Abstract
Background
Effective screening and prevention strategies for bladder cancer require accurate risk stratification models. We developed models to predict the risk of bladder cancer based on clinical and socio-demographic data on participants in the Prostate, Lung, Colon, Ovarian Cancer (PLCO) screening trial.
Methods
Baseline clinical and socio-demographic data were obtained from 149,542 PLCO participants aged 55-74 without a prior history of bladder cancer. Cox proportional hazards models were used to predict the risk of all (ABC) and of high-grade bladder cancers (HGBC) from baseline information. We used the HGBC risk model to design a hypothetical bladder cancer mortality prevention trial.
Results
Over a median follow-up of 12 years, 1124 men and 259 women developed bladder cancer (including 392 and 72 with HGBC, respectively). The incidence in men and in women was 133.6 and 29.6 cases per 100,000 person-years, respectively. Nomograms constructed for predicting the risk of ABC and HGBC had c-indices of 0.746 and 0.759, respectively. Age, race, education, smoking (intensity and duration), co-morbidity, prostatitis, syphilis, and hormone replacement therapy use were statistically significant predictors in the models. We show that our risk model can be used to design a BC mortality prevention trial half the size of a trial designed without risk stratification.
Conclusion
Models to predict the risk of ABC and HGBC have been developed and validated.
Impact
Using the upper 40th percentile from the HGBC model, a suitable cohort for a screening or chemoprevention trial could be identified, although the size and follow-up of such a trial would be costly.
Keywords: urinary bladder neoplasms, prevention and control, nomograms, prediction models, early detection of cancer, cancer screening test
INTRODUCTION
In 2013, an estimated 73,510 Americans will be diagnosed with bladder cancer and 14,880 will die making it the 10th leading cause of cancer deaths in the United States (1, 2). Approximately, 20-30% of bladder cancers are high-grade cancers and these account for nearly all cases of invasive disease and the vast majority of bladder cancer deaths. The mortality-to-incidence ratio for high-grade bladder cancer (HGBC) approaches that of lung cancer (0.25 and 0.5 deaths per new case, respectively) (1). The survival of patients with metastatic and invasive bladder cancer has largely remained unchanged over the last 3 decades despite improvements in systemic and local therapy (3). However, the outcomes of non-muscle-invasive HGBC are nowadays more favorable with either radical therapy or appropriate bladder preservation strategies utilizing transurethral resection and intravesical therapy. Early treatment or prevention of HGBC before the onset of invasive/metastatic disease has the potential to reduce the morbidity and mortality of bladder cancer.
The development of screening and chemoprevention strategies for bladder cancer is limited, in part, by its relatively low incidence in the general population. Several risk factors for bladder cancer have been identified, including cigarette smoking, exposure to arsenic, exposure to industrial chemicals, exposure to cyclophosphamide, pelvic radiation therapy, and chronic inflammatory conditions in the bladder (4-6). The incorporation of these and other clinical and sociodemographic parameters into prediction models for bladder cancer and HGBC may assist in the identification of patient cohorts of sufficiently elevated risk suitable for enrollment in screening and/or chemoprevention trials.
We developed nomograms to predict the risk of all bladder cancer (ABC) and of high-grade bladder cancer (HGBC) based on readily available clinical and sociodemographic information. Such nomograms based on clinical and sociodemographic parameters would serve as base models with which to evaluate the empiric prognostic utility of new potential biomarkers of bladder cancer risk. For this purpose we used models to predict the incidence of bladder cancer fitted to data from the prospective cohort nested within the Prostate, Lung, Colorectal, and Ovarian Cancer (PLCO) screening trial. We used the HGBC risk model to design a hypothetical bladder cancer mortality screening trial that would enroll individuals at elevated risk of HGBC based upon assumptions of mortality benefit due to screening.
MATERIALS AND METHODS
Participants
The design of PLCO trial has been described previously (7). Enrollment of 154,900 men and women aged 55 through 74 years without prior history of prostate, lung, colorectal or ovarian cancer was initiated in 1993 and completed in 2001 at 10 screening centers nationwide. Participants were followed until December 31st 2008. Our study cohort consisted of 149,542 participants without a prior history of bladder cancer at randomization and with complete baseline information and non-missing data on the incidence of bladder cancer at end of follow-up. Each PLCO center obtained annual local institutional review board approval to conduct the study, and all participants provided written informed consent. The recruitment process targeted individuals from the general population residing in the catchment area of each center. The principal recruitment strategy was mass mailing. At study entry, participants completed a self-administered baseline questionnaire inquiring about demographic and social information, medical history, smoking history and past screenings. All incident cancers diagnosed and deaths on-study were ascertained primarily by means of a mailed annual study update questionnaire which asked about survival status and type and date of any cancers diagnosed in the prior year. Participants who did not return the questionnaire were contacted by repeat mailing or telephone. To enhance the completeness of end point verification, the active follow-up was supplemented by periodic linkage to the National Death index. Time on study was censored at a maximum follow-up of 13 years as a majority of participants had complete follow-up to 13 years by December 31st, 2008. Staging and grading of bladder cancer was based on the American Joint Committee on Cancer classification.
