Abstract
Pistacia, a genus of flowering plants from the family Anacardiaceae, contains about twenty species, among them five are more popular including P. vera, P. atlantica, P. terebinthus, P. khinjuk, and P. lentiscus. Different parts of these species have been used in traditional medicine for various purposes like tonic, aphrodisiac, antiseptic, antihypertensive and management of dental, gastrointestinal, liver, urinary tract, and respiratory tract disorders. Scientific findings also revealed the wide pharmacological activities from various parts of these species, such as antioxidant, antimicrobial, antiviral, anticholinesterase, anti-inflammatory, antinociceptive, antidiabetic, antitumor, antihyperlipidemic, antiatherosclerotic, and hepatoprotective activities and also their beneficial effects in gastrointestinal disorders. Various types of phytochemical constituents like terpenoids, phenolic compounds, fatty acids, and sterols have also been isolated and identified from different parts of Pistacia species. The present review summarizes comprehensive information concerning ethnomedicinal uses, phytochemistry, and pharmacological activities of the five mentioned Pistacia species.
1. Introduction
The genus Pistacia belongs to the Anacardiaceae, a cosmopolitan family that comprise about 70 genera and over 600 species. The species of the genus Pistacia are evergreen or deciduous resin-bearing shrubs and trees which are characterized as xerophytic trees and growing to 8–10 m tall. Pistacia lentiscus L., P. atlantica Desf., P. terebinthus L., P. vera L., and P. khinjuk Stocks. are distributed from the Mediterranean basin to central Asia [1, 2]. Three Pistacia species naturally occur in Iran: P. vera L., P. khinjuk Stocks., and P. atlantica Desf.; P. atlantica has three subspecies or varieties which have been described as cabulica, kurdica, and mutica [3]. P. vera is the only species of the genus cultivated commercially, and the rest of the species are mostly used as rootstocks for P. vera [1, 2].
Different parts of Pistacia species have been investigated for various pharmacological activities. Most of the papers are devoted to the resin of P. lentiscus that is known as mastic. In addition to their therapeutic effects, Pistacia species are used in food industry, for example, consumption of pistachio (P. vera) nut as food additive [4], P. terebinthus fruit as snack food or in making coffee-like drink [5, 6], and the anthocyanin composition of P. lentiscus fruit as food colorants [7].
Chemical studies on Pistacia genus have led to discovering diverse secondary metabolites in addition to high level of vitamins and minerals.
Our review presents a comprehensive report on phytochemical aspects, pharmacological activities, and toxicity of the genus Pistacia by focusing on the data reported since the year 2000 via papers on databases including PubMed, Scopus, Google Scholar, and Web of Science.
2. Traditional Uses
Traditional uses, plant part used, and pharmacological activities of Pistacia lentiscus, P. atlantica, P. terebinthus, P. vera, and P. khinjuk from different regions are listed in Table 1.
Table 1.
Ethnomedicinal uses of selected Pistacia species.
| Species | Regions | Plant part(s) used | Traditional uses and ethnobotanical reports | Reference(s) |
|---|---|---|---|---|
| Pistacia lentiscus | Algeria | Leaf | Appetizer and astringent | [75] |
| Greece | Resin | Stomach ache, dyspepsia, stomach ulcer, intestinal disorders, hepatic inflammation, tooth disease, diabetes, hypercholesterolemia, and diuretic |
[33, 128, 129] | |
| Aerial part | Stimulant, diuretic, hypertension, kidney stones, jaundice, cough, sore throat, eczema, and stomach ache | [88] | ||
| Iraq | Resin | Abdominal pain | [130] | |
| Iran | Resin | Gum tissue strengthener, breath deodorizer, brain and liver tonic, and gastrointestinal ailments | [11, 100, 102] | |
| Italy | Leaf | Toothache, mycosis, herpes, abdominal and intestinal pain, rheumatism, antiseptic, cicatrizant, emollient, expectorant, and astringent | [131, 132] | |
| Jordan | Leaf | Jaundice | [121, 133] | |
| Resin | Heart burn and stomach ache | |||
| Morocco | Leaf | Digestive disease, evil eye | [134] | |
| Portugal | Leaf, bark | Gastric analgesic | [135] | |
| Root | Antiseptic and antiodontalgic | [135] | ||
| Seeds | Antirheumatic | [135] | ||
| Stem | Buccal antiseptic | [135] | ||
| Spain | Aerial part | Hypertension | [136] | |
| Fruit | Influenza | [71] | ||
| Leaf | Dermatophytosis in cows | [72] | ||
| Tender bud | Warts | [73] | ||
| Tunisia | Fruit | Edible usage, condiment, scabies, Rheumatism, and antidiarrheal |
[60] | |
| Turkey | leaf | Eczema, diarrhea, throat infections, paralysis, kidney stones, Jaundice, asthma, stomach ache, astringent, anti-inflammatory, antipyretic, and stimulant | [96] | |
|
| ||||
| Pistacia atlantica | Algeria | Fruit | Stomach ache, cough, stress, tonic, and antidiarrheal |
[20, 63] |
| Greek | Fruit | Mouth flavouring, tanning, and as fodder | [31] | |
| Iran | Aerial part | Veterinary | [31] | |
| Fruit | Antidiarrheal | [11] | ||
| Resin | Peptic ulcer, mouth freshener, antiseptic, gum tissue strengthener, as chewing gum, appetizer, phlegm dissolver, astringent, laxative, demulcent, diuretic, emmenagogue, carminative, visceral inflammation, scabies, stomach, liver and kidneys tonic, gastrointestinal disorders, and motion sickness | [9] | ||
| Resin, bark | Joint pains, toothache, wound healing | [137] | ||
| Jordan | Fruit | Stomach ache | [133] | |
| leaf | Antidiabetic | [109] | ||
| Morocco | Leaf | Eye infection | [134] | |
| Resin | Gum tissue strengthener, breath deodorizer, cough, chill, and stomach disease | [27] | ||
| Turkey | Fruit | Mouth disease | [138] | |
| leaf | As vegetables and food | [127] | ||
| Resin | Wound healing | [138] | ||
|
| ||||
| Pistacia terebinthus | Greece | Resin | Antidote, aphrodisiac, expectorant, and treatment of leprosy | [139] |
| Iran | Resin | Smoke of it as air purifier and antiseptic | [140] | |
| Leaf, bark | Astringent and antidiarrhea | [11] | ||
| Jordan | Resin | Diuretic, laxative, stimulant, and aphrodisiac | [18] | |
| Leaf | Diuretic, antihypertensive, and treatment of jaundice | [18] | ||
| Spain | Aerial part | Hypotensive and cephalalgic | [141] | |
| Branch | Antiseptic | [141] | ||
| Flower, leaf | Odontalgia and Dislocated joint | [142] | ||
| Fruit | Antiprostatitis | [141] | ||
| Turkey | Fruit | Cold, flu, diuretic, stomach ache, rheumatism, stimulant, antitussive, appetizer, as coffee, urinary inflammations, and soap production |
[29, 53, 138, 143] | |
| Leaf | Stomach ache, mycosis, and antidiabetic | [29, 53, 144, 145] | ||
| Resin | Urinary and respiratory antiseptic, asthma, antipyretic, and anti-inflammatory | [53] | ||
|
| ||||
| Pistacia vera | Iran | Nut shell | Tonic, sedative, and antidiarrhea | [11] |
| Fruit | Food | [10] | ||
| Jordan | Oil | Facial skin cleanser | [133] | |
| Turkey | Resin | Asthma, stomach ache, and hemorrhoids | [146] | |
|
| ||||
| Pistacia khinjuk | Iran | Aerial part | Veterinary use | [147] |
| Resin | Stomach discomfort, nausea, vomiting, and motion sickness | [148] | ||
Different parts of Pistacia species including resin, leave, fruit, and aerial part have been traditionally used for a wide range of purposes. Among them, P. lentiscus is the most commonly used in different regions and resin of that has been utilized for as long as 5000 years. Resin of P. lentiscus has been used for variety of gastric ailments in the Mediterranean and Middle East countries for the last 3000 years [8]. It was used in ancient Egypt as incense; it has also been used as a preservative and breath sweetener [4] Most of the traditional uses reports for resin of P. atlantica are from Iran and have been used for the treatment of digestive, hepatic, and kidney diseases [9]. Fruit of P. vera (pistachio) is used all over the world. Records of the consumption of pistachio as a food date to 7000 BC [4]. Pistachio is cultivated in the Middle East, United States, and Mediterranean countries. Iran is one of the biggest producers and exporters of pistachio nuts [10]. In traditional Iranian medicine (TIM), different parts of P. vera, P. atlantica, P. khinjuk P. terebinthus, and P. lentiscus have been used for a long time as useful remedies for different diseases, for example, the fruit kernel of P. vera as a cardiac, stomach, hepatic, and brain tonic; the fruits of P. atlantica, P. khinjuk, and P. terebinthus for their aphrodisiac activity and treatment of liver, kidney, heart, and respiratory system disorders, and the gum resin of P. lentiscus, P. atlantica, P. khinjuk, and P. terebinthus for their wound healing activity, and treatment of brain and gastrointestinal disorders [9, 11].
3. Phytochemical Studies
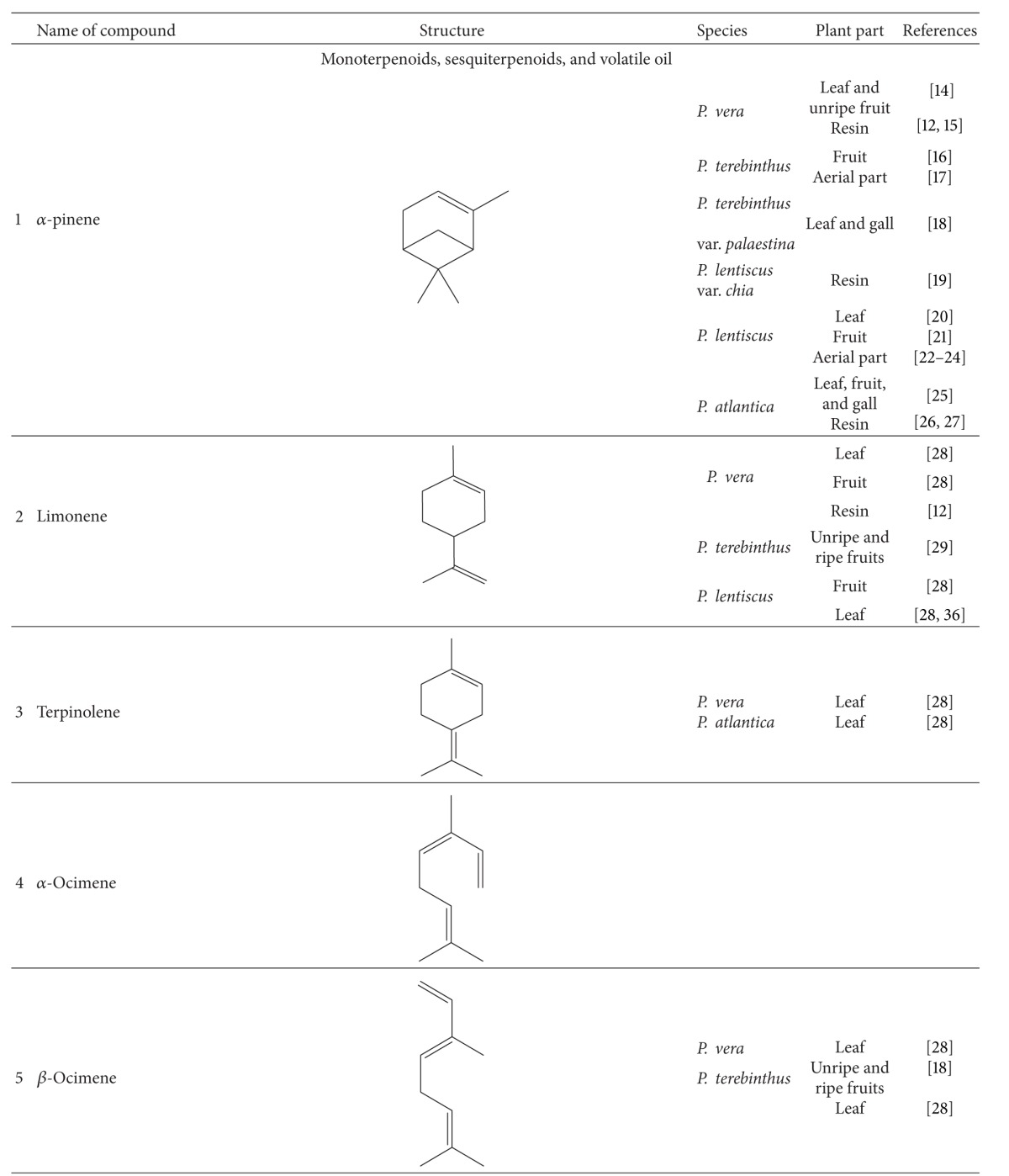
Various compounds from different phytochemical groups were identified in Pistacia species. These are summarized below and also in Table 2 based on the structure of finding components.
Table 2.
Chemical compounds isolated from selected Pistacia species.


|
3.1. Terpenoids
3.1.1. Monoterpenoids, Sesquiterpenoids, and Volatile Oil
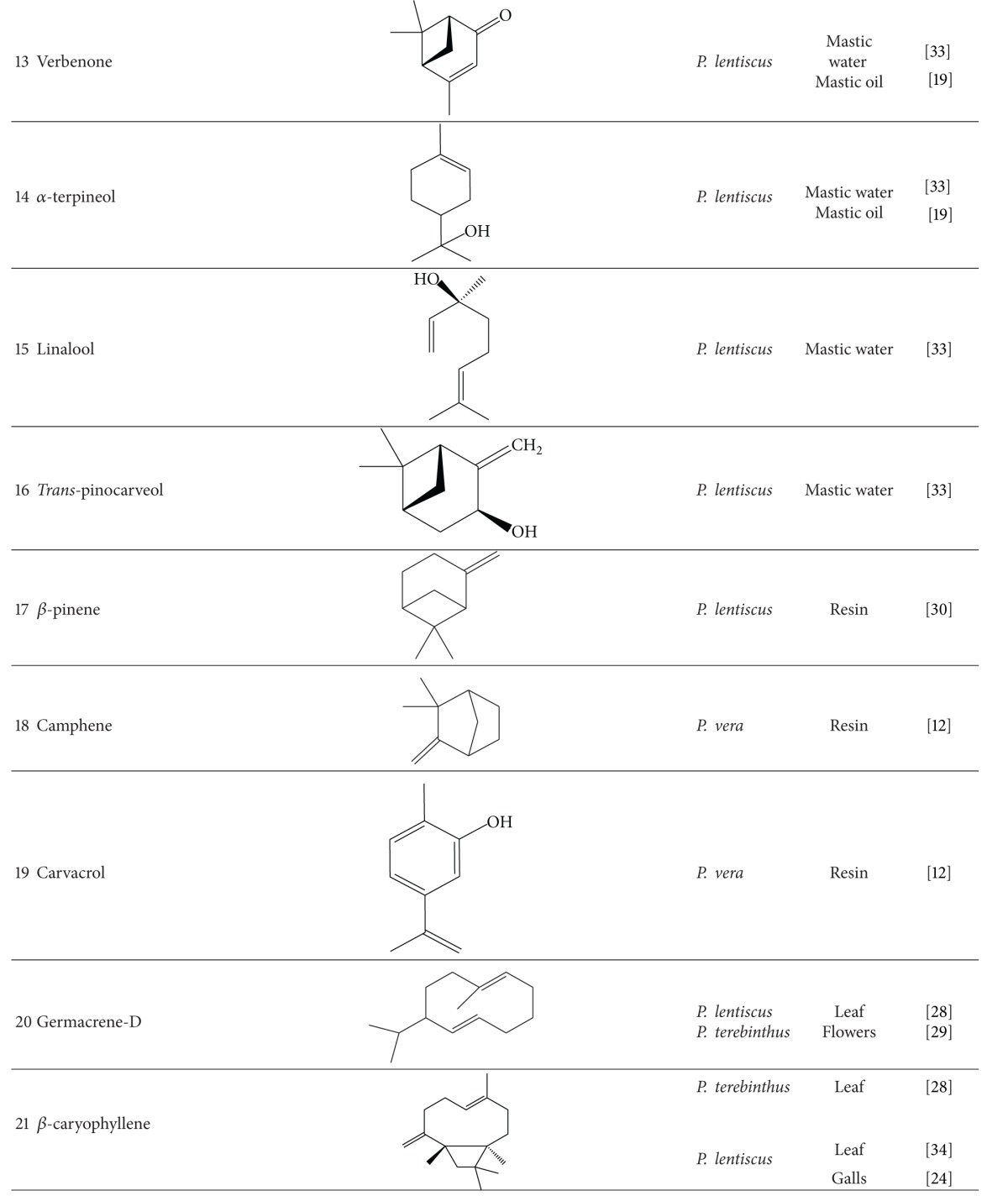
Essential oil is one of the main components reported from different parts of Pistacia species including leaves, resin, ripe and unripe fruits, galls, leaf-buds, twigs, and flowers. Analysis of essential oils is mostly performed by means of gas-chromatography (GC) based techniques. There are many qualitative and quantitative variations between the content of essential oils. These variations are related to several parameters like plant species and part, sex of cultivars, harvesting time, geographical origin, and climatic conditions [12, 13]. Hydrocarbon and oxygenated monoterpens are the major chemical constituents in essential oil and among hydrocarbon monoterpens, α-pinene (1) has been reported as the main compound of some samples like P. vera [12, 14, 15], P. terebinthus [16–18], P. lentiscus [19–24], and P. atlantica [25–27]. In addition to α-pinene, other major components isolated from different parts of Pistacia species are as follows: limonene (2), α-terpinolene, and ocimene (3,4) from fruits and leaves of P. vera [28]; (E)-β-Ocimene (5) and limonene in fruits [18, 28, 29]; (E)-β-Ocimene and terpinen-4-ol (6) in leaves and p-cymen, (7) in young shoots of P. terebinthus [28–30]; bornyl acetate (8), terpinen-4-ol, sabinene (9), and myrcene (10) in fruits, terpinen-4-ol, myrcene, p-mentha-1 (7),8 diene (11), and ocimene from leaves [27, 28, 31], sabinene and p-mentha-1 (7),8 diene in leaf buds, and Δ3-carene (12) in unripe galls of P. atlantica [31, 32]. Monoterpens are also detected in mastic water which was separated from the mastic oil during steam distillation. Verbenone (13), α-terpineol (14), linalool (15), and trans-pinocarveol (16) are the main constituents of mastic water [33]. β-pinene (17) in oleoresin, β-myrcene and sabinene in fruits [28, 30, 34], terpinen-4-ol in aerial parts [22], and limonene, myrcene, sabinene, and teroinen-4-ol in leaves of P. lentiscus were determined as the main composition [28, 30, 35, 36].
