Abstract
Field isolates of foot-and-mouth disease virus (FMDV) have been shown to use three αv integrins, αvβ1, αvβ3, and αvβ6, as cellular receptors. Binding to the integrin is mediated by a highly conserved RGD motif located on a surface-exposed loop of VP1. The RGD tripeptide is recognized by several other members of the integrin family, which therefore have the potential to act as receptors for FMDV. Here we show that SW480 cells are made susceptible to FMDV following transfection with human β8 cDNA and expression of αvβ8 at the cell surface. The involvement of αvβ8 in infection was confirmed by showing that virus binding and infection of the transfected cells are inhibited by RGD-containing peptides and by function-blocking monoclonal antibodies specific for either the αvβ8 heterodimer or the αv chain. Similar results were obtained with a chimeric αvβ8 including the β6 cytodomain (αvβ8/6), showing that the β6 cytodomain can substitute efficiently for the corresponding region of β8. In contrast, virus binding to αvβ6 including the β8 cytodomain (αvβ6/8) was lower than that of the wild-type integrin, and this binding did not lead to infection. Further, the αvβ6 chimera was recognized poorly by antibodies specific for the ectodomain of αvβ6 and displayed a relaxed sequence-binding specificity relative to that of wild-type integrin. These data suggest that the β6 cytodomain is important for maintaining αvβ6 in a conformation required for productive infection by FMDV.
Foot-and-mouth disease virus (FMDV) is the etiological agent of foot-and-mouth disease, a severe vesicular disease of cloven-hoofed animals including domesticated ruminants and pigs. The virus exists as seven serotypes, which are members of the genus Aphthovirus of the family Picornaviridae (35). The virion consists of an 8.5-kb strand of RNA enclosed within an icosahedral capsid formed from 60 copies each of four proteins, VP1 to VP4 (1).
Two classes of cell surface receptors that mediate FMDV infection have been identified (30). Theses are the integrins (7, 31, 33) and heparan sulfate (HS) proteoglycans (HSPGs) (29). The ability to use HSPGs as receptors appears to be restricted to strains of FMDV that have been multiply passaged through cultured cell lines (4, 5, 22, 41, 52, 58), and presently there is no convincing evidence of a role for HS in cell entry by field viruses. Instead, field viruses are dependent on integrin receptors to initiate infection in vitro, and integrins are believed to be the receptors used in the infected animal. Recently, two independent studies have shown that certain strains of FMDV can infect cultured cells via an entry pathway that is independent of both integrins and cellular HS, implying the existence of a third, as yet unidentified receptor family (4, 65).
Integrins are a family of integral membrane receptors with distinct ligand-binding specificities and tissue distributions. They contribute to a variety of cellular functions, including cell-cell and cell-matrix adhesion, and exist in alternative low- and high-affinity states, enabling them to transmit signals both into and out of cells (19, 25). Each receptor molecule is a heterodimer of two type 1 transmembrane subunits, α and β, each of which has a large extracellular domain and in most cases a short cytoplasmic tail. Most members of the integrin family recognize their ligands by binding to short linear peptide sequences, and several, including αvβ1, αvβ3, αvβ5, αvβ6, αvβ8, α5β1, and α8β1, recognize the arginine-glycine-aspartic acid (RGD) motif. To date, three RGD-dependent integrins, αvβ1, αvβ3, and αvβ6, have been reported to function as receptors for FMDV (7, 31, 33). Virus attachment to the integrin is mediated through a highly conserved RGD tripeptide, located at the apex of a long surface loop, the GH loop of VP1 (6, 24, 31, 32, 33, 38, 39, 42, 44, 56, 59). However, despite having an RGD, FMDV appears unable to use any of the RGD-dependent integrins as receptors to initiate infection, and evidence for αvβ5 and α5β1 as receptors has been consistently negative (4, 21, 33, 43, 52).
The integrin αvβ6 is of particular interest because, in our experience, it is a much more active receptor for FMDV than either αvβ1 or αvβ3 and is expressed exclusively in epithelial cells, which are the preferred cell type infected by FMDV in vivo. It is also unusual among integrins in binding only a small number of ligands, including the latency-associated protein (LAP) component of transforming growth factor β1 (TGF-β1) and TGF-β3 (27, 40, 50, 57, 62, 64). The amino acid sequences that immediately follow the RGD of LAP-1 (RGDLATI), LAP-3 (RGDLGRL), and FMDV (RGDLQVL) are similar to each other, which suggests that these ligands may share common integrin receptors. Recently, LAP-1 has been shown to be a ligand for αvβ8 also, and this prompted us to investigate whether αvβ8 could serve as a receptor for FMDV (49). In this report, we show that SW480 cells become susceptible to FMDV following transfection with the integrin β8 subunit and stable expression of αvβ8 at the cell surface. The involvement of αvβ8 in infection was confirmed in competition experiments showing that virus binding and infection are inhibited by function-blocking monoclonal antibodies (MAbs) specific for the αvβ8 heterodimer or the αv chain.
The cytodomains of the α and β chains are critically important for many of the functional properties of integrins, including the regulation of ligand-binding affinity, linkage to the cytoskeleton, formation of signaling complexes, and integrin-mediated uptake of ligands (8, 19, 28, 55, 60, 63). As a first step toward understanding the events that follow the attachment of a virus to its integrin receptor, we have studied the role of the β6 cytodomain in αvβ6-mediated infection (47). These studies showed that although the β6 cytodomain was not required for virus binding to αvβ6, the integrity of this domain was essential for αvβ6-mediated infection (47). In the present study, we have investigated further the role of the β-chain cytodomain by using chimeric αvβ6 and αvβ8 integrins in which the cytodomains of the β chains have been exchanged (αvβ6/8 and αvβ8/6, respectively). These studies show that the β6 cytodomain can substitute efficiently for the corresponding domain of β8. In contrast, although FMDV bound to cells expressing the αvβ6/8 chimera, and did so through an RGD-dependent interaction, this binding led only to very inefficient infection. Furthermore, the αvβ6/8 chimera was recognized poorly by antibodies specific for the ectodomain of αvβ6 and displayed an altered sequence-binding specificity for RGD-containing peptides. Together, these data suggest that the β6 cytodomain is important for maintaining αvβ6 in a conformation required for productive infection by FMDV.
MATERIALS AND METHODS
Cells and viruses.
