Abstract
Many plants exhibit antioxidant properties which may be useful in the prevention of oxidative stress reactions, such as those mediated by the formation of free radical species in different pathological situations. In recent years a number of studies have shown that whole grain products in particular have strong antioxidant activity. Primary cultures of rat hepatocytes were used to investigate whether and how a fermented powder of wheat (Lisosan G) is able to modulate antioxidant and detoxifying enzymes, and whether or not it can activate Nrf2 transcription factor or inhibit NF-kB activation. All of the antioxidant and detoxifying enzymes studied were significantly up-regulated by 0.7 mg/ml Lisosan G treatment. In particular, NAD(P)H:quinone oxidoreductase and heme oxygenase-1 were induced, although to different degrees, at the transcriptional, protein and/or activity levels by the treatment. As for the Nrf2 transcription factor, a partial translocation of its protein from the cytosol to the nucleus after 1 h of Lisosan G treatment was revealed by immunoblotting. Lisosan G was also observed to decrease H2O2-induced toxicity
Taken together, these results show that this powder of wheat is an effective inducer of ARE/Nrf2-regulated antioxidant and detoxifying genes and has the potential to inhibit the translocation of NF-kB into the nucleus.
Introduction
Humans are constantly exposed to factors causing oxidative stress including pollutants, radiation and oxidized food [1]. Oxidative stress, defined as a loss of balance between the cellular concentration of reactive oxygen species and the cell's antioxidant capacity, is implicated in the onset of various diseases [2]. The human body has several endogenous systems [3] with which it can protect itself against oxidative stress, but antioxidant factors acquired from food also play a key role. Indeed, certain micronutrients obtained from food have potent antioxidant properties and may play an important role in maintaining the oxidative/antioxidative balance, especially if the diet is rich in these constituents [4]. In recent years, a variety of vegetables that contain antioxidants potentially capable of preventing oxidative stress reactions, such as those mediated by the formation of free radical species have been studied. Tomatoes, spinach, green peppers and cabbage are important sources of vitamin C. In vivo, this vitamin acts as scavenger of oxygen radicals and also as competitive inhibitor of nitrosamine synthesis from nitrite and amines in vivo [5]. Isothiocyanates are a family of molecules which are abundant in cruciferous vegetables such as broccoli, watercress and cauliflower. Sulforaphane, the best known isothiocyanate, induces drug metabolizing enzymes such as glutathione S-transferase A1/2 isoforms and NAD(P)H:quinone oxidoreductase (NQO1) in primary hepatocytes [6]. Whole grains are good source of B group vitamins, vitamin E, some minerals (zinc, magnesium and phosphorous), and they contain a variety of phytochemicals such as phytoestrogens, phytate, proteins, polysaccharides, phenols and lignans that are able to minimize oxydive damage [7]. All these components may act synergically [8]. By contrast, refined grains have a reduced nutrient content as the milling process results in the loss of dietary fibre, vitamins, minerals, lignans, phytoestrogens, phenolic compounds and phytic acid [9]. Many wheat proteins contain reduced sulfhydryl groups, which can have some free radical scavenging activity. Phytic acid can protect tissues against oxidative reactions by sequestering and inactivating pro-oxidative transition metals [3]. In epidemiological studies, whole grain consumption is associated with improvements in body mass index (BMI) [10] and insulin sensitivity [11] as well as with lower incidences of type 2 diabetes [12], cardiovascular diseases [13], and colorectal cancer [14]. Little is known about how cereals effect cells and to our knowledge, no research has yet been done on the antioxidant properties of whole grain products in primary hepatocytes.
Several studies have shown that some phytochemicals can modulate antioxidant and phase II enzymes through the activation of nuclear factor E2-related protein (Nrf2) [15]. Nrf2 is a basic-leucine zipper transcription factor that under basal conditions, is present in an inactive form in the cytoplasm, bound to the Kelch-like ECH- associated protein 1 (Keap1) [16]. Various agents including Antioxidant Response Element (ROS) and weak electrophiles (e.g. isothiocyanates) can alter the Keap1-Nrf2 protein complex and free Nrf2 through phosphorilation or alkylation of one or more of the 27 cysteine residues in Keap1 [17]. When this occurs, Nrf2 translocates into the nucleus. Upon activation, Nrf2 dimerizes with a small Maf protein then binds to antioxidant responsive element (ARE) sites in the promoter regions of antioxidant and phase II genes, thereby inducing their transcription [18].
In recent years, many authors have suggested the existence of cross-talk between Nrf2/ARE and the nuclear factor-kappa B (NF-kB) signaling pathways in response to inflammation [19]–[21]. The Nrf2 and NF-kB signaling pathways interface at several points to control the transcription or function of downstream target proteins [22]. In addition, ROS now appear to act as second messengers in numerous signaling pathways [23]–[24]. One signaling pathway that engages in cross-talk with ROS involves NF-kB family transcription factors [25]–[27]. It had already been shown twenty years ago by Schreck and coworkers [28] that oxidative stresses, such as addition of extracellular hydrogen peroxide, can induced NF-kB nuclear translocation in several cell lines. The NF-kB family is made up of NF-kB1 (p50), NFkB2 (p52), RelA (p65), c-Rel and RelB. In the absence of stimuli, NF-kB, is associated with the inhibitor protein, IkBα, and sequestered in the cytosol. Upon stimulation with a NF-kB inducers, IkBα is rapidly phosphorylated on two serine residues (S32 and S36), which targets the inhibitor for ubiquitination and degradation by proteosome.
Lisosan G is a powder obtained from Triticum Sativum (wheat) and it is registered with the Italian Ministry of Health as a nutritional supplement. In the production process, the wholegrain is first ground to a rough powder. From this intermediary product, the bran and germ are separated and collected for further treatment which consists in the following: water is added to moisten the mix, then selected microbic starting cultures are inoculated to initiate fermentation. The starting cultures typically consist of a mix of lacto-bacillus and natural yeast strains. Once the product is sufficiently fermented, it is dried. The resulting dry powder is now Lisosan G, which is widely used in food production thanks to its rich nutritional content.
It contains vitamins, minerals and polyunsatured fatty acids as well as having significant antioxidant activity [29]. In vivo, Lisosan G protects against cisplatin induced toxicity [30], and a recent paper showed that Lisosan G helps prevent microcirculatory dysfunction [31]. The authors of these works suggested that the protective effect of Lisosan G could be associated with the attenuation of oxidative stress and the preservation of antioxidant enzymes. To date, no studies have attempted to identify the molecular mechanism that determines antioxidant properties of Lisosan G. For this reason, in the present study, we investigated the effects of Lisosan G on the antioxidant and drug-metabolising enzymes at transcriptional, catalytic and protein levels using cultures of primary rat hepatocytes.
