Abstract
The multikinase inhibitor, sorafenib (Nexavar®, BAY43-9006), which inhibits both the Raf/MEK/ERK pathway and several receptor tyrosine kinases (RTKs), has shown significantly therapeutic benefits in advanced hepatocellular carcinoma (HCC). However, not all HCC patients respond to sorafenib well and new therapeutic strategies to optimize the efficacy of sorafenib are urgently required. Overexpression of breast cancer resistance protein (BCRP/ABCG2) mediates the drug-efflux of several tyrosine kinase inhibitors (TKIs) to attenuate their efficacy. This study aimed to investigate the role of BCRP/ABCG2 in the sensitivity of HCC to sorafenib. Our data showed that BCRP/ABCG2 mediated the efflux of sorafenib. Co-treatment with a BCRP/ABCG2 inhibitor greatly augmented the cytotoxicity of sorafenib in HCC cells. Similar results were also achieved by the competitive inhibitor of BCRP/ABCG2, gefitinib, in combination with sorafenib. These results suggest not only that BCRP/ABCG2 is a potential predictor for the sorafenib sensitivity in HCC, but also that blockage of BCRP/ABCG2 may be a potential strategy to increase the response of HCC cells to sorafenib.
Background
Hepatocellular carcinoma (HCC) is a leading cause of cancer mortality in the world, especially in Asia[1], [2]. Because there is no obvious symptom during the early stage, HCC patients are often diagnosed at the advanced stage, and the advanced HCC is recognized as a difficult-to-treat disease[3], [4], [5], [6]. The multikinase inhibitor, sorafenib (Nexavar®, BAY43-9006) is now the only drug for the standard treatment of advanced HCC[7], [8]. However, HCC patients show different responses to this drug[9], [10], and the underlying mechanism remains unclear.
ATP-binding cassette (ABC) transporters mediate drug efflux to protect cells from xenobiotic- and toxin-induced damages under physiological conditions. Overexpression of ABC transporters is frequently observed in cancer patients who are unresponsive to chemotherapy, and has been proposed to account for the multidrug resistance (MDR) of cancer cells[11], [12]. Inhibition of ABC transporter activity is a potential strategy to overcome the chemoresistance. Three ABC transporters, including P-glycoprotein (P-gp, MDR1, ABCB1), multidrug resistance protein 1 (MRP1, ABCC1), and breast cancer resistance protein (BCRP, MXR, ABCG2), play important roles in most cases of MDR in cancer cells[13], [14]. In the past few years, small molecule tyrosine kinase inhibitors (TKIs) have been suggested to be potential substrates of ABC transporters and combinatory usage of these TKIs as competitive inhibitors is able to reduce ABC transporter-mediated MDR[15], [16], [17], [18]. Among these transporters, BCRP/ABCG2 overexpression was found to confer resistance to gefitinib, the epidermal growth factor receptor (EGFR) TKI, suggesting the association between ABC transporter expression and TKI resistance[19], [20], [21], [22].
BCRP/ABCG2 and MDR1 are two major regulators controlling the brain distribution of anti-cancer drugs. It has been reported that BCRP/ABCG2 plays a significant role in restricting the distribution of sorafenib across the blood-brain barrier (BBB) to the brain[24], [26], [27]. In comparison to MDR1, BCRP/ABCG2 showed higher activity in the transportation of sorafenib in vitro [23], [24], [25]. Although BCRP/ABCG2 and MDR1 have been viewed as the two most important determinants for MDR in response to chemotherapy in HCC[28], [29], however, it remains unclear whether BCRP/ABCG2 expression is associated with HCC sensitivity to sorafenib. Therefore, this study aimed to investigate the causal relationship between BCRP/ABCG2 expression and sorafenib sensitivity, and to examine whether BCRP/ABCG2 inhibition is a potential strategy to sensitize HCC cells to sorafenib.
Methods
Cell lines and reagents
Hep3B and HepG2 HCC cell lines were maintained in Dulbecco's modified Eagle's medium/F12 medium supplemented with 10% fetal bovine serum (Logan, UT). Huh-7 HCC cells were maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum. Sorafenib was kindly provided by Dr. Chao-Ming Hung (E-Da Hospital, Kaohsiung, TW) and was dissolved in dimethyl sulfoxide (DMSO) as stock concentration at 100 µM. Chrysin was purchased from Sigma-Aldrich (St. Louis, MO). Gefitinib was purchased from LC laboratory. The BCRP/ABCG2 protein level was detected by using an anti-BCRP antibody from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). The anti-phospho-ERK1/2-T202/Y204 antibody (p-ERK1/2) and anti-cleaved PARP antibody from Cell Signaling (Danvers, MA) were used. Turbofect™ siRNA transfection reagent was purchased from Fermentas (Glen Burnie, MD). TransIT-2020 transfection reagent was purchased from Mirus Bio LLC (Madison, WI).
Cell viability assay
In vitro cell viability assays were conducted by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay, crystal violet staining or bright-field imaging. For the MTT assay, cells (5×103 cells per well) were seeded in 96-well plates overnight. Cells were subjected to pre-treatment with BCRP/ABCG2 inhibitors, followed by sorafenib treatment. Three days later, relative cell amounts were determined by adding 1 µg/ml MTT to each well. Then, the medium was removed after 4-hour incubation. Formazan solubilized in 100 µl DMSO was added to each well, and the absorbance was measured at 570 nm. For the crystal violet staining assay, HCC cells, subjected to the indicated experiments, were re-seeded (1×105 cells per well) in 6-well plates overnight, followed by sorafenib treatment. Approximately one week later, relative cell amounts were determined by crystal violet staining. Briefly, cells were washed with 1X PBS once, followed by fixation and staining with 1% crystal violet dissolved in 30% ethanol for 15–30 minutes at room temperature. Then, cells were washed with tap water to eliminate background interference.