BHK cells were cultured in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum (FCS), 20 mM glutamine, penicillin (100 SI units/ml), and streptomycin (100 μg/ml). Mock- and integrin transfected SW480 cells expressing either wild-type αvβ6, wild-type αvβ8, or the chimeric β subunits were cultivated in Dulbecco's modified Eagle medium supplemented with 10% FCS, 20 mM glutamine, penicillin (100 SI units/ml), streptomycin (100 μg/ml), and 4 μg of puromycin (Sigma)/ml. Construction of cells expressing wild-type αvβ6, wild-type αvβ8, and the αvβ6/8 chimera has been described previously (15, 49). The β8/6 chimera was made by splice-overlap PCR mutagenesis using the mutagenic primers 5′-CTGATCATTAGACAGGTGATACTACAATGGAAGCTACTGGTGTCATTTCAT-3′ and 5′-ATGAAATGACACCAGTAGCTTCCATTGTAGTATCACCTGTCTAATGATCAG-3′, joining W711 to K731 of the respective β8 and β6 open reading frames. Transduction and selection were performed as described previously (15). Construction and cultivation of cells expressing deletions in the β6 cytodomain (SW480-T1, -T3, and -T5) have been described previously (2, 16, 50). The virus used in this study was FMDV strain O1Kcad2. This virus does not bind HS and is dependent on integrins as its sole receptor family. For infectivity assays, virus stocks were prepared by using primary bovine thyroid cells. In all assays, the multiplicity of infection (MOI) was based on the virus titer on BHK cells. Virus purification on sucrose gradients was performed as described previously (17).
Antibodies and peptides.
The FMDV RGD peptide, with its sequence derived from the GH loop of VP1 of type O virus (FMDV-RGD; VPNLRGDLQVLA), and the control RGE version were synthesized in the peptide synthesis facility at the Oxford Centre for Molecular Science, New Chemistry Laboratory, Oxford, United Kingdom. The GRGDSP and GRGESP peptides were purchased from Novabiochem. Anti-integrin antibodies used in these studies were10D5 (mouse immunoglobulin G2a [IgG2a]) and E7P6 (mouse IgG1) against αvβ6, R6G9 (mouse IgG2a) against β6, P1F6 (mouse IgG1) against αvβ5, and 6S6 (mouse IgG1) against β1 (all from Chemicon) and SAM-1 (mouse IgG2b) against α5β1 (Serotec). Other MAbs used were 14E5 (IgG1) and 37E5 (IgG2a) against αvβ8 and the anti-αv MAb L230 (mouse IgG1). The anti-FMDV MAbs B2 (mouse IgG1) and D9 (mouse IgG2a), which recognize antigenic site 1 of type O FMDV (45), were purified by using protein A (Pierce) according to the manufacturer's instructions.
Infectious center assay.
Cells were harvested by using EDTA and were washed in cell culture medium. One million cells were collected by centrifugation, resuspended in 100 μl of Tris-buffered saline (pH 7.4) containing 1 mM CaCl2 and 0.5 mM MgCl2, and infected with FMDV O1Kcad2 (MOI, ∼0.3) at 37°C for 1 h with continuous rotation. Following infection, virus that remained on the outsides of the cells was inactivated by addition of 1 ml of 0.1 M citric acid buffer (pH 5.2) for 2 min. The cells were washed with phosphate-buffered saline (PBS), pH 7.5, containing 2 mM CaCl2 and 1 mM MgCl2 and then resuspended in 300 μl of the same buffer supplemented with 0.5% FCS. Dilutions of the infected cells (100 μl) were layered onto subconfluent monolayers of BHK cells as described previously (33). The monolayers were incubated at 37°C for 40 to 48 h, after which the infectious centers were visualized as plaques by staining with methylene blue-4% formaldehyde in PBS (pH 7.5). In the competition experiments, anti-integrin antibodies and peptides (0.1 mM) were added to the cells for 0.5 h at room temperature prior to the addition of virus, and infection was initiated by incubation at 37°C for 45 min. Following infection, virus that remained on the outsides of the cells was acid inactivated, and the cells were plated onto BHK monolayers as described above.
Flow cytometry analysis. (i) Integrin expression.
Cells were harvested by using EDTA and were resuspended at 5 × 106 per ml in a solution containing Tris-buffered saline (pH 7.5), 1 mM CaCl2, 0.5 mM MgCl2, 2% goat serum, and 3% bovine serum albumin (buffer A). Cells (30 μl) were collected by centrifugation and incubated with primary antibodies (10 μg/ml in buffer A) on ice for 0.5 h. The cells were then washed with buffer A and incubated on ice for 25 min with secondary antibodies conjugated with R-phycoerythrin (Southern Biotechnology Associates). The cells were then washed twice with buffer A and resuspended in PBS (pH 7.5)-2 mM CaCl2-1 mM MgCl2 containing 1% paraformaldehyde. Fluorescent staining was analyzed by flow cytometry using a FACSCalibur (Becton Dickinson) and by counting 10,000 cells per sample. Background fluorescence was determined by omitting the primary antibody from the assay.
(ii) Virus binding assay.
Cells were prepared in buffer A as described above and then incubated with O1Kcad2 (10 μg/ml) for 0.5 h on ice. The cells were then washed with buffer A and incubated with the anti-type O MAb B2 (10 μg/ml), followed by an R-phycoerythrin-conjugated goat anti-mouse IgG1 antibody. Background fluorescence was determined by omitting either the virus or MAb B2 from the assay. These two control conditions gave nearly identical results.
(iii) Competition experiments.
Competing MAbs and peptides were added to the cells for 0.5 h on ice before the addition of virus (10 μg/ml) for a further 0.5 h. The cells were then washed with buffer A, and cell-bound virus was detected by using an anti-type O FMDV MAb. When 10D5, 37E5, or SAM-1 was used as a competitor, virus was detected by using MAb B2. When P1F6 was used as a competitor, virus was detected by using MAb D9. Anti-FMDV antibodies were detected by using R-phycoerythrin-conjugated goat anti-mouse IgG isotype-specific antibodies. For these experiments, additional controls were performed to verify that the R-phycoerythrin-conjugated isotype-specific antibodies were not cross-reactive for the competing MAb.
RESULTS
To determine whether integrin αvβ8 could function as a receptor for FMDV, we compared SW480 cells that had been transfected to stably express αvβ8 with cells transfected with the expression plasmid alone (mock transfected). To further understand the role of the β-chain cytodomain in integrin-mediated infection, we included in these studies cells expressing either wild-type αvβ6 or chimeric αvβ8 or αvβ6 in which the cytodomains of the β chains had been exchanged (SW480αvβ8/6 and SW480αvβ6/8, respectively).