Materials and Methods
2.1 Chemicals
Lisosan G is registered as nutritional supplement by the Italian Minister of Health and was supplied by Agrisan Company, Larciano (PT), Italy. Collagenase; dexamethasone; insulin; glucagon; penicillin/streptomycin; ampicillin/kanamycin; fetal bovine serum; 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES); Tween 20; phenylmethylsulfonyl fluoride (PMSF); leupeptin; apoprotein; pepstatin; tunicamycin; Williams E medium; bovine serum albumin (BSA); epidermal growth factor (EGF); β-nicotinamide adenine dinucleotide reduced (NADPH); ethylenediaminetetraacetic acid (EDTA); ethylene glycol tetraacetic acid (EGTA); glutathione (GSH); Glutathione disulfide (GSSG); and hydrogen peroxide (H2O2) were all supplied by Sigma-Aldrich (St. Louis, MO). Rabbit polyclonal anti-Nrf2 (sc-13032), anti-heme oxygenase-1 (sc-10789), NFkB (sc-7178), β-actin (sc-130657), PARP-1 (sc-25780) and goat anti-rabbit (1∶2000 or 1∶5000) were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Collagen (type I) was prepared by the method of Beken et al. (1998).
2.2 Primary rat hepatocytes isolation, culture and treatments
Hepatocytes were isolated from 200–300 g Wistar male rats with free access to drinking water and food and on a 12 h light/dark cycle. The research with the use of animals was approved by the Italian Ministry of Health in compliance with European Community law n. 116/92. The approved protocol number is 10/09. The animals were anesthetized with an intraperitoneally injection of Zoletil ® (40 mg/kg) and then subjected to midline laparotomy in order to exteriorize the liver and isolate the portal vein. A needle was inserted into the portal vein and then the liver was perfused as described previously [32].
After filtration and centrifugation, the cell viability was determined by trypan blue exclusion. The cells were dispersed in Williams E medium containing 39 ng/ml dexamethasone, 0.5 U/ml insulin, 0.007 µg/ml glucagon, 5 µg/ml penicillin and streptomycin, 5 µg/ml ampicillin and kanamycin and 10% fetal bovine serum. The cells were plated at a density of 4.5×106 cells/8 ml on 100 mm cell culture dish pre-coated with 3 ml of collagen (type I) solution (1 mg/ml). The cultures were maintained at 37°C in 5% CO2 in a humidifier incubator. After 5 h, the medium was replaced with serum-free Williams E medium supplemented with 2% BSA, 7.5 µg/ml hydrocortisone 21-hemisuccinate sodium salt and EGF 20 ng/ml. Cultures were maintained in this medium at 37°C and 5% CO2 for 24 h. After this period, the medium was removed and a second layer of type I collagen was added to create a collagen-gel sandwich culture [33] and after 45 minutes serum-free Williams E medium was added again. The cells were maintained for additional 24 h before treatments. Cells treatments were divided in four different groups: in the first group (control), the cells were treated with medium only; in the second one with Lisosan G 0.7 mg/ml (Lis); in the third group with H2O2 200 µM and in the last group, the H2O2 200 µM was added after 1 h pre-treatment with Lisosan G 0.7 mg/ml (Lis+H2O2). We have used different concentrations of H2O2 (10–500 µM) and we chose 200 µM (good toxicity); we also used for Lisosan G different concentrations from 0.1 to 2.8 mg/ml and 0.7 mg/ml was the best concentration in terms of maximum protective effect and without toxicity. The cytotoxic effects compared with the vehicle-only controls, was also measured by lactate dehydrogenase assay (data not shown). We have also performed experiments in function of time of treatments of H2O2 and Lisosan G and we chose the time of treatment on the basis of the best results.
2.3 Enzymatic activities
After 24 h the end of treatment, the medium was removed and a collagenase solution was added. After 30 minutes, recovered cells were centrifuged (400× g) for 3 minutes at 4°C. The cell pellet was sonicated and used for the microsomal preparation [34]. Total protein concentration was determined by the method of Lowry [35]. NAD(P)H:quinone oxidoreductase (NQO1) activity was measured by the method of Bensen et al. [36]. Glutathione-S-transferase (GST) activity was quantified as previously described by Habig et al. [37] using 1-chloro-2,4-dinitrobenzene as substrate. Catalase activity was monitored following the H2O2 decomposition at 240 nm, as described by Cao and Li [38]. Heme oxygenase-1 (HO-1) activity was determined by the method of Naughton et al. [39]. Lactate dehydrogenase activity was assayed as previously described [40]. Reduced GSH was measured using the method previously described by Hissin and Hilf [41]. Lipid peroxidation was monitored by determining the production of malondialdehyde (MDA)-like products, quantified by the reaction with thiobarbituric acid (TBA) as reported by Stacey et al. [42].
2.4 RNA Extraction and cDNA synthesis
Total cellular RNA was extracted from primary rat hepatocytes 4 h after ITC treatment, using the RNeasy Mini Kit (Qiagen, Valencia, CA), following the supplied protocol. RNA was quantified using NanoDrop (Celbio, Mi, Italy); its purity and integrity were evaluated by checking the absorbance ratio at 260–280 nm and assessing the sharpness of 18S and 28S ribosomal RNA bands on agarose gel stained with ethidium bromide. Genomic DNA elimination and reverse transcription of total RNA were performed using QuantiTect Reverse Transcription Kit (Qiagen).
2.5 RT-PCR
Two microliters of cDNA were added to a PCR Master Mix (GoTaq Green Master Mix, Promega, Madison, WI) for the amplification reaction (various cycles) performed using for each transcript 400 nM of forward–reverse primers for heme oxygenase-1 (GenBank accession no. NM_012580.2), NQO1 (GenBank accession no. NM_017000.3), β-actin, as housekeeping gene, (GenBank accession no. NM_031144.2) and the annealing temperature indicated in Table 1. The DNA fragments were separated on ethidium bromide-stained 1% agarose gel and visualized by transillumination with ultraviolet light. Bands obtained from five independent rat experiments were quantified by an Image J software. The results have been normalized to β-actin levels and are expressed as percentages of control. Results are reported as means ± SD of cells from five independent experiments using five rats.
Table 1. Primer pairs, annealing temperature and product size for RT-PCR experiments.
| Gene | Forward (5′-3′) | Reverse (5′-3′) | Annealing T. (°C) | Product size (bp) |
| NQO1 | ACTCGGAGAACTTTCAGTACC | TTGGAGCAAAGTAGACTGGT | 59 | 492 |
| HO-1 | CAGGGTGACAGAAGAGGCTAAGAC | TGAGGACCCATCGCAGGAG | 66 | 229 |
| β-actin | CCCCATTGAACACGGATT | CATCTTTTCACGGTTGGCCTTA | 67 | 150 |
2.6 Preparation of nuclear fractions
Nuclear and cytosolic extracts were prepared by previously established methods [43]. Briefly, hepatocytes were washed twice with 1× phosphate buffer saline (PBS). Cells were then harvested in 1 ml of PBS and centrifuged at 800 g for 3 min at 4°C. The pellet was carefully resuspended in 200 µl of cold hypotonic buffer, consisting of 10 mM HEPES (pH 7.9), 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 µM dithiothreitol and complete protease inhibitor cocktail (Sigma antiprotease cocktail P8340). After addition of NP40 to a final concentration of 0.3%, the cells were vortexed and centrifuged at 800 g for 3 min at 4°C. The resulting nuclear pellet was resuspended in 30 µl of cold nuclear extraction buffer (20 mM HEPES (pH 7.9), 0.4 M NaCl, 1 mM EDTA, 1 mM EGTA, 1 µM dithiothreitol, 25% glycerol and protease inhibitors) and incubated on ice for 30 min. The nuclear extract was finally centrifuged at 15000 g for 15 min at 4°C. The supernatant containing nuclei proteins was aliquoted and stored at −30°C.
