Abstract
The sophisticated circuitry of the neocortex is assembled from a diverse repertoire of neuronal subtypes generated during development under precise molecular regulation. In recent years, several key controls over the specification and differentiation of neocortical projection neurons have been identified. This work provides substantial insight into the “molecular logic” underlying cortical development, increasingly supporting a model in which individual progenitor-stage and postmitotic regulators are embedded within highly-interconnected networks that gate sequential developmental decisions. Here, we provide an integrative account of the molecular controls that direct the progressive development and delineation of subtype and area identity of neocortical projection neurons.
Introduction
The mammalian neocortex is responsible for processing multiple modalities of sensory information, controlling motor output, and mediating higher-order cognitive functions. Its organization into only six histologically-distinct layers belies an extraordinary diversity of neuronal subtypes, which serve as building blocks for computationally-powerful neural circuitry. In recent years, tremendous progress has been made toward understanding the molecular events that control the development of these diverse types of neocortical neurons.
Two major classes of neurons, interneurons and projection neurons, populate the neocortex1. Interneurons connect locally within the neocortex, are largely inhibitory, and are generated by progenitors in the subpallial (ventral) proliferative zone of the telencephalon before migrating to the neocortex2-4. In contrast, projection neurons send axons to distant brain targets, are excitatory, and are generated by progenitors in the pallial (dorsal) proliferative zone5,6. Interneuron diversity and development have been reviewed elsewhere7-9; in this article, we focus exclusively on projection neurons.
Individual phenotypic characteristics, such as dendritic morphology, electrophysiological properties, or projection patterns, have been used in the past to systematically classify projection neurons6,10-12. Although these classification schemes have facilitated investigation of projection neuron development and function, a more comprehensive understanding of neuronal diversity will require integration of these and other phenotypic data, including transcriptomic and epigenomic profiles13. Here, we group neurons primarily by the target of their axons (Box 1), both because hodology is centrally related to function, and because establishment of appropriate projections requires successful stepwise execution of elaborate developmental programs.
Box 1. Projection neuron diversity in the cerebral cortex.
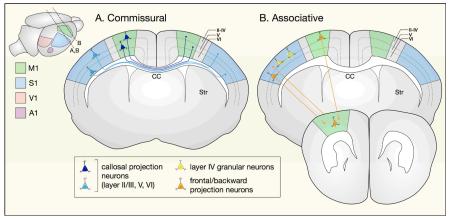
Projection neurons are classified broadly according to whether they extend axons within one cortical hemisphere (associative projection neurons), across the midline to the contralateral hemisphere (commissural projection neurons), or away from cortex (corticofugal projection neurons). Some neurons project to multiple targets and can therefore be classified into more than one broad class. Importantly, neurons of a given subtype residing in different cortical areas (motor, somatosensory, visual, and auditory) project to anatomically and functionally distinct targets138.
Commissural projection neurons, project to the contralateral cortical hemisphere. Most cross the midline through the corpus callosum (callosal projection neurons, CPN), while a smaller population crosses through the anterior commissure. CPN reside primarily in layers II/III (~80%), with fewer in layers V and VI (~20%), and extend axons to mirror-image locations in the same functional area of the contralateral hemisphere, enabling bilateral integration of modality-specific information.
Associative projection neurons, present in all layers of the neocortex, project within a single cortical hemisphere. This population includes short-distance intrahemispheric projection neurons, which extend axons within a single cortical column or to nearby cortical columns (such as layer IV granular neurons) and long-distance intrahemispheric projection neurons, which extend axons to adjacent or distant cortical areas (such as forward and backward projection neurons).
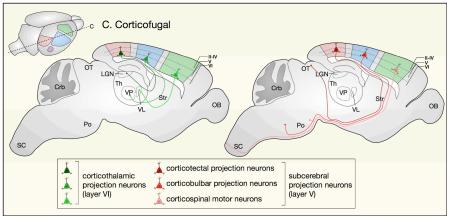
Corticofugal projection neurons (CFuPN), project away from cortex to subcortical targets and include corticothalamic projection neurons (CThPN), which reside in layer VI, and subcerebral projection neurons (SCPN), which reside in layer V.
CThPN extend axons to specific thalamic nuclei in an area-specific manner: motor cortex CThPN establish connections with the ventral lateral and ventral anterior nuclei, sensory cortex CThPN with the ventral posterior nucleus, and visual cortex CThPN with the lateral geniculate nucleus.
SCPN extend axons to different primary targets in the brainstem and spinal cord depending on their areal location. In general: motor cortex SCPN project to spinal cord (corticospinal motor neurons) and brainstem motor nuclei (cortico-brainstem motor neurons); somatosensory cortex SCPN to the trigeminal principal sensory nucleus and dorsal column medullary nuclei (corticobulbar projection neurons); and visual cortex SCPN to optic tectum (corticotectal projection neurons).
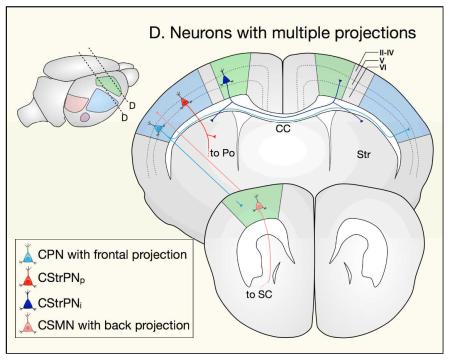
Neurons that send projections to multiple targets can sometimes be classified into more than one of the categories above. Examples include CPN with frontal projections, which extend axons to the contralateral hemisphere and to ipsilateral frontal cortex; SCPN with backward projections, which extend axons to subcerebral targets and to ipsilateral caudal cortex; and intratelencephalic corticostriatal projection neurons (CStrPNi), which extend projections to the contralateral hemisphere and to ipsilateral striatum{Sohur:2012ep}. Other neurons that project to multiple targets, such as pyramidal corticostriatal projection neurons (CStrPNp), can be classified into only one category.
CC, corpus callosum; Crb, cerebellum; LGN, lateral geniculate nucleus of thalamus;OB, olfactory bulb; OT, optic tectum; Po, pons; SC, spinal cord; Th, thalamus; VL, ventral lateral nucleus of thalamus; VP, ventral posterior nucleus of thalamus.
Projection neurons progressively acquire subtype and area identities, and their developmental trajectories can be followed along three distinct axes: time, subtype differentiation, and area differentiation. Most work to date has addressed each of these axes separately, providing descriptive analyses of individual molecular controls acting either in progenitors or in postmitotic neurons. However, it is becoming increasingly clear that specification of subtype and area identity, as well as the timing of specification decisions, are both interrelated and interdependent. In this review, we address transcriptional mechanisms controlling specification of projection neuron subtype and area identity in mice. We first examine molecular programs acting in progenitors to establish fate-restricted lineages and to impart positional information, and then analyze those acting in postmitotic neurons to direct extension of axons to appropriate targets and to refine initially promiscuous patterns of gene expression and connectivity. At each stage, we consider how genetic programs operate to establish boundaries in n-dimensional “identity space” between distinct projection neuron subtypes and between distinct cortical areas.
Progenitor specification
Progenitor diversity and corticogenesis
Early in development, the telencephalic wall is composed of undifferentiated neuroepithelial cells (Figure 1A). As these progenitors proliferate and expand in number, some begin to differentiate into radial glia (RG), establishing the ventricular zone (VZ) 14. RG, in turn, give rise to additional progenitor classes, including outer radial glia (oRG) and intermediate progenitors (IP), which together form the subventricular zone (SVZ) 15,16.
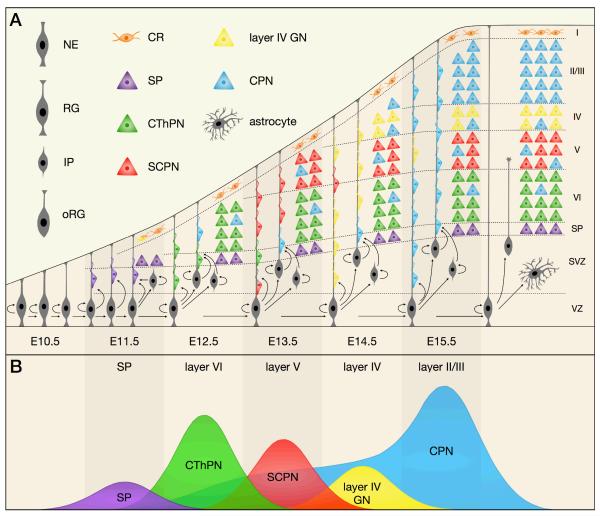
Figure 1. Neocortical projection neurons are generated in an “inside-out” fashion by diverse progenitor types in the VZ and SVZ.
This schematic depicts the sequential generation of neocortical projection neuron subtypes and their migration to appropriate layers over the course of mouse embryonic development. (a) Radial glia (RG) in the ventricular zone (VZ) begin to produce projection neurons around E11.5. At the same time, RG generate intermediate progenitors (IP) and outer radial glia (oRG), which establish the subventricular zone (SVZ) and act as transit-amplifying cells to increase neuronal production. After neurogenesis is complete, neural progenitors transition to a gliogenic mode, generating astrocytes and oligodendrocytes. Cajal-Retzius (CR) cells primarily migrate into neocortical layer I from non-cortical locations, while other projection neurons are born in the neocortical VZ / SVZ and migrate along radial glial processes to reach their final laminar destinations. (b) Distinct projection neuron subtypes are born in sequential waves over the course of neurogenesis. The peak birth of subplate (SP) neurons occurs around embryonic day (E) 11.5, with the peak birth of corticothalamic projection neurons (CThPN) and subcerebral projection neurons (SCPN) occuring at E12.5 and E13.5, respectively. Layer IV granular neurons (GN) are born around E14.5. Some callosal projection neurons (CPN) are born starting at E12.5, and those CPN born concurrently with CThPN and SCPN also migrate to deep layers. Most CPN are born between E14.5 and E16.5, and these late-born CPN migrate to superficial cortical layers. Peak sizes are proportional to the approximate number of neurons of each subtype born on each day.
NE, neuroepithelial cell; WM, white matter
Each of these progenitor populations has distinct morphological properties and follows a specific pattern of cell division. RG span the thickness of cortex from the ventricular (apical) surface to the pial (basal) surface, and are used as a scaffold by newly-born neurons as they migrate into cortex17. They primarily divide asymmetrically to self-renew, while also giving rise to oRG, IP, or neurons18,19. oRG are also unipolar, but can be distinguished from RG by their lack of an apical process20-22. They were first characterized in the outer SVZ of developing human cortex20 and, until recently, were thought to be present only in gyrencephalic animals21. However, a small population also exists in the SVZ of rodents22,23, undergoing asymmetric divisions to self-renew and generate neurons. IP have a multipolar morphology and, unlike RG and oRG, are not anchored to either the apical or basal cortical surface. They act primarily as transit-amplifying cells, undergoing limited proliferative divisions, and more often dividing symmetrically to produce two neurons14,15,24-26. A fourth class of progenitors, the short neural precursors (SNP), reside in the VZ, but they have a basal process that does not reach the pia. In other respects, SNP appear similar to IP, suggesting that they might represent RG in the process of becoming IP26.