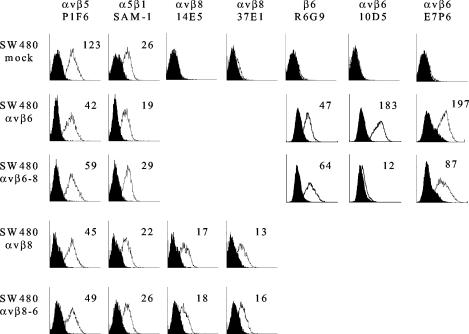
Initially, we used flow cytometry to confirm the integrin expression profiles on transfected cells. SW480 cells normally express αvβ5 and α5β1 as their only RGD-binding integrins (Fig. 1) (62). However, upon transfection with integrin β-chain cDNA, they are capable of expressing “new” αvβ combinations as functional heterodimers. Figure 1 shows that expression of αvβ5 was lower on cells transfected with β-chain cDNA than on mock-transfected cells, presumably as a result of competition between the endogenous and transfected β chains for the αv subunit, whereas expression of α5β1 was not altered. Cells transfected with either the wild-type β8 chain or the β8/6 chimera were found to express similar amounts of αvβ8 on the cell surface. Similarly, by use of an antibody specific for the ectodomain of the β6 chain, cells transfected with either wild-type β6 or the β6/8 chimera were found to express similar amounts of β6 (Fig. 1). Because β6 is expressed at the cell surface only as a heterodimer with the αv chain, this observation indicates that the transfected cells express similar amounts of αvβ6 or αvβ6/8, respectively. However, despite the fact that β6 is expressed at similar levels on transfected cells, antibodies specific for the αvβ6 heterodimer (MAbs 10D5 and E7P6) appeared to recognize the αvβ6/8 chimera less efficiently than they recognized wild-type αvβ6 (Fig. 1). This reduction in expression of the epitopes for MAbs 10D5 and E7P6 suggests that inclusion of the β8 cytodomain maintains αvβ6 in a conformation which is recognized poorly by MAbs specific for the ectodomain of αvβ6. During the course of the experiments reported here, levels of integrin expression on transfected cells were determined by flow cytometry and did not change significantly from those shown in Fig. 1.
FIG. 1.
Flow cytometric analysis of RGD-dependent integrins expressed on mock- and β-transfected SW480 cells. Mock-transfected cells (SW480 mock) and cells transfected with wild-type β6 (SW480 αvβ6), wild-type β8 (SW480 αvβ8), or the chimeric β subunit β6/8 (SW480 αvβ6-8) or β8/6 (SW480 αvβ8-6) were incubated with (open histogram) or without (solid histogram) the indicated anti-integrin antibody, followed by an R-phycoerythrin-conjugated goat anti-mouse isotype-specific secondary antibody. The mean fluorescence intensity is shown for each antibody.
Next, we compared infection of the transfected cells by using an infectious center assay, which permits the number of productive infectious events to be quantified. Table 1 shows that cells expressing αvβ8 are more susceptible to infection by FMDV than mock-transfected cells. Similar numbers of infectious centers were obtained with cells expressing either wild-type αvβ8 or the αvβ8/6 chimera; certainly there was no evidence that the domain substitution reduced the receptor activity of the integrin. As expected, expression of αvβ6 also resulted in increased susceptibility to infection relative to that of mock-transfected cells. Only a small number of infectious centers were obtained for cells transfected with the β6/8 chimera.
TABLE 1.
Infection of transfected cellsa
| Cells infected | Transfected integrin | No. of infectious centersb |
|---|---|---|
| SW480-mock | None | 17 ± 2 |
| SW480/αvβ6 | αvβ6 | 12,375 ± 867 |
| SW480/αvβ6-8 | αvβ6/8 | 444 ± 25 |
| SW480/αvβ8 | αvβ8 | 3,930 ± 350 |
| SW480/αvβ8-6 | αvβ8-6 | 6,050 ± 680 |
Mock- and integrin-transfected cells were infected with FMDV strain O1Kcad2 at an MOI of <1 PFU/cell and then layered onto monolayers of BHK indicator cells in an infectious center assay (see Materials and Methods).
Per 106 cells infected. Values are means ± standard errors of the means for at least three independent experiments.
To confirm the role of αvβ8 in infection, we performed competition experiments using function-blocking MAbs specific for either the αvβ8 heterodimer (MAb 37E1) or the αv chain (MAb L230). Figure 2 shows that preincubation of αvβ8-expressing cells with these antibodies inhibited infection, whereas MAbs to αvβ5 or the β1 chain did not have a significant inhibitory effect. A combination of the MAbs to αvβ8 and αvβ5 (10 μg/ml each) did not result in a greater inhibitory effect than that obtained with the αvβ8 MAb alone (data not shown). Similarly, preincubation of αvβ8-expressing cells with an RGD-containing peptide whose sequence is derived from the FMDV RGD site (FMDV-RGD) (see Materials and Methods) inhibited infection by more than 90% (data not shown). These data show that αvβ8 functions as a receptor for FMDV and that its ability to mediate infection is not diminished by replacing the β-chain cytodomain with the corresponding region of β6. In contrast, the ability of αvβ6 to mediate FMDV infection was almost completely inhibited (97%) by substitution of the β8 cytodomain.
FIG. 2.
Infection of β8-transfected SW480 cells is specifically inhibited by MAbs to the αv subunit and the αvβ8 heterodimer. β8-transfected cells (SW480αvβ8) were incubated with antibodies against αvβ5 (P1F6), αvβ8 (37E1), β1 (6S6), or αv (L230) prior to infection by O1Kcad2 at an MOI of ∼0.3 PFU/cell, and the infected cells were used in an infectious center assay. Numbers of infectious centers are expressed as percentages of the number obtained in the absence of competing antibodies, taken as 100%. Data are means (± standard errors of the means) from three independent experiments, each carried out in duplicate.
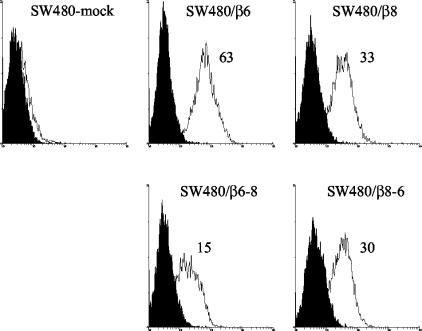
The data described above show that αvβ8 promotes infection by FMDV. To gain a better understanding of the role of αvβ8 in infection, we determined the abilities of the transfected cells to bind FMDV. Figure 3 shows that the increased susceptibility to infection of cells expressing αvβ8 is correlated with an increase in virus binding. Similar levels of virus binding were obtained with cells expressing either wild-type αvβ8 or the αvβ8/6 chimera, consistent with the similar levels of integrin expressed by these cells (Fig. 1). Figure 3 also shows that, in agreement with previous observations (33), FMDV binds to SW480 cells expressing wild-type αvβ6. Interestingly, the very poor susceptibility to infection of cells expressing the αvβ6/8 chimera was not fully reflected in the binding data, which show almost one-quarter of the virus binding remaining relative to that of cells expressing wild-type αvβ6.
FIG. 3.
Flow cytometric analysis of FMDV binding to mock- and β-transfected SW480 cells. Histograms show FMDV O1Kcad2 binding to mock-transfected (SW480-mock) and cells expressing αvβ6 (SW480/β6), αvβ8 (SW480/β8), αvβ6/8 (SW480/β6-8), or αvβ8/6 (SW480/β8-6). Virus binding (open histograms) was detected by using the anti-FMDV antibody B2 followed by an R-phycoerythrin-conjugated goat anti-mouse IgG1 secondary antibody. Mean fluorescence intensities are given. Solid histograms, background fluorescence (see Materials and Methods).
