Abstract
Background: Demographic profile and outcome can vary in pediatric intensive care unit (PICU) patients. The aim of our study was to analyze demographic profile and outcome in a Greek PICU.
Methods: Prospective observational study. Data collected: demographic profile; co morbidities; source and diagnosis at admission; Pediatric Risk of Mortality (PRISM III-24); Glasgow Coma Scale (GCS, pediatric); Injury Severity Score (ISS); procedures; treatment; mechanical ventilation (MV); MV days; length of stay (LOS) and the outcome at PICU discharge. Statistical analysis: Student’s t-test; Mann-Whitney U test; Kruskall-Wallis test; χ2 criterion with relative risk (RR) estimation; Cox regression analysis; as appropriate. Values are mean ± SD, p < 0.05.
Results: 300 patients (196 boys/104 girls), aged 54.26 ± 49.93 months, were admitted due to respiratory failure (22.3%), head trauma (15.3%), seizures (13.7%), coma (9.7%), postoperative care (7.7%), polytrauma (7%), accidents (5.3%), sepsis-septic shock (5.3%), cardiovascular diseases (4.7%), metabolic diseases (3.3%), multiple organ failure syndrome (3%) and miscellaneous diseases (2.7%). PRISM III-24 score was 8.97 ± 7.79 and predicted mortality rate was 11.16% ± 18.65. MV rate was 67.3% (58.3% at admission) for 6.54 ± 14.45 days, LOS 8.85 ± 23.28 days and actual PICU mortality rate 9.7%. Patients who died had statistically worse severity scores. Significant mortality risk factors were inotropic use, PRISM III-24 > 8, MV, arterial and central venous catheterization, nosocomial infections, complications, and cancer. COX regression analysis showed that PRISM III-24 score and inotropic use were independent predictors of mortality.
Conclusions: Demographic profile followed similar patterns to relevant studies while there were major differences in case mix and the severity of the disease. Mortality rate (9.7%) was relatively high but better than predicted and in accordance with the characteristics of our population.
Keywords: pediatric intensive care unit, pediatric risk of mortality PRISM III-24, mortality, mortality risk factors
Beyond the 4 Ds of childhood1, which stand for the developmental change, the different demographic and disease characteristics, and the dependence on adults for accessing care and implementing treatments, there are some more uniqueness connected to pediatric intensive care2,3. Critical illness is a rather rare event in childhood; doctors that face such medical cases often become more effective compared to their colleagues that face a case rarely. Pollack et al., showed a better outcome of PICU patients in units where there was a pediatric intensivist and/or a pediatric intensive care fellowship programme4,5. Moreover, there are references that support better outcome of PICU patients in tertiary centers, which led to the development of a centralized system of PICUs worldwide6,11. Significant numbers of critically ill children need to be transferred between hospitals supported by a well organized transfer system that guarantees the safety of the patient and the quality of the transfer12,14.
The Department of Pediatric Intensive Care in Hippokratio General Hospital, Thessaloniki, Greece, is a multidisciplinary 8-bed PICU of a tertiary 1000-bed hospital which serves an estimated population of Northern Greece of 3,500,000 millions. It has a 24hours/7days full coverage of a pediatric intensivist and provides admission to infants with age of > 40 days to children up to 14 years, in all diagnostic categories, except postoperative congenital heart diseases patients. Laboratory, radiological and operational facilities are 24hrs available, while there is on call coverage of all pediatric subspecialties.
Demographic profile and outcome of PICU patients can vary widely in different studies while there is a scarcity of data in Greek critically ill children. The aim of the present study was to describe the demographic profile and the outcome of our PICU patients, to evaluate the relationship of the outcome to diagnostic categories, illness severity and treatment characteristics and to investigate mortality risk and possible outcome prediction factors.
Materials and methods
Patients.
All consecutive PICU patients admitted between 1/1/2001 to 29/4/2003 were prospectively recorded, according to exclusion criteria. Exclusion criteria were: patients with missing data and patients who died during the first two hrs of admission, because PICU stay was too short to be connected to the outcome. In case of readmission, the patient was recorded only during the first admission. Due to the observational character of the study which didn’t require any deviation from routine medical care, institutional review board approval was waived and informed consent was not required. Patients were followed until death in the PICU, or discharge. On discharge patients are transferred to pediatric wards, as there is there is no intermediate step down unit available. All deaths happened in the PICU; withdrawal of life support is not a routine practice in the unit because of absence of legal guidelines on this issue in our country.
Data collection.
The following data were collected prospectively: age; gender; admission diagnosis; instead of co morbidities; elective/emergency status; operative status; clinical service of primary responsibility; admission source; previous neonatal, pediatric intensive care or hospital admission; procedures; treatment characteristics; the need for mechanical ventilation (MV) and MV days; PICU length of stay (LOS) and the outcome. Critical illness severity was estimated with the Pediatric Risk of Mortality (PRISM III-24) score whereas clinical and laboratory data needed to calculate PRISM III-24 score were reported as the worst value within 24 hrs after PICU admission15. Neurologic status was evaluated using the pediatric version of Glasgow Coma Scale (GCS)16 and patients with GCS < 8 were recorded as suffered from coma. Trauma severity was estimated through Injury Severity Score (ISS)17.
Statistical analysis.