Statistical Analysis
We computed incidence rates of ABC and of HGBC, by gender and according to smoking history. In our study, HGBC was defined as the presence of high-grade disease. Time on study was calculated as the time elapsed between completion of the baseline questionnaire and incident disease, death or end of follow-up. We modeled the incidence of ABC and the incidence of HGBC in two separate Cox proportional hazards regression analyses. Because we wanted to allow for the inclusion of effects nested within gender we incorporated gender-specific baseline hazards. Covariates analyzed in the model included well known risk factors: duration and intensity (usual packs per day) of smoking, age, race (white/black/other), personal history of any cancer, family history of bladder, renal and other cancers. The use of gender specific baseline hazards allowed us to include gender specific variables as nested effects. In men: nocturia (times per night and age at first occurrence), BPH, prostatitis, history of prostate surgery, a history of syphilis, and a history of gonorrhea (8); and in women: gravity, use of birth control and use of hormone replacement therapy (HRT), due to the known the connection between female hormones and cancer. Models also included adjustment for educational level (high-school graduate, less than high-school, more than high-school), years since quitting smoking, number of co-morbidities (0, 1, > 1), use of aspirin and use of ibuprofen. The full model included up to cubic spline terms in each continuous covariate (age, BMI, smoking duration and years since quitting). Model selection for covariates to enter into the final model was done in a data-dependent manner in a single pass using a threshold p-value of 0.20. We computed concordance indices, corrected for internal validation bias, by first computing a fully internally validated model concordance using an identical validation set and training set, and then correcting the upward bias in measures of validity following the bootstrap procedure of Efron (9). We constructed nomograms for 5 year incidence of ABC and of HGBC by gender by computing the predicted risk from the Cox regression models and then applying the bias correction factor.
Development of Hypothetical Bladder Cancer Mortality Screening Trial: Statistical Considerations
We used our validated model for risk of HGBC to design a hypothetical bladder cancer mortality prevention trial in the following way. The model would be used to identify individuals in a given upper percentile of HGBC risk for randomization into the trial. By selecting from among choices for risk threshold, statistical power, magnitude of expected benefit and duration of the trial, we devised a panel of prospective designs. For each set of choices, the expected number of HGBC deaths prevented was computed from the expected mortality benefit and the statistical power using information on post HGBC diagnostic 5 year survival. The latter was obtained from the Surveillance Epidemiology and End Results (SEER) database (a collaborative effort between associated US cancer registries, the National Center for Health Statistics and the National Cancer Institute) (10). Based upon a stipulated acceptable number needed to screen (NNS=2000) to prevent one death, we calculated the minimum acceptable number of deaths prevented as the ratio of the number screened to the NNS. The difference between expected deaths prevented and minimum acceptable deaths prevented is called the “net benefit” (11). The candidate designs were compared based upon net benefit and upon feasibility (size and duration of trial).
All statistical analyses were performed using R (version 2.14.0, 2011, R Development Core Team, Vienna Austria). All P values resulted from use of two-sided statistical tests.
RESULTS
Incidence of ABC and HGBC according to smoking intensity
The patient population had nearly equal numbers of males and females (73,562 and 75,980 respectively) and median age was 62 (IQR, 58-67). The population was predominantly Caucasian (88.4%). Nearly thirty percent of the population had no co-morbidities. Approximately half of individuals in the cohort had never smoked and one third had smoked 1 pack per day or less. Further detail on intensity and duration of smoking are described in Table 1 along with other relevant demographic information. Over a median follow-up of 12 years, incident bladder cancers were detected in 1,124 of 73,562 men (133.6 cases per 100,000 person-years) and in 259 of 75,980 women or 29.6 cases per 100,000 person-years. HGBC’s were detected in 392 men or 46.6 cases per 100,000 person-years and in 72 women or 8.2 cases per 100,000 person-years (Table 2). A 4 fold increase in the incidence of HGBC was observed between the highest category of smokers and never-smokers in men, whereas a 10 fold increase was observed between the same categories in women. The incidence of HGBC in men was 4-fold larger than that in women (Table 2B).
Table 1.