To confirm the direct involvement of αvβ8 as a virus attachment receptor, we carried out competition experiments using the anti-integrin MAbs described above. Figure 4 shows that MAbs to αvβ8 (37E5) or the αv chain (L230) inhibited virus binding to cells expressing wild-type αvβ8, whereas MAbs to αvβ5 or α5β1 did not have an inhibitory effect. Similar observations were made for cells expressing the αvβ8/6 chimera (data not shown), confirming that inclusion of the β6 cytodomain does not interfere with virus binding to αvβ8. In agreement with a previous observation (33), the anti-αvβ6 MAb, MAb 10D5, inhibited FMDV binding to cells expressing wild-type αvβ6 (data not shown). We were unable to reproduce these observations with cells expressing the αvβ6/8 chimera, because MAb 10D5 is the only currently available function-blocking MAb to αvβ6, and this MAb has a low affinity for these cells (Fig. 1). However, in view of the fact that virus binding was inhibited preferentially by the FMDV peptide (see below and Fig. 5), a characteristic of αvβ6, it is likely that the chimeric αvβ6/8 integrin is a receptor for FMDV attachment.
FIG. 4.
Anti-integrin antibodies inhibit the binding of FMDV to β8-transfected SW480 cells. β8-transfected cells (SW480αvβ8) were incubated with antibodies to αvβ5 (P1F6), αvβ8 (37E1), α5β1 (SAM-1), or αv (L230) at 6 μg/ml prior to the addition of virus (O1Kcad2; 10 μg/ml), and the cells were analyzed for bound virus by flow cytometry (see Materials and Methods). Data are expressed as percentages of the level of binding in the absence of the competing antibody (set at 100%) and are means of two independent experiments, each carried out using triplicate samples, that gave near-identical results.
FIG. 5.
Binding of FMDV to β-transfected SW480 cells is inhibited by RGD peptides. β-transfected cells expressing wild-type αvβ8 (SW480/αvβ8) (A), wild-type αvβ6 (SW480/αvβ6) (B), or chimeric αvβ6/8 (SW480/αvβ6-8) (C) were incubated with an RGD-containing peptide (VPNLRGDLQVLA [solid bars] or GRGDSP [open bars]) or the control RGE version (VPNLRGELQVLA [hatched bars] or GRGESP [checked bars]) prior to the addition of FMDV O1Kcad2 (10 μg/ml). Cell-bound virus was detected by flow cytometry using the anti-FMDV antibody B2 followed by an R-phycoerythrin-conjugated goat anti-mouse IgG1 antibody. Data are means from two independent experiments, each carried out by using triplicate samples, that gave nearly identical results.
The anti-αvβ8 MAb (MAb 37E1) was found to inhibit virus binding to cells expressing αvβ8 by ∼60% (Fig. 4) despite inhibiting infection by ∼85% (Fig. 2). Increasing the concentration of this MAb to 25 μg/ml did not inhibit virus binding beyond that shown in Fig. 4. Similar observations were made with cells expressing the αvβ8/6 chimera (data not shown). At present we do not know why the anti-αvβ8 MAb did not inhibit virus binding to cells expressing αvβ8 more efficiently. However, when used at a high concentration, MAb 37E1 (IgG2a) could be detected by the anti-IgG1 conjugated antibody used to detect virus binding (see Materials and Methods). This cross-reactivity could, in part, account for the apparent residual virus binding in the presence of MAb 37E1. Alternatively, since the anti-αv MAb inhibited virus binding to a greater extent that the anti-αvβ8 MAb, it is possible that more than one αv integrin may serve as a receptor for virus attachment to the transfected cells. However, this explanation is unlikely for two reasons. First, SW480 cells normally express only one αv integrin, αvβ5, and this integrin is not a receptor for FMDV on these cells (Table 1; Fig. 3). Second, an antibody to αvβ5 did not inhibit virus binding or infection of cells expressing wild-type αvβ8 (Fig. 4), again suggesting that αvβ5 is not involved in virus attachment.
Ligand binding to integrins is differentially regulated by divalent cations, and manganese (Mn2+) ions are known to enhance ligand binding to several integrin receptors (34, 36, 37, 48). It has been shown previously that Mn2+ ions enhance FMDV binding to αvβ1 and αvβ3 (31, 32), whereas this cation does not affect FMDV binding to αvβ6 (33). FMDV binding to cells expressing wild-type αvβ8 in the presence of 1 mM MnCl2 was not increased above that obtained in the presence of calcium and magnesium alone (data not shown).
The binding of FMDV to its integrin receptors is RGD dependent and is inhibited by synthetic peptides containing this motif; moreover, viruses with mutations at the RGD site fail to bind to cells (see the introduction). Previously it has been shown that the binding of FMDV to its integrin receptors is differentially sensitive to RGD-containing peptides. Thus, whereas an FMDV RGD peptide (VPNLRGDLQVLA) inhibits virus binding to αvβ1, αvβ3, and αvβ6, a shorter RGD-containing peptide (GRGDSP) is effective only for αvβ1 and αvβ3 and does not inhibit virus binding to αvβ6 (31, 32, 33). To determine whether FMDV binding to αvβ8 is also differentially sensitive to RGD-containing peptides, we used the two peptides described above in competition experiments to inhibit virus binding to αvβ8. Figure 5A shows that FMDV binding to αvβ8 was inhibited by either of these RGD-containing peptides in a sequence-specific manner, since the control RGE versions of these peptides had only a minimal effect on virus binding at the highest concentration used. The FMDV peptide was found to be a more potent inhibitor of virus binding to αvβ8 than the GRGDSP peptide, suggesting that high-affinity binding of the FMDV peptide to αvβ8 may also be dependent on residues that lie outside of the RGD motif.
To understand further the nature of the virus binding to the αvβ6/8 chimera, we also performed peptide competition experiments using cells expressing this integrin (Fig. 5C). As expected, virus binding to cells expressing wild-type αvβ6 was inhibited by the FMDV peptide but not by the GRGDSP peptide (Fig. 5B), confirming previous observations (31). Figure 5C shows that virus binding to cells expressing αvβ6/8 is also inhibited by the FMDV peptide; however, much less peptide was required to inhibit virus binding to these cells than to cells expressing wild-type αvβ6. It is worth recalling that the domain substitution in αvβ6/8 was also associated with a reduced ability to bind virus (Fig. 3), and it seems likely that both properties may reflect a reduced affinity of the αvβ6/8 chimera for FMDV relative to that of wild-type αvβ6. In addition, the αvβ6/8 chimera appeared to display a relaxed sequence-binding specificity relative to that of wild-type αvβ6, since the GRGDSP peptide was found to inhibit virus binding to cells expressing this chimera (Fig. 5C).
