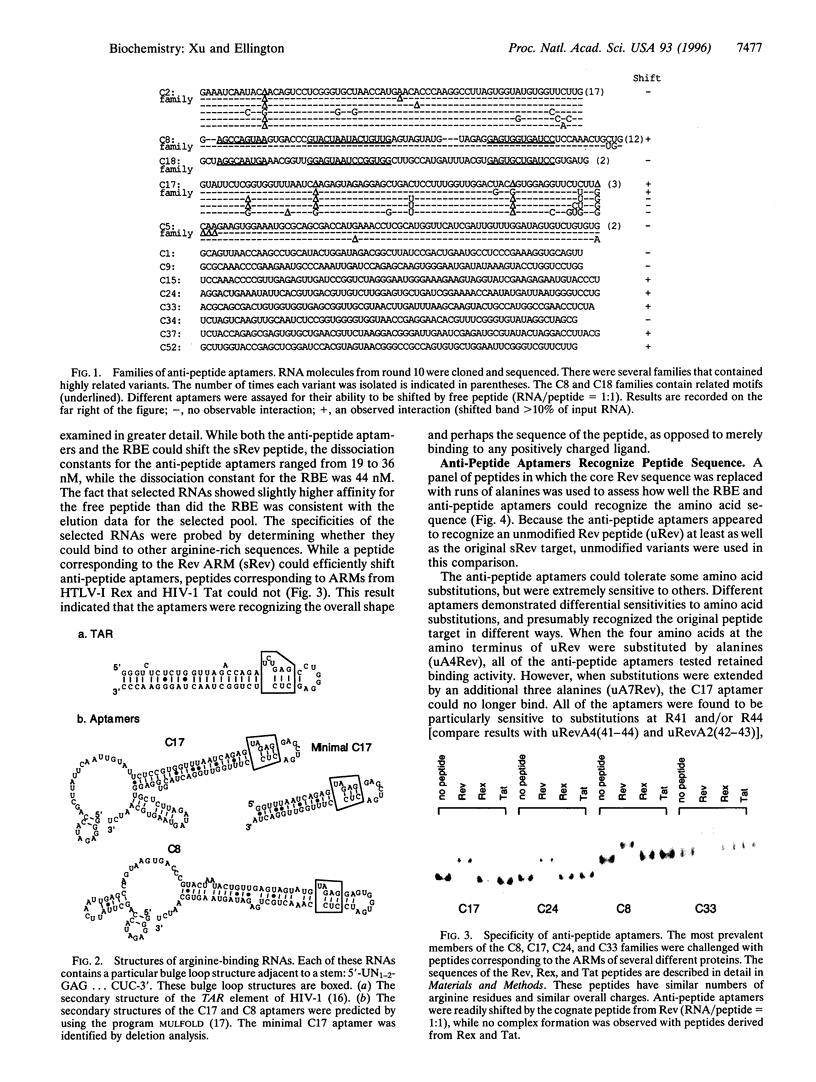
Abstract
In vitro selection of nucleic acid binding species (aptamers) is superficially similar to the immune response. Both processes produce biopolymers that can recognize targets with high affinity and specificity. While antibodies are known to recognize the sequence and conformation of protein surface features (epitopes), very little is known about the precise interactions between aptamers and their epitopes. Therefore, aptamers that could recognize a particular epitope, a peptide fragment of human immunodeficiency virus type I Rev, were selected from a random sequence RNA pool. Several of the selected RNAs could bind the free peptide more tightly than a natural RNA ligand, the Rev-binding element. In accord with the hypothesis that protein and nucleic acid binding cusps are functionally similar, interactions between aptamers and the peptide target could be disrupted by sequence substitutions. Moreover, the aptamers appeared to be able to bind peptides with different solution conformations, implying an induced fit mechanism for binding. Just as anti-peptide antibodies can sometimes recognize the corresponding epitope when presented in a protein, the anti-peptide aptamers were found to specifically bind to Rev.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltimore D. Gene therapy. Intracellular immunization. Nature. 1988 Sep 29;335(6189):395–396. doi: 10.1038/335395a0. [DOI] [PubMed] [Google Scholar]
- Baskerville S., Zapp M., Ellington A. D. High-resolution mapping of the human T-cell leukemia virus type 1 Rex-binding element by in vitro selection. J Virol. 1995 Dec;69(12):7559–7569. doi: 10.1128/jvi.69.12.7559-7569.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battiste J. L., Tan R., Frankel A. D., Williamson J. R. Binding of an HIV Rev peptide to Rev responsive element RNA induces formation of purine-purine base pairs. Biochemistry. 1994 Mar 15;33(10):2741–2747. doi: 10.1021/bi00176a001. [DOI] [PubMed] [Google Scholar]
- Chen H., Gold L. Selection of high-affinity RNA ligands to reverse transcriptase: inhibition of cDNA synthesis and RNase H activity. Biochemistry. 1994 Jul 26;33(29):8746–8756. doi: 10.1021/bi00195a016. [DOI] [PubMed] [Google Scholar]
- Conrad R. C., Baskerville S., Ellington A. D. In vitro selection methodologies to probe RNA function and structure. Mol Divers. 1995 Sep;1(1):69–78. doi: 10.1007/BF01715810. [DOI] [PubMed] [Google Scholar]
- Conrad R., Keranen L. M., Ellington A. D., Newton A. C. Isozyme-specific inhibition of protein kinase C by RNA aptamers. J Biol Chem. 1994 Dec 23;269(51):32051–32054. [PubMed] [Google Scholar]
