Abstract
The objective of the present meta-analysis was to evaluate the survival, recurrence rate, and complications in patients with stage I non-small cell lung cancer (NSCLC) who received video-assisted thoracoscopic surgery (VATS) or open lobectomy. A literature search was conducted on June 31, 2012 using combinations of the search terms video-assisted thoracic surgery, open thoracotomy, lobectomy, and non-small-cell lung cancer (NSCLC). Inclusion criteria were: 1) Compared video-assisted thoracic surgery (VATS) lobectomy with open lobectomy. 2) Stage I NSCLC. 2) No previous treatment for lung cancer. 4) Outcome data included 5-year survival rate, complication, and recurrence rate. Tests of heterogeneity, sensitivity, and publication bias were performed. A total of 23 studies (21 retrospective and 2 prospective) met the inclusion criteria. VATS was associated with a longer 5-year survival (odds ratio [OR] = 1.622, 95% confidence interval [CI] 1.272 to 2.069; P<0.001), higher local recurrence rate (OR = 2.152, 95% CI 1.349 to 3.434; P = 0.001), similar distant recurrence rate (OR = 0.91, 95% CI 0.33 to 2.48; P = 0.8560), and lower total complication rate (OR = 0.45, 95% CI 0.24 to 0.84; P = 0.013) compared to open lobectomy. VATS was also associated with lower rates arrhythmias, prolonged air leakage, and pneumonia but it did not show any statistical significance. Patients with stage I NSCLC undergoing VATS lobectomy had longer survival and fewer complications than those who received open lobectomy.
Introduction
Since the introduction of thoracoscopic surgery, video-assisted thoracoscopic surgery (VATS) has become a viable option for the treatment of early stage lung cancer. Since the initial description of VATS in 1992, the number of VATS procedures for early stage lung cancer has steadily increased [1]. The past decade has seen an increase in the use of VATS for early stage lung cancer, and a recent report based on data from the Society of Thoracic Surgeons database indicated that VATS is used for 32% of all lobectomies in the United States [2]. VATS lobectomy has been shown to be associated with less postoperative pain, less surgical morbidity, fewer complications, and shorter hospitalization [3]–[9]. However, there is still much debate with respect to the role of VATS in lobectomy for the treatment of lung cancer. Though the feasibility and the safety of VATS for the treatment of early stage lung cancer has been proven [10], [11], there are persisting doubts regarding its oncological value; i.e., the potential compromise of oncological principles during surgery.
The objective of the present meta-analysis was to evaluate the survival, recurrence rate, and complications in patients with stage I non-small cell lung cancer (NSCLC) who received VATS or open lobectomy.
Methods
Literature Search Strategy
A search was conducted of PubMed, EMBASE, Google Scholar, and the Gray Journal including annual meetings of the American Society of Clinical Oncology and the American Society of Thoracic Surgery (chest surgery) using combinations of the search terms: video-assisted thoracic surgery, open thoracotomy, lobectomy, and non-small-cell lung cancer (NSCLC). The search date was June 31, 2012. Each publication was carefully examined, including the names of all authors, to avoid duplication of data.
Selection Criteria
Studies were selected for inclusion in this analysis based on the following criteria. 1) Compared video-assisted thoracic surgery (VATS) lobectomy with open lobectomy. 2) Disease was non-small-cell lung cancer. 3) Stage I disease; no lymph node or distant metastasis. 4) No previous treatment for lung cancer. 5) Outcome data included 5-year survival rate, complication, and recurrence rate. Exclusion criteria for this analysis were as follows. 1) Abstracts, letters, editorials, and expert opinions, reviews without original data, case reports, and studies lacking control groups. 2) Studies concerned with unresectable lung cancer or recurrence after lobectomy. 3) Studies with no clearly reported outcomes of interest.
Data extraction
Two independent reviewers extracted the data from eligible studies. A third reviewer was consulted for resolution of any disagreement. Data extracted included survival rates, recurrence rates, operative time, patent gender and age, disease stage, length of hospitalization, perioperative mortality, and complications including air leakage, arrhythmias, and pneumonia. The primary outcome measure was 5-year survival rate. Secondary outcomes were local and systematic recurrence rates, complications, and operation time.
Data analysis
The 5-yr survival rate was used to evaluate treatment efficacy. The operation times, local recurrence, distant recurrence, total complications, prolonged air leakage, arrhythmia, and pneumonia were considered for safety evaluation. Proportion (%) or mean with standard deviations (SD) were summarized for the outcomes, and were compared between participants who received VATS or open lobectomy. Any χ2-based test of homogeneity was performed using Cochran's Q statistic and calculated I2, the percentage of the total variability in effect estimates among trials that is due to heterogeneity rather than chance. If the I2 statistic (>50%) indicated heterogeneity existed between studies, a random-effects model was calculated. Otherwise, fixed-effects models were used. Combined summary statistics of the odds ratios (ORs) or mean difference for individual studies were shown. All statistical assessments were 2-sided, and a P value<0.05 was considered to indicate statistical significance. Moreover, sensitivity and publication bias analysis were applied for the primary outcome, i.e., the 5-year survival rate. Sensitivity analysis was performed based on the leave-one-out approach. A Funnel plot and the fail-safe N (which indicates whether the observed significance is spurious or not) were used to assess possible publication bias. All analyses were performed using Comprehensive Meta-Analysis statistical software, version 2.0 (Biostat, Englewood, NJ, USA).
