Abstract
Cytochrome P450 (P450)-catalyzed oxidation of the aromatic ring of estradiol can result in 2- or 4-hydroxylation. Which of these products is formed is biologically important, as the 4-hydroxylated metabolite is carcinogenic, whereas the 2-hydroxylated metabolite is not. Most human P450 enzymes, including CYP1A1 and CYP1A2, exhibit a high preference for estradiol 2-hydroxylation, but human CYP1B1 greatly favors 4-hydroxylation. Here we show that heterologous expression of the human, monkey, dog, rat, and mouse CYP1B1 enzymes yields active proteins that differ in their estradiol hydroxylation specificity. The monkey and dog orthologs, like the human enzyme, preferentially catalyze 4-hydroxylation, but the rat and mouse enzymes favor 2-hydroxylation. Analysis of the CYP1B1 sequences in light of these findings suggested that one residue, Val395 in human CYP1B1, could account for the differential hydroxylation specificities. In fact, mutation of this valine in human CYP1B1 to the leucine present in the rat enzyme produces a human enzyme that has the 2-hydroxylation specificity of the rat enzyme. The converse is true when the leucine in the rat enzyme is mutated to the human valine. The role of CYP1B1 in estradiol carcinogenicity thus depends on the identity of this single amino acid residue.
Introduction
CYP1B1 is an extrahepatic cytochrome P450 (P450) that is of interest for several reasons. First, it oxidizes a variety of endogenous molecules, including retinol (Choudhary et al., 2004), arachidonic acid (Choudhary et al., 2004), melatonin (Ma et al., 2005), and steroids, particularly 17β-estradiol (Hayes et al., 1996; Shimada et al., 1998a), and thus may play a role in signaling, hormone action, and development. Support for this inference is provided by the fact that polymorphisms in the cyp1B1 gene are closely associated in humans with congenital glaucoma (Vasiliou and Gonzalez, 2008; Choudhary et al., 2009). CYP1B1 polymorphisms have also been linked to the incidence of breast and lung cancer (Watanabe et al., 2000; Zheng et al., 2000; De Vivo et al., 2002). Second, CYP1B1 oxidizes a variety of procarcinogenic compounds to their activated forms, including polycyclic aromatic hydrocarbons and aryl amines (Shimada et al., 2001a). In contrast to CYP1A1, human CYP1B1 preferentially oxidizes 17β-estradiol to the carcinogenic 4-hydroxy rather than to the noncarcinogenic 2-hydroxy derivative (Hayes et al., 1996; Shimada et al., 1998a). Studies with knockout mice have suggested that the susceptibility to 7,12-dimethylbenz[a]anthracene-induced lymphomas is determined by CYP1B1 (Buters et al., 1999). This potential role in carcinogenesis suggests that inhibitors of the enzyme may have anticarcinogenic properties (Shimada et al., 2011). Finally, using a CYP1B1 antibody that does not recognize CYP1A1 or CYP1A2, studies have shown that CYP1B1 is specifically or highly selectively expressed in tumors rather than normal tissues (Murray et al., 1997). This property makes the enzyme a highly attractive target for the activation of anticancer prodrugs.
Human CYP1B1 is a protein of 543 amino acids, of which 53 are part of an N-terminal membrane-binding domain, 10 are located in a proline-rich hinge region, and 480 make up the globular catalytic domain (Savas et al., 1994; Sutter et al., 1994). The protein shares 37% sequence identity with CYP1A1 and approximately the same with CYP1A2. The structure of human CYP1B1 complexed with α-naphthoflavone (ANF) has been determined at 2.70 Å resolution (Wang et al., 2011). Before the structures of CYP1A1 and CYP1B1 were available, homology models were constructed based on the known structure of CYP1A2 in an effort to understand why human CYP1A1 favored 2-hydroxylation of 17β-estradiol, whereas human CYP1B1 predominantly catalyzed 4-hydroxylation of the same substrate (Itoh et al., 2010). The authors argued that Ser122 and Phe258 of CYP1A1 and Ala133 and Asn265 of CYP1B1 were particularly important in substrate recognition, but these predictions are uncertain because they were based on homology models and were never experimentally tested.
The association of CYP1B1 with cancer and ocular disorders has stimulated a search for human variants, and more than 80 polymorphic forms of human CYP1B1 are now known (Vasiliou and Gonzalez, 2008; Choudhary et al., 2009). The relative substrate oxidation activities of some of these variants have been examined. Thus, a range of human CYP1B1 allelic proteins varying at positions 48, 119, and 432 have been heterologously expressed in Escherichia coli, and their activities in the oxidation of E2 and other substrates have been examined (Spink et al., 2000; Shimada et al., 2001b; Lewis et al., 2003). The wild-type is considered to be Arg48,Ala119,Leu432,Asn453 (RALN). It was found that the Arg48 variants generally had a modestly higher preference for 4- versus 2-hydroxylation than the Gly48 variants.
The CYP1B1 orthologs from mouse (Savas et al., 1997) and zebrafish (Scornaienchi et al., 2010) have been cloned and heterologously expressed. Mouse CYP1B1 was reported to not bind or oxidize 17β-estradiol. The zebrafish enzyme was shown to oxidize estradiol, but, in contrast to the human enzyme that exhibits a 4-hydroxyestradiol (4OH E2)/2-hydroxyestradiol (2OH E2) ratio of approximately 3.44 (Lee et al., 2003), the zebrafish enzyme favored 2-hydroxylation and gave a 4OH E2/2OH E2 ratio of 0.32 (Scornaienchi et al., 2010).
Two electrons are required for each normal catalytic turnover of a P450 enzyme. For most mammalian P450 enzymes, the first electron is provided by NADPH–cytochrome P450 reductase (CPR). This flavoprotein can also provide the second electron, but in some instances cytochrome b5 can function as an alternative donor of the second electron. The interaction with cytochrome b5 can therefore accelerate catalytic turnover, inhibit turnover by competing for binding with CPR, or, through allosteric interactions, alter the products that are formed; it can also have no measurable effect. Cytochrome b5 has been reported to have no effect on substrate oxidation by human CYP1B1 (Shimada et al., 1997; Yamazaki et al., 2002). In contrast, cytochrome b5 was found to enhance 7-ethoxycoumarin O-dealkylation by zebrafish CYP1B1, whereas the oxidation of 17β-estradiol by this same enzyme was inhibited by cytochrome b5 (Scornaienchi et al., 2010).