Neocortical progenitors begin to produce excitatory projection neurons around embryonic day (E) 10.5 in mice27,28. The earliest-born neurons migrate away from the ventricular surface to segregate from progenitors and form the preplate29,30. Later-born neurons migrate into the preplate, splitting it into the marginal zone and subplate, and establishing the cortical plate between the two31. Throughout the rest of corticogenesis, newly-born neurons migrate into the cortical plate, organizing themselves in an “inside-out” fashion (Figure 1B), such that early-born neurons populate deeper neocortical layers (VI, then V), and late-born neurons migrate past them to progressively populate more superficial layers (IV, then II/III).
Progenitor lineage commitment
In aggregate, neocortical progenitors generate different projection neuron subtypes in sequential waves; however, the lineages leading from progenitor cells to specific neuronal subtypes, and the molecular mechanisms that determine the fixed order in which neuronal subtypes are generated, remain largely unknown.
One widely-followed model of progenitor lineage commitment proposes that a single lineage of progenitors generates all subtypes of projection neurons, and that the competence of a given progenitor to generate specific subtypes becomes progressively limited over the course of development. In support of this model, early-stage progenitors transplanted into late-stage cortex are capable of producing all subtypes, but late-stage progenitors transplanted into early-stage cortex are competent only to produce superficial-layer subtypes32-34. In addition, retroviral lineage tracing experiments show that single progenitors labeled early in corticogenesis are competent to produce neurons of all layers35-37, whereas progenitors labeled later in corticogenesis primarily give rise to progeny residing in superficial layers38. In vitro studies of both primary dissociated and embryonic stem (ES) cell-derived cortical progenitors indicate that they are capable of autonomously recapitulating the sequential generation of neuronal subtypes characteristic of corticogenesis in vivo39-42. Although these various approaches demonstrate a narrowing of competence in the overall progenitor population over time, they do not show that every progenitor is initially capable of producing all subtype fates. These findings would also be consistent with changing relative abundance of different lineage-committed progenitor populations.
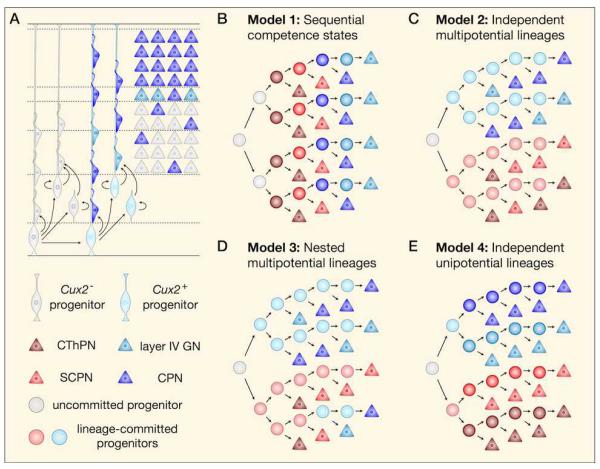
An alternative model of progenitor diversification proposes that independent, fate-restricted lineages of progenitors generate specific neuronal subtypes. Early evidence for this model came from the observation that a number of subtype-specific transcription factors are expressed in progenitors earlier in development, suggesting that different subsets of progenitors may be committed to generating particular classes of projection neurons. For example, Fezf2 (Fez family zinc finger 2; formerly Fezl) is sparsely expressed in the proliferative zones primarily during deep-layer neurogenesis, and is specific postmitotically to corticofugal projection neurons (CFuPN) 43-47. Conversely, Cux1 and Cux2 (cut-like homeobox 1 and 2) are expressed in the VZ and SVZ primarily during superficial-layer neurogenesis, and are specific postmitotically to callosal projection neurons (CPN) and other superficial-layer neurons48-50. Direct evidence for the existence of partially lineage-committed progenitors in the neocortex derives from recent genetic fate mapping experiments using mice that express Cre-ERT2 under the control of the endogenous Cux2 promoter51. This work demonstrated that a subset of progenitors that is present from the earliest stages of corticogenesis exclusively produces CPN and other superficial-layer neuron subtypes (Figure 2A). While deep-layer neurons are being generated, Cux2-positive progenitors mainly undergo proliferative divisions, expanding as a population while producing only a limited number of neurons. Later, they switch to a neurogenic mode of division and generate superficial-layer neurons. Although the authors suggest that early-born neurons derived from the Cux2 lineage become deep-layer CPN, a large number of all Cux2 fate-mapped cells in deep-layers are interneurons, significantly complicating interpretation of single marker expression analysis51. Further investigation using additional and independent genetic lineage tracing approaches are likely to uncover additional complexity in cortical progenitor lineage relationships.
Figure 2. Models of deep-layer and superficial-layer projection neuron production by distinct progenitor lineages.
(a) Fate-mapping experiments have established that most superficial-layer (commissural and associative) projection neurons derive from Cux2-positive progenitors, while deep-layer (corticofugal) neurons derive from Cux2-negative progenitors. Several models have been proposed to describe how this process occurs. (b) The “sequential competence states” model suggests that individual progenitors are able to produce a single neuronal subtype at a time as they progress through a series of competence windows, and that fate-restricted lineages do not exist. Although this model has been refuted, the precise structure of lineage trees during corticogenesis remains unknown. It is possible that progenitors commit to independent lineages before the onset of neurogenesis (c), or that some progenitors first give rise to neurons of one lineage and later commit to a different lineage (d). Similarly, progenitors might be multipotential, giving rise to more than one type of neuron (c and d), or become progressively fate restricted until they are unipotential (e).
A number of different models, ranging from strict sequential progression through competence states to immediate single-lineage commitment, can be entertained on the basis of current evidence. Of these, the sequential competence model (Figure 2B) seems least consistent with current experimental data. This model predicts that lineage-committed progenitors should not be present from the onset of corticogenesis, but Cux2-positive RG can be observed as early as E10.551. Although it is clear that at least two distinct lineages exist, it is not known whether they are entirely mutually exclusive (Figure 2C), or whether some progenitors join the Cux2-positive lineage after generating deep-layer neuronal subtypes, therefore changing their competence state (Figure 2D). This question could be experimentally addressed by identifying a gene expressed only by Cux2-negative progenitors (potentially Fezf2) and generating a Flp knock-in line, in order to simultaneously fate-map deep-layer and superficial-layer neurons. Importantly, the Cux2-positive and the Cux2-negative lineages each include multiple projection neuron subtypes, and it remains to be determined whether further fate-restricted sub-lineages emerge in progenitors (Figure 2E) or whether some fate specification decisions are resolved entirely postmitotically.
For the sake of simplicity, we have assumed here that all superficial-layer neurons are generated by Cux2-positive progenitors, and that Cux2-negative progenitors comprise a single lineage, but these remain open questions. Moreover, evidence for lineage-committed progenitors does not rule out the existence of multipotential progenitors. We anticipate that additional layers of complexity will emerge as these issues are more thoroughly investigated.
Positional information in progenitors
Neocortical arealization is initiated by expression of morphogens and signaling molecules from patterning centers at the borders of the neocortical primordium52. Beginning at E9.5 in mice, fibroblast growth factors FGF8 and FGF17 are secreted rostromedially by the commissural plate53-56, while caudomedially, Wnt and Bmp family members are secreted from the cortical hem, and, laterally, the Wnt antagonist SFRP2 and several Egf family members are secreted from the antihem57,58. Of these morphogens, only Fgf8 has been shown to function as a true organizer of area identity. Increasing Fgf8 expression by in utero electroporation causes rostromedial areas of cortex to expand caudally59,60; conversely, reduced Fgf8 expression in hypomorphic mutants causes caudal areas of cortex to expand rostrally, as does antagonism by overexpression of the cytoplasmic domain of its receptor Fgfr3c61,62. In addition, very early expression of Fgf8 from an ectopic caudal or midlateral source can cause a complete duplication of the cortical area map63.
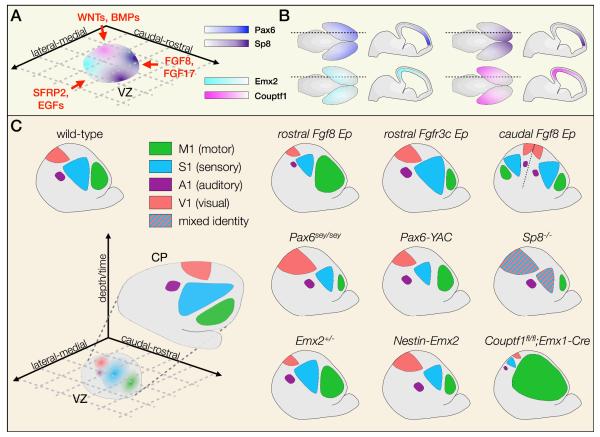
Together, these diffusible factors induce graded expression of transcription factors in VZ progenitors, which in turn control the relative size and position of cortical areas (Figure 3A). Pax6 (paired box 6) and Emx2 (empty spiracles homolog 2) are expressed in the VZ in reciprocal rostrolateral to caudomedial gradients64,65, whereas Sp8 (trans-acting transcription factor 8) and Couptf1 (chicken ovalbumin upstream promoter transcription factor 1) are expressed in reciprocal rostromedial to caudolateral gradients66-69. Because of the orthogonal orientation of these two pairs of gradients, relative expression levels of these four transcription factors (and possibly others, yet to be identified) can theoretically define any set of cortical coordinates, such that each postmitotic projection neuron might emerge from the ventricular zone poised to acquire a specific area identity.
Figure 3. Transcription factors in the VZ establish an area identity fate map.
(a) Arealization of the cerebral cortex is initiated by diffusible morphogens and signaling molecules secreted from opposing sides of the neocortical periphery (left panel). These signals induce expression of complementary and orthogonal transcription factor gradients such as Pax6/Emx2 and Sp8/Couptf1, seen in a schematized flatmount view of the ventricular zone (VZ) (b) Pax6 is expressed most highly rostrolaterally, in opposition to Emx2, which is expressed most highly caudomedially. Similarly, Sp8 is expressed most highly rostromedially, in opposition to Couptf1, which is expressed most highly caudolaterally. Gradients are shown in wholemount (left) and sagittal (right) views for each. (c) Progenitors located at different medio-lateral and rostro-caudal coordinates express specific levels of these transcription factors, which combinatorially establish a fate map of cortical areas in the ventricular zone. This fate map is later translated into a definitive area map in the cortical plate (CP), shown in flatmount view (left panel). Manipulation of morphogen signaling or VZ transcription factor expression results in dramatic changes in the size and position of cortical areas (right panel). Hatching indicates mixed area identity.
A1, primary auditory cortex; Ep, electroporation; M1, primary motor cortex; S1, primary somatosensory cortex; sey/sey, small eye hypomorphic mutant; V1, primary visual cortex; YAC, yeast artificial chromosome
Strong caudal expression of Emx2 and Couptf1 promotes specification of sensory areas. In Nestin-Emx2 transgenic mice, Emx2 is expressed more uniformly throughout the ventricular zone, leading to an increase in the size of visual cortex, and a concomitant size decrease and rostrolateral shift of somatosensory and motor areas. In the absence of one allele of Emx2, in contrast, motor areas expand, and sensory areas shift caudomedially70. Similarly, in Couptf1 conditional null mice, motor areas expand dramatically to occupy a large portion of cortex, while sensory areas are displaced to a narrow occipital band that contains compressed, but properly-configured, sensory representations71.