Results
Literature search
A total of 23 studies (21 retrospective and 2 prospective) met the inclusion criteria, and were included in this study. Briefly, 630 records were identified by the database searches and screened for relevance. After excluding non-relevant studies (n = 583) and duplicates (n = 17), 30 full text articles were assessed for eligibility. Of these 30 studies, the disease type was not specified in 2, and other stage disease was also included in 5. These 7 studies were excluded, and thus 23 were included in this meta-analysis. The included studies are listed in Table 1. Of note, data of the outcomes specified for the current analysis were not included in each of the 23 studies.
Table 1. Studies included in the meta-analysis.
| Open Lobectomy | VATS | ||||||||||
| Study Design | Reference Number | 1st Author (year) | Disease Stage | N of patients | % male | Age, y | Operation Time, min | N of patients | % male | Age, y | Operation Time, min |
| Retrospective | [12] | Park JS (2011) | pIA+IB | 136 | 51.5 | 60 | ND | 136 | 51.5 | 60 | ND |
| Retrospective | [13] | Ilonen IK (2011) | cIA+IB | 212 | 59.4 | 65 | ND | 116 | 50 | 69 | ND |
| Retrospective | [14] | Kawachi R (2009) | cIA+IB | 176 | 65.9 | 67 | ND | 73 | 42.5 | 65 | ND |
| Retrospective | [15] | Sakuraba M (2007) | cIA | 56 | 58.9 | 63 | ND | 84 | 60.7 | 66 | ND |
| Retrospective | [16] | Whitson BA (2007) | cIA+IB | 88 | 48.9 | 65 | ND | 59 | 49.1 | 67 | ND |
| Retrospective | [17] | Park BJ (2007) | cIA+IB | 122 | 31.1 | 67 | ND | 122 | 31.1 | 67 | ND |
| Retrospective | [18] | Tashima T (2005) | cIA+IB | 173 | 44.5 | 67 | 185±42 | 67 | 37.3 | 68 | 175±66 |
| Retrospective | [19] | Inada K (2000) | cIA+IB | 30 | 73.3 | 67 | 222±17.7 | 24 | 70.8 | 67 | 280±19.7 |
| Retrospective | [20] | Ohbuchi T (1998) | cIA | 35 | 57.1 | 59 | 195.1±38.4 | 35 | 54.3 | 61 | 216.6±48.2 |
| Retrospective | [21] | Flores RM (2009) | cIA | 343 | 34.1 | 67 | ND | 398 | 38.2 | 67 | ND |
| Retrospective | [22] | Shigemura N (2006) | cIA | 55 | 52.7 | 62 | 159±28 | 81 | 49.4 | ND | 216.9±40.67 |
| Retrospective | [23] | Muraoka M (2006) | cIA | 42 | 73.8 | 65 | 293±83 | 43 | 53.5 | 65 | 288±66 |
| Retrospective | [24] | Shiraishi T (2006) | cIA | 79 | 65.8 | 66 | 224.8±64.5 | 81 | 59.3 | 63 | 226.7±48.9 |
| Retrospective | [25] | Sawada S (2007) | cIA+IB | 123 | 46.1 | 65 | ND | 165 | 63.4 | 65 | ND |
| Retrospective | [26] | Tatsumi A (2003) | cIA+IB | 121 | 62.8 | 68 | 230±57.6 | 118 | 56.8 | 66 | 219±61.67 |
| Retrospective | [26] | Yang X (2009) | pIA+IB | 98 | ND | 52 | ND | 43 | ND | 54 | ND |
| Retrospective | [28] | Koizumi K (2002) | cIA | 9 | ND | 71 | ND | 25 | ND | 74 | ND |
| Retrospective | [29] | Sugiura H (1999) | cIA+IB | 22 | 27.3 | 61 | 196±64 | 22 | 45.5 | 62 | 227±47 |
| Retrospective | [30] | Nomori H (2001) | cIA+IB | 22 | 50 | 64 | 270±84.9 | 22 | 50 | 64 | 281±81 |
| Retrospective | [31] | Yim AP (2000) | cIA+IB | 18 | 55.6 | 58 | 82±27 | 18 | 66.7 | 63 | 78±36 |
| Retrospective | [32] | Tajiri M (2007) | cIA+IB | 61 | 67.2 | 64 | 254.4±65.1 | 231 | 58.4 | ND | 258.6±51.28 |
| Prospective | [33] | Sugi K (2000) | cIA | 52 | 55.8 | 65 | ND | 48 | 58.3 | 66 | ND |
| Prospective | [34] | Kirby TJ (1995) | cIA+IB | 31 | 45.2 | 62 | 175±93 | 30 | 40 | 58 | 161±61 |
ND, not derived; VATS, video-assisted thoracoscopic surgery.