Materials and Methods
Chemicals and Enzymes.
The rat (Rattus norvegicus), mouse (Mus musculus), rhesus monkey (Macaca mulatta), dog (Canis lupus familiaris), and human CYP1B1 enzymes were coexpressed in E. coli with the CPR from the corresponding species, except for monkey CYP1B1, which was coexpressed with the human CPR. These expressions of the CYP1B1 orthologs were performed by Cypex (Dundee, UK) using their corporate technology. In addition, human and rat CYP1B1 and CPR were coexpressed in E. coli in our laboratory (see below). We obtained purified human cytochrome b5 from Invitrogen (Carlsbad, CA); 17β-estradiol, 4-hydroxyestradiol, glucose-6-phosphate, and glucose-6-phosphate dehydrogenase from Sigma-Aldrich (St. Louis, MO); 2-hydroxyestradiol from Cayman Chemical (Ann Arbor, MI); N-methyl-N-(trimethylsilyl)trifluoroacetamide and N-trimethylsilylimidazole from Pierce (Rockford, IL), and potassium phosphate and chloroform from Fisher Scientific (Rockford, IL).
Expression Plasmids and Site-Directed Mutagenesis.
The bicistronic expression plasmid pCWori encoding human CYP1B1 and human CPR was a generous gift from F. P. Guengerich. The CYP1B1 sequence is that of the native protein, except that amino acids 2-4 (Gly-Thr-Ser) have been removed (Shimada et al., 1998a) to increase the yield, and five histidine residues have been added to the C terminus as a metal affinity tag.
Site-directed mutagenesis of human CYP1B1 was performed via QuikChange (Agilent Technologies, Santa Clara, CA) to create the CYP1B1 V395L mutant. The L395V mutant of rat CYP1B1 was made using overlap extension polymerase chain reaction. The CYP1B1 gene was recloned into a bicistronic plasmid that had not undergone polymerase chain reaction cycling, and its sequence was confirmed by DNA sequencing.
Rat CYP1B1 in pCWori and rat CPR in plasmid pACYC, employing synthetic genes, were provided by Cypex. CYP1B1 was constructed with an N-terminal OmpA leader sequence plus two extra linker amino acids (Ala-Pro), which remained on the CYP1B1 protein following OmpA cleavage. CPR was constructed with an N-terminal PelB leader sequence.
Enzyme Expression and Purification.
The enzymes were expressed in E. coli DH5α cells, using either dual CYP1B1 and CPR plasmids or a bicistronic construct. The 1-liter cultures in Terrific Broth medium were grown at 37°C until an optical density of 1.0 was reached (wavelength 600 nm), at which time 0.5 mM δ-aminolevulinic acid and 1 mM isopropyl β-D-1-thiogalactopyranoside were added, the temperature was reduced to 28°C, and shaking was reduced to 180 rpm. After approximately 30 hours, cells were harvested and “bactosome” membrane fractions containing CYP1B1 and CPR were prepared using previously described techniques (Shimada et al., 1998a).
Spectroscopic Characterization.
Ferrous carbonmonoxy P450 spectra were obtained from dilutions of bactosomes in 0.1 M potassium phosphate, pH 7.4, 20% glycerol. Reduction was accomplished either with sodium dithionite or under anaerobic conditions with NADPH and the endogenous CPR. The concentration of the P450 enzyme was calculated using a molar extinction coefficient of 91,000 M−1cm−1 for the maximum absorbance difference between 450 and 500 nm.
Cytochrome c Reduction Assay.
CPR activity was measured at 37°C in 0.1 M potassium phosphate, pH 7.4, using an extinction coefficient for reduced cytochrome c of 21,000 M−1cm−1 at 550 nm.
7-Ethoxyresorufin O-Deethylation Assay.
The formation of resorufin was measured against standard curves by fluorescence employing a Horiba Jobin Yvon (Kyoto, Japan) Fluorolog with excitation and emission wavelengths of 550 and 585 nm, respectively, and slit widths of 2 and 5 nm.
Estradiol Hydroxylation Assay.
Estradiol hydroxylation reactions were performed at 37°C in 0.1 M potassium phosphate, pH 7.4, with 20 µM estradiol, 5 mM sodium ascorbate, and an NADPH-regeneration system composed of 1 U/ml glucose-6-phosphate dehydrogenase, 5 mM glucose-6-phosphate, and 5 mM magnesium chloride. Bacterial membranes from the expression systems were added to a prewarmed reagent mixture and were preincubated for 3 minutes before initiating the reaction by adding 1 mM NADPH. Aliquots were quenched by adding 1/10 volume of 1 M HCl containing 1 mM sodium ascorbate, and were then immediately extracted with chloroform. The chloroform was evaporated under a stream of argon, and the residual extracts were dissolved in high-pressure liquid chromatography mobile phase.
2-Hydroxyestradiol and 4-hydroxyestradiol were analyzed by reversed-phase high-pressure liquid chromatography and quantified using calibration curves of metabolite standards that had been mock quenched. Separation was done on an Agilent Eclipse XDB-C18 column (4.6 × 150 mm, 5 µm pore) with isocratic elution at a flow rate of 0.8 ml/min of equal parts (v/v) of 0.1% acetic acid in water and 0.1% acetic acid in 3:2 acetonitrile:methanol. 2-Hydroxyestradiol was detected by fluorescence, with excitation at 290 nm and emission at 360 nm, and 4-hydroxyestradiol by absorbance at 206 nm. When present, cytochrome b5 was added under the same reaction conditions.
Sequence and Structure Analysis.
Primary sequences were aligned using ClustalW2 (Larkin et al., 2007) and Clustal Omega (Sievers et al., 2011), and Pymol Molecular Graphics System, Versions 1.2r3pre and 1.5.0.4 (Schrödinger, New York, NY) was used to view the three-dimensional structures and to generate the figures. The CYP1B1 primary sequences were National Center for Biotechnology Information RefSeq NP_000095.2 (human), NP_001253797.1 (rhesus monkey), NP_001153156.1 (dog), NP_034124.1 (mouse), NP_037072.1 (rat), and NP_001139180.1 (zebrafish). The crystal structure coordinates for CYP1B1 and CYP1A2 were PDB ID 3PM0 (Wang et al., 2011) and PDB ID 2HI4 (Sansen et al., 2007), respectively.