Rostrally, expression of Pax6 and Sp8 drives specification of motor identity. Both Sp8 and Pax6 conditional null mice, as well as Pax6sey/sey (“small eye”) hypomorphic mutants, exhibit a drastic loss of motor areas, although interpretation of these findings are complicated by a simultaneous decrease in the overall size of cortex72-74. Gain- and loss-of-function in utero electroporation experiments, however, independently support a role for Sp8 in cortical area identity, both by cell-autonomous repression of Couptf1 in neocortical progenitors and indirectly by induction of Fgf867,75.
Although manipulation of these transcription factor gradients is sufficient to change the size and position of cortical areas (Figure 3B), neuronal identity within the ectopically-located areas is largely established normally. Each respecified area expresses molecular markers that are appropriate to its new identity, attracts modality-specific thalamocortical input, and extends projections to correct targets. Taken together, these results suggest that progenitor-based controls establish a coordinate system of positional information that anchors area identity to specific rostrocaudal and mediolateral positions. This fate map in radial glia76 and intermediate progenitors77 (also known as the “proto-map”) must then be transmitted to their neuronal progeny to be interpreted and executed by a second network of transcription factors that direct postmitotic acquisition of area identity.
Postmitotic subtype specification
Although neocortical projection neurons are generated by partially fate-restricted progenitors, postmitotic controls are also necessary to specify the precise subtype identities of newly-born neurons. Over the past decade, high-throughput efforts to define laminar- and subtype-specific gene expression patterns in the neocortex45,78-83 have led to the identification of an increasing number of molecular controls over subtype development.
Delineation of SCPN and CThPN subtype identity
Subcerebral projection neurons (SCPN) and corticothalamic projection neurons (CThPN) are closely-related corticofugal projection neuron (CFuPN) subtypes that reside in the deep layers of the neocortex and are sequentially generated early in corticogenesis. Substantial plasticity exists in the specification of CFuPN into either SCPN or CThPN, and each population can expand at the expense of the other in the absence of critical controls (Figure 4).
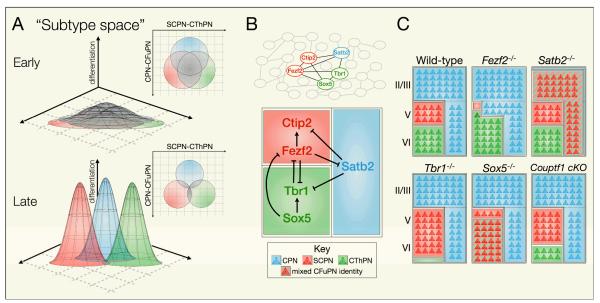
Figure 4. Competing molecular programs direct differentiation of newly-postmitotic projection neurons into one of three broad subtype identities.
(a) The subtype identities of postmitotic projection neurons are depicted within a theoretical n-dimensional “subtype space” in which individual subtype identities (as defined by gene expression, morphology, dendritic structure, projection patterns, physiology, and other characteristics) occupy distinct coordinates. Boundaries between these identities, preventing neurons of one subtype from taking on characteristics of another subtype, are established by the action of cross-repressive molecular controls. One boundary exists between neurons specified as SCPN and those specified as CThPN, and another exists between CFuPN (SCPN/CThPN) and CPN. Early in corticogenesis, undifferentiated neurons have largely overlapping subtype identities (top). As development proceeds, neurons differentiate and subtypes become more distinct from each other (bottom).
(b) Known molecular controls represent key nodes of an elaborate transcriptional network, only beginning to be elucidated (top). Arrows indicate known cases of genetic or transcriptional activation or repression, and further interactions and molecular controls remain to be identified (bottom). (c) Changes in expression of these key regulators can cause boundaries between subtypes to shift, with neurons partially or completely acquiring features characteristic of other subtypes. In some mutants, neurons acquire CFuPN identity generally, rather than a well-defined CThPN or SCPN identity. The boundaries between CFuPN and deep-layer or superficial-layer CPN may shift independently of one another, represented by the dashed line between deep-layer and superficial-layer CPN.
CFuPN, corticofugal projection neurons; CPN, callosal projection neurons; CThPN, corticothalamic projection neurons; SCPN, subcerebral projection neurons
The zinc finger transcription factor Fezf2 is critical for specification of SCPN. It is expressed by a subset of ventricular zone (VZ) progenitors while deep cortical layers are being generated, and also by postmitotic CFuPN, although it is not known whether Fezf2 functions primarily in progenitors or postmitotically. Fezf2 is expressed at high levels by SCPN, and at lower levels by CThPN and SP neurons43-47,84, and in Fezf2 null mice the large pyramidal neurons that normally define layer V are entirely absent. Even more strikingly, expression of SCPN-specific genes is lost, and no cortical neurons project to the brainstem and spinal cord46,84. Instead, expression of Tbr1, a transcription factor critical for CThPN development85,86, expands into presumptive layer V46, and many of these Tbr1-expressing neurons project to thalamus86, indicating that some SCPN are fate-converted to CThPN (while other SCPN are fate-converted to CPN, as discussed below). Thus, Fezf2 specifies SCPN identity, at least in part by repressing CThPN identity.
In addition to being a “master” regulator of SCPN development, Fezf2 also functions in the specification of CFuPN identity more broadly. CThPN and SP neurons appear disorganized in Fezf2 null mice, and a number of CThPN-specific genes, including DARPP-32, Grg4, and Foxp2, fail to be expressed46,84. These findings suggest that low-level Fezf2 expression by CThPN and SP neurons is necessary for precise differentiation of these populations. Furthermore, misexpression of Fezf2 by in utero electroporation causes layer II/III CPN to redirect their axons toward a broad set of subcortical targets, including the thalamus, brainstem, and spinal cord46,47,87,88. Taken together, these data indicate that Fezf2 instructs CFuPN identity, and not SCPN identity alone.
A second transcription factor, Ctip2 (COUPTF-interacting protein 2), functions downstream of Fezf2 to control appropriate differentiation of SCPN. Although SCPN are still born and migrate normally to layer V in the absence of Ctip2, they exhibit striking defects in axon outgrowth, fasciculation, and pathfinding. Most critically, SCPN axons fail to reach the spinal cord, as they become misrouted and defasciculated in the midbrain, only rarely reaching pons, and never reaching the pyramidal decussation45. Although activation of Ctip2 by Fezf2 is critical for SCPN development, several transcriptional controls over CPN, CThPN, and SP development (including Satb289,90, Sox591, and Couptf192) operate at least in part by repressing Ctip2 expression, indicating that Ctip2 is a critical target for transcriptional regulation during neocortical projection neuron development.
Tbr1 (T-box brain 1) acts in opposition to Fezf2 and Ctip2 to specify CThPN identity. It is expressed postmitotically by CThPN and SP neurons, and at lower levels by Cajal-Retzius cells and CPN85,86. In the absence of Tbr1, the subplate is not morphologically discernible, and subplate-specific genes fail to be expressed85. Similarly, early-born neurons that would normally develop into CThPN express aberrantly high levels of Fezf2 and Ctip2, as well as several other SCPN-specific genes, and extend axons toward subcerebral targets instead of the thalamus85,86,93. Tbr1 directly binds to highly-conserved regulatory regions to repress expression of Fezf2, therefore functioning, at least in part, by preventing SCPN specification86,93.
Temporal control over CFuPN subtype generation
CFuPN subtypes are generated in temporally-overlapping waves and share the same core developmental program; however, specific controls direct the sequential generation of SP neurons, CThPN, and SCPN, ensuring precise acquisition of molecular identity by each subtype.
The transcription factor Sox5 (SRY-box containing 5) controls the orderly emergence of CFuPN subtypes by repressing high-level expression of SCPN genes, including Fezf2 and Ctip2, until generation of subplate neurons and CThPN is complete91,94,95. Sox5 directly represses Fezf2 by binding an enhancer element required for Fezf2 expression in the forebrain95. In Sox5−/− mice, subplate neurons express inappropriately high levels of CTIP2, take an abnormal laminar position in superficial cortical layers, and project to the cerebral peduncle91. In addition, FOG2 and CTIP2, normally specific to CThPN and SCPN, respectively, are co-expressed by a single population of neurons with mixed SCPN/CThPN character, indicating imprecise differentiation94. Loss of Sox5 results in widespread CFuPN pathfinding defects, including extensive defasciculation of SCPN axons in the midbrain, and formation of an accessory subcerebral tract that projects through the external capsule91. Corticothalamic projections are also severely compromised, as reported by Golli-GFP and Fezf2-GFP transgenic labeling, as well as pancortical Emx1-Cre;CAG-Cat-GFP labeling94.
Couptf1 suppresses SCPN identity in the latest-born, most superficially-located CThPN. In the absence of Couptf1, layer VIa neurons in somatosensory cortex become “motorized,” expressing aberrantly high levels of CTIP2 and Fezf2, but maintaining expression of TBR1. Although more deep-layer neurons project subcerebrally in Couptf1 conditional nulls, only the axons of SCPN prematurely generated at E12.5 and located in layer VIa are able to reach the spinal cord. Axons of SCPN generated at E13.5 and located in layer V, in contrast, aberrantly terminate in pons before entering the spinal cord92. In a general sense, repression of SCPN subtype identity by Couptf1 represents an additional aspect of its function repressing motor identity in favor of sensory identity.
Delineation of CFuPN and CPN subtype identity
CFuPN share a developmental boundary with callosal projection neurons (CPN), and especially with deep-layer CPN, which are generated during the same temporal window, and reside intermingled with CFuPN in layers V and VI. From the time CFuPN and CPN axons exit the cortical plate, they follow dramatically divergent trajectories, either away from cortex or toward the midline96. Accordingly, some critical controls over CFuPN and CPN development function largely by repressing molecular programs that would instruct differentiation toward the alternate fate (Figure 4).
As described above, Fezf2 functions centrally to specify CFuPN identity, which requires suppression of CPN fate. Fezf2 overexpression in vivo is sufficient to redirect the axons of superficial-layer CPN toward subcortical targets46,87,88. In the absence of Fezf2, neurons expressing alkaline phosphatase from the Fezf2 locus extend axons across the corpus callosum. In addition, more neurons in layer V display electrophysiological characteristics typical of CPN and express CPN-specific genes, suggesting that many SCPN are fate-converted to CPN87. Interestingly, these neurons appear to take on a deep-layer CPN identity, expressing broad CPN identity genes, such as Satb2 and Lpl, but not expressing genes specific to superficial-layer CPN, such as Inhba and Limch150,97.
The transcription factor Satb2 (special AT-rich sequence binding protein 2) is critical for CPN specification and concomitant repression of CFuPN fate. Satb2 is expressed at high levels by CPN, and likely also by associative neurons, in all layers of cortex89,90. In the absence of Satb2, almost no axons cross through the corpus callosum, even though establishment of the midline appears normal. Instead, neurons expressing LacZ from the Satb2 locus project toward the brainstem and spinal cord89,98. Expression of several genes characteristic of CPN, including Cdh10, Dkk3, Sip1, and Cux1, is lost or severely reduced in Satb2 null mice. Conversely, superficial-layer neurons in these mice express high levels of Ctip2, as well as a number of other genes characteristic of SCPN, including Clim1, Cdh13, and Grb14. Satb2 operates by directly repressing Ctip2, rather than by upstream control of Fezf2, and, consequently, Satb2 null CPN are not fully fate-converted to SCPN89,90. Recently, the transcriptional co-regulator Ski (ski sarcoma viral oncogene homolog) has been shown to be a critical component of the repressor complex recruited by Satb2 to initiate HDAC1-dependent chromatin remodeling, and Ski−/− mice largely phenocopy Satb2−/− mice99.