Primary outcome (5-year survival rate)
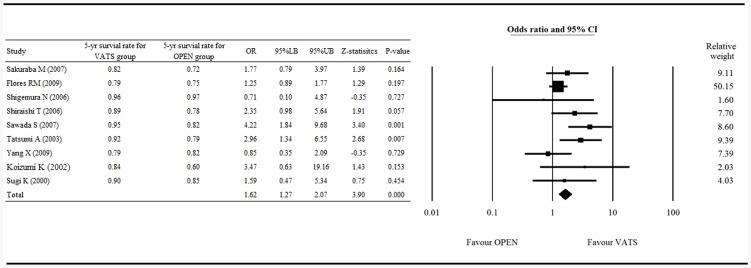
The Forrest plot of the 5-year survival rate between patients who received VATS and those who received open surgery is shown in Figure 1. Nine studies [15], [21], [22]–[27], [29], [33] with complete survival rate data were included in the analysis. The heterogeneity test showed a fixed effect model was considered with a Q statistic = 13.652, and I2 = 41.401 (P = 0.091). The result, which showed an overall OR = 1.622 (95% confidence interval [CI] 1.272 to 2.069), significantly favored VATS over open surgery with respect to 5-year survival, with a Z-statistic = 3.898 (P<0.001; Fig. 1).
Figure 1. Forest plot of the 5-year survival rates of the VATS vs. open surgery groups.
OR, odd ratio; LB, lower boundary; UB, upper boundary; CI, confidence interval.
Sensitivity analysis
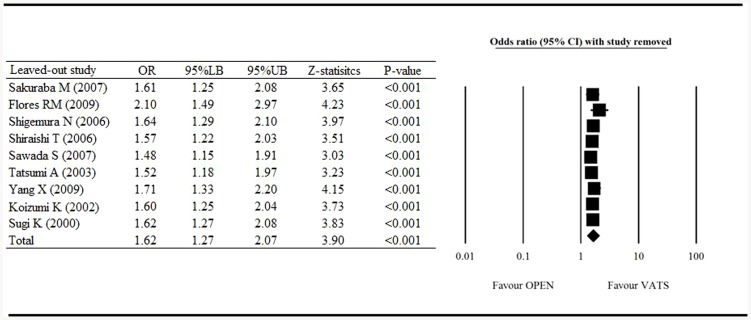
Figure 2 shows the results of the meta-analysis of the 5-year survival rate, with one study removed in turn. The results indicated that even with each of the studies removed in turn, the direction and magnitude of combined estimates did not have a large variation. This result indicates that the meta-analysis was proven to have good reliability.
Figure 2. Sensitivity analysis of the influence of individual studies on pooled estimate for 5-year survival rate.
OR, odd ratio; LB, lower boundary; UB, upper boundary; CI, confidence interval.
Publication bias
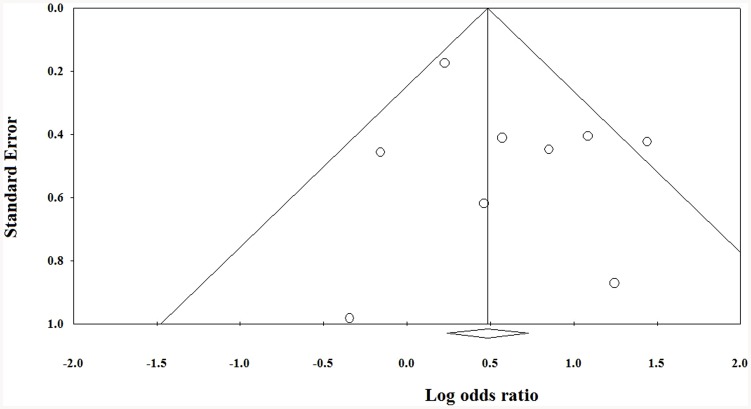
The Funnel plot for publication bias (standard error by 5-year survival rate) demonstrated marked evidence of symmetry (Fig. 3), indicating a publication bias did not exist. The combined effect size yielded a Z value of 4.051, with a corresponding P value<0.001. This result indicates that the fail-safe N value was relevant.
Figure 3. Funnel plot of the standard error by mean difference for 5-year survival rate.
