Abstract
Purpose of review
The platelet paradigm that is well established in hemostasis and thrombosis can be extended to other disease states. A consideration for some major health issues, such as inflammation, cancer, infection, and neuroscience and how platelet function impacts the pathophysiology of each clinical situation is provided.
Recent findings
Decades of research and knowledge of platelet function exists and the same is true for inflammation and cancer. The literature is full of platelet biology overlapping into other, non-thrombotic, disease states. However, major gaps exist that prevent a complete mechanistic understanding of platelet function in these other diseases. While much of the overlap provides antidotal relationships, future studies will likely uncover novel pathophysiological pathways that are highly relevant to human diseases.
Summary
Recent findings in four major disease areas, inflammation, cancer, infection and neuroscience are described with current literature linking the disease to platelet function. The availability of anti-platelet therapies, such as aspirin, exist and future consideration can be given as to whether anti-platelet therapy is potentially beneficial or harmful as mechanisms of platelet involvement are better defined.
Keywords: inflammation, cancer, infection, neural disease
Introduction
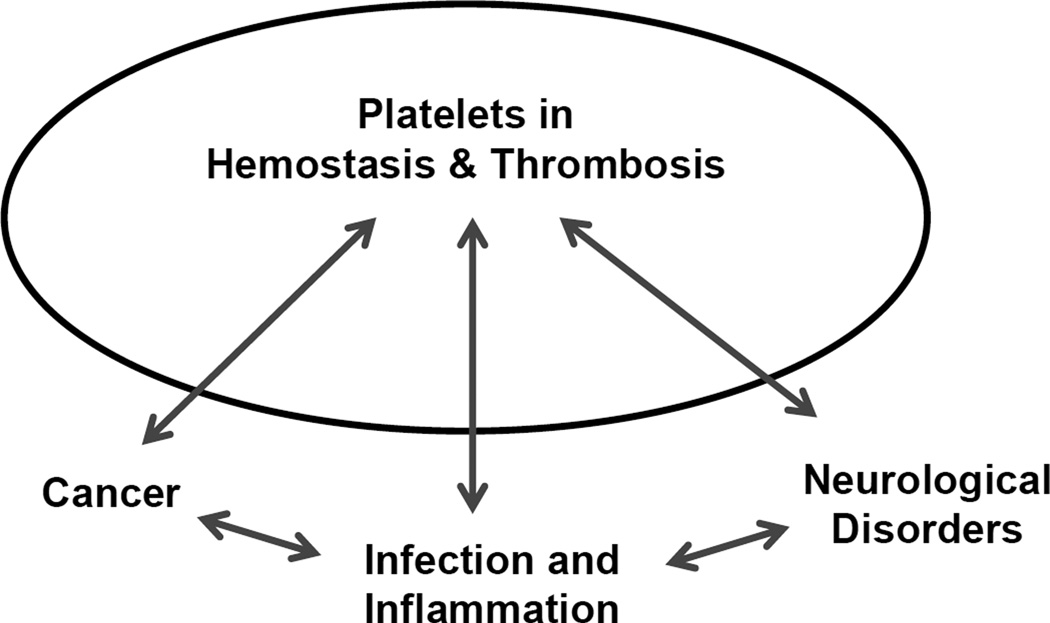
The intense investigation of platelet function over the past few decades is warranted given the platelet’s fundamental role in both hemostasis and thrombosis. Indeed, the molecular events associated with both have been described in elegant molecular detail identifying multiple therapeutic targets, some that have been exploited in drug development, and others that are still waiting to be tested. While platelet relevance in hemostasis and thrombosis is well established there exists a wide range of literature establishing platelet importance in diseases not immediately associated with either event [1]. While a role for platelets in other diseases exists the in vivo relevance has sometimes been difficult to dissect owing, in part, to whether outcomes are due to the platelet’s role in hemostasis or, as an example, the platelet’s role as an immune modulator [2]. Nevertheless, overlapping functions do exist and this review will highlight 3 different disease topics where studies have linked platelet function to disease progression, severity, and outcome. Specifically, recent highlights in inflammation and infection, cancer, and neurological disorders will be discussed (Figure 1).
