Abstract
Bradykinin (BK) is one of the most potent vasodilator agonists known and belongs to the kinin family of proinflammatory peptides. BK induces its activity via two G protein–coupled receptors: BK receptor 1 (B1R) and BK receptor 2. Although BK receptor 2 is constitutively expressed on endothelial cells (ECs), B1R is induced by IL-1β. The C1q receptor, receptor for the globular heads of C1q (gC1qR), which plays a role in BK generation, is expressed on activated ECs and is also secreted as soluble gC1qR (sgC1qR). Because sgC1qR can bind to ECs, we hypothesized that it may also serve as an autocrine/paracrine signal for the induction of B1R expression. In this study, we show that gC1qR binds to ECs via a highly conserved domain consisting of residues 174–180, as assessed by solid-phase binding assay and deconvolution fluorescence microscopy. Incubation of ECs (24 h, 37°C) with sgC1qR resulted in enhancement of B1R expression, whereas incubation with gC1qR lacking aa 174–180 and 154–162 had a diminished effect. Binding of sgC1qR to ECs was through surface-bound fibrinogen and was inhibited by anti-fibrinogen. In summary, our data suggest that, at sites of inflammation, sgC1qR can enhance vascular permeability by upregulation of B1R expression through de novo synthesis, as well as rapid translocation of preformed B1R.
Introduction
The endothelial cell (EC) receptor for high m.w. kininogen (HK)—the bradykinin (BK) precursor—consists of receptor for the globular heads of C1q (gC1qR), cytokeratin 1, and urokinase-type plasminogen activator receptor (uPAR), which are organized into two bimolecular complexes: uPAR–cytokeratin 1 and gC1qR–cytokeratin 1 (1–7). Within this complex, gC1qR serves as a strictly zinc-dependent high-affinity site for HK and, therefore, is critical for the assembly and activation of the plasma Kinin–Kallikrein system (KKS), leading to the generation of BK (1, 5). The KKS consists of three essential proteins that interact in vivo in a complex fashion once bound to a macromolecular complex formed during an inflammatory response or bound to proteins along cell surfaces (8). These are coagulation factor XII (FXII; or Hageman factor), prekallikrein (PK), and HK. Although FXII preferentially binds to the uPAR-cytokeratin 1 complex, HK, which circulates in complex with PK, binds to residues located in its domain 5 (8). Once FXII is activated to factor XIIa, it converts PK to kallikrein, a process that is enhanced by heat shock protein 90 and/or the enzyme carboxypeptidase released by ECs (9). In turn, kallikrein generated in this manner digests HK to generate the nonapeptide BK (NH2-Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg-OH) (8). BK belongs to the kinin family of proinflammatory peptides and is among the most potent vasodilator agonists known (10, 11). Once generated, it is rapidly converted to des-Arg9-BK by carboxypeptidase N, which removes the C-terminal arginine (11). BK induces its activity via two G protein–coupled receptors: BK receptor 2 (B2R) and BK receptor 1 (B1R). Although B2R is ubiquitously and constitutively expressed on many healthy cell types, including ECs, B1R is expressed at low levels, but is induced rapidly, after tissue damage by IL-1β (12). Furthermore, although BK has a higher affinity for B2R, des-Arg9-BK is a selective ligand for B1R. Activation of both receptors, in turn, is known to contribute to a plethora of acute and chronic pathological disorders, including hypotension, bronchoconstriction, pain, and inflammation (13, 14).
During the inflammatory response, activated cells, including ECs, are known to overexpress and to secrete and/or release soluble gC1qR (sgC1qR). sgC1qR, in turn, binds to intact cells in an autocrine/paracrine manner (15). Therefore, the present studies were undertaken to identify the cell surface entity that is the site for sgC1qR and to assess the possibility that bound sgC1qR is able to induce B1R expression. The results of these findings are discussed, and the biological relevance is underscored.
Materials and Methods
Chemicals and reagents
The following reagents and chemicals were used. Dulbecco’s PBS (D-PBS) with and without calcium and magnesium was obtained from Mediatech (Manassas, VA). DMEM, RPMI 1640, and 100× Penicillin/Streptomycin were obtained from Life Technologies-Invitrogen (Grand Island, NY). Heat-inactivated FBS was from HyClone (Logan, UT). Human serum albumin was obtained from Immuno U S (Rochester, MI), and Immu-Mount was from Thermo Fisher (Waltham, MA). Alexa Fluor 488–streptavidin or Alexa Fluor 594–streptavidin, Alexa Fluor 488–F(ab′)2 or Alexa Fluor 594–F(ab′)2, goat anti-mouse or anti-rabbit, FITC-conjugated goat anti-mouse IgG F(ab′)2, and sheep anti-rabbit IgG F(ab′)2 were from Invitrogen (Carlsbad, CA). p-nitrophenyl phosphate and alkaline phosphatase–conjugated rabbit anti-goat IgG were from Pierce (Rockford, IL).
Expression of recombinant gC1qR and its deletion mutants
The strategy for the construction of plasmids containing full-length gC1qR, as well as deletion mutants lacking highly charged domains identified from the crystal structure (16), were described in detail in our earlier publications (17–19).
Cultured cells
Human brain microvascular ECs were cultured in Endothelial Cell Growth Medium-2 (Lonza, Walkersville, MD) containing 5% heat-inactivated FBS, as described (20). Cells grown to confluence on 2% gelatin were treated first (30 min, 37°C) with 0.25% trypsin, 0.01% EDTA in 0.01 M TBS, to dissociate cells from the gelatin matrix by incubation. The cells were then washed and cultured in DMEM. Experiments were done with cells between passages 3 and 15.
The U937 cell line was grown in suspension in RPMI 1640 containing 10% heat-inactivated FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies-Invitrogen) and maintained in humidified air consisting of 5% CO2 and 95% air, as described (21). Prior to each experiment, the viability of cells was verified by Trypan blue exclusion, and only cultures with ≥95% viability were used for experiments.
Proteins and Abs
Fibrinogen (FGN) and rabbit anti-FGN IgG were generated in the laboratory of Dr. Dennis Galanakis (Stony Brook University). Purified rabbit IgG Abs to B1R and B2R were purchased from Abcam (Cambridge, MA), and affinity-purified rabbit IgG (designated pAb UN-15) to a synthetic gC1qR peptide was made commercially (GenScript, Piscataway, NJ).