It has been reported previously that deletions in the β6 cytodomain do not interfere with FMDV binding to αvβ6, whereas the same deletions reduce the ability of this integrin to mediate infection (47). Specifically, truncation of the 17 C-terminal residues of the β6 chain (SW480-T3) does not significantly reduce the ability of αvβ6 to mediate infection, whereas infection of cells expressing receptors lacking the entire β6 cytodomain (SW480-T1) or containing an internal deletion within this domain (SW480-T5) is greatly reduced (47). These data have suggested that the β6 cytodomain may be required for a postattachment step(s) in FMDV infection. In the present study, we have observed that cells expressing the αvβ6/8 chimera show characteristics similar to those expressing the T1 and T5 deletions, i.e., they support FMDV binding, but infection is greatly reduced. Because the binding of MAbs to the ectodomain of αvβ6 (MAbs 10D5 and E7P6) was reduced on cells expressing the αvβ6/8 chimera (Fig. 1), we investigated whether the epitopes for these MAbs are expressed on cells expressing receptors with β6 cytodomain deletions (SW480-T1, -T3, and -T5). Figure 6 shows the results of a flow cytometric analysis of αvβ6 expression on these cells. Binding of the anti-αvβ6 MAbs (MAbs 10D5 and E7P6) is shown relative to that of MAb R6G9, which recognizes the ectodomain of the β6 chain. The binding of MAbs 10D5 and E7P6 was similar for cells expressing wild-type αvβ6 or the T3 deletion. In contrast, like that for cells expressing the αvβ6/8 chimera, binding of MAbs 10D5 and E7P6 to cells expressing the T1 or T5 deletion was greatly reduced (Fig. 6). Thus, as with the αvβ6/8 chimera, the majority of αvβ6 expressed on the SW480-T1 and -T5 cells is maintained in a conformation that is both poorly recognized by MAbs for the αvβ6 heterodimer and unable to mediate infection by FMDV.
FIG. 6.
Bar graphs showing results of flow cytometric analysis of αvβ6 receptors containing deletions in the β6 cytodomains. Cells expressing either wild-type αvβ6 (wt-B6) (SW480αvβ6) or αvβ6 containing deletion T1 (SW480-T1), T3 (SW480-T3), or T5 (SW480-T5) in the β6 cytodomain were analyzed by flow cytometry for expression of epitopes present on the ectodomain of the β6 chain (MAb R6G9) or the αvβ6 heterodimer (10D5 and E7P6). Data are ratios of the mean fluorescence intensity (MFI) obtained with the anti-αvβ6 MAbs to the MFI for MAb R6G9. Means ± standard deviations from three independent experiments, each carried out in triplicate, are shown.
DISCUSSION
Field strains of FMDV use integrins as receptors to initiate infection in vitro, and integrins are believed to perform the same role in the infected animal (52). Prior to this study, three integrins, αvβ1, αvβ3, and αvβ6, had been reported to function as receptors for FMDV. In the present study we have shown that a fourth αv integrin, αvβ8, can also function as a receptor for FMDV. The main evidence in support of this finding is as follows: (i) SW480 cells are normally nonpermissive for FMDV but are made susceptible to infection by transfection with β8 cDNA and expression of αvβ8 at the cell surface; (ii) virus attachment to the transfected cells is inhibited by function-blocking MAbs specific for either the αvβ8 heterodimer or the αv chain; (iii) in agreement with the above observations, infection of cells expressing αvβ8 is also inhibited by the same antibodies.
Binding of FMDV to its integrin receptors is RGD dependent and is inhibited by synthetic peptides containing this motif (see the introduction and Fig. 5). However, the binding of FMDV to its various integrin receptors is differentially sensitive to such peptides. Specifically, whereas an FMDV-derived RGD peptide (FMDV-RGD; VPNLRGDLQVLA) inhibits virus binding to αvβ1, αvβ3, and αvβ6, a shorter RGD-containing peptide (GRGDSP) is effective only for αvβ1 and αvβ3, failing to inhibit virus binding to αvβ6 (31, 32, 33). These data suggest that residues within the FMDV peptide, in addition to RGD, may be required for high-affinity binding to αvβ6. In the present study, we have shown that both of these peptides inhibit FMDV binding to αvβ8; however, in contrast to αvβ1 and αvβ3, for which the GRDGSP peptide was found to be the more potent inhibitor, the FMDV RGD peptide is the more potent inhibitor of binding to αvβ8. These data suggest that, as for αvβ6, residues other than RGD may be required for high-affinity ligand binding to αvβ8.
Studies to determine the role of the integrin cytodomains in FMDV infection have obtained contrasting results. Neff and Baxt (53) have reported that deletion of the cytodomain from either the α or the β chain does not interfere with the ability of αvβ3 to mediate infection, whereas Miller et al. have shown that certain deletions within the β6 cytodomain result in αvβ6 receptors that, although still able to bind FMDV, are no longer competent to mediate infection (47). Specifically, a deletion mutant lacking the 17 C-terminal residues of the β6 cytodomain (the T3 deletion) binds FMDV and mediates infection similarly to the wild-type integrin (47), whereas deletion mutants either with an internal deletion in the β6 cytodomain (the T5 deletion) or lacking this domain completely (the T1 deletion) bind virus and mediate infection inefficiently (47). These data have suggested that the β6 cytodomain may be required for a postattachment event(s) in infection. In the present study, we have investigated further the role of the β-chain cytodomain in integrin-mediated infection. This study has shown that replacement of the β8 cytodomain with the corresponding region of β6 does not affect the expression of αvβ8 at the cell surface or the ability of αvβ8 to bind virus and mediate infection. In contrast, a chimeric αvβ6 including the β8 cytodomain (αvβ6/8) shared the characteristics of the αvβ6 deletion mutants (T1 and T5): it bound FMDV but was unable to mediate infection. The αvβ6/8 chimera is recognized poorly by antibodies specific for αvβ6, suggesting that the presence of the β8 cytodomain alters the conformation of the αvβ6 ectodomain. This observation led us to reexamine αvβ6 expression on SW480 cells expressing the β6 cytodomain deletion mutants (T1, T3, and T5). This study has shown that, as for the αvβ6/8 chimera, the binding of the αvβ6-specific MAbs is also reduced when αvβ6 includes the T1 or T5 modified β chain. In contrast, these MAbs recognize similarly αvβ6 containing the T3 or wild-type β6 chain.
At present we do not know why the binding of virus to cells expressing the αvβ6/8 chimera or the T1 or T5 deletion mutant does not lead to infection. The domain substitution in the αvβ6/8 chimera was associated both with a reduced ability to bind FMDV (Fig. 3) and with a reduction in the amount of RGD peptide required to inhibit this binding (Fig. 5C). In addition, the sequence-binding specificity for the αvβ6/8 chimera was altered from that for wild-type αvβ6. These properties may reflect a reduced affinity of the αvβ6/8 chimera for FMDV. Taken together, our data are consistent with the hypothesis that the β6 cytodomain is required to maintain the ectodomain of αvβ6 in a conformation that is necessary for both high-affinity binding of FMDV and subsequent infection.