Descriptive statistics were computed for all study variables. The Kolmogorov-Smirnov test was used to verify the normality of distribution of continuous variables. Discrete variables were expressed as counts (percentage) and continuous variables as means ± standard deviation (SD). Prism III-24 score and predicted PICU mortality rate was estimated with the free for 60 days PICUEs version 3.2 software trial (PICUEs v 3.2, Children’s National Medical Center, Washington, USA) while the validity of the model in the Greek population was examined with standard discrimination (Receiver Operating Characteristics, ROC curve)18 and calibration methods (Hosmer-Lemeshow goodness-of-fit test)19. Reliability of data collection was examined with a random recollection of 30 cases by a second investigator through the interobserver k score20. Our sample was divided in two groups according to death status and differences in various parameters were sought with the use of Student’s t-test, Mann-Whitney U test or Kruskall-Wallis test as appropriate. For the analysis of mortality risk factors patients were allocated into two categories according to PRISM III-24 score values > 8 and < 8, based on previously published data showing increased mortality risk in patients with PRISM III-24 > 821. Univariate analysis was performed through Chi-square test and relative risk (RR) estimation with 95% confidence intervals. Multivariate Cox regression analysis was used for mortality prediction, while Cox proportional hazards model was used for survival analysis22. Statistical significance was set at p < 0.05. Data were analyzed using SPSS 10.1 for windows (SPSS, Chicago, IL).
Results
Among 382 consecutive patients admitted in the above time period, 300 (196 boys and 104 girls), aged 54.26 ± 49.93 months, were eligible for the study. The vast majority were admitted due to medical pediatric emergencies (210pts, 69.8%) and trauma (67pts, 22.5%), where two thirds of them (204pts, 68%) were admitted from referral hospitals, either in town (81pts, 27%) or from remote geographical areas (123pts, 41%). Most patients had an excellent health prior PICU admission, but quite a lot suffered from instead of co morbidities. Diagnosis related demographic profile is shown in Table 1, while diagnosis related treatment characteristics and outcome is shown in Table 2.
Table 1. Diagnosis related demographic profile (n=300).
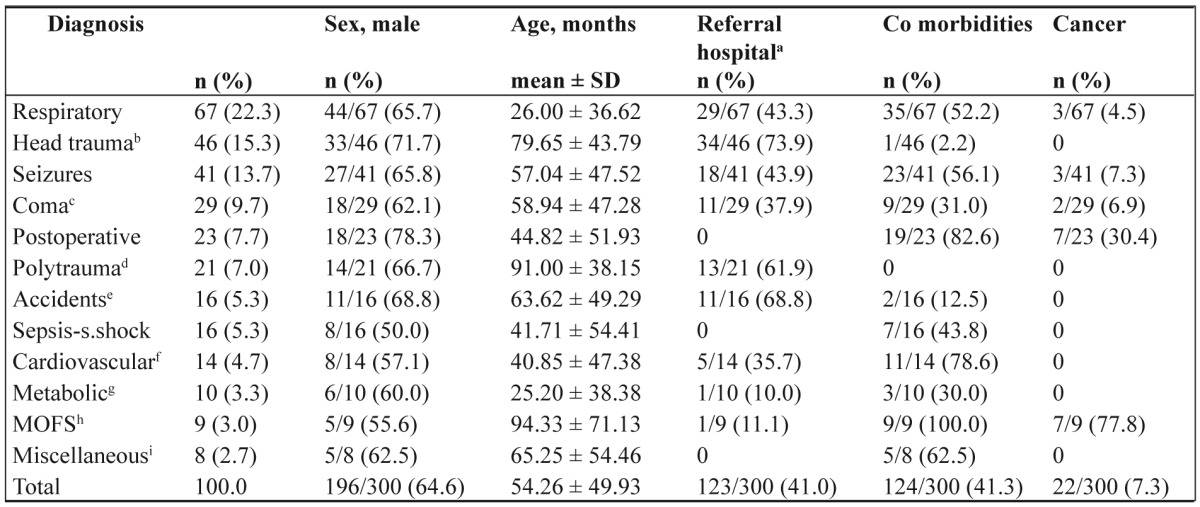
aReferral hospital, out of town remote geographical areas; bHead trauma only; cComa, central nervous system infections and tumors, stroke; dPolytrauma, with accompanying head trauma also; eAccidents, poisonings, near drownings, burns; fCardiovascular, non operative; gMetabolic diseases, inborn error of metabolism and diabetic ketoacidosis; hMOFS, multiple organ failure syndrome; iMiscellaneous, tracheostomy closure (4), Stevens-Johnson (1), uremic-hemolytic (1), acute post infectious glomerulonephritis (1), anorexia nervosa (1)
Table 2. Diagnosis related treatment characteristics and outcome (n=300; deaths 29).