Substrate Docking.
Molecular docking of 17β-estradiol with CYP1B1 (PDB ID 3PM0) was performed with Autodock Vina (Trott and Olson, 2010) and a 20 Å3 search cube centered at (−18.34, 21.5, −19.82).
Results
Coexpression of CYP1B1 Orthologs with CPR Produces Functionally Competent Enzyme Systems.
The CYP1B1 human, rhesus monkey, dog, mouse, and rat orthologuous enzymes were coxpressed with the CPR from the same species, except for the monkey, which was coexpressed with human CPR because the monkey CPR sequence was not available. Bacterial membranes from these expressions, carried out by Cypex, were donated by Cyterix Pharmaceuticals (San Carlos, CA). The resulting P450 contents, 7-ethoxyresorufin O-deethylase (EROD) activities, and cytochrome c reduction activities are shown in Table 1. The EROD activities, in combination with the reductase activities and the observation of a normal ferrous-CO absorption spectrum, confirm the presence of functional P450/CPR systems within all the membrane preparations. As a control, it was demonstrated that ANF (1 µM), a CYP1 inhibitor (Shimada et al., 1998b), abolished the EROD activity, in agreement with oxidation by CYP1B1 (data not shown).
TABLE 1.
The P450 content, protein content, and EROD activities of CYP1B1 ortholog bactosomes
Determined by ferrous CO measurements, the bicinchoninic acid method, and resorufin fluorescence, respectively.
| P450 Content |
Cytochrome c Reductase Activity |
Protein Content |
EROD Activity |
|
|---|---|---|---|---|
| pmol/mg protein | nmol/min/mg protein | mg/ml | nmol/min/nmol | |
| Human | 690 | 220 | 10.0 | 4.30 ± 0.05 |
| Monkey | 632 | 166 | 17.4 | 2.6 ± 0.6 |
| Dog | 200 | 1230 | 10.0 | 80.0 ± 10 |
| Mouse | 220 | 758 | 9.1 | 6.2 ± 0.3 |
| Rat | 36 | 1331 | 15.1 | 2.99 ± 0.01 |
| Human (BD)a | 153 | 490 | 6.5 | 15.2 ± 0.9 |
Human CYP1B1 Supersomes from BD Biosciences (San Jose, CA) included as a reference point.
CYP1A1, CYP1A2, and CYP1B1 predominantly oxidize 17β-estradiol to the 4OH E2 and 2OH E2 metabolites, but they give different product ratios. The 4OH E2:2OH E2 product ratio for human CYP1B1 has been reported to be 3.44:1, indicating that the primary product is 4-hydroxyestradiol (Lee et al., 2003). In contrast, zebrafish CYP1B1, like human CYP1A1 and CYP1A2, primarily forms the 2-hydroxylated metabolite, with a reported 4OH E2:2OH E2 ratio of 0.32:1 (Scornaienchi et al., 2010). Surprisingly, it was reported in preliminary work that purified recombinant mouse CYP1B1 did not give a detectable 17β-estradiol binding spectrum, and high level expression in microsomes did not result in detectable estradiol hydroxylation (Savas et al., 1997).
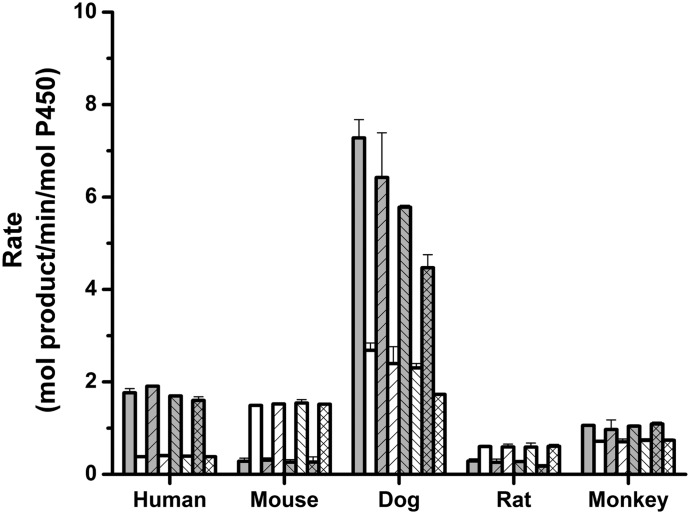
We measured 17β-estradiol hydroxylation for all five orthologs with increasing amounts of cytochrome b5, a potential modulator of the activity (Fig. 1). In the absence of b5, the relative ortholog estradiol activities paralleled the EROD activities. That is, dog CYP1B1 showed the highest activity, followed by human and mouse, and finally, rat and monkey. Cytochrome b5 added in cytochrome b5:P450 molar ratios of 1:1, 4:1 and 8:1 had minimal to no effect on 4OH E2 and 2OH E2 formation for the human, mouse, rat, and monkey systems. However, with the dog enzyme, 4OH E2 and 2OH E2 formation decreased by 38 and 35%, respectively, when the b5:P450 ratio increased from 0:1 to 8:1. Mouse CYP1B1 produced the 4OH E2 and 2OH E2 metabolites at overall rates of 0.28 ± 0.07 and 1.49 ± 0.01 mol of product per min per mol of P450, respectively. Rat CYP1B1/CPR gave similar results of 0.28 ± 0.05 min−1 and 0.60 ± 0.03 min−1. In contrast, the human, dog and rhesus monkey CYP1B1 systems preferentially produced 4OH E2 over 2OH E2, with the rates for the human enzyme being 1.76 ± 0.09 min−1 and 0.38 ± 0.01 min−1, respectively. The 4OH E2:2OH E2 product ratio was therefore highest for the human system at 4.6, followed by the dog, monkey, rat and mouse systems at 2.7, 1.5, 0.48, and 0.19. These results clearly show that the nonrodent species preferentially form the 4OH E2 product, whereas the rodent CYP1B1 enzymes, like the zebrafish enzyme (Scornaienchi et al., 2010), preferentially produce the 2OH E2 metabolite.
Fig. 1.
