Abstract
The geminivirus replication factor AL1 interacts with the plant retinoblastoma-related protein (pRBR) to modulate host gene expression. The AL1 protein of tomato golden mosaic virus (TGMV) binds to pRBR through an 80-amino-acid region that contains two highly predicted α-helices designated 3 and 4. Earlier studies suggested that the helix 4 motif, whose amino acid sequence is strongly conserved across geminivirus replication proteins, plays a role in pRBR binding. We generated a series of alanine substitutions across helix 4 of TGMV AL1 and examined their impact on pRBR binding using yeast two-hybrid assays. These experiments showed that several helix 4 residues are essential for efficient pRBR binding, with a critical residue being a leucine at position 148 in the middle of the motif. Various amino acid substitutions at leucine-148 indicated that both structural and side chain components contribute to pRBR binding. The replication proteins of the geminiviruses tomato yellow leaf curl virus and cabbage leaf curl virus (CaLCuV) also bound to pRBR in yeast dihybrid assays. Mutation of the leucine residue in helix 4 of CaLCuV AL1 reduced binding. Together, these results suggest that helix 4 and the conserved leucine residue are part of a pRBR-binding interface in begomovirus replication proteins.
Geminiviruses are DNA viruses that replicate their single-stranded genomes through double-stranded intermediates in nuclei of plant cells (reviewed in reference 28). Because of their small genomes, geminiviruses provide only the factors required to initiate rolling circle replication and depend on plant nuclear DNA polymerases to amplify their genomes. Many geminiviruses replicate in differentiated cells that have exited the cell cycle and no longer contain detectable levels of host DNA polymerases and associated factors (10, 44, 47). Geminiviruses are thought to overcome this barrier by interacting with a host protein, the retinoblastoma-related protein (pRBR), to induce transcription of genes encoding host replicative enzymes (17, 18, 38). Hence, geminiviruses are valuable tools for studying how the cell division cycle and differentiation are regulated by pRBR in plants (26).
Geminiviruses are a diverse family of plant-infecting viruses that fall into four genera based on their genome structure, insect vector, and host range (6, 58). Tomato golden mosaic virus (TGMV) is a typical begomovirus with a bipartite genome that encodes seven proteins (4). AL1 (also designated AC1, C1, or Rep) is the only viral protein that is essential for replication (19). AL1 recognizes the origin by binding to a specific DNA sequence (21), catalyzes DNA cleavage and ligation to initiate and terminate rolling circle replication (40, 52), and may act as a DNA helicase during the elongation phase of replication (24, 54). AL1 also plays a central role in reprogramming mature plant cells to support DNA replication and recruiting host replication machinery to the origin (38, 45).
Many functions of AL1 involve protein-protein interactions. Oligomerization is essential for origin recognition and may be required for other catalytic functions in vivo (51, 60). AL1 binds to the viral replication enhancer protein, AL3 (60), which in turn binds to proliferating cell nuclear antigen (PCNA), the processivity factor for DNA polymerase δ (8). Geminivirus replication proteins also interact with components of the host replication apparatus, like PCNA and the replication factor C complex, the clamp loader that transfers PCNA to the replication fork (8, 45). These interactions are likely to represent early steps in the assembly of a DNA replication complex on the geminivirus origin. AL1 also interacts with host factors involved in cell division and differentiation. AL1 binds to a mitotic kinesin and a protein kinase associated with early leaf development (37), but the roles of these interactions during infection have not been determined. In contrast, there is evidence that binding of AL1 to pRBR alters host transcriptional controls to induce the synthesis of the plant DNA replication machinery (17, 18, 38).
In animals, the retinoblastoma protein (pRb) is part of a small family that also includes p107 and p130 (30). pRb family members negatively regulate cell cycle progression and promote differentiation, in part, through their interactions with E2F transcription factors and histone deacetylase (61). These interactions repress transcription of genes encoding proteins required for entry into S phase and DNA replication (41) and facilitate maintenance of a differentiated state (29). In late G1, phosphorylation of pRb by cyclin-dependent kinases disrupts its association with E2F and allows expression of genes required for S phase (35). Mammalian DNA tumor viruses bypass this phosphorylation requirement by binding directly to pRb and disrupting its interaction with E2F (34). Several lines of evidence argue that the pRb/E2F regulatory network is conserved in plants. pRBR and E2F proteins have been identified in a variety of plant species (for reviews, see references 12, 15, and 27). pRBR is a substrate of cyclin-dependent kinases (5, 48), and its levels are high in differentiated tissues (33). E2F consensus sites have been found in the promoters of a number of plant genes (55) and shown to modulate transcription during the cell division cycle and development (7, 9, 11, 17, 39, 62). Begomoviruses are thought to overcome E2F-mediated repression of PCNA transcription in mature leaves (18), most likely through the interaction of AL1 with pRBR (38).
pRb family members display strong sequence homology across a large central domain known as the A/B pocket (46). This region is involved in a variety of protein interactions (63), including those with simian virus 40 large T antigen, Adenovirus E1A and human papillomavirus E7 (42). Each of these DNA tumor virus proteins binds to pRb through a conserved LXCXE motif (16). LXCXE motifs have also been identified in pRBR-binding proteins, like cyclin D (49), the mastrevirus RepA protein (25, 32, 43, 66), and the nanovirus Clink protein (3). However, there are plant proteins, including some E2F transcription factors (11, 14) and chromatin-remodeling proteins (2, 56, 57), that interact with pRBR through other sequences. TGMV AL1 does not have an LXCXE motif and falls into this second group of pRBR binding proteins. A previous study mapped the pRBR binding domain of AL1 between amino acids 101 to 180 and showed that mutations in this region impact AL1/pRBR interactions and tissue specificity of infection (38). In the experiments described here, we asked if a highly conserved motif within this region plays a role in pRBR binding and virus infection.
MATERIALS AND METHODS
AL1 mutants.