SDS-PAGE and Western blot analysis
Analyses by SDS-PAGE were performed on 1.5-mm-thick slab gels, according to the method of Laemmli (22), with samples being run unreduced or reduced and alkylated by boiling for 5 min in the presence of 0.1 M DTT and 0.2 M iodoacetamide. After electrophoresis, the gels were stained either with Coomassie Brilliant Blue or silver stain, destained, and dried. Samples for Western blot analysis were run first using SDS-PAGE, as above, electrotransferred to polyvinylidene difluoride nitrocellulose membranes, and blocked with 5% nonfat milk containing TBST (20 mM Tris-HCl, 150 mM NaCl, and 0.05% Tween 20); the bound proteins were probed with a predetermined dilution of a specific Ab. The bound Ab, in turn, was visualized by chemiluminescence HRP-conjugated species-specific Ab, followed by reaction with 4-chloro-1-naphthol substrate.
Identification of membrane proteins by Ag-capture assay
U937 cells were first grown in RPMI 1640, with or without heat-inactivated FBS. After the cells reached confluence (∼1 × 106/ml), the contents of each culture flask (100 ml) were washed separately three times by centrifugation (800 × g, 4°C, 10 min). Then, each cell pellet was resuspended in 5 ml 5 mM D-PBS (pH 7.5) containing 0.5 mM EDTA, 10 mM EACA, and 0.5 mM PMSF. Cell membranes were prepared by freeze-thawing in liquid nitrogen, followed by centrifugation (1 h, 45,000 × g, 4°C). The supernatant containing the cytosolic fraction was removed, and the pellet containing the cell membrane was used to prepare membrane proteins by solubilization in D-PBS containing 1% Nonidet P-40. The solubilized membrane proteins were freed from insoluble material by centrifugation (1 h, 30,000 × g, 4°C), after which the total protein concentration was determined using the detergent-compatible BCA Protein Assay kit (Pierce). The membrane proteins were kept frozen at −80°C in small aliquots until use.
To identify the membrane protein recognized by sgC1qR, 10 microtiter wells were coated with 100 μl 100 μg/ml gC1qR peptide 174–180 or 100 μl 5 μg/ml gC1qR. The wells were incubated (37°C, 2 h), after which the unbound proteins were discarded, and the unreacted sites of the well were blocked with 3% BSA (room temp, 1 h). Next, the wells were washed three times with Tris-buffered solution containing 0.1 Triton X-100, and 100 μl membrane proteins was added to each well and incubated overnight at 4°C. After removal of the unbound proteins, the wells were washed three times with Tris-buffered solution containing 0.1 Triton X-100, 100 μl glycine was added to each well, and the samples were left on a shaker for 1 h at room temperature. The glycine from each well was pooled to give a total volume of 1 ml for each protein sample, and this was added to 4 ml cold acetone. The mixture was left at −20°C for 1 h to allow precipitation of the proteins. Then, the samples were spun at 13,000 rpm (Eppendorf centrifuge) for 10 min, SDS sample buffer was added to the pellet, and the sample was analyzed by SDS-PAGE and silver staining.
To ensure that the membrane protein identified was indeed membrane associated, the above protocol was repeated using surface biotinylated U937 cells and processed as above.
Flow cytometry
U937 cells were collected and washed in PBS containing 1% BSA and 0.01% NaN3 (PBS-A). Nonspecific binding was blocked with 1 mg/ml human IgG or Fc fragments in PBS-A/1 × 106 cells (30 min, 4°C), and primary Abs or the appropriate isotype-matched controls were added to the cells (30 min, 4°C). Cells were washed twice in PBS-A and incubated with FITC-labeled secondary Abs (30 min, 4°C). The cells were washed with PBS-A, fixed in 10% formalin, and analyzed by flow cytometry using a FACSCalibur (Becton-Dickinson, Mountain View, CA). For each analysis, 5000 events were collected. The data were analyzed using CellQuest Pro software (Becton-Dickinson).
Immunofluorescence microscopy
Immunofluorescence studies were performed on either U937 cells or human brain microvascular endothelial cells. The cells were grown on glass cover slips, and the attached monolayer of cells was incubated with PBS containing 0.1% BSA and 1% heat-inactivated human serum or 1 mg/ml Fc fragments to block FcRs, followed by incubation with biotinylated gC1qR and FITC-conjugated secondary reagents, as described above. After fixing for 10 min with 10% (v/v) formalin, the cover slips were air-dried, placed face down onto microscope slides, sealed using mounting solution (Immu-Mount), and examined by three-dimensional imaging using deconvolution microscopy. Staining with anti–von Willebrand factor (vWF) was always included to ensure the authenticity of the EC phenotype.
Solid-phase binding to microplate-fixed U937 cells
The ability of gC1qR and its deletion mutants to bind to U937 cells was tested using a microtiter plate assay with fixed cells, as described elsewhere (23, 24). Briefly, intact U937 cells (2 × 105 cells /well) were attached (30 min, 22°C) to poly-l-lysine–coated (10 μg/ml in PBS [pH 7.4]) duplicate ELISA wells. Subsequently, the cells were fixed (30 min, 22°C) by addition of an equal volume of glutaraldehyde (0.5% solution in PBS), and the unreacted sites were quenched with glycine-BSA (100 mM glycine, 0.1% BSA in PBS [pH 7.4]). Next, biotinylated gC1qR or deletion mutants ranging from 0 to 5 μg/ml were added and incubated (60 min, 22°C), and the bound gC1qR proteins were detected by sequential interaction with alkaline phosphatase–conjugated Neutravidin, followed by p-nitrophenyl phosphate substrate.
Effect of gC1qR on B1R and B2R expression
To test the effect of gC1qR on the expression of B1R or B2R, ECs were grown overnight in fibronectin-coated six-well plates in 1 ml Endothelial Cell Growth Medium-2 to ensure that cell attachment was complete. After blocking FcRs, as described above, concentrations of wild-type gC1qR or gC1qR deletion mutants were added and incubated for either 2 or 24 h. After incubation, the supernatant from each well was removed, and the cells were washed in fresh medium and incubated with 5 μg/ml anti-B1R or anti-B2R Ab (1 h, room temp). After removing the unbound Ab, the cells were washed and incubated (1 h, room temp) with FITC-conjugated goat anti-rabbit IgG fixed with 10% formalin and analyzed by deconvolution microscopy.
Statistical analysis
Student t tests were performed using statistical software (Excel; Microsoft, Redmond, WA). A p value = 0.05 was considered significant (n represents separate experiments performed in duplicates).