Epistasic analysis of subtype specification
In several instances, transcription factors that specify subtype identity have been shown to repress each other directly, raising the possibility that inhibiting differentiation programs for alternate fates, rather than actively specifying a particular fate, might be their primary function. Under this model, simultaneous deletion of two competing transcription factors, such as Tbr1 and Fezf2 or Satb2 and Ctip2, might partially restore proper subtype specification. Indeed, formation of the corticospinal tract (lost in Fezf2−/−) is partially rescued in Tbr1−/−;Fezf2−/− mice, although projections to the thalamus (lost in Tbr1−/−) are still completely absent86. Similarly, formation of the corpus callosum (lost in Satb2−/−) is partially rescued in Satb2−/−;Ctip2−/− mice98. These results suggest that downstream programs are able to direct some neurons to differentiate appropriately, even in the absence of important specification controls, as long as competing controls are not active.
Progressive refinement of subtype identity
Mature deep-layer neurons exhibit strikingly divergent patterns of gene expression and axonal projection, but some of these differences begin to emerge only after several days of postmitotic refinement. Newly postmitotic neurons often extensively co-express transcription factors that later become restricted to different subtypes89,94,98,100-102. For example, between E12.5 and E14.5, neurons in the cortical plate co-express high levels of CTIP2 and TBR1/FOG2, which resolve over time to SCPN and CThPN, respectively94,101. Similarly, at E13.5, deep-layer neurons briefly co-express CTIP2 and SATB2, which later become restricted to SCPN and CPN89,98. The period of time during which deep-layer neurons co-express multiple subtype controls might correspond to a particularly plastic state, when decisions regarding subtype identity are being crystallized. This initially widespread expression of incompatible subtype controls is intriguing, however, given recent evidence of fate commitment by progenitors51.
We propose that the timing of fate specification decisions might be linked to biologically-meaningful decision points, favoring either commitment of progenitors or later resolution postmitotically (Box 2). CFuPN and deep-layer CPN begin to extend axons in different directions even as they migrate through the intermediate zone103, and, therefore, specification into one of these two broad fates might need to occur in progenitors. In contrast, CThPN and SCPN axons travel through the internal capsule together for several days before their trajectories diverge104. This coincides with a period during which newly-postmigratory CFuPN transition from co-expressing high levels of TBR1 and CTIP2 to expressing either one or the other, potentially reflecting postmitotic commitment101.
Box 2. Toward a molecular logic of neocortical development.
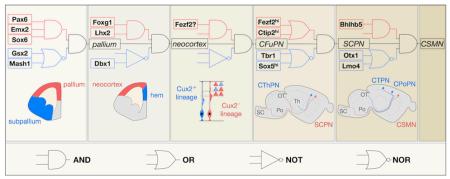
We propose that the order- and dose-dependent nature of projection neuron identity specification can be formalized using first-order Boolean logic, with decision points represented by “molecular logic gates”. Below, we illustrate this approach to schematizing the developmental trajectories of specific projection neuron subtypes, using SCPN as an archetypal population (see figure).
The neocortical domain is established by transcription factors that act combinatorially to repress subpallial programs (such as Pax6, Emx2, and Sox6) 73,139-144 and cortical hem programs (such as Lhx2 and Foxg1) 145-147. Subsequently, neocortical progenitors are further specified into at least two partially fate-restricted lineages by yet unidentified molecular controls. Progenitors that are Cux2-negative, and possibly Fezf2-positive, generate CFuPN, while progenitors that are Cux2-positive generate CPN and other neurons in superficial layers46,51.
CFuPN become committed to a specific subtype at a decision point gated by cross-repression between Fezf2, which directs SCPN specification, and Tbr1 and Sox5, which direct CThPN specification46,47,84,86,87,91,93,94. Once SCPN are specified, Ctip2 promotes subsequent differentiation steps, including axon outgrowth, fasciculation, and targeting45. Additional controls instruct further specialization of SCPN subpopulations, including collateralization and pruning decisions (e.g., Otx1110; Lmo4110).
Each sequential decision point described above is gated by the coordinated activity of multiple transcriptional regulators and chromatin-modifying proteins, which direct extensive changes in the transcriptional and epigenetic state of a cell. Although we have only considered regulation of subtype specification, other aspects of neuronal development, such as area specification and migration, proceed in parallel, orchestrated by partially-intersecting molecular programs.
Projection neuron areal specialization
Postmitotic regulators transform continuous gradients of positional information inherited from progenitors into sharp areal boundaries, instruct the formation of sensory maps, and direct projection neurons to acquire areally-appropriate phenotypic characteristics (Figure 5). Two such controls, Lmo4 and Bhlhb5, are expressed in complementary patterns and are critical for determining the precise placement of molecular boundaries between areas.
Figure 5. Postmitotic regulators set up sharp gene expression boundaries between cortical areas and direct area-specific phenotypic differentiation of projection neurons.
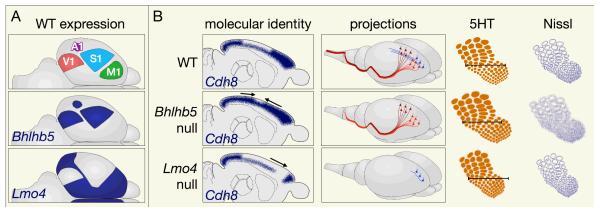
Loss of Bhlhb5 or Lmo4 function affects multiple aspects of postmitotic area identity acquisition, including gene expression, projection patterns, and cellular organization in the S1 barrel field. a) On postnatal day 7, Bhlhb5 is expressed in S1, A1, and V1, whereas Lmo4 is expressed in M1 and excluded from primary sensory areas. b) In the absence of Bhlhb5 (middle row), molecular identity of sensory areas is compromised; for example, Cdh8 expression expands into S1, from which it is normally excluded. Areally-determined projection patterns change, as CSMN in caudal motor cortex fail to reach the spinal cord. Thalamocortical axons (shown by serotonin (5-HT) immunostaining) innervate a wider area of S1 in Bhlhb5−/−, with indistinct cortical barrels (shown by Nissl staining). Conversely, in the absence of Lmo4 (bottom row), molecular identity of motor areas is compromised, and motor expression of Cdh8 and other genes is reduced. Neurons in motor cortex are inappropriately specified, and fail to send backward collaterals. Thalamocortical axons innervate a narrower area in Lmo4 conditional null mutants, although cortical cytoarchitecture has not been investigated by Nissl staining.
WT, wild-type; V1, primary visual cortex; A1, primary auditory cortex; S1, primary sensory cortex; M1, primary motor cortex
Lmo4 (Lim domain only 4) is a transcription factor that is expressed postmitotically in motor cortex and higher-order sensory areas, but excluded from primary somatosensory, visual, and auditory cortex (Figure 5) 45,105,106. Conditional loss of Lmo4 function results in a modest rostral expansion of somatosensory-specific genes, at the expense of motor-specific genes, although these defects do not suggest a dramatic failure of area identity acquisition107. In addition, the boundaries of individual barrels become blurred, and the vibrissal barrel field is slightly narrowed overall107,108. Lmo4 controls several aspects of area-specific output connectivity in motor cortex, including extension of caudal collaterals by some CPN and SCPN (backward projection neurons; BPN), as well as the ratio of brainstem- to spinal cord-projecting SCPN in rostral motor cortex106.
The basic helix-loop-helix transcription factor Bhlhb5 (BHLH domain-containing, class B5) is initially expressed in a high-caudomedial to low-rostrolateral gradient in the cortical plate, but its expression becomes progressively restricted to primary sensory areas (somatosensory, visual, and auditory). In the absence of Bhlhb5, molecular area identity is extensively disrupted in somatosensory and caudal motor cortex, and area-specific genes, including Lmo4, are aberrantly expressed. Although the position and configuration of the barrel field are unchanged, thalamocortical input appears more diffuse, and the cytoarchitectural organization of vibrissal barrels is only faintly discernible109. Bhlhb5, therefore, centrally contributes to the emergence of hallmark somatosensory cortex-specific features, including appropriate gene expression and precise cellular organization of vibrissal barrel fields.
A number of additional transcriptional regulators contribute to postmitotic acquisition of area identity. Tbr1, discussed above as a critical control over CThPN subtype identity, also contributes to area identity acquisition. It is expressed most highly in rostral areas of cortex, and, in the absence of Tbr1 function, genes typically expressed in caudal regions of cortex expand rostrally85. Notably, abnormalities in gene expression are not limited to layer VI85, suggesting that transient or low-level Tbr1 expression in superficial layers is also instructive for area identity. The homeodomain transcription factor Otx1 (orthodenticle homolog 1) is necessary for the establishment of area-specific connectivity by SCPN. It is present in the cytosol of VZ progenitors and, at later stages, in the nuclei of CThPN and SCPN, with nuclear translocation taking place during the first postnatal week110. Although expression of Otx1 is uniform across cortical areas, loss-of-function selectively affects SCPN in visual cortex, which inappropriately maintain their spinal projections, adopting a final connectivity pattern normally specific to SCPN in motor cortex110,111. Lastly, Couptf1, already discussed as an important control over arealization at the progenitor level, is also expressed postmitotically92. It is not known whether Couptf1 acts to regulate the development of area-specific gene expression and projection patterns solely by its functions in progenitors, or whether it also has continued functions in postmitotic neurons.
This emerging understanding of the expression and function of key postmitotic regulators is beginning to illuminate the molecular logic underlying area identity acquisition. For example, the division of cortex into two broad domains defined by Bhlhb5 and Lmo4 suggests that a common program controls primary sensory area development, whereas an opposing program governs acquisition of features shared by other areas, including higher-order sensory areas and motor areas. Recent evidence indicates that emergence of distinct gene expression profiles in primary and higher-order sensory areas requires thalamocortical input112, suggesting that extrinsic factors are critical for later stages of cortical area patterning. Overall, only a small number of postmitotic controls over area identity acquisition have been identified, and further important regulators likely remain to be discovered.
Integrating subtype and area identity
Early neuroanatomists first classified the neocortex into areas on the basis of regional variation in laminar morphology, cell density, and thickness113,114. These cytoarchitectural differences reflect whether an area is specialized for input, output, or integration, and arise from adjustments in the relative proportion of neurons instructed to differentiate into CThPN, SCPN, layer IV granular neurons (GN), or CPN. Therefore, areal specialization requires not only establishment of specific input and output connectivity, but also production of specific ratios of projection neuron subtypes.
Recent reports suggest that some transcription factors coordinate regulation of subtype and area specification. As discussed above, Tbr1 and Couptf1 are both important regulators of CFuPN specification, and also promote motor and sensory area identity, respectively85,86,92,93. Similarly, the transcription factor Ap2γ (activating enhancer binding protein 2 gamma) controls how many superficial-layer CPN are generated in an area-specific manner by regulating the number of Tbr2-positive IP in occipital cortex during the later stages of corticogenesis115. These findings provide initial mechanistic insight for earlier reports that progenitor cell cycle dynamics differ across cortical areas in primates116.