Secondary outcomes (local recurrence rate, distant recurrence rate)
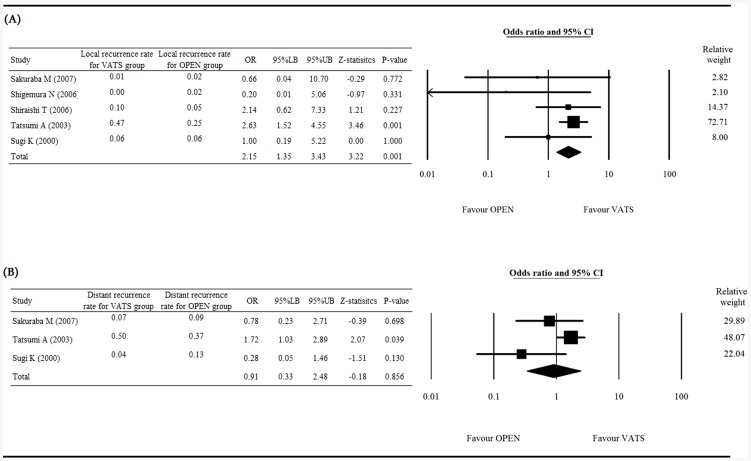
Figure 4 presents the Forest plot of local recurrence rate (Fig. 4A), and distant recurrence rate (Fig. 4B). Five studies with complete data of local recurrence rates were included in the analysis. The heterogeneity test showed a fixed effect model was considered with a Q statistic = 4.10, and I2 = 2.43% (P = 0.393). The results with an OR = 2.152 (95% CI 1.349 to 3.434) indicated that VATS was associated with a higher local recurrence rate than open surgery, with a Z-statistic = 3.216 (P = 0.001; Fig. 4A).
Figure 4. Forest plot for the VATS vs. open surgery groups.
(A) local recurrence and (B) distant recurrence rate. OR, odd ratio; LB, lower boundary; UB, upper boundary; CI, confidence interval.
Three studies with complete data of distant recurrence rates were included in the analysis of distant recurrence rate. The heterogeneity test shows a random effect model was considered with a Q statistic = 5.10 and I2 = 60.7% (P = 0.078). The results with an OR = 0.91 (95% CI 0.33 to 2.48) indicated no significant difference between the VATS and open surgery groups, with a Z-statistic = −0.18 (P = 0.856; Fig. 4B).
Safety outcomes (rate of total complications, prolonged air leakage, arrhythmia, and pneumonia)
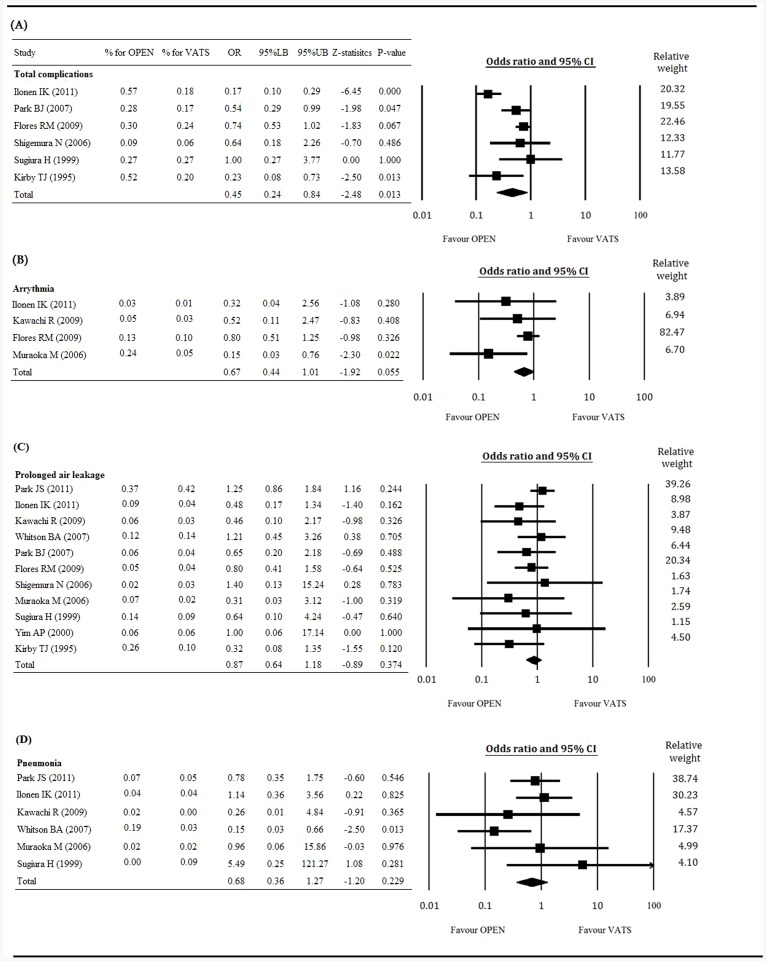
Figure 5 presents the Forest plots of total complications (Fig. 5A), arrhythmia (Fig. 5B), prolonged air leakage (Fig. 5C), and pneumonia (Fig. 5D). Six studies with complete data of the total complication rate were included in the analysis. The heterogeneity test shows a random effect model was considered with a Q statistic = 24.09 and I2 = 79.25% (P<0.001). The results with an OR = 0.45 (95% CI 0.24 to 0.84) indicated that VATS was associated with a lower rate of total complications than open surgery, with a Z-statistic = −2.48 (P = 0.013; Fig. 5A).