The plasmid pNSB148, which contains the TGMV AL1 coding sequence in a pUC118 background, was used as the template for site-directed mutagenesis as described previously (51). The oligonucleotide primers and resulting clones are listed in Table 1. Viral DNA fragments containing the mutations were verified by DNA sequence analysis. Viral replicons encoding the mutant AL1 proteins were generated by subcloning SalI/NheI fragments corresponding to ALI amino acids 120 to 232 from the mutant clones into the same sites of the wild-type replicon, pMON1565 (52), to give pNSB954 (K144), pNSB1032 (EE146), pNSB999 (A147Y), pNSB997 (L148), pNSB979 (L148V), pNSB1000 (L148G), and pNSB998 (II151). Alanine substitution mutants are designated with the wild-type amino acid(s) and the final position number. When the mutation was not to alanine, the mutant amino acid is given after the position number.
TABLE 1.
Constructs
| Protein and mutation | Mutagenesis oligonucleotidesa | Yeast dihybrid vector
|
Viral replicon | |
|---|---|---|---|---|
| Gal4 AD-AL1 | Gal4 BD | |||
| TGMV AL1 | pNSB809 | pNSB736 | pMON1565 | |
| KEE146b | pNSB894 | |||
| K144 | TCTGCAGGGCTTCTTCcgcGGAAGAAGCATTTAA | pNSB916 | pNSB954 | |
| E145 | TCTGCAGGGCcTCTgCTTTGGAAGAAGCATTTAA | pNSB917 | ||
| E146 | CTGCAGGGCTgCTTCTTTGGAAGAAGCA | pNSB975 | ||
| EE146 | TCTGCAGGGCggCcgCTTTGGAAGAAGCATTTAAC | pNSB1040 | pNSB1032 | |
| A147Y | TAATTATCTGaAGGtaTTCTTCTTTGGAAGAAGCATTTAA | pNSB1003 | pNSB999 | |
| L148 | TAATTATCTGCgcaGCTTCTTCTTTGGAAGAAGCATTTAA | pNSB1001 | pNSB997 | |
| L148V | ATTATCTGCAcGGCcTCTTCTTTGGAAGAAGCATTTAA | pNSB980 | pNSB979 | |
| L148M | pNSB979 revertant | pNSB1112 | ||
| L148G | CTAATTATCTGgccGGCTTCTTCTTTGGAAGAAGCATTTA | pNSB1004 | pNSB1000 | |
| L148I | pNSB1030 revertant | pNSB1079 | ||
| I1151 | TTTCTCTCTAgcTgcCTGaAGGGCTTCTTCTTTGGAAGA | pNSB1002 | pNSB998 | |
| REK154b | pNSB759 | |||
| CbLCV AL1 | pNSB901 | pNSB909 | ||
| Cb1-207 | pNSB974 | |||
| Cb1-207, L145A | GTGTGGAAGAGGCGgccGCAATTATAAGGGC | pNSB1114 | ||
| TYLCV-DR C1 | pTYLC102 | pTYLC103 | ||
| Maize RBR1 | 214C | |||
Lowercase letters designate mutated residues.
Data from reference 53.
Site-directed mutagenesis of cabbage leaf curl virus (CaLCuV) AL1 was performed by using a PCR-based method. Complementary oligonucleotides Cb-M1 and Cb-M2 (Table 1) were used as primers in combination with the M13 universal and reverse primers, respectively, in two separate amplification reactions with pNSB1085 as a template. pNSB1085 contains a CaLCuV A DNA fragment (positions 1499 to 33) carrying the AL1 coding sequence in a pUC118 background. The PCR products of Cb-M1/M13-universal and Cb-M2/M13-reverse amplification reactions were isolated from agarose gels and pooled for a new amplification reaction using M13-universal and reverse primers. The PCR product was digested with BamHI and BglII and cloned into the same sites of a modified pUC118 to give pNSB1097.
Yeast two-hybrid assays.
Yeast cassettes with the Gal4 DNA binding domain (DBD) were generated with pAS2-1 (Clontech, Palo Alto, Calif.). Zm214C includes a truncated maize RBR1 coding sequence consisting of the pocket and C-terminal domains fused to the DBD (1). pNSB736 contains a full-length, wild-type TGMV AL1-DBD fusion (53).
Yeast cassettes containing the Gal4 activation domain (AD) were generated by using pACT2 (Clontech). Cassettes for wild-type TGMV AL1 (pNSB809) and the mutants KEE146 (pNSB894) and REK154 (pNSB759) have been described elsewhere (53). Cassettes for the TGMV mutants K144 (pNSB916), E145 (pNSB917), E146 (pNSB975), EE146 (pNSB1040), A147Y (pNSB1003), L148 (pNSB1001), L148V (pNSB980), L148G (pNSB1004), and II151 (pNSB1002) were made by replacing the AatII-BamHI fragment of pNSB735 (53) with the equivalent fragments from pNSB946, pNSB945, pNSB968, pNSB1033, pNSB995, pNSB993, pNSB969, pNSB996, and pNSB994, respectively. Cassettes for L148M (pNSB1112) and L148I (pNSB1079) were generated by the same strategy using revertant replicons that were cloned out of plants inoculated with a wild-type B replicon and a mutant A replicon corresponding to pNSB979 or pNSB1030, respectively.