Demographic information on bladder cancer PLCO population
| Parameter | N (%) * |
|---|---|
| Age (≥65) | 53,942 (36.1) |
| Gender (M) | 73,562 (49.2) |
| Race (White) | 132,254 (88.4) |
| Education | |
| HS Graduate | 34,308 (22.9) |
| More than HS | 103,799 (69.4) |
| BMI, kg/m2 | |
| < 25 | 51,732 (34.6) |
| 25 to 30 | 62,277 (41.6) |
| ≥30 | 35,533 (23.8) |
| Personal History of any cancer | 6,525 (4.4) |
| Family History of cancer | |
| Bladder cancer | 2,552 (1.7) |
| Renal cancer | 2,092 (1.4) |
| Other malignancies | 77,652 (51.9) |
| Co-morbidities | |
| None | 43,183 (28.9) |
| One | 47,722 (31.9) |
| Two or more | 58,637 (39.2) |
| Usual Packs per Day | |
| None | 70,984 (47.5) |
| Up to 1 | 48,761 (32.6) |
| Up to 2 | 24,300 (16.2) |
| More than 2 | 5,497 (3.7) |
| Duration of Smoking (yr) | |
| Never | 70,984 (47.5) |
| 0 to 10 | 9,191 (6.1) |
| 10 to 20 | 15,462 (10.3) |
| 20 to 30 | 16,319 (10.9) |
| 30 to 40 | 18,708 (12.5) |
| 40 to 50 | 15,603 (10.4) |
| ≥50 | 3,275 (2.2) |
| Nocturia1 | |
| None | 11,938 (16.2) |
| 1x | 38,233 (52.0) |
| 2x | 15,954 (21.7) |
| 3x | 5,375 (7.3) |
| ≥4x | 2,062 (2.8) |
| BPH1 | 15,963 (21.7) |
| Prostatitis1 | 5,179 (7.0) |
| Syphilis1 | 619 (0.8) |
| Gonorrhea1 | 4,083 (5.6) |
| Use of Birth Control Pills2 | 41,131 (54.1) |
| Use of HRT2 | |
| Never | 25,921 (34.1) |
| Former | 12,833 (16.9) |
| Current | 37,226 (49.0) |
Total =149,542;
Among Men;
Among Women
Table 2.
a. ABC Incidence (per 100,000 person years) according to smoking history (pack-years)
| History of smoking (pack-years) | Women | Men | ||
|---|---|---|---|---|
| # Events | Incidence | # Events | Incidence | |
| 0 | 86 | 17.1 | 240 | 72.4 |
| 0.25-10 | 23 | 25.8 | 71 | 93.1 |
| 10-20 | 30 | 37.5 | 104 | 112.7 |
| 20-30 | 21 | 38.7 | 137 | 175.8 |
| 30-40 | 28 | 58.5 | 98 | 152.4 |
| 40-50 | 28 | 69.0 | 119 | 198.7 |
| 50-60 | 15 | 67.0 | 80 | 196.6 |
| >60 | 28 | 72.0 | 275 | 279.3 |
| Overall | 259 | 29.6 | 1124 | 133.6 |
|
| ||||
| b. HGBC Incidence (per 100,000 person years) according to smoking history (pack-years) | ||||
| History of smoking (pack-years) | Women | Men | ||
| # Events | Incidence | # Events | Incidence | |
|
| ||||
| 0 | 12 | 2.4 | 79 | 23.8 |
| 0.25-10 | 9 | 10.1 | 20 | 26.2 |
| 10-20 | 13 | 16.2 | 42 | 45.5 |
| 20-30 | 8 | 14.7 | 49 | 62.9 |
| 30-40 | 9 | 18.8 | 35 | 54.4 |
| 40-50 | 8 | 19.7 | 47 | 78.5 |
| 50-60 | 4 | 17.9 | 27 | 66.4 |
| >60 | 9 | 23.1 | 93 | 94.5 |
| Overall | 72 | 8.2 | 392 | 46.6 |
Cox proportional Hazard Regression and nomogram predicting incidence of ABC and HGBC
Results of Cox proportional hazards regression models for incidence of ABC and for HGBC are listed in table 3. The reduced models for ABC and for HGBC incidence fit the data as well as the saturated models (likelihood ratio statistics, ABC, chi-squared=25.8, 30 df, p=0.683, HGBC, chi-squared=27.0, 30 df, p=0.624). The strongest risk factors for both ABC and for HGBC were age, (ABC, RR=1.06, p < 0.000001; HGBC, RR=1.072, p < 0.000001), smoking duration (ABC, RR=1.022, p < 0.000001; HGBC, RR=1.023, p < 0.00001), smoking intensity (ABC, RR=1.318, p < 0.000001; HGBC, RR=1.422, p=0.00014), African American race (ABC, RR=0.386, p=0.0000011; HGBC, RR=0.518, p=0.022), and former HRT (ABC, RR=1.495, p=0.017; HGBC, RR=2.299, p=0.0065). Factors that were statistically significant predictors of ABC incidence but not of HGBC incidence included other race (RR=0.576, p=0.0012), education beyond high-school versus high-school degree (RR=0.820, p=0.0023), no co-morbidities versus 2 or more (RR=0.871, p=0.05) and a history of syphilis in men (RR=1.896, p=0.014). The model for ABC incidence retained adjustment for missing smoking status, family history of bladder cancer, of renal cancer and of other cancers (versus no history), and randomization center. Factors that were statistically significant predictors of HGBC incidence but not of ABC incidence included family history of bladder cancer (RR=1.825, p=0.036), and occurrence of 4 or more episodes of nocturia per night in men versus none, (RR=2.052, p=0.008). The model for HGBC incidence retained adjustment for missing smoking status, education, history of syphilis in men and randomization center. Bias corrected concordance indices for the ABC and HGBC incidence models were 0.746 and 0.756, respectively. The upward biases were 0.00831 and 0.0211, respectively. The Efron bias correction procedure was checked against an external validation by conducting full model building and selection in a randomly selected 2/3 training set and then validating using the leftover 1/3 set. This more strict external validation procedure resulted in nearly identical estimates of upward bias (ABC 0.0111, HGBC 0.0278). Nomograms for prediction of the 5 year incidence of ABC stratified by gender are shown in figures (1a) (female) and (1b) (male). Corresponding nomograms stratified by gender for HGBC are shown in figures (2a) and (2b). Calibration plots corresponding to the underlying models for ABC and for HGBC are shown in figures (1c) and (2c), respectively.