Figure 1. Platelets at the interface of disease.
The dynamics that exist between platelet function in hemostasis / thrombosis and diseases, such as cancer, inflammation, and neurological disorders are being explored. Traditional platelet function in hemostasis and thrombosis impacts each of these areas to varying degrees and some of the recent progress and insights are highlighted in this review. Further overlap between cancer / inflammation, and inflammation / neurological disorders is also known but beyond the scope of what is discussed here.
To apply the platelet paradigm beyond hemostasis and thrombosis might be best appreciated by understanding the phylogenetic origins of the platelet [3]. The anucleate human platelet is a specialized cell fragment unique to mammals. Non-mammalian vertebrates, such as fish and birds, have nucleated platelets or thrombocytes. Invertebrates have an even more primitive blood cell, the amebocyte. The amebocyte is the single blood cell of invertebrates with a multitude of functions. As different types of blood cells have appeared in phylogeny, each cell has gained a more specialized function. However, exclusivity for the specialized function seems rare [4]. Thus, as we consider mammalian platelet function beyond hemostasis and thrombosis we can often trace these functions as vestiges to the platelet’s ancestor, the thrombocyte or an amebocyte.
Platelets and Inflammation
The platelet is equipped to influence inflammation and the innate immune response at several levels [2,5,6]. First, the platelet expresses a repertoire of pattern recognition receptors, toll-like receptors (TLRs), which initiate the innate immune response [7–11]. Second, there is a platelet/leukocyte and platelet/monocyte axis where specific platelet receptors and counter receptors on the white blood cells facilitate their interaction in the blood stream [12–15]. In addition, the platelet stores and releases upon activation many inflammatory mediators, such as interleukin-1 (IL-1) that can exacerbate the immune response. In the case of IL-1β, this has been specifically linked to the pathogenesis of arthritis and systemic lupus erythematosus (SLE) [16]. In a non-classical form of platelet activation, platelets can release microparticles (less than 1 µM in diameter) and these too have been linked to the inflammatory pathways associated with rheumatoid arthritis [17,18]. So, the ability of platelets to influence inflammation is likely a dynamic process and occurring through a variety of mechanisms.
The future challenge to understanding how platelets influence inflammation must also consider the state of platelet activation and the ability of the platelet to regulate activation of the white blood cell [19*]. Much literature describes the pro-inflammatory properties of the platelet. However, understanding the dynamic life span and function of the platelet could lend itself to a more complex interpretation. Perhaps in one setting the platelet elicits an inhibitory role in inflammation but when triggered by inflammatory mediators to induce platelet activation, the platelet becomes pro-inflammatory [20]. If we consider the temporal sequence of events so well-characterized in the platelet paradigm in hemostasis, platelet function proceeds through a series of events characterized by recognition of a surface, an activation response, a platelet release reaction, recruitment of platelets, and wound repair. Considering a similar sequence of events in response to interacting with other blood cells or an inflamed endothelial cell surface, the dynamics of how a platelet contributes to the immune response is likely to be quite complex [20].
The importance of understanding platelet function in inflammation is underscored by the immune system’s complicated role in many chronic diseases. Neurodegenerative diseases [21], atherosclerosis [22–24], transfusion-related lung injury [25], rheumatoid arthritis [16], and SLE [16] represent just a sampling of the recent inflammation based pathways that have strong association with platelet activity [5].
Perhaps one of the biggest challenges to unravel is the potential relevance of platelets in the severe sepsis syndrome. The significance of improved therapies for sepsis needs little justification. Sepsis is a worldwide problem in medical management and in the U.S. accounts yearly for 250,000 deaths, ranking it in the top 10 causes of death [26]. Fibrinolytic strategies in treating severe sepsis have unfortunately not improved outcomes as originally hoped. The consensus opinions on sepsis is there exists major gaps in our understanding. Dysfunctions in coagulation during the course of sepsis are known but details beyond that statement have been elusive [27]. The recent withdrawal of Xigris and Eritoran are the latest casualties for therapeutic intervention in sepsis [28]. Thrombocytopenia associated with sepsis has been long thought to due to platelet consumption in disseminated intravascular coagulation [27], but in fact an Ashwell receptor liver clearance mechanism of platelets during the height of infection might also contribute to the thrombocytopenia [29]. An increased platelet clearance could lead to further imbalance in the inflammatory continuum at a time when dysregulated inflammation is exacerbating septic shock. Further characterizing the platelet-dependent contribution to early aspects of the innate immune response could remove a barrier to further innovation in understanding the complex disease process that is sepsis. The difficulty in sepsis treatment and development of new drugs revolves around the complex – and poorly understood – pathophysiology of sepsis. New advances will have to include animal models. While recent work has challenged the applicability of animal models in studies of inflammation [30], even if animals are less sensitive understanding the differences that exist between animals and humans is likely to be important for a complete understanding.