Some of the other monoterpenes identified as effective antibacterial components of these essential oils are camphene (18), limonene, and carvacrol (19) from P. vera resin [12].
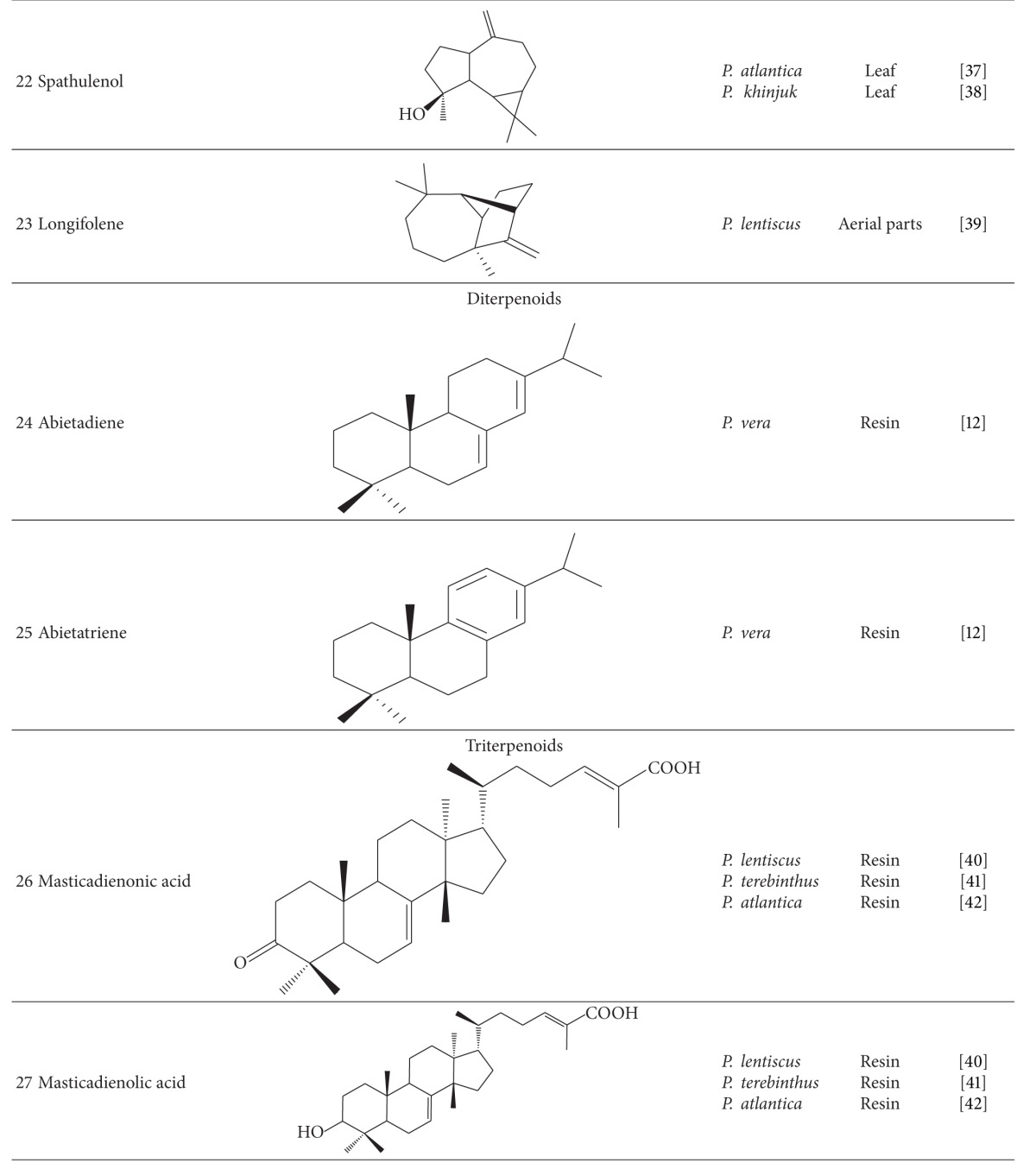
Sesquiterpenes isolated in lower amount compared with monoterpenes. Germacrene-D (20) and β-caryophyllene (21) were identified in P. lentiscus and P. terebinthus leaves with higher concentration in comparison with other sesquiterpenes [28]. Spathulenol (22), an azulenic sesquiterpene alcohol, is the predominant component of leaves of P. atlantica and P. khinjuk [37, 38]. Congiu et. al. [34] recovered Caryophyllene with the highest amount from P. lentiscus leaves by means of supercritical CO2 extraction. Germacrene-D in P. terebinthus flowers, β-caryophyllene in P. lentiscus galls, and Longifolene (23) in aerial parts of P. lentiscus are dominant [24, 29, 39].
3.1.2. Diterpenoids
Trace amounts of Diterpenoids were isolated from the essential oil of these species. Abietadiene (24) and abietatriene (25) were detected in essential oil of P. vera resin [12].
3.1.3. Triterpenoids
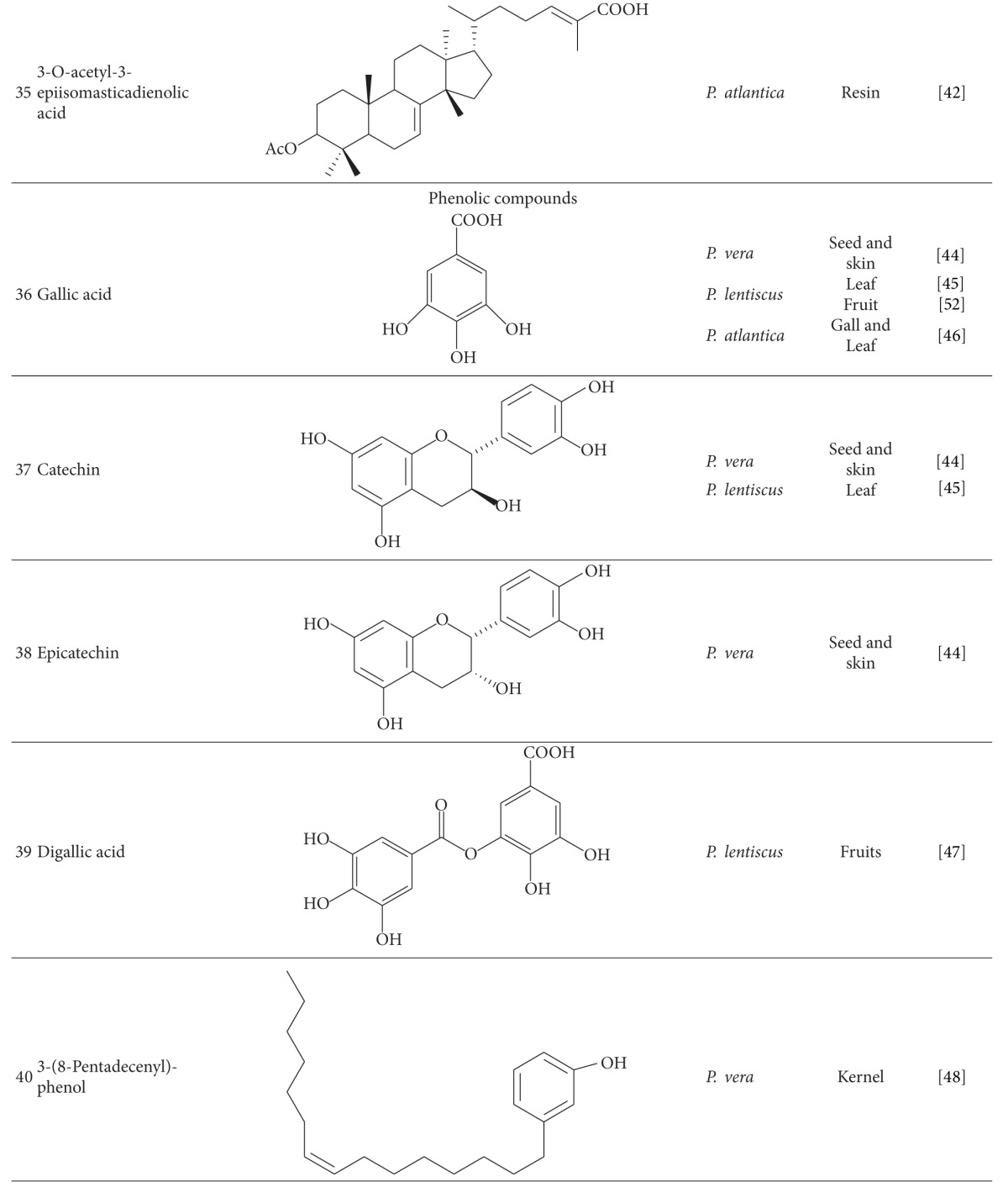
Resin of these species has been characterized by penta and tetracyclic triterpenes. Triterpenes such as masticadienonic acid (26), masticadienolic acid (27), morolic acid (28), oleanolic acid (29), ursonic acid (30) and their derivatives have been detected in acidic fractions of P. lentiscus, P. terebinthus, and P. atlantica resins [40–42]. Several triterpenoid compounds were isolated from neutral fraction of P. lentiscus and P. terebinthus resins like tirucallol (31), dammaradienone (32), β-Amyrin (33), lupeol (34), oleanolic aldehyde, and 28-norolean-12-en-3-one. Quantitative and qualitative varieties in chemical composition of resins according to the method of collection were reported [40, 41].
Anti-inflammatory properties have been reported from masticadienolic acid, masticadienonic acid, and morolic acid isolated from P. terebinthus [43]. Among triterpenes isolated from the resin of three sub-species of P. atlantica (kurdica, cabulica and mutica), 3-O-acetyl-3-epiisomasticadienolic acid (35) has been identified as the most effective antimicrobial agent [42].
3.2. Phenolic Compounds
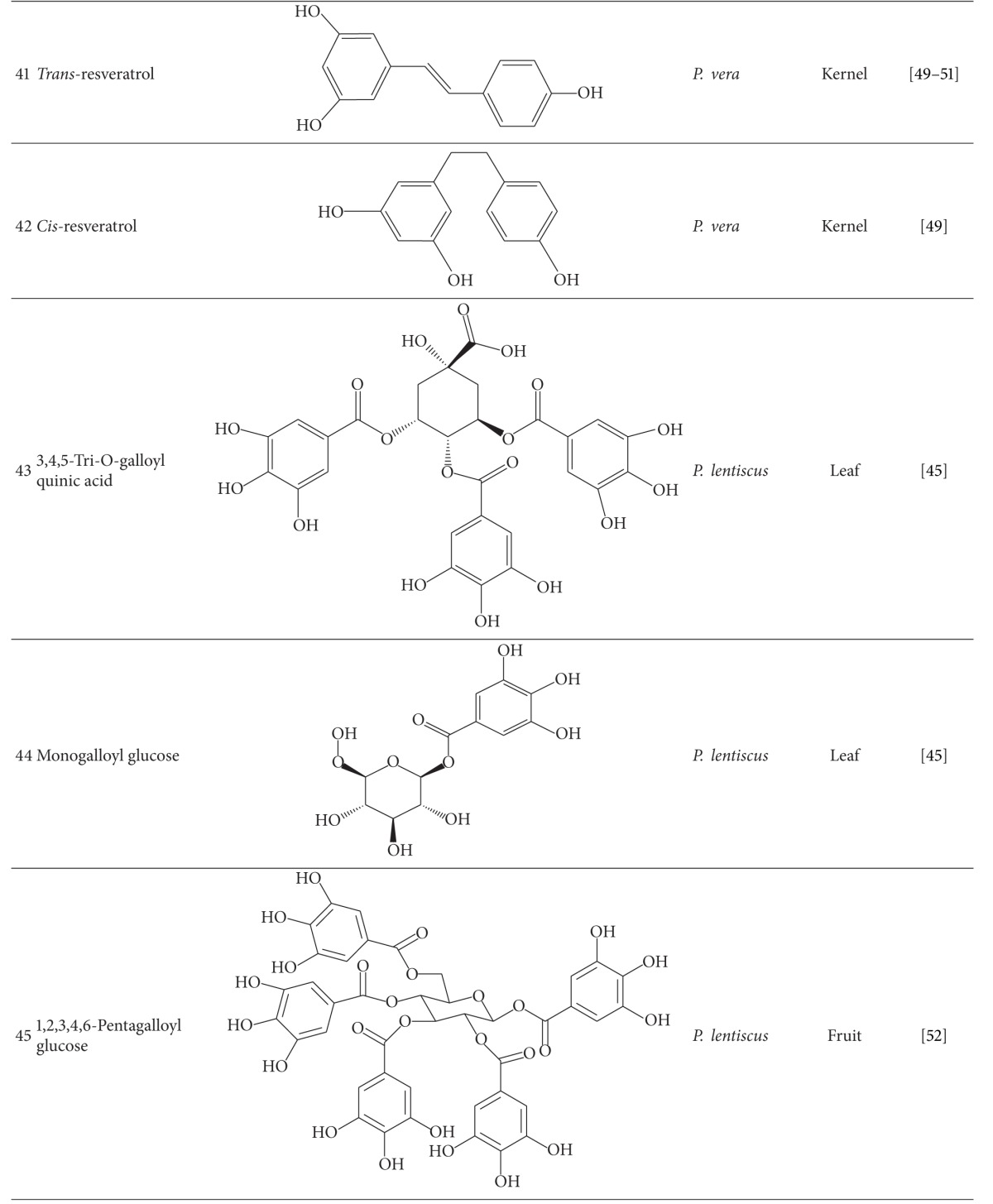
Gallic acid (36), catechin (37), epicatechin (38), and gallic acid methyl ester were identified in P. vera seed and skin, leaves of P. lentiscus and leaves and galls of P. atlantica [44–46]. Bhouri et al. [47] demonstrated that digallic acid (39) from fruits of P. lentiscus has anti-mutagenic properties. Monounsaturated, diunsaturated, and saturated cardanols have been detected in P. vera kernel. 3-(8-Pentadecenyl)-phenol (40) was the dominating cardanol in P. vera [48]. Trans and cis isomers of phytoalexin, resveratrol (3,5,4′-trihydroxystilbene) (41-42), and trans-resveratrol-3-O-β-glucoside (trans-piceid) were quantified in P. vera kernel [49–51]. P. lentiscus leaf is a rich source of polyphenol compounds (7/5% of leaf dry weight) especially galloyl derivatives like mono, di, and tri-O-galloyl quinic acid (43) and monogalloyl glucose (44) [45].
1,2,3,4,6-Pentagalloyl glucose (45) and gallic acid from fruits of P. lentiscus were introduced as antioxidant and anti-mutagenic compounds [52].
