Abstract
Objective: Autoimmune mechanisms are often involved in the pathogenesis of Dilated Cardiomyopathy (DCM) and Th1 immune response against cardiac antigens plays a pivotal role in disease development.
Methods: IL-2 receptor (CD4+/CD25+) and cytokines IL-2, IFN-γ, IL-10 were studied in 42 patients (17 with DCM - DCM group, 10 patients with hypertrophic cardiac disease - HCD group, and 15 healthy volunteers - Control group). DCM group was subdivided in: DCM-1 (9 patients with recent disease onset) and DCM-2 (8 patients with chronic DCM). The % CD4+/CD25+ T-lymphocytes were analyzed by double fluorescence flow cytometry both ex vivo and after phytohaemagglutinin (PHA)-cultures with/without 5 and 10 microgr of human cardiac myosin. The cytokines were measured using Enzyme-Linked Immunosorbant Assay (ELISA) method.
Results: Ex vivo analysis: In DCM group, CD4+/CD25+ T-cells significantly increased compared to other groups (p<0.05), due exclusively to DCM-2 subgroup (p=0.019). In PHA cultures in DCM-2 subgroup CD4+/CD25+ T-lymphocytes were significantly increased compared to all other groups (p<0.001). The addition of myosin in the cultures of DCM-2 subgroup maintained the same result. In cultures supernatants in DCM-2 subgroup, IL-2 levels were impressively increased compared to DCM-1 subgroup (p=5.91x10-6), HCD and Control groups (p<0.001). Addition of antigen decreased significantly IL-2 levels in DCM-2 subgroup (p=0.01). IFN-γ levels followed the same pattern of alterations. IL-10 levels were significantly increased in both DCM subgroups compared to HCD and Control groups (p<0.05).
Conclusions: Increased peripheral CD4+/CD25+ T-cells found in chronic DCM could be a useful prognostic marker in DCM progress. Increased synthesis of IL-2 and IFN-γ and varying IL-10 levels reflects a Th1 pattern of immune response during chronic disease and implies active cellular immunity process, related to poor prognosis. Thus, analysis of the Th1/Th2 phenotype may be useful in disease monitoring in patients with DCM.
This paper is a part of PhD thesis. It has been published at the abstract book of the Acute Cardiac Care congress 2010.
Keywords: CD4+/CD25+ T-lymphocytes, Th1/Th2 regulation, dilated cardiomyopathy, cytokines
Dilated Cardiomyopathy (DCM) is a common cause of congestive heart failure in young individuals and prognosis of the disease is poor. Autoimmune mechanisms, usually as result of infection from cardiotropic viruses (eg. adenovirus, enterovirus, parvovirus B19) are often involved in the pathogenesis of DCM 1-6.
Recent WHO definition comprises 'inflammatory cardiomyopathy' as a distinct entity where myocarditis is associated with cardiac dysfunction and is recognized to be of autoimmune and/or infectious origin 3, 7.
The involvement of a deregulated immunity is suggested early in the development of DCM. Infections often trigger myocardial injury. Initial anti-infectious immune responses can turn to responses towards self-antigens by molecular mimicry and/or epitope spreading mechanisms. T-cell responses against the most prominent heart antigen, cardiac myosin, have been well-described in mice models 5, 8, 9.
Naïve helper T-cells stimulated by antigen presented cells (APCs) differentiate into two distinct subsets: Th1 that secrete Interleukin-2 (IL-2), Interferon-γ (IFN-γ) and Tumor Necrosis Factor-α (TNFα) and promote mainly cellular immunity and Th2 that produce Interleukin-10 (IL-10), nterleukin-4 (IL-4), and nterleukin-5 (IL-5) primarily inducing humoral immunity 10. Th1 T-lymphocytes are pivotal in the autoimmune response against cardiac myosin. The well studied model of experimental myocarditis has shown that the myocardial damage in the DCM has Th1 type characteristics against certain epitopes of cardiac myosin 1, 4, 8 .
Th1 immune responses and cytokine secretion (especially IL-2 and IFN-γ) play a critical role in inflammation and healing of myocarditis. On the other hand, diminished production of Th1 cytokines has been associated with Tcell unresponsiveness and disease chronicity 2, 4, 11, 12 .
Thus, it is expected that IL-2 and its receptor (IL-2r, CD25) is critical in activating and promoting the innate (macrophages, dendritic cells) and acquired immune responses in the progression of autoimmune myocarditis to DCM. IL-2 and IL2r are very important growth and differentiation factors of T helper lymphocytes and are synthesized immediately after antigenic stimulation. This initiates Th1/Th2 cytokine regulation that determines the outcome of immune response and the development of a disease 13.
Few reports have addressed the polarization of Th1 and Th2 responses in patients with DCM and T-cell function is poorly defined. In the present study we examined the central role of IL-2 and its receptor IL-2r in Th1/Th2 regulation and cytokine synthesis in patients with DCM. We have used human cardiac myosin for the specific stimulation of T-lymphocytes.
Patients and methods
Forty two patients were studied (Table 1):
Table 1. Baseline characteristics of patients in the 3 groups.