Estradiol hydroxylation and effect of cytochrome b5. Rates of formation of 4OH E2 and 2OH E2. Reactions containing the indicated molar ratios of cytochrome b5 to P450 were quenched after 5 minutes and were analyzed directly by high-pressure liquid chromatography. Gray bars represent 4OH and white bars indicate 2OH E2. Each pair of bars corresponds to 0-, 1-, 4-, and 8-fold molar cytochrome b5 (bar patterns: none, /, \, and ×, respectively).
Identification of Candidate CYP1B1 Residues Modulating the Specificity of Estradiol Hydroxylation.
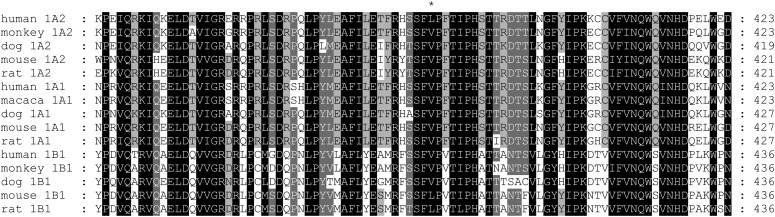
In an effort to identify structural determinants responsible for the contrasting 17β-estradiol hydroxylation specificities of human, monkey, and dog CYP1B1 (group A) versus mouse, rat, and zebrafish CYP1B1 (group B), we aligned the primary sequences of the known CYP1B1 orthologs and other CYP1 enzymes (Fig. 2). A search was then performed to identify amino acid residues that satisfied the following search criteria: a) identical or similar between mouse, rat, and zebrafish; b) different between group A and group B CYP1B1; and c) in close proximity to the active site of the CYP1B1 human crystal structure bound to ANF, a known potent inhibitor of CYP1B1 with structural similarities to estradiol.
Fig. 2.
Sequence alignment of CYP1B1, CYP1A1, and CYP1A2 orthologs. Alignment was done with ClustalW. Residue 395 of human CYP1B1 is marked with an asterisk.
Of the 21 residues that possessed at least one atom within 5 Å of the bound ANF, none of them satisfied all three criteria. Expanding the distance to 7 Å (36 residues) yielded one fitting the criteria: Val395. To account for differences in binding between ANF and 17β-estradiol, we expanded the search radius further, but at 10 Å, no other hits were obtained.
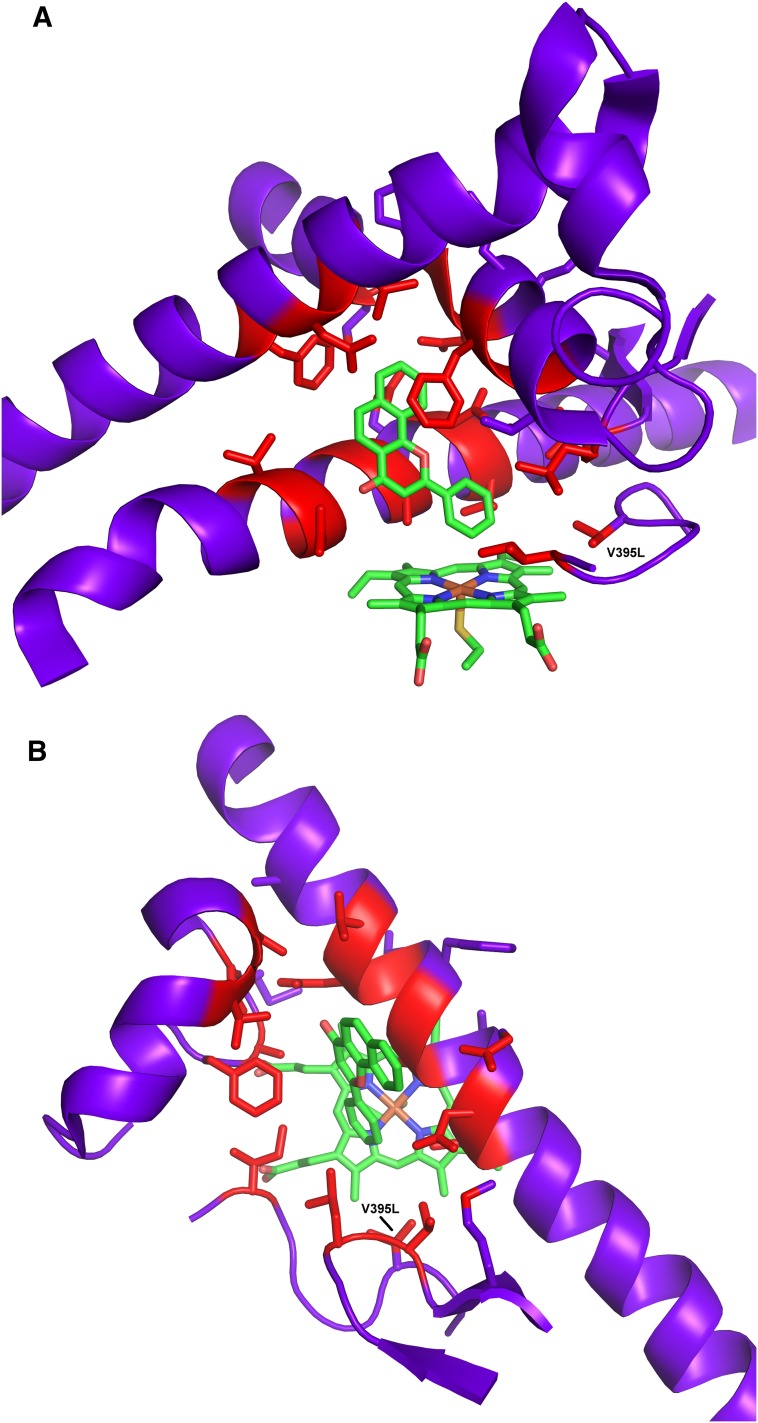
Valine 395, which is located in a loop (Fig. 3), has high homology among P450 enzymes (not shown). At its closest distance, V395 is 5.4 Å from the bound ANF. Given the structural similarity between ANF and 17β-estradiol, we thought that the residue at position 395 might be able to alter 17β-estradiol binding and therefore hydroxylation. Furthermore, CYP1A2 has a leucine at the homologous position 382 (Figs. 2 and 4A) and a 2-hydroxylation preference.