Results
The full-length gC1qR (residues 1–282) is located on the cell surface
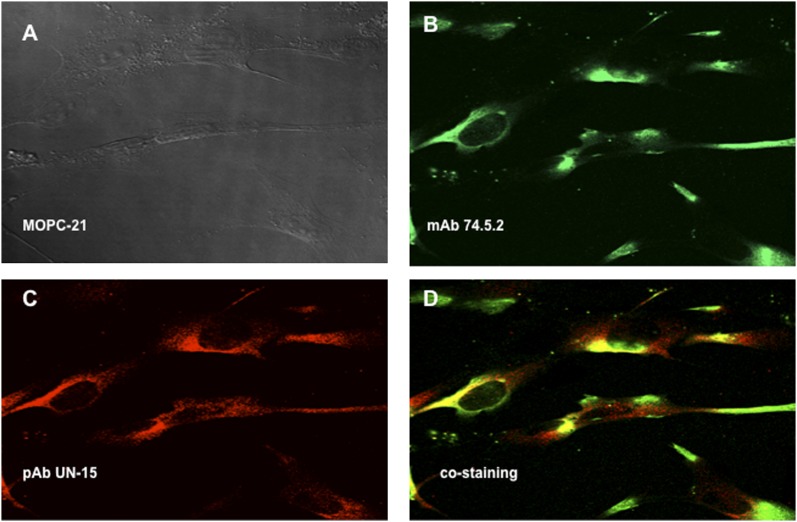
Previous studies from our laboratory (18) showed that the “mature form” of gC1qR (residues 74–282) was present on the cell surface. However, the presence of the full-length gC1qR (residues 1–282) was not studied on the premise that the full length was synthesized as a “pre-pro” protein in the mitochondria and then cleaved to generate the membrane-associated “mature” protein. To determine whether the full-length gC1qR was localized on the membrane, immunofluorescence studies were performed using monospecific polyclonal IgG (pAb UN15) recognizing a synthetic peptide within the N-terminal residues 1–74. As shown in Fig. 1, this Ab was able to stain the EC surface, and this staining colocalized with that of mAb 74.5.2, which recognizes the HK site located within residues 204–218 of gC1qR (1).
FIGURE 1.
Colocalization of the mature and full-length gC1qR on the cell surface of ECs. Human brain microvascular cells were grown on glass cover slips, and the attached monolayer of cells were incubated with PBS containing 0.1% BSA and 1 mg/ml Fc fragments to block FcRs, followed by incubation with MOPC-21 (A), mAb 74.5.2, or pAb UN15. The bound Abs were probed with either Alexa Fluor 488–F(ab′)2 anti-mouse Abs (B) or Alexa Fluor 594–F(ab′)2 anti-rabbit Abs (C). (D) The merged picture shows staining with mAb 74.5.2 and pAb UN15. Original magnification ×68. The experiment is a representative of three such experiments. The control staining with rabbit nonimmune IgG, was similar to that in (A) and is not included.
Incubation of gC1qR with ECs induces the expression of B1R
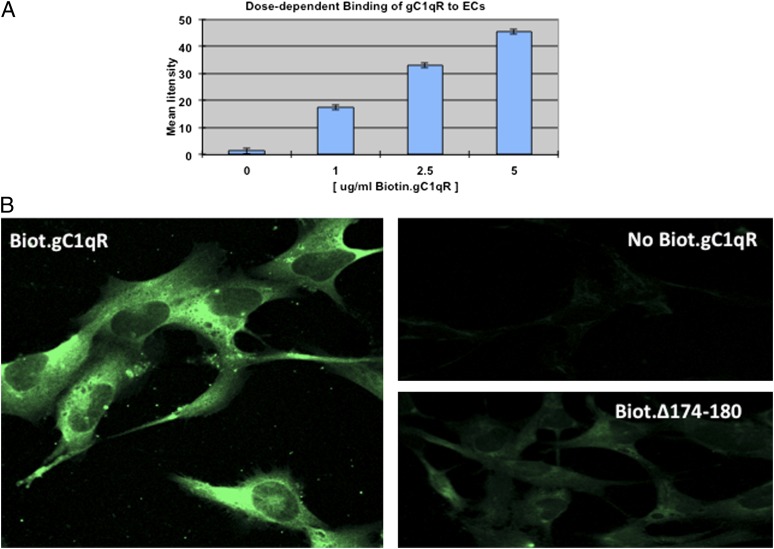
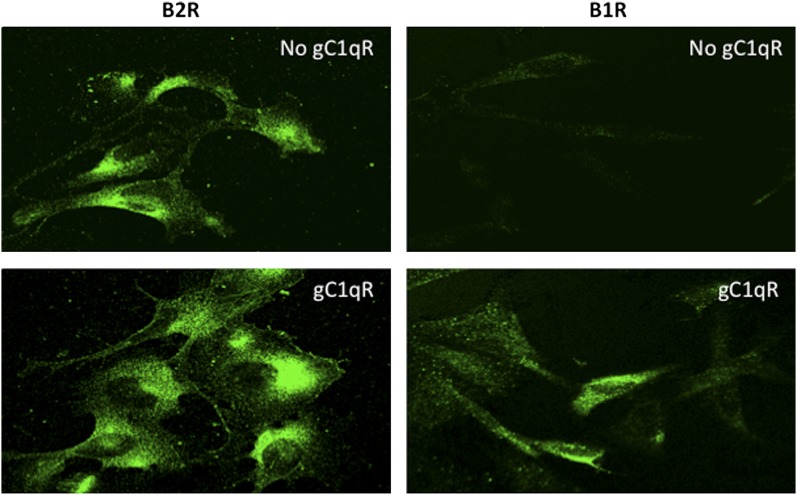
Activated cells or cells undergoing proliferation are known to secrete a soluble form of gC1qR, which is structurally and functionally similar to gC1qR purified from cell membranes (15). However the role of sgC1qR is not completely understood. We showed previously that sgC1qR, purified from culture supernatants of U937 cells, can bind to intact U937 cells, and this binding was similar to that obtained with recombinant gC1qR (15). In the present studies, we used immunofluorescence staining and deconvolution microscopy to show that recombinant and biotinylated gC1qR can also bind to ECs (Fig. 2) in a manner that is dose dependent (Fig. 2A). To assess the biologic consequence of this binding, we performed preliminary studies to determine the optimal concentration for saturation, which was in the range of 2.5–5 μg/ml (Fig. 2A). Then, experiments were performed to determine what effect such binding has on B2R and/or B1R expression. The results confirm that, although B2R is constitutively expressed, and ECs cultured alone stain very strongly with anti-B2R, there was, nonetheless, a modest and quantifiable increase (∼1.5–2-fold) in B2R surface expression when the ECs were cultured for 24 h in the presence of gC1qR (Fig. 3). In contrast, although ECs usually have small, but detectable, amounts of B1R on their surface, the staining with anti-B1R increases drastically (5–10-fold, deduced from measurement of fluorescence intensity) in the presence of increasing concentrations of gC1qR, reaching maximal staining with 5 μg/ml gC1qR (Fig. 3).