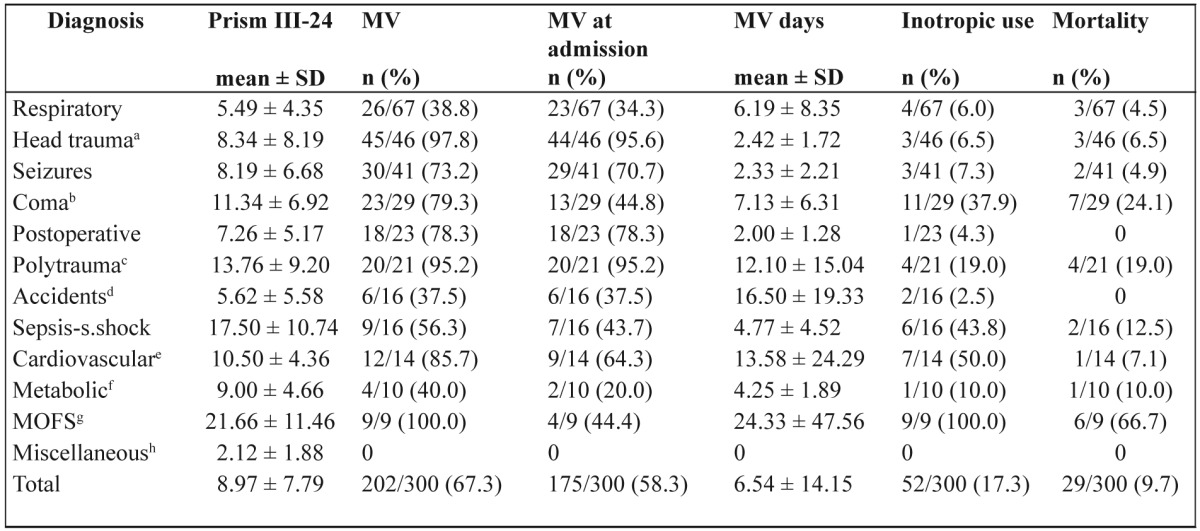
PRISM, Pediatric Risk of Mortality; MV, mechanical ventilation; aHead trauma only; bComa, central nervous system infections and tumors, stroke; cPolytrauma, with accompanying head trauma also; dAccidents, poisonings, near drownings, burns; eCardiovascular, non operative; fMetabolic diseases, inborn error of metabolism and diabetic ketoacidosis; gMOFS, multiple organ failure syndrome; hMiscellaneous, tracheostomy closure (4), Stevens-Johnson (1), uremic-hemolytic (1), acute post infectious glomerulonephritis (1), anorexia nervosa (1)
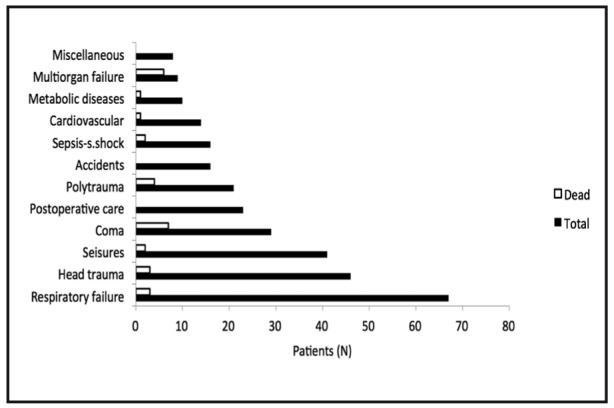
The performance of PRISM III-24 score in the Greek population was very good (area under the ROC curve 0.892 ± 0.036, p <0.001; goodness-of-fit test χ2 (8) = 1.716, p = 0.989; interobserver k score 1). Mean value of PRISM III-24 score was 8.97 ± 7.79 and mean predicted mortality rate was 11.16% ± 18.65. Only a percentage of 23.3% of our patients had mortality risk at admission lower than 1%. Mechanical ventilation rate was 67.3% (58.3% at admission) and MV duration 6.54 ± 14.15 days. Patients stayed in the PICU for 8.85 ± 23.28 days until death or discharge. Twenty nine patients died in the PICU given a mortality rate of 9.7%. Patients admitted from hospital pediatric wards as internal patients had higher mortality rate (12/96pts, 12.5%) compared to referral patients (17/204pts, 8.3%), but without statistical significance (p=0.273). Mortality across diagnostic categories is shown in Figure 1.
Figure 1. Mortality across diagnostic categories. No deaths occurred during stay in the unit in postoperative care, accidents and miscellaneous diseases patients.
Concerning the mode of death, the majority (18/29pts, 62%) died from brain death due to head trauma (7), central nervous system (CNS) infection (4), stroke (3), status epilepticus (2), inborn error of metabolism (1), and hypoxic- ischemic encephalopathy (1). Nine patients (31%) died from multiple organ failure syndrome (MOFS); 6 of them were admitted with MOFS, while 3 developed MOFS during their stay. Two patients (6.9%) died from intractable cardiac arrest and failed cardiopulmonary resuscitation (CPR).
Table 3 shows the differences in the characteristics between the patients who died and who survived. Patients who died had statistically significant worse severity scores (Figure 2). In the univariate analysis sex, infancy, source of admission, diagnosis at admission, co morbidities, presence of syndrome, prior hospital or NICU admission, gastric ulcer prophylaxis, corticosteroid use, acute renal failure and cardiac output measurements weren’t found to be significant risk factors. Relative mortality risk for the significant parameters is shown in Table 4. A multivariate analysis that followed using COX regression analysis (forward stepwise entry if p<0.05 and removal if p>0.1, with co-variates PRISM III-24 score, inotropic use and diagnosis at admission) showed that only the severity of the disease (PRISM III-24 score) and inotropic use were independent predictors of mortality (Table 5)
Table 3. Patients’ characteristics.