2.7 Immunoblot analysis
Nuclear and microsomal proteins from primary rat hepatocytes were separated according to Laemmli [44] on SDS-10% (v/v) 1.0 mm thick polyacrylamide gels and then electrophoretically transferred onto nitrocellulose membranes following the method of Towbin et al. [45]. Antibodies used were anti-Nrf2 (1: 1000, sc13032, Santa Cruz Biotechnology, Heidelberg, Germany), anti heme oxygenase-1 (1∶1000, sc-10789, Santa Cruz Biotechnology, Heidelberg, Germany), β-actin (1∶1000, sc-130657, Santa Cruz Biotecnology, Heidelberg, Germany), PARP-1 (1∶1000, sc-25780, Santa Cruz Biotecnology, Heidelberg, Germany), NF-kB (1∶1000, sc-7178, Santa Cruz Biotecnology, Heidelberg, Germany) and goat anti-rabbit (1∶2000 or 1∶5000). Immunoreactive proteins were visualized with a chemiluminescence reaction kit (EuroClone, Mi, Italy) and bands obtained from five independent rat experiments were electronically scanned and quantified by an Image J software.
2.8 Statistical analysis
Results are reported as means ± SD. Statistical significance was determined by Student's t-test for comparison between control and treated groups or the one-way ANOVA and the Dunnet tests. The statistical program used was Graphpad Prism 4. P value<0.05 was considered to be significant.
Results
3.1 Effect of Lisosan G on antioxidant and phase II enzymes
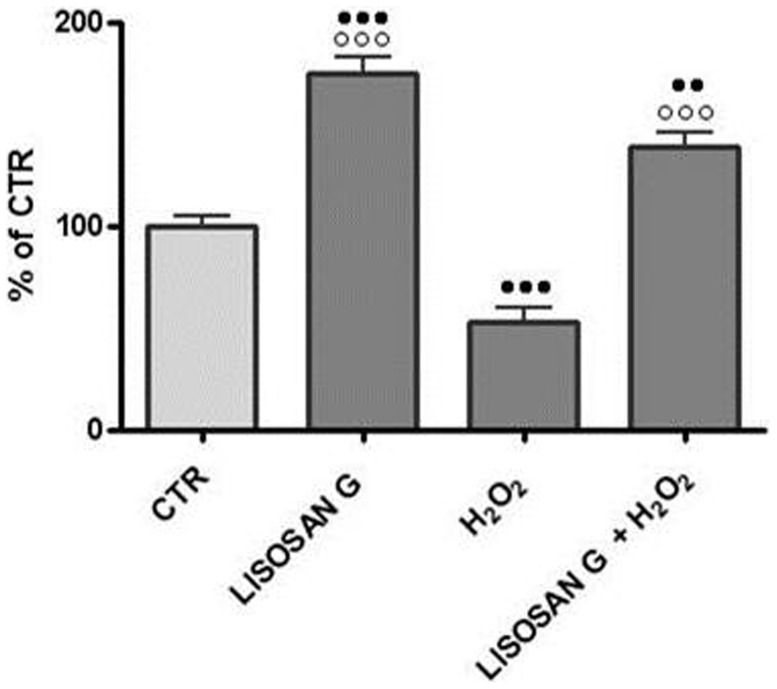
To verify the ability of Lisosan G to protect hepatocytes from damage caused by oxidative stress, we treated a first group of cells with hydrogen peroxide, while a second group was pretreated with Lisosan G prior to exposure to hydrogen peroxide. As shown in fig. 1, 200 µM H2O2 caused a decrease in reduced glutathione levels, whereas pretreatment with 0.7 mg/ml Lisosan G followed by H2O2, raised the amount of intracellular GSH to levels above control cells values. Lisosan G alone was able to increase reduced glutathione levels in hepatocytes treated for 24 h.
Figure 1. Effect of Lisosan G on GSH levels.
The activity was measured in homogenates of 24 h treated hepatocytes. Results are expressed as percentages of control activity (125.67±28.04 nmol/mg prot.). Mean ± SE of cells from five rats. ••Significantly different from control, p<0.01. ••• p<0.001. ○○○ Significantly different from H2O2, p<0.001.
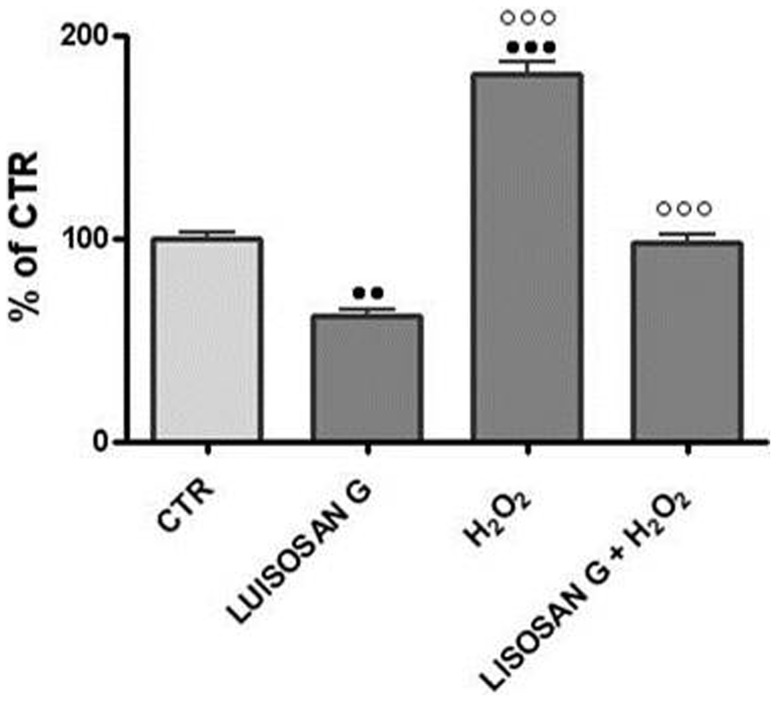
As a biomarker for lipid peroxidation, the concentration of malondialdehyde (MDA) in microsomes was measured in cells treated with H2O2 which had been pretreated for 1 h with Lisosan G. Fig. 2 shows the effect of preincubation with Lisosan G on H2O2-induced lipid peroxidation. Exposure of cells to H2O2 for 24 h significantly increased lipid peroxidation while preincubation of cells for 1 h prevented this from occurring. Also, Lisosan G on its own reduced lipid peroxidation levels compared to control (CTR) cells.
Figure 2. Effect of Lisosan G on lipid peroxidation.
The activity was measured in microsomes from 24 h treated hepatocytes. Results are expressed as percentages of control activity (32.82±2.07 mUA/mg prot.). Mean ± SE of cells from five rats. ••Significantly different from control, p<0.01. ••• p<0.001. ○○○Significantly different from H2O2, p<0.001.