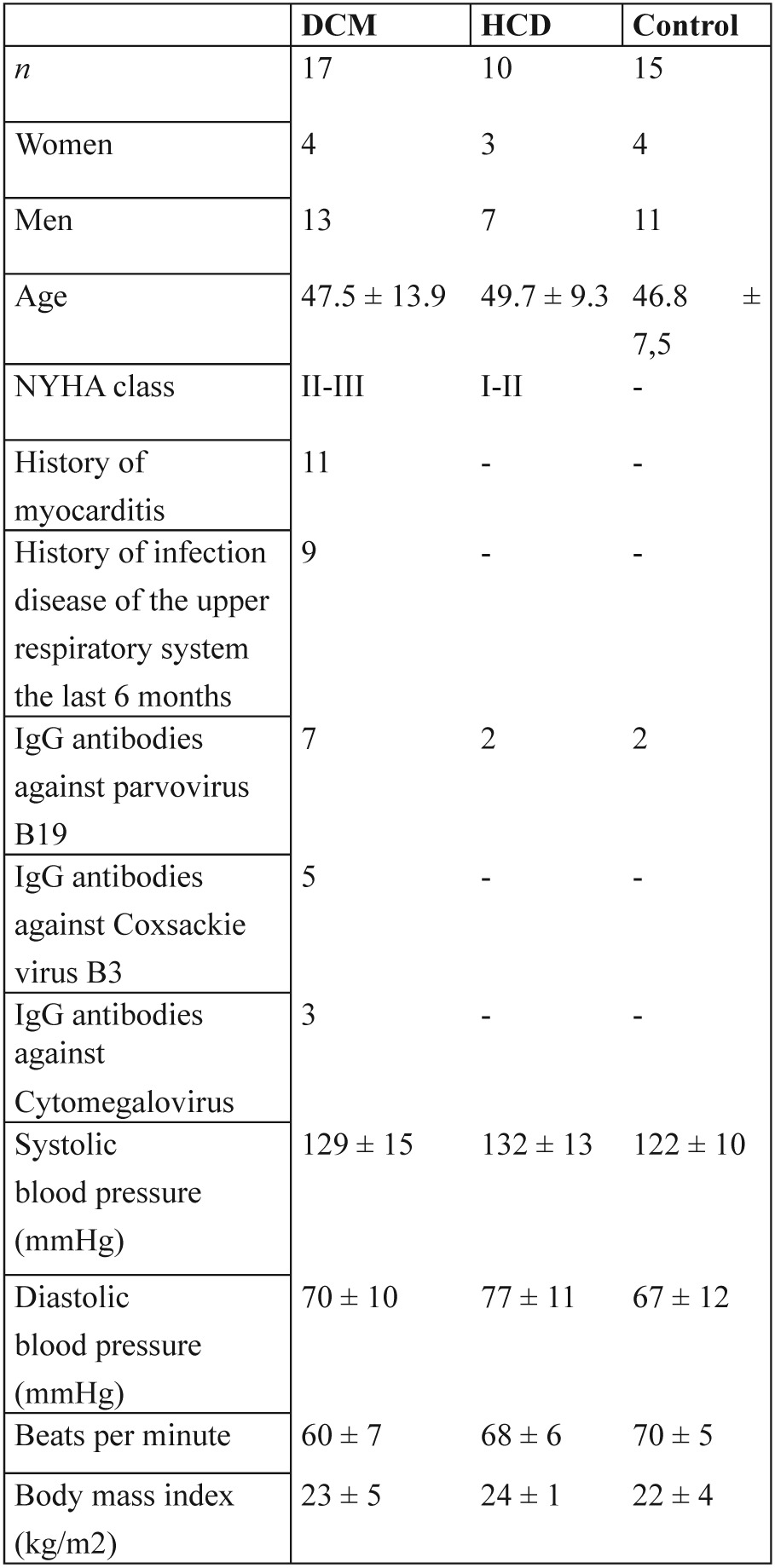
NYHA: New York Heart Association
17 patients with DCM - DCM group (13 males and 4 females aged 47.5±13.9 years). Afterwards the analysis of results of the study, we realized a differentiation of parameters that was studied, that was related with the duration of DCM. Thus, according to disease duration the DCM group was divided in two subgroups: DCM-1 subgroup consisted of 9 patients (7 males and 2 females aged 47.5±8.5 years) with a history of recent disease onset (≤ 6 months) and DCM-2 subgroup comprised 8 patients (6 males and 2 females aged 48.0±18.06 years) with chronic DCM 14.
10 patients with hypertrophic cardiac disease (HCD group) (3 patients with hypertrophic cardiomyopathy and 7 patients with hypertension, 7 males and 3 females aged 49.7±9.3) and
15 healthy volunteers (Control group) (11 males and 4 females aged 46.8±7.5 years) age and sex matched with DCM group.
Diagnosis of DCM was based on clinical criteria (history, clinical examination) and other diagnostic procedures (chest X-ray, electrocardiogram, cardiac ultrasound).
Exclusion criteria were cardiomyopathy of ischemic, alcoholic or metabolic origin, cardiomyopathies associated with systemic autoimmune diseases or cancer, systemic treatment with corticosteroids or other immunomodulatory treatment and pregnancy.
The investigation conforms with the principles outlined in the Declaration of Helsinki. All patients provided written informed consent and the protocol of this study was reviewed and approved by the Institutional Ethics Committee.
In details (Figure 1):
Figure 1. Study protocol is shown.
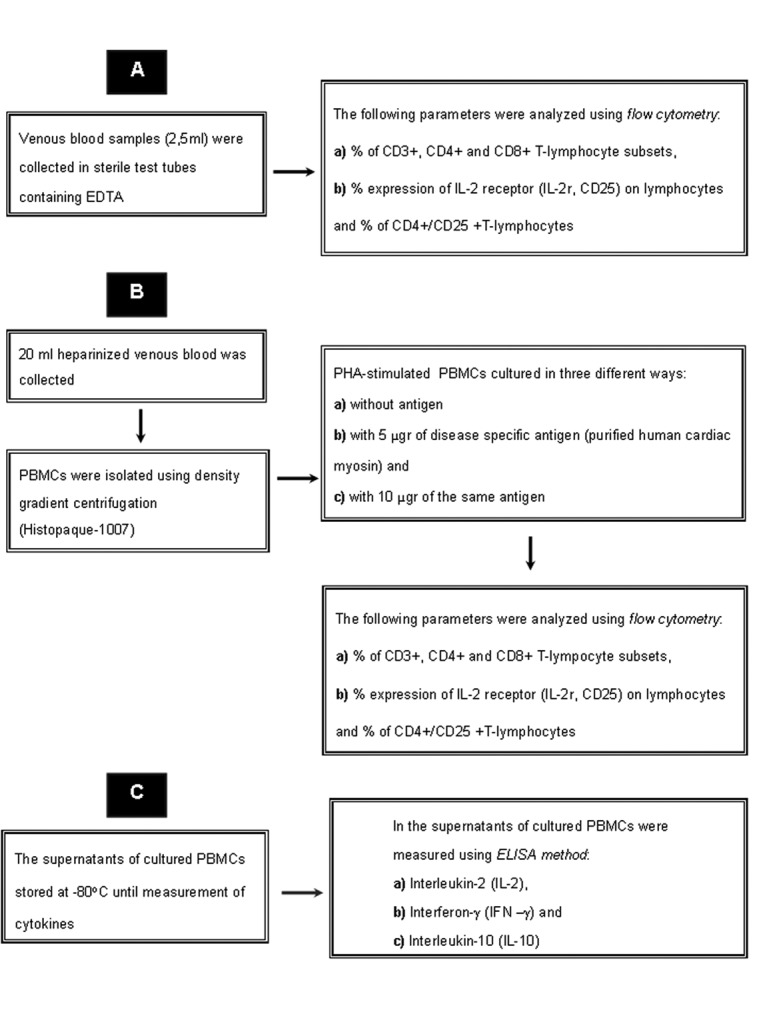
A) In all the above mentioned groups the following parameters were analyzed using double fluorescence flow cytometry (EPICS XL-MCL, Beckman Coulter, Fullerton, CA, USA) 15:
The percentage of CD3+, CD4+ and CD8+ T-lymphocyte subsets,
The percentage expression of CD25 on lymphocytes and the percentage of CD4+/CD25+ T-lymphocytes.