Drug-efflux assay
Cells were seeded in 6-cm dish and incubated overnight. The next day, cells were treated with 5 µM sorafenib for 1 h. Then, medium was refreshed without sorafenib, followed by recovery. Whole cell lysates were harvested at the indicated time points of recovery and subjected to Western blot analysis. The reversal from sorafenib inhibition during the recovery period was assessed by detecting the level of ERK1/2 activation with an anti-p-ERK1/2 antibody.
Transfection assay
Transfections of small-interfering RNA (siRNA) and DNA were conducted by using Turbofect™ siRNA transfection reagent and TransIT-2020 transfection reagent, respectively. According to the manufacturer's instruction, cells with 60–70% confluence were transfected with siRNA or DNA, followed by the indicated experiments.
Construction of expression vector
The BCRP/ABCG2 gene was obtained from A549 cells by using the forward primer (5′ BamHI-AAAGGATCCATGTCTTCCAGTAATGTCGA 3′) and the reverse primer (5′ CCCGAATTCTTAAGAATATTTTTTAAGAAATAA-EcoRI 3′). BCRP/ABCG2 gene was subsequently cloned into the pCMV-Tag2B expression vector by using the BamHI and EcoRI cutting sites. The sequence of the BCRP/ABCG2 gene was confirmed by sequencing.
Statistical analysis
The statistical analysis was performed by Student's t test. */#, p<0.05; **/##, p<0.01; ***/###, p<0.001 mean as compared to control groups.
Results
BCRP/ABCG2 was a determinant for the sensitivity of HCC cells to sorafenib treatment
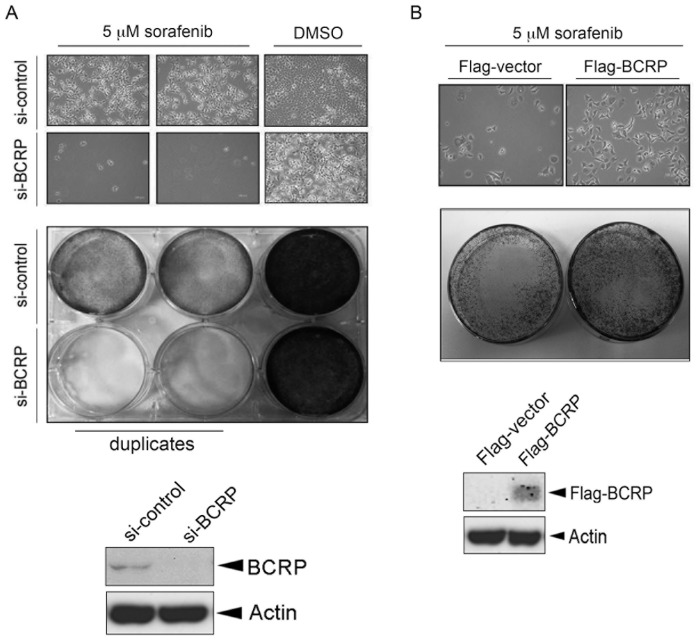
To address whether BCRP/ABCG2 expression is associated with the sensitivity of HCC to sorafenib, BCRP siRNA was employed. Hep3B HCC cells were transfected with control or BCRP siRNA for 24 hours followed by treatment with sorafenib. The effect of sorafenib on cell viability was determined by both bright-field imaging and 1% crystal violet staining. The decreased expression of BCRP/ABCG2 in the cells was confirmed by Western blot analysis, and was found to render Hep3B cells more sensitive to sorafenib (Figure 1A, bright-field image in the top panel; crystal violet staining in the middle panel). Similar result was also observed in another HepG2 HCC cell line (Figure S1 in File S1). In contrast, sorafenib-induced cytotoxicity was less obvious when BCRP/ABCG2 was overexpressed in Hep3B cells (Figure 1B). Taken together, these results suggest BCRP/ABCG2 as an important determinant for the sensitivity of HCC cells to sorafenib.
Figure 1. BCRP/ABCG2 is involved in the determination of sorafenib sensitivity in Hep3B hepatocellular carcinoma (HCC) cells.
(A–B) Hep3B cells were either knocked down with BCRP siRNA (A) or transfected with Flag-BCRP expression vector (B). One day later, cells were re-seeded at the same density, followed by treatment of 5 µM sorafenib. Three-to five days later, cell viability was measured by using bright-field imaging (top panel) and crystal violet staining assay (middle panel). BCRP/ABCG2 expression was detected by Western blot analysis (bottom panel).
