Abstract
We previously demonstrated that human cytomegalovirus (HCMV) infection induced the activation of the cellular transcription factor NF-κB. Here, we investigate the mechanism for the HCMV-induced NF-κB activation and the role that the induced NF-κB plays in transactivation of the major immediate-early promoter (MIEP) and production of immediate-early (IE) proteins. Using a dominant-negative inhibitor of NF-κB, the IκB-superrepressor, we demonstrated that active NF-κB is critical for transactivation of the HCMV MIEP. Investigation of the mechanisms of NF-κB activation following HCMV infection showed a rapid and sustained decrease in the inhibitors of NF-κB, IκBα and IκBβ. Because the IκB kinases (IKKs) regulate the degradation of the IκBs, virus-mediated changes in the IKKs were examined next. Using dominant-negative forms of the IKKs, we showed significant decreases in transactivation of the MIEP in the presence of these mutants. In addition, protein levels of members of the IKK complex and IKK kinase activity were upregulated throughout the time course of infection. Lastly, the role NF-κB plays in HCMV IE mRNA and protein production during infection was examined. Using aspirin and MG-132, we demonstrated that production of IE protein and mRNA was significantly decreased and delayed in infected cells treated with these drugs. Together, the results of these studies suggest that virus-mediated NF-κB activation, through the dysregulation of the IKK complex, plays a primary role in the initiation of the HCMV gene cascade in fibroblasts and may provide new targets for therapeutic intervention.
Human cytomegalovirus (HCMV), a ubiquitous betaherpesvirus, is a significant pathogen of immunocompromised individuals, including AIDS patients, transplant recipients, and congenitally infected neonates (reviewed in reference 12). HCMV is also a pathogen of immunocompetent individuals, as it causes infectious mononucleosis (48) and is associated with the development of cardiovascular diseases (reviewed in references 58 and 81). In addition, HCMV infection has been linked to the development of malignant gliomas (23) and cervical cancers (18, 80). A critical feature of HCMV-mediated pathogenesis is the replication of the virus in infected tissue and the overt disease caused by this viral replication (reviewed in reference 12).
For a productive HCMV infection, three ordered classes of viral genes are transcribed: first, the immediate-early (IE) genes are transcribed; second, the early genes are transcribed; third, the late genes are transcribed (61). Because the IE genes are essential for viral replication, investigating the mechanisms of their regulation is required to understand HCMV pathogenesis. IE gene expression is regulated by the major immediate-early promoter (MIEP) (8, 85). Transactivation of the MIEP appears to be essential for the development of CMV-mediated disease, as murine CMV mutants lacking the MIEP, which is similar to the MIEP of HCMV (79), were not pathogenic in mice (31). Furthermore, it has been shown that the HCMV MIEP is required for efficient viral replication as well as IE gene transcription (40, 57). The specific mechanisms surrounding the regulation of the MIEP during infection are not well understood, although NF-κB appears to be a critical regulatory factor due to the presence of four consensus NF-κB binding sites (20, 61, 70).
We, and others, previously demonstrated that HCMV infection results in the dysregulation of the tightly regulated cellular transcription factor NF-κB (49, 70, 95-98). Classical NF-κB is a heterodimer consisting of a 50-kDa subunit (p50) and a 65-kDa subunit (p65). Under normal physiological conditions, NF-κB forms a complex with its inhibitors, the IκBs, and is maintained in the cytosol in this inactive state (reviewed in reference 47). NF-κB can be freed from its inhibitors through the direct action of protein kinases, termed the IκB kinases (IKK) (28, 46, 59, 99, 100), that form a complex consisting of three subunits, IKKα, IKKβ, and IKKγ (69, 91). IKKα and IKKβ are the catalytic subunits, while IKKγ serves as a scaffolding protein to hold the complex together (24, 69). Multiple signaling pathways converge at the level of IKK activation to mediate the induction of NF-κB; however, the mechanisms surrounding the activation of the IKK complex are not completely understood (26, 63, 93, 99). Evidence suggests that IKKα may be phosphorylated first by an upstream regulator, which in turn phosphorylates IKKβ, resulting in an active IKK complex (93). Therefore, heterodimeric complexes of IKKα and IKKβ appear to be the major upstream regulators of the IκBs, although homodimers of both IKKα and IKKβ also exist within the cell and may play a role in NF-κB activation (39, 99). Activation of the IKK complex leads to the phosphorylation of the IκBs, thus targeting them for polyubiquitination and degradation by the 26S proteosome complex (19, 27). Freed from its inhibitor, NF-κB enters the nucleus and transactivates NF-κB-responsive genes.
Early studies by Sambucetti et al. into the regulation of the MIEP first proposed the possibility that NF-κB was involved in HCMV replication, as deletions of the 18-bp repeats in the HCMV MIEP, to which NF-κB binds, resulted in decreased MIEP transactivation in chloramphenicol acetyltransferase (CAT) assays (20, 70). Likewise, initial studies into the HCMV-mediated activation of NF-κB by Kowalik et al. demonstrated an increase in nuclear NF-κB activity in HCMV-infected fibroblasts (49). In a more detailed examination of the HCMV-regulated induction of NF-κB, we showed a biphasic increase in the induction of NF-κB following HCMV infection: one increase was seen immediately following infection, and a second increase was observed 8 to 12 h postinfection (hpi) (97). The initial increase in NF-κB occurred in the absence of protein synthesis, suggesting that this increase in NF-κB was the result of the release of preformed stores of NF-κB. In contrast, the second increase in NF-κB activity was, at least in part, the result of de novo protein synthesis of the NF-κB subunits (p65 and p105/p50) (97). The unique viral induction of p65 mRNA highlights the importance of NF-κB for the viral life cycle as, to date, HCMV infection is the only reported stimulus in which p65 mRNA induction is detected. To account for the rapid initial NF-κB induction, we investigated the possibility that binding of HCMV glycoproteins to their cognate cellular receptors activated a signaling pathway through a receptor-ligand interaction. Purified viral glycoproteins were shown to induce NF-κB activity (95, 96), suggesting that viral binding induces a cellular regulatory pathway that leads to the activation of NF-κB. Additional studies have confirmed that purified HCMV glycoproteins are capable of activating cellular signaling pathways (9, 75). Taken together, these studies suggest that HCMV has a vested interest in inducing the rapid and sustained activation of NF-κB. Based on our previous studies, combined with published reports demonstrating impaired replication of CMV strains with a deletion in the MIEP region (31, 40, 57), we hypothesized that the induction of NF-κB following infection drives the transactivation of the MIEP and is, thus, critical for the entire viral gene cascade.
Because of the importance of understanding the regulation of the MIEP, we initiated molecular studies to examine mechanisms by which HCMV mediates the activation of NF-κB, as well as the biological importance of this induced NF-κB activation during viral infection. Here, we demonstrate that NF-κB is essential for the maximal transactivation of the HCMV MIEP in human fibroblasts and that, during HCMV infection, the upstream regulators of the NF-κB pathway were dysregulated. The data showed that both IκBα and IκBβ protein levels rapidly decreased following infection with HCMV. Conversely, protein levels of the IKKs were increased in response to HCMV infection. Furthermore, IKK kinase activity was induced following infection with HCMV. Finally, we demonstrated that NF-κB plays a vital role in the production of the IE proteins, as levels of IE proteins and mRNA were significantly decreased and delayed in the presence of the NF-κB inhibitors MG-132 and aspirin. Together, the results of these studies underline the importance of NF-κB for HCMV replication and provide evidence that HCMV usurps cellular pathways to mediate the rapid and sustained activation of NF-κB seen during viral infection.
MATERIALS AND METHODS
Cell culture and virus.
Life-extended human foreskin fibroblasts (obtained as a generous gift from Thomas Shenk, Princeton University, Princeton, N.J. [11]) or human embryonic lung (HEL) fibroblasts were used for all experiments (96). Cells were grown in Eagle's minimal essential medium supplemented with 10% heat-inactivated fetal bovine serum (Gemini, Woodland, Calif.), penicillin (100 IU/ml), and streptomycin (100 μg/ml). A low-passage HCMV Towne/E strain (passage 35 to 42) was used in all experiments (96) and was grown in HEL fibroblasts cultured in Eagle's minimal essential medium supplemented with 4% heat-inactivated fetal bovine serum (Gemini), penicillin (100 IU/ml), and streptomycin (100 μg/ml). For all experiments involving infected cells, a multiplicity of infection (MOI) of 3 to 5 was used.
Transfections and CAT assays.