Figure 5. Forest plot for the VATS vs. open surgery groups.
(A) total complications, (B) arrhythmias, (C) prolonged air leakage, and (D) pneumonia. OR, odd ratio; LB, lower boundary; UB, upper boundary; CI, confidence interval.
There were 4, 11, and 6 studies with complete data of arrhythmias, prolonged air leakage, and pneumonia, respectively. The results of the analyses indicated that VATS was associated with lower rates of arrhythmias (OR = 0.67, 95% CI 0.44 to 1.01, P = 0.055; Fig. 5B), prolonged air leakage (OR = 0.87, 95% CI 0.64 to 1.18, P = 0.374; Fig. 5C), and pneumonia (OR = 0.68, 95% CI 0.36 to 1.27, P = 0.229; Fig. 5D) than open surgery. However, there was not any statistically significant difference in these specific complications between the VATS and open lobectomy groups.
Discussion
The results of this meta-analysis showed that VATS was associated with a better 5-year survival rate than open lobectomy in patients with stage I NSCLC, although VATS patients had a higher local recurrence rate than did those who received open surgery. Furthermore, there was no difference in distant recurrence rate between the 2 groups, while VATS was associated with lower rates of total complications, arrhythmias, prolonged air leakage, and pneumonia.
Six prior meta-analyses [5]–[9], [35] have examined VATS vs. open lobectomy in the treatment of early stage lung cancer (Table 2). While the disease stages and outcomes assessed are not exactly the same among the studies, the results of our analysis are generally in agreement with the prior studies which indicated that VATS is associated with a more favorable 5-year survival rate, either similar or lower rates of complications, and similar or lower rates of systemic recurrence as open lobectomy. In our study, however, VATS was not associated with a reduced local recurrence rate, which is different from the results of the other meta-analyses [5], [6], [8]. The inclusion and exclusion criteria of our study differed from those of the other meta-analyses, which may explain the disparate finding. Specifically, the meta-analyses reported by Yan et al [5] and Zhang et al [8] involved the results from studies of patients with early stage (stage I–IIIA) NSCLC, whereas the meta-analysis reported by Li et al [6] involved the results from studies of patients with stage I lung cancer (ie, not exclusively NSCLC).
Table 2. Comparison with prior meta-analyses.
| Parameter | Current Study | Yan, 2009 [5] | Li, 2012 [6] | Cao, 2013 [7] | Zhang, 2013 [8] | Taioli, 2013 [9] | Chen, 2013 [35] |
| Disease type | Stage I NSCLC | Early-stage NSCLC | Stage I lung cancer | NSCLC* | Early-stage NSCLC | Lung cancer | Stage I NSCLC |
| Favor treatment | Favor VATS | Favor VATS | Favor VATS | – | Favor VATS | Favor VATS | Favor VATS |
| (Survival rate) | (5 year) | (5 year) | (5 year) | (5 year) | (5 year) | ||
| Length of hospitalization | - | - | - | VATS lower | - | - | VATS lower |
| Total LND or LNS procedures | - | - | - | - | No difference | - | - |
| Local recurrence | VATS higher | No difference | No difference | - | VATS lower | - | - |
| Systemic recurrence | No difference | VATS lower | VATS lower | - | VATS lower | - | - |
| Total complications | VATS lower | - | VATS lower | VATS lower | - | - | VATS lower |
| Prolonged air leakage | No difference | No difference | - | VATS lower | - | - | No difference |
| Arrhythmia | No difference | No difference | - | VATS lower | - | - | No difference |
| Pneumonia | No difference | No difference | - | VATS lower | - | - | VATS lower |
| Renal failure | - | - | - | VATS lower | - | - | - |
| Perioperative mortality | - | No difference | - | No difference | - | - | - |
propensity score-matched.
In the Eastern Cooperative Oncology Group 3590 study, lymphadenectomy was defined as the removal of ≥10 lymph nodes from at least 2 or more mediastinal lymph nodes stations [36]. One of the chief concerns of VATS lobectomy is that it provides insufficient lymph node dissection. However, these concerns seem to be unjustified as studies have indicated that a standard lobectomy with lymph node dissection can be performed via VATS [37], [38]. Denliger et al. [39] reported that fewer lymph nodes were sampled with VATS lobectomy compared with open lobectomy; however, there was no survival difference. The authors believed that the reason fewer nodes were sampled with VATS lobectomy was because the subcarinal space does not have to be exposed in upper lobe lobectomies, thus dissection of the subcarinal lymph nodes is more challenging than that of other stations.
Our analysis showed that VATS was associated with a better the 5-year survival than open surgery, a result that is consistent with those of other meta-analyses and other studies [5], [6], [8], [9], [35]. Tahara et al. [36] reported that up to 25% of patients with T1 tumors had N+ disease at final postoperative pathological examination. However, studies have shown that patients who underwent VATS lobectomy who were stage N0 at clinical staging and who were found to have lymph node involvement at surgery or postoperative pathological examination have favorable outcomes [26], [40]. Kim et al. [40] reported that patients with pathological N1 or N2 disease after VATS lobectomy had a 3-year overall survival rate of 98% and 89%, respectively; rates comparable to that of open lobectomy. These data suggest that even in lymph node involvement is found during VATS lobectomy for clinical stage I disease, conversion to an open procedure is not necessary.