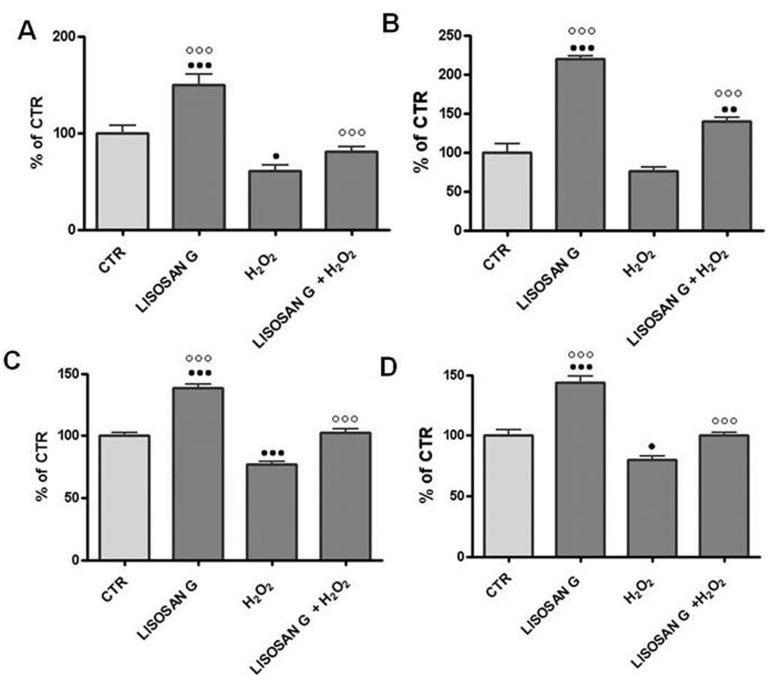
In the next set of experiments, the effect of Lisosan G on antioxidant and phase II enzyme activity was evaluated in microsomes and the cytosol of control hepatocytes and hepatocytes treated for 24 h. The enzymes chosen were NQO1, HO-1, glutathione-S-transferase (GST) and catalase. H2O2 treatment reduced NQO1 activity, but NQO1 activity remained high when a 1 hour pre-treatment with (fig. 3A) Lisosan G preceeded the H2O2 treatment. It is interesting to note that Lisosan G alone caused an increase in NQO1 activity (about 1.5 fold of control value). H2O2 also caused a decrease in HO-1 activity compared to control cells, but pretreatment with Lisosan G before H2O2 treatment elevated the activity above the level of CTR. HO-1 activity was significantly induced by Lisosan G treatment (about 2.2 fold of control value) (fig. 3B). As for GST, its activity decreased after the 24 h treatment with H2O2, but pretreatment with Lisosan G restored it nearly to control values (fig. 3C). This phase II activity was induced to about 1.2 times the control value by the Lisosan G treatment. We observed a 1,4 fold increase in catalase activity (fig. 3D), following treatment with Lisosan G and a significant decrease after the 24 h H2O2 treatment. However, the pretreatment with Lisosan G before exposure to H2O2, restored its activity to the same level of the CTR.
Figure 3. Effect of Lisosan G on activity of antioxidant and phase II drug-metabolizing enzymes.
NAD(P)H:quinone oxidoreductase (control value: 53.23±2.5 nmol/min/mg prot) (A); Heme oxygenase-1 (control value: 9.57±3.86 pmol/min/mg prot) (B); Glutathione-S-transferase (control value: 239.5±12.12 nmol/min/mg prot) (C); Catalase (control value: 147.41±11.1 nmol/min/mg prot) (D). Activities were measured in microsomes or cytosol of control cells (CTR) and 24 h treated cells. Results are expressed as percentages of control values. Mean ± SE of cells from five rats. • Significantly different from controls, p<0,05. ••p<0.01. ••• p<0.001. ○○○ Significantly different from H2O2, p<0.001.
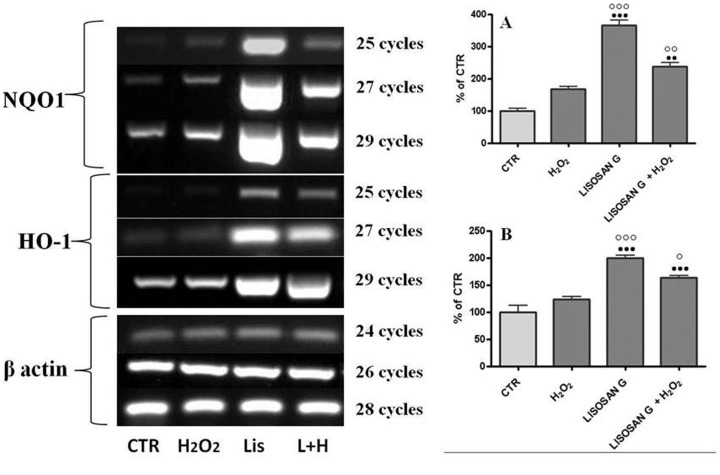
NQO1 and HO-1 were chosen to analysis of the response to Lisosan G at the transcriptional level. The expression of their transcripts in primary rat hepatocytes were analyzed by semi-quantitative RT-PCR, using the sets of primers listed in Table 1. Cells were treated with 0.7 mg/ml Lisosan G and 200 µM H2O2 for 4 h. As shown in fig. 4A, H2O2 didn't cause any significant change in NQO1 expression, but both the treatment with Lisosan G only and Lis+H2O2 increased its expression compared to the control levels.
Figure 4. A representative RT-PCR analysis.
NQO1 (A), HO-1 (B) genes performed with 25, 27 and 29 cycles in primary rat hepatocytes of control (CTR) and treated with Lisosan G or Lisosan G+H2O2. PCR products were separated by electrophoresis on agarose gels and stained with ethidium bromide. Quantitative representation of the RT-PCR analysis is reported in the histograms and the results have been normalized to β-actin levels and are expressed as percentages of control. Mean ± SE of cells from five independent experiments using five rats. •• Significantly different from controls, p<0.01. ••• p<0.001. ○ Significantly different from H2O2, p<0.05. ○○○ p<0.001.
A similar trend was found for HO-1 expression, although to different extents (Fig. 4B). For this gene, both Lisosan G alone and Lisosan G followed by H2O2 caused a rise in HO-1 expression but in this case, H2O2 alone also induced the gene.
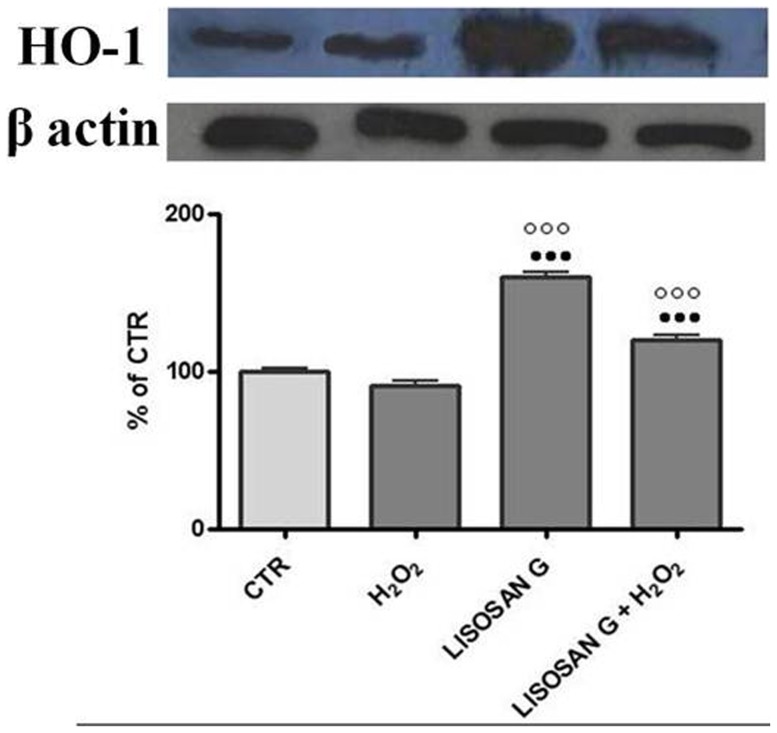
The effect of Lisosan G on heme oxygenase-1 was also assessed at the protein level by western blot (fig. 5). In microsomes from all hepatocytes treated with H2O2 no effect was noticed after 24 h treatment. On the contrary, the pre-treatment with Lisosan G before H2O2, raised the HO-1 protein level 1.6 fold above the CTR value, in agreement with results from the activity assays and transcription analysis.