Transfections of fibroblasts were performed using the calcium phosphate method as previously reported (97). DNA to be transfected was purified from bacteria using the Qiagen Maxi kit (Qiagen, Inc., Valencia, Calif.). The reporter plasmid (10 μg) containing the HCMV MIEP construct fused to the CAT gene (92) was cotransfected into fibroblasts along with 10 μg of plasmids encoding the IκB superrepressor (IκB-SR) (89) or the dominant-negative forms of IKKα and IKKβ (obtained as a generous gift from Richard Gaynor, University of Texas Southwestern Medical Center, Dallas, Tex. [67]), as indicated. In addition, cells were cotransfected with 1 μg of a β-galactosidase expression plasmid, and the harvested lysate was assayed for β-galactosidase activity as a means to control for transfection efficiency and to normalize CAT assay results, as previously performed (97, 98). pCDNA3 plasmid was used as filler DNA to ensure that an equal amount of DNA was transfected into all cells (total DNA, 21 μg/transfection mixture). Transfected cells were incubated for 24 h, washed, and infected with HCMV (MOI, 3 to 5). Cells were harvested at 48 hpi, and CAT assays were performed. Acetylated product was extracted using ethyl acetate, spotted onto silica plates, and subjected to thin-layer chromatography. Thin-later chromatography plates were then exposed to film, and data were quantified by measuring levels of incorporated 14C in each sample using a scintillation counter.
Antibodies and Western blotting.
Cultures of fibroblasts were harvested for Western blot analysis in Laemmli sample buffer (Bio-Rad Laboratories, Hercules, Calif.). Cell lysates were boiled and subjected to sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to ImmunoBlot polyvinylidene difluoride membranes (Bio-Rad Laboratories). Equal protein amounts were loaded in each lane. Following transfer, membranes were incubated in a blocking buffer (5% skim milk, 0.1% Tween 20, 1× phosphate-buffered saline), followed by incubation of the primary antibody diluted in blocking buffer. The primary antibodies (monoclonal IκBα [H-4; catalog no. sc-1643], polyclonal IκBβ [C-20; catalog no. sc-945], monoclonal IKKα [B-8; catalog no. sc-7606], monoclonal IKKβ [H-4; catalog no. sc-8014], and polyclonal IKKγ [FL-419; catalog no. sc-8330]) were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). In addition, monoclonal antibodies specific for HCMV IE1 (6E1) and IE2 (12E2) were used to detect these proteins and have been previously described (78, 96). Blots were washed with a 1× phosphate-buffered saline-0.1% Tween 20 solution and incubated with a horseradish peroxidase-conjugated secondary antibody (Amersham Biosciences, Piscataway, N.J.) diluted in blocking buffer. Blots were washed and then developed using the ECL+ system (Amersham Biosciences) according to the manufacturer's protocol.
RNA isolation and reverse transcription-PCR (RT-PCR).
Total cellular RNA from infected fibroblasts was harvested using the Qiagen RNeasy kit (Qiagen, Inc.). RNA samples were reverse transcribed using 400 U of Moloney murine leukemia virus reverse transcriptase (Invitrogen Corp., Carlsbad, Calif.) in 1× reverse transcriptase buffer supplemented with 80 U of RNasin (Promega Corp., Madison, Wis.), 0.1 μg of random hexamers (Invitrogen Corp.)/μl, and 1 mM deoxynucleoside triphosphates (Amersham Biosciences). After incubation at 37°C for 1 h, 1 U of RNase H (Stratagene, La Jolla, Calif.) was added.
RT products were amplified by PCR performed in 1× Thermo Pol buffer (New England BioLabs, Inc., Beverly, Mass.) containing 1.25 U of Deep Vent polymerase (New England Biolabs, Inc.) and a 50 μM concentration of each deoxynucleoside triphosphate. Primers specific for IE1-72 (sense, ACACGATGGAGTCCTCTGCC; antisense, TTCTATGCCGCACCATGTCC [30]; Integrated DNA Technologies, Coralville, Iowa) and IE2-86 (sense, TCCTCCTGCAGTTCGGCTTC; antisense, TTTCATGATATTGCGCACCT [17, 38]; Integrated DNA Technologies) were used to amplify regions of these genes. Following an initial denaturing step at 94°C for 5 min, the cDNA was amplified for 35 cycles (94°C for 1 min, 56°C for 1 min, and 72°C for 1.5 min). PCR products were analyzed by electrophoresis on a 2.5% agarose gel. Equal RNA loading was confirmed by repeating the PCR using glyceraldehyde-3-phosphate dehydrogenase (GAPDH)-specific primers (sense, GAAGGTGAAGGTCGGAGTC; antisense, GAAGATGGTGATGGGATTTC [44]; Integrated DNA Technologies).
GST protein induction and purification.
Plasmids expressing a wild-type IκBα construct fused to a glutathione S-transferase (GST) tag (pGEX-2T GST-wt-IκBα) (52), or a mutated truncated form of IκBα fused to a GST tag (pGEX-2T GST-IκBα 1-54 SS→ AA) (52) used as a negative control, were transformed into competent Escherichia coli BL21 bacteria (both constructs were obtained as a generous gift from John Hiscott, Institut Lady Davis de Recherches Medicales, Montreal, Quebec, Canada). Transformed bacteria were cultured overnight in 2X-YTG medium (1.6% tryptone, 1% yeast extract, 0.5% NaCl, 2% glucose) and pelleted by centrifugation. Pellets were resuspended in 2X-YT medium (1.6% tryptone, 1% yeast extract, 0.5% NaCl), and protein synthesis was induced by the addition of isopropyl-β-d-thiogalactopyranoside (0.25 mM final concentration; Novagen, Madison, Wis.). GST-bound proteins were purified from lysed bacteria using the GST-bind kit (Novagen), following the manufacturer's protocol. Aliquots of purified proteins were subjected to SDS-PAGE and stained with Coomassie blue to determine protein purity and concentration.
Protein kinase assays.
Fibroblasts were grown to confluency, infected with HCMV (MOI, 3 to 5), and harvested at various times postinfection in a detergent lysis buffer (10 mM Tris [pH 7.4], 1.0% Triton X-100, 0.5% Nonidet P-40, 150 mM NaCl) supplemented with protease inhibitor cocktail I and II (Sigma, St. Louis, Mo.) and phosphatase inhibitor cocktail (Sigma), following the manufacturer's specifications. Samples were cleared by centrifugation, and the supernatant was incubated with 3 μg of a polyclonal antibody specific for IKKγ (FL-419; catalog no. sc-8330; Santa Cruz Biotechnology, Inc.) and rocked overnight at 4°C. A 10-μl aliquot of a 50% protein A-protein G-Sepharose bead (Oncogene Research Products, San Diego, Calif.) suspension was then added and, following incubation, immunocomplexes bound to beads were pelleted by centrifugation and washed with kinase buffer (150 mM NaCl, 10 mM Tris [pH 7.4], 10 mM MgCl2, 0.5 mM dithiothreitol) supplemented with protease inhibitor cocktail I and II (Sigma) and phosphatase inhibitor cocktail (Sigma). Pellets were incubated at 30°C for 15 min with 40 μl of the kinase buffer containing 25 μM ATP, 2.5 μCi of [γ-32P]ATP (ICN Biomedicals, Inc., Irvine, Calif.), and the GST-wt-IκBα substrate (or negative control substrate, GST-IκBα 1-54 SS→AA) at a concentration of 1.0 mg/ml. Following incubation, samples were boiled and loaded on an SDS-10% PAGE gel. Gels were then stained with Coomassie blue, dried, and exposed to X-ray film.
NF-κB inhibitory drugs.
Aspirin (acetylsalicylic acid; Sigma) and MG-132 (Z-Leu-Leu-Leu-al; Sigma) were used to inhibit the activation of NF-κB in infected cells. Fibroblasts were treated with Eagle's minimal essential medium supplemented with 4% heat-inactivated fetal bovine serum, penicillin (100 IU/ml), and streptomycin (100 μg/ml) and containing 5 mM aspirin or 50 μM MG-132 for 1 h prior to infection. The medium was then changed, and fresh aspirin or MG-132 was added. The cell cultures were then infected with HCMV (MOI, 3 to 5) for 1 h, followed by replacement of the medium every hour to control for the shortened half-life of the drugs in serum. Cells treated with aspirin and MG-132 were tested for cytotoxicity at the specific concentrations used. At all time points tested, greater than 95% of the cells were viable as determined by trypan blue (Cellgro Mediatech, Inc., Herndon, Va.) exclusion staining. For all experiments containing aspirin and MG-132, HCMV-infected fibroblasts were treated with the drug solvents (1 M Tris [pH 8.0] for aspirin; dimethyl sulfoxide for MG-132), and no changes in IE mRNA or protein expression were detected in the presence of the solvent-alone controls (data not shown).