Fig. 3.
Structure of human CYP1B1 with ANF bound. Residues in red are those within 7 Å of ANF. (A) The F and G helices are at top and the I helix is behind ANF. The BC helix has been removed from the foreground. (B) Top-down view compared with A. The BC helix is shown upper left, and the F and G helices have been removed from the foreground.
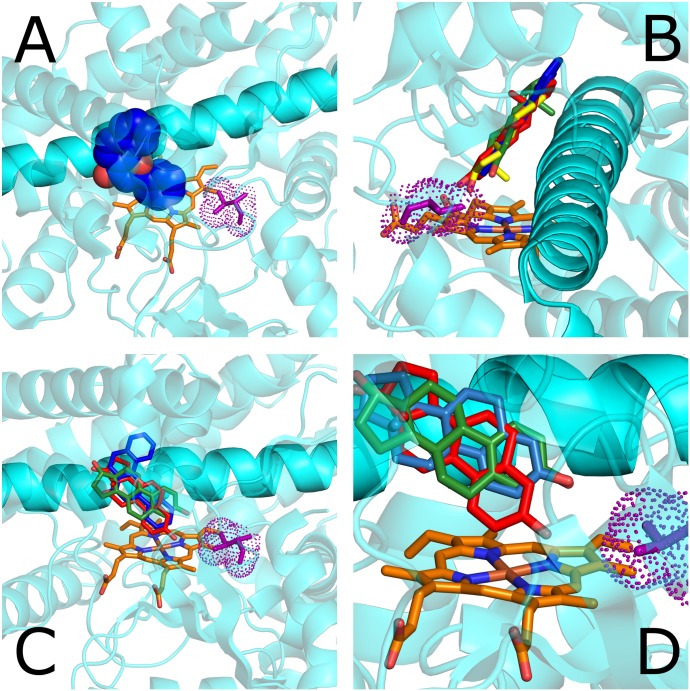
Fig. 4.
Structures of CYP1A2 and CYP1B1. (A) Crystal structure of CYP1A2 with bound ANF (blue). Leu382 is shown in purple, ANF in blue, and heme in orange. The I helix is nontransparent. (B) Crystal structure of CYP1B1 with bound ANF (blue) and docked 17β-estradiol in three conformations, two with ring A near heme (red, β plane away from I helix; green, β plane toward I helix) and one with ring D near heme (yellow). (C) Rotated view of B. (D) Closer view of the three poses of docked estradiol with ring A near heme (additional pose in light blue).
Molecular Docking of 17β-Estradiol with CYP1B1.
The structure of CYP1B1 with bound ANF was used for the docking of estradiol. The docking program Autodock Vina (Scripps Research Institute, La Jolla, CA) produced five poses, all of which had steroid rings lying roughly coplanar with ANF and with either ring D or ring A directed toward V395. Two had ring D toward the heme, of which one is shown in Fig. 4B (yellow), and the other was flipped in the orientation of the alpha and beta planar faces. Of the other three with ring A directed toward the heme, one was flipped in α/β orientation, with the 18β-methyl group directed toward the I-helix (Fig. 4, B–D, green). The other two poses were similar, differing slightly in the angle at which the phenol group was directed (Fig. 4D, red and light blue) toward V395.
Amino Acid 395 Is the Major Determinant of CYP1B1 17β-Estradiol Hydroxylation Regiospecificity.
A human CYP1B1 V395L mutant was constructed in which the human valine residue was mutated into a rodent leucine residue. The complementary reverse rat CYP1B1 L395V mutant was also constructed. These mutant enzymes were coexpressed with the corresponding human or rat CPR and the P450 content, cytochrome c reductase activity, and 17β-estradiol metabolite patterns of the resulting bacterial membranes were determined (Table 2). Cypex used a proprietary expression system with two vectors for coexpression of the various ortholog CYP1B1 proteins with P450 reductase, whereas our expressions of wild-type and mutant human CYP1B1 proteins used a single bicistronic expression plasmid. Estradiol metabolism by wild-type human CYP1B1 bactosomes from Cypex and from our laboratory were compared. Not unexpectedly, the optimized commercial system had higher 4-hydroxylation (8.6 ± 0.1 versus 3.1 ± 0.3) and 2-hydroxylation rates (1.60 ± 0.08 versus 0.62 ± 0.06, at 20 μM estradiol). However, the product ratios were identical (5.4 ± 0.3 and 5.0 ± 0.7).
TABLE 2.
The P450 content, protein content and cytochrome c reductase activities of in-house bactosome preparations of human wild-type, rat wild-type, and mutants
| P450 Content |
Cytochrome c Reductase Activity |
Protein Content |
|
|---|---|---|---|
| pmol/mg | nmol/min/mg protein | mg/ml | |
| Human | 31.1 | 640 ± 30 | 26.1 |
| Human V395L | 33.4 | 800 ± 30 | 17.0 |
| Rat L395V | 10.2 | 7600 ± 1100 | 35.4 |
| Rat | 47.0 | 410 ± 60 | 33.0 |
The cytochrome c reductase activities, which measure the CPR activities, are shown in Fig. 5A. Most of the membranes had activities ranging from 360 to 800 nmol min−1 mg−1. However, the CYP1B1 L395V mutant bactosome preparation had significantly higher activity: 7600 nmol min−1 mg−1. Comparison with Fig. 5B shows that this higher cytochrome c reduction activity did not correlate with a higher rate of 17β-estradiol hydroxylation Fig. 5B and Table 3, presumably because the cytochrome P450 enzyme was saturated with reductase at even the lower concentrations. Furthermore, we compared the 17β-estradiol hydroxylation from independently obtained bactosome samples of recombinantly coexpressed human CYP1B1 with human CPR from a commercial source (Cypex) and from in-house preparations. Although the samples differed in their cytochrome c reductase activities by 2-fold (150 and 300 nmol/min/mg protein, respectively), hydroxylation rates and product ratios were the same, with the product ratio from 5–150 μM estradiol varying between 4.2 ± 0.4 and 8 ± 2.
Fig. 5.
Cytochrome c reductase (A) and estradiol hydroxylation (B) activities of bactosomes with coexpressed CYP1B1 and CPR.