Figure 5. Western blot analysis heme oxygenase-1 protein.
In microsomes (50 µg) of control (CTR) cells and cells treated for 24 h with Lisosan G or Lisosan G+H2O2. Microsomal samples were subjected to SDS-PAGE, electrophoretically transferred to a nitrocellulose membrane, and probed with polyclonal antibodies raised against rat HO-1. Densitometric analysis of the western blot data are shown in the histogram. The results have been normalized to β-actin levels and are expressed as percentages of control. Mean ± SE of cells from five independent experiments using five rats. ••• Significantly different from controls, p<0.001. ○○○ Significantly different from H2O2, p<0.001.
3.2 Effect of Lisosan G on Nrf2 and NF-kB
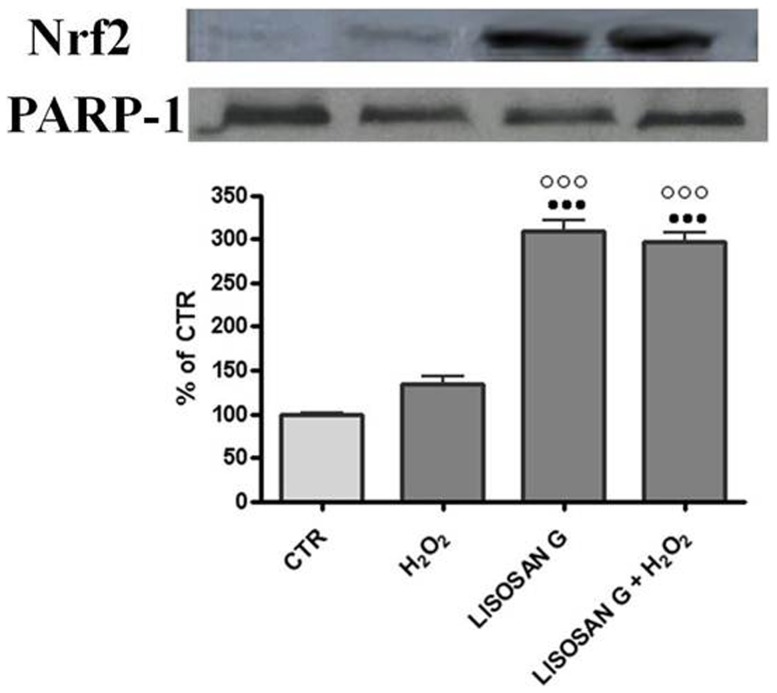
Since a major component of cellular defense against oxidative or electrophilic stress is the activation of the Nrf2/ARE signaling pathway [46], we verified whether Lisosan G was able to activate this important transcription factor in primary rat hepatocytes after 1 h of treatment. We analyzed by western blot nuclear fractions prepared from control and treated-cells (fig. 6). The protein band was faint in control and H2O2 nuclei. On the contrary, Nrf2 was clearly visible in the nuclear extracts of hepatocytes treated with Lisosan G and with Lisosan G+H2O2. The results have been normalized to PARP-1 levels.
Figure 6. Western Blotting analysis of Nrf2 in nuclear extracts.
Control cells (CTR) and cells treated with Lisosan G or Lisosan G+H2O2. Protein samples (30 µg) were subjected to SDS-PAGE, electrophoretically transferred to a nitrocellulose membrane, and probed with polyclonal antibodies raised against rat Nrf2. Densitometric analysis of the western blot data are shown in the histogram. The results have been normalized to PARP-1 levels and are expressed as percentages of control. Mean ± SE of cells from five independent experiments using five rats. ••• Significantly different from controls, p<0.001. ○○○ Significantly different from H2O2, p<0.001.
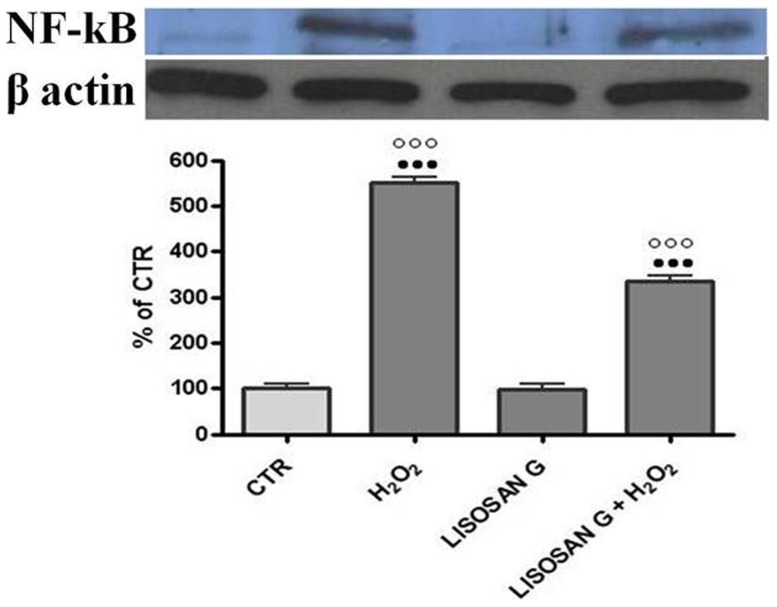
As cross talk between Nrf2 and NF-kB is an area of intense interest, we investigated whether Lisosan G treatment would prevent the NF-kB translocation to the nucleus, caused by hydrogen peroxide. We analyzed the nuclear fraction by western blot (fig. 7). NF-kB was distinctly induced in H2O2 treated hepatocytes. The signal in control cells was similar to that of cells treated with Lisosan G, both very weak. The signal from nuclear extracts of hepatocytes treated with Lisosan G+H2O2 is fainter than that obtained in hydrogen peroxide treated cells. The results have been normalized to β-actin levels.
Figure 7. Western Blotting analysis of NF-kB.
In nuclear extracts of control cells (CTR) and cells treated with Lisosan G or Lisosan G+H2O2. Protein samples (30 µg) were subjected to SDS-PAGE, electrophoretically transferred to a nitrocellulose membrane, and probed with polyclonal antibodies raised against rat NF-kB. Densitometric analysis of the western blot data are shown in the histogram. The results have been normalized to β-actin levels and are expressed as percentages of control. Mean ± SE of cells from five independent experiments using five rats. ••• Significantly different from controls, p<0.001. ○○○ Significantly different from H2O2, p<0.001.