Table 3.
Cox proportional hazards models for ABC and for HGBC. Reduced model, baseline hazard stratified on gender with gender specific effects. (a) Smoking intensity capped at 2 packs, (b) smoking duration is linear, (c) years quit is 0 for current smokers, current age for non-smokers. Baseline cumulative hazard at 5 years: ABC females, 1.438e-05; ABC males, 6.374e-05; HGBC females, 1.262e-06; HGBC males, 6.642e-06
| Parameter | Any Bladder Cancer | High-Grade Bladder Cancer | ||
|---|---|---|---|---|
| RR (95% CI) | p-value | RR (95%CI) | p-value | |
| Age | 1.060 (1.05-1.07) | < 0.000001 | 1.072 (1.05-1.09) | < 0.000001 |
| Race | ||||
| White (ref) | 1 | 1 | ||
| Black | 0.386 (0.26-0.57) | 1.10E-06 | 0.518 (0.30-0.91) | 0.022 |
| Other | 0.576 (0.41-0.80) | 0.0012 | 0.621 (0.36-1.07) | 0.086 |
| Educational Level | ||||
| Less than HS | 0.826 (0.67-1.02) | 0.069 | 0.684 (0.47-1.00) | 0.051 |
| HS graduate (ref) | 1 | 1 | ||
| More than HS | 0.820 (0.72-0.93) | 0.0023 | 0.902 (0.72-1.13) | 0.37 |
| Smoking | ||||
| min(packs/day, 2) | 1.318 (1.18-1.47) | < 0.000001 | 1.422 (1.19-1.70) | 0.00014 |
| Duration (18) | 1.022 (1.02-1.03) | < 0.000001 | 1.023 (1.02-1.03) | < 0.000001 |
| Years since quitting | 1.003 (1.00-1.01) | 0.072 | 1.003 (1.00-1.01) | 0.26 |
| Family History of Cancer | ||||
| None (ref) | 1 | 1 | ||
| Bladder | 1.283 (0.87-1.89) | 0.21 | 1.825 (1.04-3.20) | 0.036 |
| Renal | 1.102 (0.70-1.74) | 0.68 | 1.376 (0.68-2.79) | 0.38 |
| Other Malignancies | 1.103 (0.99-1.23) | 0.075 | 1.024 (0.85-1.23) | 0.80 |
| Co-morbidities | ||||
| 2 or more (ref) | 1 | |||
| 1 | 1.033 (0.91-1.17) | 0.61 | ||
| None | 0.871 (0.76-1.00) | 0.05 | ||
| Nocturia (x night) | ||||
| None (ref) | 1 | |||
| 1x | 1.090 (0.80-1.49) | 0.59 | ||
| 2x | 1.194 (0.85-1.69) | 0.31 | ||
| 3x | 1.454 (0.95-2.22) | 0.084 | ||
| ≥4x | 2.052 (1.21-3.49) | 0.008 | ||
| Syphilis | 1.896 (1.14-3.17) | 0.014 | 1.945 (0.86-4.39) | 0.11 |
| Hormone replacement use | ||||
| Never (ref) | 1 | 1 | ||
| Former | 1.495 (1.08-2.08) | 0.017 | 2.299 (1.26-4.19) | 0.0065 |
| Current | 1.107 (0.83-1.47) | 0.48 | 1.206 (0.68-2.14) | 0.52 |
Figure 1.