- Crameri A., Stemmer W. P. 10(20)-fold aptamer library amplification without gel purification. Nucleic Acids Res. 1993 Sep 11;21(18):4410–4410. doi: 10.1093/nar/21.18.4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R., Malim M. H. The HIV-1 Rev protein: prototype of a novel class of eukaryotic post-transcriptional regulators. Trends Biochem Sci. 1991 Sep;16(9):346–350. doi: 10.1016/0968-0004(91)90141-h. [DOI] [PubMed] [Google Scholar]
- Geysen H. M., Meloen R. H., Barteling S. J. Use of peptide synthesis to probe viral antigens for epitopes to a resolution of a single amino acid. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3998–4002. doi: 10.1073/pnas.81.13.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giver L., Bartel D., Zapp M., Pawul A., Green M., Ellington A. D. Selective optimization of the Rev-binding element of HIV-1. Nucleic Acids Res. 1993 Nov 25;21(23):5509–5516. doi: 10.1093/nar/21.23.5509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold L., Polisky B., Uhlenbeck O., Yarus M. Diversity of oligonucleotide functions. Annu Rev Biochem. 1995;64:763–797. doi: 10.1146/annurev.bi.64.070195.003555. [DOI] [PubMed] [Google Scholar]
- Jaeger J. A., Turner D. H., Zuker M. Predicting optimal and suboptimal secondary structure for RNA. Methods Enzymol. 1990;183:281–306. doi: 10.1016/0076-6879(90)83019-6. [DOI] [PubMed] [Google Scholar]
- Kjems J., Calnan B. J., Frankel A. D., Sharp P. A. Specific binding of a basic peptide from HIV-1 Rev. EMBO J. 1992 Mar;11(3):1119–1129. doi: 10.1002/j.1460-2075.1992.tb05152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lato S. M., Boles A. R., Ellington A. D. In vitro selection of RNA lectins: using combinatorial chemistry to interpret ribozyme evolution. Chem Biol. 1995 May;2(5):291–303. doi: 10.1016/1074-5521(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Lerner R. A. Antibodies of predetermined specificity in biology and medicine. Adv Immunol. 1984;36:1–44. doi: 10.1016/s0065-2776(08)60898-6. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Nieuwlandt D., Wecker M., Gold L. In vitro selection of RNA ligands to substance P. Biochemistry. 1995 Apr 25;34(16):5651–5659. doi: 10.1021/bi00016a041. [DOI] [PubMed] [Google Scholar]
- Paborsky L. R., McCurdy S. N., Griffin L. C., Toole J. J., Leung L. L. The single-stranded DNA aptamer-binding site of human thrombin. J Biol Chem. 1993 Oct 5;268(28):20808–20811. [PubMed] [Google Scholar]
- Puglisi J. D., Tan R., Calnan B. J., Frankel A. D., Williamson J. R. Conformation of the TAR RNA-arginine complex by NMR spectroscopy. Science. 1992 Jul 3;257(5066):76–80. doi: 10.1126/science.1621097. [DOI] [PubMed] [Google Scholar]
- Rini J. M., Schulze-Gahmen U., Wilson I. A. Structural evidence for induced fit as a mechanism for antibody-antigen recognition. Science. 1992 Feb 21;255(5047):959–965. doi: 10.1126/science.1546293. [DOI] [PubMed] [Google Scholar]