There are several potential explanations for the better 5-year survival with VATS compared with open surgery. One potential explanation is decreased release of cytokines with this approach, which would reduce the level of perioperative immunosuppression [41]–[43]. Another potential explanation is that patients who undergo VATS may be better able to tolerate postoperative chemotherapy [44], [45].
Our analysis indicated that VATS lobectomy for early stage NSCLC is associated with a lower rate of total complications, as well as lower rates of the individual complications of prolonged air leakage, arrhythmia, and pneumonia. These results are consistent with those of prior meta-analyses [5]–[9], [35].
Our meta-analysis has a number of limitations that must be considered when interpreting the findings described herein. The primary limitation is that the majority of the studies included were retrospective in nature. Another limitation is the fact that some of the studies included had a primary focus on safety or feasibility, rather than survival and/or recurrence. The methodologies employed in these studies may not have been appropriately rigorous regarding the assessment of survival and/or recurrence. We also acknowledge that various differences in between study factors that were not reported (eg, institutional VATS reliability, potential avoidance of central tumor location) may have affected the outcomes described and hence the results of our meta-analysis. Clearly, large-scale, multicenter, prospective studies would be warranted to account for these potential biasing factors.
Conclusions
In summary, patients with stage I NSCLC undergoing VATS lobectomy had longer survival and fewer complications than those who received open lobectomy. These results suggest that VATS is an effective and safe approach for the treatment of early stage NSCLC.
Supporting Information
PRISMA 2009 Checklist.
(DOC)
Funding Statement
The authors have no support or funding to report.
References
- 1. Paoletti L, Pastis NJ, Denlinger CE, Silvestri GA (2011) A decade of advances in treatment of early-stage lung cancer. Clin Chest Med 32: 827–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Boffa DJ, Allen MS, Grab JD (2008) Data from the Society of Thoracic Surgeons General Thoracic Surgery Database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg 135: 247–254. [DOI] [PubMed] [Google Scholar]
- 3. Murthy S (2012) Video-assisted thoracoscopic surgery for the treatment of lung cancer. Cleve Clin J Med 79 Electronic Suppl 1: eS23–25. [DOI] [PubMed] [Google Scholar]
- 4. Whitson BA, Groth SS, Duval SJ, Swanson SJ, Maddaus MA (2008) Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 86: 2008–16. [DOI] [PubMed] [Google Scholar]
- 5. Yan TD, Black D, Bannon PG, McCaughan BC (2009) Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 27: 2553–2562. [DOI] [PubMed] [Google Scholar]
- 6. Li Z, Liu H, Li L (2012) Video-assisted thoracoscopic surgery versus open lobectomy for stage I lung cancer: A meta-analysis of long-term outcomes. Exp Ther Med 3: 886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cao C, Manganas C, Ang SC, Peeceeyen S, Yan TD (2013) Video-assisted thoracic surgery versus open thoracotomy for non-small cell lung cancer: a meta-analysis of propensity score-matched patients. Interact Cardiovasc Thorac Surg 16: 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Z, Zhang Y, Feng H, Yao Z, Teng J, et al. (2013) Is video-assisted thoracic surgery lobectomy better than thoracotomy for early-stage non-small-cell lung cancer? A systematic review and meta-analysis. Eur J Cardiothorac Surg Jan 30. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 9. Taioli E, Lee DS, Lesser M, Flores R (2013) Long-term survival in video-assisted thoracoscopic lobectomy vs open lobectomy in lung-cancer patients: a meta-analysis. Eur J Cardiothorac Surg Feb 14. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 10. McKenna RJ, Houck W, Fuller CB (2006) Video-assisted thoracic surgery lobectomy: experience with 1,100 cases. Ann Thorac Surg 81: 421–426. [DOI] [PubMed] [Google Scholar]