BCRP/ABCG2-mediated sorafenib efflux was observed in HCC cells
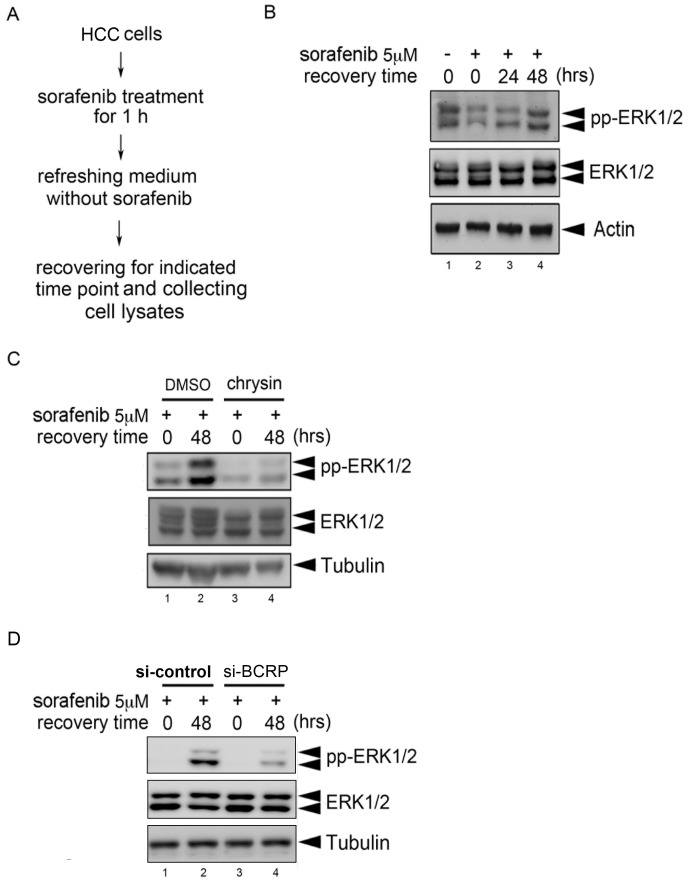
We further examined whether the drug-efflux effect of BCRP/ABCG2 affects the anti-tumor effect of sorafenib in HCC cells. Therefore, a drug-efflux assay was designed and performed. Briefly, Hep3B cells were treated with sorafenib for 1 hr followed by medium refreshment without sorafenib and further incubation for 24 or 48 hours to recover cells from the inhibition by sorafenib. Whole cell lysates were then collected at the indicated time points (Figure 2A). Because sorafenib is an inhibitor of Raf-MEK1-ERK1/2 pathway, the phosphorylation level of ERK1/2 was used as an indicator of sorafenib activity. As shown in Figure 2B, inhibition of ERK1/2 phosphorylation by sorafenib (lane 2) was gradually recovered in a time-dependent manner (lanes 3–4), suggesting the existence of sorafenib efflux in Hep3B cells. To determine whether this drug-efflux effect was mediated by BCRP/ABCG2, the BCRP/ABCG2 inhibitor, chrysin, was used. The results showed that the recovery of ERK1/2 phosphorylation from inhibition by sorafenib was observed in Hep3B cells treated with vehicle DMSO (Figure 2C, compared lane 2 with lane 1). However, this recovery was not observed when BCRP/ABCG2 activity was blocked by chrysin (Figure 2C, compared lane 4 with lane 3). Furthermore, our data showed that chrysin itself did not directly inhibit basal ERK1/2 phosphorylation in HCC cells (data not shown). Therefore, it excluded the possibility that chrysin prevents the recovery of ERK activity from sorafenib withdrawal is due to the directly inhibitory effect of chrysin on ERK activation. Similar results were also obtained in HepG2 and Huh-7 HCC cell lines (Figures S2A-B in File S1). To strengthen the importance of BCRP/ABCG2 in this regulation, the BCRP siRNA was used. As shown in Figure 2D, the recovery induction of ERK1/2 phosphorylation from inhibition by sorafenib was dramatically attenuated when the BCRP/ABCG2 expression in HepG2 cells was suppressed by BCRP siRNA (compared lanes 3–4 with lanes 1–2). Consistently, the similar result was also observed in Huh-7 cells (Figure S2C in File S1). Collectively, these results suggest that the anti-cancer activity of sorafenib was attenuated at least in part by BCRP/ABCG2-mediated drug efflux in HCC cells.
Figure 2. BCRP/ABCG2 mediates the drug efflux of sorafenib in HCC cells.
(A) The experimental procedure of the drug-efflux assay was illustrated. (B) Hep3B cells were subjected to drug-efflux assay. The expression levels of phosphorylated ERK1/2, ERK1/2 and Tubulin were examined by Western blot analysis. (C) Hep3B cells were pre-treated with 25 µM chrysin for 1 h, followed by the drug-efflux assay. The expression levels of phosphorylated ERK1/2, ERK1/2 and Tubulin were examined by Western blot analysis. (D) HepG2 cells were transiently transfected with either control siRNA or BCRP siRNA for 4 days, followed by the drug-efflux assay. The expression levels of phosphorylated ERK1/2, ERK1/2 and Tubulin were examined by Western blot analysis.