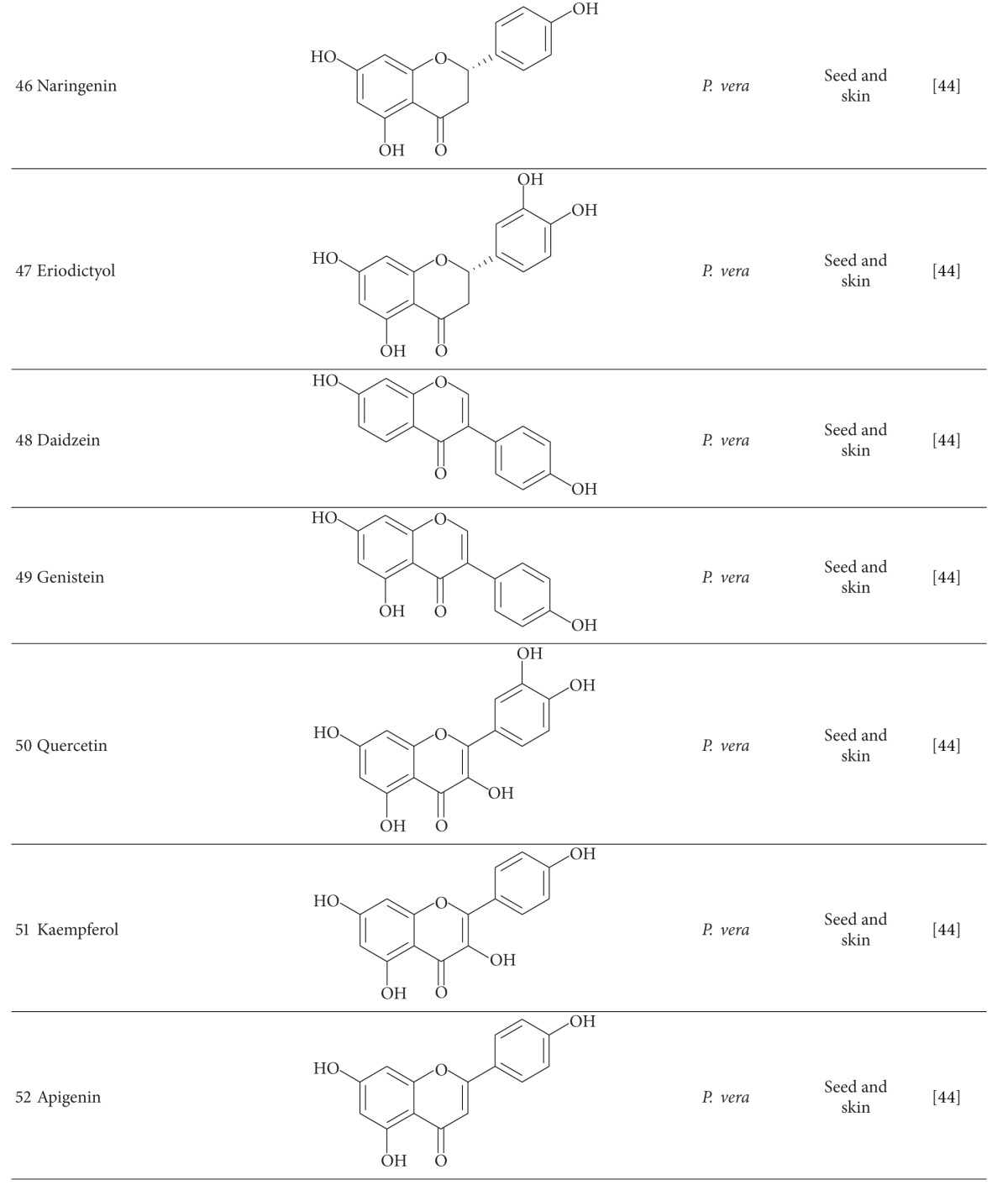
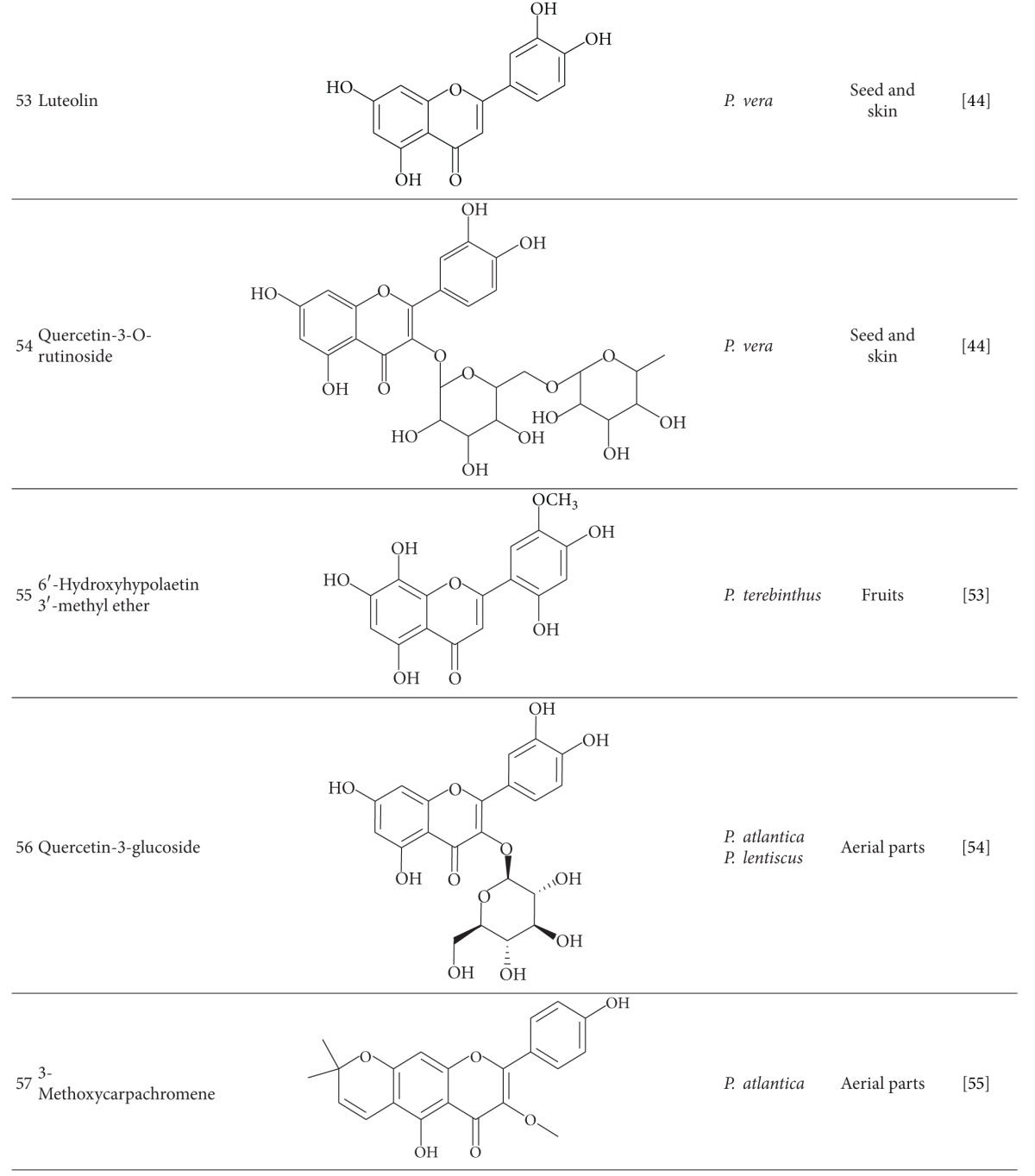
Flavonoid compounds have been detected in different parts of these species. Naringenin (46), eriodyctyol (47), daizein (48), genistein (49), quercetin (50), kaempferol (51), apigenin (52), and luteolin (53) were isolated from P. vera fruit, and quercetin-3-O-rutinoside (54) is the main constituent of seed [44]. Decrease in flavonoid content of P. vera has been reported during the fruit ripening [51]. In addition to some known flavonoids isolated from P. terebinthus and P. atlantica fruits, 6′-hydroxyhypolaetin 3′-methyl ether (55) has been identified in fruits of P. terebinthus [46, 53]. Flavonoids were also isolated from aerial parts of P. atlantica and P. lentiscus, and quercetin-3-glucoside (56) was reported as the most abundant one [54]. 3-Methoxycarpachromene (57), a flavone with antiplasmodial activity, was isolated from aerial parts of P. atlantica [55].
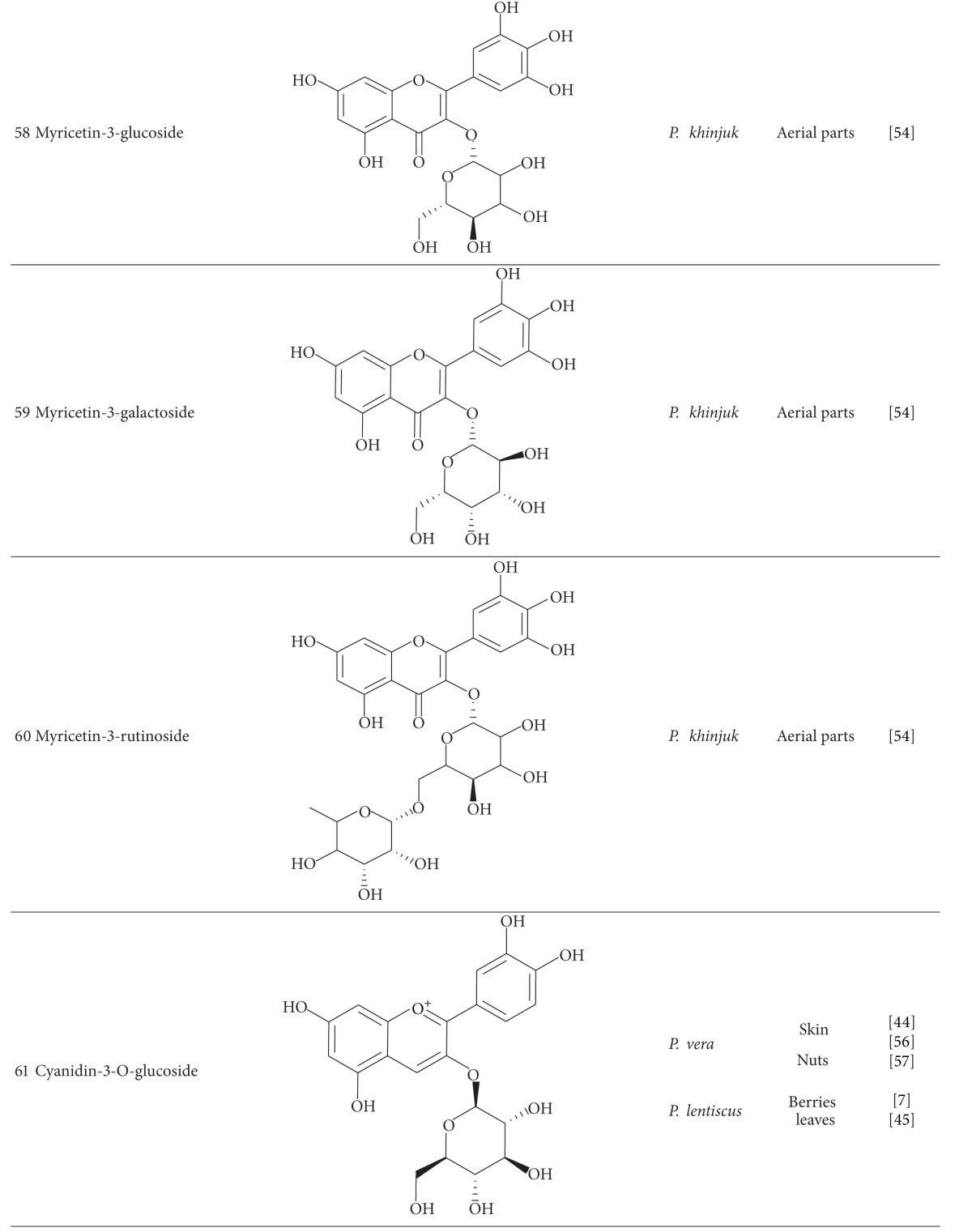
Myricetin-3-glucoside (58), myricetin-3-galactoside (59), and myricetin-3-rutinoside (60) are the major flavonoid glycosides from P. khinjuk [54]. Myricetin derivatives also were determined as 20% of the total polyphenol amount of P. lentiscus leaves [45].
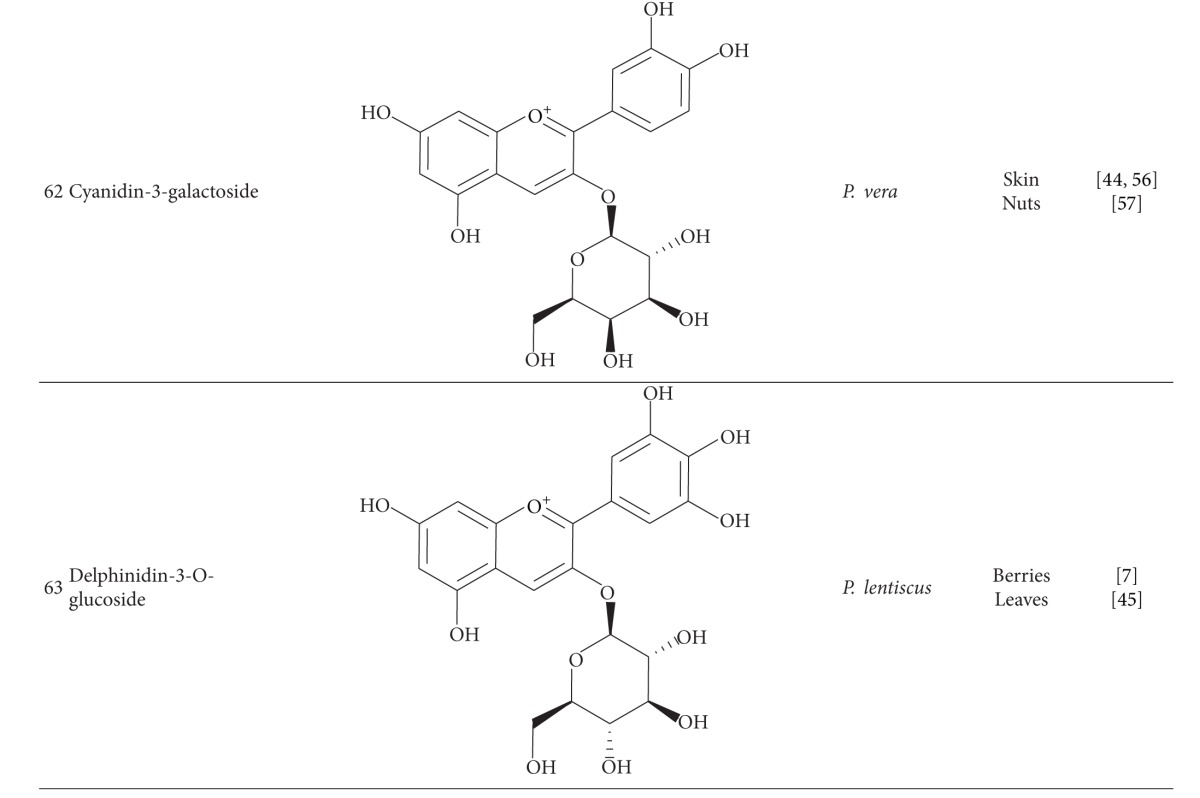
Anthocyanins have been reported from some Pistacia species. Cyanidin-3-O-glucoside (61), cyanidin-3-galactoside (62), and quercetin-3-O-rutinoside are the main anthocyanins of P. vera fruit [44, 56, 57]. Cyanidin-3-O-glucoside and delphinidin-3-O-glucoside (63) have been detected in P. lentiscus berries and leaves [7, 45].
3.3. Fatty Acids and Sterols
Pistacia species have oleaginous fruits considered by several researchers. The oil content in P. vera kernel and seed is about 50–60% [58, 59] and in ripe fruits of P. lentiscus, P. terebinthus, and P. atlantica is 32.8–45% [60–63]. The main fatty acid in seed and kernel of P. vera is oleic acid [58, 64, 65]. Oleic acid has been also determined as the most abundant fatty acid in oil of P. atlantica and P. terebinthus fruits [62, 66, 67]. Increase of oleic acid and decrease of linoleic acid have been recorded during ripening of P. lentiscus fruits [60]. Other fatty acids identified in these species are linolenic, palmitic, palmitoleic, stearic, myristic, eicosanoic, behenic, lignoceric, arachidonic, pentadecanoic, hexadecanoic, octadecanoic, and margaric acid [58, 66, 68].
The most abundant sterol reported in fruits of P. vera, P. atlantica, P. lentiscus, and P. terebinthus is β-sitosterol fallowed by campesterol, Δ5-avenasterol, stigmasterol, brassicasterol, and cholesterol [59, 60, 69, 70].
The oil from fruits of P. atlantica, P. lentiscus, and P. terebinthus, in addition to its desirable odor and taste, has been recommended as a new source for production of vegetable oils concerning the high amount of mono-unsaturated and omega-3 fatty acids like oleic acid and linolenic acid and high quantity of phytosterols like β-sitosterol [60, 68].
3.4. Miscellaneous
Chlorophylls a and b and lutein are the major colored components of P. vera nuts [56]. Pheophytin, β-carotene, neoxanthin, luteoxanthin, and violaxanthin were also determined in different samples of P. vera nuts [71]. α-tocopherol was determined in leaves of P. lentiscus, P. lentiscus var. chia, and P. terebithus [72]. Tocopherols and tocotrienols are the most abundant constituents of unsaponifiable matter of P. atlantica hull oil [73]. Different isomers of tocopherol, tocotrienol, and plastochromanol-8 have been identified in seed oil of P. terebinthus [70]. Evaluating the nutritional composition of P. terebinthus fruits illustrates the richness of this fruit in protein, oil, minerals, and fiber [62, 68].
4. Pharmacological Aspects
Different pharmacological activities of five mentioned Pistacia species have been described in detail in Table 3.
Table 3.
Pharmacological activities of selected Pistacia species.