The C1 open reading frame was amplified from a tomato yellow leaf curl virus (TYLCV-DR [Dominican Republic isolate]) genomic clone (a gift from Monsanto) using the oligonucleotides, 5′-GGACACCGATTggaTcCAgCATGCCTC and CCACAGTCgAatTCCCCggGCTTACGC. (The lowercase letters indicate mutated nucleotides.) The resulting PCR product (positions 1686 to 12 of the TYLCV-DR genome) was digested with BamHI and SmaI and cloned into pUC119 to give pTYLC78. The corresponding yeast AD vector was constructed by cloning the BamHI (trimmed)/SmaI C1 fragment from pTYLC78 into pACT2 digested with SmaI to give pTYLC102.
pCPCaLCuVA.001, which contains a single copy of CaLCuV A DNA (64), was modified by PCR mutagenesis using the oligonucleotide 5′-CCTAAATAagatcTACAAGgATcCCACGAAACCCTA to introduce a BamHI site at the 5′ end of the AL1 open reading frame. The resulting clone, pNSB900, was digested with BamHI, and the fragment containing the full-length AL1 sequence was cloned into the BamHI site of pACTII to give pNSB958. The NcoI fragment from pNSB958 encoding amino acids 2 to 178 of AL1 was then subcloned into pACTII to give pNSB974. The L145A mutation was introduced into the CaLCuV AL1 coding sequence of pNSB900 by using the oligonucleotide 5′-GTGTGGAAGAGGCGgccGCAATTATAAGGGC for PCR mutagenesis. A mutant fragment from the resulting plasmid (pNSB1097) with AatII/NcoI (repaired) ends was subcloned into pNSB974 with AatII/XhoI (repaired) ends to give pNSB1114.
Interactions between the Gal4 fusion proteins were evaluated in Saccharomyces cerevisiae strain Y187 by measuring β-galactosidase activity as described previously (53). Protein concentrations were measured by Bradford assays (Bio-Rad, Hercules, Calif.). The enzyme specific activity (1 U = 1.0 μM product/min at pH 7.3 and 37°C) was determined by using purified β-galactosidase (Sigma, St. Louis, Mo.) as the standard. The different constructs were tested in a minimum of four independent transformants in at least two experiments. The relative activities of the mutant proteins were normalized against wild-type AL1, which was set to 100%.
Replication and infectivity assays.
TGMV A replicons carrying mutant AL1 coding sequences were made using pMON1565, a pUC-based plasmid that contains 1.5 copies of wild-type TGMV A DNA (52). The mutant replicons in Table 1 were generated by replacing the SalI/NheI fragment encoding AL1 amino acids 120 to 313 of pMON1565 with the equivalent fragment of mutant AL1 open reading frames described above. pTG1.4B, which includes 1.4 copies of wild-type TGMV B, has been described previously (21).
For replication assays, protoplasts were isolated from Nicotiana tabacum (BY-2) suspension cells, electroporated, and cultured according to published methods (20). Cells were transfected with 5 μg of wild-type or mutant TGMV A replicon DNA and 25 μg of sheared salmon sperm DNA. Total DNA was extracted 72 h after transfection, digested with DpnI and XhoI, and examined for double- and single-stranded viral DNA accumulation by DNA gel blot analysis using a TGMV A specific probe (21). Viral DNA was quantified by phosphorimage analysis. Each protoplast assay was performed in at least three independent experiments.
For infectivity assays, Nicotiana benthamiana plants at the six-leaf stage were inoculated with a biolistics device as described previously (47). Wild-type or mutant TGMV A replicon DNA (10 μg) was precipitated onto 1-μm microprojectiles in the presence of a wild-type TGMV B replicon (pTG1.4B) and used to bombard plants. Total DNA was extracted from leaf tissue 14 days after bombardment (13), linearized with XhoI, and analyzed on DNA gel blots.
RESULTS
Mutations in the KEE sequence of AL1 differentially alter pRBR binding.
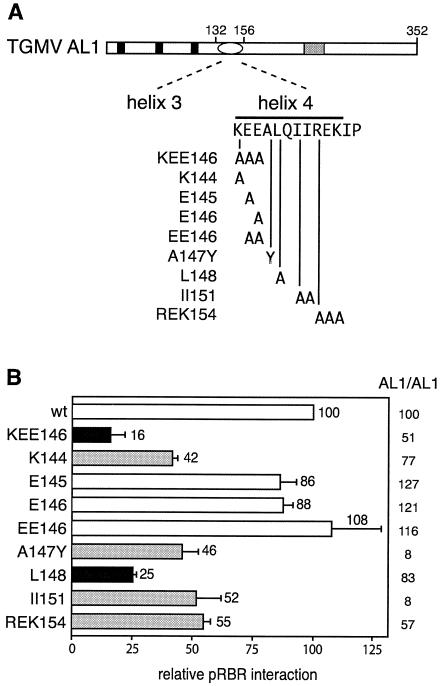
The geminivirus TGMV interacts with pRBR, a plant homolog of the mammalian Rb protein, via a novel mechanism. An earlier study showed that mutations in KEE146 in the pRBR binding domain of TGMV AL1 impair interaction (38). The KEE146 mutant contains three consecutive alanine substitutions, which may act alone or together to confer the mutant phenotype. To better understand the basis of the phenotype, we generated four additional alanine substitution mutants: K144, E145, E146, and EE146 (Fig. 1A). The mutant AL1 open reading frames were fused to the coding sequence of the Gal4 AD and expressed in yeast. Mutant proteins accumulated to levels comparable to those of an AD-wild-type AL1 fusion (data not shown), consistent with previous experiments showing that mutations in this region do not impact AL1 fusion protein stability in yeast (53). The AL1 oligomerization properties of the mutant proteins were assessed in two-hybrid assays using a wild-type TGMV AL1 protein fused to the Gal4 DBD (53). The influence of the mutations on AL1/AL1 interactions was minimal (Fig. 1B) and not statistically significant, indicating that the mutations do not have a global effect on AL1 function. However, technical constraints precluded testing the mutants in AL1/AL3 binding assays.
FIG. 1.