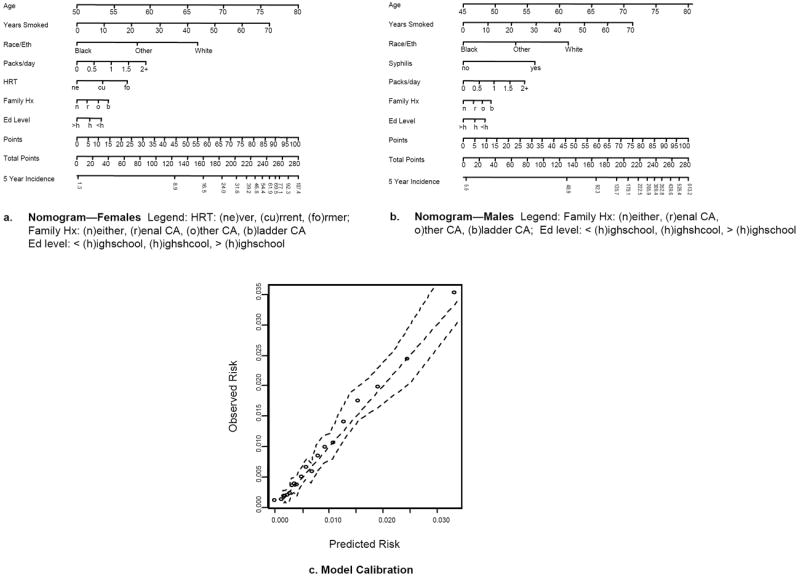
Nomograms predicting 5-year Incidence of ABC per 10,000 persons (A. Female, B. Male), and calibration plot for ABC model (C). Instructions for nomogram: Locate the patient’s age on the respective axis. Draw a straight line down to the points axis to determine how many points towards disease incidence that the patient receives for his/her Age. Repeat this process for all other risk factors included in the nomogram. Sum the points and locate this value on the total points axis. Draw a straight line down to find the patient’s 5-year probability of incident BC. Calibration plots: straight dashed line is the line of perfect prediction, points are observed model calibration points and jagged dashed lines are pointwise 95% confidence intervals.
Figure 2.
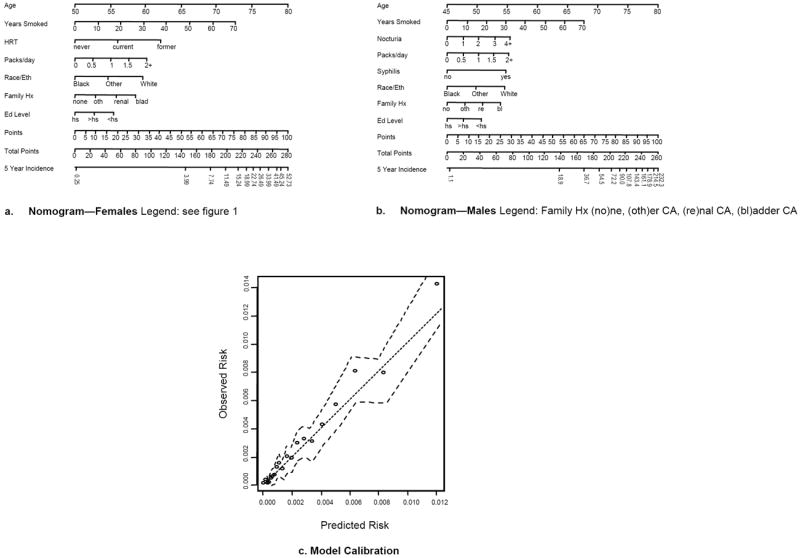
Nomograms predicting 5-year Incidence of HGBC per 10,000 persons (A. Female, B. Male), and calibration plot for ABC model (C). Instructions for nomogram: see caption to figure 1.
Hypothetical Screening Bladder Cancer Trial considerations
To determine the feasibility of designing a bladder cancer mortality screening study using these models, we derived the risk threshold, size of risk-assessed cohort, size of resulting trial, expected deaths averted, minimum tolerable deaths averted and net benefit for a given set of design parameters, (power, relative risk, expected proportion of HGBC randomized, duration of trial and maximum tolerable number needed to screen to prevent 1 HGBC death). We filtered for designs with positive net benefit and trial size less than 110,000. These are displayed in table 4. Of the designs listed, the most favorable is the design shown in the fourth row from the bottom. This trial, of size 94,701, would recruit individuals in the upper 40th percentile of HGBC risk from a source population of size 225,356 to include 80% of all HGBC incidence expected in the source population during the follow-up period. The trial would have 80% power to detect a relative risk of 0.65 with a probability type I error 0.05. This design is expected to prevent 37.6 deaths, a mortality that is 13.9 more (net benefit) than the ratio of number screened to the stipulated NNS to prevent one death. The incidence of HGBC overall is 27.0 cases per 100,000 person years, and the incidence of HGBC in the cohort identified at elevated risk is 51.5 cases per 100,000 person years so that the efficiency of the design is 1.9.
Table 4.