Deciphering neocortical evolution
Over the course of cortical evolution, radial and tangential expansion have been accompanied by neuronal diversification and regional specialization, allowing both for increased sophistication of cortical circuitry and for the emergence of a growing repertoire of functionally-specialized areas117. It has been proposed that the dorsal pallium of ancestral amniotes, like that of modern-day sauropsids, possessed only subcortically-projecting neurons118 and was divided into two major functional areas119. Although the mammalian lineage has retained this basic organization, it has also incorporated additional neuronal subtypes specialized for receiving and processing input, as well as for intra- and interhemispheric integration120. Further, specific cognitive tasks have been compartmentalized into well-defined primary, secondary, and higher-order motor and sensory areas.
Expansion of cortical thickness and elaboration of projection neuron diversity in mammals were facilitated by the appearance of IP, which are not present in sauropsids121. These transit-amplifying cells establish a supplementary progenitor compartment, the subventricular zone (SVZ), that contributes neurons to all layers of the neocortex, exponentially increasing the neurogenic capacity of the cortical germinal zone26. In primates and other mammals with well-developed cortices, such as ferrets, the SVZ is distinguished by its internal organization into an inner and an outer subcompartment (inner and outer SVZ; ISVZ and OSVZ) 21. Although mice do not possess a compartmentalized SVZ, oRG-like cells are nonetheless present, suggesting that the well-organized primate OSVZ represents an expansion of a preexisting population of progenitors22. The emergence of the SVZ likely enabled the elaboration of the six-layered mammalian neocortex23, as well as the the ability to produce new cortical neuron subtypes, including CPN.
CPN are an evolutionary innovation of placental mammals and have become the most abundant and diverse class of cortical projection neurons in eutherians. There has been considerable expansion and diversification of this population in rodents, and even more in primates118,122. CPN located in different layers, and even in different sublaminae, have remarkably different patterns of gene expression, suggesting that subpopulations of CPN have diverged to acquire specialized functions50. Consistent with the hypothesis that CPN were derived from pre-existing corticofugal populations123, repression of CFuPN programs by Satb2 is absolutely necessary for the emergence of callosal projections89,90,98. Further molecular controls over the differentiation of individual CPN subpopulations remain to be identified.
The area plan of ancestral amniotes is thought to have consisted of a sensorimotor area immediately adjacent to a primary visual area, as in many modern sauropsids119. In placental mammals, the ancestral sensorimotor area has diverged into distinct primary somatosensory and motor areas124, while V1 and S1 have become tangentially separated by the addition of a host of novel secondary and higher-order sensory areas125. These territories are all marked by expression of Lmo4, suggesting that the complementary expression patterns of Bhlhb5 and Lmo4106,109,126 reflect the distinct evolutionarily origins of these two portions of the mammalian area plan. Interestingly, Lmo4 may be further specialized in humans, as it is expressed differentially between the right and left hemispheres of human embryos, and may mediate some aspects of left-right asymmetry between the two cortical hemispheres127.
In summary, our current knowledge supports a model in which, over the course of evolution, a growing number of transcription factors was progressively recruited to control cortical development, gradually adding layers of neuronal diversity and areal specialization to a simpler ancestral framework.
Perspectives
Implications for disease and repair
Studies of cortical development have uncovered important genetic determinants that might provide mechanistic insights into neurodegenerative disease. For instance, recent work on hSOD1G93A mice, a model of amyotrophic lateral sclerosis (ALS), has shown that there is widespread degeneration not only of corticospinal motor neurons (CSMN), but of SCPN more broadly, as identified by expression of subtype-specific developmental control genes45,128. Therefore, degeneration of SCPN across multiple cortical areas might be a significant source of non-motor ALS symptoms in humans. Future work could seek to identify developmentally-specified determinants of SCPN susceptibility to degeneration, and perhaps leverage this knowledge toward development of treatments for ALS.
The developmental history of neuron subpopulations may also provide insight into closely-related neurodegenerative diseases with distinct pathologies. Progressive loss of descending cortical motor output is a prominent feature of both primary lateral sclerosis (PLS) and hereditary spastic paraplegia (HSP), but SCPN are differentially affected in these two motor neuron diseases. In PLS, SCPN projecting to bulbar, cervical, thoracic, and lumbar segments of the brainstem and spinal cord broadly degenerate, leading to generalized progressive weakness of voluntary muscles129,130. In HSP, in contrast, lumbar-projecting CSMN selectively degenerate, leading to difficulty walking131,132. The molecular basis for differential pathology of SCPN subpopulations in these diseases is not known, but is likely related to genetic determinants of SCPN located in distinct cortical regions that target specific rostro-caudal segments of the brainstem and spinal cord.
A more sophisticated understanding of the molecular controls that direct subtype-specific neuronal differentiation could also enable novel strategies for nervous system repair. In fact, lessons from development have already been adapted to in vitro systems, using morphogen signaling to guide the differentiation of ES cells into neocortical progenitors40-42,133. Progenitors derived using these protocols generate heterogeneous neuron populations that can project axons to a range of targets and integrate into host cortical circuits when transplanted41,134. Future work might direct differentiation of these ES cell-derived progenitors into large quantities of a specific neuronal subtype by leveraging recently-identified developmental controls. “Master” regulators such as Fezf2, which is sufficient both to program46,47,87 and reprogram88,135,136 neuronal subtype identity in vivo, are particularly promising candidates for instructing ES cell differentiation in vitro.
Future directions
In recent years, several important controls over the specification and differentiation of long-distance neocortical projection neurons have been identified. Although neurons that extend axons to the contralateral hemisphere, to thalamus, or to subcerebral targets have been extensively studied, much less is known about specific molecular controls or markers of associative cortical neuron subtypes, including intracortical projection neurons and layer IV GN. Furthermore, considerable uninvestigated diversity exists among neurons that project to the same target. Some subpopulations, such as CSMN that target different spinal segments137, are defined areally and have been identified based on hodology, while others, such as CPN in different sublaminae, are known only by gene expression50. It will be of great interest to identify genetic determinants responsible for engineering these additional levels of complexity.
Current models of cortical development are restricted to a handful of regulators, which account for a limited subset of key nodes within a broader regulatory network that is likely to be considerably more complex. In future studies, large-scale cell type-specific proteomic and genomic approaches should make it possible to analyze network dynamics, rather than epistatic relationships between pairs of genes. New methods for genome-wide methylation mapping might enable investigation of changes in the epigenetic landscape that accompany lineage commitment decisions and progressive specification of neuronal identity. In addition, relationships between transcription factors and effectors that determine the terminal differentiated state of a neuron, such as cell adhesion molecules and axon guidance receptors, are mostly unknown. Such a comprehensive understanding of developmental mechanisms might provide insights necessary to overcome barriers to the programming and reprogramming of specific cortical neuron types.
Online summary.
The sophisticated circuitry of the neocortex is assembled from a diverse repertoire of neuronal subtypes generated during development under precise molecular regulation, and forming distinct functional areas within the tangential expanse of the neocortex. This collection of specialized neurons is produced by a variety of progenitors with distinct morphological and molecular properties, and with distinct patterns of cell division.
The lineages leading from progenitor cells to specific neuronal subtypes, and the molecular mechanisms that determine the fixed order in which neuronal subtypes are generated, remain largely unknown. Recent work suggests that some subtypes of neurons are produced by lineage-committed progenitors, although a number of models of lineage commitment can be entertained on the basis of current evidence.
Area identity acquisition is initiated by diffusible factors released from the periphery of the neocortical domain, and subsequent induction of graded expression of arealizing transcription factors in ventricular zone progenitors. These progenitor-based controls establish a coordinate system of positional information that anchors area identity to specific rostrocaudal and mediolateral positions, which must then be transmitted to their neuronal progeny to be interpreted by a second network of transcription factors that direct postmitotic acquisition of area identity.
Projection neuron subtype identity is progressively established by extensive transcriptional cross-repression between genetic programs driving the development of one subtype of projection neuron and those driving the development of alternate subtypes. These competing regulators sort newly-postmitotic projection neurons into one of three broad subtype identities: corticothalamic, subcerebral, and callosal.
Postmitotic regulators, including Lmo4 and Bhlhb5, transform continuous gradients of positional information inherited from progenitors into sharp areal boundaries, instruct the formation of sensory maps, and direct projection neurons to acquire areally-appropriate phenotypic characteristics.
Over the course of evolution, a growing number of transcription factors was progressively recruited to control cortical development, gradually adding layers of neuronal diversity and areal specialization to a simpler ancestral framework.
The emerging understanding of the expression and function of key molecular regulators is beginning to illuminate a molecular logic underlying subtype and area identity acquisition. We propose that the order- and dose-dependent nature of projection neuron identity specification can be formalized by analogy to first-order Boolean logic, with decision points represented by “molecular logic gates”.
Glossary
- Hodology
The path followed by axons to reach their targets.
- Neuroepithelial cells
Neuroectodermal progenitors that are the main proliferative cell type of the early neocortex. They later differentiate into radial glial cells.
- Gyrencephalic
Having a folded cerebral cortex, with gyri (ridges) and sulci (furrows).
- Lineage
The shared ancestry of cells that can be traced back to a common progenitor through sequential cell divisions.
- Competence
The differentiation potential of a cell, as determined by its intrinsic molecular state.
- Fate mapping
Labeling a progenitor cell with a permanent and heritable mark to identify all its progeny.
- Flp knock-in line
A mouse line in which expression of Flp recombinase is driven by the promoter of a gene of interest.
- Morphogen
A secreted factor that can induce at least two different cell fates in a concentration-dependent manner by forming a gradient.
- Fasciculation
Bundling together of axons that project to a common final or intermediate target through adhesive interactions.
- Cajal-Retzius cells
Early-born cortical neurons that express the glycoprotein Reelin and reside in layer I.
- Enhancer element
A short region of DNA, typically occupied by multiple transcription factors, that is sufficient to drive expression of a gene with temporal and/or cell-type specificity.
- Chromatin remodeling
Changes in the three-dimensional structure of chromatin brought about by epigenetic modifications. These structural changes can result in either transcriptional activation or silencing of genes located in the involved chromatin segment.
- Barrel
A cylindrical column of neurons in layer IV of the neocortex that receives and processes sensory input from a single whisker. The topographical organization of the barrels in cortex corresponds precisely to the arrangement of whisker follicles on the snout.
Biographies
Luciano Custo Greig is an M.D.-Ph.D. student at Harvard Medical School, Boston, Massachusetts, USA. He received his B.S. and M.S. in Molecular, Cellular and Developmental Biology from Yale University, New Haven, Connecticut, USA, pursuing his M.S. thesis research on the signaling and function of axon guidance receptors in the laboratory of Elke Stein. He is currently in the fifth year of his Ph.D. studies in the laboratory of Jeffrey D. Macklis, investigating transcriptional regulation of neocortical projection neuron subtype and area identity acquisition.
Mollie Woodworth recently obtained a Ph.D. from Harvard University, Cambridge, Massachusetts, USA. She received S.B. degrees in Biology and in Brain and Cognitive Sciences from M.I.T., Cambridge, Massachusetts, USA, where she worked with Morgan Sheng on synapse biology. During her Ph.D. work with Jeffrey D. Macklis, she investigated transcriptional controls over neocortical projection neuron subtype and area identity. She is currently a postdoctoral fellow in the laboratory of Christopher Walsh at Boston Children’s Hospital and Harvard Medical School, Boston, Massachusetts, USA, investigating the development of the human neocortex.