Measurements were performed on: a) peripheral whole blood (ex-vivo), b) PHA-stimulated peripheral blood mononuclear cells (PBMCs) cultured in three different ways:
without antigen
with 5 µgr of disease specific antigen (purified human cardiac myosin) and
with 10 µgr of the same antigen
The monoclonal reagents used in the study were CD3 (clone UCHT1, FITC), CD4 (clone 13B8.2, FITC), CD8 (clone B9.11, PE), CD25 (clone BI.49.9, PE), IgG1 (mouse) control (clone 679.1Mc7, FITC), and IgG1 (mouse) control (clone 679.1Mc7, PE), (Immunotech, Marseille, France).
B) In the supernatants of cultured PBMCs, IL-2 (human), IFN-γ (human) and IL-10 (human) (Bender Medsystems, Vienna, Austria) were measured using Enzyme- Linked Immunosorbant Assay (ELISA) method.
Ex vivo analysis
Venous blood samples (2,5ml) were collected in sterile test tubes containing K3 ethylenediamine tetraacetic acid (EDTA) and immediate staining with monoclonal antibodies (mAbs) for Fluorescence-activated cell sorting (FACS) analysis followed.
Whole blood (100 µl) was incubated at room temperature for 15 min with 20 µl of each mAb. The dual staining method (synchronous two-color fluorescence analysis) was used. The erythrocytes were lysed and the leucocytes were fixed by using Immunoprep/Q-prep protocol (Coulter, Hialeah, FL, USA), which lyses red blood cells with dilute formic acid, returns the solution to neutral pH and then fixes the remaining cells with 0.1% paraformaldehyde at the final concentration.
Cell cultures
Simultaneously with the ex vivo analysis, 20 ml heparinized venous blood was collected in sterile tubes and PBMCs were isolated using density gradient centrifugation (Histopaque-1007, Sigma Laboratories, St. Louis, MO, USA). The viability of PBMC was determinated to be greater than 95%, as indicated by Trypan blue dye exclusion (Sigma, St. Luis, MO, USA). Preliminary experiments in PBMCs from healthy volunteers were carried out to standardize the time and dose of PHA.
PBMC were cultured in triplicate in the presence of 5 µg/ml PHA (Seromed, Berlin, Germany) alone, 5 µg/ml PHA plus 5 µg/ml of cardiac myosin and 5 µg/ml PHA plus 10 µg/ml of cardiac myosin in 24-well plates (Costar, Boston, MA, USA). PBMCs were 1x106 cells per well of culture plate. Each well contained 1 ml culture suspension. Culture medium consisted of RPMI-1640 (GIBCO laboratories, Paisley, UK) supplemented with 10% fetal calf serum (GIBCO), 2 mmol/l L-glutamine (Sigma), 100 IU/ml penicillin (Sigma) and 100 µg/ml streptomycin (Sigma). The cultures were kept at 37℃ in a humidified 5% CO2 atmosphere for 48 hrs. Approximately 3 x 106 cells per ml of sample were pelleted in a round-bottomed centrifuge tube and washed in RPMI-1640. The pellet was resuspended and 100 µl (~3 x 105 PBMC) was stained immediately with 20 µl of the appropriate antibody.
Flow cytometry
Lymphocytes were gated using forward scatter (FS) versus side scatter (SS) dot plots.
Mitogen and antigens
Phytohemagglutinine (PHA): For the not specific stimulation of T-lymphocytes a strong classic pan T-lymphocyte mitogen, PHA was selected 16.
Human Myosin: For the specific stimulation of Tlymphocytes purified human cardiac myosin was prepared. Cardiac myosin was prepared from the ventricular muscle of human heart, following a modified Murakami et al method 17. Human left ventricular heart tissue was obtained from autopsied patient who had died of road accident and had no history of coronary disease, myocarditis or congestive heart failure. The cardiac tissue was taken within 1-2 hours from the time of death and was kept at -80 ℃ until use.
The purity of this preparation was checked by SDS (sodium dodecyl-polyacrylamide), gel electrophoresis. Cytokine levels (IL-2, IFN-γ, and IL-10) in T-cell culture supernatants.
Cytokine levels were measured using ELISA method. The supernatants of cultured PBMCs stored at -80℃ until measurement of cytokines. Levels of IL-2, IFN-γ and IL- 10 were measured by an immunoassay kit (Bender Medsystems, Vienna, Austria).
Statistics
Data are expressed as mean ± SD for continuous variables or percentages for categorical variables. In order to identify statistically significant differences of the mean values between groups for variables measured over time, Generalized Linear Models Repeated Measures ANOVA were used. Post hoc analysis (Tuckey HSD) was utilized when appropriate to assess differences among these groups. The p- value of < 5% has been considered indicative of a statistically significant result. SPSS 14 was used for all statistical procedure
Results
A. Peripheral blood:
We noted no alterations in the percentage of CD3+, CD4+ and CD8+ T-lymphocyte subsets in any of the groups (Table 2). After the division of DCM group in two subgroups (DCM-1 and DCM-2), there was a significant increase in CD4+ T-lymphocytes in DCM-2 subgroup in comparison to control group (p=0.023) (Table 2).