Net benefit for various trial design scenarios, for power 80% or 90%, relative risk (RR) 0.60, 0.65 or 0.70, duration 10, 11, 12 or 13 years and threshhold risk set to a value so that the proportion of incident HGBC expected during follow-up that is included in the trial is 75%, 80%, 85% or 90%. Filtered on designs with positive net benefit and size of trial less than 110,000.
| Power(%) | RR | Expected % of HGBC Randomized | Duration of the trial | Upper pct’ile of HBC risk | HGBC Risk Threshold | Size of Source Cohort (N) | Size of Trial (N) | Deaths Prevented | Minimum Acceptable Deaths Prevented | Net Benefit |
|---|---|---|---|---|---|---|---|---|---|---|
| 80 | 0.60 | 75 | 11 | 35 | 0.00332 | 264,849 | 98,656 | 32.1 | 24.7 | 7.4 |
| 80 | 0.60 | 75 | 12 | 35 | 0.00332 | 231,148 | 86,102 | 32.1 | 21.5 | 10.5 |
| 80 | 0.60 | 75 | 13 | 35 | 0.00332 | 204,375 | 76,129 | 32.1 | 19.0 | 13.0 |
| 80 | 0.60 | 80 | 11 | 40 | 0.00282 | 248,296 | 104,341 | 32.1 | 26.1 | 6.0 |
| 80 | 0.60 | 80 | 12 | 40 | 0.00282 | 216,701 | 91,064 | 32.1 | 22.8 | 9.3 |
| 80 | 0.60 | 80 | 13 | 40 | 0.00282 | 191,602 | 80,517 | 32.1 | 20.1 | 11.9 |
| 80 | 0.60 | 85 | 12 | 45 | 0.00221 | 203,954 | 99,789 | 32.1 | 24.9 | 7.1 |
| 80 | 0.60 | 85 | 13 | 45 | 0.00221 | 180,331 | 88,231 | 32.1 | 22.1 | 10.0 |
| 80 | 0.60 | 90 | 13 | 50 | 0.00156 | 170,313 | 97,410 | 32.1 | 24.4 | 7.7 |
| 80 | 0.65 | 75 | 12 | 35 | 0.00332 | 271,869 | 101,271 | 37.6 | 25.3 | 12.3 |
| 80 | 0.65 | 75 | 13 | 35 | 0.00332 | 240,380 | 89,541 | 37.6 | 22.4 | 15.2 |
| 80 | 0.65 | 80 | 12 | 40 | 0.00282 | 254,878 | 107,107 | 37.6 | 26.8 | 10.8 |
| 80 | 0.65 | 80 | 13 | 40 | 0.00282 | 225,356 | 94,701 | 37.6 | 23.7 | 13.9 |
| 80 | 0.65 | 85 | 13 | 45 | 0.00221 | 212,100 | 103,775 | 37.6 | 25.9 | 11.6 |
| 90 | 0.60 | 75 | 13 | 35 | 0.00332 | 273,601 | 101,915 | 43.0 | 25.5 | 17.5 |
| 90 | 0.60 | 80 | 13 | 40 | 0.00282 | 256,500 | 107,789 | 43.0 | 26.9 | 16.0 |
DISCUSSION
Despite a relatively short latency stage, approximately 30 % of patients with high-grade bladder cancer present with either muscle-invasive and/or metastatic disease at diagnosis. The ability of current therapies to achieve long-term disease control is limited (50-65% for the former and 5-10% for the latter) and have remained relatively unchanged over the last 3 decades despite improvements in local and systemic therapy. Screening tests such as dipstick urinalysis and/or urine-based markers may enable early diagnosis of bladder cancer at a stage where treatments can be more effective. However, available bladder cancer screening studies have been hampered by relatively low incidence of bladder cancer in the screened population, particularly cancers that pose a threat to the individual in terms of progression and mortality (12, 13). Screening and chemo preventive strategies require the identification of cohorts at elevated bladder cancer risk appropriate for these interventions. To this end, we have developed and validated two prediction models using clinical and sociodemographic parameters from a large population-based prospective cohort nested within the PLCO screening trial to predict the risk of bladder cancer (and HGBC) within 5 years. Despite robust models to identify a high-risk cohort using clinical parameters, the sample size and follow-up required for screening trials based on several design assumptions are prohibitive.
Improvements in BC mortality could be achieved by screening and/or primary and secondary prevention strategies. Cigarette smoking was the strongest risk factor for incidence of BC in our models. Risk correlates well with the usual number of cigarettes smoked (3 fold increased risk among heavy smokers) and the duration of smoking but, unlike lung cancer or cardiovascular diseases, smoking cessation does not uniformly reduce the risk. A relative decrease in incidence is seen at 5 to 7 years after quitting smoking, but unfortunately, even after 10 years, the risk of being diagnosed with BC continues to be almost double that of individuals who never smoked (4). A previous meta-analysis (14) based on 11 case control studies from six European countries, established four fold increase risk of BC for smokers over a 40 year period. Investigators identified 15-20 cigarettes per day as threshold for increased risk of BC.
