcinoma of the PSI.
Removal of two genes—Cyp1a1 plus Cyp1b1—protects against CYP1B1-mediated immunosuppression and death at high daily oral BaP doses, and at 10-fold lower daily oral BaP doses leads to no PSI adenocarcinoma but rather SCC formation in the PGD. This paradigm should be attractive as a teaching model to emphasize the effects of gene-environment interactions on pharmacokinetics of PAH carcinogens and toxicants, including route of administration, rate of administration, ultimate target-organ specificity, and whether toxicity or malignancy is the end result.
Consideration of Human Epidemiologic Studies.
Human exposure to PAHs in cigarettes and charcoal-grilled meat is well known to stimulate carcinogen as well as drug metabolism (Welch et al., 1968, 1969; Nebert et al., 1969; Pantuck et al., 1972; Conney et al., 1976). Such human exposures to PAHs occur not only in the GI tract and lung but can also be important topically: PAH-induced AHH activity and BaP metabolic activation and detoxication are well known to occur in human skin (Alvares et al., 1973).
It is noteworthy that the three CYP1 enzymes are very highly conserved between mice and humans. Similar PAHs and other inducers, via AHR, upregulate these three genes in the same tissues of both species. Also, each of the three CYP1 enzymes of mice and humans handles substrates with a great deal of similarity (Nebert and Dalton, 2006). In fact, oral BaP-treated Cyp1a1/1a2(−/−) mice having the human CYP1A1 and CYP1A2 genes were shown to function the same as oral BaP-treated wild-type mice—that is, human CYP1A1 in the mouse GI tract is inducible and detoxifies oral BaP (Dragin et al., 2007). Hence, it is quite likely that our findings in the mouse might be relevant to human clinical studies. For example, is it possible that eating oil slick–contaminated seafood, or coal or road tar, or BaP-contaminated dust on a playground or highway is not as dangerous to adults or children as one might think?
Innumerable epidemiologic studies have attempted to show correlations between cancers of several types and various single-nucleotide variants in and near the CYP1A1, CYP1A2, and/or CYP1B1 genes (reviewed in Nebert and Dalton, 2006). However, cancer represents a multifactorial trait involving most likely thousands of “small-effect” genes; that is, each gene contributes between 0.0001 and 0.25% to the trait, so cohorts of perhaps 50,000 or more would likely be necessary to generate sufficient statistical power to show a truly significant association, making prediction of risk of cancer in the individual patient virtually impossible (Nebert et al., 2013b). Moreover, not a single CYP1A1 variant allele has been shown to lead to significant differences in clinical function (Nebert et al., 2004, 2013a; Nebert and Dalton, 2006). It has already been concluded (Nebert and Dalton, 2006; Nebert et al., 2013b) that—due to lack of statistical power—no study to date shows unequivocally a clinically useful relationship between any DNA sequence variants, alone or in combination, in any of the three CYP1 genes for prediction of a specific form of cancer.
The findings described in the present review also introduce more complexity into the relationship between CYP1 induction and toxicity or cancer. In other words, the route of administration, rate of administration, and target-organ response all depend on whether the CYP1 enzyme in any specific tissue or cell type might play a predominant role in metabolic activation or in detoxication. Thus, CYP1 induction per se is not all good, nor is it all bad.
Future Directions.
Studies of PAH exposure in humans have included cooked meat at high temperatures (Anderson et al., 2005; Sinha et al., 2005; Li et al., 2007; De Stefani et al., 2009), an aluminum smelter cohort (Friesen et al., 2007; Gibbs et al., 2007), consumption of fried chicken (BaP ∼5.4 μg/kg) and smoked dried beef (BaP ∼5.5 μg/kg), smokers inhaling daily ∼0.26 μg of BaP per pack of 20 cigarettes (Piccardo et al., 2010), and charcoal-broiled steak containing BaP levels of ∼9.0 μg/kg (http://www.atsdr.cdc.gov/toxprofiles/tp.asp?id=122&tid=25). Summarizing all available data leads to an extrapolated environmental dose of perhaps ∼40–50 ng/kg/d. BaP concentrations have been reported as high as 19 μg/kg in smoked meat in Austria (Tiefenbacher et al., 1982) and 69 μg/kg in rape seed oil (Pupin and Toledo, 1996); ingestion of these foods would increase BaP amounts to 80–380 ng/kg/d. The daily median total of ingested BaP exceeds the daily inhalation dose by ∼16-fold in winter and ∼120-fold in summer (Buckley et al., 1995). In New Jersey, the daily oral PAH intake from food per capita was estimated at 1.6-16 μg/d—with ∼10% as BaP (Santodonato et al., 1981).