Alternatively, given the role of β-chain cytodomains in endocytosis (60, 63), the loss of susceptibility to infection observed for cells expressing the αvβ6/8 chimera or the T1 or T5 deletion mutation may be due to a defect in virus internalization. However, sequences required for integrin-mediated virus internalization are missing from the β8 cytodomain. The β8 cytodomain is almost completely divergent in primary sequence from the other β integrin cytodomains, which in general are highly homologous (54, 60). Thus, it is possible that αvβ8 mediates productive FMDV infection through a mechanism independent of its β-chain cytodomain. This idea is supported by previous studies which demonstrate that αvβ8 is functionally competent as a TGF-β-activating receptor even in the absence of its cytodomain (49).
A third possibility is that the cytodomain of the β6 chain is required for membrane penetration, thereby permitting entry of the viral RNA genome into the cellular cytoplasm. This role has been proposed for the cytodomain of the β5 chain in αvβ5-mediated infection by adenovirus (61). In the case of αvβ5, it is the 3 C-terminal residues of the β5 chain that are most important for membrane penetration. However, these residues are different in the β6 chain, and their deletion does not inhibit αvβ6-mediated infection by FMDV (47). Therefore, it would appear that, if the β6 cytodomain is needed for virus penetration into the cell, it works by a mechanism distinct from that used by αvβ5.
FMDV is one of the most infectious animal pathogens known. It causes a severe vesicular disease of cloven-hoofed animals, which spreads by aerosol, sometimes over long distances. In vivo, FMDV shows a strong tropism for epithelial cells. The primary site of infection is thought to be epithelial cells in the upper respiratory tract (3, 10, 11, 14, 51), and during the development of disease, the virus is widely disseminated throughout the body, with secondary sites of replication in many epithelial tissues (3, 12, 13, 14). The ability of FMDV to use multiple, different integrin species to initiate infection could, in part, account for the great success of this virus. However, although integrins are believed to be the receptors used to initiate FMDV infection in an animal, presently we do not know which, if any, of the integrins identified in vitro function in this way. Similarly, very little is known of the tissue distribution and cell type expression of integrins in the natural hosts of FMDV. Studies of other mammalian species have shown that αvβ3 normally predominates in endothelial rather than epithelial cells (9, 18, 20, 26, 46), whereas, by contrast, αvβ6 is expressed exclusively in the latter cell type. Recently, αvβ8 has also been identified on airway epithelial cells (15, 23). If these observations were repeated in the natural hosts of FMDV, they would point to an important role for αvβ6 and αvβ8 in the tropism and pathogenesis of FMDV during the initial phase of infection.
Acknowledgments
We thank M. Pitkeathly and S. Shah for the peptides.
This work was supported by DEFRA (project SE2717).
REFERENCES
- 1.Acharya, R., E. Fry, D. Stuart, G. Fox, D. Rowlands, and F. Brown. 1989. The three-dimensional structure of foot-and-mouth disease virus at 2.9 Å resolution. Nature 337:709-716. [DOI] [PubMed] [Google Scholar]
- 2.Agrez, M., A. Chen, R. I. Cone, R. Pytela, and D. Sheppard. 1994. The αvβ6 integrin promotes proliferation of colon carcinoma cells through a unique region of the β6 cytoplasmic domain. J. Cell Biol. 127:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alexandersen, A., M. B. Oleksiewicz, and A. I. Donaldson. 2001. The early pathogenesis of foot-and-mouth disease virus in pigs infected by contact: a quantitative time-course study using TaqMan RT-PCR. J. Gen. Virol. 82:747-755. [DOI] [PubMed] [Google Scholar]
- 4.Baranowski, E., C. M. Ruiz-Jarabo, N. Sevilla, D. Andreu, E. Beck, and E. Domingo. 2000. Cell recognition by foot-and-mouth disease virus that lacks the RGD integrin-binding motif: flexibility in aphthovirus receptor usage. J. Virol. 74:1641-1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baranowski, E., N. Sevilla, N. Verdaguer, C. M. Ruiz-Jarabo, E. Beck, and E. Domingo. 1998. Multiple virulence determinants of foot-and-mouth disease virus in cell culture. J. Virol. 72:6362-6372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baxt, B., and Y. Becker. 1990. The effect of peptides containing the arginine-glycine aspartic acid sequence on the adsorption of foot-and-mouth disease virus to tissue culture cells. Virus Genes 4:73-83. [DOI] [PubMed] [Google Scholar]
- 7.Berinstein, A., M. Roivainen, T. Hovi, P. W. Mason, and B. Baxt. 1995. Antibodies to the vitronectin receptor (integrin αvβ3) inhibit binding and infection of foot-and-mouth disease virus to cultured cells. J. Virol. 69:2664-2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blystone, S. D., M. P. Williams, S. E. Slater, and E. J. Brown. 1997. Requirement of integrin β3 tyrosine 747 for β3 tyrosine phosphorylation and regulation of αvβ3 avidity. J. Biol. Chem. 272:28757-28761. [DOI] [PubMed] [Google Scholar]
- 9.Breuss, J. M., N. Gillett, L. Lu, D. Sheppard, and R. Pytela. 1993. Restricted distribution of integrin β6 mRNA in primate epithelial tissues. J. Histochem. Cytochem. 41:1521-1527. [DOI] [PubMed] [Google Scholar]
- 10.Brown, C. C., R. F. Meyer, H. J. Olander, C. House, and C. A. Mebus. 1992. A pathogenesis study of foot-and-mouth disease virus in cattle, using in situ hybridisation. Can. J. Vet. Res. 56:189-193. [PMC free article] [PubMed] [Google Scholar]
- 11.Brown, C. C., H. J. Olander, and R. F. Meyer. 1991. A preliminary study of the pathogenesis of foot-and-mouth disease virus, using in situ hybridisation. Vet. Pathol. 28:216-222. [DOI] [PubMed] [Google Scholar]
- 12.Brown, C. C., H. J. Olander, and R. F. Meyer. 1995. Pathogenesis of foot-and-mouth disease virus in swine, studied by in-situ hybridisation. J. Comp. Pathol. 113:51-58. [DOI] [PubMed] [Google Scholar]
- 13.Brown, C. C., M. E. Piccone, P. W. Mason, T. S.-C. McKenna, and M. J. Grubman. 1996. Pathogenesis of wild-type and leaderless foot-and-mouth disease virus in cattle. J. Virol. 70:5638-5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burrows, R.,. J. A. Mann, A. J. M. Garland, A. Greig, and D. Goodridge. 1981. The pathogenesis of natural and stimulated natural foot-and-mouth disease virus infection in cattle. J. Comp. Pathol. 91:599-609. [DOI] [PubMed] [Google Scholar]