RESULTS
NF-κB is required for HCMV MIEP transactivation.
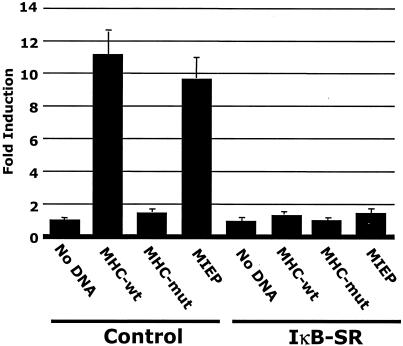
Because our laboratory previously showed that NF-κB was induced very early following HCMV infection (97), we hypothesized that this virus-mediated NF-κB induction drives the transactivation of the MIEP, which contains four NF-κB binding sites (61). To examine a possible direct role for NF-κB in the transactivation of the HCMV MIEP, we performed transfection-infection assays (98) using the dominant-negative IκB construct, the IκB-SR, to prevent NF-κB induction. HEL fibroblasts were cotransfected with the HCMV MIEP-CAT construct (92) along with the IκB-SR (89), followed by infection. The IκB-SR is a mutated form of IκBα in which serines 32 and 36, residues normally phosphorylated by the IKK complex, have been replaced with alanines (89); thus, this construct blocks NF-κB release from IκB and its translocation to the nucleus (13, 14, 19, 27, 86, 89). In the presence of the IκB-SR, transactivation of the MIEP was significantly decreased to less than 2% of the activity observed when the MIEP was cotransfected into cells with the control plasmid (Fig. 1), suggesting that the activation of NF-κB is required for MIEP transactivation. The IκB-SR also significantly decreased the known NF-κB-responsive major histocompatibility complex class I (MHC-wt) promoter, which served as a positive control (41); as a negative control the known NF-κB sites within the MHC promoter were mutated (MHC-mut). A β-galactosidase expression vector was also cotransfected into cells, and the resulting β-galactosidase activity was utilized to control for transfection efficiencies and to normalize CAT assay results. These data suggested that the regulation of the NF-κB/IκB complex plays a critical role in the transactivation of the HCMV MIEP in fibroblasts.
FIG. 1.
Inhibition of NF-κB activity prevents MIEP transactivation. Transfection-infection assays were performed in HEL fibroblasts. Cells were cotransfected with the promoter CAT constructs indicated (MIEP, MHC-wt, and MHC-mut) and the IκB-SR or the control construct and then infected with HCMV (MOI, 3 to 5), and CAT assays were performed on the harvested cell lysates. The MHC-wt promoter was used as a positive control, and a mutated MHC promoter with the NF-κB binding sites mutated (MHC-mut) was used as a negative control. Fold induction represents the difference between the percent acetylation of the test sample and that of the vector-alone control. CAT assays were repeated with similar results.
IκBα and IκBβ protein levels are decreased following HCMV infection.
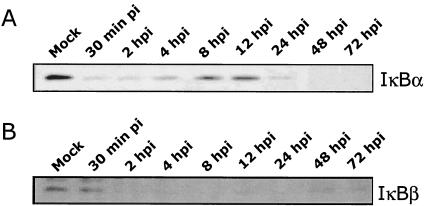
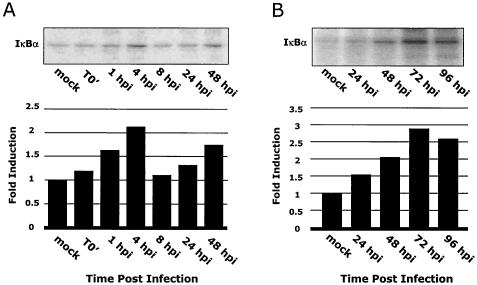
Because the results shown in Fig. 1 suggested that the NF-κB/IκB complex was required for MIEP transactivation, we next investigated virus-mediated changes in the IκB proteins as a mechanism to account for the increase in NF-κB activity. Protein levels of IκBα and IκBβ were monitored by Western blot analysis following a time course of infection in fibroblasts (Fig. 2). Within 30 min of infection, levels of IκBα were reduced to near undetectable levels; IκBα protein levels remained low until 4 to 8 hpi, when increased IκBα levels were observed (Fig. 2A). This de novo IκBα protein synthesis was not surprising, as the IκBα promoter is autoregulated by NF-κB due to the presence of NF-κB binding sites within the IκBα promoter (42). Shortly after this increase, IκBα protein levels again dropped to near undetectable levels, suggesting that HCMV was specifically targeting IκBα for sustained degradation. The decrease of IκBβ protein was delayed compared to that of IκBα, with no changes observed at 30 min postinfection. However, by 2 hpi, IκBβ protein levels were significantly reduced and remained at low levels throughout the time course of infection (Fig. 2B). The observation that HCMV targeted a sustained decrease in both of the primary inhibitors of NF-κB (IκBα and IκBβ) suggests that HCMV infection promotes maximal NF-κB release.
FIG. 2.
HCMV infection induces a decrease in IκBα and IκBβ protein levels. HEL fibroblasts were infected with HCMV (MOI, 3 to 5) and harvested at the time points shown postinfection. Western blot analysis was performed on the harvested cell lysates using a monoclonal antibody specific for IκBα (A) and a polyclonal antibody specific for IκBβ (B). “Mock” represents uninfected fibroblasts. Equal protein was loaded in each lane. Western blot analyses were repeated with similar results.
IKK activity is required for maximal transactivation of the MIEP following HCMV infection.
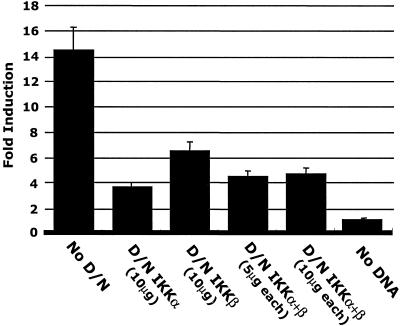
Because of the decrease in IκBα and IκBβ protein levels seen following HCMV infection (Fig. 2), we next examined if HCMV mediated changes in the IKK complex as a mechanism to regulate the NF-κB/IκB complex. To initially investigate the role of HCMV-mediated signaling through the IKK complex in NF-κB activation, we performed transfection-infection assays using the MIEP-CAT construct (92) in the presence of the dominant-negative forms of the catalytic subunits of the IKK complex, IKKα and IKKβ (59).
The results of these experiments (Fig. 3) demonstrated that dominant-negative IKKα or IKKβ constructs, when cotransfected with the MIEP-CAT construct, significantly decreased MIEP transactivation. These results suggested that both of the IKK catalytic subunits are utilized during HCMV infection to promote NF-κB activity and MIEP transactivation. There was a greater decrease in MIEP transactivation when the dominant-negative IKKα (10 μg) was used than when the dominant-negative IKKβ (10 μg) was used (74 versus 56%, respectively), suggesting that IKKα activity plays a larger role than IKKβ activity in NF-κB activation during HCMV infection. Because β-galactosidase activity was utilized to control for transfection efficiency and to normalize CAT assay results, this larger role for IKKα suggests that IKKα homodimers (39, 99), independently of the IKKα/IKKβ heterodimers, could be involved in HCMV-mediated signaling, although this possibility has not yet been investigated. MIEP transactivation was decreased to similar levels when either dominant-negative IKKα alone or both dominant-negative IKKα and dominant-negative IKKβ (5 or 10 μg of each) were cotransfected into cells together. Because the dominant-negative IKK constructs failed to inhibit MIEP transactivation to the same degree as the IκB-SR (74 or 56% decrease in MIEP transactivation in the presence of D/N IKKα or D/N IKKβ, respectively [Fig. 3 ] versus a >98% decrease in MIEP transactivation in the presence of the IκB-SR [Fig. 1]), additional mechanisms of NF-κB activation, such as CK2 (formerly casein kinase II), not involving the IKK pathway could be involved in the remaining promoter activity (5, 52, 56, 72). Nevertheless, the data suggest that IKK activation plays a dominant role in the activation of NF-κB and in the transactivation of the MIEP following HCMV infection of fibroblasts.
FIG. 3.