Hence, we guesstimate that the daily oral BaP doses of 125 and 12.5 mg/kg/d in mouse studies described herein might be ∼7,500 and ∼750 times, respectively, beyond known human exposure levels. It would be informative to see what effects might occur in this mouse Cyp1 paradigm if daily oral BaP were administered at 1.25 (∼75-fold higher), 0.125 (∼7.5-fold higher), 0.0125 (∼75% of highest human exposures), or even 0.00125 mg/kg/d (∼7.5% of highest known human exposures). It would also be useful to know the BaP metabolite profiles of tissues that develop toxicity or malignancy. Do they change as a function of these suggested regimens of dosage?
Finally, epigenetics is undoubtedly involved in gene-environment interactions leading to toxicity and neoplasia. The presently available genomewide DNA-methylation maps and assays (Boerno et al., 2010; He et al., 2011) could examine the role of hypermethylation versus hypomethylation in some of the oncogenes and tumor-suppressor genes described herein. With the recent availability of current microRNA chips (Baer et al., 2013), the involvement of all known miRNAs could be assessed during the development of immunosuppression and toxic chemical depression of the bone marrow, as well as during PSI adenocarcinoma and PGD SCC formation. We predict additional exciting advances with this Cyp1 paradigm in the near future.
Acknowledgments
The authors thank their colleagues over the years—especially Timothy P. Dalton, Lei He, Bin Wang, and Elliot S. Vesell—and many other coworkers and two anonymous reviewers for helpful discussions and/or thorough readings of this review. The authors appreciate the generous gift of Cyp1b1(−/−) knockout mice from Frank J. Gonzalez more than a decade ago. The authors thank Marian L. Miller for selfless help with graphics.
Abbreviations
- AHH
aryl hydrocarbon hydroxylase
- AHR
aryl hydrocarbon receptor
- BaP
benzo[a]pyrene
- CYP1A1
enzyme encoded by the mouse Cyp1a1 gene
- CYP1A2
enzyme encoded by the mouse Cyp1a2 gene
- CYP1B1
enzyme encoded by the mouse Cyp1b1 gene
- Cyp1a1(−/−)
Cyp1a1 knockout mouse
- Cyp1a2(−/−)
Cyp1a2 knockout mouse
- Cyp1b1(−/−)
Cyp1b1 knockout mouse
- DME
drug-metabolizing enzyme
- GI
gastrointestinal
- P450
cytochrome P450
- PAH
polycyclic aromatic hydrocarbon
- PGD
preputial gland duct
- PSI
proximal small intestine
- SCC
squamous cell carcinoma
- TCDD/“dioxin”
2,3,7,8-tetrachlorodibenzo-p-dioxin
- XME
xenobiotic-metabolizing enzyme
Authorship Contributions
Participated in research design: Nebert, Shi, Gálvez-Peralta, Uno, Dragin.
Conducted experiments: Nebert, Shi, Gálvez-Peralta, Uno, Dragin.
Contributed new reagents or analytic tools: Nebert, Shi, Gálvez-Peralta, Uno, Dragin.
Performed data analysis: Nebert, Shi, Gálvez-Peralta, Uno, Dragin.
Wrote or contributed to the writing of the manuscript: Nebert, Shi, Gálvez-Peralta.
Footnotes
This work was supported, in part, by the National Institutes of Health National Institute of Environmental Health Sciences [Grant T32 ES016646] (to M.G.-P.) and [Grants R01 ES008147, R01 ES014403, and P30 ES006096] (to D.W.N.).