Table 2. The percentage of CD3+, CD4+ and CD8+ T lymphocyte subsets and the percentage of CD4+/CD25+ T lymphocytes in peripheral blood.
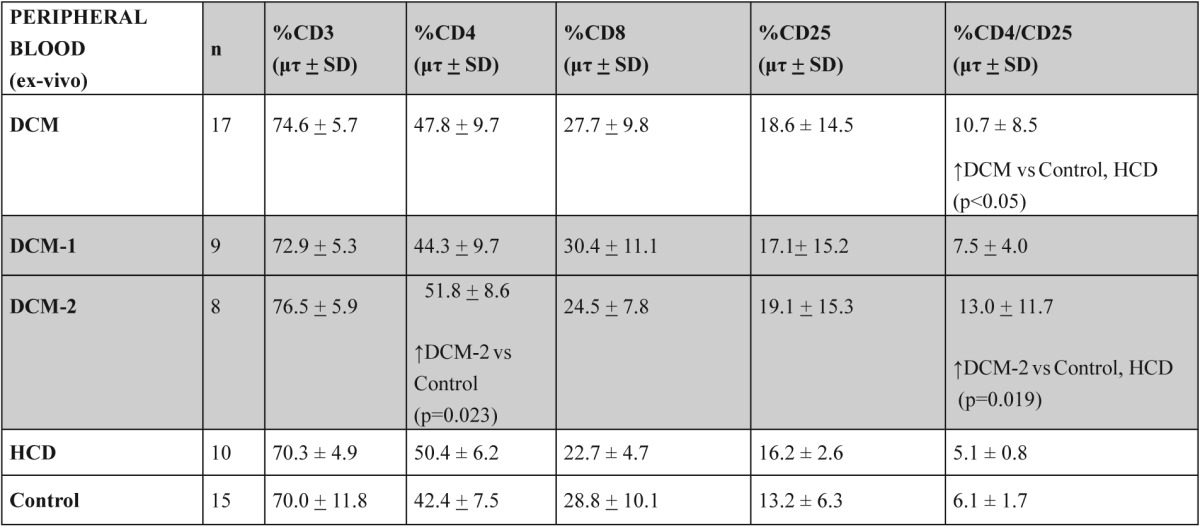
vs: versus
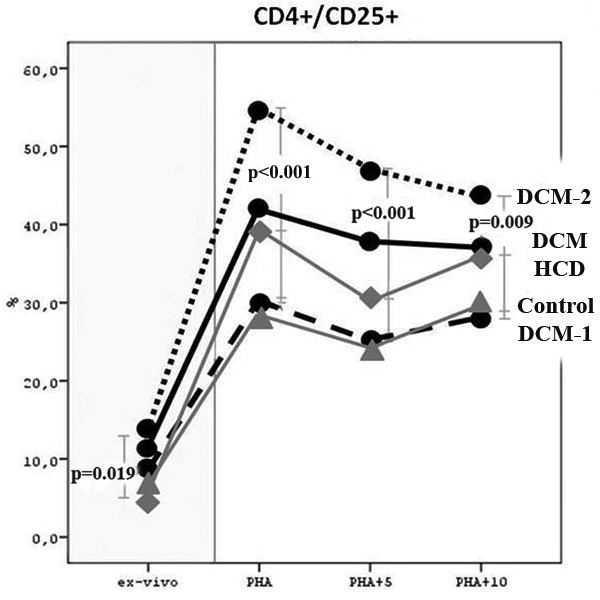
In DCM patients there was a significant increase in CD4+/CD25+ T-lymphocytes in comparison to Control and HCD groups (p<0.05). This increase was due to DCM-2 subgroup when compared to Control and HCD groups (p=0.019) (Figure 2, table 2).
Figure 2. The percentage of CD4/CD25 double positive T-lymphocytes in peripheral whole blood (ex vivo) and in PHA-stimulated PBMCs cultures in studied groups and subgroups.
B. PHA cultures (in vitro stimulation):
An important finding was that in DCM-2 subgroup there was a significant increase in IL-2r (CD25) expression on total and especially on CD4+ T-lymphocytes, in comparison to DCM-1, Control and HCD groups (p<0.001) (Figure 2, table 3).
Table 3. The percentage of CD4+/CD25+ T lymphocytes in PBMC cultures with PHA alone and with PHA plus 5µg and 10 µg of human heart myosin in groups and subgroups of the study.