Mutations in the helix 4 motif of AL1 impair interactions with pRBR. (A) Schematic of TGMV AL1. Solid boxes mark the location of the three motifs conserved among RCR initiator proteins, the oval indicates a predicted set of α-helices, and the stippled box shows the location of the ATP binding domain. The AL1 sequence between amino acids 132 to 156 is shown, with the locations of the predicted α-helices 3 and 4 indicated. Vertical lines mark the positions of the alanine substitutions. Mutations are designated by the corresponding wild-type sequence and the position of the last amino acid that was modified. (B) Yeast was cotransformed with an expression cassette (Zm214C) encoding amino acids 214 to the C terminus of ZmRBR fused to the Gal4 DBD and cassettes for either wild-type or mutant AL1 fused to the Gal4 AD. Protein interactions were assayed by measuring β-galactosidase activity in total protein extracts and normalized to wild-type values (100%). Filled bars indicate mutants strongly impaired in pRBR binding, grey bars mark moderately impaired mutants, and open bars indicate mutants with activity similar to or greater than that of wild-type AL1. Error bars correspond to 2 standard errors. The effects of the mutations on AL1/AL1 interactions (oligomerization activity) are indicated on the right.
The impact of the mutations on AL1/pRBR interactions was analyzed in two hybrid assays. Previous studies established that TGMV AL1 interacts with maize and Arabidopsis RBR proteins similarly but that binding to maize pRBR is stronger in yeast assays (1, 38). As a consequence, we used a truncated version (Zm214C) of maize pRBR1 from amino acid 214 to the C terminus fused to the Gal4 DBD (1, 38) for these experiments. This region contains the A/B pocket domain and the C-terminal domain of pRBR. The E145, E146, and EE146 mutations did not alter AL1/pRBR binding significantly (Fig. 1B), suggesting that the E residues are not essential for wild-type binding activity. In contrast, the K144 mutation reduced pRBR interactions to 42% of the wild-type levels (Fig. 1B), indicating that this residue is required for full binding activity. However, the reduction in pRBR binding activity was less for K144 than KEE146 (16% of wild-type levels; Fig. 1B), uncovering a role for one or both of the E residues in combination with K144 in pRBR interaction.
Mutations in the helix 4 motif of AL1 impair pRBR interactions.
The KEE sequence constitutes the first three residues of an 11-amino-acid motif designated helix 4 (Fig. 1A; also, see Fig. 5). The helix 4 motif is conserved across all begomovirus, curtovirus, and topocuvirus replication proteins and mastrevirus RepA/Rep proteins with respect to both amino acid sequence and predicted α-helical structure (53). Because of this strong conservation, we hypothesized that other residues in helix 4 also contribute to pRBR binding. This hypothesis is supported by previous results showing that the pRBR binding activity of an REK154 mutant, which contains three alanine substitutions in the last three amino acids of the motif, is reduced twofold (Fig. 1B) (38). To further explore the role of helix 4 in pRBR binding, we generated alanine substitutions at the conserved L148 and II151 residues and a tyrosine substitution at the invariant A147 position to mimic the conserved tyrosine at the equivalent position in mastrevirus RepA/Rep proteins. The Q149 position, which is highly variable, was not mutated. The mutant AL1 coding sequences were fused to the Gal4 AD coding sequence and analyzed in yeast two-hybrid assays as described above. The A147Y and II151 mutants displayed significantly lower pRBR binding activities than wild-type AL1 (Fig. 1B). However, these mutations also reduced AL1 oligomerization activity (Fig. 1B, right), indicating that their effects were not specific for pRBR binding. In contrast, the L148 mutation reduced the AL1/pRBR interactions to 25% of wild-type levels without a concomitant loss in AL1 oligomerization activity (Fig. 1B), establishing the specificity of the mutation for pRBR binding. Together, these results indicated that several of the amino acid residues in the helix 4 motif are important for both AL1/pRBR and AL1/AL1 interactions in yeasts and that both the KEE146 and the L148 residues contribute to pRBR binding. It was not technically feasible to verify the impact of the mutations on AL1/pRBR interactions in infected plant cells or tissues. The AL1 protein is expressed at very low levels and is not extracted efficiently under native conditions required to maintain protein complexes.
FIG. 5.
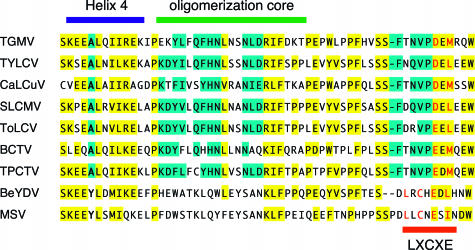
Sequence conservation in the pRBR binding domain of geminivirus proteins. Geminivirus AL1/C1 proteins are aligned with the equivalent regions of mastrevirus RepA/Rep proteins. Residues shaded in blue are conserved in AL1/C1 proteins of begomoviruses (TGMV, TYLCV, CaLCuV, Sri Lankan cassava mosaic virus [SLCMV], and tomato leaf curl virus [ToLCV]), curtoviruses (beet curly top virus [BCTV]), and topocuviruses (tomato pseudo-curly top virus [TPCTV]). Residues shaded in yellow are also conserved in the RepA/Rep proteins of mastreviruses (BeYDV and maize streak virus [MSV]). Together, these geminiviruses represent all four genera and five continents. The aligned region corresponds to amino acids 143 to 200 of TGMV AL1. The helix 4 (blue bar) and LXCXE (red bar and red type) motifs are marked. The helix 4 A and Y residues that have diverged between mastreviruses and other geminivirus genera are in bold type. The LXCXE motif includes a bulky hydrophobic residue in the 8th position (relative to L) that also directly contacts pRb (42). The oligomerization core of TGMV AL1 (green bar) is also marked (53). Accession numbers for the viruses in alignment are as follows: TGMV, K02029; TYLCV, X15656; CaLCuV, U65529; SLCMV, AJ314737; ToLCV, S53251; BCTV, AF379337; TPCTV, X84735; BeYDV, Y11023; and MSV, K02026.
The L148 mutation reduces viral DNA accumulation and symptom severity.