TABLE 3.
Estradiol 4- and 2-hydroxylation by wild-type human, rat, and mutant CYP1B1
Activities are mol product produced per mol P450 per minute.
| 4OH E2 Activity | 2OH E2 Activity | 4OH E2:2OH E2 Ratio | |
|---|---|---|---|
| min−1 | |||
| Wild-type human | 3.1 ± 0.3 | 0.62 ± 0.06 | 5.0 ± 0.7 |
| V395L human | 0.40 ± 0.03 | 0.87 ± 0.06 | 0.46 ± 0.05 |
| L395V rat | 0.95 ± 0.02 | 0.54 ± 0.01 | 1.78 ± 0.05 |
| Wild-type rat | 0.44 ± 0.12 | 1.17 ± 0.04 | 0.38 ± 0.10 |
In confirmation of the predicted importance of this residue, mutation of Val395 to a Leu in human CYP1B1 inverted the 4OH E2:2OH E2 hydroxylation preference from 5.1 to 0.45, a value resembling that of the rat (0.38). Conversely, mutating Leu395 to a Val in rat CYP1B1 shifted the 4OH E2:2OH E2 hydroxylation ratio from 0.38 to 1.8, now favoring a human-like preference for 4-hydroxylation. The rates of 2-hydroxyestradiol formation by wild-type and the V395L human mutant were similar at 0.62 ± 0.06 and 0.87 ± 0.05 min−1, but the 4-hydroxylation rates varied 8-fold between wild-type and the V395L mutant, with values of 3.1 ± 0.30 and 0.40 ± 0.03 min−1, respectively. For the wild-type and L395V rat mutant, the differences were about 2-fold for both 2-hydroxylation, with values of 1.17 ± 0.04 and 0.54 ± 0.01 min−1, and 4-hydroxylation, with values of 0.44 ± 0.11 and 0.95 ± 0.01 min−1. The differences in 4OH E2:2OH E2 product ratios thus reflect changes in 4-hydroxylation rather than 2-hydroxylation. In addition to 4- and 2-hydroxylation, estradiol metabolism by human P450 enzymes results in 16α-hydroxylation, primarily by CYP3A4 and CYP1A2 (Yamazaki et al., 1998; Badawi et al., 2001). In the case of CYP1B1, for which this is a minor reaction, the human and mouse enzymes produced less 16α-hydroxyestradiol compared with the rat, L395V rat, and V395L human enzymes. There was no clear association of 16α-hydroxyestradiol formation with the 4OH E2:2OH E2 ratios.
Discussion
CYP1B1 and CPR were matched, based on the source organism, to achieve the optimal electron transfer pairing (except monkey CPR, for which the sequence was not available). All five CYP1B1 orthologs heterologously coexpressed in E. coli with the appropriate CPR enzymes are active in the deethylation of 7-ethoxyresorufin, a common marker activity, and in the 2- and 4-hydroxylation of 17β-estradiol (Fig. 1; Table 1). To our knowledge, this is the first recombinant expression and characterization of the rat, dog, and rhesus monkey CYP1B1 enzymes.
Mouse CYP1B1 was previously expressed and purified by Savas et al. (1997), who reported its binding and metabolism of several polycyclic aromatic hydrocarbons. However, these authors were unable to detect either spectral changes due to the binding of estradiol or the hydroxylation of 17β-estradiol by E. coli-expressed mouse CYP1B1. Nevertheless, the metabolism of dimethylbenz(a)anthracene was inhibited by 17β-estradiol, which suggests that 17β-estradiol was bound even if no spectroscopic signature was observed. In contrast, we have readily observed and quantified the hydroxylation of 17β-estradiol by mouse CYP1B1/CPR. The observed 4OH E2:2OH E2 ratio of 0.19 contrasted sharply with the corresponding value of 4.6 for the human enzyme, 2.7 for the dog, and 1.5 for the monkey. Zebrafish CYP1B1 was reported earlier to exhibit a mouse-like inverse 4OH E2:2OH E2 ratio of 0.48. These results highlight the existence of significant interspecies differences among CYP1B1 orthologs that may complicate the interpretation of studies with animal models of the carcinogenicity of 17β-estradiol and other CYP1B1-activated agents.
Our primary sequence alignment in conjunction with our analysis of the relationship between the alignment and the crystal structure of human CYP1B1 pinpointed a single residue that was common (valine) in all the orthologs that favored 4-hydroxylation (human, dog, monkey) but differed in the orthologs that favored 2-hydroxylation (mouse, leucine; rat, leucine; zebrafish, threonine). Mutation of this single amino acid residue from Val to Leu in human CYP1B1 altered the 4OH E2:2OH E2 product ratio from >4 to 0.5:1, a value identical to that observed for the rat enzyme, which has a Leu at that position. Conversely, mutation of the native Leu in rat CYP1B1 to Val shifted the 4OH E2:2OH E2 product ratio to a more human-like 4OH E2 preference of 2:1. The individual 4OH E2 and 2OH E2 rates of formation showed that the mutations had a greater effect on 4-hydroxylation, which is consistent with interference of residue 395 with positioning of 17β-estradiol for 4-hydroxylation. Thus, the amino acid at position 395 is the principal determinant of the 17β-estradiol hydroxylation regiospecificity CYP1B1 enzymes. Notably, for human CYP1B1, the rates were similar for samples with different levels of CPR activity, and product ratios were similar across a range of estradiol concentrations, from 5–150 μM.
Comparison with Other CYP1 Family Enzymes.
Human CYP1A1 and CYP1A2, the two other CYP1 family enzymes, preferentially catalyze the 2-hydroxylation of 17β-estradiol. Alignment of the sequences of the CYP1A1, CYP1A2, and CYP1B1 orthologs from human, monkey, dog, mouse, and rat shows that human CYP1A2 is unique in having a Leu at the relevant position (Leu382), whereas the other CYP1A1 and CYP1A2 isozymes have a Val at that location. The presence of a Leu in human CYP1A2 and its preference for 2-hydroxylation are consistent with our results for the CYP1B1 V395L mutant. We have not found published data on 17β-estradiol hydroxylation by purified CYP1A2 orthologs from the other mammals and therefore cannot comment on whether nonhuman orthologs that possess a Val at the equivalent position have a preference for 4-hydroxylation.