Platelets and Cancer
The interrelationship between platelets and cancer dates to the 19th century with observations by Armand Trousseau (Trousseau’s syndrome). His keen insights linked venous thrombus formation as a potential predictor of an undiagnosed malignancy [31,32]. However, the association is not restricted to platelets with basic mechanisms of coagulation linked to various stages of tumor growth and metastasis [33]. Platelet participation is tumorigenesis can occur at many levels but most striking is how the tumor cells appear to have hijacked the normal wound healing properties of platelets to promote cellular growth, metastasis and angiogenesis.[34*]
A vast literature exists on the relevance of circulating platelets in many different animals models of cancer.[35] But perhaps the best proof of principle for human platelet relevance in cancer can be attributed to data mined following the wide spread use of aspirin for its cardiovascular protective effects [36–38]. The efficacy of aspirin in reducing colon cancer seems compelling, but what is less compelling is the mechanism of action [39]. The anti-platelet effects of aspirin are well established and occur via cyclooxygenase-1 (COX-1) inhibition and an inability to convert arachidonic acid into prostaglandins. Thus, the COX-1 inhibition provides a potent anti-platelet activation effect, so much, that in some cases there can be serious bleeding [40]. This highlights the challenge of maintaining the hemostatic balance even in light of potential benefits in other disease processes [41*].
The challenge in mechanistically explaining aspirin therapy efficacy in cancer patients is dissecting aspirin’s effects on platelets from a similar inhibition of COX-2 which is expressed by a wide range of cell types, including tumor cells [39,42]. Indeed, aspirin effects could be wide ranging and if properly balanced with its anti-hemostatic effects could be one of the easiest first lines of prevention in many seemingly unrelated cancers. With a growing interest in personalized medicine, understanding the individuality of aspirin resistance and aspirin sensitivity might identify a safer approach to aspirin therapy [43].
One of the better described platelet properties effecting tumor growth is the angiogenic pathway [44,45]. Again, the wound healing activation release of a wide range of stored proteins can dramatically influence new blood vessel growth to satisfy the insatiable tumor appetite for nutrients [46]. One of the bigger dilemmas in understanding the proangiogenic properties of platelets is also appreciating the presence of anti-angiogenic properties stored in similar platelet granules. Some have proposed the presence of distinct subpopulations of platelet granules where some degree of segregation might exist between pro- and anti-angiogenic proteins [47,48]. Others suggest the segregated storage is a more stochastic event[49]. The pathophysiologic situation might be even more complex with consideration for the relative amounts of each protein, the pro- or anti-angiogenic strength of each protein, and the ability of older platelets to have sequestered more growth factor from the plasma during its lifetime in the bloodstream. Thus, a complete mechanistic explanation of the platelet’s regulation of angiogenesis is still waiting, but clearly one with important therapeutic implications.
Many solid tumors can also display a degree of thrombocytosis, or elevated platelet count that correlates with a worsening prognosis [50]. Again, the effect of thrombocytosis could be multifactorial but would likely include increased levels of platelet-secreted growth factors and increased metastatic potential. Either effect would challenge intervention. A long held observation of tumor cells supporting platelet aggregation highlights the tumor cells ability to exert a thrombotic effect likely contributing to the Trousseau syndrome [32]. The aggregation mechanisms are the well characterized receptor-ligand interactions and pathways that are exquisitely described for hemostasis, such as glycoprotein (GP) Ib-IX, GPIIb/IIIa, ADP, and thromboxane [51].