| Pharmacological activity | Plant | Plant part | Assay | Extract/essential oil/isolated component | Dose or concentration |
Observations | Ref. |
|---|---|---|---|---|---|---|---|
| Antioxidant | P. lentiscus | Fruits | In vitro DPPH method |
Polyphenols: galic acid (GA) and 1,2,3,4,6 pentagalloyl-glucose (PGA) |
1, 3, 10, 30, and 100 µg/mL |
Dose dependent radical scavenging activity of GA (IC50: 2 µg/mL) and PGA (IC50: 1 µg/mL) | [52] |
| Xanthine oxidase inhibition | 100, 200, and 300 µg/mL | ↑formation of uric acid and superoxide anions (O2-) by increasing concentrations of both GA and PGA | |||||
| Inhibition of lipid peroxidation induced by H2O2 in K562 cell line | 200, 400, and 800 µg/mL for GA and 100, 200, and 400 µg/mL for PGA | Dose dependent inhibition by GA (IC50: 220 µg/mL) and PGA (IC50: 200 µg/mL) | |||||
| Leaf | Reducing power |
Seven different extracts (1) Ethanol, (2) Ethyl acetate, (3) Aqueous/ethyl acetate, (4) Hexane, (5) Aqueous/hexane, (6) Chloroform, (7) Aqueous/chloroform |
100 µg/mL | Higher activity of aqueous fractions from hexane and chloroform than standards (BHA and α-tocopherol) | [75] | ||
| Linoleic acid peroxidation | 100 µg/mL | Inhibition of linoleic acid peroxidation by aqueous extracts from chloroform and hexane comparable to those of the standard (BHA) | |||||
| DPPH method | 10–100 µg/mL | High scavenging activity (90%) equivalent to that of the standard BHA (89%) by all extracts except chloroform | |||||
| Scavenging activity against hydrogen peroxide | 100 µg/mL | High scavenging capacity against H2O2 comparable to standards (α-tocopherol and BHA) | |||||
| Aerial parts | DPPH method | Essential oil | 0.2, 0.4, 1.0, 2.0, and 4.0 mM | Antioxidant activity ranged between 0.52 and 4.61 mmol/L | [74] | ||
| DPPH method | Methanolic extracts |
100, 80, 50, 30, 20, 10, and 5 mg/L | IC50 ranged between 5.09 and 11.0 mg/L | [23] | |||
| FRAP assay | 5000 mg/L | Activity ranged between 84.6 and 131.4 mmol Fe2+/L plant extract; IC50: 5.09–11.0 (mg/L) | |||||
| P. lentiscus var. chia | Resin | Oil oxidation assay by the oven test | Resin solution in dichloromethane | 0.05, 0.1, and 0.15% w/w | Significant antioxidant activity | [149] | |
| P. lentiscus | Fruit | ABTS | Digallic acid | 0.05, 0.1, 0.15, and 0.2 mg/mL | Free radical scavenging activity towards the ABTS + radical was 99% at 0.2 mg/mL | [47] | |
| Xanthine oxidase (XO) inhibition and superoxide scavenging activity | 50, 100, and 150 µg/mL | 21% XO inhibitory activity at 150 µg/mL; 28% reduction of superoxide anion activity | |||||
| TBARs | 200, 400, and 800 µg/mL | ↓lipid peroxidation (IC50: 178 µg/mL) | |||||
| Gum | Electron-spin resonance Spectroscopy for the determination of hydroxyl radical by Fenton reaction | Mastic in water | ND | Effectively scavenged hydroxyl radical generated by the Fenton reaction |
[76] | ||
| Nitrate/nitrite colorimetric assay |
0–3 mg/mL | No nitric oxide scavenging activity | |||||
| P. lentiscus var. chia, P. terebinthus. var. chia | Gum | Copper-induced LDL oxidation | Hexane and methanol/water extracts | 2.5, 5, 10, 25, and 50 mg/2 mL | LDL protective activity; methanol/water extract of P. lentiscus showed the most LDL protection |
[77] | |
| P. lentiscus | Leaf | Reduction power activity | Ethanolic extract | 0.25; 0.5; 0.75; 1; 2; 3 mg/mL |
Reducing power comparable to ascorbic acid | [88] | |
| Pyrogallol autoxidation method | ND | Superoxide anions scavenging activity | |||||
| P. atlantica. | Leaf | Reduction power activity | Ethanolic extracts | 0.25; 0.5; 0.75; 1; 2; 3 mg/mL |
Reducing power close to values observed by ascorbic acid | [88] | |
| Pyrogallol autoxidation method | ND | Superoxide anions scavenger at a concentration as low as 0.0625 mg/mL | |||||
|
P. atlantica
subsp. mutica |
Hull | FRAP test | The unsaponifiable matter (USM) of fruit's hull oil | 100 mg in 10 mL of n-hexane | Significant reducing power; the highest reducing power amongst the USM fractions belonged to the tocopherols and tocotrienols and linear and triterpenic alcohols respectively | [80] | |
| DPPH radical-scavenging assay | ND |
EC50 value significantly lower than α-tocopherol | |||||
| Oven test | ND | Significant stabilizing effect | |||||
| P. atlantica | Leaf | (1) Reducing power (2) Chelating abilities on metallic ions (3) Radical scavenging Activity (DPPH) (4) The total antioxidant activity (thiocyanate method in linoleic acid emulsion) (5) Hydrogen peroxide scavenging activity |
Decoction | (1) 20–100 μg/mL (2) 0.25, 0.50, 0.75, and 1.0 mg/mL (3) 5–25 μg/mL (4) 100 μg/mL (5) 100 μg/mL |
(1) Reducing power of significantly higher than α-tocopherol and BHT and nearly similar to BHA (2) The chelating activity of 1.0 mg/mL was nearly fourfold less than EDTA at 0.037 mg/mL and has slightly effective capacity for iron binding (3) 85% inhibition rate at 15 μg/mL. nearly similar to ascorbic acid and BHA (4) Higher antioxidant activity than α-tocopherol and similar to BHA, BHT, and trolox (5) Concentration-dependent scavenging compared to BHA, BHT, and α-tocopherol |
[78] | |
|
P. atlantica
subsp. mutica |
Fruit hull | Rancimat test |
n-Hexane extract | Different percentages (up to 15%) | The antioxidant activity of hull oil was exactly the same as that of TBHQ at low concentrations | [79] | |
| P. atlantica | Leaf | DPPH test | Essential oil | 50 µL | Weak radical scavenging activity | [32] | |
| FRAP test | ND | Higher antioxidant capacity relative to ascorbic acid | |||||
| P. vera | Fruit hull | Oven test | Water and methanol extracts | 0.02%, 0.04%, and 0.06% in soybean oil | Effective in retarding oil deterioration at 60°C; at concentration of 0.06%, similar to BHA and BHT added at 0.02%. | [81] | |
| P. vera L., var. Bronte | Kernel | ABTS radical cation decolorization assay | Methanol/water or Dichloromethane |
ND | The antioxidant activity of the lipophilic extract was much lower than hydrophilic one | [82] | |
| Lipid peroxidation (TBARS assay) |
Hydrophilic extract | 0.25, 0.5, or 1.0 mg/mL | Radical scavenging activity in a dose-dependent manner | ||||
| Copper-mediated LDL oxidation | Hydrophilic extract | Extracts from 30, 60, or 100 µg of nut | Inhibition of LDL oxidation | ||||
| Seed and skin (hull) | DPPH assay | Methanol/water extract | 0.050–12.00 mg/mL | Radical scavenging activity | [44] | ||
| Trolox equivalent antioxidant capacity (TEAC) assay (ABTS radical) |
ND | Antioxidant power: 0.015 ± 0.001 and 2.19 ± 0.14 mmol Trolox/g of seeds and skins, respectively | |||||
| Scavenging activity against the superoxide anion | ND | IC50 of 3.25 ± 0.19 and 0.25 ± 0.02 mg for seeds and skins, respectively | |||||
| P. vera | Gum | TBARS and FRAP in rat | Extract | 0.1–0.5 g/kg | ↓brain MDA level by 63% and ↑antioxidant power of brain by 235% | [83] | |
| Hull | DPPH assay | Aqueous | 1, 1.5, 2,5, 3,5 and 4 μg/mL | Concentration-dependent radical scavenging activity | [150] | ||
| ABTS assay | ND | Scavenging capacity of crude and purified extracts was higher than standards compounds (TBHQ and BHT) | |||||
| β-carotene bleaching method | 0.48–9.5 μg/mL | Concentration-dependent antioxidant capacity | |||||
| P. terebinthus | Leaf | Trolox equivalent antioxidant capacity assay (ABTS/K2S8O2 method) | Ethanol-water extract | ND | Considerably higher antioxidant activity compared with BHA and ascorbic acid | [84] | |
| Fruits | DPPH test | Acetone and methanol extracts | 25, 50 and 100 µg/mL | High radical scavenging activity | [53] | ||
| Total antioxidant activity in β-carotene-linoleic acid system | 25, 50 and 100 µg | Isolated pure 60-hydroxyhypolaetin-30-methyl Ether showed higher antioxidant activity than both extracts and BHT |
|||||
| Superoxide anion scavenging activity | 50 µg | Both extracts had scavenging activity near to ascorbic acid; higher activity of methanol extract than acetone extract | |||||
| FRAP | 0.2–1 µg/mL | Higher reducing power of methanol extract than α-tocopherol; acetone extract reducing power was equal to that of α-tocopherol | |||||
| Metal chelating activity | 1000–4000 µg/mL | Methanol extract had higher activity than acetone extract | |||||
| Fruits and 4 terebinth coffee brands | DPPH radical scavenging activity | Ethyl acetate and methanol extracts | 250, 500, 1000 and 2000 µg/mL | High scavenging effect especially at 2000 μg/mL | [85] | ||
| DMPD radical scavenging activity | Scavenging effect lower than that of quercetin | ||||||
| H2O2 radical scavenging activity | Inactive in scavenging H2O2 radical | ||||||
| Metal-chelation effect | Remarkable metal-chelation properties as compared to EDTA |
||||||
| FRAP assay | High reducing power | ||||||
| PRAP assay | High reducing power | ||||||
|
| |||||||
| Antimutagenic | P. lentiscus | Leaf | Aflatoxin B1 (AFB1)-induced mutagenicity in S. typhimurium TA 100 | Essential oil | 250, 500 and 1000 µg/plate | Mutagenic inhibition of 76.7% by 250, 82.8% by 500, and 96.5% by 1000 µg/plate | [86] |
| (AFB1)-induced mutagenicity in S. typhimurium TA100 or TA98 | Essential oil | 0.3, 250, 500, 1000 µg/plate |
In TA100: 76, 82.8, and 96.5%, mutagenic inhibition rate for 250, 500, and 1000 µg/plate, respectively; in TA98: 99 and 100% mutagenic inhibition rate with 250 and 500 µg/plate | [87] | |||
| Aqueous extract | 0.3, 50, 300, 600 µg/plate | 50 µg/plate: 23% inhibition in TA100 and 52.2% in TA98; 300 and 600 µg/plate: 67.7 and 87.8% for TA100 and 58–76.8% for TA98 | |||||
| Flavonoid-enriched extract extracts | 50, 300, 600 µg/plate | TA100: 47, 75.3, and 88.6% inhibition by 50, 300, and 600 µg/plate, respectively; TA98: 62.5, 77, and 93.5% inhibition by 50, 300, and 600 µg/plate, respectively | |||||
| Sodium azide- induced mutagenicity in S. typhimurium TA1535 and TA100 |
Essential oil | 1.5, 10, 15, 30 µg/Plate | TA100: 79, 83, and 94% inhibition by 10, 15, and 30 µg/plate, respectively; TA1535:, 62, 76, and 93% inhibition by 10, 15, and 30 µg/plate, respectively | ||||
| Aqueous extract | 1.5, 50, 300, 600 µg/plate | TA100: 92, 96, and 98% inhibition by 50, 300, and 600 µg, respectively; TA 1535: 62, 80, and 94% for the same concentrations | |||||
| Flavonoid-enriched extract extracts | 50, 300, 600 µg/plate | 50 and 300 µg/plate: from 54 to 68% inhibition in TA1535 and from 84 to 93% in TA100 | |||||
|
| |||||||
| Anitmicrobial and antiviral | P. lentiscus | Leaf | Disc diffusion | Essential oil | 0.03, 0.15, 0.62, 2.5, 10.0, 40.0 mg/mL | Noticeable activity against S. enteritidis (MIC: 30 µg/mL) and St. aureus (30 µg/mL); less important activity against S. typhimurium, (MIC: 150 µg/mL); No significant inhibitory activity towards Escherichia coli, Pseudomonas aeruginosa, and Enterococcus faecalis |
[86] |
| Disc diffusion | Ethanolic extract | 5 and 10 μL | No effect on Klebsiella pneumoniae and Escherichia coli. Significant inhibition against Candida albicans, Staphylococcus aureus, and Salmonella typhi | [88] | |||
| Disc diffusion | Ethanolic extract | 50, 100, 500 μL, and 1 mL | Inhibiting activity on Trichoderma sp and Fusarium sp | [88] | |||
| Disc diffusion | Aqueous extract | ND | Most active against S. typhimurium, (MIC: 4 μg/mL), significant inhibitory activity towards P. aeruginosa and S. enteritidis (MIC: 40 μg/mL), and no activity against S. aureus, E. coli, and Ent. faecalis up to 1000 μg/mL | [87] | |||
| Disc diffusion | Total oligomer flavonoid-enriched extract | ND | TOF extract exhibited antibacterial activity only against S. typhimurium (MIC: 100 μg/mL) | ||||
| Microdilution agar | Essential oil | ND | Activity against S. enteritidis, S. typhimurium, and S. aureus (MICs between 30 and 620 μg/mL). No effect on Ent. foecalis, P. aeruginosa, and E. coli up to 1000 μg/mL | ||||
| P. lentiscus var. chia | Gum | Disc diffusion | Essential oil and its fractions and components | ND |
Escherichia coli, Staphylococcus aureus, and Bacillus subtilis were resistant to α-pinene. E. coli is resistant to β-myrcene, S. aureus showed an intermediate response, and B. subtilis is sensitive to it. p-Cymene, β-caryophyllene, methyl isoeugenol, limonene, γ-terpinene, and trans-anethole showed moderate antibacterial activity, and in some cases, the bacteria were resistant to them. E. coli and S. aureus were resistant to β-pinene, slightly inhibited B. subtilis. Verbenone, R-terpineol, and linalool showed higher antibacterial activity than other components |
[19] | |
| Gum | Disc diffusion | Mastic gum water (MWR) and its major constituents | MWR (58 mg/mL), (−)-trans-pinocarveol (13 mg/mL), (−)-linalool (37.6 mg/mL), (±)-linalool (36.6 mg/mL), (−)-verbenone (29.5 mg/mL), and (+)-α-terpineol (29.2 mg/mL) | The broadest average inhibition zones were for E. coli and S. aureus by (+)-α-terpineol and (±)-linalool compared to the positive control (gentamicin 10 µg); significant antifungal activity against Candida albicans by MWR | [33] | ||
| Microdilution | 4%, 2%, 1%, 0.5%, 0.25%, 0.125%, 0.063%, and 0.032% (v/v) |
The most potent antimicrobial constituents were (±)-linalool and α-terpineol against E. coli and S. aureus. Significant antifungal activity of MWR, (±)-linalool, (−)-verbenone, and (+)-α-terpineol against C. albicans | |||||
| P. lentiscus | Gum | ND | Liquid mastic | 2% liquid mastic | Activity against Porphyromonas gingivalis and Prevotella melaninogenica | [76] | |
| Human T-cell leukemia MT-4 cells infected with HIV-1IIIB; viable cell number determination by MTT assay | Solid and liquid mastic | Solid mastic: 0–200 μg/mL; liquid mastic: 0–0.0006% | Neither solid nor liquid mastic had any anti-HIV activity compared to positive controls | ||||
| Pistacia lentiscus var. chia | Gum | Microdilution | Total mastic extract without polymer (TMEWP), acidic and neutral fractions |
MEWP: 0.049 to 1.560 mg/mL, fractions: 0.060 to 1.920 mg/mL | The acidic fraction exhibited the highest activity against Helicobacter pylori followed by the TMEWP and neutral fraction | [33] | |
| In vivo administration of extract in infected mice with H. pylori | Total mastic extract without polymer (TMEWP) | 180 µg/mL | Moderately reduced H. pylori colonization in the antrum and corpus of the mice stomach. Visible reduction in H. pylori colonization observed in histopathology evaluations | ||||
|
P. lentiscus,
P. atlantica (sp. cabulica, kurdica, and mutica) |
Gum | Broth microdilution | Isolated components of the acidic fractions of the gum | ND | The MIC values for the components ranged from 0.1 to 50 μg/mL against the strains of H. pylori and all Gram-negative bacteria including Escherichia coli, Salmonella typhimurium, Serratia marcescens, Pseudomonas aeruginosa, Alcaligenes faecalis, Enterobacter aerogenes Pseudomonas fluorescens, Porphyromonas gingivalis, and Proteus vulgaris and ranged from 2 to 100 μg/mL against Gram-positive bacteria including Bacillus cereus, Staphylococcus aureus, Streptococcus faecalis, Staphylococcus epidermidis, Bacillus subtilis, and Corynebacterium sp | [151] | |
|
P. atlantica
(sp. kurdica) |
Gum | ND | Essential oil, α-pinene |
ND | Against all tested bacteria mentioned in previous row, MIC values for essential oil and pure α-pinene ranged 500–1000 mg/mL | [152] | |
| P. atlantica | Leaf and twig | Modified [3H]-hypoxanthine incorporation assay |
Flavone 3-methoxycarpachromene from ethyl acetate extract | 0.8 and 4.9 µg/mL | IC50 of 3.4 µM against P. falciparum K1 strain where the positive controls artemisinin and chloroquine had IC50s of 3.6 and 89 nM, respectively | [55] | |
| Leaf and fruit derm | Disk diffusion method | Methanol, ethanol, ethanol + water, and water extracts |
25, 50 and 75 mg/mL | Dose dependent activity against E. coli, Staphylococcus aureus, and Staphylococcus epidermidis; less activity in comparison with gentamicin (10 μg/disk), tobramycin (10 μg/disk), and kanamycin (30 μg/disk) | [91] | ||
| Leaf | Disc diffusion | Ethanolic extract | 5 and 10 μL | Klebsiella pneumoniae and Escherichia coli were not sensitive to the extract. Candida albicans, Staphylococcus aureus, and Salmonella typhi showed a sensitizing effect at the 5 μL and a very significant effect at 10 μL | [88] | ||
| Disc diffusion | Ethanolic extract | (50, 100, 500 μL, and 1 mL) of ethanolic extract (0.338 g/mL) | No inhibiting activity was observed against Aspergillus flavus, Rhizopus stolonifer, Trichoderma sp, Fusarium sp and Aspergillus flavus | ||||
| Gall | Disc diffusion | Aqueous extract | 4.9 mg | Activity against the Bacillus species and Pseudomonas aeruginosa | [92] | ||
| Leaf and gall | Disc diffusion | Essential oils | Final 0.1% v/v | Delayed not block fungal growth in Fomitopsis pinicola and Penicillium sp. by volatile constituents of galls; volatile constituents of leaf inhibited only the growth of Penicillium sp | |||
| Gum | Agar disc diffusion | Essential oil | 10−1, 10−2, 10−3, and 10−4 μg/mL | Most active against E. coli followed by S. aureus and S. pyogenes. | [90] | ||
| Inhibitory quantity (MIQ) method | 0.5, 1, 1.5, and 2 μg/mL | S. aureus and S. pyogenes were susceptible to 0.5 μg/mL, and E. coli was tolerant to this concentration | |||||
| Maruzzella method | 10−1, 10−2, 10−3 μg/mL | E. coli, Staphylococcus aureus, and Streptococcus pyogenes were sensitive to 10−1 μg/mL | |||||
| P. atlantica var. kurdica | Gum | Mice infected with Leishmania major | Gum | Locally rubbed on lesions | ↓Skin lesion size in mice infected with L. major compared with control (P < 0.01); ↓number of parasitologicaly positive mice (P < 0.05) | [93] | |
| P. terebinthus | Leaf | Microdilution | Hydroalocholic extract | 0.024, 0.049, 0.097, 0.19, 0.78, 1.56, and 25 mg/mL (for S. aureus) 0.049–12.5 mg/mL (for E. coli) |
Activity against S. aureus with a MIC: ≤1.56 mg/mL. No antimicrobial effect on E. coli. |
[84] | |
| Gum | Disc diffusion, microdilution |
Essential oil and gum smoke | ND | Activity of essential oil against all tested bacteria including Bacillus subtilis, Salmonella typhi, Escherichia coli, Staphylococcus epidermidis, and Pseudomonas aeruginosa; activity of nonpolar smoke fraction on all of strains especially on S. dysenteriae, E. coli, B. subtilis, and P. aeruginosa | [140] | ||
| P. khinjuk | Not mentiond | Disc diffusion, microdilution |
Ethanolic extract and its fractions |
ND | Active against Gram-positive and Gram-negative bacteria especially n-butanolic fraction |
[153] | |
| Leaf | Microdilution | Chloroform, ethyl acetate, ethyl alcohol, and diethyl ether extracts |
ND | Activity against bacteria including Bacillus subtilis, Enterococcus faecalis, Staphylococcus aureus Staphylococcus epidermidis, Escherichia coli, and Klebsiella pneumoniae (MIC = 0.02–0.5 mg/mL) and fungi including Candida albicans and Saccharomyces cerevisiae (MIC = 0.06–0.4 mg/mL). Chloroform extract inhibited growth of fungi more than others |
[38] | ||