- 11. Onaitis MW, Petersen RP, Balderson SS, Toloza E, Burfeind WR, et al. (2006) Thoracoscopic lobectomy is a safe and versatile procedure: experience with 500 consecutive patients. Ann Surg 244: 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Park JS, Kim K, Choi MS, Chang SW, Han WS (2011) Video-assisted thoracic surgery (VATS) lobectomy for pathologic stage I non-small cell lung cancer: a comparative study with thoracotomy lobectomy. Korean J Thorac Cardiovasc Surg 44: 32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ilonen IK, Räsänen JV, Knuuttila A, Salo JA, Sihvo EI (2011) Anatomic thoracoscopic lung resection for non-small cell lung cancer in stage I is associated with less morbidity and shorter hospitalization than thoracotomy. Acta Oncol 50: 1126–1132. [DOI] [PubMed] [Google Scholar]
- 14. Kawachi R, Tsukada H, Nakazato Y, Takei H, Koshi-ishi Y, et al. (2009) Morbidity in video-assisted thoracoscopic lobectomy for clinical stage I non-small cell lung cancer: is VATS lobectomy really safe? Thorac Cardiovasc Surg 57: 156–159. [DOI] [PubMed] [Google Scholar]
- 15. Sakuraba M, Miyamoto H, Oh S, Shiomi K, Sonobe S, et al. (2007) Video-assisted thoracoscopic lobectomy vs. conventional lobectomy via open thoracotomy in patients with clinical stage IA non-small cell lung carcinoma. Intera ct Cardiovasc Thorac Surg 6: 614–617. [DOI] [PubMed] [Google Scholar]
- 16. Whitson BA, Andrade RS, Boettcher A, Bardales R, Kratzke RA, et al. (2007) Video-assisted thoracoscopic surgery is more favorable than thoracotomy for resection of clinical stage I non-small cell lung cancer. Ann Thorac Surg 83: 1965–1970. [DOI] [PubMed] [Google Scholar]
- 17. Park BJ, Zhang H, Rusch VW, Amar D (2007) Video-assisted thoracic surgery does not reduce the incidence of postoperative atrial fibrillation after pulmonary lobectomy. J Thorac Cardiovasc Surg 133: 775–779. [DOI] [PubMed] [Google Scholar]
- 18. Tashima T, Yamashita J, Nakano S, Joutsuka T, Hayashi N, et al. (2005) Comparison of video-assisted minithoracotomy and standard open thoracotomy for the treatment of non-small cell lung cancer. Minim Invasive Ther Allied Technol 14: 203–208. [DOI] [PubMed] [Google Scholar]
- 19. Inada K, Shirakusa T, Yoshinaga Y, Yoneda S, Shiraishi T, et al. (2000) The role of video-assisted thoracic surgery for the treatment of lung cancer: lung lobectomy by thoracoscopy versus the standard thoracotomy approach. Int Surg 85: 6–12. [PubMed] [Google Scholar]
- 20. Ohbuchi T, Morikawa T, Takeuchi E, Kato H (1998) Lobectomy: video-assisted thoracic surgery versus posterolateral thoracotomy. Jpn J Thorac Cardiovasc Surg 46: 519–522. [DOI] [PubMed] [Google Scholar]
- 21. Flores RM, Park BJ, Dycoco J, Aronova A, Hirth Y, et al. (2009) Lobectomy by video-assisted thoracic surgery (VATS) versus thoracotomy for lung cancer. J Thorac Cardiovasc Surg 138: 11–18. [DOI] [PubMed] [Google Scholar]
- 22. Shigemura N, Akashi A, Funaki S, Nakagiri T, Inoue M, et al. (2006) Long-term outcomes after a variety of video-assisted thoracoscopic lobectomy approaches for clinical stage IA lung cancer: a multi-institutional study. J Thorac Cardiovasc Surg 132: 507–512. [DOI] [PubMed] [Google Scholar]
- 23. Muraoka M, Oka T, Akamine S, Tagawa T, Nakamura A, et al. (2006) Video-assisted thoracic surgery lobectomy reduces the morbidity after surgery for stage I non-small cell lung cancer. Jpn J Thorac Cardiovasc Surg 54: 49–55. [DOI] [PubMed] [Google Scholar]
- 24. Shiraishi T, Shirakusa T, Hiratsuka M, Yamamoto S, Iwasaki A (2006) Video-assisted thoracoscopic surgery lobectomy for c-T1N0M0 primary lung cancer: its impact on locoregional control. Ann Thorac Surg 82: 1021–1026. [DOI] [PubMed] [Google Scholar]
- 25. Sawada S, Komori E, Yamashita M, Nakata M, Nishimura R, et al. (2007) Comparison in prognosis after VATS lobectomy and open lobectomy for stage I lung cancer: retrospective analysis focused on a histological subgroup. Surg Endosc 21: 1607–1611. [DOI] [PubMed] [Google Scholar]
- 26. Tatsumi A, Ueda Y (2003) Video-assisted thoracic surgery for lung cancer: is it a feasible operation for stage I lung cancer? Jpn J Thorac Cardiovasc Surg 51: 646–650. [DOI] [PubMed] [Google Scholar]
- 27. Yang X, Wang S, Qu J (2009) Video-assisted thoracic surgery (VATS) compares favorably with thoracotomy for the treatment of lung cancer: a five-year outcome comparison. World J Surg 33: 1857–1861. [DOI] [PubMed] [Google Scholar]