FIGURE 2.
sgC1qR binds to ECs. (A) Cells were stained with biotinylated gC1qR (ranging from 0 to 5 μg/ml), and the fluorescence intensity of each staining (n = 2) was plotted to show dose dependence. (B) The comparative immunofluorescence staining between biotinylated gC1qR (5 μg/ml) and biotinylated gC1qR deletion mutant lacking residues 174–180 (Δ174–180). Original magnification ×68. The negative control was ECs incubated with 5 μg/ml unbiotinylated gC1qR.
FIGURE 3.
sgC1qR induces the expression of B1R. ECs were grown for 24 h, as described Fig. 1, until full attachment and cobblestone formation were achieved. The cells were then cultured or not with 5 μg/ml gC1qR for an additional 24 h. The expression of B2R or B1R was examined by incubation of the cells with the corresponding Abs and analyzed by deconvolution immunofluorescence microscopy. Original magnification ×68.
Domain 174–180 is the primary attachment site for sgC1qR
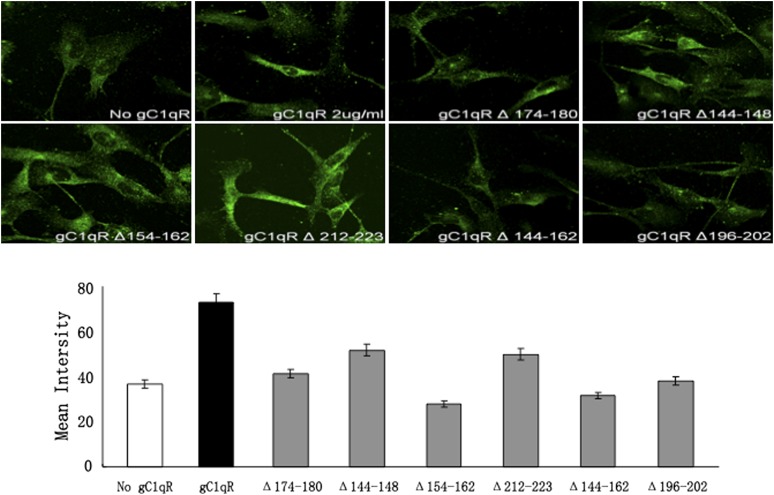
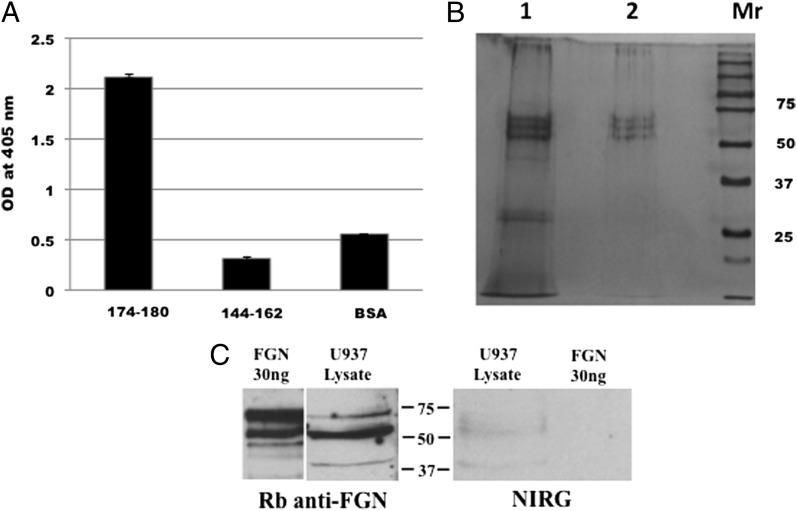
The above experiments, together with data from previous studies, conclusively showed that sgC1qR is able to bind to various cell types, including U937 cells (15) and ECs, as shown in this study. However, neither the domain of gC1qR that allows for such binding nor the cellular entity that is the putative site for gC1qR is known. In an effort to identify the gC1qR cell-binding domain, we compared the binding of wild-type gC1qR with 10 types of gC1qR deletion (Δ) mutants lacking highly charged surface-exposed domains. As shown in Fig. 4, gC1qR lacking residues 174–180 or 144–162, 154–162, and 196–202 consistently showed diminished binding to ECs, suggesting that at least two sites exist: one at 174–180 and another in an overlapping site between 144–162 and 154–162. Because subsequent binding studies using microtiter plate–fixed U937 cells showed diminished binding with gC1qRΔ174–180, we generated two synthetic peptides: one corresponding to residues 174–180 and another to 144–162. These peptides were then used in an Ag-capture ELISA to pull down solubilized membrane protein(s) from surface biotinylated U937 cells. As shown in Fig. 5A, only peptide 174–180 was able to pull down biotinylated membrane protein(s). To visualize and examine the structure of the captured protein, pull-down experiments were performed using intact gC1qR and peptide 174–180. When the captured U937 membrane proteins were analyzed by SDS-PAGE and silver staining under denaturing and reducing conditions (Fig. 5B), a predominant molecule with a chain structure and total m.w. that resembled FGN was visualized. In addition, two low m.w. bands, which have structural similarity to C1q, were captured by peptide 174–180. Therefore, subsequent pull-down experiments were designed to verify that the gC1qR-bound U937 membrane proteins were indeed FGN. As shown in Fig. 5C, the protein bands captured by gC1qR reacted strongly with anti-FGN. The specificity of this interaction is supported by the fact that neither the captured bands nor highly purified FGN used as control reacted with isotype- and species-matched IgG (Fig. 5C).
FIGURE 4.
Comparison of the effect of WT gC1qR and its deletion mutants on B1R expression. The binding of biotinylated (2 μg/ml) gC1qR and various deletion mutants (Δ) to ECs was assessed by immunofluorescence microscopy (upper panels). Original magnification ×68. The mean intensity of each staining is plotted (lower panel) for comparison. The experiment is a representative of three experiments run in triplicates.
FIGURE 5.
Intact gC1qR and gC1qR peptide 174–180 recognize the same membrane protein. (A) ELISA-based Ag capture assay in which duplicate wells of a microtiter plate were coated with 100 μl (100 μg/ml) of either gC1qR peptide 174–180 or peptide 144–162. After blocking with 1% BSA, the wells were incubated (overnight, 4°C) with 100 μl of surface biotinylated U937 membrane proteins, and the captured protein(s) were detected with alkaline phosphatase–conjugated Neutravidin and developed following standard ELISA procedures. (B) Pull-down experiment in which membrane proteins were captured by either peptide 174–180 (lane 1) or intact gC1qR (lane 2). The captured proteins were eluted with glycine, acetone precipitated, and, after addition of SDS sample buffer, analyzed by SDS-PAGE under reducing conditions. Protein bands were visualized by silver staining. (C) The three bands between 75 and 50 kDa in lanes 1 and 2 in (B) are the α, β, and γ-chains of FGN and are recognized by anti-FGN IgG and not by nonimmune IgG (NIRG).