Most of our knowledge regarding BC screening is based on two studies of a urine-based chemical reagent strip test for the presence of hemoglobin (hematuria). Messing et al (12) screened 1,575 men aged 50 years and older with hematuria home testing, finding 21 ABC (1.3%), 9 HGBC and 1 invasive BC. A follow-up report compared HGBC incidence in their screening study with overall rates in a cancer registry and reported a lower proportion of invasive high-grade cancers in screened men (4.8%) than among men in the general population (23.9%; p=0.007). At 14 years follow-up, no men with screen-detected BC died of the disease, whereas 16.4% of men with BC in the general population had died of BC (p=0.025) (13). During the same time period, other authors (15) tested urine from 2356 men and reported repeated hematuria in 474 men (20.1%) and diagnosis of BC in 17 men (0.7%). The false positive rate was 7% with a positive predictive value of 9%. At 7 years follow-up, no patients with low-grade BC died of the disease or from progression, although three of the nine patients with high-grade or invasive tumors died from BC (16). These studies highlight the very low incidence of BC in the general population thus the difficulty of applying screening protocols to general population. Unfortunately, these studies were not controlled so that the reported benefit from screening could have been affected by confounding.
Recently, Vickers et al presented an analysis of the PLCO BC population with the intent to derive candidate screening trial designs based upon the identification of a high risk population. The authors of that report modeled the incidence of any BC and used the results of their analysis to design a hypothetical HGBC prevention trial which would randomize individuals at elevated predicted risk of any BC (11). In our analysis, we used more complete follow-up and modeled the incidence of HGBC and used the results of our analysis to design a hypothetical BC mortality prevention trial which would randomize individuals at elevated predicted risk of HGBC.
At present, many urine molecular markers have been proposed to assist in BC screening. However, none of them seem sensitive enough to fulfill screening requirements. Mainly, NMP22 was shown to be elevated in a large cohort of patients at elevated risk for BC with a sensitivity, specificity and positive predictive value of 55.7%, 85.7% and 19.7%, respectively. Recently, a study was conducted on 1,303 patients at risk for BC undergoing cystoscopy, urine cytology and measurement of urinary NMP22 levels. In all, 72 patients (5.5%) were found to have BC. In multivariable prediction models, NMP22 improved the predictive accuracy (AUC 80.1%) of the base model by 8.2% and of the base model plus cytology by 4.2% (17). To date, the best improvement in dipstick hematuria accuracy has been achieved with the addition of the biomarker NMP22 to predictive models. Our nomograms will be the baseline models by which the clinical utility of these and other biomarkers for screening will be evaluated.
The nomogram developed here has certain limitations. First, inherent in any predictive model is prediction error, the area under the receiver operating characteristics curve on the validation sample was 0.75. Second, PLCO participants may not represent the overall population since participants may have been subjected to additional diagnostic tests which may have increased BC incidence relative to that experienced in the general population. In order to test for possible confounding between screening related events and associations reported in our final models, we entered PLCO screening events into final ABC and HGBC models as time dependent covariates. The risk of incident ABC was increased by detection of prostate cancer (RR=1.32, p=0.015) and there was a borderline statistically significant effect due to detection of ovarian cancer (RR=1.34, p=0.063). The final model for ABC fit the data nearly as well as the model to which the PLCO event variables were added (likelihood ratio statistic: chi-squared=13.4, 10df, p-value=0.2015). This possible confounding appears to be less troublesome in the case of the HGBC model, for which the effect of detection of prostate cancer was effectively zero (RR=0.89, p=0.59) but the effect of detection of ovarian cancer remained borderline significant (RR=1.89, p=0.068). The final model for HGBC fit the data as well as the model to which the PLCO event variables were added (likelihood ratio statistic: chi-squared=8.92, 10df, p-value= 0.54). Despite this caveat, the present data and analysis should be considered a major advance in providing validated ABC and HGBC nomograms, especially in light of the difficulty in identification of a cohort at sufficiently elevated risk of BC for the purpose of screening/chemoprevention, as the impact of our cohort being nested in a screening trial appears to be small.
In summary, the novelty of our study is that it is based upon a prospective cohort and includes baseline information for all patients as well as accurate follow-up to BC diagnosis. Thus it provides an invaluable tool that allows a clinician to determine individualized risk in order to determine which subpopulations would benefit the most from further screening.
Acknowledgments
The authors would like to acknowledge Timothy R. Church for several useful discussions.
Footnotes
All have contributed substantially to the design, conduct and writing of this manuscript A.B. and G.I. are employees of the United States Government and therefore this work is public domain.