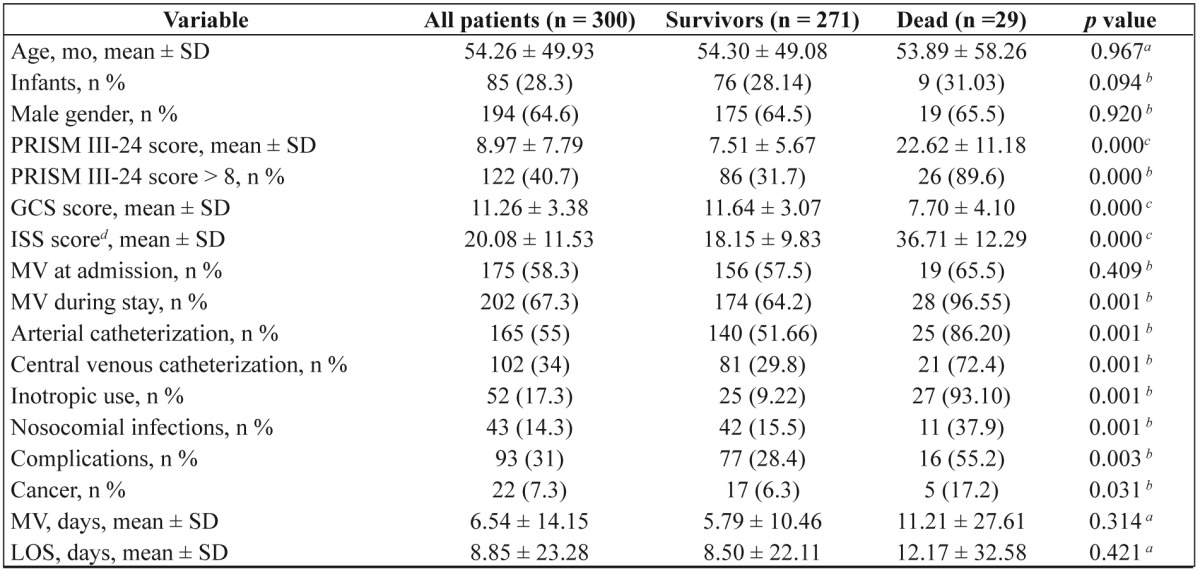
PRISM, Pediatric Risk of Mortality; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MV, mechanical ventilation; LOS, length of stay in the unit
aStudent’s t-test; bChi square test; cMann-Whitney U test; dtrauma patients only
Figure 2. Severity scores in patients who died and survived. Differences were always statistically significant (p=0.000). GCS, Glasgow coma scale; ISS, injury severity score; PRISM, pediatric risk of mortality.
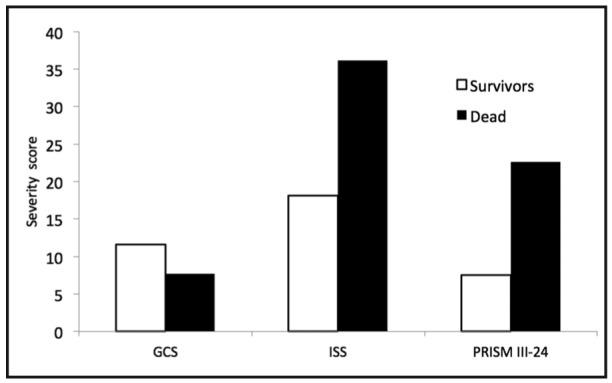
Table 4. Univariate analysis of risk factors for PICU mortality (n=300; deaths 29).
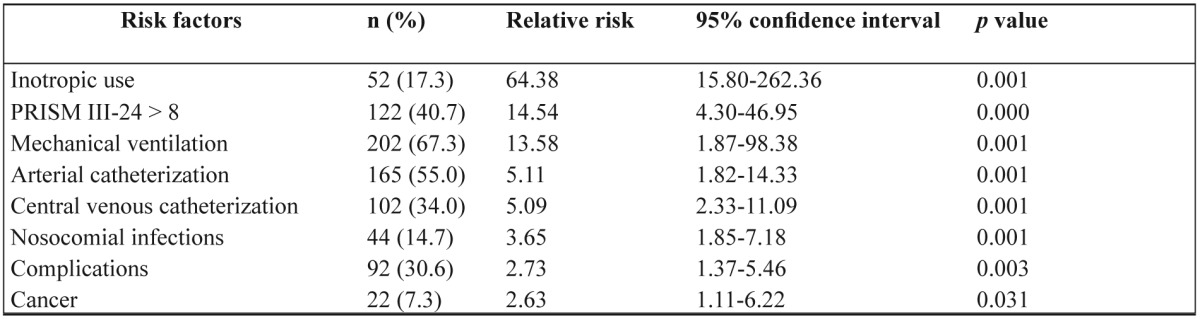
Table 5. Cox regression analysis of PICU mortality.