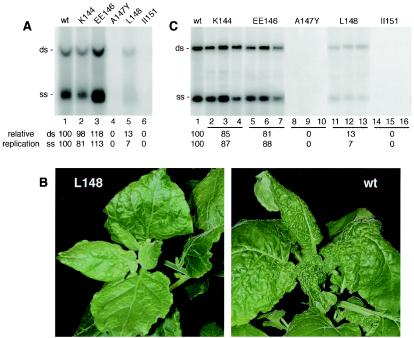
The KEE146 mutation alters the level of TGMV DNA accumulation in cultured cells and the tissue specificity and symptoms of TGMV infection in plants (38). To determine if mutations in other helix 4 residues also have an impact on these viral processes, we examined the newly generated mutants in transient-replication and infectivity assays. The mutations were transferred into the AL1 open reading frame of a TGMV A replicon, and viral DNA accumulation in N. tabacum BY-2 protoplasts was assessed on DNA gel blots. The levels of double-stranded and single-stranded DNA that accumulated for the K144 (Fig. 2A, lane 2) and EE146 (lane 3) mutants were essentially wild type (lane 1). A similar result was observed with the E145 and E146 mutants (data not shown). The L148 mutant (Fig. 2A, lane 5) also supported viral DNA synthesis but at levels significantly lower than that for wild-type TGMV A (lane 1) and similar to those previously reported for KEE146 (38). The reduction in L148 DNA accumulation was observed for both double- and single-stranded forms of TGMV DNA. In contrast, the A147Y (Fig. 2A, lane 4) and II151 (lane 6) mutants, both of which were severely impaired in AL1 oligomerization (Fig. 1B), failed to replicate to detectable levels in cultured cells. The same viral DNA accumulation patterns were observed when BY-2 protoplasts were cotransfected with a TGMV B replicon and plant expression cassettes for AL1 and AL3 (data not shown).
FIG. 2.
The L148 mutation reduces replication and symptom severity. (A) DNA replication of TGMV AL1 mutants was analyzed in tobacco protoplasts. DNA was isolated 72 h posttransfection, and 10 μg of total DNA was analyzed by DNA gel blot hybridization by using a radiolabeled TGMV A probe. Lanes 1 to 6, transfections with TGMV A replicons with either wild-type (wt) or mutant AL1 open reading frames corresponding to the indicated mutations. The positions of double-stranded (ds) and single-stranded (ss) forms of TGMV A DNA are indicated. (B) N. benthamiana plants infected with the pRBR binding mutant L148 developed chlorosis along the veins but no leaf curling or stunting characteristic of wild-type TGMV infection. These milder symptoms were maintained over a 5-week infection period. (C) N. benthamiana plants were cobombarded with DNAs corresponding to TGMV A and B replicons. The AL1 open reading frames of the A components were wild type (wt) or carried the indicated mutations. Total DNA (2.5 μg/lane) was isolated from young leaves from three plants for each construct at 14 days postinfection and analyzed on DNA gel blots. Viral DNA was detected with a radiolabeled probe specific for TGMV A. In panels A and C, the average relative accumulation of double- and single-stranded DNA for three independent samples is given below each lane, with the wild-type value set at 100. No signals were detected in mutants A147Y and II51 even with longer exposures.
Plant infection experiments were carried out by cobombarding either wild-type or mutant A component DNA with a TGMV B replicon onto N. benthamiana plants. Plants inoculated with the wild-type virus developed clear symptoms by 6 to 7 days postinoculation, exhibiting leaf curling, general chlorosis, and stunting of new growth (Fig. 2B, wt). The K144, E145, E146, and EE146 mutants caused symptoms that were indistinguishable from those caused by the wild type, indicating that these mutations do not visibly alter the infection process. In contrast, plants inoculated with the L148 mutant only developed chlorosis along the veins (Fig. 2B) between 14 and 21 days postinfection and never displayed stunted growth or leaf curling. These symptoms, which resemble those reported for KEE146-infected plants (38), were observed in all 12 inoculated plants and were maintained over a 5-week infection period. The A147Y and II151 mutants produced no detectable symptoms even at 5 weeks postinoculation, consistent with their inability to replicate in tobacco protoplasts.
We also examined TGMV DNA accumulation in the N. benthamiana plants inoculated with either wild-type or mutant virus. Total DNA was isolated from systemically infected leaves 14 days postinoculation and analyzed on DNA gel blots by using a TGMV A probe. Viral DNA was detected in extracts of plants infected with all mutant viruses that produced symptoms (Fig. 2C) but not in asymptomatic plants inoculated with the A147Y (lanes 8 to 10) and II151 (lanes 14 to 16) mutants. Plants infected with the K144 (Fig. 2C, lanes 2 to 4) or EE146 (lanes 5 to 7) mutant contained essentially wild-type levels of single- and double-stranded DNA (lane1). Again, the same results were obtained with E145- and E146-infected plants (data not shown). In contrast, both DNA forms were reduced in L148-inoculated plants relative to wild-type-inoculated plants (Fig. 2C, cf. lane 1 and lanes 11 to 13). DNA gel blot analysis at 7, 14, and 21 days postinoculation showed that the differences in TGMV DNA levels between L148- and wild-type-infected plants are stable over time (data not shown). Reduced accumulation of viral DNA (3 to 4% of wild-type values) was previously observed for KEE146-infected plants (38). In these earlier experiments, an E- -N140 mutant accumulated to 1 to 2% of wild-type levels but caused wild-type symptoms, establishing that there is no direct relationship between the altered symptoms and reduced viral DNA accumulation during infection (38). Hence, the altered symptoms caused by the L148 mutation are likely to be due to reduced pRBR binding, as has been hypothesized for KEE146.
AL1/pRBR binding activity is differentially affected by substitutions at L148.