Discussion
In the present study, we investigated whether a fermentated wheat powder, Lisosan G, was able to protect against H2O2 induced oxidative stress and whether it was able to modulate phase 2 enzymes by activating the Nrf2 protein and causing its translocation into the nucleus. We also analyzed its ability to prevent NF-kB nuclear translocation. We used sandwich cultures of primary rat hepatocytes, a unique in vitro system that preserves hepatic cytomorphology, as well as its drug metabolism, deposition and toxicity, to allow close resemblance with in vivo parameters [47]. When cultured between two layers of gelled collagen, hepatocytes also retain their ability to form intact canalicular networks and they retain their polarized excretory function [48].
Lisosan G defended cells against damage induced by H2O2, and increased NQO1, HO-1, GST and catalase activity, suggesting that Lisosan G has antioxidant properties and confirming earlier in vivo studies which had demonstrated the ability of Lisosan G to elevate GST, NQO1, catalase and GSH peroxidase activity [29], [30]. The induction of HO-1 represents an important event in adaptive cellular response to different oxidative stimuli [20]. Indeed, we detected an increase in HO-1 at the catalytic, transcriptional and protein level, both in response to Lisosan G treatment alone and to Lisosan G pretreatment followed by H2O2 induced oxidative stress. Several classes of phytochemicals such as phenols, flavonoids, isothiocyanates, organosulfurs, and indoles can induce detoxifying enzymes such as NQO1 and HO-1 [49]. Curcumin, for example, was observed to exert hepatoprotective properties against ethanol-induced oxidative stress, via dose- and time-dependent induction of HO-1, in primary rat hepatocytes [50]. We noticed that Lisosan G increased GSH levels both when used on its own and as pre-treatment before H2O2. GSH is a multifunctional intracellular non-enzymatic antioxidant and it is considered to be the major thiol-disulphide redox buffer of the cell [51]. The protective role of GSH against oxidative stress depends on the equilibrium between thiol reduced (GSH) and disulfide-oxidized forms [52]. Cellular GSH depletion has been found to be associated with decreased cell proliferation in vascular endothelial cells [53]. Since the level of GSH is an important factor in the protection of cells, we believe that Lisosan G has excellent cytoprotective ability.
NQO1, HO-1 and GST gene expression is regulated by several key transcriptional factors located in the upstream region [20]. We found that in rat hepatocytes the induction of these enzymes occurred through the activation of the Nrf2 protein and its subsequent translocation into the nucleus. This mechanism has also been observed in hepatic cell lines in previous studies [54]. In this paper, we have shown by immunoblotting that this fermented wheat powder was able to activate Nrf2 after just 1 h of treatment.
It is important to note that the Nrf2 and NF-kB signaling pathways interface at several points to control the transcription or function of downstream target proteins. We therefore tried to understand if Lisosan G was able to regulate NF-kB. It is known that released NF-kB translocates into the nucleus where it regulates the transcription of genes for chemokines, cytokines, immunoreceptors, cell-adhesion molecules, growth factors, tumor necrosis factor α (TNFα), inducible NOS (iNOS), interleukin-1 (IL-1), interleukin-6 (IL-6) and cyclooxygenase (COX-2) [55]. Some phytochemicals can prevent the activation of NF-kB. Sulforaphane, for exemple, reduces the DNA binding of NF-kB in Raw 264.7 macrophages without affecting IkB [56]. Several chemopreventive agents trigger Nrf2 signaling with a concomitant repression of NF-kB and its target genes [22]. Chalcone (a flavonoid) has been shown to induce Nrf2 while inhibiting the activation of NF-kB in endothelial cells [57]. 3H-1,2-Dithiole-3-thione reduces the nuclear translocation and DNA binding of NF-kB, and also induces changes in phosphorilation of IkB in rat hepatocytes [58]. Our results show that the hydrogen peroxide treatment increases the amount of NF-kB protein in nucleus and the pretreatment with Lisosan G decreased it.
The ability of Lisosan G to induce phase II enzymes via Nrf2 and inhibit NF-kB activation, may be linked to its composition [29]. Lisosan G contains has linoleic (20: 4n-6) and linolenic (18: 3n-3) acids, which are defined “essential” fatty acids since they are not synthesized in the human body and are mostly obtained from diet [59]. They appear to play an important role in the prevention and treatment of a number of diseases (coronary disease, arthritis, inflammatory disorders) [60]. Pal and Ghosh [61] have shown that activity of antioxidant enzymes such as catalase, superoxide dismutase, glutathione peroxidase in liver and kidney decrease significantly in response to oxidative stress generated by methylmercury (MeHg); but histopathology of liver and kidney cells showed that administration of α-linolenic acid restored, in rat, all the altered parameters and also reduced lipid peroxidation. A recent paper showed a consistently presence of flavonoids and phenolic components in Lisosan G [62].
Lisosan G also contains minerals such as iron, zinc, copper and it is rich in vitamins (B1, B2, B6 and E). Vitamin E is an important natural antioxidant, and its most common and biologically active form is α-tocopherol. Unpublished results have shown that α-lipoic acid (ALA) is among the various components in Lisosan G, and that it is present in a concentration of 66 mg/kg.
ALA is a thiol antioxidant found in vegetables, including broccoli, spinach and tomatoes [63]. In human leukemia HL-60 cells and neuroblastoma SH-SY5Y cells, ALA upregulates NQO1 gene transcription [64], [65]. In addition, ALA, as sulforaphane, increases phase II protein levels in Clone 9 cells [66]. Ogborne et al. [67], have shown that in human monocytic cells, ALA induces HO-1 expression via Nrf2. It is interesting to note that ALA is an inhibitor of NF-kB [63] and it inhibits the NF-kB-dependent expression of metalloproteinase-9 in vitro [68]. We can suppose that components of Lisosan G, such as linoleic and linolenic acids, α-lipoic acid, flavonoids and phenols, which are known to cross the membrane, could be responsible of activation of NRF2 and of the inhibition of NF-KB in the primary hepatocyte cells.
This study has established for the first time in primary rat hepatocytes that Lisosan G can modulate phase 2 enzymes through the activation of Nrf2 pathway. We have demonstrated the Lisosan G decreases the H2O2-induced translocation of NF-kB to the nucleus. It seems likely that the beneficial effects of Lisosan G derive from its high phytochemical and vitamin content.
Funding Statement
No current external funding sources for this study.