Dominant-negative IKK constructs block transactivation of the HCMV MIEP. Kinase mutant IKK constructs (D/N IKKα and D/N IKKβ) were cotransfected into HEL fibroblasts along with the HCMV MIEP-CAT construct. Transfected cells were infected with HCMV (MOI, 3 to 5) and harvested after 48 h, and CAT assays were then performed on the collected cell lysates. Fold induction represents the difference between the percent acetylation of the test sample and that of vector-alone controls. CAT assays were repeated with similar results.
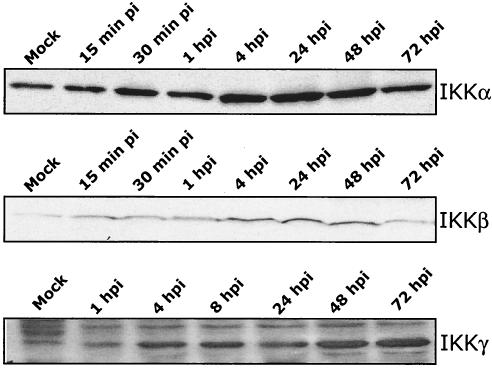
FIG. 4.
HCMV induces an increase in IKK complex (IKKα, IKKβ, and IKKγ) protein levels. HEL fibroblasts were infected with HCMV (MOI, 3 to 5), and cells were harvested at the times indicated postinfection. Western blot analysis was performed using antibodies specific for IKKα, IKKβ, and IKKγ. “Mock” represents uninfected fibroblasts. Equal protein was loaded in each lane. Western blot analyses were repeated with similar results.
Increased protein levels of the IKK complex are detected in HCMV-infected cells.
The involvement of the IKK complex in MIEP transactivation led us to examine if changes in the IKK protein levels occurred during HCMV infection. Total cellular lysates from a time course of infected fibroblasts were harvested and subjected to SDS-PAGE followed by Western blot analysis using antibodies specific for each member of the IKK complex (Fig. 4). Intracellular protein levels of IKKα, IKKβ, and IKKγ all increased upon HCMV infection, suggesting that HCMV increased the protein levels of the entire IKK complex.
IKK function is increased following HCMV infection.
Because IKK activity is mediated at the functional level, we next investigated HCMV-induced changes in IKK activity. In vitro kinase assays using purified GST-IκBα as a substrate were performed on IKK complexes immunoprecipitated from whole-cell lysates using a polyclonal antibody specific for IKKγ (Santa Cruz Biotechnology, Inc.). As a negative control, GST-IκBα containing mutations in the IKK phosphorylation sites was used. An equal amount of IKK protein was used for each sample tested. The results of these studies (Fig. 5) demonstrated that IKK kinase activity increased at least 2.8-fold in response to HCMV infection. IKK activation occurred in a biphasic manner, with the first peak in IKK activity occurring at 4 hpi (Fig. 5A). IKK kinase activity appeared to reach maximal levels at 72 hpi (Fig. 5B).
FIG. 5.
IKK activity increases following HCMV infection. Fibroblasts were infected with HCMV and harvested at the times shown postinfection. The IKK complex was immunoprecipitated using a polyclonal antibody specific for IKKγ from cell lysates harvested at early times postinfection (A) and later times of infection (B). In vitro kinase assays were then performed on purified immunocomplexes using a wild-type GST-IκBα as the substrate (GST-wt-IκBα). An IκBα with the IKK phosphorylation sites mutated (GST-IκBα SS→AA) was used as a negative control. Densitometry analysis was performed on autoradiographs from in vitro kinase assays. Results are presented as the fold induction of kinase activity compared to that in mock-infected cells. T0′ represents time zero. Experiments were repeated with similar results.
NF-κB is required for efficient production of HCMV IE mRNA and protein.
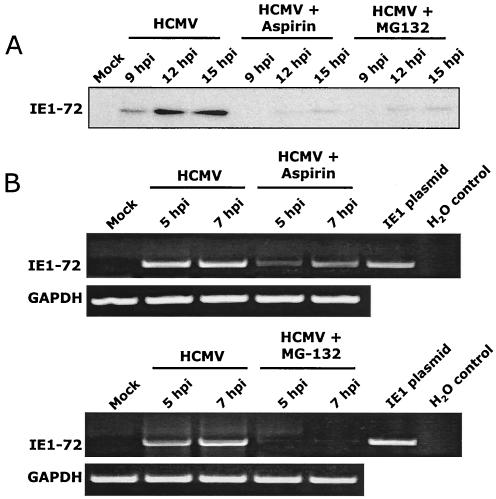
The results from the experiments presented above, along with our group's earlier studies (96-98), suggest that HCMV infection induces NF-κB to drive viral gene expression. Specifically, we hypothesized that HCMV infection induces NF-κB activation to transactivate the MIEP and subsequently induce IE mRNA and protein production. To test this hypothesis, we examined the role that NF-κB plays in the viral life cycle by investigating the levels of IE mRNA and protein expression following treatment with NF-κB inhibitory drugs. We used two drugs widely used to block NF-κB activation: aspirin, a general NF-κB inhibitor (94), and MG-132, a specific proteasome inhibitor (65). Titrations were performed (data not shown) to determine optimal doses of these drugs in our system. Fibroblasts were incubated in the presence of the NF-κB inhibitory drugs for 1 h prior to the addition of virus, and cells were harvested at the times indicated and subjected to Western blot analysis (Fig. 6A) and RT-PCR (Fig. 6B).
FIG. 6.
NF-κB inhibitory drugs block IE gene expression. Cells were treated with aspirin or MG-132 for 1 h prior to infection with HCMV (MOI, 3 to 5). (A) Protein levels were analyzed by Western blot analysis using antibodies specific for IE1-72. Replicate studies were performed using IE2-86-specific reagents with similar results (data not shown). Equal protein was loaded per lane. (B) Steady-state mRNA levels were analyzed by RT-PCR analysis. RT-PCR was performed using primers specific for IE1-72. Equal RNA loading was determined by repeating the PCR using GAPDH-specific primers. Replicate studies were performed using IE2-86-specific reagents with similar results (data not shown). Fibroblasts treated with the drug solvents (1 M Tris [pH 8.0] for aspirin and dimethyl sulfoxide for MG-132) and infected with HCMV were used as controls, which showed no effect due to the solvents (data not shown). Experiments were repeated with similar results.
We first addressed changes in IE1-72 protein levels in response to NF-κB inhibitory drugs. As shown in Fig. 6A, IE1-72 protein levels were decreased by >95%, as determined by Western blot analysis followed by densitometry analysis. Similar results were observed when samples were tested for IE2-86 protein production (data not shown). Next, to determine if the decrease in IE protein expression was regulated at the transcriptional level, we examined changes in steady-state IE mRNA levels by RT-PCR following treatment with the NF-κB inhibitory drugs. As shown in Fig. 6B, aspirin and MG-132 significantly decreased and delayed IE1-72 mRNA expression (>95%). Likewise, IE2-86 mRNA expression was similarly decreased and delayed (data not shown). Mock-treated control cells (treated with only the drug solvents, as stated in Materials and Methods) exhibited normal levels of IE1-72 and IE2-86 mRNA expression as determined by RT-PCR. Primers specific for GAPDH were used to confirm that equal amounts of cDNA were used in each PCR.
Because NF-κB can drive expression of antiapoptotic proteins (reviewed in reference 4) and has been shown to have an antiapoptotic effect following herpes simplex virus type 1 infection (33), cells treated with aspirin or MG-132 were tested for viability by trypan blue dye exclusion staining. No significant decrease in cell viability was observed at any of the time points tested in our experiments (data not shown). Lastly, Western blotting was performed on the harvested lysates at 1 hpi, and the blots were examined for IκBα expression. The control experiments showed that IκBα levels did not change in cells treated with aspirin and MG-132, demonstrating that these drugs do block NF-κB activation in fibroblasts and that the inhibition of NF-κB activity correlates with the decrease in IE expression. Taken together, these results suggest that the viral induction of NF-κB drives the transactivation of the HCMV MIEP in fibroblasts, and they begin to delineate the important role that this transcription factor plays in the HCMV life cycle.