| Leaf, fruits derm | Disc diffusion | Methanolic extract | 25, 50, 75 mg/mL | Hydroalcoholic extract of fruits derm on E. coli, water extract on S. epidermidis, and methanolic extract on S. aureus (all in 75 mg/mL) had higher antibacterial activity than tobramycin and same as gentamicin and kanamycin | [91] | ||
| P. vera | Leaf, branch, stem, seed |
In vitro study on four parasitic protozoa | Lipophylic extracts | 0.8 to 9.7 µg/mL | No inhibitory activity against Trypanosoma brucei rhodesiense | [94] | |
| Not any significant inhibitory potential against Trypanosoma cruzi | |||||||
| Remarkable activity of branches extract at 4.8 µg/mL against Leishmania donovani | |||||||
| Dried leaf extract displayed notable activity against Plasmodium falciparum at 4.8 µg/mL | |||||||
| Gum | Hole-plate, agar dilution |
Essential oil | 1/10, 1/20, 1/40, 1/80, and 1/100 v/v |
All isolates of Helicobacter pylori were sensitive to the essential oil (MIC: 1.55 µg/mL) | [15] | ||
| Agar-disc diffusion, broth microdilution, and broth susceptibility | Essential oil | of 2 and 4 µL |
Dose dependent activities against Corynebacterium xerosis, Bacillus brevis, B. megaterium, Mycobacterium smegmatis, St. aureus, Klebsiella oxytoca, Enterococcus faecalis, Micrococcus luteus, Escherichia coli, Yersinia enterocolitica, Kluyveromyces fragilis Rhodotorula rubra, and Candida albicans |
[12] | |||
| Hull | Disk diffusion test | Aqueous | 1200 μg/plate | Gram positive bacteria were the most sensitive | [150] | ||
| Agar dilution method | 0.5 to 10 mg/mL | ||||||
| Leaf, branch, stem, kernel, shell skins, and seeds |
Microdilution | Lipophylic extracts | 256 and 512 mg/mL | Greater activity against Gram positive bacteria than Gram-negative; remarkable antifungal activity against C. albicans and C. parapsilosis | [89] | ||
| In vitro antiviral assay | Extracts of shell skin and fresh kernel had significant activity against Parainfluenza virus and Herpes simplex virus same as the acyclovir | ||||||
|
| |||||||
| Anti-inflammatory | P. terebinthus | Gall | Phospholipase A2 (PLA2) induced hind-paw mouse edema |
Methanolic extract | 200 mg/kg | Inhibition of edema | [95] |
| Ethyl phenylpropiolate (EPP) induced mouse ear edema |
1 mg/ear | Inhibition of edema by 44%. | |||||
| 12-O-Tetradecanoylphorbol-13-acetate (TPA)-induced mouse ear edema |
1 mg/ear | Nonsignificant effect | |||||
| Mouse ear edema induced by multiple topical applications of TPA | 1 mg/ear | 58% inhibition of chronic inflammatory swelling | |||||
| In vitro phospholipase A2 activity assay | ND | ↓activity of the enzyme by 75% | |||||
| Myeloperoxidase assay | ND | ↓activity of the enzyme by 73% | |||||
| Phospholipase A2 (PLA2)-induced hind-paw mouse edema | Masticadienonic acid, masticadienolic acid, and morolic acid from methanolic extract | 30 mg/kg | Inhibition of edema by all triterpenes | [95] | |||
| Ethyl phenylpropiolate (EPP) induced mouse ear edema |
1 mg/ear | 31% and 38% nonsignificant inhibition of edema by masticadienolic acid and morolic acid, whereas masticadienonc acid was inactive | |||||
| Mouse ear edema induced by multiple topical applications of TPA |
0.3 mg/ear | Inhibition of swelling and neutrophil infiltration by all compounds | |||||
| Myeloperoxidase assay | 10–100 µg/mL | 80% inhibition of enzyme activity by all the compounds | |||||
| Inhibition of the production of LTB4 from rat polymorphonuclear leukocytes (PMNL) | 12.5–100 µM | Inhibition of leukotriene B4 production in rat PMNL by all compounds | |||||
| Ethyl phenylpropiolate-induced mouse ear oedema | Oleanolic acid and its semisynthetic 3-oxo-analogue | 1 mg/ear | No activity on the edema | [95] | |||
| Mouse ear edema induced by TPA | 0.5 mg/ear | A nonsignificant 28% inhibition | |||||
| Mouse edema induced by DPP | 0.5 mg/ear | ↓swelling by 40% similar to standard (carbamazepine) | |||||
| Delayed type hypersensitivity induced by fluorobenzene in mouse ear |
Oleanolic and oleanonic acids | 0.5 mg/ear | Oleanonic acid: ineffective at both 24 and 96 h; oleanolic acid: ↓edema nonsignificantly at 96 h by 32% | ||||
| Mouse ear inflammation induced by multiple topical applications of TPA | 0.3 mg/ear | Oleanonic acid: significant effect with 45% inhibition; oleanolic acid: inactive | |||||
| Myeloperoxidase assay | ND | Inhibition of neutrophil infiltration by oleanonic and oleanolic 84% and 67%, respectively | |||||
| Phospholipase A2-induced hind paw mouse edema | 30 mg/kg | ↓edema by both compounds | |||||
| Bradykinin-induced mouse paw edema | Oleanonic acid | 30 mg/kg | ↓edema by 61% | ||||
| Inhibition of leukotriene B4 production from rat polymorphonuclear leukocytes |
ND | ↓leukotriene B4 (IC50: 17 µM) | |||||
| P. vera | Fruits, leaf, branches, peduncles, and oleoresin |
Carrageenan-induced hind paw edema | Ethanolic and aqueous extracts | 250, 500 mg/kg | Among all extracts, only the oleoresin exhibited a dose-dependent anti-inflammatory activity | [146] | |
| p-Benzoquinone-induced abdominal constriction test in mice |
250, 500 mg/kg | Among all extracts, only the oleoresin displayed antinociceptive activity with 32.1% inhibition at 500 mg/kg and 21.7% inhibition at 250 mg/kg | |||||
| Leaf | Hot plate test | Aqueous extract, ethanolic extract |
0.4 and 0.5 g/Kg | Dose-dependent antinociceptive activity after 30–60 min of treatment | [97] | ||
| Xylene-induced ear edema | Aqueous extract | 0.4, 0.16, 0.28 g/kg | Significant anti-inflammatory activities | ||||
| Chronic anti-inflammatory activity (granuloma pouch method) | Aqueous extract, ethanolic extract | 0.4 g/Kg 0.35, 0.5 g/Kg |
Significant and dose-dependent anti-inflammatory activity | ||||
| Writhing test | Aqueous extract ethanolic extract |
0.4, 0.28 g/kg 0.35, 0.5 g/Kg |
↓number of mouse abdominal constrictions induced by acetic acid | ||||
| P. lentiscus var. chia | Gum | Modification of VCAM-1 and ICAM1 expression by ELAISA |
Neutral extract and isolated phytosterol tirucallol |
Extract: 25, 50, 100, 200 µg/mL Tirucallol: 0.1, 1, 10, 100 µM |
significant dose-dependent ↓in vascular adhesion molecule 1 (VCAM-1) and intracellular adhesion molecule 1 (ICAM-1) expression | [98] | |
| U937 cell adhesion assay | ↓adhesion of U937 cells to TNF-α-stimulated human aortic endothelial cells | ||||||
| Measurement of NFkB p65 phosphorylation by ELISA | ↓phosphorylation of NFkB p65 | ||||||
|
| |||||||
| Effects on Gastrointestinal disorders | P. lentiscus | Resin | Pyloric ligation-, Aspirin-, phenylbutazone-, and reserpine-induced and cold-restraint stress ulcer in rat |
Powder finely suspended in corn oil | An oral dose of 500 mg/kg |
↓intensity of gastric mucosal damage in all models | [103] |
| P. lentiscus | Resin | TNBS-induced colitis in rats | Powder in polyherbal formulation | 50, 100, and 200 mg/kg of formula with 4% P. lentiscus resin | ↓macroscopic and microscopic colonic damage; ↓TNF-α, IL-1β, MPO, and lipid peroxidation; not significantly increase in antioxidant power of colon | [106] | |
| P. lentiscus var. chia. | Resin | 3-week double-blind randomised placebo controlled study on patients with functional dyspepsia | Powder | 350 mg TID | Improved the feeling of symptoms significantly | [104] | |
| P. lentiscus var. chia. | Resin | Dextran-sulfate sodium (DSS) model of colitis in mice | Powder | 0.20 g/kg chow (0.02%) 2.0 g/kg chow (0.20%) |
Delayed the onset and progression of acute colitis and ↓weight loss caused by the disease | [105] | |
| P. lentiscus var. chia. | Resin | 4-week pilot study on 10 patients with Crohn's disease and 8 controls | Capsules of fine powder |
2.22 g/day (6 caps/d, 0.37 g/cap) | ↓Crohn's disease activity index and plasma inflammatory mediators such as C-reactive protein, interleukin-6 (IL-6) without any side effects; immunomodulatory effect by ↓ tumor necrosis factor-alpha (TNF-α) and ↑macrophage migration inhibitory factor | [107] | |
| P. lentiscus var. chia | Resin | 4-week pilot study on 10 patients with crohn's disease and 8 controls | Capsules of fine powder |
2.22 g/day (6 caps/d, 0.37 g/cap) | Immunomodulatory activity ↓TNF-α and ↑macrophage migration inhibitory factor (MIF) in these patients | [108] | |
|
| |||||||
| Antidiabetic | P. atlantica | Leaf | In vitro and in vivo (normoglycemic and streptozocin-induced hyperglycemic rats) | Aqueous extract | 2 mL plant extract equivalent to 200 mg of starting material | Significant inhibitory effect on α-amylase in vitro; no significant hypoglycemic activity in normoglycemic and hyperglycemic rats | [109] |
| In vitro enzymatic starch digestion and rat model | Aqueous extract | 1, 5, 10, 12.5, 25, 50, and 100 mg/mL 125, 250, and 500 mg/kg |
In vitro: significant dose dependent dual inhibition of α-amylase and α-glucosidase comparable to acarbose In vivo: significant acute postprandial antihyperglycemic activity comparable to metformin and glipizide and improved glucose intolerance in oral starch tolerance test |
[110] | |||
| P. lentiscus var. chia | Resin | Human study | Powder diluted in 250 mL of water | 0.7 g per day | Significantly decrease (3.1 mg/dL per month, P = 0.003) in serum glucose level among male subjects | [111] | |
|
| |||||||
| Antitumor | P. lentiscus var. chia | Resin | In vitro study on human colon cancer cells (HCT116) |
Ethanol extract | ND | Inhibited proliferation and induced apoptosis of human colorectal tumor cells | [112] |
| P. lentiscus | Resin | In vitro study on human leukemic cell line | Liquid and solid resin | 0–200 μg/mL (solid mastic) or 0–2 (v/v)% of liquid mastic |
The most cytotoxic effect against promyelocytic leukemia HL-60 among 13 human cell types; inhibition of natural apoptosis of oral polymorphonuclear leukocytes | [76] | |
| In vivo human colon cancer/immunodeficient mouse model | Hexane extract | 200 mg/kg administered daily for 4 consecutive days (followed by 3 days without treatment) |
Anticancer activity via its delay effect on the growth of colorectal tumors developed from HCT116 xenografted into mice | [8] | |||
| Human cell line (androgen-responsive prostate cancer cell line) | ND | 2, 4, 6, 8, 10, and 12 µg/mL | Remarkable potency to decrease the expression and function of the androgen receptor in androgen-responsive prostate cancer cell line (LNCaP) | [154] | |||
| Human prostate cancer cell lines (LNCaP and DU-145), RT-PCR, and Western blotting were used to detect maspin expression | ND | 2, 4, 6, and 8 µg/mL | Increased maspin expression in LNCaP cells | [113] | |||
| The human prostate cancer cell lines (PC-3), MTT assay, gene assay, RT-PCR, and Western blotting |
ND | 10, 20, and 30 µg/mL | Inhibited proliferation and blocked the cell cycle progression in androgen-independent prostate cancer PC-3 cells by suppressing NF-κB activity and the NF-κB signal pathway | [114] | |||
| Lewis lung carcinoma cells | Essential oil | 0.01% v/v | A time-dependent modification in the expression of 925 genes and phenomena in Lewis lung carcinoma cells by its antiproliferative, proapoptotic, and anti-inflammatory activities | [155] | |||
| P. atlantica sub. kurdica | Fruit | Immunocompetent mice | Essential oil | 45 mg/kg intraperitoneally, 3 times a week for 3 weeks | Significant inhibition on tumor growth without signs of toxicity related to apoptosis induction, reduced neovascularization, and inhibiting chemokine expression | [115] | |
| Cells line and the in vivo chicken embryo CAM angiogenesis model |
Essential oil | 0.01–0.1% v/v | Antiproliferative and proapoptotic effect on K562 human leukemia cells; inhibited the release of vascular endothelial growth factor from K562 and B16 mouse melanoma cell; concentration-dependent inhibition of endothelial cell proliferation without affecting cell survival; significant decrease of microvessel formation | [116] | |||
| Rat liver medium-term carcinogenesis bioassay (Ito-test) | Powder in diet | 0, 0.01, 0.1 and 1% | Promoted the preneoplastic lesions development in rat liver with increasing liver relative weight | [117] | |||
| human colon carcinoma HT29 cells | Ethanol : H2O (70 : 30) | 0.7 mg/mL | 50% growth inhibition similar to 500 nM of doxorubicin | [119] | |||
| P. vera | Resin | In vitro cytotoxic activity against human cell lines | Crud methanolic extract fractionated against petroleum ether, chloroform, and n-butanol |
ND | Moderate cytotoxic effect against breast cancer cell line (MCF7), hepatocellular carcinoma cell line (HEPG2), cervix cancer cell line (HELA), and normal melanocytes (HFB4); n-hexane fraction showed strong cytotoxic effect (IC50: 3.15–4.17 µg/mL) against all of the tested cell lines, except for MCF7 (IC50: 13.5 µg/mL) |
[120] | |
|
| |||||||
| Effects on liver and serum biochemical parameters | P. lentiscus | Leaf | Rat model using Carbon tetrachloride | Aqueous extract | 4 mL/kg (contained 1.946 g of solid matter) |
↓bilirubin and activity of 3 enzymes including alkaline phosphatase (ALP), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) | [121] |
| Rat model using Thioacetamide | Aqueous extract | 15 mg/kg and 75 mg/kg | Hepatic fibrosis, an inflammatory response, mild cholestasis, and depletion of reduced glutathione associated with an increase in its oxidized form for five weeks administration in healthy rats; in thioacetamide-induced rat liver lesions, it aggravated the inflammatory, fibrotic, and glutathione depleting responses without affecting the extent of lipid peroxidation | [122] | |||
| P. lentiscus var. chia | Resin | Human model | Powder diluted in one glass (250 mL) of water | 5 g | Serum total cholesterol, LDL, total cholesterol/HDL ratio, lipoprotein, apolipoprotein A-1, apolipoprotein B, AST, ALP, and gamma-GT were reduced in human subjects | [111] | |
| P. lentiscus | Seeds oil | Rabbit model, mercury induced toxicity | Pistacia oil | 5% | Mercury induced toxicity in rabbits caused increase in the level of ALP, AST, and urea serum, while it was reported that P. lentiscus oil-treated rabbits showed none of those changes | [156] | |
| P. vera | Fruit (roasted, unsalted pistachio nuts) | Human model (10 patients with moderate hypercholesterolemia) | Nut | 20% in diet | ↓total cholesterol, total cholesterol/HDL ratio, and LDL/HDL ratio and ↑HDL after 3 weeks use | [124] | |
| P. terebinthus | Fruit | Rabbit model | Fruit | 1 g/kg | Inhibited the development of hydropic degeneration and fatty changes in the liver and demonstrated hypolipidemic effect | [125] | |
|
| |||||||
| Effects on atherosclerosis | P. vera | Fruit | Rabbit model | Methanolic and cyclohexane extracts | Methanolic extract (1% v/w) cyclohexane extract (5% v/w) |
Beneficial effects on HDL, LDL, and aortic intimal thickness. The methanolic extract additionally showed an antioxidant activity and remarkable decrease in aortic surface lesions | [123] |
| P. terebinthus | Fruit | Rabbit model | Fruit | 1 g/kg | Inhibited the development of the atherosclerotic lesions in the thoracic artery | [125] | |
| P. lentiscus | Resin | Cell culture (peripheral blood mononuclear cell, PBMC); cell viability assessed via MTT assay |
Total polar extract | 2.7, 27, and 270 µg/mL | Restored intracellular antioxidant glutathione (GSH) levels and downregulated CD36 mRNA expression resulted in antioxidant and antiatherogenic effects | [126] | |
|
| |||||||
| Anticholinesterase activity | P. atlantica | leaf | TLC bioautography assay, Ellman's colorimetric method | Aqueous extract | 5, 10, 15, 20, and 25 µg/mL | Strong acetylcholinesterase (AChE) inhibition | [13] |
| P. atlantica | Leaf | Ellman's colorimetric method | Methanol and ethyl acetate extracts | 0.1 mg/mL | Relatively weak AChE inhibitory activity | [127] | |
| P. terebinthus | Fruit | Ellman's colorimetric method and the modified dopachrome method | Ethyl acetate and methanol extracts | 25, 50, 100, and 200 µg/mL | No inhibitory activity against AChE and tyrosinase while selectively inhibited butyrylcholinesterase (BChE) at moderate levels (below 50%) at the tested concentrations | [85] | |
4.1. Antioxidant Activity
Different parts and constituents from P. lentiscus have been shown in vitro radical scavenging properties [23, 47, 52, 74–76]. Pistacia lentiscus var. chia and P. terebinthus var. chia resins were effective in protecting human LDL from oxidation in vitro [77]. P. atlantica leaf and fruit have shown antioxidant activity similar to or significantly higher than those of standard antioxidant compounds in different in vitro antioxidant assays [78–80]. However, the essential oil from P. atlantica leaf showed weak antioxidant activity in DPPH test compared to synthetic antioxidants [32]. P. vera fruit revealed significant antioxidant activity similar to the synthetic antioxidant [81]. Lipophilic extract from P. vera nuts showed lower antioxidant potential that than of hydrophilic extract [82]. One survey showed P. vera skins had a better antioxidant activity compared to seeds by means of four different assays because of higher content of antioxidant phenolic compounds in skins [44]. Antioxidant activity has been also reported from other parts of P. vera [83].
In one study, the extract from P. terebinthus leaf had nearly 12-fold higher antioxidant capacity than those of BHA and ascorbic acid [84]. P. terebinthus fruits showed noticeable metal-chelation properties as compared to EDTA and high radical scavenging activity similar to the standards. Antioxidant activity of the fruits may be elevated by roasting process [85].
4.2. Antimutagenic Activity
Essential oil and different extracts from P. lentiscus leaves indicated significant inhibitory effect on mutagenicity in vitro [86, 87]. Gallic acid, digallic acid, and 1,2,3,4,6-pentagalloylglucose, polyphenols isolated from the fruits of P. lentiscus, induced an inhibitory activity against mutagenicity and genotoxicity in in vitro assays [47, 52].
4.3. Antimicrobial and Antiviral Activities
Pistacia species have demonstrated significant antibacterial activity against various Gram positive and Gram negative bacteria as shown in Table 3. Antimicrobial activity of Pistacia lentiscus resin, the essential oil and gum from P. atlantica var. kurdica and its major constituent α-pinene and P. vera gum against Helicobacter pylori were recorded [15, 33]. A study indicated that antibacterial activity of P. lentiscus gum oil can be attributed to combination of several components rather than to one particular compound. Verbenone, R-terpineol, and linalool showed high antibacterial activity against Escherichia coli, Staphylococcus aureus, and Bacillus subtilis which is comparable to that of mastic oil itself [19]. P. lentiscus gum revealed selective antibacterial activity against Porphyromonas gingivalis and Prevotella melaninogenica and had antiplaque activity on teeth by inhibiting bacterial growth in saliva [76].