Cell surface–expressed FGN is the binding site for sgC1qR
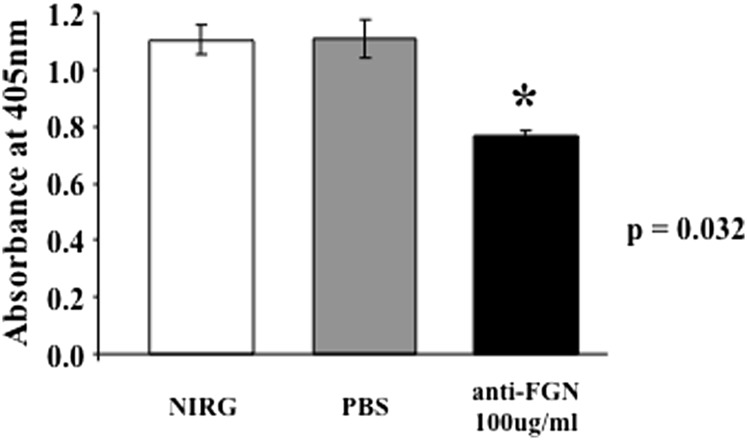
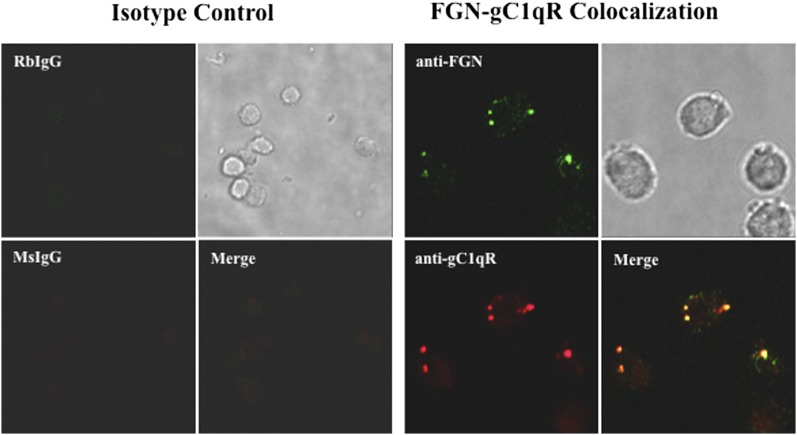
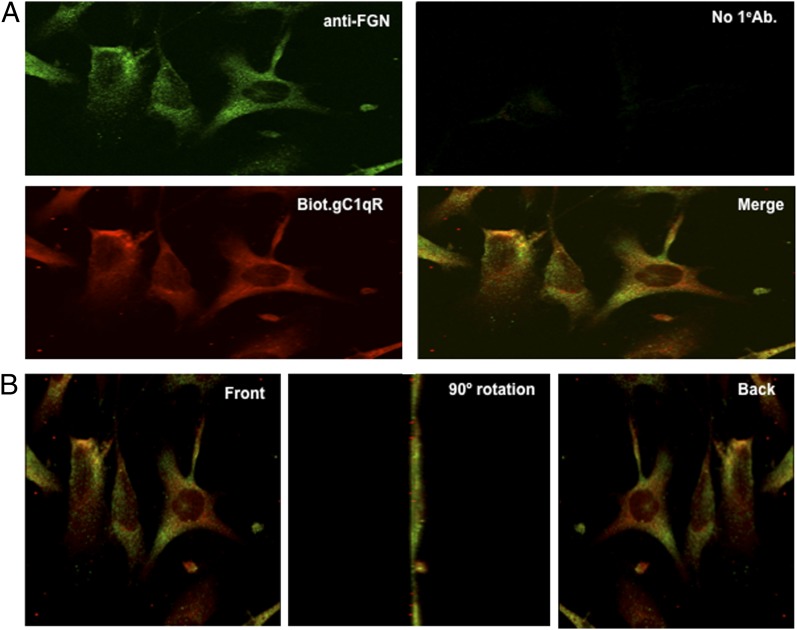
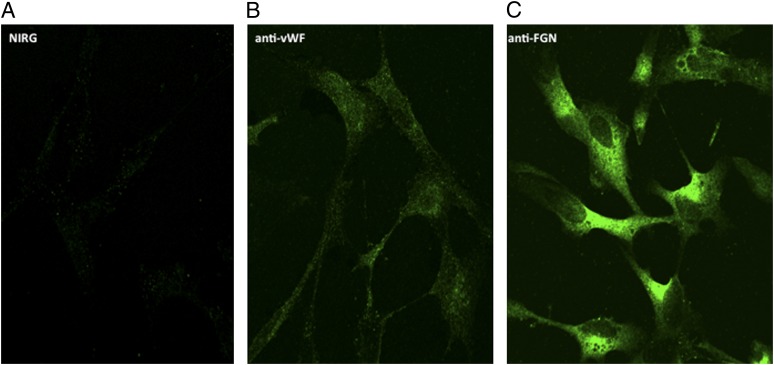
To our knowledge, the presence of FGN on cell surfaces has not been reported before. Therefore, based on the experiments depicted in Fig. 5, we proceeded to look for the presence of FGN on the cell surface. The initial strategy was to use highly specific anti-FGN IgG to detect the presence of FGN on microtiter plate–fixed U937 cells grown either in complete medium containing FBS or serum-free growth medium. This preliminary experiment showed a strong anti-FGN binding compared with isotype- and species-matched IgG. Subsequent experiments were designed to determine whether anti-FGN Abs could inhibit gC1qR binding to microtiter plate–fixed U937 cells. As shown in Fig. 6, a statistically significant inhibition (p = 0.032) was observed when cells were treated with anti-FGN IgG prior to incubation with gC1qR. Immunofluorescence staining experiments further confirmed the presence of FGN on U937 cells (Fig. 7) and ECs (Fig. 8). In addition, it was interesting to note that, at least on U937 cells, the expression of both FGN and gC1qR was “patchy” and both colocalized in these patchy sections of the cell membrane (Fig. 7). Furthermore, not all cells stained positive, and cells that appeared to undergo cell division stained more robustly, which may indicate that FGN is expressed at specific stages of cell division and proliferation. Controls were always included to verify that the ECs that were positive for FGN were also positive for vWF (Fig. 9).
FIGURE 6.
Anti-FGN IgG inhibits gC1qR binding to U937 cells. The binding of biotinylated gC1qR to microtiter plate–fixed U937 cells was assessed by standard ELISA in the presence or absence of anti-FGN IgG or nonimmune rabbit IgG as control.
FIGURE 7.