References
- Alvares AP, Kappas A, Levin W, Conney AH. (1973) Inducibility of benzo(a)pyrene hydroxylase in human skin by polycylic hydrocarbons. Clin Pharmacol Ther 14:30–40 [DOI] [PubMed] [Google Scholar]
- Alvares AP, Schilling G, Levin W, Kuntzman R. (1967) Studies on the induction of CO-binding pigments in liver microsomes by phenobarbital and 3-methylcholanthrene. Biochem Biophys Res Commun 29:521–526 [DOI] [PubMed] [Google Scholar]
- Anderson KE, Kadlubar FF, Kulldorff M, Harnack L, Gross M, Lang NP, Barber C, Rothman N, Sinha R. (2005) Dietary intake of heterocyclic amines and benzo(a)pyrene: associations with pancreatic cancer. Cancer Epidemiol Biomarkers Prev 14:2261–2265 [DOI] [PubMed] [Google Scholar]
- Baer C, Claus R, Plass C. (2013) Genome-wide epigenetic regulation of miRNAs in cancer. Cancer Res 73:473–477 [DOI] [PubMed] [Google Scholar]
- Bauer E, Guo Z, Ueng YF, Bell LC, Zeldin D, Guengerich FP. (1995) Oxidation of benzo[a]pyrene by recombinant human cytochrome P450 enzymes. Chem Res Toxicol 8:136–142 [DOI] [PubMed] [Google Scholar]
- Bock KW, Köhle C. (2006) Ah receptor: dioxin-mediated toxic responses as hints to deregulated physiologic functions. Biochem Pharmacol 72:393–404 [DOI] [PubMed] [Google Scholar]
- Bock KW, Köhle C. (2009) The mammalian aryl hydrocarbon (Ah) receptor: from mediator of dioxin toxicity toward physiological functions in skin and liver. Biol Chem 390:1225–1235 [DOI] [PubMed] [Google Scholar]
- Boerno ST, Grimm C, Lehrach H, Schweiger MR. (2010) Next-generation sequencing technologies for DNA methylation analyses in cancer genomics. Epigenomics 2:199–207 [DOI] [PubMed] [Google Scholar]
- Buckley TJ, Waldman JM, Dhara R, Greenberg A, Ouyang Z, Lioy PJ. (1995) An assessment of a urinary biomarker for total human environmental exposure to benzo[a]pyrene. Int Arch Occup Environ Health 67:257–266 [DOI] [PubMed] [Google Scholar]
- Buters JT, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, Doehmer J, Ward JM, Jefcoate CR, Gonzalez FJ. (1999) Cytochrome P450 CYP1B1 determines susceptibility to 7,12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad Sci USA 96:1977–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JF, Brown JP, Dawson SV, Marty MA. (1991) Risk assessment for benzo[a]pyrene. Regul Toxicol Pharmacol 13:170–184 [DOI] [PubMed] [Google Scholar]
- Conney AH, Chang RL, Jerina DM, Wei SJ. (1994) Studies on the metabolism of benzo[a]pyrene and dose-dependent differences in the mutagenic profile of its ultimate carcinogenic metabolite. Drug Metab Rev 26:125–163 [DOI] [PubMed] [Google Scholar]
- Conney AH, Miller EC, Miller JA. (1956) The metabolism of methylated aminoazo dyes. V. Evidence for induction of enzyme synthesis in the rat by 3-methylcholanthrene. Cancer Res 16:450–459 [PubMed] [Google Scholar]
- Conney AH, Miller EC, Miller JA. (1957) Substrate-induced synthesis and other properties of benzpyrene hydroxylase in rat liver. J Biol Chem 228:753–766 [PubMed] [Google Scholar]
- Conney AH, Pantuck EJ, Hsiao KC, Garland WA, Anderson KE, Alvares AP, Kappas A. (1976) Enhanced phenacetin metabolism in human subjects fed charcoal-broiled beef. Clin Pharmacol Ther 20:633–642 [DOI] [PubMed] [Google Scholar]
- Dalton TP, Dieter MZ, Matlib RS, Childs NL, Shertzer HG, Genter MB, Nebert DW. (2000) Targeted knockout of Cyp1a1 gene does not alter hepatic constitutive expression of other genes in the mouse [Ah] battery. Biochem Biophys Res Commun 267:184–189 [DOI] [PubMed] [Google Scholar]
- De Stefani E, Boffetta P, Deneo-Pellegrini H, Ronco AL, Aune D, Acosta G, Brennan P, Mendilaharsu M, Ferro G. (2009) Meat intake, meat mutagens and risk of lung cancer in Uruguayan men. Cancer Causes Control 20:1635–1643 [DOI] [PubMed] [Google Scholar]