DISCUSSION
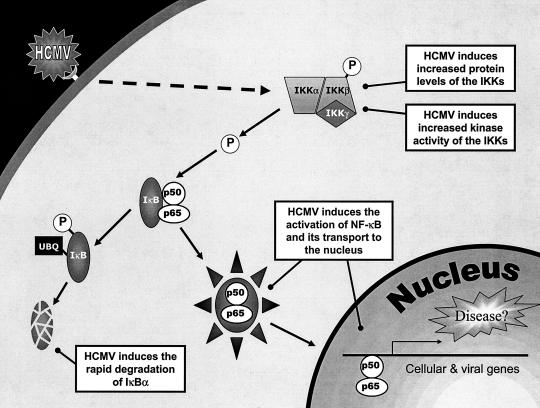
The goal of our present study was to investigate the potential mechanisms by which HCMV infection activates NF-κB and the role that this virus-induced NF-κB plays in the regulation of the HCMV gene cascade. Our data suggest that activation of NF-κB is specifically induced by HCMV to drive transactivation of the HCMV MIEP (summarized in our model in Fig. 7). Together, our results provide strong evidence that NF-κB is a critical player for the initiation of the HCMV gene cascade and, thus, the entire viral life cycle.
FIG. 7.
Model showing the interaction of HCMV with the NF-κB pathway. HCMV infection induces increased levels of the IKK proteins as well as increased IKK kinase activity, leading to a rapid and sustained decrease in IκBα and IκBβ protein levels. This in turn leads to the prolonged activation of NF-κB and transactivation of the HCMV MIEP, resulting in the production of viral transcripts necessary for HCMV replication.
Previously, our investigators showed that NF-κB activation following HCMV infection is biphasic, with one increase initiating within 5 min of viral infection and a second increase observed around 12 hpi (97). Our present studies support this model for NF-κB activation, as the degradation of IκBα following infection followed a similar biphasic pattern (Fig. 2). Our data suggest that the biphasic activation of NF-κB is critical for efficient viral replication, as the first tier of NF-κB activation, due to a receptor-ligand-mediated signaling effect of the HCMV gB and gH glycoproteins (96), serves to prepare the cell for immediate production of viral transcripts, while the second tier of NF-κB activation, which involves the de novo synthesis of new NF-κB molecules, serves to maintain high levels of NF-κB in the infected cell during later times of viral replication (97). We hypothesize that the prolonged activation of NF-κB is important for viral replication, as it would not only transactivate multiple classes of viral promoters but would also serve a protective function by inducing the expression of antiapoptotic genes (4) at later times postinfection. No decrease in cell viability was observed in the presence of NF-κB inhibitors at early times postinfection; however, we are now investigating the protective role of the induced NF-κB in HCMV-infected cells at later times of infection as has been shown for herpes simplex virus infection (33). A possible protective role for NF-κB during HCMV infection is intriguing because of the extended life cycle of the virus seen in vivo, where it can take weeks to complete the viral life cycle (61).
IκBα is generally thought to be the major inhibitor of NF-κB activation; however, our data suggest that both IκBα and IκBβ are involved in the HCMV-mediated activation of NF-κB. IκBβ is thought to be involved in the chronic release of NF-κB (82, 84, 87), and the viral targeting of IκBβ points to a possible mechanism for the sustained NF-κB activity observed in HCMV-infected cells. IκBβ levels are not autoregulated by NF-κB and, thus, the virus-mediated decrease in IκBβ levels early in infection allows the virus to only have to contend with the regulation of IκBα levels during the course of infection. The sustained decrease in IκBβ in response to HCMV infection points to multiple virus-mediated signaling pathways being induced early after infection, because IκBβ degradation has been reported to require two signals (21). Based on our previous studies showing that both gB and gH signal by binding to their cellular receptors (95, 96), it is intriguing to propose that the signaling induced by both HCMV glycoproteins may act in concert to target IκBβ. Because the IκB proteins are rapidly degraded following phosphorylation by the IKKs (19, 27), we propose that similar events occur upon HCMV infection and that the decrease in IκBα and IκBβ levels is due to proteolytic degradation.
MIEP transactivation was decreased to near basal levels in the presence of the IκB-SR; therefore, we hypothesized that a similar decrease in MIEP transactivation would be observed when the dominant-negative forms of IKKα and IKKβ were used. Surprisingly, while MIEP transactivation was significantly decreased in the presence of these dominant-negative IKK proteins, at least 25% of MIEP transactivation remained. These results suggest that additional pathways are involved in the targeting of the NF-κB/IκB complex in response to HCMV infection. While most NF-κB signaling pathways are thought to converge at the level of the IKK complex, additional kinases, such as CK2, have been demonstrated to directly phosphorylate IκBα and induce its degradation, resulting in NF-κB activation (5, 52, 56, 72). Changes in CK2 activity have not yet been examined during HCMV infection, although the promoters of both subunits of CK2 contain Sp1 binding sites (66), which our investigators previously have shown are induced in response to HCMV infection (96). Alternatively, because MIEP activity remained following treatment of the cells with the dominant-negative IKK constructs (Fig. 3), the data could point to the role other transcription factors play in MIEP transactivation. However, because we have shown that the IκB-SR inhibits nearly all MIEP transactivation (Fig. 1), we would argue that NF-κB activity is essential for MIEP activity while the IKK pathway only accounts for a majority of the induced NF-κB activity.
We demonstrated that IKK complex activity is increased by greater than twofold following infection with HCMV (Fig. 5), consistent with previously published reports demonstrating the activation of the IKK complex in response to herpes simplex virus infection (1). It should be noted, however, that this twofold increase in IKK activity underrepresents the true increase in IKK activity, because the IKK kinase assays were performed via immunoprecipitation, with equal molar amounts of IKK being pulled down. A twofold increase in IKK kinase activity for each IKK complex (Fig. 5), combined with the significant increase in IKK protein levels (three to fivefold increase) observed following HCMV infection (Fig. 4), would be expected to result in a substantial increase in overall cellular IKK kinase activity (possibly a true 6- to 10-fold increase) during the course of infection.
HCMV pathogenesis is largely dependent on viral replication (31) and, thus, inhibition of the HCMV life cycle provides an effective means of combating HCMV-related disease. Our data demonstrated that production of HCMV IE protein and mRNA was significantly decreased and delayed in the presence of the NF-κB inhibitory drugs, aspirin and MG-132. Aspirin is a general NF-κB inhibitor whose mechanism of action is not completely understood, but it is thought to inhibit IKKβ (94) as well as other upstream factors (60, 76). MG-132 specifically inhibits the 26S proteasome, thereby preventing the degradation of the IκBs (43). Because both IE1-72 (29, 34, 62) and IE2-86 (54) are required for efficient HCMV replication in vitro, our results suggest that the inhibition of NF-κB activity, and consequently IE expression, would significantly block viral replication. However, by 15 hpi low levels of both IE1-72 and IE2-86 proteins were detected in fibroblasts. Because the MIEP contains multiple transcription factor binding sites (61), it is possible that additional transcription factors are capable of inducing MIEP transactivation at later times postinfection. Alternatively, the increase in IE protein production observed at 15 hpi could be the result of increased toleration of the cells to the drugs used. Aspirin and MG-132, in addition to their NF-κB inhibitory effects, could affect other aspects of cellular physiology. Nevertheless, because we used a combination of approaches to inhibit NF-κB in our system, the results strongly support our proposed model for the importance of NF-κB activation in the transactivation of the HCMV MIEP and suggest that MIEP regulation could be a potential target for therapeutic intervention.
The aberrant regulation of the NF-κB regulatory pathway by HCMV may provide important clues to the mechanisms of viral pathology. NF-κB is central to the inflammatory response (reviewed in reference 88), and inflammation is a key factor in HCMV-mediated diseases, including atherosclerosis (22, 51). Independent studies have demonstrated an increased prevalence of atherosclerosis in individuals infected with HCMV (7, 35, 53, 77, 102), and NF-κB is activated in tissues from atherosclerotic lesions in contrast to control nonatherosclerotic tissue (10, 68, 90). Because many of the genes that are found elevated in atherosclerotic plaques (including those encoding proinflammatory cytokines, chemotactic proteins, and cell adhesion molecules) are regulated by NF-κB (25), our study provides a molecular model that is consistent with HCMV's proposed role in atherosclerotic disease. In addition, HCMV is the leading viral cause of congenital birth defects in the United States (12, 55). Interestingly, aberrant regulation of members of the NF-κB activation pathway has also been shown to be responsible for congenital deformities (reviewed in reference 2), and deficiencies in the IKK proteins result in severe birth defects (6, 16, 36, 37, 45, 50, 73, 74, 83, 103). Because, as we have reported here, IKK complex protein levels and activity are dysregulated in response to HCMV infection, it is possible that HCMV-mediated alterations in these important developmental proteins may contribute to the congenital deformities observed during prenatal HCMV infection.