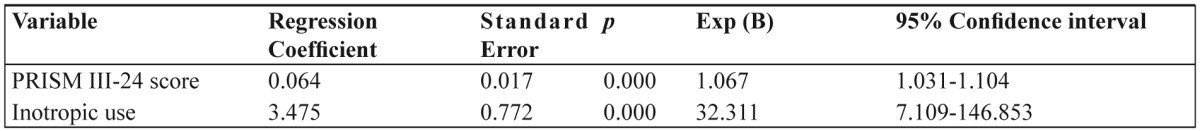
Exp (B), the predicted change in the hazard for a unit increase in the predictor
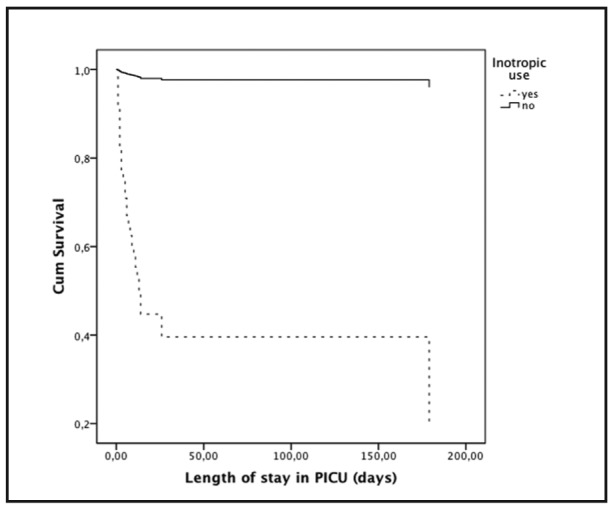
Survival analysis for selected parameters, according to PRISM III-24 categories and inotropic use are shown in Figure 3 and Figure 4.
Figure 3. Survival curve (n=300, deaths 29) into two PRISM III-24 categories using Cox proportional hazards model. Relative risk of mortality when PRISM III-24 > 8 was 14.54 (95% CI 4.30-46.95). PRISM, pediatric risk of mortality.
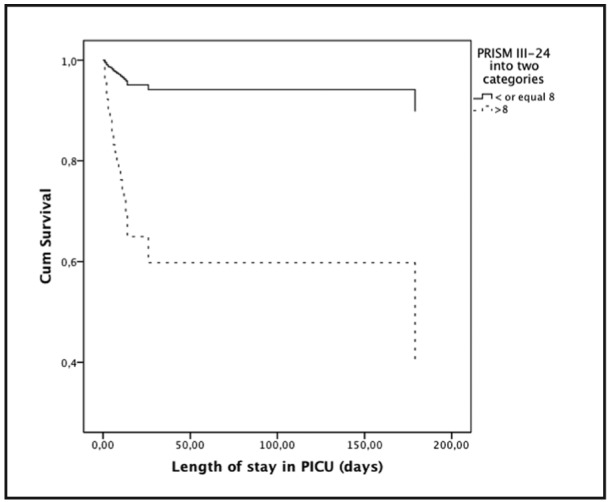
Figure 4. Survival curve (n=300; deaths 29) according to inotropic use using Cox proportional hazards model. Relative risk of mortality in the presence of inotropic use was 64.38 (95% CI 15.80-262.36).
Discussion
We presented the demographic profile and the outcome of Greek PICU patients, the relationship of the outcome to diagnostic categories, illness severity and treatment characteristics and the investigation of relative mortality risk and possible outcome prediction factors. Our major finding was that the severity of critical illness and inotropic use were independent predictors of PICU mortality.
Mean age of our population (54.26 ± 49.93 mo), as well as the proportion of infants (28%), was within the reference values of mean age (3-6yrs or 36-72mo) of PICU patients7,9,23-27. Age distribution across diagnostic categories was quite diverse. The preponderance of male sex (64.6%) was somehow higher than the relevant values of 54-61.1%23-26,28, and rather uniform in all diagnosis. Our unit seems also to follow the centralization profile of PICUs worldwide6-11 as the majority of our patients (68%) were admitted from referral hospitals. Co morbidities (41.3%) were similar to related studies with reported rates of 29-45%7,25,26,29,29-30. Although the principal demographic profile of our population is analogous to linked studies, it is difficult to make comparisons across diagnostic categories, due to lack of information in this field.
The majority of admissions were pathologic pediatric emergencies (92.3%), and only 7.7% were admitted for postoperative care. This is opposite to associated studies where surgical patients represent a big proportion of PICU patients ranged from 16-60%9,10,23,30. Additionally, trauma patients in our unit (22.3%) were higher than the reference values of 6.5-11.5%9,10,23-26. Surgical patients have generally a better prognosis whether the opposite is true for trauma patients, especially for patients with severe traumatic brain injury31. The different case mix of our study should be taken into account when interpreting PICU mortality rate.
For the purposes of our study we validated PRISM III-24, an international mortality prediction model which enabled us not only to estimate the severity of illness of our population but to compare also our results to international data15. We found that PRISM III-24 had a very good performance with high discrimination and calibration capabilities. The severity of critical illness in our population was higher than the reference values; only a percentage of 23.3% of our patients had a mortality risk at admission lower than 1%, compared to values from 15.8-67.5%7,15,23,27,32. Patients with mortality risk < 1% are considered low risk patients, their proportion is crucial in outcome studies, and should be taken into account as well in mortality assessment. The higher the percentage of low risk patients the better the prognosis and vice versa. Furthermore, patients with mortality risk > 1% (76.7% in present study) are representative of PICU efficiency30.