Significant antifungal activity was seen from essential oil of P. lentiscus leaf and gum, different extracts of P. khinjuk leaf, and essential oil of P. vera gum [15, 19, 38, 88]. Evaluating the effect of P. vera gum essential oil on growth of 13 bacteria and 3 yeasts demonstrated inhibitory effect on all of them except Bacillus cereus, Pseudomonas aeruginosa, and Klebsiella pneumonia and more effective yeasticide than nystatin. Carvacrol was found to be the most effective constituent [12, 15]. Lipophylic extracts from different parts of P. vera showed a little antibacterial activity and noticeable antifungal one against C. albicans and C. parapsilosis. Kernel and seed extracts showed significant antiviral activity [89].
Some active constituents of essential oil from the aerial parts of P. khinjuk responsible for its antibacterial and antifungal activity are α-pinene, β-pinene, myrcene, beta-caryophyllene, Germacrene B, and Spathulenol [38].
Organic fraction of mastic water obtained during the steam distillation of resin from Pistacia lentiscus var. chia indicated acceptable antifungal activity but moderate antibacterial effect. Among some of its major compounds, (±)-linalool and α-terpineol had the highest antimicrobial effect [33].
Essential oil from leaf and gum of P. atlantica showed acceptable antibacterial and antifungal activities [90–92]. However, leaf ethanolic extract had no distinct antimicrobial activity [88].
A remarkable inhibitory activity of different extracts and essential oil from P. lentiscus leaves was observed against Salmonella typhimurium; additionally, essential oil showed significant inhibitory effects against S. enteritidis and Staphylococcus aureus [86, 87].
As reported by Adams et al. [55], the leaves and twigs of P. atlantica and its active substance 3-methoxycarpachromene showed antiprotozoal activity against Plasmodium falciparum. P. atlantica var. kurdica gum controlled cutaneous leishmaniasis in mice infected with Leishmania major [93]. Extract from P. vera branch had significant inhibitory activity against Leishmania donovani and leaf extract inhibited Plasmodium falciparum without cytotoxicity on mammalian cells [94].
4.4. Anti-Inflammatory and Antinociceptive Activity
Anti-inflammatory and antinociceptive activity of five mentioned Pistacia species have been shown in Table 3.
P. terebinthus gall showed anti-inflammatory activity in different in vivo models of acute and chronic inflammation [95]. Masticadienonic acid (26), masticadienolic acid (27), and morolic acid (28), three triterpene isolated from P. terebinthus gall, seem to be responsible for its anti-inflammatory activity [43]. Additionally, oleanonic acid (29) from the galls of P. terebinthus, reduced the production of leukotriene B4 from rat peritoneal leukocytes and showed antiedematous activity in mice [96]. Oleoresin and leaf extract from P. vera showed significant anti-inflammatory and antinociceptive activity [97].
Extract of the resin of P. lentiscus var. Chia and its isolated phytosterol tirucallol (31) showed anti-inflammatory activity on human aortic endothelial cells and had significant inhibitory activity on adhesion molecules expression in TNF-α-stimulated human aortic endothelial cells [98]. It was proposed that the anti-inflammatory effect of P. lentiscus var. chia gum may be related to inhibition of protein kinase C which leads to decrease in superoxide and H2O2 production by NADPH oxidase [99].
4.5. Effects on Gastrointestinal Disorders
One of the most important traditional uses of gums from Pistacia species is for management of gastrointestinal disorders. Moreover, there are several scientific studies that confirm this property [100–102]. Resin of P. lentiscus significantly reduced the intensity of gastric mucosal damage induced by pyloric ligation, aspirin, phenylbutazone, reserpine, and restraint with cold stress via its antisecretory and cytoprotective activities [103]. In one double-blind placebo controlled trial, P. lentiscus gum improved the feeling of symptoms significantly in patients with functional dyspepsia [104]. Moreover, Pistacia species exerted significant antibacterial activity on Helicobacter pylori [15, 33]. Supplementation with P. lentiscus oil in experimental model of colitis delayed the onset and progression of acute colitis and led to decrease weight loss caused by the disease [105]. A polyherbal formula that contains P. lentiscus gum caused significant decrease in colonic damage and biochemical markers related to pathophysiology of IBS in rat model of colitis [106]. Adminstration of P. lentiscus var. chia resin to patients with established mild to moderate active crohn's disease (CD) for 4 weeks caused significant reduction in CD activity index and plasma inflammatory mediators without any side effects and also as an immunomodulator resulted in significantly reduction in tumor necrosis factor-alpha (TNF-α) and enhanced macrophage migration inhibitory factor in these patients [107, 108].
4.6. Antidiabetic Activity
Aqueous leaf extract from P. atlantica showed significant inhibitory effect on α-amylase and α-glucosidase in vitro [109, 110]. It demonstrated significant acute postprandial antihyperglycemic activity comparable to metformin and glipizide in starch-fed rats. It also improved glucose intolerance [110]. However, another study on this extract did not show significant hypoglycemic activity when tested in normoglycemic and streptozocin-induced hyperglycemic rats [109]. Administration of P. lentiscus var. chia gum to human subjects for 12 months caused significantly decrease in serum glucose level among male subjects. Serum glucose in women was not affected [111].
4.7. Antitumor Activity
Among mentioned species of Pistacia, P. lentiscus is the most investigated for antitumor activity (Table 3). P. lentiscus var. chia gum inhibited proliferation and induced apoptosis of human colorectal tumor cells in vitro [112]. The resin exerted the most cytotoxic effect against promyelocytic leukemia among 13 human cell types and also inhibited the natural apoptosis of oral polymorphonuclear leukocytes [76]. The gum demonstrated anticancer activity via delaying the growth of colorectal tumors developed from human colon cancer cells xenografted into mice [8]. It also increased maspin (a mammary serine protease inhibitor with tumor suppressive activity for prostate cancers) expression in responsive prostate cancer cells and inhibited cell proliferation and blocked the cell cycle progression [113, 114]. Essential oil of P. lentiscus demonstrated significant inhibition on tumor growth in immunocompetent mice without signs of toxicity, related to apoptosis induction, reduced neovascularization, and inhibiting chemokine expression [115]. In addition, it had antiproliferative and proapoptotic effect on human leukemia cells and inhibited the release of vascular endothelial growth factor from these cells [116]. Despite many reports on antitumor activities of P. lentiscus, one in vivo study showed that the high dose of P. lentiscus gum promoted the preneoplastic lesions development in rat liver with increasing liver relative weight which proposed that desirable anticarcinogenic effects of mastic could be obtained at relatively low doses [117]. In one recent study, the current data on the anticancer activities of gum, oil, and extracts of P. lentiscus L. and its major constituent, have been reviewed comprehensively with special attention to the probable anticancer mechanisms [118].
The fruit extract of P. atlantica sub. kurdica showed growth inhibition in human colon carcinoma cells similar to Doxorubicin [119]. P. vera oleoresin demonstrated moderate cytotoxic effect against breast cancer cell line, hepatocellular carcinoma cell line, cervix cancer cell line, and normal melanocytes [120].
4.8. Effects on Liver and Serum Biochemical Parameters
P. lentiscus leaf demonstrated significant hepatoprotective activity against carbon tetrachloride induced hepatotoxicity in rats by reducing the level of bilirubin and activity of liver enzymes [121]. However, another study reported hepatic fibrosis, mild cholestasis, and depletion of reduced glutathione by long-term administration of aqueous leaf extract in healthy rats [122]. Administration of P. lentiscus var. chia gum for 18 months in healthy volunteers caused reduction in liver enzymes and exerted hypolipidemic effect [111]. Extracts from P. vera fruits have shown beneficial effects on HDL and LDL level in rabbit model of atherosclerosis [123]. Positive changes in lipid profile were recorded after three-week use of P. vera nuts in patients with moderate hypercholesterolemia. The decrease in triglyceride and LDL levels was not significant [124]. P. terebinthus fruit demonstrated hypolipidemic effect in hypercholesterolemic rabbits [125].
4.9. Effects on Atherosclerosis
More over than the antihyperlipidemic activity that described above, Pistacia species exerts their antiathesclerotic effects by direct activity on atherosclerotic lesions moreover than their antihyperlipidemic activity. Both methanolic and cyclohexane extracts from P. vera fruits have shown beneficial effects on HDL, LDL, and aortic intimal thickness in rabbit model of atherosclerosis. The methanolic extract additionally showed an antioxidant activity and remarkable decrease in aortic surface lesions [123]. P. terebinthus fruits inhibited the development of the atherosclerotic lesions in the thoracic artery [125]. P. lentiscus resin that downregulated CD36 mRNA expression (as the oxLDL receptor in macrophages that play a pivotal role in atherosclerotic foam cell formation) resulted in antiatherogenic effects [126].
4.10. Anticholinesterase Activity
Aqueous extracts from P. atlantica and P. lentiscus leaves showed strong acetylcholinesterase (AChE) inhibition [13]; additionally, both the methanol and ethyl acetate extracts of P. atlantica leaf showed relatively weak AchE inhibitory activity [127]. However, one study showed that ethyl acetate and methanol extracts of various commercially terebinth coffee brands (an oily brown-coloured powder produced from the dried and roasted fruits of P. terebinthus) and the unprocessed fruits of P. terebinthus did not have inhibitory activity against AChE and tyrosinase, while they selectively inhibited butyrylcholinesterase (BChE) at moderate levels [85].
5. Conclusion
In traditional Iranian medicine textbooks and papers, five species of Pistacia genus including P. vera, P. lentiscus, P. terebinthus, P. atlantica, and P. khinjuk had been introduced for treating the wide range of ailments. These species until now have been utilized in Iran by people for different nutritional and medicinal proposes. This review considered findings about phytochemical and pharmacological properties of these five species and presents comprehensive analysis of papers published since the year 2000. Ethnopharmacological data about these species may help us to know that many pharmacological aspects proposed nowadays for these species have been derived from traditional uses like antiseptic and antimicrobial, anti-inflammatory and anti-nociceptive, antihepatotoxic, and anticancer activities and their beneficial effects in gastrointestinal disorders. Furthermore, there are several pharmacological activities discussed in traditional medicine such as diuretic, lithontripic, anti-tussive, antirheumatic, antiasthmatic, antihypertensive, and aphrodisiac activities which are not supported by any current scientific documents, and so, they could be considered for investigation by researchers.
Phytochemical studies provided evidence for traditional applications of these species. With respect to phytochemical assays, triterpenes found in the resin and monoterpens are the most abundant composition of the essential oil from different parts of these species. Essential oil constituents might be valuable chemotaxonomic marker to ascertain different Pistacia chemotypes. Considering the therapeutic effect of isolated components, it can be concluded that terpenoids including mono, di-, and triterpenoids are associated with anti-inflammatory and antimicrobial effects. High amount of natural phenols and flavonoids is related to potent antioxidant and anticancer activities.
Review on current researches about the genus Pistacia L. highlighting pharmacological studies on crude plant parts, extracts, and some pure metabolites has provided scientific evidence for traditional uses and has revealed this genus to be a valuable source for medicinally important molecules.
So many studies were carried out on antioxidant activity of this genus considering their flavonoids, anthocyanins, and other phenolic compounds as preventive factors against cancer and cardiovascular diseases. P. lentiscus is the most studied species for antioxidant effects followed by P. atlantica, P. vera, P. terebinthus and P. khinjuk.
Most of the studies showed antimicrobial activity of these species especially P. lentiscus on a wide range of microorganisms including Gram-positive and -negative, aerobic and aerobic bacteria, viruses and fungi. The findings indicated that α-pinene, verbenone, R-terpineol, linalool, carvacrol and flavones are major compounds related to antibacrial activity.
Conflict of Interests
The authors declare that they have no conflict of interests.
Abbreviations
- ABTS:
2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)
- ALP:
Alkalinephosphatase
- ALT:
Alanineaminotransferase
- AST:
Aspartateaminotransferase
- B(a)p:
Benzo(a)pyrene
- BHA:
Butylatedhydroxyanisole
- BHT:
Butylatedhydroxytoluene
- DMPD:
N,N-dimethyl-p-phenylendiamine
- DPPH:
2,2-Diphenyl-1-picrylhydrazyl
- EC50:
Halfmaximaleffectiveconcentration
- EDTA:
Ethylenediaminetetraaceticacid
- EPP:
Ethylphenylpropiolate
- FRAP:
Ferricreducingantioxidantpower
- Gamma-GT:
Gamma-glytamyltranspeptidase
- IC50:
Thehalfmaximalinhibitoryconcentration
- LOX:
Lipoxygenase
- MBC:
MinimumBactericidalConcentration
- MDA:
Malonaldehyde
- MIC:
MinimuminhibitoryConcentration
- NF-kB:
Nuclearfactorkappa-light-chain-enhancerofactivatedBcells
- OxLDL:
OxidizedLowdensitylipoprotein
- PLA2:
PhospholipaseA2
- SGOT:
Serumglutamicoxaloacetictransaminase
- SGPT:
Serumglutamic-pyruvictransaminase
- SOD:
Superoxidedismutase
- TBARS:
Thiobarbituricacidreactivesubstances
- TBHQ:
TertiaryButylhydroquinone
- TPA:
12-O-Tetradecanoylphorbol-13-acetate.