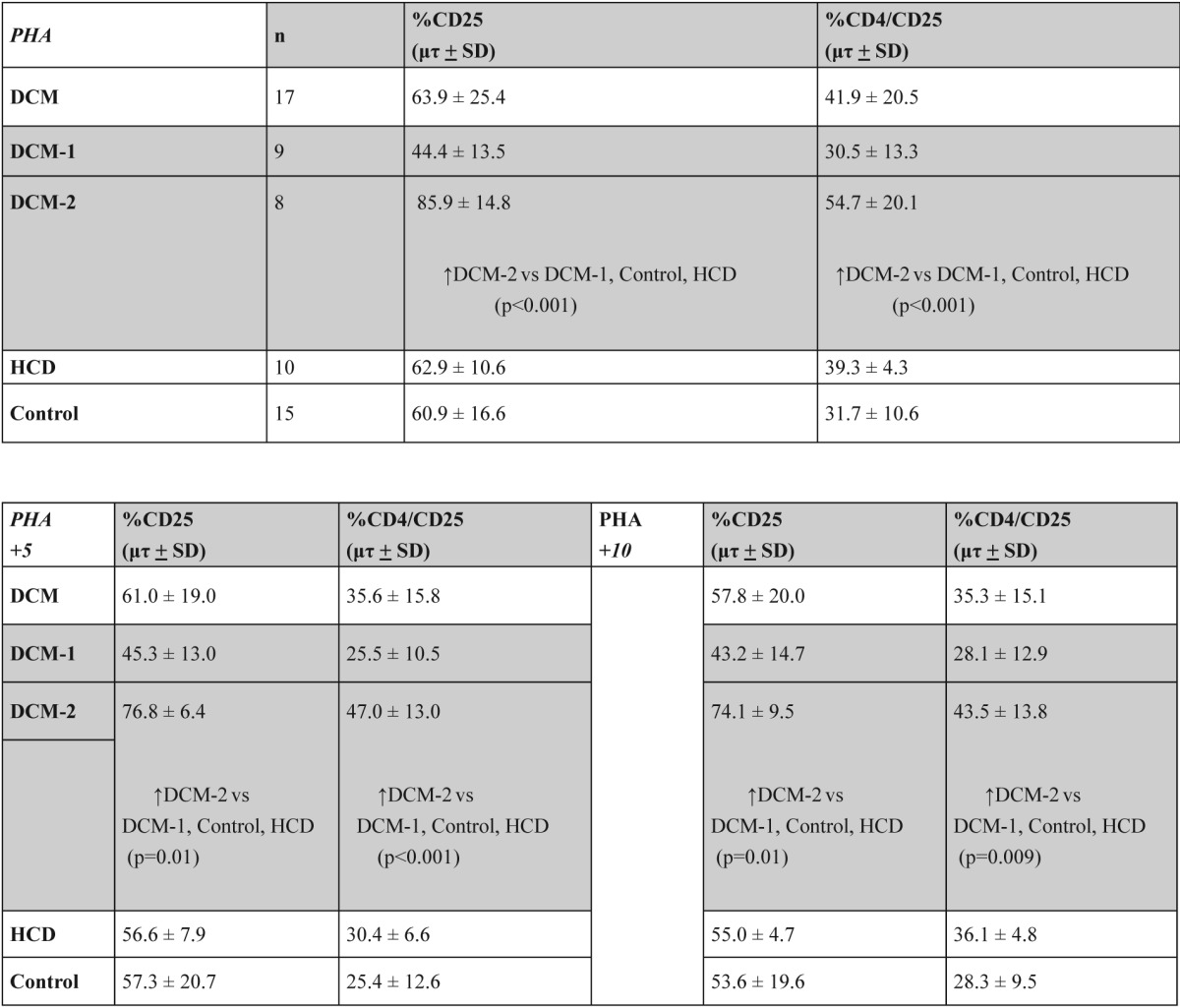
The addition of 5 µg antigen (human cardiac myosin) in the cultures of patients of DCM-2 subgroup lead to a blocking (decrease) of the expression of CD25 on total and especially on CD4+/CD25+ T-lymphocytes (Figure 2, table 3). Moreover, the addition of 10 µg antigen in the cultures of patients of DCM-2 subgroup maintained the above result. Comparisons of the expression of CD4+/ CD25+ T-lymphocytes with two different quantities of cardiac antigen showed no differences.
C. Cytokine profile in cultures supernatants:
In IL-2 levels there were no significant differences in DCM, Control and HCD groups
However, after the division of DCM group in DCM-1 and DCM-2 subgroups an impressive differentiation between the subgroups appeared: In DCM-2 subgroup an important increase of IL-2 levels was observed in comparison to DCM-1 subgroup (p=5.91 x 10-6). The difference in comparison to Control and HCD groups remained significant (p<0.001) (Table 4). The addition of antigen on PHA-cultures shared similar results (Table 4). In DCM-2 subgroup the addition of antigen in PHA-cultures decreased the levels of IL-2 significantly (p=0.01) (data not shown).
Table 4. IL-2, IFN-γ and IL-10 levels in cultures supernatants with PHA, with PHA plus 5 µgr myosin and with PHA plus 10gr myosin, in the groups and subgroups of the study.
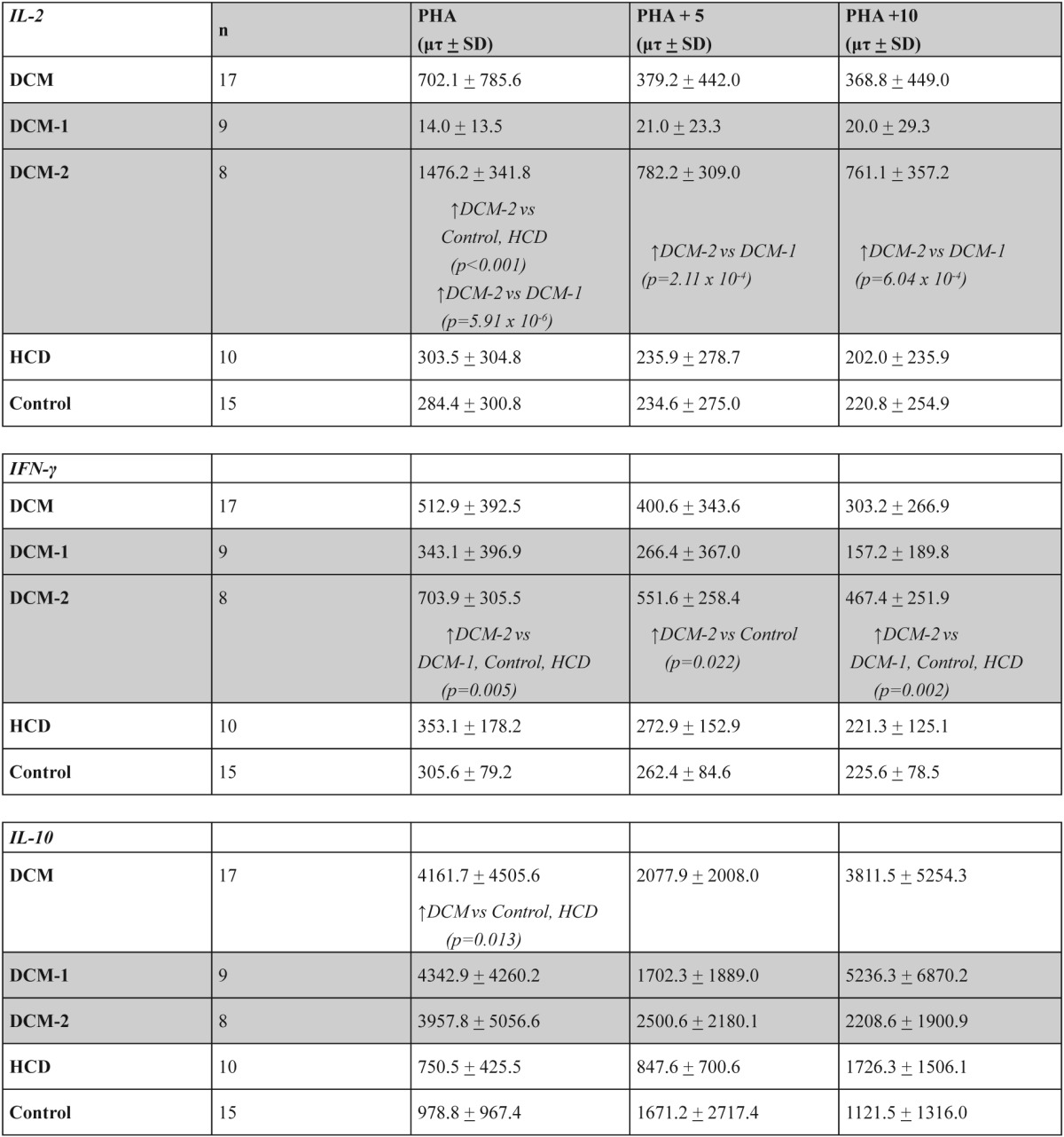
IFN-γ levels followed almost a similar model of changes like IL-2: there were no significant differences in DCM, Control and HCD groups.