- Rodda S. J., Geysen H. M., Mason T. J., Schoofs P. G. The antibody response to myoglobin--I. Systematic synthesis of myoglobin peptides reveals location and substructure of species-dependent continuous antigenic determinants. Mol Immunol. 1986 Jun;23(6):603–610. doi: 10.1016/0161-5890(86)90096-9. [DOI] [PubMed] [Google Scholar]
- Rowlands D. J. How can peptide vaccines work? FEMS Microbiol Lett. 1992 Dec 15;100(1-3):479–481. doi: 10.1111/j.1574-6968.1992.tb14080.x. [DOI] [PubMed] [Google Scholar]
- Stanfield R. L., Fieser T. M., Lerner R. A., Wilson I. A. Crystal structures of an antibody to a peptide and its complex with peptide antigen at 2.8 A. Science. 1990 May 11;248(4956):712–719. doi: 10.1126/science.2333521. [DOI] [PubMed] [Google Scholar]
- Stanfield R. L., Wilson I. A. Protein-peptide interactions. Curr Opin Struct Biol. 1995 Feb;5(1):103–113. doi: 10.1016/0959-440x(95)80015-s. [DOI] [PubMed] [Google Scholar]
- Tan R., Chen L., Buettner J. A., Hudson D., Frankel A. D. RNA recognition by an isolated alpha helix. Cell. 1993 Jun 4;73(5):1031–1040. doi: 10.1016/0092-8674(93)90280-4. [DOI] [PubMed] [Google Scholar]
- Tan R., Frankel A. D. Costabilization of peptide and RNA structure in an HIV Rev peptide-RRE complex. Biochemistry. 1994 Dec 6;33(48):14579–14585. doi: 10.1021/bi00252a025. [DOI] [PubMed] [Google Scholar]
- Tan R., Frankel A. D. Structural variety of arginine-rich RNA-binding peptides. Proc Natl Acad Sci U S A. 1995 Jun 6;92(12):5282–5286. doi: 10.1073/pnas.92.12.5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Adya N., Wagner S., Giam C. Z., Green M. R., Ellington A. D. Dissecting protein:protein interactions between transcription factors with an RNA aptamer. RNA. 1995 May;1(3):317–326. [PMC free article] [PubMed] [Google Scholar]
- Tsai D. E., Kenan D. J., Keene J. D. In vitro selection of an RNA epitope immunologically cross-reactive with a peptide. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):8864–8868. doi: 10.1073/pnas.89.19.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Regenmortel M. H. The conformational specificity of viral epitopes. FEMS Microbiol Lett. 1992 Dec 15;100(1-3):483–487. doi: 10.1111/j.1574-6968.1992.tb14081.x. [DOI] [PubMed] [Google Scholar]
- Weeks K. M., Crothers D. M. RNA recognition by Tat-derived peptides: interaction in the major groove? Cell. 1991 Aug 9;66(3):577–588. doi: 10.1016/0092-8674(81)90020-9. [DOI] [PubMed] [Google Scholar]
- Wien M. W., Filman D. J., Stura E. A., Guillot S., Delpeyroux F., Crainic R., Hogle J. M. Structure of the complex between the Fab fragment of a neutralizing antibody for type 1 poliovirus and its viral epitope. Nat Struct Biol. 1995 Mar;2(3):232–243. doi: 10.1038/nsb0395-232. [DOI] [PubMed] [Google Scholar]