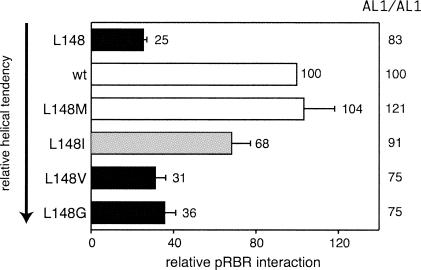
Because of the strong phenotypic effect of the L148 mutation, we examined the role of L148 in AL1/pRBR interactions in greater detail. The leucine residue may facilitate pRBR binding by contributing molecular contacts and/or by stabilizing the predicted structure of the helix 4 motif. To address these possibilities, we substituted a series of amino acids at position L148 with different side chains and tendencies to occur in α-helices. The impact of the different mutations on the pRBR binding and oligomerization activities of TGMV AL1 was analyzed in yeast two-hybrid assays (Fig. 3). An L148 M mutation had no detectable effect on pRBR binding activity, whereas an L148I substitution resulted in a moderate reduction. Like the L148 mutant, the L148V and L148G mutants displayed significantly less pRBR binding activity than wild-type AL1. In general, the binding activities of the mutants declined with the decreasing probability of the substituted amino acid to occur in an α-helix. However, the low activity of the L148 mutant, which is predicted to readily form an α-helical structure, supports the involvement of side chain contacts. Together, these results suggest that L148 contributes both structural and specific contacts to pRBR binding. None of the mutations had a strong effect on AL1 oligomerization activity, indicating that the reduced pRBR binding activities are not due to a general destabilization of AL1. It was not possible to use the L148V and L148G mutants to assess further the impact of AL1/pRBR interactions on the disease process due to instability or lack of functionality of these mutations during infection (unpublished data).
FIG. 3.
The pRBR binding activity of AL1 is differentially affected by substitutions at position 148. Yeast was cotransformed with the ZmRBR 214C cassette fused to the Gal4 DBD and Gal4 AD cassettes for either wild-type (L148) or mutant AL1 coding sequences (on the left) and analyzed as described for Fig. 1B. The arrow indicates decreasing α-helical tendency of the amino acid substitutions (50). Bars are as defined for Fig. 1. The effect of the mutations on AL1 oligomerization activity is indicated on the right.
The replication proteins of TYLCV and CaLCuV bind to pRBR.
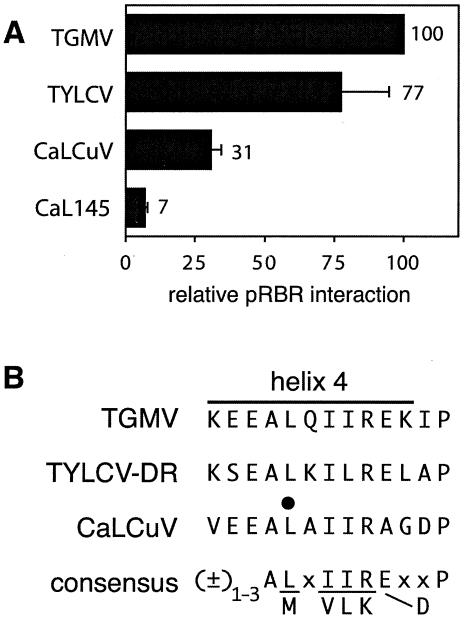
To date, TGMV AL1 is the only begomovirus replication protein that has been shown to interact with pRBR (1). The begomovirus genus includes many viruses with distinct host ranges, symptoms, and tissue tropisms, and it not known if pRBR binding is a general property of begomovirus replication proteins. As a consequence, we asked if the C1/AL1 proteins of other begomoviruses interact with pRBR. For these experiments, we chose two geminiviruses—TYLCV (TYLCV-DR) and CaLCuV (also referred to as CbLCV)—that are evolutionarily distant from TGMV and each other. TYLCV-DR, which has a single genome component, is representative of Old World begomoviruses (59). CaLCuV is from a small group of New World begomoviruses whose AL1 proteins lack a highly conserved sequence of unknown function between the DNA cleavage motif III and the predicted helix 3 (31, 53). We generated Gal4 AD fusions corresponding to full-length TYLCV C1 and CaLCuV AL1 and tested the fusions for interaction with a DBD-pRBR fusion in yeast. The pRBR binding activity of TYLCV C1 was similar to that detected for TGMV AL1 in parallel assays (Fig. 4A). In contrast, we were unable to recover colonies carrying the expression cassette corresponding to the full-length CaLCuV AL1 fusion. We have encountered similar expression problems in bacterial and insect cell systems with full-length CaLCuV AL1, indicating that its accumulation is detrimental. To overcome this problem, we generated a Gal4 AD fusion corresponding to amino acids 2 to 178 of CaLCuV AL1. Using this fusion, we detected a reduced but significant level of pRBR binding by the CaLCuV AL1 N terminus (Fig. 4A).
FIG. 4.
pRBR interacts with the replication proteins of CaLCuV and TYLCV. (A) Yeast was cotransformed with the ZmRBR 214C cassette fused to the Gal4 DBD and Gal4 AD cassettes for TGMV AL1, TYLCV C1, CaLCuV AL1, or mutant CaLCuV AL1 (CaL145). Protein interactions were assayed by measuring β-galactosidase activity in total protein extracts and normalized to wild-type TGMV AL1 (100%). The error bars correspond to 2 standard errors. (B) Helix 4 motifs of TGMV AL1 (amino acids 144 to 156), TYLCV to DR C1 (amino acids 142 to 154), and CaLCuV AL1 (amino acids 141 to 153). The conserved leucine residue in the helix center that was mutated to an alanine in CaL145 is marked with a dot. A consensus for begomovirus AL1/C1 proteins is shown at the bottom.
We compared the AL1/C1 sequences from 78 begomoviruses of both Old and New World descent to derive a consensus sequence for the helix 4 motif (Fig. 4B). These comparisons revealed that the motif consists of a conserved hydrophobic core flanked by charged residues. The core includes an invariant alanine residue followed by a leucine in 67 of the examined AL1/C1 proteins and methionine in the remaining 11 proteins. The L/M position corresponds to L148 in TGMV and is represented by a leucine in both TYLCV and CaLCuV. We asked if an alanine substitution at residue L145 in CaLCuV AL1 impairs pRBR binding analogous to the TGMV L148 mutant. The L145 mutation was introduced into the Gal4 AD fusion vector carrying amino acids 2 to 178 of the CaLCuV AL1 coding sequence and tested for pRBR binding in yeast two-hybrid assays. As shown in Fig. 4A, the CaL145 mutation caused a significant reduction in pRBR binding activity. The CaLCuV L145 and TGMV L148 mutations reduced pRBR binding to 23% (Fig. 4A) and 25% (Fig. 1B) of their respective wild-type controls. Together, these data show that diverse begomovirus replication proteins interact with pRBR through a conserved motif.