- Dragin N, Shi Z, Madan R, Karp CL, Sartor MA, Chen C, Gonzalez FJ, Nebert DW. (2008) Phenotype of the Cyp1a1/1a2/1b1(−/−) triple-knockout mouse. Mol Pharmacol 73:1844–1856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragin N, Uno S, Wang B, Dalton TP, Nebert DW. (2007) Generation of ‘humanized’ hCYP1A1_1A2_Cyp1a1/1a2(−/−) mouse line. Biochem Biophys Res Commun 359:635–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duran-Reynals ML, Lilly F, Bosch A, Blank KJ. (1978) The genetic basis of susceptibility to leukemia induction in mice by 3-methylcholanthrene applied percutaneously. J Exp Med 147:459–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen MC, Demers PA, Spinelli JJ, Lorenzi MF, Le ND. (2007) Comparison of two indices of exposure to polycyclic aromatic hydrocarbons in a retrospective aluminium smelter cohort. Occup Environ Med 64:273–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara M, Sun B, Kato K, Kimura M, Yamazaki S. (1999) Cytochrome P450 isoforms catalyzing benzo[a]pyrene metabolism in the Chinese hamster liver. Toxicol Lett 110:85–93 [DOI] [PubMed] [Google Scholar]
- Galván N, Page TJ, Czuprynski CJ, Jefcoate CR. (2006) Benzo(a)pyrene and 7,12-dimethylbenz(a)anthrecene differentially affect bone marrow cells of the lymphoid and myeloid lineages. Toxicol Appl Pharmacol 213:105–116 [DOI] [PubMed] [Google Scholar]
- Galván N, Teske DE, Zhou G, Moorthy B, MacWilliams PS, Czuprynski CJ, Jefcoate CR. (2005) Induction of CYP1A1 and CYP1B1 in liver and lung by benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene do not affect distribution of polycyclic hydrocarbons to target tissue: role of AhR and CYP1B1 in bone marrow cytotoxicity. Toxicol Appl Pharmacol 202:244–257 [DOI] [PubMed] [Google Scholar]
- Gálvez-Peralta M, Shi Z, Chen J, Miller ML, Nebert DW. (2013) Oral benzo[a]pyrene in Cyp1a1/1b1(−/−) double-knockout mice: microarray analysis during squamous cell carcinoma formation in preputial gland duct. Int J Cancer 132:2065–2075 [DOI] [PubMed] [Google Scholar]
- Gibbs GW, Armstrong B, Sevigny M. (2007) Mortality and cancer experience of Quebec aluminum reduction plant workers. Part 2: mortality of three cohorts hired on or before January 1, 1951. J Occup Environ Med 49:1105–1123 [DOI] [PubMed] [Google Scholar]
- Gielen JE, Goujon FM, Nebert DW. (1972) Genetic regulation of aryl hydrocarbon hydroxylase induction. II. Simple Mendelian expression in mouse tissues in vivo. J Biol Chem 247:1125–1137 [PubMed] [Google Scholar]
- Grover PL, Sims P. (1968) Enzyme-catalysed reactions of polycyclic hydrocarbons with deoxyribonucleic acid and protein in vitro. Biochem J 110:159–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Shimada T. (1998) Activation of procarcinogens by human cytochrome P450 enzymes. Mutat Res 400:201–213 [DOI] [PubMed] [Google Scholar]
- He G, Elling AA, Deng XW. (2011) The epigenome and plant development. Annu Rev Plant Biol 62:411–435 [DOI] [PubMed] [Google Scholar]
- Hu D, Zheng H, Liu H, Li M, Ren W, Liao W, Duan Z, Li L, Cao Y. (2008) Immunoglobulin expression and its biological significance in cancer cells. Cell Mol Immunol 5:319–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MO, Lou Z, Rowland-Faux L, Celander MC. (2005) Properties and regional expression of a CYP3A-like protein in channel catfish intestine. Aquat Toxicol 72:361–371 [DOI] [PubMed] [Google Scholar]
- Järup L. (2003) Hazards of heavy metal contamination. Br Med Bull 68:167–182 [DOI] [PubMed] [Google Scholar]