The challenge going forward is to understand a plethora of pathways where cancer and platelets converge. While retrospective analysis have supported aspirin effects in limiting metastasis, the inhibition of P2Y12 for which large data sets exists because of its widespread use in cardiovascular disease has been more controversial [52,53]. Recent evidence linking P2Y12 inhibition and increased growth of solid tumors is alarming [52]. Others have developed anti-platelet antibodies to recognize the activated platelet and then further fragment the platelet potentially reducing metastatic potential [54,55]. Clearly, better mechanistic insights are needed to understand the translation of animal model studies to the human clinical situation. In most cases, it will likely be an example of where the tumor cell is exploiting the normal specialized physiology of the circulating platelet, an ability to adhere, activate, recruit and repair damage. Because the normal platelet response is a temporal sequence of events with platelet properties changing at each step, focusing on any single event may not afford the greatest improvement in therapy. A multi-faceted approach may prove beneficial but will also be challenging given the heterogeneity in the human population at each level of intervention. Will a personalized medicine approach provide answers and benefits [56*]? The potential for personalized medicine to improve care has shown some success but it is worthy to never underestimate biological complexities. When one approach looks obvious it many times underscores a simplistic view that is later proven to be poorly understood. Thus, while garnered enthusiasm is great, caution is needed too.
Platelets and Infection
Similar to the dynamic role of platelets in cancer biology, similar interactions and dynamic relevance also exists in models of cerebral malaria [57]. Here, the situation is far from simple with the “state” of the platelet affording very different protective or deleterious outcomes during infection. In the early phase of infection, platelets can limit parasite growth by killing Plasmodium falciparum through the release of platelet factor 4 [58,59*]. Later as the disease progresses, platelet activation begins to significantly contribute to the malaria-associated inflammation presumably through many of the pathways outlined above [57].
Platelet bacterial interactions have also been documented and support the universal theme of intrinsic platelet adhesion [60]. The pathophysiologic consequences in situations such as infective endocarditis would significantly worsen cardiovascular disease and congestive heart failure [61]. The major platelet adhesion receptor, GP Ib, has been implicated as a binding site on the platelet surface to Streptococcus sanguis, and as a consequence of binding can induce platelet activation and aggregation [61,62]. While S. sanguis studies are relevant to thrombus formation on a cardiac valve, one could speculate on similar consequences in other cases of severe bacterial infection, as briefly mentioned above related to the severe sepsis syndrome. Understanding the dynamics of platelet interactions with bacteria, immune cells, the state of platelet activation, is likely to be a daunting task but clearly one where antidotal pathophysiologic significance seems obvious but needs more detailed mechanistic investigation.
The Circulating Neuron
The idea of the platelet as a circulating neuron contains some truth but can also be somewhat misleading. The truth stems from similarities between the platelet release reaction of stored agonists following stimulation, and neurotransmitter release following an action potential stimulation of a neuron. The similarities and dissimilarities comparing platelet and neuron exocytosis has been reviewed [63]. What is clear is a wealth of literature examining platelet function from individuals with a range of diagnosed psychiatric syndromes. A recent search of www.pubmed.org with the search terms “platelet and mental illness” returned 3569 citations. Further refining the search to schizophrenia or other disorders still returns a plethora of information. An obvious examination of platelet function relates to ease of obtaining a venipuncture vial of blood for analysis and analyzing the platelet as a window into what might be dysfunctional in the neuron.
What is most compelling in these searches is the majority of the literature is pre-1980s. Thus, given the significant platelet mechanistic insights that have been realized in the last 30 years, a re-examination of this older work is likely to provide some very interesting new insights into the circulating neuron hypothesis. Given the widespread use of serotonin-uptake inhibitors to treat depression and the platelet as a circulating regulator of serotonin [64], this is a beyond hemostasis/thrombosis topic that clearly deserves attention.
Conclusion
Significant insights into platelet mechanisms in hemostasis and thrombosis have been made. Platelet targets for inhibition of thrombosis have been identified and many have been used. Less appreciated is how the same platelet mediated interactions are influencing inflammation, cancer, infection. Moving forward such investigations should quickly provide new and exciting information thanks to the immediate benefit of established mouse models, antibodies, and sophisticated techniques to examine hemodynamic properties and the platelet response.