Comparison of the CYP1B1 and CYP1A2 structures identified common residues that contact ANF, despite the different binding orientations of this ligand in the two proteins (1B1/1A2: F134/F125, F231/F226, F268/F260, D326/D313, G329/G316, A330/A317, D333/D320, T334/T321, I399/I386, and L509/L497). Thus, based on ANF binding, CYP1A2 Leu382 might be a significant contributor to hydroxylation preference. However, this single residue does not control the hydroxylation specificity for all CYP1A isoforms, as all the CYP1A1 orthologs have Val at the relevant position yet, based on the reported human activity, CYP1A1 exhibits a preference for 2-hydroxylation. In the absence of a structure for CYP1A1 to compare with those for CYP1B1 and CYP1A2, it is not possible to know what other active site differences reconcile the presence of a Val with the observed 2-hydroxylation preference.
The E2 molecule exhibits high conservation in its conformation when observed bound to estrogen dehydrogenase (PDB ID 1FDS), sex hormone-binding globulin (PDB ID 1LHU), and estrogen receptor (PDB ID 1ERE). This molecular conformation is likely to be shared when bound to CYP1B1.
The CYP1A2 and CYP1B1 active sites, when ANF is bound, possess certain ligand binding features that we believe to exist when E2 is bound. The I helix in P450s is an essential core helix that bears the commonly observed distal threonine. In the ANF-bound structures, the I helix provides three binding features: a surface upon which the planar tri-ring of ANF lies, and two “bracketing” aspartates (CYP1B1 D326 and D333)—conserved among CYP1A1, 1A2, and 1B1 from human, monkey, dog, mouse, and rat (except rat 1A2 E318 instead of D333)—on either side of the ANF planar edges. By nature of its importance in catalysis and heme binding, the I helix structure should be relatively unchanged regarding the binding of E2 in place of ANF, placing E2 in a similar planar orientation as ANF.
The CYP1A2 and CYP1B1 structures have ANF bound in flipped orientations of the benzo[h]chromen-4-one rings. While these rings in ANF have planar symmetry, estradiol possesses an 18β-methyl group and a 17β-hydroxyl group on one side of the steroid plane that add to the planar asymmetry of the estrogen rings. When the planes of ANF and the estrogens are approximately aligned with ring A in a similar position to the phenyl ring of ANF near the heme, the 17β-hydroxy and 18β-methyl groups are either directed toward or away from the I helix.
Three explanations for the observed shift in hydroxylation preference in going from the human wild-type to the V395L mutant can be envisioned. First, flipping of the sterol structure causes one orientation to place the C4 and the other the C2 carbon near the ferryl oxygen, as illustrated by the poses observed in the docked estradiol (Fig. 4D, red and blue for C2, green for C4). Second, side-wise sliding of a single sterol orientation caused by the amino acid change results in a shift of the preferred site of oxidation. Third, the V395L substitution specifically interferes with C4 hydroxylation. The latter rationale is consistent with the observed lowering of C4 hydroxylation rate and relatively unchanged C2 hydroxylation rate by the V395L substitution. Likewise, the L395V mutation of rat 1B1 could open access to the closer C4 position due to the decrease in side-chain bulk.
Acknowledgments
The authors thank Cyterix for the gift of Cypex-expressed enzymes.
Abbreviations
- 2OH E2
2-hydroxyestradiol
- 4OH E2
4-hydroxyestradiol
- ANF
α-naphthoflavone
- CPR
NADPH–cytochrome P450 reductase
- EROD
7-ethoxyresorufin O-deethylase
- P450
cytochrome P450
Authorship Contributions
Participated in research design: Nishida, Everett, Ortiz de Montellano.
Conducted experiments: Nishida.
Contributed new reagents or analytic tools: Everett.
Performed data analysis: Nishida, Ortiz de Montellano.
Wrote or contributed to the writing of the manuscript: Nishida, Ortiz de Montellano.
Footnotes
This work was supported by the National Institutes of Health National Institute of General Medical Sciences [Grant GM25515]; and by a gift from Cyterix Pharmaceuticals, Inc.
References
- Badawi AF, Cavalieri EL, Rogan EG. (2001) Role of human cytochrome P450 1A1, 1A2, 1B1, and 3A4 in the 2-, 4-, and 16alpha-hydroxylation of 17beta-estradiol. Metabolism 50:1001–1003 [DOI] [PubMed] [Google Scholar]
- Buters JTM, Sakai S, Richter T, Pineau T, Alexander DL, Savas U, Doehmer J, Ward JM, Jefcoate CR, Gonzalez FJ. (1999) Cytochrome P450 CYP1B1 determines susceptibility to 7, 12-dimethylbenz[a]anthracene-induced lymphomas. Proc Natl Acad Sci USA 96:1977–1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Schenkman JB. (2009) CYP1B1, a developmental gene with a potential role in glaucoma therapy. Xenobiotica 39:606–615 [DOI] [PubMed] [Google Scholar]
- Choudhary D, Jansson I, Stoilov I, Sarfarazi M, Schenkman JB. (2004) Metabolism of retinoids and arachidonic acid by human and mouse cytochrome P450 1b1. Drug Metab Dispos 32:840–847 [DOI] [PubMed] [Google Scholar]
- De Vivo I, Hankinson SE, Li L, Colditz GA, Hunter DJ. (2002) Association of CYP1B1 polymorphisms and breast cancer risk. Cancer Epidemiol Biomarkers Prev 11:489–492 [PubMed] [Google Scholar]
- Hayes CL, Spink DC, Spink BC, Cao JQ, Walker NJ, Sutter TR. (1996) 17 β-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA 93:9776–9781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T, Takemura H, Shimoi K, Yamamoto K. (2010) A 3D model of CYP1B1 explains the dominant 4-hydroxylation of estradiol. J Chem Inf Model 50:1173–1178 [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- Lee AJ, Cai MX, Thomas PE, Conney AH, Zhu BT. (2003) Characterization of the oxidative metabolites of 17β-estradiol and estrone formed by 15 selectively expressed human cytochrome p450 isoforms. Endocrinology 144:3382–3398 [DOI] [PubMed] [Google Scholar]