References
- 1. Halliwell B, Aeschbach R, Löliger J, Aruoma OI (1995) The characterization of antioxidants. Food Chem Toxicol 33: 601–617. [DOI] [PubMed] [Google Scholar]
- 2. Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44–84. [DOI] [PubMed] [Google Scholar]
- 3. Baublis AJ, Lu C, Clydesdale FM, Decker EA (2000) Potential of wheat-based breakfast cereals as a source of dietary antioxidants. Journal of the American College of Nutrition 19: 308S–311S. [DOI] [PubMed] [Google Scholar]
- 4. Weisburger JH (1999) Mechanisms of action of antioxidants as exemplified in vegetables, tomatoes and tea. Food and chemical toxicology 37: 943–948. [DOI] [PubMed] [Google Scholar]
- 5. Oliveira SQ, Dal-Pizzol F, Gosmann G, Guillaume D, Moreira JC, et al. (2003) Antioxidant activity of Bacchairs articulata extracts: isolation of a new compound with antioxidant activity. Free Radic Res 37: 555–559. [DOI] [PubMed] [Google Scholar]
- 6. Payen L, Courtois A, Loewert K, Guillouzo A, Fardel O (2001) Reactive oxygen species-related induction of multidrug resistance-associated protein 2 expression in primary hepatocytes exposed to sulforaphane. Biochem Biophys Res Comm 282: 257–263. [DOI] [PubMed] [Google Scholar]
- 7. Venn B, Thies F, O'Neil C (2012) Whole Grains, Legumes, and Health. Journal of Nutrition and Metabolism 2012: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slavin JL, Jacobs D, Marquart L, Wiemer K (2001) The role of whole grains in disease prevention. Journal of the American Dietetic Association 101: 780–785. [DOI] [PubMed] [Google Scholar]
- 9. Slavin J (2004) Whole grains and human health. Nutrition research reviews 17: 99–110. [DOI] [PubMed] [Google Scholar]
- 10. Gaesser GA (2007) Carbohydrate quantity and quality in relation to body mass index. Journal of the American Dietetic Association 107: 1768–1780. [DOI] [PubMed] [Google Scholar]
- 11. Steffen LM, Jacobs DR Jr, Stevens J, Shahar E, Carithers T, et al. (2003) Associations of Whole-grain, Refined-grain, and Fruit and Vegetable Consumption with Risks of All-cause Mortality and Incident Coronary Artery Disease and Ischemic Stroke: The Atherosclerosis Risk in Communities (ARIC) Study. The American Journal of Clinical Nutrition 78: 383–390. [DOI] [PubMed] [Google Scholar]
- 12. McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF (2002) Whole-grain Intake Is Favorably Associated with Metabolic Risk Factors for Type 2 Diabetes and Cardiovascular Disease in the Framingham Offspring Study. The American Journal of Clinical Nutrition 76: 390–398. [DOI] [PubMed] [Google Scholar]
- 13. Flight I, Clifton P (2006) Cereal grains and legumes in the prevention of coronary heart disease and stroke: a review of the literature. European journal of clinical nutrition 60: 1145–1159. [DOI] [PubMed] [Google Scholar]
- 14. Randi G, Edefonti V, Ferraroni M, La Vecchia C, Decarli A (2010) Dietary patterns and the risk of colorectal cancer and adenomas. Nutr Rev 68: 389–408. [DOI] [PubMed] [Google Scholar]
- 15. Xiang W, Qiah-hua Z, Ke X (2009) Are isothiocyanates potential anti-cancer drugs? Acta Pharmacol Sin 30: 501–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, et al. (1999) Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev 13: 76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Alam J, Wicks C, Stewart D, Gong P, Touchard C, et al. (2000) Mechanism of heme oxygenase-1 gene activation by cadmium in MCF-7 mammary epithelial cells. Role of p38 kinase and Nrf2 transcription factor. J Biol Chem 275: 27694–702. [DOI] [PubMed] [Google Scholar]
- 18. Buckley BJ, Marshall ZM, Whorton AR (2003) Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem Biophys Res Commun 307: 973–979. [DOI] [PubMed] [Google Scholar]
- 19. Lee SH, Geom SS, Ji YK, Jin XY, Kim HD, et al. (2006) Heme oxygenase 1 mediates anti-inflammatory effects of 2′,4′,6′-tris(methoxymethoxy) chalcone. European journal of pharmacology 532: 178–186. [DOI] [PubMed] [Google Scholar]
- 20. Prawan A, Saw CL, Khor TO, Keum YS, Yu S, et al. (2009) Anti-NF-kappaB and anti-inflammatory activities of synthetic isothiocyanates: effect of chemical structures and cellular signaling. Chemico-biological interactions 179: 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Saw CL, Qing W, Ah-Ng TK (2010) Anti-cancer and potential chemopreventive actions of ginseng by activating Nrf2 (NFE2L2) anti-oxidative stress/anti-inflammatory pathways. Chinese Medicine 5: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wakabayashi N, Slocum SL, Skoko JJ, Shin S, Kensler TW (2010) When NRF2 talks, who's listening? Antioxidants & redox signaling 13: 1649–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Curtin JF, Donovan M, Cotter TG (2002) Regulation and measurement of oxidative stress in apoptosis. Journal of immunological methods 265: 49–72. [DOI] [PubMed] [Google Scholar]
- 24. Storz P (2006) Reactive oxygen species-mediated mitochondria-to-nucleus signaling: a key to aging and radical-caused diseases. Science's STKE 332: re3. [DOI] [PubMed] [Google Scholar]
- 25. Sakon S, Xue X, Takekawa M, Sasazuki T, Okazaki T, et al. (2003) NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. The EMBO journal 22: 3898–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kamata H, Honda S, Maeda S, Chang L, Hirata H, et al. (2005) Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120: 649–661. [DOI] [PubMed] [Google Scholar]
- 27. Karin M (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441: 431–436. [DOI] [PubMed] [Google Scholar]
- 28. Schreck R, Baeuerle PA (1991) A role for oxygen radicals as second messengers. Trends Cell Biol 1: 39–42. [DOI] [PubMed] [Google Scholar]
- 29. Longo V, Chirulli V, Gervasi PG, Nencioni S, Pellegrini M (2007) Lisosan G, a powder of grain, does not interfere with the drug metabolizing enzymes and has a protective role on carbon tetrachloride-induced hepatotoxicity. Biotechnology letters 29: 1155–1159. [DOI] [PubMed] [Google Scholar]
- 30. Longo V, Gervasi PG, Lubrano V (2011) Cisplatin induced toxicity in rat tissues: the protective effect of Lisosan G. Food and chemical toxicology 49: 233–237. [DOI] [PubMed] [Google Scholar]
- 31. Lubrano V, Baldi S, Napoli D, Longo V (2012) Beneficial effect of Lisosan G on cultured human microvascular endothelial cells exposed to oxidised low density lipoprotein. The Indian journal of medical research 136: 82–88. [PMC free article] [PubMed] [Google Scholar]
- 32. De Smet K, Beken S, Vanhaecke T, Pauwels M, Vercruysse A, et al. (1998) Isolation of rat hepatocytes. Methods Mol Biol 107: 295–301. [DOI] [PubMed] [Google Scholar]
- 33.Beken S, Vanhaecke T, De Smet K, Pauwels M, Vercruysse A, et al.. (1998) Collagen-gel cultures of rat hepatocytes. In: Phillips, I.R., Shephard, E.A., Humana Press (Eds.), Cytochrome P450 protocols. E-Publishing Inc., New York, vol. 107 pp. 303–309.