- Koley AP, Buters JT, Robinson RC, Markowitz A, Friedman FK. (1997) Differential mechanisms of cytochrome P450 inhibition and activation by α-naphthoflavone. J Biol Chem 272:3149–3152 [DOI] [PubMed] [Google Scholar]
- Kuntzman R, Levin W, Jacobson M, Conney AH. (1968) Studies on microsomal hydroxylation and the demonstration of a new carbon monoxide binding pigment in liver microsomes. Life Sci II 7:215–224 [DOI] [PubMed] [Google Scholar]
- Legraverend C, Harrison DE, Ruscetti FW, Nebert DW. (1983) Bone marrow toxicity induced by oral benzo[a]pyrene: protection resides at the level of the intestine and liver. Toxicol Appl Pharmacol 70:390–401 [DOI] [PubMed] [Google Scholar]
- Li D, Day RS, Bondy ML, Sinha R, Nguyen NT, Evans DB, Abbruzzese JL, Hassan MM. (2007) Dietary mutagen exposure and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev 16:655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang HC, Li H, McKinnon RA, Duffy JJ, Potter SS, Puga A, Nebert DW. (1996) Cyp1a2(−/−) null mutant mice develop normally but show deficient drug metabolism. Proc Natl Acad Sci USA 93:1671–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meehan RR, Speed RM, Gosden JR, Rout D, Hutton JJ, Taylor BA, Hilkens J, Kroezen V, Hilgers J, Adesnik M. (1988) Chromosomal organization of the mouse cytochrome Cyp2c gene subfamily: locus associated with constitutive aryl hydrocarbon hydroxylase. Proc Natl Acad Sci USA 85:2662–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KP, Ramos KS. (2001) Impact of cellular metabolism on the biological effects of benzo[a]pyrene and related hydrocarbons. Drug Metab Rev 33:1–35 [DOI] [PubMed] [Google Scholar]
- Mitsumori K, Elwell MR. (1988) Proliferative lesions in the male reproductive system of F344 rats and B6C3F1 mice: incidence and classification. Environ Health Perspect 77:11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’jai AU, Larsen M, Shi L, Jefcoate CR, Czuprynski CJ. (2010) Bone marrow lymphoid and myeloid progenitor cells are suppressed in 7,12-dimethylbenz(a)anthracene (DMBA) treated mice. Toxicology 271:27–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW. (1970) Microsomal cytochromes b5 and P450 during induction of aryl hydrocarbon hydroxylase activity in mammalian cell culture. J Biol Chem 245:519–527 [PubMed] [Google Scholar]
- Nebert DW. (1989) The Ah locus: genetic differences in toxicity, cancer, mutation, and birth defects. Crit Rev Toxicol 20:153–174 [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. (2006) The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer 6:947–960 [DOI] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP, Okey AB, Gonzalez FJ. (2004) Role of aryl hydrocarbon receptor-mediated induction of the CYP1 enzymes in environmental toxicity and cancer. J Biol Chem 279:23847–23850 [DOI] [PubMed] [Google Scholar]
- Nebert DW, Gelboin HV. (1969) The in vivo and in vitro induction of aryl hydrocarbon hydroxylase in mammalian cells of different species, tissues, strains, and developmental and hormonal states. Arch Biochem Biophys 134:76–89 [DOI] [PubMed] [Google Scholar]
- Nebert DW, Goujon FM, Gielen JE (1971). Genetic regulation of monooxygenase activity, in Fonds de la Recherche Scientifique Medicale, Groupes de Contact (Heusghem C, ed), pp 240–270, Brussels [Google Scholar]
- Nebert DW, Jensen NM. (1979) Benzo[a]pyrene-initiated leukemia in mice. Association with allelic differences at the Ah locus. Biochem Pharmacol 28:149–151 [DOI] [PubMed] [Google Scholar]
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. (2000) Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol 59:65–85 [DOI] [PubMed] [Google Scholar]