BCRP/ABCG2 inhibitors augmented the anti-cancer activity of sorafenib in HCC cells
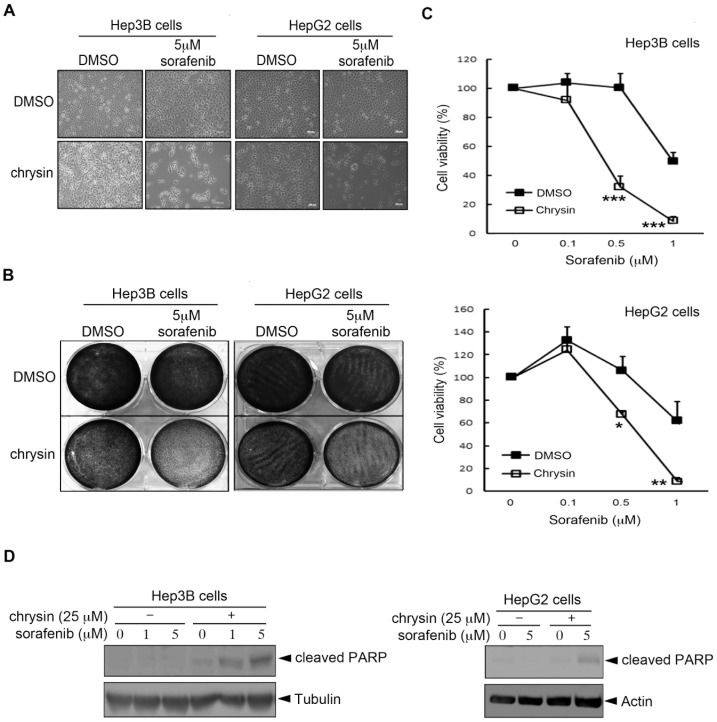
Since our results indicated that BCRP/ABCG2-mediated drug efflux reduced the anti-tumor activity of sorafenib in HCC cells (Figures 1 and 2), we next addressed whether combination with BCRP/ABCG2 inhibitors is a potential strategy to increase the sensitivity of HCC cells to sorafenib. Indeed, our results showed that co-treatment with chrysin synergized the sorafenib-mediated inhibition of cellular viability in both Hep3B and HepG2 HCC cells (Figure 3A). In addition to the bright-field imaging assay, this synergistic effect of chrysin was observed by crystal violet staining (Figure 3B) and MTT assay (Figure 3C). Similar results were also obtained in Huh-7 HCC cells (Figure S3 in File S1). Furthermore, sorafenib only slightly induced the protein cleavage of poly ADP-ribose polymerase (PARP), an apoptotic marker, in Hep3B and HepG2 cells, and this effect was obviously enhanced by co-treatment with chrysin (Figure 3D). Altogether, these results suggest that a combination of BCRP/ABCG2 inhibitor may provide a way to enhance the sensitivity of HCC cells to sorafenib.
Figure 3. Co-treatment with the BCRP/ABCG2 inhibitor, chrysin, greatly enhances the cytotoxicity of sorafenib in Hep3B and HepG2 HCC cells.
(A-D) HCC cells were pre-treated with 25 µM chrysin for 1 h, followed by sorafenib treatment. Cell viability was examined by using a bright-field imaging assay after 1 day (A), crystal violet staining assay after 1 day (B) and MTT assay after 3 days (C). The expression of the apoptotic marker, cleaved PARP, was examined by Western blot analysis (D).
Gefitinib acted as a competitive BCRP/ABCG2 inhibitor to improve the therapeutic efficacy of sorafenib in HCC cells
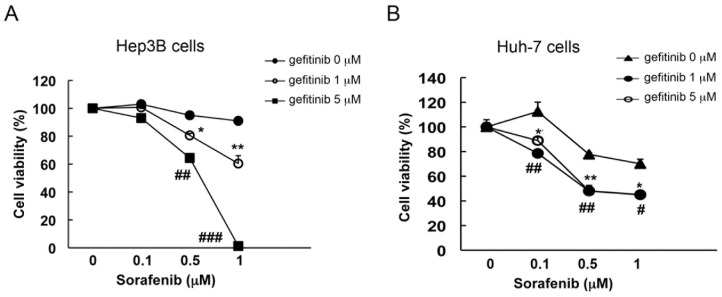
Based on the aforementioned results, simultaneous inhibition of BCRP/ABCG2 activity was suggested to enhance the anti-tumor activity of sorafenib in HCC cells. Due to the binding competition, some substrates for BCRP/ABCG2 have also been recognized as inhibitors of BCRP/ABCG2 when other BCRP/ABCG2 substrates were used simultaneously[30]. Therefore, co-treatment with other anti-cancer drugs, which were also defined as BCRP/ABCG2 substrate, may be an alternative way to enhance the anti-tumor efficacy of sorafenib in HCC cells. EGFR TKI gefitinib was demonstrated as a potential substrate for BCRP/ABCG2[15], [16], [17], [18], and combinatory treatment with gefitinib was also found to overcome the BCRP/ABCG2-mediated resistance to some anti-cancer drugs[31], [32], [33], [34], [35]. Accordingly, we assessed whether gefitinib could be used as an alternative BCRP/ABCG2 inhibitor to increase the cytotoxicity induced by sorafenib in HCC cells. We found that cell viability inhibition by sorafenib was greatly enhanced as the concentration of gefitinib was increased in Hep3B cells (Figure 4A). Consistently, similar result was also observed in both Huh-7 (Figure 4B) and HepG2 cells (Figure S4 in File S1). Taken together, these results suggest that gefitinib may be a promising combinatory therapy for increasing the therapeutic efficacy of sorafenib in HCC.
Figure 4. Co-treatment with the BCRP/ABCG2 substrate, gefitinib, enhances the cytotoxicity of sorafenib in Hep3B and Huh-7 HCC cells.
(A–B) HCC cells were pre-treated with 1 or 5 µM gefitinib for 1 h, followed by various doses of sorafenib treatment. Three days later, cell viability was examined by MTT assay.