Mechanical ventilation (67.3%) approximated the upper reference values of 31.5-67% 21,23,24,26,29 while the majority of our patients (58.3%) were already mechanical ventilated at admission. Inotropic support started only after full fluids resuscitation and was performed under international guidelines33. Mechanical ventilation is a unique PICU therapy and together or not with inotropic use, in some studies, is considered too as an index of PICU efficiency. Efficiency of our unit based on unique PICU therapies (mechanical ventilation and/or inotropic use) was 69.3%, whilst, as mentioned above, based on patients with mortality risk at admission > 1% was 76.7%. Both values are within the reference efficiency values of 32.5-84.2%, and close to the efficiency goal of 80% set by Pollack et al.7,30,32,34.
The mortality rate in our patients was 9.7%, within the reference values of 4.2-13%, but relatively high compared to the most recent ones9,15,21,23,24,27-29,30,32,35. PICU’s main goal is the reduction in mortality, yet special consideration should be given to mortality studies; reports on mortality rates alone, without risk adjustment, could make their results misinterpreted36. In our study, the high proportion of emergency patients and admissions from remote areas, the case mix of our population with low percentage of surgical and high percentage of trauma patients, the high severity of illness and the high proportion of mechanically ventilated patients, could account for the relative high mortality. However, the mortality rate of our patients is better than the predicted PRISM III-24 PICU mortality of 11.16%, indicating the high effectiveness of our unit as well.
Contrary to references3,10 that attribute higher mortality to internal patients (OR 1.66-1.78), mortality rate in internal patients of the present study, although higher, was not statistically significant (12.5% vs. 8.3%, p=0.273), probably due to the small size of our sample. Even though there are many mortality studies in the whole cohort of PICU patients, data are not so abundant for mortality across diagnosis. In our study, mortality was worse for patients with MOFS; the majority of those patients suffered from co morbidities, mainly cancer and syndrome pathology. Next, followed patients with coma, sepsis-septic shock, trauma, metabolic diseases, cardiovascular failure, seizures and respiratory failure, while best prognosis was found in postoperative care, accidents and miscellaneous diseases patients. Our diagnosis related mortality is approximate to reference mortality values for non operative cardiovascular disease (9.5-11.4%), head trauma (9.4-10.1%) and respiratory failure (3.1-4.5%) reported by Pollack et al. in big multicenter studies of USA patients15,27. Because of lack of reported data it is hard to compare mortality across the rest diagnostic categories.
Brain death was the main mode of death (62%) in our study; all trauma patients that died did so because of severe traumatic brain injury. Rest brain dead patients could be related to the high proportion of CNS pathology in admission; if coma, seizures and metabolic patients that often have CNS involvement are put together with trauma patients, they account for 49% of all admissions, and could explain the unfavorable progress of CNS damage to brain death. A remarkable note on these patients is that the majority (12/18pts, 66.7%) didn’t have previous health problems. All patients that died from MOFS (31%) did so despite maximal treatment due to terminal organ failure and refractory shock, while the two patients (6.9%) that died from intractable cardiac arrest did so in the ground of congenital heart diseases. Our findings on the mode of death are quite different from the literature where it is reported that approximately 28-65% of deaths in the PICU follow limitation or withdrawal of life sustaining treatment with a proportion of brain dead patients of 23-38%37-39. The different death profile of our patients could be attributed to the differences in the case mix and the lack of guidelines on forgoing life-sustaining medical treatment in our country40-41.
As expected, patients who died had statistically significant worse severity scores. Relative mortality risk was higher for inotropic use, PRISM III-24 score > 8, MV, arterial and central venous catheterization, nosocomial infections, complications and cancer. The multivariate analysis that followed showed that only the severity of the disease (PRISM III-24 score) and inotropic use were independent predictors of mortality. Our findings are in accordance with relevant studies reported that critical illness severity is the main outcome prediction factor9,15,21. Tan and al., showed that relative risk for mortality in the presence of PRISM III-24 scores > 8 and MODS were 15.8 (95% CI 2.0-127.8) and 11.3 (95% CI 3.3-38.3) respectively, and reported that only PRISM III-24 score was found to be an independent predictor of mortality21. In the original PRISM III predictive model study by Pollack and al., the authors reported that PRISM III score value contributed 95% to the variance explained by the model, while the additional risk variables contributed only 5% 15. Tilford et al.9, on the initial PRISM model found that an increase of PRISM score of 10 to 20 was accompanied by a more than sevenfold increase in mortality risk (OR 7.6).
In conclusion, our study is one of the first to provide thorough data on Greek PICU patients, and the first to perform a validation of a mortality prediction model for critically ill children in the Greek population. We found that PRISM III-24 has a very good performance in our patients, which permitted the estimation of the severity of illness and the probability of death, and the comparison of our data to international standards. The demographic profile of our patients showed that although age, sex, source of admission and co morbidities follow the general pattern of PICU patients worldwide, there are major differences in case mix and the severity of the disease. Outcome analysis showed that PICU mortality rate (9.7%) was higher than in relevant recent studies but in accordance with the case mix and the severity of the disease, and better than predicted based on PRISM III- 24 predictive model, whereas mortality key factors were severity of critical illness and inotropic use.