Maria J. Galazo is a postdoctoral research fellow in the Stem Cell and Regenerative Biology Department, Harvard University, Cambridge, Massachusetts, USA. She graduated in biology from Complutense University, Madrid, Spain, and obtained a Ph.D. in Neuroscience from Autonoma University of Madrid, Spain. She joined the laboratory of Jeffrey D. Macklis in 2007 for postdoctoral training. Since then, she has been investigating molecular controls over development of cortical projection neuron subtypes, with special interest in the specification of cortical output neuron subtypes.
Hari Padmanabhan is a postdoctoral fellow in the laboratory of Jeffrey D. Macklis at Harvard University, Cambridge, Massachusetts, USA. His research focuses on applying principles of brain development to questions of regeneration. He received his Ph.D. from the Tata Institute of Fundamental Research, Mumbai, India with Shubha Tole, where he studied the role of transcription factors in neuronal development in mouse cerebral cortex and in fruit fly brain.
Jeffrey D. Macklis’ laboratory is focused on the development, diversity, selective degeneration, regeneration, and directed differentiation of projection neuron subtypes in the cerebral cortex. His lab studies neocortical projection neuron differentiation; cortical progenitor biology; induction of neurogenesis within murine neocortex; directed cortical neuron subtype differentiation from neural progenitors and pluripotent cells, functional repair of brain and spinal cord circuitry, and connections between development and degeneration of specific neuron types. He is a Professor of Stem Cell and Regenerative Biology at Harvard University, Cambridge, Massachusetts, Professor of Neurology [Neuroscience] at Harvard Medical School, Boston, Massachusetts, and was founding Program Head of Neuroscience in the Harvard Stem Cell Institute. He attended the Massachusetts Institute of Technology (M.I.T.), Cambridge, Massachusetts, Harvard Medical School, and graduate school at M.I.T. within the Harvard–M.I.T. Division of Health Sciences and Technology. He trained in developmental neuroscience with Richard Sidman. Until 2011, he was Director of the Massachusetts General Hospital–Harvard Medical School Center for Nervous System Repair.
References
- 1.Parnavelas JG. The origin and migration of cortical neurones: new vistas. Trends Neurosci. 2000;23:126–131. doi: 10.1016/s0166-2236(00)01553-8. [DOI] [PubMed] [Google Scholar]
- 2.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 3.Cobos I, Puelles L, Martinez S. The avian telencephalic subpallium originates inhibitory neurons that invade tangentially the pallium (dorsal ventricular ridge and cortical areas) Dev Biol. 2001;239:30–45. doi: 10.1006/dbio.2001.0422. [DOI] [PubMed] [Google Scholar]
- 4.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 5.Gorski JA, et al. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molyneaux BJ, Arlotta P, Menezes JRL, Macklis JD. Neuronal subtype specification in the cerebral cortex. Nat Rev Neurosci. 2007;8:427–437. doi: 10.1038/nrn2151. [DOI] [PubMed] [Google Scholar]
- 7.Batista-Brito R, Fishell G. The developmental integration of cortical interneurons into a functional network. Curr Top Dev Biol. 2009;87:81–118. doi: 10.1016/S0070-2153(09)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu Rev Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbin JG, Butt SJB. Developmental mechanisms for the generation of telencephalic interneurons. Dev Neurobiol. 2011;71:710–732. doi: 10.1002/dneu.20890. [DOI] [PubMed] [Google Scholar]
- 10.Migliore M, Shepherd GM. Opinion: an integrated approach to classifying neuronal phenotypes. Nat Rev Neurosci. 2005;6:810–818. doi: 10.1038/nrn1769. [DOI] [PubMed] [Google Scholar]
- 11.Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- 12.Oberlaender M, et al. Cell type-specific three-dimensional structure of thalamocortical circuits in a column of rat vibrissal cortex. Cereb Cortex. 2012;22:2375–2391. doi: 10.1093/cercor/bhr317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Defelipe J, et al. New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat Rev Neurosci. 2013;14:202–216. doi: 10.1038/nrn3444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noctor SC, Martínez-Cerdeño V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- 16.Noctor SC, Martínez-Cerdeño V, Kriegstein AR. Contribution of intermediate progenitor cells to cortical histogenesis. Arch. Neurol. 2007;64:639–642. doi: 10.1001/archneur.64.5.639. [DOI] [PubMed] [Google Scholar]
- 17.Rakic P. Guidance of neurons migrating to the fetal monkey neocortex. Brain Res. 1971;33:471–476. doi: 10.1016/0006-8993(71)90119-3. [DOI] [PubMed] [Google Scholar]
- 18.Noctor SC, Flint AC, Weissman TA, Dammerman RS, Kriegstein AR. Neurons derived from radial glial cells establish radial units in neocortex. Nature. 2001;409:714–720. doi: 10.1038/35055553. [DOI] [PubMed] [Google Scholar]
- 19.Miyata T, Kawaguchi A, Okano H, Ogawa M. Asymmetric inheritance of radial glial fibers by cortical neurons. Neuron. 2001;31:727–741. doi: 10.1016/s0896-6273(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 20.Hansen DV, Lui JH, Parker PRL, Kriegstein AR. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature. 2010;464:554–561. doi: 10.1038/nature08845. [DOI] [PubMed] [Google Scholar]
- 21.Fietz SA, et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci. 2010;13:690–699. doi: 10.1038/nn.2553. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Tsai J-W, LaMonica B, Kriegstein AR. A new subtype of progenitor cell in the mouse embryonic neocortex. Nat Neurosci. 2011;14:555–561. doi: 10.1038/nn.2807. References # and # identify a novel subpopulation of radial glia that lacks an apical process and has expanded dramatically in primates to establish an outer SVZ.
- 23.Martínez-Cerdeño V, et al. Comparative analysis of the subventricular zone in rat, ferret and macaque: evidence for an outer subventricular zone in rodents. PLoS ONE. 2012;7:e30178. doi: 10.1371/journal.pone.0030178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu S-X, et al. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci USA. 2005;102:17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sessa A, Mao C-A, Hadjantonakis A-K, Klein WH, Broccoli V. Tbr2 directs conversion of radial glia into basal precursors and guides neuronal amplification by indirect neurogenesis in the developing neocortex. Neuron. 2008;60:56–69. doi: 10.1016/j.neuron.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kowalczyk T, et al. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex. 2009;19:2439–2450. doi: 10.1093/cercor/bhn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Angevine JB, Sidman RL. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- 28.Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974;183:425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- 29.Marin-Padilla M. Dual origin of the mammalian neocortex and evolution of the cortical plate. Anat. Embryol. 1978;152:109–126. doi: 10.1007/BF00315920. [DOI] [PubMed] [Google Scholar]
- 30.Raedler E, Raedler A. Autoradiographic study of early neurogenesis in rat neocortex. Anat. Embryol. 1978;154:267–284. doi: 10.1007/BF00345657. [DOI] [PubMed] [Google Scholar]
- 31.Luskin MB, Shatz CJ. Studies of the earliest generated cells of the cat’s visual cortex: cogeneration of subplate and marginal zones. J Neurosci. 1985;5:1062–1075. doi: 10.1523/JNEUROSCI.05-04-01062.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McConnell SK. Fates of visual cortical neurons in the ferret after isochronic and heterochronic transplantation. J Neurosci. 1988;8:945–974. doi: 10.1523/JNEUROSCI.08-03-00945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McConnell SK, Kaznowski CE. Cell cycle dependence of laminar determination in developing neocortex. Science. 1991;254:282–285. doi: 10.1126/science.254.5029.282. [DOI] [PubMed] [Google Scholar]
- 34.Frantz GD, McConnell SK. Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron. 1996;17:55–61. doi: 10.1016/s0896-6273(00)80280-9. [DOI] [PubMed] [Google Scholar]
- 35.Luskin MB, Pearlman AL, Sanes JR. Cell lineage in the cerebral cortex of the mouse studied in vivo and in vitro with a recombinant retrovirus. Neuron. 1988;1:635–647. doi: 10.1016/0896-6273(88)90163-8. [DOI] [PubMed] [Google Scholar]
- 36.Walsh C, Cepko CL. Clonally related cortical cells show several migration patterns. Science. 1988;241:1342–1345. doi: 10.1126/science.3137660. [DOI] [PubMed] [Google Scholar]
- 37.Price J, Thurlow L. Cell lineage in the rat cerebral cortex: a study using retroviral-mediated gene transfer. Development. 1988;104:473–482. doi: 10.1242/dev.104.3.473. [DOI] [PubMed] [Google Scholar]
- 38.Reid CB, Liang I, Walsh C. Systematic widespread clonal organization in cerebral cortex. Neuron. 1995;15:299–310. doi: 10.1016/0896-6273(95)90035-7. [DOI] [PubMed] [Google Scholar]
- 39.Shen Q, et al. The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci. 2006;9:743–751. doi: 10.1038/nn1694. [DOI] [PubMed] [Google Scholar]
- 40.Eiraku M, et al. Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell. 2008;3:519–532. doi: 10.1016/j.stem.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 41.Gaspard N, et al. An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature. 2008;455:351–357. doi: 10.1038/nature07287. The authors of references # and # direct differentiation of mouse ES cells into telencephalic progenitors in monolayer cultures relying exclusively on pharmacological agents and morphogens. ES cell-derived cortical progenitors are able to generate different projection neuron subtypes in the appropriate temporal order.
- 42.Nasu M, et al. Robust formation and maintenance of continuous stratified cortical neuroepithelium by laminin-containing matrix in mouse ES cell culture. PLoS ONE. 2012;7:e53024. doi: 10.1371/journal.pone.0053024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Inoue K, Terashima T, Nishikawa T, Takumi T. Fez1 is layer-specifically expressed in the adult mouse neocortex. Eur J Neurosci. 2004;20:2909–2916. doi: 10.1111/j.1460-9568.2004.03763.x. [DOI] [PubMed] [Google Scholar]
- 44.Hirata T, et al. Zinc finger gene fez-like functions in the formation of subplate neurons and thalamocortical axons. Dev Dyn. 2004;230:546–556. doi: 10.1002/dvdy.20068. [DOI] [PubMed] [Google Scholar]
- 45.Arlotta P, et al. Neuronal subtype-specific genes that control corticospinal motor neuron development in vivo. Neuron. 2005;45:207–221. doi: 10.1016/j.neuron.2004.12.036. The authors purify individual neuronal populations on the basis of their axonal projections to define subtype-specific developmental programs. By focusing on genes expressed by corticospinal motor neurons, but not closely related callosal projection neurons, the authors identify candidate molecular controls over subtype development, including the transcription factor Ctip2.
- 46.Molyneaux B, Arlotta P, Hirata T, Hibi M, Macklis JD. Fezl is required for the birth and specification of corticospinal motor neurons. Neuron. 2005;47:817–831. doi: 10.1016/j.neuron.2005.08.030. [DOI] [PubMed] [Google Scholar]
- 47.Chen J-G, Rasin M-R, Kwan KY, Sestan N. Zfp312 is required for subcortical axonal projections and dendritic morphology of deep-layer pyramidal neurons of the cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17792–17797. doi: 10.1073/pnas.0509032102. Fezf2 was the first transcription factor identified to specify the identity of one neocortical projection neuron subtype (subcerebral projection neurons).