In DCM group the addition of antigen in PHA-cultures decreased the IFN-γ levels significantly (p<0.001) (data not shown).
After the division of DCM group in DCM-1 and DCM-2 subgroups there was a significant increase of IFN-γ levels in DCM-2 subgroup compared to other groups DCM-1, Control and HCD (p=0.005) (Table 4). In DCM-2 subgroup the addition of antigen in PHAcultures decreased the IFN-γ levels significantly (p<0.01) (data not shown) although they remained significantly increased compared to Control and HCD groups (p=0.022 and 0.002 equivalents) (Table 4).
IL-10 levels were increased significantly in DCM group compared to Control and HCD groups (p=0,013) (Table 4). This increase concerned both DCM-1 and DCM-2 subgroups in comparison to Control and HCD groups (p<0.05). In DCM-1 subgroup the addition of antigen in PHA-cultures decreased the levels of IL-10 significantly (p=0.049) (data not shown).
Discussion
In this study we assessed peripheral T-lymphocyte subsets and CD4+ T-lymphocytes expressing CD25 (CD4+/CD25+) in two subgroups of DCM patients (DCM-1 / recent onset and DCM-2 / long lasting) to investigate their role in DCM outcome and the effect on main Th1/Th2 cytokine network (IL-2, IFN-γ and IL-10). We found a significant increase in expression of CD4+ / CD25+ (IL-2r) T-lymphocytes in patients with DCM and specifically in patients with long lasting disease. Expression of IL-2 receptor on T-cell has been shown to increase during antigenic stimulation and inflammation, particularly during infections18-20.
Moreover, in the ex-vivo study, patients with long lasting disease had significantly increased CD4+ T-cells compared to healthy controls. It could be suggested that this increase reflected CD4+ T-cell clone expansion accompanying the increased expression of IL-2r and increased IL-2-levels (in the supernatants), in the context of chronic inflammation and disease perpetuation.
Thus, our findings suggest that unregulated IL-2r could be considered as an activation marker of peripheral T-lymphocytes and clinically, increased expression in DCM could be used as a marker of persistent antigenic stimulation that reflects continuation of myocardial inflammation.
Our findings are in accordance with Limas et al who came to similar conclusions and suggest that increased soluble IL-2r levels in DCM patients could be an independent predictor of a more aggressive clinical course 21.
PBMC cultures with a strong T-cell mitogen like PHA, constitute a functional assay of the blastogenic capacity of lymphocytes that can induce the expression of CD25, related to the activation of T-lymphocytes 16. CD4+/CD25+ T lymphocytes were significantly increased in PHA-cultures.
After subgrouping patients to recent and chronic DCM, it was showed that DCM-1 subgroup was not affected by human cardiac myosin addition, while DCM-2 dropped significantly. Particularly interesting was this finding of a significant drop of PHA-cultured CD4+/ CD25+ T-lymphocytes, in the presence of antigen. It seems that the addition of myosin in the cultures partially 'blocked' IL2-r expression and/or decreased (antagonized) the mitogenic effect of PHA itself on T-cells.
This 'myosin effect' on cultured T cells from DCM-2 patients could reflect a disturbed Th1/Th2 cytokine profile during disease revolution. It is known that a positive outcome of a myocardial infection requires sufficient Th1 cellular responses and cytokine regulation 22. Contrastingly, the inflammatory process perpetuates and induces autoimmunity and organ specific (myocardium) autoimmune damage. Sustained myocardial inflammation/infection beyond 14-28 days or repeated antigenic stimulation e.g. with myosin could lead T-lymphocyte clones to autosensitization against myosin resulting to DCM 3, 4, 23.
T-cell-mediated heart injury in the evolution of DCM is very much influenced by the release of cytokines and vice-versa, since cytokines, expressed by APCs, lymphocytes and also cardiomyocytes, are pivotal in intracellular signaling of immunocompetent cells 1, 2, 4, 22. Changes of certain cytokine levels can determinately affect the cellular immune response process in myocardium. The consequences of Th1/Th2 cytokine deregulation have been demonstrated by several studies of patients with heart failure20, 24-26.
Antigen specific proliferation of helper T-lymphocytes following stimulation, is critically dependent on IL-2 expression, secretion, and binding to IL-2 receptor induced. In addition, IL-2 modulates the expression of IFN-γ and other cytokines 13, 27.