- Nebert DW, Wikvall K, Miller WL. (2013a) Human cytochromes P450 in health and disease. Philos Trans R Soc Lond B Biol Sci 368:20120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Winker J, Gelboin HV. (1969) Aryl hydrocarbon hydroxylase activity in human placenta from cigarette smoking and nonsmoking women. Cancer Res 29:1763–1769 [PubMed] [Google Scholar]
- Nebert DW, Zhang G, Vesell ES. (2013b) Genetic risk prediction: individualized variability in susceptibility to toxicants. Annu Rev Pharmacol Toxicol 53:355–375 [DOI] [PubMed] [Google Scholar]
- Pantuck EJ, Kuntzman R, Conney AH. (1972) Decreased concentration of phenacetin in plasma of cigarette smokers. Science 175:1248–1250 [DOI] [PubMed] [Google Scholar]
- Phillips DH, Grover PL, Sims P. (1978) The covalent binding of polycyclic hydrocarbons to DNA in the skin of mice of different strains. Int J Cancer 22:487–494 [DOI] [PubMed] [Google Scholar]
- Piccardo MT, Stella A, Valerio F. (2010) Is the smokers exposure to environmental tobacco smoke negligible? Environ Health 9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poland AP, Glover E. (1974) Comparison of 2,3,7,8-tetrachlorodibenzo-p-dioxin, a potent inducer of aryl hydrocarbon hydroxylase, with 3-methylcholanthrene. Mol Pharmacol 10:349–359 [PubMed] [Google Scholar]
- Poland AP, Palen D, Glover E. (1994) Analysis of the four alleles of the murine aryl hydrocarbon receptor. Mol Pharmacol 46:915–921 [PubMed] [Google Scholar]
- Poland AP, Glover E, Robinson JR, Nebert DW. (1974) Genetic expression of aryl hydrocarbon hydroxylase activity. Induction of monooxygenase activities and cytochrome P1-450 formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin in mice genetically “nonresponsive” to other aromatic hydrocarbons. J Biol Chem 249:5599–5606 [PubMed] [Google Scholar]
- Pupin AM, Toledo MC. (1996) Benzo(a)pyrene in Brazilian vegetable oils. Food Addit Contam 13:639–645 [DOI] [PubMed] [Google Scholar]
- Robinson JR, Felton JS, Levitt RC, Thorgeirsson SS, Nebert DW. (1975) Relationship between “aromatic hydrocarbon responsiveness” and the survival times in mice treated with various drugs and environmental compounds. Mol Pharmacol 11:850–865 [PubMed] [Google Scholar]
- Rozman KK, Klaassen CD. (2007) Absorption, distribution and excretion of toxicants, in Casarett and Doull’s Toxicology: The Basic Science of Poisons (Klaassen CD, ed) pp 107–132, McGraw-Hill, New York [Google Scholar]
- Rubin H. (2001) Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: a bio-historical perspective with updates. Carcinogenesis 22:1903–1930 [DOI] [PubMed] [Google Scholar]
- Santodonato J, Howard P, Basu D. (1981) Health and ecological assessment of polynuclear aromatic hydrocarbons. J Environ Pathol Toxicol 5:1–364 [PubMed] [Google Scholar]
- Schlede E, Conney AH. (1970) Induction of benzol(α)pyrene hydroxylase activity in rat skin. Life Sci II 9:1295–1303 [DOI] [PubMed] [Google Scholar]
- Schlede E, Kuntzman R, Haber S, Conney AH. (1970) Effect of enzyme induction on the metabolism and tissue distribution of benzo(α)pyrene. Cancer Res 30:2893–2897 [PubMed] [Google Scholar]
- Shi Z, Dragin N, Gálvez-Peralta M, Jorge-Nebert LF, Miller ML, Wang B, Nebert DW. (2010a) Organ-specific roles of CYP1A1 during detoxication of dietary benzo[a]pyrene. Mol Pharmacol 78:46–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Dragin N, Miller ML, Stringer KF, Johansson E, Chen J, Uno S, Gonzalez FJ, Rubio CA, Nebert DW. (2010b) Oral benzo[a]pyrene-induced cancer: two distinct types in different target organs depend on the mouse Cyp1 genotype. Int J Cancer 127:2334–2350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims P, Grover PL. (1968) Quantitative aspects of the metabolism of 7,12-dimethylbenz[a]anthracene by liver homogenates from animals of different age, sex and species. Biochem Pharmacol 17:1751–1758 [DOI] [PubMed] [Google Scholar]