- 48.Nieto M, et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II-IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- 49.Zimmer C, Tiveron M-C, Bodmer R, Cremer H. Dynamics of Cux2 expression suggests that an early pool of SVZ precursors is fated to become upper cortical layer neurons. Cereb Cortex. 2004;14:1408–1420. doi: 10.1093/cercor/bhh102. [DOI] [PubMed] [Google Scholar]
- 50.Molyneaux BJ, et al. Novel subtype-specific genes identify distinct subpopulations of callosal projection neurons. J Neurosci. 2009;29:12343–12354. doi: 10.1523/JNEUROSCI.6108-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franco SJ, et al. Fate-restricted neural progenitors in the mammalian cerebral cortex. Science. 2012;337:746–749. doi: 10.1126/science.1223616. Genetic fate-mapping of Cux2-expressing cells enables the authors to establish that a subset of cortical progenitors, present from the earliest stages of corticogenesis, is committed to generating “upper-layer” (commissural and associative) neurons.
- 52.Grove EA, Fukuchi-Shimogori T. Generating the cerebral cortical area map. Annu Rev Neurosci. 2003;26:355–380. doi: 10.1146/annurev.neuro.26.041002.131137. [DOI] [PubMed] [Google Scholar]
- 53.Crossley PH, Martin GR. The mouse Fgf8 gene encodes a family of polypeptides and is expressed in regions that direct outgrowth and patterning in the developing embryo. Development. 1995;121:439–451. doi: 10.1242/dev.121.2.439. [DOI] [PubMed] [Google Scholar]
- 54.Shimamura K, Rubenstein JL. Inductive interactions direct early regionalization of the mouse forebrain. Development. 1997;124:2709–2718. doi: 10.1242/dev.124.14.2709. [DOI] [PubMed] [Google Scholar]
- 55.Maruoka Y, et al. Comparison of the expression of three highly related genes, Fgf8, Fgf17 and Fgf18, in the mouse embryo. Mech Dev. 1998;74:175–177. doi: 10.1016/s0925-4773(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 56.Bachler M, Neubüser A. Expression of members of the Fgf family and their receptors during midfacial development. Mech Dev. 2001;100:313–316. doi: 10.1016/s0925-4773(00)00518-9. [DOI] [PubMed] [Google Scholar]
- 57.Grove EA, Tole S, Limon J, Yip L, Ragsdale CW. The hem of the embryonic cerebral cortex is defined by the expression of multiple Wnt genes and is compromised in Gli3-deficient mice. Development. 1998;125:2315–2325. doi: 10.1242/dev.125.12.2315. [DOI] [PubMed] [Google Scholar]
- 58.Assimacopoulos S, Grove EA, Ragsdale CW. Identification of a Pax6-dependent epidermal growth factor family signaling source at the lateral edge of the embryonic cerebral cortex. J Neurosci. 2003;23:6399–6403. doi: 10.1523/JNEUROSCI.23-16-06399.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–1074. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- 60.Toyoda R, et al. FGF8 acts as a classic diffusible morphogen to pattern the neocortex. Development. 2010;137:3439–3448. doi: 10.1242/dev.055392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garel S, Huffman KJ, Rubenstein JLR. Molecular regionalization of the neocortex is disrupted in Fgf8 hypomorphic mutants. Development. 2003;130:1903–1914. doi: 10.1242/dev.00416. [DOI] [PubMed] [Google Scholar]
- 62.Cholfin JA, Rubenstein JLR. Patterning of frontal cortex subdivisions by Fgf17. Proc Natl Acad Sci USA. 2007;104:7652–7657. doi: 10.1073/pnas.0702225104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Assimacopoulos S, Kao T, Issa NP, Grove EA. Fibroblast growth factor 8 organizes the neocortical area map and regulates sensory map topography. J Neurosci. 2012;32:7191–7201. doi: 10.1523/JNEUROSCI.0071-12.2012. The authors use early microelectroporations of Fgf8 into ectopic sites to demonstrate that the mature cortical area pattern is organized during development by a finely-tuned FGF8 signaling gradient.
- 64.Walther C, Gruss P. Pax-6, a murine paired box gene, is expressed in the developing CNS. Development. 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 65.Gulisano M, Broccoli V, Pardini C, Boncinelli E. Emx1 and Emx2 show different patterns of expression during proliferation and differentiation of the developing cerebral cortex in the mouse. Eur J Neurosci. 1996;8:1037–1050. doi: 10.1111/j.1460-9568.1996.tb01590.x. [DOI] [PubMed] [Google Scholar]
- 66.Waclaw RR, et al. The Zinc Finger Transcription Factor Sp8 Regulates the Generation and Diversity of Olfactory Bulb. Neuron. 2006;49:503–516. doi: 10.1016/j.neuron.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 67.Sahara S, Kawakami Y, Izpisua Belmonte JC, O’Leary DDM. Sp8 exhibits reciprocal induction with Fgf8 but has an opposing effect on anterior-posterior cortical area patterning. Neural Dev. 2007;2:10. doi: 10.1186/1749-8104-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Q, Dwyer ND, O’Leary DD. Differential expression of COUP-TFI, CHL1, and two novel genes in developing neocortex identified by differential display PCR. J Neurosci. 2000;20:7682–7690. doi: 10.1523/JNEUROSCI.20-20-07682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou C, Tsai SY, Tsai MJ. COUP-TFI: an intrinsic factor for early regionalization of the neocortex. Genes Dev. 2001;15:2054–2059. doi: 10.1101/gad.913601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hamasaki T, Leingärtner A, Ringstedt T, O’Leary DDM. EMX2 regulates sizes and positioning of the primary sensory and motor areas in neocortex by direct specification of cortical progenitors. Neuron. 2004;43:359–372. doi: 10.1016/j.neuron.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 71.Armentano M, et al. COUP-TFI regulates the balance of cortical patterning between frontal/motor and sensory areas. Nat Neurosci. 2007;10:1277–1286. doi: 10.1038/nn1958. Using conditional deletion of Couptf1 in the neocortex, the authors demonstrate that the size and position of cortical areas are primarily determined by cortex-autonomous genetic programs.
- 72.Bishop KM, Goudreau G, O’Leary DD. Regulation of area identity in the mammalian neocortex by Emx2 and Pax6. Science. 2000;288:344–349. doi: 10.1126/science.288.5464.344. [DOI] [PubMed] [Google Scholar]
- 73.Muzio L, Mallamaci A. Emx1, emx2 and pax6 in specification, regionalization and arealization of the cerebral cortex. Cereb Cortex. 2003;13:641–647. doi: 10.1093/cercor/13.6.641. [DOI] [PubMed] [Google Scholar]
- 74.Zembrzycki A, Griesel G, Stoykova A, Mansouri A. Genetic interplay between the transcription factors Sp8 and Emx2 in the patterning of the forebrain. Neural Dev. 2007;2:8. doi: 10.1186/1749-8104-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Borello U, et al. Sp8 and COUP-TF1 Reciprocally Regulate Patterning and Fgf Signaling in Cortical Progenitors. Cereb Cortex. 2013 doi: 10.1093/cercor/bhs412. doi:10.1093/cercor/bhs412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- 77.Elsen GE, et al. The protomap is propagated to cortical plate neurons through an Eomes-dependent intermediate map. Proc Natl Acad Sci USA. 2013;110:4081–4086. doi: 10.1073/pnas.1209076110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gray PA, et al. Mouse brain organization revealed through direct genome-scale TF expression analysis. Science. 2004;306:2255–2257. doi: 10.1126/science.1104935. [DOI] [PubMed] [Google Scholar]
- 79.Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–6. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Magdaleno S, et al. BGEM: an in situ hybridization database of gene expression in the embryonic and adult mouse nervous system. PLoS Biol. 2006;4:e86. doi: 10.1371/journal.pbio.0040086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lein ES, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 82.Sugino K, et al. Molecular taxonomy of major neuronal classes in the adult mouse forebrain. Nat Neurosci. 2006;9:99–107. doi: 10.1038/nn1618. [DOI] [PubMed] [Google Scholar]
- 83.Visel A, et al. A high-resolution enhancer atlas of the developing telencephalon. Cell. 2013;152:895–908. doi: 10.1016/j.cell.2012.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen B, Schaevitz LR, McConnell SK. Fezl regulates the differentiation and axon targeting of layer 5 subcortical projection neurons in cerebral cortex. Proc Natl Acad Sci USA. 2005;102:17184–17189. doi: 10.1073/pnas.0508732102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bedogni F, et al. Tbr1 regulates regional and laminar identity of postmitotic neurons in developing neocortex. Proc Natl Acad Sci USA. 2010;107:13129–13134. doi: 10.1073/pnas.1002285107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McKenna WL, et al. Tbr1 and Fezf2 regulate alternate corticofugal neuronal identities during neocortical development. J Neurosci. 2011;31:549–564. doi: 10.1523/JNEUROSCI.4131-10.2011. The authors show that Tbr1 regulates corticothalamic projection neuron development in large part by repressing transcription of Fezf2.
- 87.Chen B, et al. The Fezf2-Ctip2 genetic pathway regulates the fate choice of subcortical projection neurons in the developing cerebral cortex. Proc Natl Acad Sci USA. 2008;105:11382–11387. doi: 10.1073/pnas.0804918105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rouaux C, Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat Cell Biol. 2013;15:214–221. doi: 10.1038/ncb2660. References # and # demonstrate that Fezf2 can postmitotically reprogram the input connectivity of layer IV granular neurons and the output connectivity of superficial-layer CPN, respectively.
- 89.Alcamo EA, et al. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron. 2008;57:364–377. doi: 10.1016/j.neuron.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 90.Britanova O, et al. Satb2 is a postmitotic determinant for upper-layer neuron specification in the neocortex. Neuron. 2008;57:378–392. doi: 10.1016/j.neuron.2007.12.028. These back-to-back papers identified Satb2 as the first transcriptional regulator to control the generation of callosal projection neurons.
- 91.Lai T, et al. SOX5 controls the sequential generation of distinct corticofugal neuron subtypes. Neuron. 2008;57:232–247. doi: 10.1016/j.neuron.2007.12.023. [DOI] [PubMed] [Google Scholar]
- 92.Tomassy GS, et al. Area-specific temporal control of corticospinal motor neuron differentiation by COUP-TFI. Proc Natl Acad Sci USA. 2010;107:3576–3581. doi: 10.1073/pnas.0911792107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han W, et al. TBR1 directly represses Fezf2 to control the laminar origin and development of the corticospinal tract. Proc Natl Acad Sci USA. 2011;108:3041–3046. doi: 10.1073/pnas.1016723108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kwan KY, et al. SOX5 postmitotically regulates migration, postmigratory differentiation, and projections of subplate and deep-layer neocortical neurons. Proc Natl Acad Sci USA. 2008;105:16021–16026. doi: 10.1073/pnas.0806791105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shim S, Kwan KY, Li M, Lefebvre V, Sestan N. Cis-regulatory control of corticospinal system development and evolution. Nature. 2012;486:74–79. doi: 10.1038/nature11094. The authors identify an evolutionarily-conserved cortex-specific enhancer for Fezf2 expression, together with the SoxC transcription factors that bind this enhancer to drive Fezf2 expression.