All of the authors have no conflict of interest related to this manuscript.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA: a cancer journal for clinicians. 2013 Jan;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Abdollah F, Gandaglia G, Thuret R, Schmitges J, Tian Z, Jeldres C, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: A trend analysis. Cancer epidemiology. 2013 Jun;37(3):219–25. doi: 10.1016/j.canep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 3.Bosetti C, Bertuccio P, Chatenoud L, Negri E, La Vecchia C, Levi F. Trends in mortality from urologic cancers in Europe, 1970-2008. Eur Urol. 2011 Jul;60(1):1–15. doi: 10.1016/j.eururo.2011.03.047. [DOI] [PubMed] [Google Scholar]
- 4.Rink M, Zabor EC, Furberg H, Xylinas E, Ehdaie B, Novara G, et al. Impact of Smoking and Smoking Cessation on Outcomes in Bladder Cancer Patients Treated with Radical Cystectomy. European urology. 2012 Nov 27; doi: 10.1016/j.eururo.2012.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Howe GR, Burch JD, Miller AB, Cook GM, Esteve J, Morrison B, et al. Tobacco use, occupation, coffee, various nutrients, and bladder cancer. J Natl Cancer Inst. 1980 Apr;64(4):701–13. [PubMed] [Google Scholar]
- 6.Zeegers MP, Goldbohm RA, van den Brandt PA. A prospective study on active and environmental tobacco smoking and bladder cancer risk (The Netherlands) Cancer Causes Control. 2002 Feb;13(1):83–90. doi: 10.1023/a:1013954932343. [DOI] [PubMed] [Google Scholar]
- 7.Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials. 2000 Dec;21(6 Suppl):273S–309S. doi: 10.1016/s0197-2456(00)00098-2. [DOI] [PubMed] [Google Scholar]
- 8.Michaud DS, Platz EA, Giovannucci E. Gonorrhoea and male bladder cancer in a prospective study. British journal of cancer. 2007 Jan 15;96(1):169–71. doi: 10.1038/sj.bjc.6603510. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.E B. Bootstrap methods: another look at the jackknife. Annals of Statistics. 1979;7(1):1–26. [Google Scholar]
- 10.Lynch CF, D J, Platz CE. Cancer of urinary bladder. In: Ries LAG, Young JL, Keel GE, et al., editors. SEER Survival Monograph: Cancer Survival Among Adult: U S SEER PROGRAM, 1998-2001, Patient and Tumor Characteristics. National Cancer Institute, SEER Program, NIH Pub No 07-6215; Bethesda, MD: 2007. [Google Scholar]
- 11.Vickers AJ, Bennette C, Kibel AS, Black A, Izmirlian G, Stephenson AJ, et al. Who should be included in a clinical trial of screening for bladder cancer?: A decision analysis of data from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Cancer. 2012 Jun 26; doi: 10.1002/cncr.27692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messing EM, Young TB, Hunt VB, Roecker EB, Vaillancourt AM, Hisgen WJ, et al. Home screening for hematuria: results of a multiclinic study. J Urol. 1992 Aug;148(2 Pt 1):289–92. doi: 10.1016/s0022-5347(17)36575-8. [DOI] [PubMed] [Google Scholar]
- 13.Messing EM, Madeb R, Young T, Gilchrist KW, Bram L, Greenberg EB, et al. Long-term outcome of hematuria home screening for bladder cancer in men. Cancer. 2006 Nov;107(9):2173–9. doi: 10.1002/cncr.22224. [DOI] [PubMed] [Google Scholar]
- 14.Brennan P, Bogillot O, Greiser E, Chang-Claude J, Wahrendorf J, Cordier S, et al. The contribution of cigarette smoking to bladder cancer in women (pooled European data) Cancer Causes Control. 2001 Jun;12(5):411–7. doi: 10.1023/a:1011214222810. [DOI] [PubMed] [Google Scholar]
- 15.Britton JP, Dowell AC, Whelan P, Harris CM. A community study of bladder cancer screening by the detection of occult urinary bleeding. J Urol. 1992 Sep;148(3):788–90. doi: 10.1016/s0022-5347(17)36720-4. [DOI] [PubMed] [Google Scholar]
- 16.Mayfield MP, Whelan P. Bladder tumours detected on screening: results at 7 years. Br J Urol. 1998 Dec;82(6):825–8. doi: 10.1046/j.1464-410x.1998.00879.x. [DOI] [PubMed] [Google Scholar]
- 17.Barbieri CE, Cha EK, Chromecki TF, Dunning A, Lotan Y, Svatek RS, et al. Decision curve analysis assessing the clinical benefit of NMP22 in the detection of bladder cancer: secondary analysis of a prospective trial. BJU Int. 2012 Mar;109(5):685–90. doi: 10.1111/j.1464-410X.2011.010419.x. [DOI] [PubMed] [Google Scholar]