In our study, IL-2 levels were found to be significantly increased in the supernatants from PHA cultured T cells in the chronic/long lasting DCM group of patients. In contrast, no alteration (low levels) of IL-2 was found in the recent-onset DCM group of patients. These findings are in accordance to our results concerning low IL2-r expression in the same group.
These findings support the contention that the low grade activity of the IL-2/L-2r system at the beginning of the disease (recent-onset DCM), is a double-edged sword. It might either protect from disease chronicity (mild inflammation) or, might result in a decreased Th1 immune response, which might lead to chronicity. On the other hand, increased IL-2/L-2r system capacity in the chronic/ long lasting DCM group as described above, in an effort to overcome the inflammation state, might be ineffective and sustains inflammation, leading to chronicity.
Fuse et al examined changes of Th subsets in a case of human acute viral myocarditis, as well as in a rat model of experimental autoimmune myocarditis and showed that Th1/Th2 ratio in peripheral blood followed the clinical course: Th1 was dominant in the acute inflammatory phase, while Th2 increased during the recovery phase 28, 29.
The role of IFN-γ as a significant Th1 cytokine in controlling the fate of myocarditis, is unequivocal. IFN-γ activates macrophages, induces MHC class I and II expression, recruits T cells at the site of inflammation and plays a critical role in antiviral activity and pathogen clearance at myocarditis onset 2, 5, 27, 30, 31.
In the present study, IFN-γ variations in the supernatants of cultured T-cells from DCM patients followed the IL-2 changes with consistency. IFN-γ levels remained unchanged in recent-onset disease and were found significantly increased in long lasting disease. Moreover, in the presence of cardiac myosin IFN-γ levels decreased, but were still significantly higher compared to controls. Increased IFN-γ levels in long lasting DCM, in combination to increased IL-2 levels in the same subgroup of patients, also reflects the Th1/Th2 imbalance in the disease course and a Th1 cytokine pattern in the chronic state, which favours disease persistence. However, in the chronic disease state, IFN-γ is not needed anymore. It is undesirable because it promotes IL-1 and TNF-α production which may have detrimental effect at long lasting disease.
Few studies have explored the significant pathways of inflammation resolution at sites of injury. IL-10 is known to be significant in the termination of inflammation. It seems to control immune responses and tolerance. IL-10 directly inhibits IL-2 synthesis and vise versa. It is also suggested to play a role in peripheral tolerance and in protection against autoimmunity 31-33 . In the present study, IL-10 levels were found to be increased in the supernatants from PHA-cultured T cells in both DCM patients subgroups, maybe in an effort to drive a Th2 response in order to terminate inflammation and heart injury.
Conclusion
Our findings support that an imbalance of Th1/Th2 regulation exists in DCM patients. This is supported by an insufficient Th1 regulation and Th2 predominance at the onset of DCM. As the disease progressed, autoimmune mechanisms against myosin and Th1 switch were established. Thus, the increased % IL-2r expression and the analysis of Th1/Th2 phenotype can be useful markers in patients' follow-up and postmyocarditis DCM development.
From the immunopathophysiological point of view, there appears to be an incorrect 'timing' of immunological changes that leads in Th2 predominance in the recent onset DCM and Th1 predominance in chronic DCM. There seems to be a need to modulate the cellular immune response of chronic DCM patients towards a Th2 pattern and vice versa.
Conflict of interest:
None declared.
References
- 1.Izumi T, Takehana H, Matsuda C, Yokohama H, Kohno K, Suzuki K, et al. Experimental Autoimmune Myocarditis and Its Pathomechanism. Herz. 2000:274–278. doi: 10.1007/s000590050020. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham MW. Cardiac Myosin and the TH1/TH2 Paradigm in Autoimmune Myocarditis. AJP July. 2001;159:5–12. doi: 10.1016/S0002-9440(10)61665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maisch B, Richter A, Sandmöller A, Portig I, Pankuweit S. Inflammatory dilated cardiomyopathy (DCMI) Herz. 2005;30:535–544. doi: 10.1007/s00059-005-2730-5. [DOI] [PubMed] [Google Scholar]
- 4.Kallwellis-Opara A, Dorner A, Poller WC, Noutsias M, Kóhl U, Schultheiss HP, et al. Autoimmunological features in inflammatory cardiomyopathy. Clin Res Cardiol. 2007;96:469–480. doi: 10.1007/s00392-007-0524-x. [DOI] [PubMed] [Google Scholar]
- 5.Lindner J, Noutsias M, Lassner D, Wenzel J, Schultheiss HP, Kuehl U, et al. Adaptive immune responses against parvovirus B19 in patients with myocardial disease. J Clin Virol. 2009;44:27–32. doi: 10.1016/j.jcv.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Kaya Z, Katus HA. Role of autoimmunity in dilated cardiomyopathy. Basic Res Cardiol. 2010;105:7–8. doi: 10.1007/s00395-009-0069-4. [DOI] [PubMed] [Google Scholar]
- 7.Elliott P, Andersson B, Arbustini E, Bilinska Z, Cecchi F, Charron P, et al. Classification of the cardiomyopathies: a position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2008;29:270–276. doi: 10.1093/eurheartj/ehm342. [DOI] [PubMed] [Google Scholar]