Key points.
Platelets are highly specialized mammalian cellular fragments, the origin of which can be traced to multi-functional cells, such as thrombocytes and amebocytes found in lower organisms.
The adhesive nature of platelets supports interactions with cells participating in a wide range of disease processes, such as cancer, inflammation, and neurological disorders.
Extending the platelet paradigm that is so well described in hemostasis and thrombosis to other disease states remains an important direction where major gaps exist in our knowledge.
Acknowledgements
Funding from the National Institutes of Health (HL50545 and AR 061991) to jw and an AHA Predoctoral Award to ac is acknowledged.
Abbreviations
- TLR
toll-like receptor
- SLE
systemic lupus erythematosus
- IL-1
interleukin-1
- COX
cyclooxygenase
- GP
glycoprotein
Footnotes
Conflict of Interest: The authors have no financial interests to disclose.
References and recommended reading
- 1.Leslie M. Cell biology. Beyond clotting: the powers of platelets. Science. 2010;328(5978):562–564. doi: 10.1126/science.328.5978.562. [DOI] [PubMed] [Google Scholar]
- 2.Semple JW, Italiano JE, Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274. doi: 10.1038/nri2956. [DOI] [PubMed] [Google Scholar]
- 3.Levin J. The evolution of mammalian platelets. In: Michelson AD, editor. Platelets. 3rd ed. Vol. 3. New York: Elsevier Inc.; 2013. p. 25. [Google Scholar]
- 4.St Paul M, Paolucci S, Barjesteh N, et al. Characterization of chicken thrombocyte responses to toll-like receptor ligands. PLoS One. 2012;7(8):e43381. doi: 10.1371/journal.pone.0043381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang HS, Chang HH. Platelets in inflammation and immune modulations: functions beyond hemostasis. Arch Immunol Ther Exp (Warsz) 2012;60(6):443–451. doi: 10.1007/s00005-012-0193-y. [DOI] [PubMed] [Google Scholar]
- 6.Ghasemzadeh M, Hosseini E. Platelet-leukocyte crosstalk: Linking proinflammatory responses to procoagulant state. Thromb Res. 2013;131(3):191–197. doi: 10.1016/j.thromres.2012.11.028. [DOI] [PubMed] [Google Scholar]
- 7.Andonegui G, Kerfoot SM, McNagny K, et al. Platelets express functional Toll-like receptor-4. Blood. 2005;106(7):2417–2423. doi: 10.1182/blood-2005-03-0916. [DOI] [PubMed] [Google Scholar]
- 8.Cognasse F, Hamzeh H, Chavarin P, et al. Evidence of Toll-like receptor molecules on human platelets. Immunol Cell Biol. 2005;83(2):196–198. doi: 10.1111/j.1440-1711.2005.01314.x. [DOI] [PubMed] [Google Scholar]
- 9.Cognasse F, Hamzeh-Cognasse H, Lafarge S, et al. Toll-like receptor 4 ligand can differentially modulate the release of cytokines by human platelets. Br J Haematol. 2008;141(1):84–91. doi: 10.1111/j.1365-2141.2008.06999.x. [DOI] [PubMed] [Google Scholar]
- 10.Berthet J, Damien P, Hamzeh-Cognasse H, et al. Toll-like receptor 4 signal transduction in platelets: novel pathways. Br J Haematol. 2010;151(1):89–92. doi: 10.1111/j.1365-2141.2010.08292.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang G, Han J, Welch EJ, et al. Lipopolysaccharide stimulates platelet secretion and potentiates platelet aggregation via TLR4/MyD88 and the cGMP-dependent protein kinase pathway. J Immunol. 2009;182(12):7997–8004. doi: 10.4049/jimmunol.0802884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zago AC, Simon DI, Wang Y, et al. The importance of the interaction between leukocyte integrin Mac-1 and platelet glycoprotein Ibα for leukocyte recruitment by platelets and for the inflammatory response to vascular injury. Arq Bras Cardiol. 2008;90(1):54–63. doi: 10.1590/s0066-782x2008000100009. [DOI] [PubMed] [Google Scholar]
- 13.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17(11):1381–1390. doi: 10.1038/nm.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Projahn D, Koenen RR. Platelets: key players in vascular inflammation. J Leukoc Biol. 2012;92(6):1167–1175. doi: 10.1189/jlb.0312151. [DOI] [PubMed] [Google Scholar]
- 15.Seizer P, Gawaz M, May AE. Platelet-monocyte interactions--a dangerous liaison linking thrombosis, inflammation and atherosclerosis. Curr Med Chem. 2008;15(20):1976–1980. doi: 10.2174/092986708785132852. [DOI] [PubMed] [Google Scholar]