Because aberrantly high levels of NF-κB activation have deleterious cellular effects (3, 32, 64, 71, 101) as discussed above, it is likely that HCMV also encodes proteins to inhibit the activation of NF-κB in order to maintain NF-κB activation at levels that are advantageous for viral replication yet that would allow the maintenance of a healthy cell. A recent study by Browne and Shenk suggested that the major HCMV tegument protein, pp65, functions to inhibit NF-κB activation for just this purpose (15), suggesting that a combination of HCMV-mediated mechanisms is required to maintain a fine balance of NF-κB activation in infected cells.
Collectively, our results provide a molecular understanding of the central role that NF-κB plays in the initiation of the viral life cycle. Because HCMV pathogenesis is related to the ability of the virus to replicate (31) and because the MIEP drives viral replication, our study identifies the NF-κB pathway as a potentially attractive target for the development of novel antiviral therapies to combat HCMV disease.
Acknowledgments
This work was supported in part by research grant 1-FYO1-332 from the March of Dimes Birth Defects Foundation, by research grant 0160239B from the American Heart Association, and by research grant 1-P20-RR018724-01 from the National Institutes of Health.
We thank Angela DeMeritt and Rona Scott for helpful discussions and careful reading of the manuscript.
REFERENCES
- 1.Amici, C., G. Belardo, A. Rossi, and M. G. Santoro. 2001. Activation of IκB kinase by herpes simplex virus type 1. A novel target for anti-herpetic therapy. J. Biol. Chem. 276:28759-28766. [DOI] [PubMed] [Google Scholar]
- 2.Aradhya, S., and D. L. Nelson. 2001. NF-κB signaling and human disease. Curr. Opin. Genet. Dev. 11:300-306. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin, A. S., Jr. 1996. The NF-κB and IκB proteins: new discoveries and insights. Annu. Rev. Immunol. 14:649-683. [DOI] [PubMed] [Google Scholar]
- 4.Barkett, M., and T. D. Gilmore. 1999. Control of apoptosis by Rel/NF-κB transcription factors. Oncogene 18:6910-6924. [DOI] [PubMed] [Google Scholar]
- 5.Barroga, C. F., J. K. Stevenson, E. M. Schwarz, and I. M. Verma. 1995. Constitutive phosphorylation of IκBα by casein kinase II. Proc. Natl. Acad. Sci. USA 92:7637-7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bell, S., K. Degitz, M. Quirling, N. Jilg, S. Page, and K. Brand. 2003. Involvement of NF-κB signalling in skin physiology and disease. Cell. Signal. 15:1-7. [DOI] [PubMed] [Google Scholar]
- 7.Blum, A., M. Giladi, M. Weinberg, G. Kaplan, H. Pasternack, S. Laniado, and H. Miller. 1998. High anti-cytomegalovirus (CMV) IgG antibody titer is associated with coronary artery disease and may predict post-coronary balloon angioplasty restenosis. Am. J. Cardiol. 81:866-868. [DOI] [PubMed] [Google Scholar]
- 8.Boshart, M., F. Weber, G. Jahn, K. Dorsch-Hasler, B. Fleckenstein, and W. Schaffner. 1985. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell 41:521-530. [DOI] [PubMed] [Google Scholar]
- 9.Boyle, K. A., R. L. Pietropaolo, and T. Compton. 1999. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol. Cell. Biol. 19:3607-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brand, K., S. Page, G. Rogler, A. Bartsch, R. Brandl, R. Knuechel, M. Page, C. Kaltschmidt, P. A. Baeuerle, and D. Neumeier. 1996. Activated transcription factor nuclear factor-κB is present in the atherosclerotic lesion. J. Clin. Investig. 97:1715-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bresnahan, W. A., G. E. Hultman, and T. Shenk. 2000. Replication of wild-type and mutant human cytomegalovirus in life-extended human diploid fibroblasts. J. Virol. 74:10816-10818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 13.Brockman, J. A., D. C. Scherer, T. A. McKinsey, S. M. Hall, X. Qi, W. Y. Lee, and D. W. Ballard. 1995. Coupling of a signal response domain in IκBα to multiple pathways for NF-κB activation. Mol. Cell. Biol. 15:2809-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown, K., S. Gerstberger, L. Carlson, G. Franzoso, and U. Siebenlist. 1995. Control of IκBα proteolysis by site-specific, signal-induced phosphorylation. Science 267:1485-1488. [DOI] [PubMed] [Google Scholar]
- 15.Browne, E. P., and T. Shenk. 2003. Human cytomegalovirus UL83-coded pp65 virion protein inhibits antiviral gene expression in infected cells. Proc. Natl. Acad. Sci. USA 100:11439-11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bushdid, P. B., D. M. Brantley, F. E. Yull, G. L. Blaeuer, L. H. Hoffman, L. Niswander, and L. D. Kerr. 1998. Inhibition of NF-κB activity results in disruption of the apical ectodermal ridge and aberrant limb morphogenesis. Nature 392:615-618. [DOI] [PubMed] [Google Scholar]
- 17.Chang, M. H., H. H. Huang, E. S. Huang, C. L. Kao, H. Y. Hsu, and C. Y. Lee. 1992. Polymerase chain reaction to detect human cytomegalovirus in livers of infants with neonatal hepatitis. Gastroenterology 103:1022-1025. [DOI] [PubMed] [Google Scholar]
- 18.Chang, S. F., M. F. Chao, S. L. Yang, G. M. Lin, W. W. Chang, C. W. Wu, M. S. Yen, H. T. Ng, J. C. Thomas, and C. Y. Shen. 1993. High rate of concurrent genital infections with human cytomegalovirus and human papillomaviruses in cervical cancer patients. J. Med. Virol. 41:24-29. [DOI] [PubMed] [Google Scholar]
- 19.Chen, Z., J. Hagler, V. J. Palombella, F. Melandri, D. Scherer, D. Ballard, and T. Maniatis. 1995. Signal-induced site-specific phosphorylation targets IκBα to the ubiquitin-proteasome pathway. Genes Dev. 9:1586-1597. [DOI] [PubMed] [Google Scholar]
- 20.Cherrington, J. M., and E. S. Mocarski. 1989. Human cytomegalovirus IE1 transactivates the alpha promoter-enhancer via an 18-base-pair repeat element. J. Virol. 63:1435-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheshire, J. L., and A. S. Baldwin, Jr. 1997. Synergistic activation of NF-κB by tumor necrosis factor alpha and gamma interferon via enhanced IκBα degradation and de novo IκBβ degradation. Mol. Cell. Biol. 17:6746-6754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cinatl, J., Jr., J. U. Vogel, R. Kotchetkov, M. Scholz, and H. W. Doerr. 1999. Proinflammatory potential of cytomegalovirus infection. Specific inhibition of cytomegalovirus immediate-early expression in combination with antioxidants as a novel treatment strategy? Intervirology 42:419-424. [DOI] [PubMed] [Google Scholar]
- 23.Cobbs, C. S., L. Harkins, M. Samanta, G. Y. Gillespie, S. Bharara, P. H. King, L. B. Nabors, C. G. Cobbs, and W. J. Britt. 2002. Human cytomegalovirus infection and expression in human malignant glioma. Cancer Res. 62:3347-3350. [PubMed] [Google Scholar]
- 24.Cohen, L., W. J. Henzel, and P. A. Baeuerle. 1998. IKAP is a scaffold protein of the IκB kinase complex. Nature 395:292-296. [DOI] [PubMed] [Google Scholar]
- 25.Collins, T., and M. I. Cybulsky. 2001. NF-κB: pivotal mediator or innocent bystander in atherogenesis? J. Clin. Investig. 107:255-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delhase, M., M. Hayakawa, Y. Chen, and M. Karin. 1999. Positive and negative regulation of IκB kinase activity through IKKβ subunit phosphorylation. Science 284:309-313. [DOI] [PubMed] [Google Scholar]
- 27.DiDonato, J., F. Mercurio, C. Rosette, J. Wu-Li, H. Suyang, S. Ghosh, and M. Karin. 1996. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol. Cell. Biol. 16:1295-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 29.Gawn, J. M., and R. F. Greaves. 2002. Absence of IE1 p72 protein function during low-multiplicity infection by human cytomegalovirus results in a broad block to viral delayed-early gene expression. J. Virol. 76:4441-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerna, G., D. Zipeto, E. Percivalle, M. Parea, M. G. Revello, R. Maccario, G. Peri, and G. Milanesi. 1992. Human cytomegalovirus infection of the major leukocyte subpopulations and evidence for initial viral replication in polymorphonuclear leukocytes from viremic patients. J. Infect. Dis. 166:1236-1244. [DOI] [PubMed] [Google Scholar]
- 31.Ghazal, P., M. Messerle, K. Osborn, and A. Angulo. 2003. An essential role of the enhancer for murine cytomegalovirus in vivo growth and pathogenesis. J. Virol. 77:3217-3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gilmore, T. D., M. Koedood, K. A. Piffat, and D. W. White. 1996. Rel/NF-κB/IκB proteins and cancer. Oncogene 13:1367-1378. [PubMed] [Google Scholar]
- 33.Goodkin, M. L., A. T. Ting, and J. A. Blaho. 2003. NF-κB is required for apoptosis prevention during herpes simplex virus type 1 infection. J. Virol. 77:7261-7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greaves, R. F., and E. S. Mocarski. 1998. Defective growth correlates with reduced accumulation of a viral DNA replication protein after low-multiplicity infection by a human cytomegalovirus IE1 mutant. J. Virol. 72:366-379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hendrix, M. G., M. M. Salimans, C. P. van Boven, and C. A. Bruggeman. 1990. High prevalence of latently present cytomegalovirus in arterial walls of patients suffering from grade III atherosclerosis. Am. J. Pathol. 136:23-28. [PMC free article] [PubMed] [Google Scholar]
- 36.Hu, Y., V. Baud, M. Delhase, P. Zhang, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science 284:316-320. [DOI] [PubMed] [Google Scholar]
- 37.Hu, Y., V. Baud, T. Oga, K. I. Kim, K. Yoshida, and M. Karin. 2001. IKKα controls formation of the epidermis independently of NF-κB. Nature 410:710-714. [DOI] [PubMed] [Google Scholar]
- 38.Huang, E. S., and T. F. Kowalik. 1993. Diagnosis of human cytomegalovirus infection: laboratory approach, p. 225-256. In Y. Becker and G. Darai (ed.), Molecular aspects of human cytomegalovirus disease. Springer-Verlag, Berlin, Germany.