- Sinha R, Kulldorff M, Gunter MJ, Strickland P, Rothman N. (2005) Dietary benzo[a]pyrene intake and risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev 14:2030–2034 [DOI] [PubMed] [Google Scholar]
- Sun B, Fukuhara M, Takanaka A. (1995) Characterization of benzo[a]pyrene metabolism and related cytochrome P-450 isozymes in Syrian hamster livers. J Toxicol Environ Health 46:47–55 [DOI] [PubMed] [Google Scholar]
- Talalay P. (2000) Chemoprotection against cancer by induction of phase 2 enzymes. Biofactors 12:5–11 [DOI] [PubMed] [Google Scholar]
- Thomas PE, Kouri RE, Hutton JJ. (1972) The genetics of aryl hydrocarbon hydroxylase induction in mice: a single gene difference between C57BL-6J and DBA-2J. Biochem Genet 6:157–168 [DOI] [PubMed] [Google Scholar]
- Tiefenbacher K, Pfannhauser W, Woidich H. (1982) Investigation on contamination of food by polycyclic aromatic hydrocarbons, in Recent Developments in Food Analysis: Proceedings of the First European Conference on Food Chemistry (Baltes B, Czedik-Eysenberg PB, Pfannhauser W. eds) pp 76-82, Verlag Chemie, Deerfield Beach, FL [Google Scholar]
- Uno S, Dalton TP, Derkenne S, Curran CP, Miller ML, Shertzer HG, Nebert DW. (2004) Oral exposure to benzo[a]pyrene in the mouse: detoxication by inducible cytochrome P450 is more important than metabolic activation. Mol Pharmacol 65:1225–1237 [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Dragin N, Curran CP, Derkenne S, Miller ML, Shertzer HG, Gonzalez FJ, Nebert DW. (2006) Oral benzo[a]pyrene in Cyp1 knockout mouse lines: CYP1A1 important in detoxication, CYP1B1 metabolism required for immune damage independent of total-body burden and clearance rate. Mol Pharmacol 69:1103–1114 [DOI] [PubMed] [Google Scholar]
- Uno S, Dalton TP, Shertzer HG, Genter MB, Warshawsky D, Talaska G, Nebert DW. (2001) Benzo[a]pyrene-induced toxicity: paradoxical protection in Cyp1a1(-/-) knockout mice having increased hepatic BaP-DNA adduct levels. Biochem Biophys Res Commun 289:1049–1056 [DOI] [PubMed] [Google Scholar]
- Uno S, Dragin N, Miller ML, Dalton TP, Gonzalez FJ, Nebert DW. (2008) Basal and inducible CYP1 mRNA quantitation and protein localization throughout the mouse gastrointestinal tract. Free Radic Biol Med 44:570–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wattenberg LW, Leong JL, Strand PJ. (1962) Benzpyrene hydroxylase activity in the gastrointestinal tract. Cancer Res 22:1120–1125 [PubMed] [Google Scholar]
- Welch RM, Harrison YE, Conney AH, Poppers PJ, Finster M. (1968) Cigarette smoking: stimulatory effect on metabolism of 3,4-benzpyrene by enzymes in human placenta. Science 160:541–542 [DOI] [PubMed] [Google Scholar]
- Welch RM, Harrison YE, Gommi BW, Poppers PJ, Finster M, Conney AH. (1969) Stimulatory effect of cigarette smoking on the hydroxylation of 3,4-benzpyrene and the N-demethylation of 3-methyl-4-monomethylaminoazobenzene by enzymes in human placenta. Clin Pharmacol Ther 10:100–109 [DOI] [PubMed] [Google Scholar]
- Wogan GN, Hecht SS, Felton JS, Conney AH, Loeb LA. (2004) Environmental and chemical carcinogenesis. Semin Cancer Biol 14:473–486 [DOI] [PubMed] [Google Scholar]
- Yun CH, Shimada T, Guengerich FP. (1992) Roles of human liver cytochrome P4502C and 3A enzymes in the 3-hydroxylation of benzo(a)pyrene. Cancer Res 52:1868–1874 [PubMed] [Google Scholar]