FGN and gC1qR are colocalized on the surface of U937 cells. U937 cells that were grown to confluence in serum-free medium were incubated with 5 μg/ml rabbit nonimmune IgG (RbIgG) or MOPC-21 (MsIgG) or with rabbit anti-FGN or mAb74.5.2 anti-gC1qR. The bound Ab was visualized using Alexa Fluor 488–conjugated (green) or Alexa Fluor 594–conjugated (red) F(ab′)2 secondary Ab. Original magnification ×68. After fixing, the cells were analyzed by immunofluorescence staining and deconvolution microscopy.
FIGURE 8.
Colocalization of gC1qR and FGN on the EC surface. (A) ECs were grown on cover slips and incubated with anti-FGN, biotinylated-gC1qR (Biot.gC1qR), or both (merge). Cells incubated without primary Ab (No 1eAb) were used as negative control. (B) Cells showing colocalization of FGN and gC1qR (merge) were subjected to three-dimensional rotation to show staining of cells as seen from the front (or top), the side (90° rotation), or back (360° rotation). Original magnification ×68.
FIGURE 9.
Comparison of the expression of FGN and vWF on EC surface. Cells were grown on cover slips and incubated with 5 μg/ml anti-FGN (C), anti-vWF (B), or species-matched IgG (A). The bound Ab was visualized using Alexa Fluor 488–F(ab′)2 secondary Ab, as described in Materials and Methods. Original magnification ×68.
Discussion
Earlier molecular-cloning studies suggested that gC1qR is synthesized as a pre-pro or “full-length” protein of 282 residues, which is then cleaved at position 73–74 to generate the “mature form” (residues 74–282) found on the cell membrane (18). However, when a monospecific polyclonal Ab recognizing a peptide (residues 50–63) in the N terminus of gC1qR was used in immunofluorescence studies, a robust membrane staining was observed (Fig. 1), indicating the presence of either the full-length protein or a fragment that contains residues 1–73. This staining was not only comparable to the staining observed with mAb 74.5.2, which recognizes residues 204–218, but more importantly, the molecule(s) recognized by both Abs were colocalized on the membrane, indicating that these Abs recognize the same molecule. Therefore, this experiment clearly shows that, in addition to its localization inside the cell, the full-length molecule is present on the cell membrane, probably in the lipid rafts bound to cholesterol (25) via a cholesterol recognition amino acid consensus motif, which is present on gC1qR. This, in turn, suggests that the membrane-bound mature protein (residues 74–282) may be generated from the full-length protein during cell activation or stimulation.
Most proliferating cells, such as cancer, inflammatory, or activated cells, are known to overexpress, as well as secrete, gC1qR into the pericellular milieu, where it can potentially modulate a diverse range of physiological and immunological functions, including angiogenesis (26), vascular permeability (9, 27), and inflammation (27), through activation of the complement system and KKS. This is due, in part, to the susceptibility of gC1qR to proteolytic cleavage by enzymes in plasma or membrane-associated enzymes, such as MT1-MMP, as shown earlier (28). Because previous studies (12) showed that sgC1qR (either recombinant or purified from cell cultures) can bind to U937 cells, the present studies were designed to examine the function of sgC1qR by addressing three interrelated questions. Does gC1qR bind to ECs? And if so, what surface molecule(s) serves as the gC1qR site? And does the binding of gC1qR serve to stimulate or suppress B1R, which, unlike B2R, is induced at inflammatory sites by IL-1β and/or other factors (13, 14, 29, 30). The rationale for using both ECs and U937 cells is two-fold. First, both are gC1qR-expressing proinflammatory cells in which the effect of the gC1qR-mediated response could be easily monitored. Second, we can grow U937 cells in large numbers for flow cytometry, as well as for fixation to microtiter plates for ELISA-type experiments.
The data obtained in the present studies show that gC1qR is able to bind to ECs in a dose-dependent manner (Fig. 2), as well to enhance the expression of B2R and, more importantly, induce the expression of B1R (Fig. 3). To identify the region or domain of gC1qR responsible for this binding, experiments were performed in which the binding of wild-type gC1qR to U937 cells or ECs was compared with the binding of several gC1qR deletion mutants, each lacking a highly charged domain exposed on the surface of the molecule (16, 17). The results of these experiments showed that the binding of gC1qR deletion mutants lacking residues 174–180, 144–162, and 154–162 were significantly reduced compared with wild-type gC1qR (Fig. 4). However, subsequent experiments showed that only peptide 174–180, and not peptide 144–162, was able to recognize a membrane protein from U937 cells. These results suggest that the primary or initial gC1qR interaction site may be through domain 174–180, with subsequent multisite and stable binding occurring through other sites, including a site found within residues 144–162. In support of this hypothesis is the finding that, although both gC1qR and peptide 174–180 captured a membrane protein from U937 cells, peptide 144–162 did not. The captured protein(s), in turn, appears to be surface-bound FGN, because pull-down experiments followed by silver staining (Fig. 5) analysis showed a three-chain molecule whose chain structure and m.w. appeared to be remarkably similar to FGN; anti-FGN Abs inhibited binding of gC1qR to U937 cells; and both U937 cells (Fig. 7) and ECs (Fig. 8) stained strongly for FGN and less strongly for vWF (Fig. 9) when analyzed by immunofluorescence staining. In earlier unrelated studies, it was shown that recombinant gC1qR bound to human FGN and impaired its polymerization (31). The binding site for gC1qR was found to be on the D domain of FGN/fibrin, with the carboxyl-terminal segment of at least the FGN/fibrin γ-chain being important for this interaction. These observations suggested a potential role for gC1qR in modulating fibrin formation, particularly at local sites of immune injury or inflammation (31). Therefore, although the observation of gC1qR/FGN interaction is not surprising, the fact that this interaction occurs at the cell surface is, and it represents a novel biological function for sgC1qR.