References
- 1.Mozaffarian V. Trees and Shrubs of Iran. 1st edition. Tehran, Iran: Farhang Moaser; 2005. [Google Scholar]
- 2.Kole C. Wild Crop Relatives: Genomic and Breeding Resources Legume Crops and Forages. Heidelberg, Germany: Springer; 2011. [Google Scholar]
- 3.Mozaffarian V. A Dictionary of Iranian Plant Names. Tehran, Iran: Farhang Moaser; 1998. [Google Scholar]
- 4.derMarderosian A, Beutler JA. The Review of Natural Products. 6th edition. Missouri, Mo, USA: Wolters Kluwer Health; 2010. [Google Scholar]
- 5.Durmaz G, Gökmen V. Changes in oxidative stability, antioxidant capacity and phytochemical composition of Pistacia terebinthus oil with roasting. Food Chemistry. 2011;128(2):410–414. doi: 10.1016/j.foodchem.2011.03.044. [DOI] [PubMed] [Google Scholar]
- 6.Gogus F, Ozel MZ, Kocak D, Hamilton JF, Lewis AC. Analysis of roasted and unroasted Pistacia terebinthus volatiles using direct thermal desorption-GCxGC-TOF/MS. Food Chemistry. 2011;129(3):1258–1264. doi: 10.1016/j.foodchem.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Longo L, Scardino A, Vasapollo G. Identification and quantification of anthocyanins in the berries of Pistacia lentiscus L., Phillyrea latifolia L. and Rubia peregrina L. . Innovative Food Science and Emerging Technologies. 2007;8(3):360–364. [Google Scholar]
- 8.Dimas K, Hatziantoniou S, Wyche JH, Pantazis P. A mastic gum extract induces supression of growth of human colorectal tumor xenografts in immunodeficient mice. In Vivo. 2009;23(1):63–68. [PubMed] [Google Scholar]
- 9.Avicenna. The Canon. Tehran, Iran: Soroush Press; 2008. Translated by: A. Shrafkandi. [Google Scholar]
- 10.Kashaninejad M, Mortazavi A, Safekordi A, Tabil LG. Some physical properties of Pistachio (Pistacia vera L.) nut and its kernel. Journal of Food Engineering. 2006;72(1):30–38. [Google Scholar]
- 11.Aghili MH. Makhzan-al-Advia. Tehran, Iran: Tehran University of Medical Sciences; 2009. Edited by R. Rahimi, M.R. Shams Ardekani and F. Farjadmand. [Google Scholar]
- 12.Alma MH, Nitz S, Kollmannsberger H, Digrak M, Efe FT, Yilmaz N. Chemical composition and antimicrobial activity of the essential oils from the gum of Turkish Pistachio (Pistacia vera L.) Journal of Agricultural and Food Chemistry. 2004;52(12):3911–3914. doi: 10.1021/jf040014e. [DOI] [PubMed] [Google Scholar]
- 13.Benamar H, Rached W, Derdour A, Marouf A. Screening of Algerian medicinal plants for acetylcholinesterase inhibitory activity. Journal of Biological Sciences. 2010;10(1):1–9. [Google Scholar]
- 14.Tsokou A, Georgopoulou K, Melliou E, Magiatis P, Tsitsa E. Composition and enantiomeric analysis of the essential oil of the fruits and the leaves of Pistacia vera from Greece. Molecules. 2007;12(6):1233–1239. doi: 10.3390/12061233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramezani M, Khaje-Karamoddin M, Karimi-Fard V. Chemical composition and anti-Helicobacter pylori activity of the essential oil of Pistacia vera . Pharmaceutical Biology. 2004;42(7):488–490. [Google Scholar]
- 16.Özcan M, Tzakou O, Couladis M. Essential oil composition of the turpentine tree (Pistacia terebinthus L.) fruits growing wild in Turkey. Food Chemistry. 2009;114(1):282–285. [Google Scholar]
- 17.Usai M, Pintore G, Chessa M, Tirlllini B. Essential oil composition of different aerial parts of Pistacia terebinthus L. growing wild in Sardinia. Journal of Essential Oil Research. 2006;18(4):383–385. [Google Scholar]
- 18.Flamini G, Bader A, Cioni PL, Katbeh-Bader A, Morelli I. Composition of the essential oil of leaves, galls, and ripe and unripe fruits of Jordanian Pistacia palaestina Boiss. Journal of Agricultural and Food Chemistry. 2004;52(3):572–576. doi: 10.1021/jf034773t. [DOI] [PubMed] [Google Scholar]
- 19.Koutsoudaki C, Krsek M, Rodger A. Chemical composition and antibacterial activity of the essential oil and the gum of Pistacia lentiscus var. chia . Journal of Agricultural and Food Chemistry. 2005;53(20):7681–7685. doi: 10.1021/jf050639s. [DOI] [PubMed] [Google Scholar]
- 20.Mecherara-Idjeri S, Hassani A, Castola V, Casanova J. Composition and chemical variability of the essential oil from Pistacia lentiscus L. growing wild in Algeria part I: leaf oil. Journal of Essential Oil Research. 2008;20(2):32–38. [Google Scholar]
- 21.Mecherara-Idjeri S, Hassani A, Castola V, Casanova J. Composition and chemical variability of the essential oil from Pistacia lentiscus L. growing wild in Algeria: part II: fruit oil. Journal of Essential Oil Research. 2008;20(2):104–107. [Google Scholar]
- 22.Zrira S, Elamrani A, Benjilali B. Chemical composition of the essential oil of Pistacia lentiscus L. from Morocco—a seasonal variation. Flavour and Fragrance Journal. 2003;18(6):475–480. [Google Scholar]
- 23.Gardeli C, Vassiliki P, Athanasios M, Kibouris T, Komaitis M. Essential oil composition of Pistacia lentiscus L. and Myrtus communis L.: evaluation of antioxidant capacity of methanolic extracts. Food Chemistry. 2008;107(3):1120–1130. [Google Scholar]
- 24.Fernández A, Camacho A, Fernández C, Altarejos J. Composition of the essential oils from galls and aerial parts of Pistacia lentiscus L. . Journal of Essential Oil Research. 2000;12(1):19–23. [Google Scholar]
- 25.Mecherara-Idjeri S, Hassani A, Castola V, Casanova J. Composition of leaf, fruit and gall essential oils of algerian Pistacia atlantica desf. Journal of Essential Oil Research. 2008;20(3):215–219. [Google Scholar]
- 26.Delazar A, Reid RG, Sarker SD. GC-MS analysis of the essential oil from the oleoresin of Pistacia atlantica var. mutica . Chemistry of Natural Compounds. 2004;40(1):24–27. [Google Scholar]
- 27.Barrero AF, Herrador MM, Arteaga JR, et al. Chemical composition of the essential oils of Pistacia atlantica Desf. Journal of Essential Oil Research. 2005;17(1):52–54. [Google Scholar]
- 28.Roitman JN, Merrill GB, Beck JJ. Survey of ex situ fruit and leaf volatiles from several Pistacia cultivars grown in California. Journal of the Science of Food and Agriculture. 2011;91(5):934–942. doi: 10.1002/jsfa.4268. [DOI] [PubMed] [Google Scholar]
- 29.Couladis M, Özcan M, Tzakou O, Akgül A. Comparative essential oil composition of various parts of the turpentine tree (Pistacia terebinthus L) growing wild in Turkey. Journal of the Science of Food and Agriculture. 2003;83(2):136–138. [Google Scholar]
- 30.Duru ME, Cakir A, Kordali S, et al. Chemical composition and antifungal properties of essential oils of three Pistacia species. Fitoterapia. 2003;74(1-2):170–176. doi: 10.1016/s0367-326x(02)00318-0. [DOI] [PubMed] [Google Scholar]
- 31.Tzakou O, Bazos I, Yannitsaros A. Volatile metabolites of Pistacia atlantica Desf. from Greece. Flavour and Fragrance Journal. 2007;22(5):358–362. [Google Scholar]
- 32.Gourine N, Yousfi M, Bombarda I, Nadjemi B, Stocker P, Gaydou EM. Antioxidant activities and chemical composition of essential oil of Pistacia atlantica from Algeria. Industrial Crops and Products. 2010;31(2):203–208. [Google Scholar]
- 33.Paraschos S, Magiatis P, Gousia P, et al. Chemical investigation and antimicrobial properties of mastic water and its major constituents. Food Chemistry. 2011;129(3):907–911. doi: 10.1016/j.foodchem.2011.05.043. [DOI] [PubMed] [Google Scholar]
- 34.Congiu R, Falconieri D, Marongiu B, Piras A, Porcedda S. Extraction and isolation of Pistacia lentiscus L. essential oil by supercritical CO2 . Flavour and Fragrance Journal. 2002;17(4):239–244. [Google Scholar]
- 35.Benyoussef E-H, Charchari S, Nacer-Bey N, NabilaYahiaoui N, Chakou A, Bellatreche M. The essential oil of Pistacia lentiscus L. from Algeria. Journal of Essential Oil Research. 2005;17(6):642–644. [Google Scholar]
- 36.Castola V, Bighelli A, Casanova J. Intraspecific chemical variability of the essential oil of Pistacia lentiscus L. from Corsica. Biochemical Systematics and Ecology. 2000;28(1):79–88. doi: 10.1016/s0305-1978(00)00044-2. [DOI] [PubMed] [Google Scholar]
- 37.Ait Said S, Fernandez C, Greff S, Derridj A, Gauquelin T, Mevy J-P. Inter-population variability of leaf morpho-anatomical and terpenoid patterns of Pistacia atlantica Desf. ssp. atlantica growing along an aridity gradient in Algeria. Flora. 2011;206(4):397–405. [Google Scholar]
- 38.Taran M, Sharifi M, Azizi E, Khanahmadi M. Antimicrobial activity of the leaves of Pistacia khinjuk. Journal of Medicinal Plants. 2010;9(6):81–85. [Google Scholar]
- 39.Dob T, Dahmane D, Chelghoum C. Chemical composition of the essential oils of Pistacia lentiscus L. from Algeria. Journal of Essential Oil Research. 2006;18(3):335–338. [Google Scholar]
- 40.Assimopoulou AN, Papageorgiou VP. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part I. Pistacia lentiscus var. Chia . Biomedical Chromatography. 2005;19(4):285–311. doi: 10.1002/bmc.454. [DOI] [PubMed] [Google Scholar]
- 41.Assimopoulou AN, Papageorgiou VP. GC-MS analysis of penta- and tetra-cyclic triterpenes from resins of Pistacia species. Part II. Pistacia terebinthus var. Chia . Biomedical Chromatography. 2005;19(8):586–605. doi: 10.1002/bmc.484. [DOI] [PubMed] [Google Scholar]
- 42.Sharifi MS, Hazell SL. Isolation, analysis and antimicrobial activity of the acidic fractions of mastic, Kurdica, Mutica and Cabolica gums from Genus Pistacia . Global Journal of Health Science. 2012;4(1):217–228. doi: 10.5539/gjhs.v4n1p217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giner-Larza EM, Máñez S, Giner RM, et al. Anti-inflammatory triterpenes from Pistacia terebinthus galls. Planta Medica. 2002;68(4):311–315. doi: 10.1055/s-2002-26749. [DOI] [PubMed] [Google Scholar]
- 44.Tomaino A, Martorana M, Arcoraci T, Monteleone D, Giovinazzo C, Saija A. Antioxidant activity and phenolic profile of pistachio (Pistacia vera L., variety Bronte) seeds and skins. Biochimie. 2010;92(9):1115–1122. doi: 10.1016/j.biochi.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 45.Romani A, Pinelli P, Galardi C, Mulinacci N, Tattini M. Identification and quantification of galloyl derivatives, flavonoid glycosides and anthocyanins in leaves of Pistacia lentiscus L. . Phytochemical Analysis. 2002;13(2):79–86. doi: 10.1002/pca.627. [DOI] [PubMed] [Google Scholar]
- 46.Yousfi M, Djeridane A, Bombarda I, Chahrazed-Hamia C-H, Duhem B, Gaydou EM. Isolation and characterization of a new hispolone derivative from antioxidant extracts of Pistacia atlantica . Phytotherapy Research. 2009;23(9):1237–1242. doi: 10.1002/ptr.2543. [DOI] [PubMed] [Google Scholar]
- 47.Bhouri W, Derbel S, Skandrani I, et al. Study of genotoxic, antigenotoxic and antioxidant activities of the digallic acid isolated from Pistacia lentiscus fruits. Toxicology in Vitro. 2010;24(2):509–515. doi: 10.1016/j.tiv.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 48.Saitta M, Giuffrida D, La Torre GL, Potortì AG, Dugo G. Characterisation of alkylphenols in pistachio (Pistacia vera L.) kernels. Food Chemistry. 2009;117(3):451–455. [Google Scholar]
- 49.Tokuşoğlu Ö, Ünal MK, Yemiş F. Determination of the phytoalexin resveratrol (3,5,4′-Trihydroxystilbene) in peanuts and pistachios by High-Performance Liquid Chromatographic Diode Array (HPLC-DAD) and Gas Chromatography-Mass Spectrometry (GC-MS) Journal of Agricultural and Food Chemistry. 2005;53(12):5003–5009. doi: 10.1021/jf050496+. [DOI] [PubMed] [Google Scholar]
- 50.Grippi F, Crosta L, Aiello G, et al. Determination of stilbenes in Sicilian pistachio by high-performance liquid chromatographic diode array (HPLC-DAD/FLD) and evaluation of eventually mycotoxin contamination. Food Chemistry. 2008;107(1):483–488. [Google Scholar]
- 51.Ballistreri G, Arena E, Fallico B. Influence of ripeness and drying process on the polyphenols and tocopherols of Pistacia vera L. . Molecules. 2009;14(11):4358–4369. doi: 10.3390/molecules14114358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdelwahed A, Bouhlel I, Skandrani I, et al. Study of antimutagenic and antioxidant activities of Gallic acid and 1,2,3,4,6-pentagalloylglucose from Pistacia lentiscus. Confirmation by microarray expression profiling. Chemico-Biological Interactions. 2007;165(1):1–13. doi: 10.1016/j.cbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 53.Topçu G, Ay M, Bilici A, Sarıkürkcü C, Öztürk M, Ulubelen A. A new flavone from antioxidant extracts of Pistacia terebinthus . Food Chemistry. 2007;103(3):816–822. [Google Scholar]
- 54.Kawashty SA, Mosharrafa SAM, El-Gibali M, Saleh NAM. The flavonoids of four Pistacia species in Egypt. Biochemical Systematics and Ecology. 2000;28(9):915–917. doi: 10.1016/s0305-1978(99)00113-1. [DOI] [PubMed] [Google Scholar]
- 55.Adams M, Plitzko I, Kaiser M, Brun R, Hamburger M. HPLC-profiling for antiplasmodial compounds-3-Methoxycarpachromene from Pistacia atlantica . Phytochemistry Letters. 2009;2(4):159–162. [Google Scholar]
- 56.Bellomo MG, Fallico B. Anthocyanins, chlorophylls and xanthophylls in pistachio nuts (Pistacia vera) of different geographic origin. Journal of Food Composition and Analysis. 2007;20(3):352–359. [Google Scholar]
- 57.Wu X, Prior RL. Identification and characterization of anthocyanins by high-performance liquid chromatography-electrospray ionization-tandem mass spectrometry in common foods in the United States: vegetables, nuts, and grains. Journal of Agricultural and Food Chemistry. 2005;53(8):3101–3113. doi: 10.1021/jf0478861. [DOI] [PubMed] [Google Scholar]
- 58.Satil F, Azcan N, Baser KHC. Fatty acid composition of pistachio nuts in Turkey. Chemistry of Natural Compounds. 2003;39(4):322–324. [Google Scholar]
- 59.Arena E, Campisi S, Fallico B, Maccarone E. Distribution of fatty acids and phytosterols as a criterion to discriminate geographic origin of pistachio seeds. Food Chemistry. 2007;104(1):403–408. [Google Scholar]
- 60.Trabelsi H, Cherif OA, Sakouhi F, et al. Total lipid content, fatty acids and 4-desmethylsterols accumulation in developing fruit of Pistacia lentiscus L. growing wild in Tunisia. Food Chemistry. 2012;131(2):434–440. [Google Scholar]
- 61.Charef M, Yousfi M, Saidi M, Stocker P. Determination of the fatty acid composition of Acorn (Quercus), Pistacia lentiscus seeds growing in algeria. Journal of the American Oil Chemists’ Society. 2008;85(10):921–924. [Google Scholar]
- 62.Özcan M. Characteristics of fruit and oil of terebinth (Pistacia terebinthus L.) growing wild in Turkey. Journal of the Science of Food and Agriculture. 2004;84(6):517–520. [Google Scholar]
- 63.Yousfi M, Nedjmi B, Bellal R, Ben Bertal D, Palla G. Fatty acids and sterols of Pistacia atlantica fruit oil. Journal of the American Oil Chemists’ Society. 2002;79(10):1049–1050. [Google Scholar]
- 64.Phillips KM, Ruggio DM, Ashraf-Khorassani M. Phytosterol composition of nuts and seeds commonly consumed in the United States. Journal of Agricultural and Food Chemistry. 2005;53(24):9436–9445. doi: 10.1021/jf051505h. [DOI] [PubMed] [Google Scholar]
- 65.Aslan M, Orhan I, Şener B. Comparison of the seed oils of Pistacia vera L. of different origins with respect to fatty acids. International Journal of Food Science and Technology. 2002;37(3):333–335. [Google Scholar]
- 66.Farhoosh R, Tavakoli J, Khodaparast MHH. Chemical composition and oxidative stability of kernel oils from two current subspecies of Pistacia atlantica in Iran. Journal of the American Oil Chemists’ Society. 2008;85(8):723–729. [Google Scholar]
- 67.Benhassaini H, Bendahmane M, Benchalgo N. The chemical composition of fruits of Pistacia atlantica desf. subsp. atlantica from Algeria. Chemistry of Natural Compounds. 2007;43(2):121–124. [Google Scholar]
- 68.Kizil S, Turk M. Microelement contents and fatty acid compositions of Rhus coriaria L. and Pistacia terebinthus L. fruits spread commonly in the South Eastern Anatolia region of Turkey. Natural Product Research. 2010;24(1):92–98. doi: 10.1080/14786410903132555. [DOI] [PubMed] [Google Scholar]
- 69.Sharayei P, Farhoosh R, Poorazrang H, Khodaparast MHH. Improvement of canola oil frying stability by bene kernel oil’s unsaponifiable matter. Journal of the American Oil Chemists’ Society. 2011;88(7):993–1000. [Google Scholar]
- 70.Matthäus B, Özcan MM. Quantitation of fatty acids, sterols, and tocopherols in turpentine (Pistacia terebinthus Chia) growing wild in Turkey. Journal of Agricultural and Food Chemistry. 2006;54(20):7667–7671. doi: 10.1021/jf060990t. [DOI] [PubMed] [Google Scholar]
- 71.Giuffrida D, Saitta M, La Torre L, Bombaci L, Dugo G. Carotenoid, chlorophyll and chlorophyll-derived compounds in pistachio kernels (Pistacia vera L.) from Sicily. Italian Journal of Food Science. 2006;18(3):309–316. [Google Scholar]
- 72.Kivçak B, Akay S. Quantitative determination of α-tocopherol in Pistacia lentiscus, Pistacia lentiscus var. chia, and Pistacia terebinthus by TLC-densitometry and colorimetry. Fitoterapia. 2005;76(1):62–66. doi: 10.1016/j.fitote.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 73.Farhoosh R, Kafrani MHT. Frying performance of the hull oil unsaponifiable matter of Pistacia atlantica subsp. mutica. European Journal of Lipid Science and Technology. 2010;112(3):343–348. [Google Scholar]
- 74.Barra A, Coroneo V, Dessi S, Cabras P, Angioni A. Characterization of the volatile constituents in the essential oil of Pistacia lentiscus L. from different origins and its antifungal and antioxidant activity. Journal of Agricultural and Food Chemistry. 2007;55(17):7093–7098. doi: 10.1021/jf071129w. [DOI] [PubMed] [Google Scholar]
- 75.Atmani D, Chaher N, Berboucha M, et al. Antioxidant capacity and phenol content of selected Algerian medicinal plants. Food Chemistry. 2009;112(2):303–309. [Google Scholar]
- 76.Sakagami H, Kishino K, Kobayashi M, et al. Selective antibacterial and apoptosis-modulating activities of mastic. In Vivo. 2009;23(2):215–224. [PubMed] [Google Scholar]
- 77.Andrikopoulos NK, Kaliora AC, Assimopoulou AN, Papapeorgiou VP. Biological activity of some naturally occurring resins, gums and pigments against in vitro LDL oxidation. Phytotherapy Research. 2003;17(5):501–507. doi: 10.1002/ptr.1185. [DOI] [PubMed] [Google Scholar]
- 78.Peksel A. Antioxidative properties of decoction of Pistacia atlantica Desf. leaves. Asian Journal of Chemistry. 2008;20(1):681–693. [Google Scholar]
- 79.Farhoosh R, Khodaparast MHH, Sharif A. Bene hull oil as a highly stable and antioxidative vegetable oil. European Journal of Lipid Science and Technology. 2009;111(12):1259–1265. [Google Scholar]
- 80.Farhoosh R, Tavassoli-Kafrani MH, Sharif A. Antioxidant activity of the fractions separated from the unsaponifiable matter of bene hull oil. Food Chemistry. 2011;126(2):583–589. [Google Scholar]
- 81.Goli AH, Barzegar M, Sahari MA. Antioxidant activity and total phenolic compounds of pistachio (Pistachia vera) hull extracts. Food Chemistry. 2005;92(3):521–525. [Google Scholar]
- 82.Gentile C, Tesoriere L, Butera D, et al. Antioxidant activity of Sicilian pistachio (Pistacia vera L. var. Bronte) nut extract and its bioactive components. Journal of Agricultural and Food Chemistry. 2007;55(3):643–648. doi: 10.1021/jf062533i. [DOI] [PubMed] [Google Scholar]