DISCUSSION
Geminiviruses do not encode the DNA polymerases and accessory factors required for their replication; instead, they rely on plant DNA replication machinery. Earlier studies showed that TGMV and CaLCuV alter host transcriptional controls to induce PCNA synthesis, a component of the host DNA replication complex (17, 18). Interaction of TGMV AL1 with pRBR plays a key role in this induction process (38), but it was not known if other begomovirus replication proteins also bind to pRBR. In this paper, we show that like TGMV AL1, CaLCuV AL1 and TYLCV C1 bind to pRBR. Mutational studies established that the helix 4 motif, which is distinct from the canonical LXCXE motif (Fig. 5), contributes several important contacts for pRBR binding. Together, these studies demonstrated conservation of the pRBR binding activity of begomovirus AL1/C1 proteins through the helix 4 motif.
The helix 4 motif is an 11-amino-acid sequence that is located within residues 140 to 170 of geminivirus replication proteins and is predicted to form an α-helix with more than 90% probability (53). It contains clusters of charged amino acids at its ends separated by a conserved hydrophobic core (Fig. 4B). These properties are characteristic of many protein binding surfaces that rely on a combination of ionic bonds and buried hydrophobic residues to stabilize protein interactions (22). Earlier studies showed that the helix 4 motif in TGMV AL1 is located within the overlapping oligomerization and pRBR binding domains and indicated that the charged residues at the edges of the motif are involved in protein interactions (38, 53). The C-terminal residues REK154 contribute to AL1/AL1 and AL1/pRBR interactions equally, while the N-terminal residues KEE146 are more important for pRBR binding. Analysis of individual mutations in the KEE sequence revealed that pRBR binding is more dependent on K144 than on E145 and E146. The wild-type phenotype of the double mutant EE146 ruled out the possibility that the individual E residues are redundant with each other. The KEE146 mutation was stronger than any of the individual or double mutants, indicating that at least two residues in the sequence are essential for wild-type pRBR binding. The involvement of multiple charged residues is consistent with the presence of two or three charged amino acids at the N-terminal edge of helix 4 in most geminivirus replication proteins (Fig. 4B).
Analysis of the hydrophobic core of helix 4 further supported its involvement in pRBR binding and oligomerization. Mutation of A147Y and II151 affected both activities of TGMV AL1. Both mutations were significantly more detrimental to oligomerization activity, suggesting that pRBR binding is not strictly dependent on AL1/AL1 interactions as previously proposed (38, 53). In contrast, substitutions at TGMV L148 specifically reduced pRBR binding without having a significant impact on oligomerization. The importance of the leucine residue was underscored by the reduced pRBR binding activity of the equivalent CaLCuV L145 mutant. Different amino acid substitutions for TGMV L148 indicated that the leucine residue contributes both side chain contacts and to the maintenance of structural integrity of the helix 4 motif. Reduction of pRBR binding activity by the alanine replacement most likely reflected the loss of a key side chain contact, while the lower α-helical tendencies of the isoleucine and valine substitutions may have induced local structural changes that interfered with pRBR binding. The only substitution at L148 that was phenotypically normal is methionine, which has a large hydrophobic side chain and a high probability of occurring in an α-helical region. Phylogenetic analysis showed that methionine is the only naturally occurring alternative to leucine at this position in geminivirus replication proteins (Fig. 4B).
Helix 4 mutations differentially altered virus replication and infectivity. The A147Y and II151 mutations blocked both viral replication in protoplasts and infection. Both of these mutants are severely impaired in TGMV AL1 oligomerization, which is essential for origin recognition, the first step in viral replication (51). The results with K144 and EE146, both of which were wild type for replication and infection, differed from the mutant phenotype reported previously for KEE146 (38). In contrast, the L148 mutant closely resembled KEE146 in that it showed reduced replication in protoplasts and chlorosis only along the veins. It was proposed that the altered tissue specificity of KEE146 infection is a result of reduced pRBR binding and reflected a lower threshold for induction in vascular versus mesophyll and epidermal cells (38). The correlation between pRBR binding and symptoms observed for K144, EE146, and L148 further supports this hypothesis and provides insight into the nature of the proposed threshold. K144 retained 42% of wild-type pRBR binding activity, while KEE146 and L148 had 16 and 25% of the wild-type activity, respectively. These results suggested that 42% but not 25% pRBR binding activity is sufficient to allow infection of mesophyll and epidermal cells. Our data also indicated that pRBR binding may play a role in virus replication in protoplasts isolated from mid-log-phase BY-2 cells. pRBR binding mutants of WDV RepA also display reduced replication in cultured maize cells (67). These results differ significantly from those obtained with mammalian DNA tumor viruses, which do not require pRb binding activity to replicate efficiently in actively cycling cells (23), and suggest a unique role for pRBR during the plant cell division cycle.