The results described in this article are significant at many levels. First, as we showed previously (32), sgC1qR can bind ECs. Although recombinant gC1qR, which is not glycosylated, was used in these studies, the soluble form of gC1qR that is naturally secreted by activated cells, including ECs, is glycosylated, and this form is likely to be even more effective than the gC1qR used in these studies. Highly activated cells, especially tumor cells or cells at inflammatory sites, overexpress gC1qR on their surface and release a soluble form of the molecule into the pericellular milieu. Each form of the gC1qR molecule—surface expressed or soluble—was shown to play a role in the induction and/or perpetuation of the inflammatory process by activation of KKS, as well as by activation of the classical pathway of the complement system (15). Second, activation of KKS leads to the generation of BK (amino acid sequence Arg-Pro-Pro-Gly-Phe-Ser-Pro-Phe-Arg), which acts on B2R to initiate vascular permeability. In humans, the half-life of BK is extremely short because it is degraded rapidly by three kininases: angiotensin-converting enzyme, aminopeptidase P, and the carboxypeptidases N or M (membrane-bound enzyme), which cleave the 7–8, 1–2, and 8–9 positions, respectively (33, 34). Of these, the first degradation product of BK—des-Arg9-BK—is more stable and possesses high affinity for B1R. Although a detectable amount of B1R exists on the cell surface, B1R is induced by IL-1β produced by monocytes recruited to the inflammatory site. Third, sgC1qR is capable of inducing B1R expression and, thus, enhancing BK-mediated vascular permeability, and it is also able to recruit C1q, a powerful chemoattractant (35, 36), which like activated HK, is able to generate proinflammatory cytokines, such as TNF-α, IL-1β, and IL-6, and the chemokines IL-8 and MCP-1 (37, 38). Thus, at sites of inflammation or diseases states, such as angioedema, in which vascular permeability plays a central role, gC1qR can serve as an important catalyst by orchestrating various proinflammatory cascades. Therefore, it represents a suitable target for the generation of peptide-based or Ab-based therapy to prevent local or systemic inflammation.
This work was supported in part by grants from the National Institute of Allergy and Infectious Diseases (R01 AI 060866 and R01 AI-084178 [to B.G.] and R01 GM-063769 [to R.R.K.]).
- BK
- bradykinin
- B1R
- bradykinin receptor 1
- B2R
- bradykinin receptor 2
- D-PBS
- Dulbecco’s PBS
- EC
- endothelial cell
- FGN
- fibrinogen
- FXII
- factor XII
- gC1qR
- receptor for the globular heads of C1q
- HK
- high m.w. kininogen
- KKS
- Kinin–Kallikrein system
- PBS-A
- PBS containing 1% BSA and 0.01% NaN3
- PK
- prekallikrein
- sgC1qR
- soluble receptor for the globular heads of C1q
- uPAR
- urokinase-type plasminogen activator receptor
- vWF
- von Willebrand factor.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Joseph K., Ghebrehiwet B., Peerschke E. I. B., Reid K. B. M., Kaplan A. P. 1996. Identification of the zinc-dependent endothelial cell binding protein for high molecular weight kininogen and factor XII: identity with the receptor that binds to the globular “heads” of C1q (gC1q-R). Proc. Natl. Acad. Sci. USA 93: 8552–8557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herwald H., Dedio J., Kellner R., Loos M., Müller-Esterl W. 1996. Isolation and characterization of the kininogen-binding protein p33 from endothelial cells. Identity with the gC1q receptor. J. Biol. Chem. 271: 13040–13047 [DOI] [PubMed] [Google Scholar]
- 3.Hasan A. A., Zisman T., Schmaier A. H. 1998. Identification of cytokeratin 1 as a binding protein and presentation receptor for kininogens on endothelial cells. Proc. Natl. Acad. Sci. USA 95: 3615–3620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colman R. W., Pixley R. A., Najamunnisa S., Yan W., Wang J., Mazar A., McCrae K. R. 1997. Binding of high molecular weight kininogen to human endothelial cells is mediated via a site within domains 2 and 3 of the urokinase receptor. J. Clin. Invest. 100: 1481–1487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pixley R. A., Espinola R. G., Ghebrehiwet B., Joseph K., Kao A., Bdeir K., Cines D. B., Colman R. W. 2011. Interaction of high-molecular-weight kininogen with endothelial cell binding proteins suPAR, gC1qR and cytokeratin 1 determined by surface plasmon resonance (BiaCore). Thromb. Haemost. 105: 1053–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahdi F., Shariat-Madar Z., Todd R. F., III, Figueroa C. D., Schmaier A. H. 2001. Expression and colocalization of cytokeratin 1 and urokinase plasminogen activator receptor on endothelial cells. Blood 97: 2342–2350 [DOI] [PubMed] [Google Scholar]
- 7.Joseph K., Tholanikunnel B. G., Ghebrehiwet B., Kaplan A. P. 2004. Interaction of high molecular weight kininogen binding proteins on endothelial cells. Thromb. Haemost. 91: 61–70 [DOI] [PubMed] [Google Scholar]
- 8.Kaplan A. P. 2004. Mechanisms of bradykinin generation. In Urticaria and angioedema. Greaves M. W., Kaplan A. P., eds. Marcel Decker Inc., New York, p. 51–72 [Google Scholar]
- 9.Joseph K., Kaplan A. P. 2005. Formation of bradykinin: a major contributor to the innate inflammatory response. Adv. Immunol. 86: 159–208 [DOI] [PubMed] [Google Scholar]
- 10.Regoli D., Barabé J. 1980. Pharmacology of bradykinin and related kinins. Pharmacol. Rev. 32: 1–46 [PubMed] [Google Scholar]
- 11.Bhoola K. D., Figueroa C. D., Worthy K. 1992. Bioregulation of kinins: kallikreins, kininogens, and kininases. Pharmacol. Rev. 44: 1–80 [PubMed] [Google Scholar]
- 12.Leeb-Lundberg L. M., Kang D. S., Lamb M. E., Fathy D. B. 2001. The human B1 bradykinin receptor exhibits high ligand-independent, constitutive activity. Roles of residues in the fourth intracellular and third transmembrane domains. J. Biol. Chem. 276: 8785–8792 [DOI] [PubMed] [Google Scholar]
- 13.Menke J. G., Borkowski J. A., Bierilo K. K., MacNeil T., Derrick A. W., Schneck K. A., Ransom R. W., Strader C. D., Linemeyer D. L., Hess J. F. 1994. Expression cloning of a human B1 bradykinin receptor. J. Biol. Chem. 269: 21583–21586 [PubMed] [Google Scholar]