- 39.Huynh, Q. K., H. Boddupalli, S. A. Rouw, C. M. Koboldt, T. Hall, C. Sommers, S. D. Hauser, J. L. Pierce, R. G. Combs, B. A. Reitz, J. A. Diaz-Collier, R. A. Weinberg, B. L. Hood, B. F. Kilpatrick, and C. S. Tripp. 2000. Characterization of the recombinant IKK1/IKK2 heterodimer. Mechanisms regulating kinase activity. J. Biol. Chem. 275:25883-25891. [DOI] [PubMed] [Google Scholar]
- 40.Isomura, H., and M. F. Stinski. 2003. The human cytomegalovirus major immediate-early enhancer determines the efficiency of immediate-early gene transcription and viral replication in permissive cells at low multiplicity of infection. J. Virol. 77:3602-3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Israel, A., O. Le Bail, D. Hatat, J. Piette, M. Kieran, F. Logeat, D. Wallach, M. Fellous, and P. Kourilsky. 1989. TNF stimulates expression of mouse MHC class I genes by inducing an NF-κB-like enhancer binding activity which displaces constitutive factors. EMBO J. 8:3793-3800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ito, C. Y., A. G. Kazantsev, and A. S. Baldwin, Jr. 1994. Three NF-κB sites in the IκB-α promoter are required for induction of gene expression by TNF-α. Nucleic Acids Res. 22:3787-3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jensen, T. J., M. A. Loo, S. Pind, D. B. Williams, A. L. Goldberg, and J. R. Riordan. 1995. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell 83:129-135. [DOI] [PubMed] [Google Scholar]
- 44.Kanai, Y., S. Ushijima, Y. Nakanishi, and S. Hirohashi. 1999. Reduced mRNA expression of the DNA demethylase, MBD2, in human colorectal and stomach cancers. Biochem. Biophys. Res. Commun. 264:962-966. [DOI] [PubMed] [Google Scholar]
- 45.Kanegae, Y., A. T. Tavares, J. C. Izpisua Belmonte, and I. M. Verma. 1998. Role of Rel/NF-κB transcription factors during the outgrowth of the vertebrate limb. Nature 392:611-614. [DOI] [PubMed] [Google Scholar]
- 46.Karin, M. 1999. How NF-κB is activated: the role of the IκB kinase (IKK) complex. Mol. Cell. Biol. 19:4547-4551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 48.Klemola, E., and L. Kaariainen. 1965. Cytomegalovirus as a possible cause of a disease resembling infectious mononucleosis. Br. Med. J. 5470:1099-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowalik, T. F., B. Wing, J. S. Haskill, J. C. Azizkhan, A. S. Baldwin, Jr., and E. S. Huang. 1993. Multiple mechanisms are implicated in the regulation of NF-κB activity during human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 90:1107-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li, Q., D. Van Antwerp, F. Mercurio, K. F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 51.Libby, P. 2002. Inflammation in atherosclerosis. Nature 420:868-874. [DOI] [PubMed] [Google Scholar]
- 52.Lin, R., P. Beauparlant, C. Makris, S. Meloche, and J. Hiscott. 1996. Phosphorylation of IκBα in the C-terminal PEST domain by casein kinase II affects intrinsic protein stability. Mol. Cell. Biol. 16:1401-1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Maisch, B., U. Schonian, M. Crombach, I. Wendl, C. Bethge, M. Herzum, and H. H. Klein. 1993. Cytomegalovirus associated inflammatory heart muscle disease. Scand. J. Infect. Dis. Suppl. 88:135-148. [PubMed] [Google Scholar]
- 54.Marchini, A., H. Liu, and H. Zhu. 2001. Human cytomegalovirus with IE-2 (UL122) deleted fails to express early lytic genes. J. Virol. 75:1870-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marshall, E. 2002. Viral threat to newborns under radar. Science 295:1631. [DOI] [PubMed] [Google Scholar]
- 56.McElhinny, J. A., S. A. Trushin, G. D. Bren, N. Chester, and C. V. Paya. 1996. Casein kinase II phosphorylates IκBα at S-283, S-289, S-293, and T-291 and is required for its degradation. Mol. Cell. Biol. 16:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Meier, J. L., and J. A. Pruessner. 2000. The human cytomegalovirus major immediate-early distal enhancer region is required for efficient viral replication and immediate-early gene expression. J. Virol. 74:1602-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melnick, J. L., E. Adam, and M. E. DeBakey. 1995. Cytomegalovirus and atherosclerosis. Bioessays 17:899-903. [DOI] [PubMed] [Google Scholar]
- 59.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, A. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 60.Mitchell, J. A., M. Saunders, P. J. Barnes, R. Newton, and M. G. Belvisi. 1997. Sodium salicylate inhibits cyclo-oxygenase-2 activity independently of transcription factor (nuclear factor κB) activation: role of arachidonic acid. Mol. Pharmacol. 51:907-912. [DOI] [PubMed] [Google Scholar]
- 61.Mocarski, E. S. 1996. Cytomegalovirus and their replication, p. 2447-2492. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 62.Mocarski, E. S., G. W. Kemble, J. M. Lyle, and R. F. Greaves. 1996. A deletion mutant in the human cytomegalovirus gene encoding IE1(491aa) is replication defective due to a failure in autoregulation. Proc. Natl. Acad. Sci. USA 93:11321-11326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O'Mahony, A., X. Lin, R. Geleziunas, and W. C. Greene. 2000. Activation of the heterodimeric IκB kinase α (IKKα)-IKKβ complex is directional: IKKα regulates IKKβ under both basal and stimulated conditions. Mol. Cell. Biol. 20:1170-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.O'Neill, L. A., and C. Kaltschmidt. 1997. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 20:252-258. [DOI] [PubMed] [Google Scholar]
- 65.Palombella, V. J., O. J. Rando, A. L. Goldberg, and T. Maniatis. 1994. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell 78:773-785. [DOI] [PubMed] [Google Scholar]
- 66.Pyerin, W., and K. Ackermann. 2001. Transcriptional coordination of the genes encoding catalytic (CK2α) and regulatory (CK2β) subunits of human protein kinase CK2. Mol. Cell. Biochem. 227:45-57. [PubMed] [Google Scholar]
- 67.Ren, H., A. Schmalstieg, N. S. van Oers, and R. B. Gaynor. 2002. I-κB kinases α and β have distinct roles in regulating murine T cell function. J. Immunol. 168:3721-3731. [DOI] [PubMed] [Google Scholar]
- 68.Ritchie, M. E. 1998. Nuclear factor-κB is selectively and markedly activated in humans with unstable angina pectoris. Circulation 98:1707-1713. [DOI] [PubMed] [Google Scholar]
- 69.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKK-γ is an essential regulatory subunit of the IκB kinase complex. Nature 395:297-300. [DOI] [PubMed] [Google Scholar]
- 70.Sambucetti, L. C., J. M. Cherrington, G. W. Wilkinson, and E. S. Mocarski. 1989. NF-κB activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 8:4251-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schmidt-Ullrich, R., T. Aebischer, J. Hulsken, W. Birchmeier, U. Klemm, and C. Scheidereit. 2001. Requirement of NF-κB/Rel for the development of hair follicles and other epidermal appendices. Development 128:3843-3853. [DOI] [PubMed] [Google Scholar]