- 8.Izumi T, Kohno K, Inomata T, Takagaki Y. Myocardiotogenic epitopes and autoimmune myocarditis. Intern Med. 2003;42:3–6. doi: 10.2169/internalmedicine.42.3. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham MW. T cell mimicry in inflammatory heart disease. Mol Immunol. 2004;40:1121–1127. doi: 10.1016/j.molimm.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Rich RR. The human immune response. In: Rich RR, Shearer WT, Fleisher TA, Schroeder HW Jr, Frew A, Weyand C, editors. Clinical Imunology: Principles and Practice. 3rd ed. London: Elsevier; 2008. [Google Scholar]
- 11.Kurrer MO, Kopfb M, Penninger JM, Eriksson U. Cytokines that regulate autoimmune myocarditis. Swiss MED WKLY. 2002;132:408–413. doi: 10.4414/smw.2002.10054. [DOI] [PubMed] [Google Scholar]
- 12.Yoshikawa T, Baba A, Nagatomo Y. Autoimmune Mechanisms Underlying Dilated Cardiomyopathy. Circ J. 2009;73:602–607. doi: 10.1253/circj.cj-08-1151. [DOI] [PubMed] [Google Scholar]
- 13.Gaffen SL, Goldsmith MA, Greene WC. Interleukin-2 and the interleukin-2 receptor. In: Thomson AW, editor. The cytokine handbook. 3rd edition. London: Academic Press; 1998. [Google Scholar]
- 14.McNamara DM, Holubkov R, Starling RC, Dec GW, Loh E, Torre-Amione G, et al. Controlled Trial of Intravenous Immune Globulin in Recent-Onset Dilated Cardiomyopathy. Circulation. 2001;103:2254–2259. doi: 10.1161/01.cir.103.18.2254. [DOI] [PubMed] [Google Scholar]
- 15.Ormerod MG. Flow cytometry: a practical approach. 3rd ed. New York: Oxford University Press; 2003. [Google Scholar]
- 16.Abbas AK, Lichtman AH. Basic Immunology: Functions and Disorders of the Immune System. 3rd ed. Philadelphia: WB Saunders; 2009. [Google Scholar]
- 17.Kodama M, Matsumoto Y, Fujiwara M, Masani F, Izumi T, Shibata A. A novel experimental model of Giant Cell Myocarditis induced in rats by immunization with cardiac myosin fraction. Clin Immunol Immunopathol. 1990;57:250–262. doi: 10.1016/0090-1229(90)90039-s. [DOI] [PubMed] [Google Scholar]
- 18.Stephens DS, Shafer WM. Immune response to extracellular bacteria. In: Rich RR, editor. Clinical Imunology. Principles and Practice. 3rd ed. London: Mosby; 2008. [Google Scholar]
- 19.Mueller SN, Rouse BT. Immune response to viruses. In: Rich RR, editor. Clinical Imunology. Principles and Practice. 3rd ed. London: Mosby; 2008. [Google Scholar]
- 20.Zhao P, Sharma AC, Ren J. Pathogenesis and therapy of autoimmunity-induced dilated cardiomyopathy. Front Biosci. 2009;14:1708–1715. doi: 10.2741/3334. [DOI] [PubMed] [Google Scholar]
- 21.Limas CJ, Hasikidis C, Iakovou J, Kroupis C, Haidaroglou A, Cokkinos DV. Prognostic significance of soluble interleukin-2 receptor levels in patients with dilated cardiomyopathy. Eur J Clin Invest. 2003;33:443–448. doi: 10.1046/j.1365-2362.2003.01111.x. [DOI] [PubMed] [Google Scholar]
- 22.Bach JF. Infections and autoimmunity. Rev Med Interne. 2005;26(Spec No 1):32–4. [PubMed] [Google Scholar]
- 23.Jane-wit D, Tuohy VK. Autoimmune cardiac-specific T cell responses in dilated cardiomyopathy. Int J Cardiol. 2006;112:2–6. doi: 10.1016/j.ijcard.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Boura P, Lefkos N, Boudonas G, Kountouras J, Zacharioudaki E, Efthimiadis A, et al. Antigenic stimulation in T-cells cultures in cardiomyopathies: differences in cytokine profiles. Eur J Immunogenet. 1999;26:285–291. doi: 10.1046/j.1365-2370.1999.00152.x. [DOI] [PubMed] [Google Scholar]
- 25.Noutsias M, Pauschinger M, Poller WC, Schultheiss HP, Kuhl U. Immunomodulatory treatment strategies in inflammatory cardiomyopathy: current status and future perspectives. Expert Rev Cardiovasc Ther. 2004;2:37–51. doi: 10.1586/14779072.2.1.37. [DOI] [PubMed] [Google Scholar]
- 26.Fukunaga T, Soejima H, Irie A, Sugamura K, Oe Y, Tanaka T, et al. Relation between CD4+ T-cell activation and severity of chronic heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 2007;100:483–488. doi: 10.1016/j.amjcard.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 27.Afanasyeva M, Georgakopoulos D, Belardi DF, Bedja D, Fairweather D, Wang Y, et al. Impaired up-regulation of CD25 on CD4+ T cells in IFN-{gamma{ knockout mice is associated with progression of myocarditis to heart failure. PNAS. 2005;102:180–185. doi: 10.1073/pnas.0408241102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuse K, Kodama M, Aizawa Y, Yamaura M, Tanabe Y, Takahashi K. Th1/Th2 Balance Alteration in the Clinical Course of a Patient With Acute Viral Myocarditis. Jpn Circ J. 2001;65:1082–1084. doi: 10.1253/jcj.65.1082. [DOI] [PubMed] [Google Scholar]
- 29.Fuse K, Kodama M, Ito M, Okura Y, Kato K, Hanawa H, et al. Polarity of helper T cell subsets represents disease nature and clinical course of experimental autoimmune myocarditis in rats. Clin Exp Immunol. 2003;134:403–408. doi: 10.1111/j.1365-2249.2003.02312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eriksson U, Kurrer MO, Sebald W, Brombacher F, Kopf M. Dual Role of the IL -12/IFN-γ Axis in the Development of Autoimmune Myocarditis: Induction by IL-12 and Protection by IFN. J Immunol. 2001;167:5464–5469. doi: 10.4049/jimmunol.167.9.5464. [DOI] [PubMed] [Google Scholar]
- 31.Lindberg E, Andersson B, Hörnquist EH, Magnusson Y. Impaired activation of IFN-γ CD4+ T cells in peripheral blood of patients with dilated cardiomyopathy. Cell Immunol. 2010;263:224–229. doi: 10.1016/j.cellimm.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 32.Li Y, Heuser JS, Kosanke SD, Hemric M, Cunningham MW. Protection against Experimental Autoimmune Myocarditis Is Mediated by Interleukin-10-Producing T Cells that Are Controlled by Dendritic Cells. AJP. 2005;167:5–15. doi: 10.1016/S0002-9440(10)62948-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mosser DM, Zhang X. Interleukin-10: new perspectives on an old cytokine. Immunol Rev. 2008;226:205–218. doi: 10.1111/j.1600-065X.2008.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