- 16.Boilard E, Blanco P, Nigrovic PA. Platelets: active players in the pathogenesis of arthritis and SLE. Nat Rev Rheumatol. 2012;8(9):534–542. doi: 10.1038/nrrheum.2012.118. [DOI] [PubMed] [Google Scholar]
- 17.Boilard E, Nigrovic PA, Larabee K, et al. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327(5965):580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zimmerman GA, Weyrich AS. Immunology. Arsonists in rheumatoid arthritis. Science. 2010;327(5965):528–529. doi: 10.1126/science.1185869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mueller K. Inflammation. Inflammation's yin-yang. Introduction. Science. 2013;339(6116):155. doi: 10.1126/science.339.6116.155. An issue of Science dedicated to inflammation imposing the yin-yang theory highlighting the balance between inflammatory pathways that provide benefit and those that worsen disease.
- 20.O'Brien M. The reciprocal relationship between inflammation and coagulation. Top Companion Anim Med. 2012;27(2):46–52. doi: 10.1053/j.tcam.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Langer HF, Choi EY, Zhou H, et al. Platelets contribute to the pathogenesis of experimental autoimmune encephalomyelitis. Circ Res. 2012;110(9):1202–1210. doi: 10.1161/CIRCRESAHA.111.256370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Totani L, Evangelista V. Platelet-leukocyte interactions in cardiovascular disease and beyond. Arterioscler Thromb Vasc Biol. 2010;30(12):2357–2361. doi: 10.1161/ATVBAHA.110.207480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fuentes QE, Fuentes QF, Andres V, et al. Role of platelets as mediators that link inflammation and thrombosis in atherosclerosis. Platelets. 2012;24(4):255–262. doi: 10.3109/09537104.2012.690113. [DOI] [PubMed] [Google Scholar]
- 24.Li N. CD4+ T cells in atherosclerosis: Regulation by platelets. Thromb Haemost. 2013;109(5) doi: 10.1160/TH12-11-0819. [DOI] [PubMed] [Google Scholar]
- 25.Caudrillier A, Looney MR. Platelet-neutrophil interactions as a target for prevention and treatment of transfusion-related acute lung injury. Curr Pharm Des. 2012;18(22):3260–3266. doi: 10.2174/1381612811209023260. [DOI] [PubMed] [Google Scholar]
- 26.Levinson AT, Casserly BP, Levy MM. Reducing mortality in severe sepsis and septic shock. Semin Respir Crit Care Med. 2011;32(2):195–205. doi: 10.1055/s-0031-1275532. [DOI] [PubMed] [Google Scholar]
- 27.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, et al. The pathogenesis of sepsis. Annu Rev Pathol. 2011;6:19–48. doi: 10.1146/annurev-pathol-011110-130327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams SC. After Xigris, researchers look to new targets to combat sepsis. Nat Med. 2012;18(7):1001. doi: 10.1038/nm0712-1001. [DOI] [PubMed] [Google Scholar]
- 29.Grewal PK, Uchiyama S, Ditto D, et al. The Ashwell receptor mitigates the lethal coagulopathy of sepsis. Nat Med. 2008;14(6):648–655. doi: 10.1038/nm1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A. 2013;110(9):3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goubran HA, Burnouf T, Radosevic M, El-Ekiaby M. The platelet-cancer loop. Eur J Intern Med. 2013 doi: 10.1016/j.ejim.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 32.Dammacco F, Vacca A, Procaccio P, et al. Cancer-related coagulopathy (Trousseau's syndrome): review of the literature and experience of a single center of internal medicine. Clin Exp Med. 2013;13(2):85–97. doi: 10.1007/s10238-013-0230-0. [DOI] [PubMed] [Google Scholar]
- 33.Yapijakis C, Bramos A, Nixon AM, et al. The interplay between hemostasis and malignancy: the oral cancer paradigm. Anticancer Res. 2012;32(5):1791–1800. [PubMed] [Google Scholar]
- 34. Labelle M, Hynes RO. The initial hours of metastasis: the importance of cooperative host-tumor cell interactions during hematogenous dissemination. Cancer Discov. 2012;2(12):1091–1099. doi: 10.1158/2159-8290.CD-12-0329. An excellent review on the early events associated with metastasis and tumorigenesis.