- 28. Koizumi K, Haraguchi S, Hirata T, Hirai K, Mikami I, et al. (2002) Video-assisted lobectomy in elderly lung cancer patients. Jpn J Thorac Cardiovasc Surg 2002;50: 15–22. [DOI] [PubMed] [Google Scholar]
- 29. Sugiura H, Morikawa T, Kaji M, Sasamura Y, Kondo S, et al. (1999) Long-term benefits for the quality of life after video-assisted thoracoscopic lobectomy in patients with lung cancer. Surg Laparosc Endosc Percutan Tech 9: 403–408. [PubMed] [Google Scholar]
- 30. Nomori H, Horio H, Naruke T, Suemasu K (2001) What is the advantage of a thoracoscopic lobectomy over a limited thoracotomy procedure for lung cancer surgery? Ann Thorac Surg 2: 879–884. [DOI] [PubMed] [Google Scholar]
- 31. Yim AP, Wan S, Lee TW, Arifi AA (2000) VATS lobectomy reduces cytokine responses compared with conventional surgery. Ann Thorac Surg 70: 243–247. [DOI] [PubMed] [Google Scholar]
- 32. Tajiri M, Maehara T, Nakayama H, Sakamoto K (2007) Decreased invasiveness via two methods of thoracoscopic lobectomy for lung cancer compared with open thoracotomy. Respirology 12: 207–211. [DOI] [PubMed] [Google Scholar]
- 33. Sugi K, Kaneda Y, Esato K (2000) Video-assisted thoracoscopic lobectomy achieves a satisfactory long-term prognosis in patients with clinical stage IA lung cancer. World J Surg 24: 27–30. [DOI] [PubMed] [Google Scholar]
- 34. Kirby TJ, Mack MJ, Landreneau RJ, Rice TW (1995) Lobectomy–video-assisted thoracic surgery versus muscle-sparing thoracotomy. A randomized trial. J Thorac Cardiovasc Surg 109: 997–1001. [DOI] [PubMed] [Google Scholar]
- 35. Chen FF, Zhang D, Wang YL, Xiong B (2013) Video-assisted thoracoscopic surgery lobectomy versus open lobectomy in patients with clinical stage I non-small cell lung cancer: A meta-analysis. Eur J Surg Oncol 39 (9) 957–963 doi: 10.1016/j.ejso.2013.06.016 [DOI] [PubMed] [Google Scholar]
- 36. Tahara RW, Lackner RP, Graver LM (2000) Is there a role for routine mediastinoscopy in patients with peripheral T1 lung cancers? Am J Surg 180: 488–491. [DOI] [PubMed] [Google Scholar]
- 37. Sagawa M, Sato M, Sakurada A, Matsumura Y, Endo C, et al. (2000) A prospective trial of systematic nodal dissection for lung cancer by videoassisted thoracic surgery: can it be perfect? Ann Thorac Surg 73: 900–904. [DOI] [PubMed] [Google Scholar]
- 38. Kondo T, Sagawa M, Tanita T, Sato M, Ono S, et al. (1998) Is complete systematic nodal dissection by thoracoscopic surgery possible? A prospective trial of video-assisted lobectomy for cancer of the right lung. J Thorac Cardiovasc Surg 116: 651–652. [DOI] [PubMed] [Google Scholar]
- 39. Denlinger CE, Fernandez F, Meyers BF, Pratt W, Zoole JB, et al. (2010) Lymph node evaluation in video-assisted thoracoscopic lobectomy versus lobectomy by thoracotomy. Ann Thorac Surg 89: 1730–1736. [DOI] [PubMed] [Google Scholar]
- 40. Kim HK, Choi YS, Kim J, Shim YM, Kim K (2010) Outcomes of unexpected pathologic N1 and N2 disease after video-assisted thoracic surgery lobectomy for clinical stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 140: 1288–1293. [DOI] [PubMed] [Google Scholar]
- 41. Sugi K, Kaneda Y, Esato K (2000) Video-assisted thoracoscopic lobectomy reduces cytokine production more than conventional open lobectomy. Jpn J Thorac Cardiovasc Surg 48: 161–165. [DOI] [PubMed] [Google Scholar]
- 42. Ng CS, Lee TW, Wan S, Wan IY, Sihoe AD, et al. (2005) Thoracotomy is associated with significantly more profound suppression in lymphocytes and natural killer cells than video-assisted thoracic surgery following major lung resections for cancer. J Invest Surg 18: 81–88. [DOI] [PubMed] [Google Scholar]
- 43. Whitson BA, D'Cunha J, Maddaus MA (2007) Minimally invasive cancer surgery improves patient survival rates through less perioperative immunosuppression. Med Hypotheses 68: 1328–1332. [DOI] [PubMed] [Google Scholar]
- 44. Jiang G, Yang F, Li X, Liu J, Li J, et al. (2011) Video-assisted thoracoscopic surgery is more favorable than thoracotomy for administration of adjuvant chemotherapy after lobectomy for non-small cell lung cancer. World J Surg Oncol 21: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Petersen RP, Pham D, Burfeind WR, Hanish SI, Toloza EM, et al. (2007) Thoracoscopic lobectomy facilitates the delivery of chemotherapy after resection for lung cancer. Ann Thorac Surg 83: 1245–1250. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Checklist.
(DOC)