- 15.Cambier, S., D. Mu, D. O'Connell, K. Boylen, W. Travis, W. Liu, V. C. Broaddus, and S. L. Nishimura. 2000. A role for the integrin αvβ8 in the negative regulation of epithelial cell growth. Can. Res. 60:7084-7093. [PubMed] [Google Scholar]
- 16.Cone, R. I., A. Weinacker, A. Chen, and D. Sheppard. 1994. Effects of β subunit cytoplasmic domain deletions on the recruitment of the integrin αvβ6 to focal contacts. Cell Adhesion Commun. 2:101-113. [DOI] [PubMed] [Google Scholar]
- 17.Curry, S., E. Fry, W. E. Blakemore, R. Abu-Ghazaleh, T. Jackson, A. King, S. Lea, J. Newman, D. Rowlands, and D. Stuart. 1996. Perturbations in the surface structure of A22 Iraq foot-and-mouth disease virus accompanying coupled changes in host cell specificity and antigenicity. Structure 4:135-145. [DOI] [PubMed] [Google Scholar]
- 18.Damjanovich, L., S. M. Albelda, S. A. Mette, and C. A. Buck. 1992. Distribution of integrin cell adhesion receptors in normal and malignant lung tissue. Am. J. Respir. Cell Mol. Biol. 6:197-206. [DOI] [PubMed] [Google Scholar]
- 19.Dedhar, S., and G. E. Hannigan. 1996. Integrin cytoplasmic interactions and bidirectional transmembrane signalling. Curr. Opin. Cell Biol. 8:657-669. [DOI] [PubMed] [Google Scholar]
- 20.Delporte, C., R. S. Redman, and B. J. Baum. 1997. Relationship between the cellular distribution of the αvβ3/5 integrins and adenoviral infection in salivary glands. Lab. Investig. 77:167-173. [PubMed] [Google Scholar]
- 21.Duque, H., and B. Baxt. 2003. Foot-and-mouth disease virus receptors: comparison of bovine αv integrin utilization by type A and O viruses. J. Virol. 77:2500-2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Escarmis, C., E. C. Carrillo, M. Ferrer, J. F. G. Arriaza, N. Lopez, C. Tami, N. Verdaguer, E. Domingo, and M. T. Franze-Fernandez. 1998. Rapid selection in modified BHK-21 cells of a foot-and-mouth disease virus variant showing alterations in cell tropism. J. Virol. 72:10171-10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fjellbirkeland, L., S. Cambier, V. C. Broaddus, A. Hill, P. Brunetta, G. Dolganov, D. Jablons, and S. L. Nishimura. 2003. Integrin αvβ8-mediated activation of transforming growth factor-β inhibits human airway epithelial proliferation in intact bronchial tissue. Am. J. Pathol. 163:533-542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fox, G., N. R. Parry, P. V. Barnett, B. McGinn, D. J. Rowlands, and F. Brown. 1989. Cell attachment site on foot-and-mouth disease virus includes the amino acid sequence RGD (arginine-glycine-aspartic acid). J. Gen. Virol. 70:625-637. [DOI] [PubMed] [Google Scholar]
- 25.Giancotti, F. G., and E. Ruoslahtil. 1999. Integrin signalling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 26.Haapasalmi, K., K. Zhang, M. Tonnesen, J. Olerud, D. Sheppard, T. Salo, R. Krammer, R. Clark, V. Uitto, and H. Larjava. 1996. Keratinocytes in human wounds express αvβ6 integrin. J. Investig. Dermatol. 106:42-48. [DOI] [PubMed] [Google Scholar]
- 27.Huang, X., J. F. Wu, S. Spong, and D. Sheppard. 1998. The integrin αvβ6 is critical for keratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J. Cell Sci. 111:2189-2195. [DOI] [PubMed] [Google Scholar]
- 28.Hynes, R. O. 1992. Integrins: versatility, modulation, and signalling in cell adhesion. Cell 69:11-25. [DOI] [PubMed] [Google Scholar]
- 29.Jackson, T., F. M. Ellard, R. Abu-Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. I. Newman, and A. M. Q. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jackson, T., A. M. Q. King, D. I. Stuart, and E. Fry. 2003. Structure and receptor binding. Virus Res. 91:33-46. [DOI] [PubMed] [Google Scholar]
- 31.Jackson. T., A. P. Mould, D. Sheppard, and A. M. Q. King. 2002. The integrin αvβ1 is a receptor for foot-and-mouth disease virus. J. Virol. 76:935-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jackson, T., A. Sharma, R. Abu-Ghazaleh, W. E. Blakemore, F. M. Ellard, D. L. Simmons, J. W. I. Newman, D. I. Stuart, and A. M. Q. King. 1997. Arginine-glycine-aspartic acid-specific binding by foot-and-mouth disease virus to the purified integrin αvβ3 in vitro. J. Virol. 71:8357-8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackson, T., D. Sheppard, M. Denyer, W. E. Blakemore, and A. M. Q. King. 2000. The epithelial integrin αvβ6 is a receptor for foot-and-mouth disease virus. J. Virol. 74:4949-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirchhofer, D., J. Grzesiak, and M. D. Pierschbacher. 1991. Calcium as a potential physiological regulator of integrin-mediated cell adhesion. J. Biol. Chem. 266:4471-4477. [PubMed] [Google Scholar]
- 35.Knowles, N. J., and A. R. Samuel. 2003. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 91:65-80. [DOI] [PubMed] [Google Scholar]
- 36.Lee, J. O., L. A. Bankston, M. A. Arnaout, and R. C. Liddington. 1995. Two conformations of the integrin A-domain (I-domain): a pathway for activation? Structure 3:1333-1340. [DOI] [PubMed] [Google Scholar]
- 37.Li, R., P. Rieu, D. L. Griffith, D. Scott, and M. A. Arnaout. 1998. Two functional states of the CD11b A-domain: correlations with key features of two Mn2+-complexed crystal structures. J. Cell Biol. 143:1523-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liebermann, H., R. Dolling, D. Schmidt, and G. Thalmann. 1991. RGD-containing peptides of VP1 of foot-and-mouth disease virus (FMDV) prevent virus infection in vitro. Acta Virol. 35:90-93. [PubMed] [Google Scholar]
- 39.Logan, D., R. Abu-Ghazaleh, W. E. Blakemore, S. Curry, T. Jackson, A. King, S. Lea, R. Lewis, J. W. I. Newman, N. Parry, D. Rowlands, D. Stuart, and E. Fry. 1993. Structure of a major immunogenic site on foot-and-mouth disease virus. Nature 362:566-568. [DOI] [PubMed] [Google Scholar]