- 35.Kerr BA, McCabe NP, Feng W, Byzova TV. Platelets govern pre-metastatic tumor communication to bone. Oncogene. 2012 doi: 10.1038/onc.2012.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rothwell PM, Price JF, Fowkes FG, et al. Short-term effects of daily aspirin on cancer incidence, mortality, and non-vascular death: analysis of the time course of risks and benefits in 51 randomised controlled trials. Lancet. 2012;379(9826):1602–1612. doi: 10.1016/S0140-6736(11)61720-0. [DOI] [PubMed] [Google Scholar]
- 37.Rothwell PM, Wilson M, Price JF, et al. Effect of daily aspirin on risk of cancer metastasis: a study of incident cancers during randomised controlled trials. Lancet. 2012;379(9826):1591–1601. doi: 10.1016/S0140-6736(12)60209-8. [DOI] [PubMed] [Google Scholar]
- 38.Algra AM, Rothwell PM. Effects of regular aspirin on long-term cancer incidence and metastasis: a systematic comparison of evidence from observational studies versus randomised trials. Lancet Oncol. 2012;13(5):518–527. doi: 10.1016/S1470-2045(12)70112-2. [DOI] [PubMed] [Google Scholar]
- 39.Kaiser J. Wondering how the wonder drug works. Science. 2012;337(6101):1472. doi: 10.1126/science.337.6101.1472. [DOI] [PubMed] [Google Scholar]
- 40.De Berardis G, Lucisano G, D'Ettorre A, et al. Association of aspirin use with major bleeding in patients with and without diabetes. JAMA. 2012;307(21):2286–2294. doi: 10.1001/jama.2012.5034. [DOI] [PubMed] [Google Scholar]
- 41. Kaiser J. Will an aspirin a day keep cancer away? Science. 2012;337(6101):1471–1473. doi: 10.1126/science.337.6101.1471. Highlights a wealth of available clinical data for aspirin that can be applied to the progression of other diseases, such as cancer.
- 42.Bruno A, Dovizio M, Tacconelli S, Patrignani P. Mechanisms of the antitumoural effects of aspirin in the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2012;26(4):e1–e13. doi: 10.1016/j.bpg.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 43.Casado-Arroyo R, Gargallo C, Lanas AA. Balancing the risk and benefits of low-dose aspirin in clinical practice. Best Pract Res Clin Gastroenterol. 2012;26(2):173–184. doi: 10.1016/j.bpg.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Pinedo HM, Verheul HM, D'Amato RJ, Folkman J. Involvement of platelets in tumour angiogenesis? Lancet. 1998;352(9142):1775–1777. doi: 10.1016/s0140-6736(98)05095-8. [DOI] [PubMed] [Google Scholar]
- 45.Patzelt J, Langer HF. Platelets in angiogenesis. Curr Vasc Pharmacol. 2012;10(5):570–577. doi: 10.2174/157016112801784648. [DOI] [PubMed] [Google Scholar]
- 46.Peterson JE, Zurakowski D, Italiano JE, Jr, et al. Normal ranges of angiogenesis regulatory proteins in human platelets. Am J Hematol. 2010;85(7):487–493. doi: 10.1002/ajh.21732. [DOI] [PubMed] [Google Scholar]
- 47.Italiano JE, Jr, Richardson JL, Patel-Hett S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111(3):1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: modulation of physiologic and pathologic angiogenesis. Blood. 2011;118(5):1359–1369. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamykowski J, Carlton P, Sehgal S, Storrie B. Quantitative immunofluorescence mapping reveals little functional coclustering of proteins within platelet α-granules. Blood. 2011;118(5):1370–1373. doi: 10.1182/blood-2011-01-330910. [DOI] [PubMed] [Google Scholar]