- 34. Jagow R, Kampffmeyer H, Kinese M (1965) The Preparation of Microsomes. Naunyn-Schmiedebergs Arch Pharmak 251: 73–87. [DOI] [PubMed] [Google Scholar]
- 35. Lowry OH, Rosenbrough NJ, Farr Al, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265–276. [PubMed] [Google Scholar]
- 36. Bensen AM, Hunkeler MS, Talalay P (1980) Increase of NAD(P)H: quinone reductase by dietary antioxidants: possible role in protection against carcinogenesis and toxicity. Proc Natl Acad Sci 77: 5216–5220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-Transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem 249: 7130–7139. [PubMed] [Google Scholar]
- 38. Cao Z, Li Y (2002) Chemical induction of cellular antioxidants affords marked protection against oxidative injury in vascular smooth muscle cells. Biochem Biophys Res Commun 292: 50–57. [DOI] [PubMed] [Google Scholar]
- 39. Naughton P, Foresti R, Bains SK, Hoque M, Green CJ, et al. (2002) Induction of heme oxygenase 1 by nitrosative stress. J Biol Chem 277: 40666–40674. [DOI] [PubMed] [Google Scholar]
- 40. Deker T, Lohmann-Matthes M (1988) A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Imunology Met 15: 61–69. [DOI] [PubMed] [Google Scholar]
- 41. Hissin PJ, Hilf R (1976) A fluorometric method for determination of oxidized and reduced glutathione in tissues. Analytical biochemistry 74: 214–226. [DOI] [PubMed] [Google Scholar]
- 42. Stacey NH, Cantilena LR, Klaassen CD (1980) Cadmiumtoxicity and lipidperoxidation in isolatedrathepatocytes. Toxicol Appl Pharmacol 53: 470–480. [DOI] [PubMed] [Google Scholar]
- 43. Balogun E, Hoque M, Gong P, Killeen E, Green CJ, et al. (2003) Curcumin activates the heme oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J 371: 887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685. [DOI] [PubMed] [Google Scholar]
- 45. Towbin HT, Staehelin P, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gel to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci 76: 4350–4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen T, Sherratt PJ, Pickett CB (2003) Regulatory mechanisms controlling gene expression. Mediated by the antioxidant response element. Annu Rev Pharmacol Toxicol 43: 233–60. [DOI] [PubMed] [Google Scholar]
- 47. Swift B, Pfeifer ND, Brower KLR (2010) Sandwich-cultured hepatocytes: an in vitro model to evaluate hepatobiliary transporter-based drug interactions and hepatotoxicity. Drug Metab Rev 42: 446–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Dunn JCY, Tompkins RG, Yarmush ML (1991) Long-term in vitro function of adult hepatocytes in a collagen sandwich configuration. Biotechnol Prog 7: 237–245. [DOI] [PubMed] [Google Scholar]
- 49. Chen C, Kong AN (2004) Dietary chemopreventive compounds and ARE/EpRE signaling. Free Radic Biol Med 36: 1505–1516. [DOI] [PubMed] [Google Scholar]
- 50. Bao W, Li K, Rong S, Yao P, Hao L, et al. (2010) Curcumin alleviates ethanol-induced hepatocytes oxidative damage involving heme oxygenase-1 induction. Journal of ethnopharmacology 128: 549–553. [DOI] [PubMed] [Google Scholar]
- 51. Masella R, Di Benedetto R, Vari R, Filesi C, Giovannini C (2005) Novel mechanisms of natural antioxidant compounds in biological system: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem 16: 577–586. [DOI] [PubMed] [Google Scholar]
- 52. Pastore A, Federici G, Bertini E, Piemonte F (2003) Analysis of glutathione: implication in redox and detoxification. Clinica chimica acta 333: 19–39. [DOI] [PubMed] [Google Scholar]
- 53. Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M (2006) Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chemico-biological interactions 160: 1–40. [DOI] [PubMed] [Google Scholar]
- 54. Jeong WS, Keum YS, Chen C, Jain MR, Shen G, et al. (2005) Differential Expression and Stability of Endogenous Nuclear Factor E2-related Factor 2 (Nrf2) by Natural Chemopreventive Compounds in HepG2 Human Hepatoma Cells. J Biochem and Mol Biol 38: 167–176. [DOI] [PubMed] [Google Scholar]
- 55. Gilmore TD (2006) Introduction to NF-kB: players, pathways, perspectives. Oncogene 25: 6680–6684. [DOI] [PubMed] [Google Scholar]
- 56. Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhäuser C (2001) Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem 276: 32008–32015. [DOI] [PubMed] [Google Scholar]
- 57. Liu YC, Hsieh CW, Wu CC, Wung BS (2007) Chalcone inhibits the activation of NF-kappaB and STAT3 in endothelial cells via endogenous electrophile. Life Sci 80: 1420–1430. [DOI] [PubMed] [Google Scholar]
- 58. Karuri AR, Huang Y, Bodreddigari S, Sutter CH, Roebuck BD, et al. (2006) 3H-1,2-dithiole-3-thione targets nuclear factor kappaB to block expression of inducible nitric-oxide synthase, prevents hypotension, and improves survival in endotoxemic rats. The Journal of pharmacology and experimental therapeutics 317: 61–67. [DOI] [PubMed] [Google Scholar]
- 59. Russo GL (2009) Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochemical pharmacology 77: 937–946. [DOI] [PubMed] [Google Scholar]
- 60. Kuhnt K, Degen C, Jaudszus A, Jahreis G (2012) Searching for health beneficial n-3 and n-6 fatty acids in plant seeds. European journal of lipid science and technology 114: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Pal M, Ghosh M (2012) Studies on comparative efficacy of α-linolenic acid and α-eleostearic acid on prevention of organic mercury-induced oxidative stress in kidney and liver of rat. Food and chemical toxicology 50: 1066–1072. [DOI] [PubMed] [Google Scholar]
- 62. Packer L, Witt EH, Tritschler HJ (1995) Alpha-Lipoic acid as a biological antioxidant. Free radical biology & medicine 19: 227–250. [DOI] [PubMed] [Google Scholar]
- 63. Laus MN, Denoth F, Ciardi M, Giorgetti L, Pastore D, et al. (2013) The antioxidant-rich food supplement Lisosan G induces reversion of hepatic steatosis. Medycyna Weterynaryjna 69 4: 235–240. [Google Scholar]
- 64. Jia Z, Hallur S, Zhu H, Misra HP (2008) Potent Upregulation of Glutathione and NAD(P)H:Quinone Oxidoreductase 1 by Alpha-lipoic Acid in Human Neuroblastoma SH-SY5Y Cells: Protection Against Neurotoxicant-elicited Cytotoxicity. Neurochemical Research 33: 790–800. [DOI] [PubMed] [Google Scholar]
- 65. Elangovan S, Hsieh TC (2008) Control of cellular redox status and upregulation of quinone reductase NQO1 via Nrf2 activation by alpha-lipoic acid in human leukemia HL-60 cells. International journal of oncology 33: 833–838. [PubMed] [Google Scholar]
- 66. Lii CK, Liu KL, Cheng YP, Lin AH, Chen HW, et al. (2010) Sulforaphane and α-Lipoic Acid Upregulate the Expression of the π Class of Glutathione S-Transferase Through c-Jun and Nrf2 Activation. The Journal of Nutrition 140: 885–892. [DOI] [PubMed] [Google Scholar]
- 67. Ogborne RM, Rushworth SA, O'Connell MA (2005) α-Lipoic Acid–Induced Heme Oxygenase-1 Expression Is Mediated by Nuclear Factor Erythroid 2-Related Factor 2 and P38 Mitogen-Activated Protein Kinase in Human Monocytic Cells. Arteriosclerosis, Thrombosis, and Vascular Biology 25: 2100–2105. [DOI] [PubMed] [Google Scholar]
- 68. Kim HS, Kim HJ, Park KG, Kim YN, Kwon TK, et al. (2007) Alpha-lipoic acid inhibits matrix metalloproteinase-9 expression by inhibiting NF-kappaB transcriptional activity. Experimental & molecular medicine 39: 106–113. [DOI] [PubMed] [Google Scholar]