Discussion
The approval of sorafenib is a breakthrough for the treatment of HCC. Although it benefits some HCC patients, the most optimized use of sorafenib is not achieved[36]. Thus, many efforts have been made to identify the potential predictors for sorafenib response in order to further elevate its therapeutic efficacy in HCC patients. To date, only a few biomarkers predicting the sensitivity to sorafenib have been identified[37]. Constitutive activation of Akt and signal transducer and activator of transcription 3 (STAT3) have been reported to be associated with sorafenib resistance in HCC[38], [39]. Furthermore, favorable response to sorafenib has been observed in HCC patients with higher level of basal ERK1/2 phosphorylation[40], [41]. More recently, it has been reported that EGFR and HER3 (also known as EGFR3, ErbB3) determine the sensitivity of HCC cells to sorafenib treatment[42], [43]. In the current study, we identified BCRP/ABCG2 as another potential biomarker that might be used to predict the therapeutic efficacy of sorafenib in HCC cells. It has been reported that Akt activity regulates the membrane distribution of BCRP/ABCG2, which may affect its extrusion ability[44]. Our previous studies demonstrated that the induction of BCRP/ABCG2 expression by constitutively activated Akt accounts for the acquired gefitinib resistance[19], [20]. It is worthy to further investigate whether BCRP/ABCG2 expression is also induced in response to chronic treatment with sorafenib and thereby contributes to the Akt-mediated intrinsic and acquired resistance to sorafenib in HCC [39].
In the past three decades, the major focus of researches regarding ABC efflux pumps has been on their roles in mediating chemo-resistance, which is an important challenge for cancer therapy. Many efforts are being made to develop specific and selective inhibitors for ABC transporter, which could not only circumvent the severe MDR to chemotherapy but also have fewer side effects on normal cells. However, none of them have been successfully performed in clinic to date[45]. The successful development of targeted therapy by using small molecule TKIs against critical oncogenes in tumors has brought great improvements to cancer therapy. In addition to chemotherapeutic agents, ABC transporters also efficiently mediate the drug efflux of these TKIs, including gefitinib and sorafenib, and thereby lead to the TKI resistance in tumors[15], [19]. In the current study, we observed that BCRP/ABCG2 mediated the efflux of sorafenib, which in turn, attenuated the response of HCC cells to sorafenib. Co-treatment with a BCRP/ABCG2 inhibitor or substrate greatly augmented the therapeutic efficacy of sorafenib. These results provide the evidence supporting BCRP/ABCG2 as a potential determinant for the sensitivity of HCC to sorafenib. Interestingly, a more recent study suggests that the attenuation of exposure to sorafenib over time in HCC patients may be due to an induction of expression of the efflux transporter in the gut wall. Therefore, an increase in the dose of sorafenib may be considered to elevate its anti-tumor efficacy[46]. Coupling this study with our findings strongly suggests that simultaneous inhibition of BCRP/ABCG2 activity is a potential strategy to augment the sorafenib efficacy in HCC. While BCRP/ABCG2 is demonstrated as a major transporter for the efflux of sorafenib in this and in other studies[24], [26], [27], the possibility of the involvement of other ABC transporters cannot be completely ruled out.
Like the action of chrysin in inhibiting BCRP/ABCG2, our findings showed that the use of gefitinib in combination with sorafenib also enhanced sorafenib-induced cytotoxicity in HCC cells, providing an alternative way to enhance the effectiveness of sorafenib in HCC. Gefitinib is a safe and well-tolerated TKI originally approved for non-small cell lung cancer (NSCLC). More importantly, it has been reported that direct activation of EGFR/HER3 by either autocrine or paracrine signaling circuit is both an adaptive process and a driving force to maintain ERK and Akt activities and subsequent HCC cell growth under sorafenib treatment[42], [43]. As mentioned above, EGFR downstream signaling Akt may regulate both protein expression and membrane distribution of BCRP/ABCG2 to affect its efflux ability[20], [44]. Accordingly, gefitinib may not only function as an EGFR inhibitor to block EGFR/HER3 activation and its downstream survival signaling, but also act as a BCRP/ABCG2 inhibitor by reducing its protein expression and pump activity to prevent the sorafenib efflux from HCC cells. These findings suggest that combination with gefitinib may reduce or prevent the acquisition of sorafenib resistance due to these functions. In supporting to this notion, the results from a phase I clinical trial of sorafenib in combination of gefitinib reveals not only the safety and well tolerance, but also the promising efficacy in recurrent NSCLC patients[47]. Since there is no BCRP/ABCG2 inhibitor approved for clinical use due to the severe side effects on normal cells to date, the combination therapy of gefitinib and sorafenib accordingly seems to be a potential strategy for treatment of advanced HCC.
Conclusion
Our study shows that BCRP/ABCG2 may be a biomarker for the determination of response to sorafenib in HCC in vitro. Simultaneous inhibition of BCRP/ABCG2 activity increases the cytotoxicity of sorafenib in HCC cells. Our findings not only identify a potential predictor for sorafenib sensitivity but also provide a strategy to enhance sorafenib efficacy and decrease the resistance to sorafenib in HCC.