- 40.Ludbrook, S. B., S. T. Barry, C. J. Delves, and C. M. T. Horgan. 2003. The integrin αvβ3 is a receptor for the latency-associated peptides of transforming growth factors β1 and β3. Biochem. J. 369:311-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez, M. A., N. Verdaguer, M. Mateu, and E. Domingo. 1997. Evolution subverting essentiality: dispensability of the cell attachment Arg-Gly-Asp motif in multiply passaged foot-and-mouth disease virus. Proc. Natl. Acad. Sci. USA 94:6798-6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mason, P. W., E. Rieder, and B. Baxt. 1994. RGD sequence of foot-and-mouth disease virus is essential for infecting cells via the natural receptor but can be bypassed by an antibody-dependent enhancement pathway. Proc. Natl. Acad. Sci. USA 91:1932-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mason. P. W., B. Baxt, F. Brown, J. Harber, A. Murdin, and E. Wimmer. 1993. Antibody-complexed foot-and-mouth disease virus, but not poliovirus, can infect cells via the Fc receptor. Virology 192:568-577. [DOI] [PubMed] [Google Scholar]
- 44.Mateu, M. G., M. Luz Valero, D. Andreu, and E. Domingo. 1996. Systematic replacement of amino acid residues within an Arg-Gly-Asp-containing loop of foot-and-mouth disease virus and effects on cell recognition. J. Biol. Chem. 271:12814-12819. [DOI] [PubMed] [Google Scholar]
- 45.McCahon, D., J. R. Crowther, G. J. Belsham, J. D. A. Kitson, M. Duchesne, P. Have, R. H. Meloen, D. O. Morgan, and F. de Simone. 1989. Evidence for at least four antigenic sites on type O foot-and-mouth disease virus involved in neutralization; identification by single and multiple site monoclonal antibody-resistant mutants. J. Gen. Virol. 70:639-645. [DOI] [PubMed] [Google Scholar]
- 46.Mette, S. A., J. Pilewski, C. A. Buck, and S. M. Albelda. 1993. Distribution of integrin cell adhesion receptors in normal bronchial epithelial cells and lung cancer cells in vitro and in vivo. Am. J. Respir. Cell Mol. Biol. 8:562-572. [DOI] [PubMed] [Google Scholar]
- 47.Miller, L. C., W. E. Blakemore, D. Sheppard, A. Atakilit, A. M. Q. King, and T. Jackson. 2001. Role of the cytoplasmic domain of the β-subunit of integrin αvβ6 in infection by foot-and-mouth disease virus. J. Virol. 75:4158-4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mould, A. P., S. K. Akiyama, and M. J. Humphries. 1995. Regulation of integrin α5β1-fibronectin interactions by divalent cations. J. Biol. Chem. 270:26270-26277. [DOI] [PubMed] [Google Scholar]
- 49.Mu, D., S. Cambier, L. Fjellbirkeland, J. L. Baron, J. S. Munger, H. Kawakatsu, D. Sheppard, V. C. Broaddus, and S. L. Nishimura. 2002. The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J. Cell Biol. 157:493-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munger, J. S., X. Huang, H. Kawakatsu, M. D. J. Griffiths, S. L. Dalton, J. Wu, J. F. Pittet, N. Kaminski, C. Garat, M. A. Matthay, D. B. Rifkin, and D. Sheppard. 1999. The integrin αvβ6 binds and activates latent TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 96:319-328. [DOI] [PubMed] [Google Scholar]
- 51.Murphy, P. M. L., M. A. Forsyth, G. J. Belsham, and J. S. Salt. 1999. Localization of foot-and-mouth disease virus RNA by in situ hybridisation within bovine tissues. Virus Res. 62:67-76. [DOI] [PubMed] [Google Scholar]
- 52.Neff, S., D. Sa-Carvalho, E. Rieder, P. W. Mason, S. D. Blystone, E. J. Brown, and B. Baxt. 1998. Foot-and-mouth disease virus virulent for cattle utilizes the integrin αvβ3 as its receptor. J. Virol. 72:3587-3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neff, S., and B. Baxt. 2001. The ability of integrin αvβ3 to function as a receptor for foot-and-mouth disease virus is not dependent on the presence of complete subunit cytoplasmic domains. J. Virol. 75:527-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nishimura, S. L., D. Sheppard, and R. Pytela. 1994. Integrin αvβ8. Interaction with vitronectin and functional divergence of the β8 cytoplasmic domain. J. Biol. Chem. 269:28708-28715. [PubMed] [Google Scholar]
- 55.O'Toole, T. E., J. Ylanne, and B. M. Culley. 1995. Regulation of integrin affinity states through an NPXY motif in the β subunit cytoplasmic domain. J. Biol Chem. 270:8553-8558. [DOI] [PubMed] [Google Scholar]
- 56.Pfaff, E., H.-J. Thiel, E. Beck, K. Strohmaier, and H. Schaller. 1988. Analysis of neutralizing epitopes on foot-and-mouth disease virus. J. Virol. 62:2033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prieto, A. L., G. M. Edelman, and K. L. Crossin. 1993. Multiple integrins mediate cell attachment to cytotactin/tenascin. Proc. Natl. Acad. Sci. USA 90:10154-10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sa-Carvalho, D., E. Rieder, B. Baxt, R. Rodarte, A. Tanuri, and P. W. Mason. 1997. Tissue culture adaptation of foot-and-mouth disease virus selects viruses that bind to heparin and are attenuated in cattle. J. Virol. 71:5115-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Surovoi, A. Y., V. T. Ivanov, A. V. Chepurkin, V. N. Ivanyuschenkov, and N. N. Dryagalin. 1988. Is the Arg-Gly-Asp sequence the site for foot-and-mouth disease virus binding with cell receptor? Sov. J. Bioorg. Chem. 14:965-968. [PubMed] [Google Scholar]
- 60.Van Nhieu, G. T., E. S. Krukonis, A. A. Reszka, A. F. Horwitz, and R. R. Isberg. 1996. Mutations in the cytoplasmic domain of the integrin β1 chain indicate a role for endocytosis factors in bacterial internalisation. J. Biol. Chem. 271:7665-7672. [DOI] [PubMed] [Google Scholar]
- 61.Wang, K., T. Guan, D. A. Cheresh, and G. R. Nemerow. 2000. Regulation of adenovirus membrane penetration by the cytoplasmic tail of integrin β5. J. Virol. 74:2731-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weinacker, A., A. Chen, M. Agrez, R. I. Cone, S. Nishimura, E. Wayner, R. Pytela, and D. Sheppard. 1994. Role of the integrin αvβ6 in cell attachment to fibronectin. J. Biol. Chem. 269:6940-6948. [PubMed] [Google Scholar]
- 63.Ylänne, J., J. Huuskonen, T. E. O'Toole, M. H. Ginsberg, I. Virtanen, and C. G. Gahmberg. 1995. Mutation of the cytoplasmic domain of the integrin β3 subunit. Differential effects on cell spreading, recruitment to adhesion plaques, endocytosis and phagocytosis. J. Biol. Chem. 270:9550-9557. [DOI] [PubMed] [Google Scholar]
- 64.Yokosaki, Y., H. Monis, J. Chen, and D. Sheppard. 1996. Differential effects of the integrins α9β1, αvβ3 and αvβ6 on cell proliferative responses to tenascin. J. Biol. Chem. 271:24144-24150. [DOI] [PubMed] [Google Scholar]
- 65.Zhao, Q., J. M. Pacheco, and P. W. Mason. 2003. Evaluation of genetically engineered derivatives of a Chinese strain of foot-and-mouth disease virus reveals a novel cell-binding site which functions in cell culture and in animals. J. Virol. 77:3269-3280. [DOI] [PMC free article] [PubMed] [Google Scholar]