- 14.Hess J. F., Borkowski J. A., Young G. S., Strader C. D., Ransom R. W. 1992. Cloning and pharmacological characterization of a human bradykinin (BK-2) receptor. Biochem. Biophys. Res. Commun. 184: 260–268 [DOI] [PubMed] [Google Scholar]
- 15.Peterson K. L., Zhang W., Lu P. D., Keilbaugh S. A., Peerschke E. I., Ghebrehiwet B. 1997. The C1q binding membrane proteins cC1q-R and gC1q-R are released from activated cells: Subcellular distribution and immunochemical characterization. Clin. Immunol. Immunopathol. 84: 17–26 [DOI] [PubMed] [Google Scholar]
- 16.Jiang J. Y., Zhang Y., Krainer A. R., Xu R. M. 1999. Crystal structure of human p32, a doughnut-shaped acidic mitochondrial matrix protein. Proc. Natl. Acad. Sci. USA. 96: 3572–3577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghebrehiwet B., Jesty J., Peerschke E. I. B. 2002. gC1qR/p33: structure-function predictions from the crystal structure. Immunobiology 205: 421–432 [DOI] [PubMed] [Google Scholar]
- 18.Ghebrehiwet B., Lim B.-L., Peerschke E. I., Willis A. C., Reid K. B. M. 1994. Isolation, cDNA cloning, and overexpression of a 33-kD cell surface glycoprotein that binds to the globular “heads” of C1q. J. Exp. Med. 179: 1809–1821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lim, B. L., K. B. Reid, B. Ghebrehiwet, E. I. Peerschke, L. A. Leigh, and K. T. Preissner. 1996. The binding protein for globular heads of complement C1q, gC1qR. Functional expression and characterization as a novel vitronectin binding factor. J. Biol. Chem. 271: 26739–26744. [DOI] [PubMed]
- 20.Yin W., Ghebrehiwet B., Weksler B., Peerschke E. I. 2007. Classical pathway complement activation on human endothelial cells. Mol. Immunol. 44: 2228–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Randazzo B. P., H. B. FleitKaplan A. P., Ghebrehiwet B. 1988. Expression of functional cell surface C1-inactivator by U937 cells. Clin. Immunol. Immunopathol. 49: 463–477 [DOI] [PubMed] [Google Scholar]
- 22.Laemmli U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 [DOI] [PubMed] [Google Scholar]
- 23.Kennett R. H. 1980. Enzyme-linked antibody assay with cells attached to polyvinyl chloride plates. In Monoclonal Antibodies. Kennett R. H., McKearn T. J., Bechtol K. B., eds. Plenum Press, New York, p. 376–377. [Google Scholar]
- 24.Ghebrehiwet B., Lu P. D., Zhang W., Lim B.-L., Eggleton P., Leigh L. E., Reid K. B. M., Peerschke E. I. B. 1996. Identification of functional domains on gC1Q-R, a cell surface protein that binds to the globular “heads” of C1Q, using monoclonal antibodies and synthetic peptides. Hybridoma 15: 333–342 [DOI] [PubMed] [Google Scholar]
- 25.Kim K.-B., Kim B.-W., Choo H.-J., Kwon Y. C., Ahn B. Y., Choi J. S., Lee J. S., Ko Y. G. 2009. Proteome analysis of adipocyte lipid rafts reveals that gC1qR plays essential roles in adipogenesis and insulin signal transduction. Proteomics 9: 2373–2382 [DOI] [PubMed] [Google Scholar]
- 26.Bossi F., Rizzi L., Bulla R., Tripodo C., Guarnota C., Novati F., Ghebrehiwet B., Tedesco F. 2011. C1q induces in vivo angiogenesis and wound healing. Mol. Immunol. 48: 1676–1677 (abstract) [Google Scholar]
- 27.Ghebrehiwet B., Jesty J., Vinayagasundaram R., Vinayagasundaram U., Ji Y., Valentino A., Tumma N., Hosszu K. H., Peerschke E. I. 2013. Targeting gC1qR domains for therapy against infection and inflammation. Adv. Exp. Med. Biol. 734: 97–110 [DOI] [PubMed] [Google Scholar]
- 28.Rozanov D. V., Ghebrehiwet B., Postnova T. I., Eichinger A., Deryugina E. I., Strongin A. Y. 2002. The hemopexin-like C-terminal domain of membrane type 1 matrix metalloproteinase regulates proteolysis of a multifunctional protein, gC1qR. J. Biol. Chem. 277: 9318–9325 [DOI] [PubMed] [Google Scholar]
- 29.McEachern A. E., Shelton E. R., Bhakta S., Obernolte R., Bach C., Zuppan P., Fujisaki J., Aldrich R. W., Jarnagin K. 1991. Expression cloning of a rat B2 bradykinin receptor. Proc. Natl. Acad. Sci. USA 88: 7724–7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marceau F., Regoli D. 2004. Bradykinin receptor ligands: therapeutic perspectives. Nat. Rev. Drug Discov. 3: 845–852 [DOI] [PubMed] [Google Scholar]
- 31.Lu P. D., Galanakis D. D., Ghebrehiwet B., Peerschke E. I. B. 1999. The receptor for the globular “heads” of C1q, gC1q-R, binds to fibrinogen/fibrin and impairs its polymerization. Clin. Immunol. Immunopathol. 90: 360–367 [DOI] [PubMed] [Google Scholar]
- 32.Guo W.-X., Ghebrehiwet B., Weksler B., Schweitzer K., Peerschke E. I. B. 1999. Up-regulation of endothelial cell binding proteins/receptors for complement component C1q by inflammatory cytokines. J. Lab. Clin. Med. 133: 541–550 [DOI] [PubMed] [Google Scholar]
- 33.Dendorfer A., Wolfrum S., Wagemann M., Qadri F., Dominiak P. 2001. Pathways of bradykinin degradation in blood and plasma of normotensive and hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 280: H2182–H2188 [DOI] [PubMed] [Google Scholar]
- 34.Kuoppala A., Lindstedt K. A., Saarinen J., Kovanen P. T., Kokkonen J. O. 2000. Inactivation of bradykinin by angiotensin-converting enzyme and by carboxypeptidase N in human plasma. Am. J. Physiol. Heart Circ. Physiol. 278: H1069–H1074 [DOI] [PubMed] [Google Scholar]
- 35.Leigh L. E., Ghebrehiwet B., Perera T. P., Bird I. N., Strong P., Kishore U., Reid K. B. M., Eggleton P. 1998. C1q-mediated chemotaxis by human neutrophils: involvement of gClqR and G-protein signalling mechanisms. Biochem. J. 330: 247–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vegh Z., Kew R. R., Gruber B. L., Ghebrehiwet B. 2006. Chemotaxis of human monocyte-derived dendritic cells to complement component C1q is mediated by the receptors gC1qR and cC1qR. Mol. Immunol. 43: 1402–1407 [DOI] [PubMed] [Google Scholar]
- 37.Khan M. M., Bradford H. N., Isordia-Salas I., Liu Y., Wu Y., Espinola R. G., Ghebrehiwet B., Colman R. W. 2006. High-molecular-weight kininogen fragments stimulate the secretion of cytokines and chemokines through uPAR, Mac-1, and gC1qR in monocytes. Arterioscler. Thromb. Vasc. Biol. 26: 2260–2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van den Berg R. H., Faber-Krol M. C., Sim R. B., Daha M. R. 1998. The first subcomponent of complement, C1q, triggers the production of IL-8, IL-6, and monocyte chemoattractant peptide-1 by human umbilical vein endothelial cells. J. Immunol. 161: 6924–6930 [PubMed] [Google Scholar]