The authors have not disclosed any potential conflicts of interest.
References
- 1.Forrest BC, Shipman AS, Dougherty D, Miller RM. Outcome research in pediatric settings: Recent trends and future directions. Pediatrics. 2003;111:171–178. doi: 10.1542/peds.111.1.171. [DOI] [PubMed] [Google Scholar]
- 2.Bennett NR. Paediatric intensive care. Br J Anaesth. 1999;83:139–156. doi: 10.1093/bja/83.1.139. [DOI] [PubMed] [Google Scholar]
- 3.Epstein D, Brill JE. A history of pediatric critical care medicine. Pediatr Res. 2005;58:987–996. doi: 10.1203/01.PDR.0000182822.16263.3D. [DOI] [PubMed] [Google Scholar]
- 4.Pollack MM, Cuerdon TT, Patel KM, Ruttimann UE, Getson PR, Levetown M. Impact of quality-of-care factors on pediatric intensive care unit mortality. Jama. 1994;272:941–946. [PubMed] [Google Scholar]
- 5.Pollack MM, Patel KM, Ruttimann E. Pediatric critical care training programs have a positive effect on pediatric intensive care mortality. Crit Care Med. 1997;25:1637–1642. doi: 10.1097/00003246-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Pollack MM, Alexander SR, Clarke N, Ruttimann UE, Tesselaar HM, Bachulis AC. Improved outcomes from tertiary center pediatric intensive care: a statewide comparison of tertiary and nontertiary care facilities. Crit Care Med. 1991;19:150–159. doi: 10.1097/00003246-199102000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Gemke RJ, Bonsel GJ. Comparative assessment of pediatric intensive care: a national multicenter study. Pediatric Intensive Care Assessment of Outcome (PICASSO) Study Group. Crit Care Med. 1995;23:238–245. doi: 10.1097/00003246-199502000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Pearson G, Shann F, Barry P, Vyas J, Thomas D, Powel C, et al. Should pediatric intensive care be centralised? Trend versus Victoria. Lancet. 1997;349:1213–1237. doi: 10.1016/S0140-6736(96)12396-5. [DOI] [PubMed] [Google Scholar]
- 9.Tilford JM, Simpson PM, Green JW, Lensing S, Fiser DH. Volume-outcome relationships in pediatric intensive care units. Pediatrics. 2000;106:289–294. doi: 10.1542/peds.106.2.289. [DOI] [PubMed] [Google Scholar]
- 10.Ruttimann UE, Patel KM, Pollack MM. Relevance of diagnostic diversity and patient volumes for quality and length of stay in pediatric intensive care units. Pediatr Crit Care Med. 2000;1:133–139. doi: 10.1097/00130478-200010000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Watson R, Hartmann M. Volume and Outcome in Pediatric Critical Care: How much is enough? In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine. Berlin Heidelberg: Springer – Verlag. 2003 [Google Scholar]
- 12.Vos G, Ramsay G. Interhospital Intensive Care Transport. In: Vincent JL, editor. Yearbook of Intensive Care and Emergency Medicine. Berlin Heidelberg: Springer – Verlag. 2003 [Google Scholar]
- 13.American Academy of Pediatrics TFoIT. Guidelines for Air and Ground Transport of Neonatal and Pediatric Patients. Elk Grove Village. 1999 [Google Scholar]
- 14.White M, Weir PM, Garland L, Edees S, Henderson AJ. Outcome of critically ill children before and after the establishment of a pediatric retrieval service as a component of a national strategy for pediatric intensive care. Pediatr Crit Care Med. 2002;3:255–260. doi: 10.1097/00130478-200207000-00010. [DOI] [PubMed] [Google Scholar]
- 15.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated Pediatric Risk of Mortality score. Crit Care Med. 1996;24:743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Reilly PL, Simpson DA, Sprod R, Thomas L. Assessing the conscious level in infants and young children: a paediatric version of the Glasgow Coma Scale. Childs Nerv Syst. 1988;4:30–33. doi: 10.1007/BF00274080. [DOI] [PubMed] [Google Scholar]
- 17.Baker SP, O’Neill B, Haddon W Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14:187–196. [PubMed] [Google Scholar]
- 18.Ηanley J, McNeil B. The meaning and use of the area under a receiver operating characteristic curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer D, Lemeshow S. Applied logistic regression. New York: John Wiley & Sons, Inc. 1989 [Google Scholar]
- 20.Soeken KL, Prescott PA. Issues in the use of kappa to estimate reliability. Med Care. 1986;24:733–744. doi: 10.1097/00005650-198608000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Tan GH, Tan TH, Goh DY, Yap HK. Risk factors for predicting mortality in a paediatric intensive care unit. Ann Acad Med Singapore. 1998;27:813–818. [PubMed] [Google Scholar]
- 22.Cox DR. Regression models and life tables. Journal of the Royal Statistical Society Series B. 1972;34:187–220. [Google Scholar]