- 96.Koester SE, O’Leary DD. Connectional distinction between callosal and subcortically projecting cortical neurons is determined prior to axon extension. Dev Biol. 1993;160:1–14. doi: 10.1006/dbio.1993.1281. [DOI] [PubMed] [Google Scholar]
- 97.Lodato S, et al. Excitatory projection neuron subtypes control the distribution of local inhibitory interneurons in the cerebral cortex. Neuron. 2011;69:763–779. doi: 10.1016/j.neuron.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Srinivasan K, et al. A network of genetic repression and derepression specifies projection fates in the developing neocortex. Proc Natl Acad Sci USA. 2012;109:19071–19078. doi: 10.1073/pnas.1216793109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Baranek C, et al. Protooncogene Ski cooperates with the chromatin-remodeling factor Satb2 in specifying callosal neurons. Proc Natl Acad Sci USA. 2012;109:3546–3551. doi: 10.1073/pnas.1108718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Azim E. Dissertation: Molecular Controls over Neocortical Neuronal Diversity and Oligodendrocyte Development. 2009:1–381. [Google Scholar]
- 101.Deck M, et al. Pathfinding of Corticothalamic Axons Relies on a Rendezvous with Thalamic Projections. Neuron. 2013;77:472–484. doi: 10.1016/j.neuron.2012.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sohur US, Padmanabhan HK, Kotchetkov IS, Menezes JRL, Macklis JD. Anatomic and Molecular Development of Corticostriatal Projection Neurons in Mice. Cereb Cortex. 2012 doi: 10.1093/cercor/bhs342. doi:10.1093/cercor/bhs342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lickiss T, Cheung AFP, Hutchinson CE, Taylor JSH, Molnár Z. Examining the relationship between early axon growth and transcription factor expression in the developing cerebral cortex. J Anat. 2012;220:201–211. doi: 10.1111/j.1469-7580.2011.01466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Molnár Z, Cordery P. Connections between cells of the internal capsule, thalamus, and cerebral cortex in embryonic rat. J Comp Neurol. 1999;413:1–25. doi: 10.1002/(sici)1096-9861(19991011)413:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 105.Azim E, Shnider SJ, Cederquist GY, Sohur US, Macklis JD. Lmo4 and Clim1 progressively delineate cortical projection neuron subtypes during development. Cereb Cortex. 2009;19(Suppl 1):i62–9. doi: 10.1093/cercor/bhp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Cederquist GY, Azim E, Shnider SJ, Padmanabhan H, Macklis JD. Lmo4 Establishes Rostral Motor Cortex Projection Neuron Subtype Diversity. J Neurosci. 2013;33:6321–6332. doi: 10.1523/JNEUROSCI.5140-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Huang Z, et al. Transcription factor Lmo4 defines the shape of functional areas in developing cortices and regulates sensorimotor control. Dev Biol. 2009;327:132–142. doi: 10.1016/j.ydbio.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kashani AH, et al. Calcium activation of the LMO4 transcription complex and its role in the patterning of thalamocortical connections. J Neurosci. 2006;26:8398–8408. doi: 10.1523/JNEUROSCI.0618-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Joshi PS, et al. Bhlhb5 regulates the postmitotic acquisition of area identities in layers II-V of the developing neocortex. Neuron. 2008;60:258–272. doi: 10.1016/j.neuron.2008.08.006. The authors demonstrate that the transcription factor Bhlhb5 acts postmitotically to regulate refinement of molecular area identity and extension of corticospinal axons.
- 110.Weimann JM, et al. Cortical neurons require Otx1 for the refinement of exuberant axonal projections to subcortical targets. Neuron. 1999;24:819–831. doi: 10.1016/s0896-6273(00)81030-2. [DOI] [PubMed] [Google Scholar]
- 111.Ando K, et al. Establishment of framework of the cortical area is influenced by Otx1. Neurosci Res. 2008;60:457–459. doi: 10.1016/j.neures.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 112.Chou S-J, et al. Geniculocortical input drives genetic distinctions between primary and higher-order visual areas. Science. 2013;340:1239–1242. doi: 10.1126/science.1232806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. J. A. Barth; 1909. [Google Scholar]
- 114.Brodmann K, Garey LJ. Brodmann’s. Springer Verlag; 2006. [Google Scholar]
- 115.Pinto L, et al. AP2gamma regulates basal progenitor fate in a region- and layer-specific manner in the developing cortex. Nat Neurosci. 2009;12:1229–1237. doi: 10.1038/nn.2399. [DOI] [PubMed] [Google Scholar]
- 116.Lukaszewicz A, et al. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rakic P. Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci. 2009;10:724–735. doi: 10.1038/nrn2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Molnár Z. Evolution of cerebral cortical development. Brain Behav Evol. 2011;78:94–107. doi: 10.1159/000327325. [DOI] [PubMed] [Google Scholar]
- 119.Medina L, Reiner A. Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci. 2000;23:1–12. doi: 10.1016/s0166-2236(99)01486-1. [DOI] [PubMed] [Google Scholar]
- 120.Aboitiz F, Montiel J. One hundred million years of interhemispheric communication: the history of the corpus callosum. Braz J Med Biol Res. 2003;36:409–420. doi: 10.1590/s0100-879x2003000400002. [DOI] [PubMed] [Google Scholar]
- 121.Cheung AFP, et al. The subventricular zone is the developmental milestone of a 6-layered neocortex: comparisons in metatherian and eutherian mammals. Cereb Cortex. 2010;20:1071–1081. doi: 10.1093/cercor/bhp168. [DOI] [PubMed] [Google Scholar]
- 122.Abdel-Mannan O, Cheung AFP, Molnár Z. Evolution of cortical neurogenesis. Brain Res Bull. 2008;75:398–404. doi: 10.1016/j.brainresbull.2007.10.047. [DOI] [PubMed] [Google Scholar]
- 123.Fame RM, MacDonald JL, Macklis JD. Development, specification, and diversity of callosal projection neurons. Trends Neurosci. 2011;34:41–50. doi: 10.1016/j.tins.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kaas JH. Evolution of somatosensory and motor cortex in primates. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2004;281:1148–1156. doi: 10.1002/ar.a.20120. [DOI] [PubMed] [Google Scholar]
- 125.Kaas JH. Reconstructing the areal organization of the neocortex of the first mammals. Brain Behav Evol. 2011;78:7–21. doi: 10.1159/000327316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bulchand S, Subramanian L, Tole S. Dynamic spatiotemporal expression of LIM genes and cofactors in the embryonic and postnatal cerebral cortex. Dev Dyn. 2003;226:460–469. doi: 10.1002/dvdy.10235. [DOI] [PubMed] [Google Scholar]
- 127.Sun T, et al. Early asymmetry of gene transcription in embryonic human left and right cerebral cortex. Science. 2005;308:1794–1798. doi: 10.1126/science.1110324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ozdinler PH, et al. Corticospinal motor neurons and related subcerebral projection neurons undergo early and specific neurodegeneration in hSOD1G9 3A transgenic ALS mice. J Neurosci. 2011;31:4166–4177. doi: 10.1523/JNEUROSCI.4184-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Strong MJ, Gordon PH. Primary lateral sclerosis, hereditary spastic paraplegia and amyotrophic lateral sclerosis: discrete entities or spectrum? Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2005;6:8–16. doi: 10.1080/14660820410021267. [DOI] [PubMed] [Google Scholar]
- 130.Singer MA, Statland JM, Wolfe GI, Barohn RJ. Primary lateral sclerosis. Muscle Nerve. 2007;35:291–302. doi: 10.1002/mus.20728. [DOI] [PubMed] [Google Scholar]
- 131.Harding AE. Classification of the hereditary ataxias and paraplegias. Lancet. 1983;1:1151–1155. doi: 10.1016/s0140-6736(83)92879-9. [DOI] [PubMed] [Google Scholar]
- 132.Salinas S, Proukakis C, Crosby A, Warner TT. Hereditary spastic paraplegia: clinical features and pathogenetic mechanisms. Lancet Neurol. 2008;7:1127–1138. doi: 10.1016/S1474-4422(08)70258-8. [DOI] [PubMed] [Google Scholar]
- 133.Watanabe K, et al. Directed differentiation of telencephalic precursors from embryonic stem cells. Nat Neurosci. 2005;8:288–296. doi: 10.1038/nn1402. [DOI] [PubMed] [Google Scholar]
- 134.Espuny-Camacho I, et al. Pyramidal Neurons Derived from Human Pluripotent Stem Cells Integrate Efficiently into Mouse Brain Circuits In Vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. Neurons derived from human IPS cells can establish appropriate patterns of axonal connectivity, dendritic arborization, and functional circuit integration when transplanted into mouse brains.
- 135.Rouaux C, Arlotta P. Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nat Neurosci. 2010;13:1345–1347. doi: 10.1038/nn.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.la Rossa, De A, et al. In vivo reprogramming of circuit connectivity in postmitotic neocortical neurons. Nat Neurosci. 2013;16:193–200. doi: 10.1038/nn.3299. [DOI] [PubMed] [Google Scholar]
- 137.Joosten EA, Gribnau AA, Dederen PJ. An anterograde tracer study of the developing corticospinal tract in the rat: three components. Brain Res. 1987;433:121–130. doi: 10.1016/0165-3806(87)90070-8. [DOI] [PubMed] [Google Scholar]
- 138.Woodworth MB, Greig LC, Kriegstein AR, Macklis JD. SnapShot: Cortical Development. Cell. 2012;151:918–918.e1. doi: 10.1016/j.cell.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Toresson H, Potter SS, Campbell K. Genetic control of dorsal-ventral identity in the telencephalon: opposing roles for Pax6 and Gsh2. Development. 2000;127:4361–4371. doi: 10.1242/dev.127.20.4361. [DOI] [PubMed] [Google Scholar]
- 140.Stoykova A, Treichel D, Hallonet M, Gruss P. Pax6 modulates the dorsoventral patterning of the mammalian telencephalon. J Neurosci. 2000;20:8042–8050. doi: 10.1523/JNEUROSCI.20-21-08042.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Muzio L, et al. Conversion of cerebral cortex into basal ganglia in Emx2(−/−) Pax6(Sey/Sey) double-mutant mice. Nat Neurosci. 2002;5:737–745. doi: 10.1038/nn892. [DOI] [PubMed] [Google Scholar]
- 142.Yoshida M, et al. Emx1 and Emx2 functions in development of dorsal telencephalon. Development. 1997;124:101–111. doi: 10.1242/dev.124.1.101. [DOI] [PubMed] [Google Scholar]
- 143.Schuurmans C, Guillemot F. Molecular mechanisms underlying cell fate specification in the developing telencephalon. Curr Opin Neurobiol. 2002;12:26–34. doi: 10.1016/s0959-4388(02)00286-6. [DOI] [PubMed] [Google Scholar]
- 144.Azim E, Jabaudon D, Fame RM, Macklis JD. SOX6 controls dorsal progenitor identity and interneuron diversity during neocortical development. Nat Neurosci. 2009;12:1238–1247. doi: 10.1038/nn.2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- 146.Chou S-J, Perez-Garcia CG, Kroll TT, O’Leary DDM. Lhx2 specifies regional fate in Emx1 lineage of telencephalic progenitors generating cerebral cortex. Nat Neurosci. 2009;12:1381–1389. doi: 10.1038/nn.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hanashima C, Li SC, Shen L, Lai E, Fishell G. Foxg1 suppresses early cortical cell fate. Science. 2004;303:56–59. doi: 10.1126/science.1090674. [DOI] [PubMed] [Google Scholar]