- 72.Schwarz, E. M., D. Van Antwerp, and I. M. Verma. 1996. Constitutive phosphorylation of IκBα by casein kinase II occurs preferentially at serine 293: requirement for degradation of free IκBα. Mol. Cell. Biol. 16:3554-3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seitz, C. S., H. Deng, K. Hinata, Q. Lin, and P. A. Khavari. 2000. Nuclear factor κB subunits induce epithelial cell growth arrest. Cancer Res. 60:4085-4092. [PubMed] [Google Scholar]
- 74.Seitz, C. S., Q. Lin, H. Deng, and P. A. Khavari. 1998. Alterations in NF-κB function in transgenic epithelial tissue demonstrate a growth inhibitory role for NF-κB. Proc. Natl. Acad. Sci. USA 95:2307-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simmen, K. A., J. Singh, B. G. Luukkonen, M. Lopper, A. Bittner, N. E. Miller, M. R. Jackson, T. Compton, and K. Fruh. 2001. Global modulation of cellular transcription by human cytomegalovirus is initiated by viral glycoprotein B. Proc. Natl. Acad. Sci. USA 98:7140-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith, W. L., D. L. DeWitt, and E. A. Meade. 1993. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J. Biol. Chem. 268:6610-6614. [PubMed] [Google Scholar]
- 77.Sorlie, P. D., F. J. Nieto, E. Adam, A. R. Folsom, E. Shahar, and M. Massing. 2000. A prospective study of cytomegalovirus, herpes simplex virus 1, and coronary heart disease: the atherosclerosis risk in communities (ARIC) study. Arch. Intern. Med. 160:2027-2032. [DOI] [PubMed] [Google Scholar]
- 78.Speir, E., R. Modali, E. S. Huang, M. B. Leon, F. Shawl, T. Finkel, and S. E. Epstein. 1994. Potential role of human cytomegalovirus and p53 interaction in coronary restenosis. Science 265:391-394. [DOI] [PubMed] [Google Scholar]
- 79.Stenberg, R. M. 1996. The human cytomegalovirus major immediate-early gene. Intervirology 39:343-349. [DOI] [PubMed] [Google Scholar]
- 80.Stevenson, K., and J. C. Macnab. 1989. Cervical carcinoma and human cytomegalovirus. Biomed. Pharmacother. 43:173-176. [DOI] [PubMed] [Google Scholar]
- 81.Streblow, D. N., S. L. Orloff, and J. A. Nelson. 2001. Do pathogens accelerate atherosclerosis? J. Nutr. 131:2798S-2804S. [DOI] [PubMed] [Google Scholar]
- 82.Suyang, H., R. Phillips, I. Douglas, and S. Ghosh. 1996. Role of unphosphorylated, newly synthesized IκBβ in persistent activation of NF-κB. Mol. Cell. Biol. 16:5444-5449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Takeda, K., O. Takeuchi, T. Tsujimura, S. Itami, O. Adachi, T. Kawai, H. Sanjo, K. Yoshikawa, N. Terada, and S. Akira. 1999. Limb and skin abnormalities in mice lacking IKKα. Science 284:313-316. [DOI] [PubMed] [Google Scholar]
- 84.Thompson, J. E., R. J. Phillips, H. Erdjument-Bromage, P. Tempst, and S. Ghosh. 1995. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell 80:573-582. [DOI] [PubMed] [Google Scholar]
- 85.Thomsen, D. R., R. M. Stenberg, W. F. Goins, and M. F. Stinski. 1984. Promoter-regulatory region of the major immediate early gene of human cytomegalovirus. Proc. Natl. Acad. Sci. USA 81:659-663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Traenckner, E. B., H. L. Pahl, T. Henkel, K. N. Schmidt, S. Wilk, and P. A. Baeuerle. 1995. Phosphorylation of human IκB-α on serines 32 and 36 controls IκB-α proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 14:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tran, K., M. Merika, and D. Thanos. 1997. Distinct functional properties of IκBα and IκBβ. Mol. Cell. Biol. 17:5386-5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valen, G., Z. Q. Yan, and G. K. Hansson. 2001. Nuclear factor κB and the heart. J. Am. Coll. Cardiol. 38:307-314. [DOI] [PubMed] [Google Scholar]
- 89.Wang, C. Y., M. W. Mayo, and A. S. Baldwin, Jr. 1996. TNF- and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274:784-787. [DOI] [PubMed] [Google Scholar]
- 90.Wilson, S. H., P. J. Best, W. D. Edwards, D. R. Holmes, Jr., P. J. Carlson, D. S. Celermajer, and A. Lerman. 2002. Nuclear factor-κB immunoreactivity is present in human coronary plaque and enhanced in patients with unstable angina pectoris. Atherosclerosis 160:147-153. [DOI] [PubMed] [Google Scholar]
- 91.Woronicz, J. D., X. Gao, Z. Cao, M. Rothe, and D. V. Goeddel. 1997. IκB kinase-β: NF-κB activation and complex formation with IκB kinase-α and NIK. Science 278:866-869. [DOI] [PubMed] [Google Scholar]
- 92.Wu, G. J., E. S. Huang, F. Y. Wu, C. W. Wu, and C. Y. Yuo. 1992. Stable expression of functional human cytomegalovirus immediate-early proteins IE1 and IE2 in HeLa cells. Intervirology 34:94-104. [DOI] [PubMed] [Google Scholar]
- 93.Yamamoto, Y., M. J. Yin, and R. B. Gaynor. 2000. IκB kinase α (IKKα) regulation of IKKβ kinase activity. Mol. Cell. Biol. 20:3655-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yin, M. J., Y. Yamamoto, and R. B. Gaynor. 1998. The anti-inflammatory agents aspirin and salicylate inhibit the activity of IκB kinase-β. Nature 396:77-80. [DOI] [PubMed] [Google Scholar]
- 95.Yurochko, A. D., and E. S. Huang. 1999. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J. Immunol. 162:4806-4816. [PubMed] [Google Scholar]
- 96.Yurochko, A. D., E. S. Hwang, L. Rasmussen, S. Keay, L. Pereira, and E. S. Huang. 1997. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J. Virol. 71:5051-5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yurochko, A. D., T. F. Kowalik, S. M. Huong, and E. S. Huang. 1995. Human cytomegalovirus upregulates NF-κB activity by transactivating the NF-κB p105/p50 and p65 promoters. J. Virol. 69:5391-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yurochko, A. D., M. W. Mayo, E. E. Poma, A. S. Baldwin, Jr., and E. S. Huang. 1997. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-κB promoters. J. Virol. 71:4638-4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zandi, E., Y. Chen, and M. Karin. 1998. Direct phosphorylation of IκB by IKKα and IKKβ: discrimination between free and NF-κB-bound substrate. Science 281:1360-1363. [DOI] [PubMed] [Google Scholar]
- 100.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 101.Zawia, N. H., R. Sharan, M. Brydie, T. Oyama, and T. Crumpton. 1998. Sp1 as a target site for metal-induced perturbations of transcriptional regulation of developmental brain gene expression. Brain Res. Dev. Brain Res. 107:291-298. [DOI] [PubMed] [Google Scholar]
- 102.Zhu, J., G. M. Shearer, J. E. Norman, L. A. Pinto, F. M. Marincola, A. Prasad, M. A. Waclawiw, G. Csako, A. A. Quyyumi, and S. E. Epstein. 2000. Host response to cytomegalovirus infection as a determinant of susceptibility to coronary artery disease: sex-based differences in inflammation and type of immune response. Circulation 102:2491-2496. [DOI] [PubMed] [Google Scholar]
- 103.Zonana, J., M. E. Elder, L. C. Schneider, S. J. Orlow, C. Moss, M. Golabi, S. K. Shapira, P. A. Farndon, D. W. Wara, S. A. Emmal, and B. M. Ferguson. 2000. A novel X-linked disorder of immune deficiency and hypohidrotic ectodermal dysplasia is allelic to incontinentia pigmenti and due to mutations in IKK-γ (NEMO). Am. J. Hum. Genet. 67:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]