- 50.Buergy D, Wenz F, Groden C, Brockmann MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130(12):2747–2760. doi: 10.1002/ijc.27441. [DOI] [PubMed] [Google Scholar]
- 51.Lian L, Li W, Li ZY, et al. Inhibition of MCF-7 breast cancer cell-induced platelet aggregation using a combination of antiplatelet drugs. Oncol Lett. 2013;5(2):675–680. doi: 10.3892/ol.2012.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Serebruany V, Floyd J, Chew D. Excess of Solid Cancers After Prasugrel: The Food and Drug Administration Outlook. Am J Ther. 2010 doi: 10.1097/MJT.0b013e31824ea5f9. [DOI] [PubMed] [Google Scholar]
- 53.Cattaneo M. Prasugrel and cancer. Arch Intern Med. 2010;170(21):1944. doi: 10.1001/archinternmed.2010.421. [DOI] [PubMed] [Google Scholar]
- 54.Zhang W, Dang S, Hong T, et al. A humanized single-chain antibody against beta 3 integrin inhibits pulmonary metastasis by preferentially fragmenting activated platelets in the tumor microenvironment. Blood. 2012;120(14):2889–2898. doi: 10.1182/blood-2012-04-425207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ware J. Fragmenting the platelet to reduce metastasis. Blood. 2012;120(14):2779–2780. doi: 10.1182/blood-2012-08-450072. [DOI] [PubMed] [Google Scholar]
- 56. Wistuba II, Gelovani JG, Jacoby JJ, et al. Methodological and practical challenges for personalized cancer therapies. Nature Reviews. 2013;8:135–141. doi: 10.1038/nrclinonc.2011.2. Personalized medicine is the new "buzz" word in translational science but an ojective asssessment of potential and limitations is provided.
- 57.Aggrey AA, Srivastava K, Ture S, et al. Platelet Induction of the Acute-Phase Response Is Protective in Murine Experimental Cerebral Malaria. J Immunol. 2013 doi: 10.4049/jimmunol.1202672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McMorran BJ, Wieczorski L, Drysdale KE, et al. Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science. 2012;338(6112):1348–1351. doi: 10.1126/science.1228892. [DOI] [PubMed] [Google Scholar]
- 59. Engwerda CR, Good MF. Immunology. Platelets kill the parasite within. Science. 2012;338(6112):1304–1305. doi: 10.1126/science.1232439. A world wide health issue with a recently revealed platelet relevance.
- 60.Kerrigan SW, Cox D. Platelet-bacterial interactions. Cell Mol Life Sci. 2010;67(4):513–523. doi: 10.1007/s00018-009-0207-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tilley DO, Arman MA, Smolenski A, et al. Glycoprotein Ibα and FcγRIIa play key roles in platelet activation by the colonizing bacterium, Streptococcus oralis. J Thromb Haemost. 2013 doi: 10.1111/jth.12175. [DOI] [PubMed] [Google Scholar]
- 62.Plummer C, Wu H, Kerrigan SW, et al. A serine-rich glycoprotein of Streptococcus sanguis mediates adhesion to platelets via GPIb. Br J Haematol. 2005;129(1):101–109. doi: 10.1111/j.1365-2141.2005.05421.x. [DOI] [PubMed] [Google Scholar]
- 63.Reed GL, Fitzgerald ML, Polgár J. Molecular mechanisms of platelet exocytosis: insights into the "secrete" life of thrombocytes. Blood. 2000;96:3334–3342. [PubMed] [Google Scholar]
- 64.Ziu E, Mercado CP, Li Y, et al. Down-regulation of the serotonin transporter in hyperreactive platelets counteracts the pro-thrombotic effect of serotonin. J Mol Cell Cardiol. 2012;52(5):1112–1121. doi: 10.1016/j.yjmcc.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]