- Lewis DFV, Gillam EMJ, Everett SA, Shimada T. (2003) Molecular modelling of human CYP1B1 substrate interactions and investigation of allelic variant effects on metabolism. Chem Biol Interact 145:281–295 [DOI] [PubMed] [Google Scholar]
- Ma X, Idle JR, Krausz KW, Gonzalez FJ. (2005) Metabolism of melatonin by human cytochromes p450. Drug Metab Dispos 33:489–494 [DOI] [PubMed] [Google Scholar]
- Murray GI, Taylor MC, McFadyen MCE, McKay JA, Greenlee WF, Burke MD, Melvin WT. (1997) Tumor-specific expression of cytochrome P450 CYP1B1. Cancer Res 57:3026–3031 [PubMed] [Google Scholar]
- Sansen S, Yano JK, Reynald RL, Schoch GA, Griffin KJ, Stout CD, Johnson EF. (2007) Adaptations for the oxidation of polycyclic aromatic hydrocarbons exhibited by the structure of human P450 1A2. J Biol Chem 282:14348–14355 [DOI] [PubMed] [Google Scholar]
- Savas U, Bhattacharyya KK, Christou M, Alexander DL, Jefcoate CR. (1994) Mouse cytochrome P-450EF, representative of a new 1B subfamily of cytochrome P-450s. Cloning, sequence determination, and tissue expression. J Biol Chem 269:14905–14911 [PubMed] [Google Scholar]
- Savas U, Carstens CP, Jefcoate CR. (1997) Biological oxidations and P450 reactions. Recombinant mouse CYP1B1 expressed in Escherichia coli exhibits selective binding by polycyclic hydrocarbons and metabolism which parallels C3H10T1/2 cell microsomes, but differs from human recombinant CYP1B1. Arch Biochem Biophys 347:181–192 [DOI] [PubMed] [Google Scholar]
- Scornaienchi ML, Thornton C, Willett KL, Wilson JY. (2010) Cytochrome P450-mediated 17β-estradiol metabolism in zebrafish (Danio rerio). J Endocrinol 206:317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, El-Bayoumy K, Upadhyaya P, Sutter TR, Guengerich FP, Yamazaki H. (1997) Inhibition of human cytochrome P450-catalyzed oxidations of xenobiotics and procarcinogens by synthetic organoselenium compounds. Cancer Res 57:4757–4764 [PubMed] [Google Scholar]
- Shimada T, Murayama N, Tanaka K, Takenaka S, Guengerich FP, Yamazaki H, Komori M. (2011) Spectral modification and catalytic inhibition of human cytochromes P450 1A1, 1A2, 1B1, 2A6, and 2A13 by four chemopreventive organoselenium compounds. Chem Res Toxicol 24:1327–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Oda Y, Gillam EMJ, Guengerich FP, Inoue K. (2001a) Metabolic activation of polycyclic aromatic hydrocarbons and other procarcinogens by cytochromes P450 1A1 and P450 1B1 allelic variants and other human cytochromes P450 in Salmonella typhimurium NM2009. Drug Metab Dispos 29:1176–1182 [PubMed] [Google Scholar]
- Shimada T, Watanabe J, Inoue K, Guengerich FP, Gillam EMJ. (2001b) Specificity of 17β-oestradiol and benzo[a]pyrene oxidation by polymorphic human cytochrome P4501B1 variants substituted at residues 48, 119 and 432. Xenobiotica 31:163–176 [DOI] [PubMed] [Google Scholar]
- Shimada T, Wunsch RM, Hanna IH, Sutter TR, Guengerich FP, Gillam EMJ. (1998a) Recombinant human cytochrome P450 1B1 expression in Escherichia coli. Arch Biochem Biophys 357:111–120 [DOI] [PubMed] [Google Scholar]
- Shimada T, Yamazaki H, Foroozesh M, Hopkins NE, Alworth WL, Guengerich FP. (1998b) Selectivity of polycyclic inhibitors for human cytochrome P450s 1A1, 1A2, and 1B1. Chem Res Toxicol 11:1048–1056 [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spink DC, Spink BC, Zhuo X, Hussain MM, Gierthy JF, Ding X. (2000) NADPH- and hydroperoxide-supported 17β-estradiol hydroxylation catalyzed by a variant form (432L, 453S) of human cytochrome P450 1B1. J Steroid Biochem Mol Biol 74:11–18 [DOI] [PubMed] [Google Scholar]
- Sutter TR, Tang YM, Hayes CL, Wo YY, Jabs EW, Li X, Yin H, Cody CW, Greenlee WF. (1994) Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J Biol Chem 269:13092–13099 [PubMed] [Google Scholar]
- Trott O, Olson AJ. (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem 31:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasiliou V, Gonzalez FJ. (2008) Role of CYP1B1 in glaucoma. Annu Rev Pharmacol Toxicol 48:333–358 [DOI] [PubMed] [Google Scholar]
- Wang A, Savas U, Stout CD, Johnson EF. (2011) Structural characterization of the complex between α-naphthoflavone and human cytochrome P450 1B1. J Biol Chem 286:5736–5743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Shimada T, Gillam EMJ, Ikuta T, Suemasu K, Higashi Y, Gotoh O, Kawajiri K. (2000) Association of CYP1B1 genetic polymorphism with incidence to breast and lung cancer. Pharmacogenetics 10:25–33 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Nakamura M, Komatsu T, Ohyama K, Hatanaka N, Asahi S, Shimada N, Guengerich FP, Shimada T, Nakajima M, et al. (2002) Roles of NADPH-P450 reductase and apo- and holo-cytochrome b5 on xenobiotic oxidations catalyzed by 12 recombinant human cytochrome P450s expressed in membranes of Escherichia coli. Protein Expr Purif 24:329–337 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Shaw PM, Guengerich FP, Shimada T. (1998) Roles of cytochromes P450 1A2 and 3A4 in the oxidation of estradiol and estrone in human liver microsomes. Chem Res Toxicol 11:659–665 [DOI] [PubMed] [Google Scholar]
- Zheng W, Xie DW, Jin F, Cheng JR, Dai Q, Wen WQ, Shu XO, Gao YT. (2000) Genetic polymorphism of cytochrome P450-1B1 and risk of breast cancer. Cancer Epidemiol Biomarkers Prev 9:147–150 [PubMed] [Google Scholar]