- 23.Seferian EG, Carson SS, Pohlman A, Hall J. Comparison of resource utilization and outcome between pediatric and adult intensive care unit patients. Pediatr Crit Care Med. 2001;2:2–8. doi: 10.1097/00130478-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Brady AR, Harrison D, Black S, Jones S, Rowan K, Pearson G, et al. Assessment and optimization of mortality prediction tools for admissions to pediatric intensive care in the United kingdom. Pediatrics. 2006;117:e733–742. doi: 10.1542/peds.2005-1853. [DOI] [PubMed] [Google Scholar]
- 25.Fiser DH. Assessing the outcome of pediatric intensive care. J Pediatr. 1992;121:68–74. doi: 10.1016/s0022-3476(05)82544-2. [DOI] [PubMed] [Google Scholar]
- 26.Bertolini G, Ripamonti D, Cattaneo A, Apolone G. Pediatric risk of mortality: an assessment of its performance in a sample of 26 Italian intensive care units. Crit Care Med. 1998;26:1427–1432. doi: 10.1097/00003246-199808000-00031. [DOI] [PubMed] [Google Scholar]
- 27.Pollack MM. PRISM III Mortality Risk Recalibration. [Children’s national medical center homepage in the internet] Washington: Children’s National Medical Center. 2006 Available from: http://www.cnmc.org/picues/scientific_mortality.aspx. [Google Scholar]
- 28.Lopez AM, Tilford JM, Anand KJ, Jo CH, Green JW, Aitken ME, et al. Variation in pediatric intensive care therapies and outcomes by race, gender, and insurance status. Pediatr Crit Care Med. 2006;7:2–6. doi: 10.1097/01.pcc.0000192319.55850.81. [DOI] [PubMed] [Google Scholar]
- 29.Martinot A, Leteurtre S, Grandbastien B, Duhamel A, Leclerc F. Characteristics of patients and use of resource in French pediatric intensive care units. Le groupe francophone de Reanimation et urgences pediatriques. Arch Pediatr. 1997;4:730–736. doi: 10.1016/s0929-693x(97)83410-0. [DOI] [PubMed] [Google Scholar]
- 30.Pollack MM, Getson PR, Ruttimann UE, Steinhart CH, Kanter RK, Katz RW, et al. Efficiency of intensive care. A comparative analysis of eight pediatric intensive care units. Jama. 1987;258:1481–1486. [PubMed] [Google Scholar]
- 31.Carcillo JA. What’s new in pediatric intensive care. Crit Care Med. 2006;34:S183–190. doi: 10.1097/01.CCM.0000232492.44019.E6. [DOI] [PubMed] [Google Scholar]
- 32.Gemke RJ, Bonsel GJ, van Vught AJ. Effectiveness and efficiency of a Dutch pediatric intensive care unit: validity and application of the Pediatric Risk of Mortality score. Crit Care Med. 1994;22:1477–1484. doi: 10.1097/00003246-199409000-00020. [DOI] [PubMed] [Google Scholar]
- 33.Carcillo JA, Fields AI. Clinical practice parameters for hemodynamic support of pediatric and neonatal patients in septic shock. Crit Care Med. 2002;30:1365–1378. doi: 10.1097/00003246-200206000-00040. [DOI] [PubMed] [Google Scholar]
- 34.Ruttimann UE, Patel KM, Pollack MM. Length of stay and efficiency in pediatric intensive care units. J Pediatr. 1998;133:79–85. doi: 10.1016/s0022-3476(98)70182-9. [DOI] [PubMed] [Google Scholar]
- 35.Slater A, Shann F. The suitability of the Pediatric Index of Mortality (PIM), PIM2, the Pediatric Risk of Mortality (PRISM), and PRISM III for monitoring the quality of pediatric intensive care in Australia and New Zealand. Pediatr Crit Care Med. 2004;5:447–454. doi: 10.1097/01.PCC.0000138557.31831.65. [DOI] [PubMed] [Google Scholar]
- 36.Marcin JP, Pollack MM. Review of the methodologies and applications of scoring systems in neonatal and pediatric intensive care. Pediatr Crit Care Med. 2000;1:20–27. doi: 10.1097/00130478-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Martinot A, Lejeune C, Hue V, Fourier C, Beyaert C, Diependaele JF, et al. Modality and causes of 259 deaths in a pediatric intensive care unit. Arch Pediatr. 1995;2:735–741. doi: 10.1016/0929-693x(96)81242-5. [DOI] [PubMed] [Google Scholar]
- 38.ten Berge J, de Gast-Bakker DA, Plotz FB. Circumstances surrounding dying in the paediatric intensive care unit. BMC Pediatrics. 2006;6:22. doi:10.1186/1471-2431-6-22 doi: 10.1186/1471-2431-6-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garros D, Rosychuk JR, Cox NP. Circumstances surrounding end of life in a pediatric intensive care unit. Pediatrics. 2003;112:e371. doi: 10.1542/peds.112.5.e371. [DOI] [PubMed] [Google Scholar]
- 40.American Academy of Pediatrics. Commitee on Bioethics. Guidelines on forgoing life-sustaining medical treatment. Pediatrics. 1994;93:532–536. Statement of reaffirmation on October 1, 2004 and on May 1, 2009. [PubMed] [Google Scholar]
- 41.Devictor DJ, Nouven DT, et al. Groupe francophone de reanimation et d’urgences pediatriques. Forgoing life-sustaining treatments: how the decision is made in French pediatric intensive care units. Crit Care Med. 2001;29:1356–1359. doi: 10.1097/00003246-200107000-00010. [DOI] [PubMed] [Google Scholar]