Supporting Information
Supporting Information. Figure S1, BCRP/ABCG2 is involved in the determination of sorafenib sensitivity in HepG2 HCC cells. HepG2 cells were transfected with control siRNA or BCRP siRNA. One day later, cells were re-seeded at the same density, followed by treatment of 5 µM sorafenib. Three days later, cell viability was measured by using crystal violet staining assay (left panel). BCRP/ABCG2 expression was detected by Western blot analysis (right panel). Figure S2, BCRP/ABCG2 mediates the drug efflux of sorafenib in HepG2 and Huh-7 cells. (A–B) HepG2 (A) and Huh-7 (B) cells were pre-treated with 25 µM chrysin for 1 h. Then, the medium was changed to medium lacking sorafenib. Cells were allowed to recover at 0 and 48 hrs time points. The expression levels of phosphorylated ERK1/2, ERK1/2 and Tubulin were examined by Western blot analysis. Fold degree of reversal of sorafenib inhibition on ERK1/2 phosphorylation was shown in right panel. (C) Huh-7 cells were transiently transfected with either control siRNA or BCRP siRNA for 4 days, followed by the drug-efflux assay. The expression levels of phosphorylated ERK1/2, ERK1/2 were examined by Western blot analysis. Figure S3, Co-treatment with the BCRP/ABCG2 inhibitor, chrysin, significantly enhances the cytotoxicity of sorafenib in Huh-7 cells. (A–B) Huh-7 cells were pre-treated with 25 µM chrysin for 1 h, followed by sorafenib treatment. Cell viability was examined by using crystal violet staining assay after 2 day (A) and MTT assay after 3 days (B). Figure S4, Co-treatment with the BCRP/ABCG2 substrate, gefitinib, enhances the cytotoxicity of sorafenib in HepG2 cells. HepG2 cells were pre-treated with 1 or 5 µM gefitinib for 1 h, followed by various doses of sorafenib treatment. Three days later, cell viability was examined by MTT assay.
(DOC)
Funding Statement
This work was supported by grants from the National Science Council of Taiwan (NSC-101-2911-I-002-303, NSC-101-2320-B-214-005, NSC-102-2320-B-039-054-MY3, NSC-102-2320-B-039-052), E-Da Hospital, Taiwan (EDAHT100026, EDPJ101039), I-Shou University, Taiwan (ISU101-S-06), the National Health Research Institutes of Taiwan (NHRI-EX-101-9812BC) and China Medical University and Hospital (CMU101-S-28). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sanyal AJ, Yoon SK, Lencioni R (2010) The etiology of hepatocellular carcinoma and consequences for treatment. Oncologist 15 Suppl 414–22. [DOI] [PubMed] [Google Scholar]
- 2. Raza SA, Clifford GM, Franceschi S (2007) Worldwide variation in the relative importance of hepatitis B and hepatitis C viruses in hepatocellular carcinoma: a systematic review. Br J Cancer 96: 1127–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, et al. (2000) Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology 32: 1224–1229. [DOI] [PubMed] [Google Scholar]
- 4. Nagashima I, Hamada C, Naruse K, Osada T, Nagao T, et al. (1996) Surgical resection for small hepatocellular carcinoma. Surgery 119: 40–45. [DOI] [PubMed] [Google Scholar]
- 5. Qian J, Feng GS, Vogl T (2003) Combined interventional therapies of hepatocellular carcinoma. World J Gastroenterol 9: 1885–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Muller C (2006) Hepatocellular carcinoma—rising incidence, changing therapeutic strategies. Wien Med Wochenschr 156: 404–409. [DOI] [PubMed] [Google Scholar]
- 7. Wilhelm SM, Carter C, Tang L, Wilkie D, McNabola A, et al. (2004) BAY 43-9006 exhibits broad spectrum oral antitumor activity and targets the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in tumor progression and angiogenesis. Cancer Res 64: 7099–7109. [DOI] [PubMed] [Google Scholar]
- 8. Carlomagno F, Anaganti S, Guida T, Salvatore G, Troncone G, et al. (2006) BAY 43-9006 inhibition of oncogenic RET mutants. J Natl Cancer Inst 98: 326–334. [DOI] [PubMed] [Google Scholar]
- 9. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378–390. [DOI] [PubMed] [Google Scholar]
- 10. Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25–34. [DOI] [PubMed] [Google Scholar]
- 11. Perez-Tomas R (2006) Multidrug resistance: retrospect and prospects in anti-cancer drug treatment. Curr Med Chem 13: 1859–1876. [DOI] [PubMed] [Google Scholar]
- 12. Glavinas H, Krajcsi P, Cserepes J, Sarkadi B (2004) The role of ABC transporters in drug resistance, metabolism and toxicity. Curr Drug Deliv 1: 27–42. [DOI] [PubMed] [Google Scholar]
- 13. Borst P, Evers R, Kool M, Wijnholds J (1999) The multidrug resistance protein family. Biochim Biophys Acta 1461: 347–357. [DOI] [PubMed] [Google Scholar]
- 14. Gottesman MM, Fojo T, Bates SE (2002) Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer 2: 48–58. [DOI] [PubMed] [Google Scholar]