- 83.Hosseinzadeha H, Abolghasem S, Tabassib S, Moghadamc NM, Rashediniac M, Mehri S. Antioxidant activity of Pistacia vera fruits, leaves and gum extracts. Iranian Journal of Pharmaceutical Research. 2012;11(3):879–887. [PMC free article] [PubMed] [Google Scholar]
- 84.Kavak DD, Altiok E, Bayraktar O, Ülkü S. Pistacia terebinthus extract: as a potential antioxidant, antimicrobial and possible β-glucuronidase inhibitor. Journal of Molecular Catalysis B. 2010;64(3-4):167–171. [Google Scholar]
- 85.Orhan IE, Senol FS, Gulpinar AR, Sekeroglu N, Kartal M, Sener B. Neuroprotective potential of some terebinth coffee brands and the unprocessed fruits of Pistacia terebinthus L. and their fatty and essential oil analyses. Food Chemistry. 2012;130(4):882–888. [Google Scholar]
- 86.Douissa FB, Hayder N, Chekir-Ghedira L, et al. New study of the essential oil from leaves of Pistacia lentiscus L. (Anacardiaceae) from Tunisia. Flavour and Fragrance Journal. 2005;20(4):410–414. [Google Scholar]
- 87.Hayder N, Ammar RB, Abdelwahed A, et al. Antibacterial and antimutagenic activitiy of extracts and essential oil from (Tunisian) Pistacia lentiscus . Toxicological & Environmental Chemistry. 2005;87(4):567–573. [Google Scholar]
- 88.Benhammou N, FA B, Panovska TK. Antioxidant and antimicrobial activities of the Pistacia lentiscus and Pistacia atlantica extracts. African Journal of Pharmacy and Pharmacology. 2008;2(2):22–28. [Google Scholar]
- 89.Özçelik B, Aslan M, Orhan I, Karaoglu T. Antibacterial, antifungal, and antiviral activities of the lipophylic extracts of Pistacia vera . Microbiological Research. 2005;160(2):159–164. doi: 10.1016/j.micres.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 90.Ghalem BR, Mohamed B. Essential oil from gum of Pistacia atlantica Desf.: screening of antimicrobial activity. African Journal of Pharmacy and Pharmacology. 2009;3(1):13–15. [Google Scholar]
- 91.Tohidi M, Khayami M, Nejati V, Meftahizade H. Evaluation of antibacterial activity and wound healing of Pistacia atlantica and Pistacia khinjuk. Journal of Medicinal Plants Research. 2011;5(17):4310–4314. [Google Scholar]
- 92.Gerchman Y, Inbar M. Distinct antimicrobial activities in aphid galls on Pistacia atlantica . Plant Signaling & Behavior. 2011;6(12):2008–2012. doi: 10.4161/psb.6.12.18031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Taran M, Mohebali M, Esmaeli J. In vivo efficacy of gum obtained Pistacia atlantica in experimental treatment of cutaneous leishmaniasis. Iranian Journal of Public Health. 2010;39(1):36–41. [PMC free article] [PubMed] [Google Scholar]
- 94.Orhan I, Aslan M, Sener B, Kaiser M, Tasdemir D. In vitro antiprotozoal activity of the lipophilic extracts of different parts of Turkish Pistacia vera L. . Phytomedicine. 2006;13(9-10):735–739. doi: 10.1016/j.phymed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 95.Giner-Larza EM, Máñez S, Giner-Pons RM, Carmen Recio M, Ríos JL. On the anti-inflammatory and anti-phospholipase A2 activity of extracts from lanostane-rich species. Journal of Ethnopharmacology. 2000;73(1-2):61–69. doi: 10.1016/s0378-8741(00)00276-2. [DOI] [PubMed] [Google Scholar]
- 96.Giner-Larza EM, Máez S, Recio MC, et al. Oleanonic acid, a 3-oxotriterpene from Pistacia, inhibits leukotriene synthesis and has anti-inflammatory activity. European Journal of Pharmacology. 2001;428(1):137–143. doi: 10.1016/s0014-2999(01)01290-0. [DOI] [PubMed] [Google Scholar]
- 97.Hosseinzadeh H, Behravan E, Soleimani MM. Antinociceptive and anti-inflammatory effects of Pistacia vera leaf extract in mice. Iranian Journal of Pharmaceutical Research. 2011;10(4):821–828. [PMC free article] [PubMed] [Google Scholar]
- 98.Loizou S, Paraschos S, Mitakou S, Chrousos GP, Lekakis I, Moutsatsou P. Chios mastic gum extract and isolated phytosterol tirucallol exhibit anti-inflammatory activity in human aortic endothelial cells. Experimental Biology and Medicine. 2009;234(5):553–561. doi: 10.3181/0811-RM-338. [DOI] [PubMed] [Google Scholar]
- 99.Triantafyllou A, Bikineyeva A, Dikalova A, Nazarewicz R, Lerakis S, Dikalov S. Anti-inflammatory activity of Chios mastic gum is associated with inhibition of TNF-alpha induced oxidative stress. Nutrition Journal. 2011;10(1, article 64) doi: 10.1186/1475-2891-10-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rahimi R, Shams-Ardekani MR, Abdollahi M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World Journal of Gastroenterology. 2010;16(36):4504–4514. doi: 10.3748/wjg.v16.i36.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rahimi R, Mozaffari S, Abdollahi M. On the use of herbal medicines in management of inflammatory bowel diseases: a systematic review of animal and human studies. Digestive Diseases and Sciences. 2009;54(3):471–480. doi: 10.1007/s10620-008-0368-x. [DOI] [PubMed] [Google Scholar]
- 102.Farzaei MH, Rahimi R, Abbasabadi Z, Abdollahi M. An evidence-based review on medicinal plants used for the treatment of peptic ulcer in traditional Iranian medicine. International Journal of Pharmacology. 2013;9(2):108–124. [Google Scholar]
- 103.Al-Said MS, Ageel AM, Parmar NS, Tariq M. Evaluation of mastic, a crude drug obtained from Pistacia lentiscus for gastric and duodenal anti-ulcer activity. Journal of Ethnopharmacology. 1986;15(3):271–278. doi: 10.1016/0378-8741(86)90165-0. [DOI] [PubMed] [Google Scholar]
- 104.Dabos KJ, Sfika E, Vlatta LJ, Frantzi D, Amygdalos GI, Giannikopoulos G. Is Chios mastic gum effective in the treatment of functional dyspepsia? A prospective randomised double-blind placebo controlled trial. Journal of Ethnopharmacology. 2010;127(2):205–209. doi: 10.1016/j.jep.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 105.Kim H-J, Neophytou C. Natural anti-inflammatory compounds for the management and adjuvant therapy of inflammatory bowel disease and its drug delivery system. Archives of Pharmacal Research. 2009;32(7):997–1004. doi: 10.1007/s12272-009-1704-1. [DOI] [PubMed] [Google Scholar]
- 106.Rahimi R, Baghaei A, Baeeri M, et al. Promising effect of Magliasa, a traditional Iranian formula, on experimental colitis on the basis of biochemical and cellular findings. World Journal of Gastroenterology. 2013;19(12):1901–1911. doi: 10.3748/wjg.v19.i12.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaliora AC, Stathopoulou MG, Triantafillidis JK, Dedoussis GVZ, Andrikopoulous NK. Chios mastic treatment of patients with active Crohn’s disease. World Journal of Gastroenterology. 2007;13(5):748–753. doi: 10.3748/wjg.v13.i5.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kaliora AC, Stathopoulou MG, Triantafillidis JK, Dedoussis GVZ, Andrikopoulos NK. Alterations in the function of circulating mononuclear cells derived from patients with Crohn’s disease treated withmastic. World Journal of Gastroenterology. 2007;13(45):6031–6036. doi: 10.3748/wjg.v13.45.6031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hamdan II, Afifi FU. Studies on the in vitro and in vivo hypoglycemic activities of some medicinal plants used in treatment of diabetes in Jordanian traditional medicine. Journal of Ethnopharmacology. 2004;93(1):117–121. doi: 10.1016/j.jep.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 110.Kasabri V, Afifi FU, Hamdan I. In vitro and in vivo acute antihyperglycemic effects of five selected indigenous plants from Jordan used in traditional medicine. Journal of Ethnopharmacology. 2011;133(2):888–896. doi: 10.1016/j.jep.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 111.Triantafyllou A, Chaviaras N, Sergentanis TN, Protopapa E, Tsaknis J. Chios mastic gum modulates serum biochemical parameters in a human population. Journal of Ethnopharmacology. 2007;111(1):43–49. doi: 10.1016/j.jep.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 112.Balan KV, Prince J, Han Z, et al. Antiproliferative activity and induction of apoptosis in human colon cancer cells treated in vitro with constituents of a product derived from Pistacia lentiscus L. var. chia . Phytomedicine. 2007;14(4):263–272. doi: 10.1016/j.phymed.2006.03.009. [DOI] [PubMed] [Google Scholar]
- 113.He M-L, Chen W-W, Zhang P-J, et al. Gum mastic increases maspin expression in prostate cancer cells. Acta Pharmacologica Sinica. 2007;28(4):567–572. doi: 10.1111/j.1745-7254.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 114.He M-L, Li A, Xu C-S, et al. Mechanisms of antiprostate cancer by gum mastic: NF-κB signal as target. Acta Pharmacologica Sinica. 2007;28(3):446–452. doi: 10.1111/j.1745-7254.2007.00536.x. [DOI] [PubMed] [Google Scholar]
- 115.Magkouta S, Stathopoulos GT, Psallidas I, et al. Protective effects of mastic oil from Pistacia lentiscus variation chia against experimental growth of lewis lung carcinoma. Nutrition and Cancer. 2009;61(5):640–648. doi: 10.1080/01635580902825647. [DOI] [PubMed] [Google Scholar]
- 116.Loutrari H, Magkouta S, Pyriochou A, et al. Mastic oil from Pistacia lentiscus var. chia inhibits growth and survival of human K562 leukemia cells and attenuates angiogenesis. Nutrition and Cancer. 2006;55(1):86–93. doi: 10.1207/s15327914nc5501_11. [DOI] [PubMed] [Google Scholar]
- 117.Doi K, Wei M, Kitano M, Uematsu N, Inoue M, Wanibuchi H. Enhancement of preneoplastic lesion yield by Chios Mastic Gum in a rat liver medium-term carcinogenesis bioassay. Toxicology and Applied Pharmacology. 2009;234(1):135–142. doi: 10.1016/j.taap.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 118.Giaginis C, Theocharis S. Current evidence on the anticancer potential of chios mastic gum. Nutrition and Cancer. 2011;63(8):1174–1184. doi: 10.1080/01635581.2011.607546. [DOI] [PubMed] [Google Scholar]
- 119.Rezaei PF, Fouladdel S, Hassani S, et al. Induction of apoptosis and cell cycle arrest by pericarp polyphenol-rich extract of Baneh in human colon carcinoma HT29 cells. Food and Chemical Toxicology. 2012;50(3-4):1054–1059. doi: 10.1016/j.fct.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 120.Almehdar H, Abdallah HM, Osman A-MM, Abdel-Sattar EA. In vitro cytotoxic screening of selected Saudi medicinal plants. Journal of Natural Medicines. 2012;66(2):406–412. doi: 10.1007/s11418-011-0589-8. [DOI] [PubMed] [Google Scholar]
- 121.Janakat S, Al-Merie H. Evaluation of hepatoprotective effect of Pistacia lentiscus, Phillyrea latifolia and Nicotiana glauca . Journal of Ethnopharmacology. 2002;83(1-2):135–138. doi: 10.1016/s0378-8741(02)00241-6. [DOI] [PubMed] [Google Scholar]
- 122.Ljubuncic P, Song H, Cogan U, Azaizeh H, Bomzon A. The effects of aqueous extracts prepared from the leaves of Pistacia lentiscus in experimental liver disease. Journal of Ethnopharmacology. 2005;100(1-2):198–204. doi: 10.1016/j.jep.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 123.Marinou KA, Georgopoulou K, Agrogiannis G, et al. Differential effect of Pistacia vera extracts on experimental atherosclerosis in the rabbit animal model: an experimental study. Lipids in Health and Disease. 2010;9(73):1–9. doi: 10.1186/1476-511X-9-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Edwards K, Kwaw I, Matud J, Kurtz I. Effect of pistachio nuts on serum lipid levels in patients with moderate hypercholesterolemia. Journal of the American College of Nutrition. 1999;18(3):229–232. doi: 10.1080/07315724.1999.10718856. [DOI] [PubMed] [Google Scholar]
- 125.Bakirel T. The investigation of the effects of Pistacia terebinthus L. upon experimentally induced hypercholesterolemia and atherosclerosis in rabbits. Turkish Journal of Veterinary and Animal Sciences. 2003;27:1283–1292. [Google Scholar]
- 126.Dedoussis GVZ, Kaliora AC, Psarras S, et al. Antiatherogenic effect of Pistacia lentiscus via GSH restoration and downregulation of CD36 mRNA expression. Atherosclerosis. 2004;174(2):293–303. doi: 10.1016/j.atherosclerosis.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 127.Peksel A, Arisan-Atac I, Yanardag R. Evaluation of antioxidant and antiacetylcholinesterase activities of the extracts of Pistacia lentiscus Desf. leaves. Journal of Food Biochemistry. 2010;34(3):451–476. [Google Scholar]
- 128.Wellmann M. Pedanii Dioscuridis Anazarbei, de Materia Medica Libri Quinque. Berlin, Germany: Weidmann; 1907. [Google Scholar]
- 129.Hanlidou E, Karousou R, Kleftoyanni V, Kokkini S. The herbal market of Thessaloniki (N Greece) and its relation to the ethnobotanical tradition. Journal of Ethnopharmacology. 2004;91(2-3):281–299. doi: 10.1016/j.jep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 130.Mati E, de Boer H. Ethnobotany and trade of medicinal plants in the Qaysari Market, Kurdish Autonomous Region, Iraq. Journal of Ethnopharmacology. 2011;133(2):490–510. doi: 10.1016/j.jep.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 131.Scherrer AM, Motti R, Weckerle CS. Traditional plant use in the areas of Monte Vesole and Ascea, Cilento National Park (Campania, Southern Italy) Journal of Ethnopharmacology. 2005;97(1):129–143. doi: 10.1016/j.jep.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 132.Palmese MT, Uncini Manganelli RE, Tomei PE. An ethno-pharmacobotanical survey in the Sarrabus district (South-East Sardinia) Fitoterapia. 2001;72(6):619–643. doi: 10.1016/s0367-326x(01)00288-x. [DOI] [PubMed] [Google Scholar]
- 133.Lev E, Amar Z. Ethnopharmacological survey of traditional drugs sold in the Kingdom of Jordan. Journal of Ethnopharmacology. 2002;82(2-3):131–145. doi: 10.1016/s0378-8741(02)00182-4. [DOI] [PubMed] [Google Scholar]
- 134.El-Hilaly J, Hmammouchi M, Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco) Journal of Ethnopharmacology. 2003;86(2-3):149–158. doi: 10.1016/s0378-8741(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 135.Novais MH, Santos I, Mendes S, Pinto-Gomes C. Studies on pharmaceutical ethnobotany in Arrabida Natural Park (Portugal) Journal of Ethnopharmacology. 2004;93(2-3):183–195. doi: 10.1016/j.jep.2004.02.015. [DOI] [PubMed] [Google Scholar]
- 136.Sanz MJ, Terencio MC, Paya M. Isolation and hypotensive activity of a polymeric procyanidin fraction from Pistacia lentiscus L. . Pharmazie. 1992;47(6):466–467. [PubMed] [Google Scholar]
- 137.Mosaddegh M, Naghibi F, Moazzeni H, Pirani A, Esmaeili S. Ethnobotanical survey of herbal remedies traditionally used in Kohghiluyeh va Boyer Ahmad province of Iran. Journal of Ethnopharmacology. 2012;141(1):80–95. doi: 10.1016/j.jep.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 138.Altundag E, Ozturk M. Ethnomedicinal studies on the plant resources of East Anatolia, Turkey. Procedia-Social and Behavioral Sciences. 2011;19:756–777. [Google Scholar]
- 139.Duke J. Medicinal Plants of the Bible. New York, NY, USA: Conch Puplications; 1983. [Google Scholar]
- 140.Mohagheghzadeh A, Faridi P, Ghasemi Y. Analysis of Mount Atlas mastic smoke: a potential food preservative. Fitoterapia. 2010;81(6):577–580. doi: 10.1016/j.fitote.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 141.Agelet A, Vallès J. Studies on pharmaceutical ethnobotany in the region of Pallars (Pyrenees, Catalonia, Iberian Peninsula). Part II. New or very rare uses of previously known medicinal plants. Journal of Ethnopharmacology. 2003;84(2-3):211–227. doi: 10.1016/s0378-8741(02)00319-7. [DOI] [PubMed] [Google Scholar]
- 142.Benítez G, González-Tejero MR, Molero-Mesa J. Pharmaceutical ethnobotany in the western part of Granada province (Southern Spain): ethnopharmacological synthesis. Journal of Ethnopharmacology. 2010;129(1):87–105. doi: 10.1016/j.jep.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 143.Cakilcioglu U, Khatun S, Turkoglu I, Hayta S. Ethnopharmacological survey of medicinal plants in Maden (Elazig-Turkey) Journal of Ethnopharmacology. 2011;137(1):469–486. doi: 10.1016/j.jep.2011.05.046. [DOI] [PubMed] [Google Scholar]
- 144.Sezik E, Yeşilada E, Honda G, Takaishi Y, Takeda Y, Tanaka T. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. Journal of Ethnopharmacology. 2001;75(2-3):95–115. doi: 10.1016/s0378-8741(00)00399-8. [DOI] [PubMed] [Google Scholar]
- 145.Ugurlu E, Secmen O. Medicinal plants popularly used in the villages of Yunt Mountain(Manisa-Turkey) Fitoterapia. 2008;79(2):126–131. doi: 10.1016/j.fitote.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 146.Orhan I, Küpeli E, Aslan M, Kartal M, Yesilada E. Bioassay-guided evaluation of anti-inflammatory and antinociceptive activities of pistachio, Pistacia vera L. . Journal of Ethnopharmacology. 2006;105(1-2):235–240. doi: 10.1016/j.jep.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 147.Bahmani M, Eftekhari Z. An ethnoveterinary study of medicinal plants in treatment of diseases and syndromes of herd dog in southern regions of Ilam province, Iran. Comparative Clinical Pathology. 2012;22(3):1–5. doi: 10.1007/s00580-012-1423-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salehi- Surmaghi M. Medicinal Plants and Phytotherapy. Tehran, Iran: Tehran University of Medical Sciences; 2010. [Google Scholar]
- 149.Assimopoulou AN, Zlatanos SN, Papageorgiou VP. Antioxidant activity of natural resins and bioactive triterpenes in oil substrates. Food Chemistry. 2005;92(4):721–727. [Google Scholar]
- 150.Rajaei A, Barzegar M, Mobarez AM, Sahari MA, Esfahani ZH. Antioxidant, anti-microbial and antimutagenicity activities of pistachio (Pistachia vera) green hull extract. Food and Chemical Toxicology. 2010;48(1):107–112. doi: 10.1016/j.fct.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 151.Sharifi MS, Ebrahimi D, Hibbert DB, Hook J, Hazell SL. Bio-activity of natural polymers from the genus pistacia: a validated model for their antimicrobial action. Global Journal of Health Science. 2012;4(1):149–161. doi: 10.5539/gjhs.v4n1p149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sharifi MS, Hazell SL. Isolation, analysis and antimicrobial activity of the acidic fractions of Mastic, Kurdica, Mutica and Cabolica gums from genus Pistacia . Global Journal of Health Science. 2011;4(1):217–228. doi: 10.5539/gjhs.v4n1p217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shojaei A, Javidnia K, Miri R. Antioxidant and antimicrobial activity of ethanolic extract of Pistacia khinjuk (anacardiaceae) European Journal of Pharmacology. 2011;668:e43–e44. [Google Scholar]
- 154.He M-L, Yuan H-Q, Jiang A-L, et al. Gum mastic inhibits the expression and function of the androgen receptor in prostate cancer cells. Cancer. 2006;106(12):2547–2555. doi: 10.1002/cncr.21935. [DOI] [PubMed] [Google Scholar]
- 155.Moulos P, Papadodima O, Chatziioannou A, Loutrari H, Roussos C, Kolisis FN. A transcriptomic computational analysis of mastic oil-treated Lewis lung carcinomas reveals molecular mechanisms targeting tumor cell growth and survival. BMC Medical Genomics. 2009;2:1–15. doi: 10.1186/1755-8794-2-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Tounes M, Abdennour C, Houaine N. Influence of Pistacia lentiscus oil on serum biochemical parameters of domestic rabbit Oryctolagus cuniculus in mercury induced toxicity. European Journal of Scientific Research. 2008;24(4):591–600. [Google Scholar]