- 15. Brozik A, Hegedus C, Erdei Z, Hegedus T, Ozvegy-Laczka C, et al. (2011) Tyrosine kinase inhibitors as modulators of ATP binding cassette multidrug transporters: substrates, chemosensitizers or inducers of acquired multidrug resistance? Expert Opin Drug Metab Toxicol 7: 623–642. [DOI] [PubMed] [Google Scholar]
- 16. Shi Z, Parmar S, Peng XX, Shen T, Robey RW, et al. (2009) The epidermal growth factor tyrosine kinase inhibitor AG1478 and erlotinib reverse ABCG2-mediated drug resistance. Oncol Rep 21: 483–489. [PMC free article] [PubMed] [Google Scholar]
- 17. Tiwari AK, Sodani K, Wang SR, Kuang YH, Ashby CR Jr, et al. (2009) Nilotinib (AMN107, Tasigna) reverses multidrug resistance by inhibiting the activity of the ABCB1/Pgp and ABCG2/BCRP/MXR transporters. Biochem Pharmacol 78: 153–161. [DOI] [PubMed] [Google Scholar]
- 18. Sodani K, Tiwari AK, Singh S, Patel A, Xiao ZJ, et al. (2012) GW583340 and GW2974, human EGFR and HER-2 inhibitors, reverse ABCG2- and ABCB1-mediated drug resistance. Biochem Pharmacol 83: 1613–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen YJ, Huang WC, Wei YL, Hsu SC, Yuan P, et al. (2011) Elevated BCRP/ABCG2 expression confers acquired resistance to gefitinib in wild-type EGFR-expressing cells. PLoS One 6: e21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang WC, Chen YJ, Li LY, Wei YL, Hsu SC, et al. (2011) Nuclear translocation of epidermal growth factor receptor by Akt-dependent phosphorylation enhances breast cancer-resistant protein expression in gefitinib-resistant cells. J Biol Chem 286: 20558–20568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Elkind NB, Szentpetery Z, Apati A, Ozvegy-Laczka C, Varady G, et al. (2005) Multidrug transporter ABCG2 prevents tumor cell death induced by the epidermal growth factor receptor inhibitor Iressa (ZD1839, Gefitinib). Cancer Res 65: 1770–1777. [DOI] [PubMed] [Google Scholar]
- 22. Ozvegy-Laczka C, Cserepes J, Elkind NB, Sarkadi B (2005) Tyrosine kinase inhibitor resistance in cancer: role of ABC multidrug transporters. Drug Resist Updat 8: 15–26. [DOI] [PubMed] [Google Scholar]
- 23. Haouala A, Rumpold H, Untergasser G, Buclin T, Ris HB, et al. (2010) siRNA-Mediated Knock-Down of P-Glycoprotein Expression Reveals Distinct Cellular Disposition of Anticancer Tyrosine Kinases Inhibitors. Drug Metab Lett 4: 114–119. [DOI] [PubMed] [Google Scholar]
- 24. Agarwal S, Sane R, Ohlfest JR, Elmquist WF (2011) The role of the breast cancer resistance protein (ABCG2) in the distribution of sorafenib to the brain. J Pharmacol Exp Ther 336: 223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lagas JS, van Waterschoot RA, Sparidans RW, Wagenaar E, Beijnen JH, et al. (2010) Breast cancer resistance protein and P-glycoprotein limit sorafenib brain accumulation. Mol Cancer Ther 9: 319–326. [DOI] [PubMed] [Google Scholar]
- 26. Poller B, Wagenaar E, Tang SC, Schinkel AH (2011) Double-transduced MDCKII cells to study human P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) interplay in drug transport across the blood-brain barrier. Mol Pharm 8: 571–582. [DOI] [PubMed] [Google Scholar]
- 27. Asakawa C, Ogawa M, Kumata K, Fujinaga M, Kato K, et al. (2011) [11C]sorafenib: radiosynthesis and preliminary PET study of brain uptake in P-gp/Bcrp knockout mice. Bioorg Med Chem Lett 21: 2220–2223. [DOI] [PubMed] [Google Scholar]
- 28. Li G, Chen X, Wang Q, Xu Z, Zhang W, et al. (2007) The roles of four multi-drug resistance proteins in hepatocellular carcinoma multidrug resistance. J Huazhong Univ Sci Technolog Med Sci 27: 173–175. [DOI] [PubMed] [Google Scholar]
- 29. Sun Z, Zhao Z, Li G, Dong S, Huang Z, et al. (2010) Relevance of two genes in the multidrug resistance of hepatocellular carcinoma: in vivo and clinical studies. Tumori 96: 90–96. [DOI] [PubMed] [Google Scholar]
- 30. Van Bambeke F, Pages JM, Lee VJ (2006) Inhibitors of bacterial efflux pumps as adjuvants in antibiotic treatments and diagnostic tools for detection of resistance by efflux. Recent Pat Antiinfect Drug Discov 1: 157–175. [DOI] [PubMed] [Google Scholar]
- 31. Noguchi K, Kawahara H, Kaji A, Katayama K, Mitsuhashi J, et al. (2009) Substrate-dependent bidirectional modulation of P-glycoprotein-mediated drug resistance by erlotinib. Cancer Sci 100: 1701–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Takigawa N, Takeyama M, Kozuki T, Shibayama T, Hisamoto A, et al. (2007) Combination of SN-38 with gefitinib or imatinib overcomes SN-38-resistant small-cell lung cancer cells. Oncol Rep 17: 983–987. [PubMed] [Google Scholar]
- 33. Braun AH, Stark K, Dirsch O, Hilger RA, Seeber S, et al. (2005) The epidermal growth factor receptor tyrosine kinase inhibitor gefitinib sensitizes colon cancer cells to irinotecan. Anticancer Drugs 16: 1099–1108. [DOI] [PubMed] [Google Scholar]
- 34. Nakamura Y, Oka M, Soda H, Shiozawa K, Yoshikawa M, et al. (2005) Gefitinib (“Iressa”. ZD1839), an epidermal growth factor receptor tyrosine kinase inhibitor, reverses breast cancer resistance protein/ABCG2-mediated drug resistance. Cancer Res 65: 1541–1546. [DOI] [PubMed] [Google Scholar]
- 35. Yanase K, Tsukahara S, Asada S, Ishikawa E, Imai Y, et al. (2004) Gefitinib reverses breast cancer resistance protein-mediated drug resistance. Mol Cancer Ther 3: 1119–1125. [PubMed] [Google Scholar]
- 36. Kelley RK, Venook AP (2008) Sorafenib in hepatocellular carcinoma: separating the hype from the hope. J Clin Oncol 26: 5845–5848. [DOI] [PubMed] [Google Scholar]
- 37.Gauthier A, Ho M (2012) Role of sorafenib in the treatment of advanced hepatocellular carcinoma: An update. Hepatol Res. [DOI] [PMC free article] [PubMed]
- 38. Tai WT, Cheng AL, Shiau CW, Huang HP, Huang JW, et al. (2011) Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J Hepatol 55: 1041–1048. [DOI] [PubMed] [Google Scholar]
- 39. Chen KF, Chen HL, Tai WT, Feng WC, Hsu CH, et al. (2011) Activation of phosphatidylinositol 3-kinase/Akt signaling pathway mediates acquired resistance to sorafenib in hepatocellular carcinoma cells. J Pharmacol Exp Ther 337: 155–161. [DOI] [PubMed] [Google Scholar]
- 40. Abou-Alfa GK, Schwartz L, Ricci S, Amadori D, Santoro A, et al. (2006) Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol 24: 4293–4300. [DOI] [PubMed] [Google Scholar]
- 41. Zhang Z, Zhou X, Shen H, Wang D, Wang Y (2009) Phosphorylated ERK is a potential predictor of sensitivity to sorafenib when treating hepatocellular carcinoma: evidence from an in vitro study. BMC Med 7: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blivet-Van Eggelpoel MJ, Chettouh H, Fartoux L, Aoudjehane L, Barbu V, et al. (2012) Epidermal growth factor receptor and HER-3 restrict cell response to sorafenib in hepatocellular carcinoma cells. J Hepatol 57: 108–115. [DOI] [PubMed] [Google Scholar]
- 43. Ezzoukhry Z, Louandre C, Trecherel E, Godin C, Chauffert B, et al. (2012) EGFR activation is a potential determinant of primary resistance of hepatocellular carcinoma cells to sorafenib. Int J Cancer 131: 2961–2969. [DOI] [PubMed] [Google Scholar]
- 44. Takada T, Suzuki H, Gotoh Y, Sugiyama Y (2005) Regulation of the cell surface expression of human BCRP/ABCG2 by the phosphorylation state of Akt in polarized cells. Drug Metab Dispos 33: 905–909. [DOI] [PubMed] [Google Scholar]
- 45. Modok S, Mellor HR, Callaghan R (2006) Modulation of multidrug resistance efflux pump activity to overcome chemoresistance in cancer. Curr Opin Pharmacol 6: 350–354. [DOI] [PubMed] [Google Scholar]
- 46. Arrondeau J, Mir O, Boudou-Rouquette P, Coriat R, Ropert S, et al. (2012) Sorafenib exposure decreases over time in patients with hepatocellular carcinoma. Invest New Drugs 30: 2046–2049. [DOI] [PubMed] [Google Scholar]
- 47. Adjei AA, Molina JR, Mandrekar SJ, Marks R, Reid JR, et al. (2007) Phase I trial of sorafenib in combination with gefitinib in patients with refractory or recurrent non-small cell lung cancer. Clin Cancer Res 13: 2684–2691. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information. Figure S1, BCRP/ABCG2 is involved in the determination of sorafenib sensitivity in HepG2 HCC cells. HepG2 cells were transfected with control siRNA or BCRP siRNA. One day later, cells were re-seeded at the same density, followed by treatment of 5 µM sorafenib. Three days later, cell viability was measured by using crystal violet staining assay (left panel). BCRP/ABCG2 expression was detected by Western blot analysis (right panel). Figure S2, BCRP/ABCG2 mediates the drug efflux of sorafenib in HepG2 and Huh-7 cells. (A–B) HepG2 (A) and Huh-7 (B) cells were pre-treated with 25 µM chrysin for 1 h. Then, the medium was changed to medium lacking sorafenib. Cells were allowed to recover at 0 and 48 hrs time points. The expression levels of phosphorylated ERK1/2, ERK1/2 and Tubulin were examined by Western blot analysis. Fold degree of reversal of sorafenib inhibition on ERK1/2 phosphorylation was shown in right panel. (C) Huh-7 cells were transiently transfected with either control siRNA or BCRP siRNA for 4 days, followed by the drug-efflux assay. The expression levels of phosphorylated ERK1/2, ERK1/2 were examined by Western blot analysis. Figure S3, Co-treatment with the BCRP/ABCG2 inhibitor, chrysin, significantly enhances the cytotoxicity of sorafenib in Huh-7 cells. (A–B) Huh-7 cells were pre-treated with 25 µM chrysin for 1 h, followed by sorafenib treatment. Cell viability was examined by using crystal violet staining assay after 2 day (A) and MTT assay after 3 days (B). Figure S4, Co-treatment with the BCRP/ABCG2 substrate, gefitinib, enhances the cytotoxicity of sorafenib in HepG2 cells. HepG2 cells were pre-treated with 1 or 5 µM gefitinib for 1 h, followed by various doses of sorafenib treatment. Three days later, cell viability was examined by MTT assay.
(DOC)