The helix 4 motif is highly conserved across all geminivirus genera and is likely to be involved in interaction with pRBR by begomoviruses, curtoviruses, and topocuviruses, whose AL1/C1 proteins lack LXCXE motifs. In contrast, the role of the helix 4 motif in mastrevirus RepA and Rep proteins is less clear because of the presence of the canonical LXCXE motif that is required for efficient pRBR binding in vitro (43, 67). However, recent studies in animal systems showed that the contacts between pRb and LXCXE-containing proteins are extensive and that other protein domains are necessary for functional interaction. Simian virus 40 large T antigen contacts pRb through two α-helices in addition to the LXCXE sequence (36). A nonapeptide containing the LXCXE motif of human papillomavirus binds to pRb but is not sufficient to interfere with E2F/pRb interactions (42, 65). Recent crystallographic studies showed that E2F, which lacks an LXCXE motif, contacts pRb through an extended region including the marked box and the transactivation domain (65). The idea that geminiviruses also interact with pRBR through a larger protein domain is supported by the observations that the pRBR binding domain of TGMV AL1 encompasses 80 amino acids and that mutations outside of helix 4 impact binding (38). Hence, it is likely that the mastrevirus pRBR-binding domain extends beyond the LXCXE motif and may encompass the conserved helix 4 motif. The involvement of a more extensive domain that stabilizes the RepA/pRBR complex in vivo may explain why LXCXE mutations have no obvious phenotypic effect on bean yellow dwarf virus (BeYDV) infection (43).
The evolutionary conservation of the LXCXE motif in plant and animal systems suggests that it is an ancient pRb/pRBR binding motif that arose before divergence of the kingdoms. In contrast, a search of the Arabidopsis and human protein databases did not uncover any known or potential pocket domain-binding proteins with homology to the helix 4 consensus sequence (unpublished result), indicating that this motif is of more recent origin and unique to geminiviruses. As a consequence, we hypothesize that the ancestral geminivirus pRBR binding domain contained the LXCXE motif and that the helix 4 sequence evolved before divergence of the genera. Later in geminivirus evolution, begomoviruses, curtoviruses, and topocuviruses lost the LXCXE motif but retained the helix 4 motif as their primary pRBR binding site. Sequence comparison across the different geminivirus genera supports this hypothesis. In addition to the conservation of the helix 4 motif, the C-terminal half of the LXCXE motif is conserved in all genera (Fig. 5). In begomoviruses, curtoviruses, and topocuviruses, the N-terminal half of the LXCXE motif appears to have undergone concerted evolution away from the consensus. In addition, helix 4 may have evolved by the replacement of a tyrosine residue conserved in mastrevirus RepA/Rep proteins with an invariant alanine in other geminivirus genera, leading to enhanced pRBR binding through this motif. Recent studies (32) of the pRBR binding properties of the RepA and Rep proteins may provide insight into the basis of the selective pressure for these changes. Even though these proteins are splicing variants that share the same 200-amino-acid N terminus and include the LXCXE and helix 4 motifs, only RepA is able to bind to pRBR. It was proposed that the inability of Rep to interact with pRBR reflects steric hindrance induced by its C-terminal domain that is absent in RepA but shared by the AL1/C1 proteins of other geminivirus genera. Hence, it is possible that when the ancestral C1-C2 gene rearranged and lost the ability to produce two proteins, there was strong pressure to maintain the ability to bind to pRBR and alter plant cell cycle controls, leading to evolution of the pRBR binding domain of the AL1/C1 proteins of begomoviruses, curtoviruses, and topocuviruses.
Acknowledgments
This research was supported by a grant from the National Science Foundation (MCB-0110536 to LHB) and a postdoctoral fellowship from the PEW Foundation (P0291SC to GAA).
We thank Dominique Robertson and Gerardus Dambrauskas for critical reading of the manuscript.
REFERENCES
- 1.Ach, R. A., T. Durfee, A. B. Miller, P. Taranto, L. Hanley-Bowdoin, P. C. Zambriski, and W. Gruissem. 1997. RRB1 and RRB2 encode maize retinoblastoma-related proteins that interact with a plant D-type cyclin and geminivirus replication protein. Mol. Cell. Biol. 17:5077-5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ach, R. A., P. Taranto, and W. Gruissem. 1997. A conserved family of WD-40 proteins binds to the retinoblastoma protein in both plants and animals. Plant Cell 9:1595-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aronson, M. N., A. D. Meyer, J. Gyorgyey, L. Katul, H. J. Vetten, B. Gronenborn, and T. Timchenko. 2000. Clink, a nanovirus-encoded protein, binds both pRB and SKP1. J. Virol. 74:2967-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bisaro, D. M., W. D. O. Hamilton, R. H. A. Coutts, and K. W. Buck. 1982. Molecular cloning and characterization of the two DNA components of tomato golden mosaic virus. Nucleic Acids Res. 10:4913-4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boniotti, M. B., and C. Gutierrez. 2001. A cell-cycle-regulated kinase activity phosphorylates plant retinoblastoma protein and contains, in Arabidopsis, a CDKA/cyclin D complex. Plant J. 28:341-350. [DOI] [PubMed] [Google Scholar]
- 6.Briddon, R. W., I. D. Bedford, J. H. Tsai, and P. G. Markham. 1996. Analysis of the nucleotide sequence of the treehopper-transmitted geminivirus, tomato pseudo-curly top virus, suggests a recombinant origin. Virology 219:387-394. [DOI] [PubMed] [Google Scholar]
- 7.Castellano, M. M., J. C. del Pozo, E. Ramirez-Parra, S. Brown, and C. Gutierrez. 2001. Expression and stability of Arabidopsis CDC6 are associated with endoreplication. Plant Cell 13:2671-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castillo, A. G., D. Collinet, S. Deret, A. Kashoggi, and E. R. Bejarano. 2003. Dual interaction of plant PCNA with geminivirus replication accessory protein (REn) and viral replication protein (Rep). Virology 312:381-394. [DOI] [PubMed] [Google Scholar]
- 9.Chaboute, M. E., B. Clement, and G. Philipps. 2002. S phase and meristem-specific expression of the tobacco RNR1b gene is mediated by an E2F element located in the 5′ leader sequence. J. Biol. Chem. 277:17845-17851. [DOI] [PubMed] [Google Scholar]
- 10.Coello, P., R. Rodriguez, E. Garcia, and J. M. Vazquez-Ramos. 1992. A DNA polymerase from maize axes—its purification and possible role. Plant Mol. Biol. 20:1159-1168. [DOI] [PubMed] [Google Scholar]
- 11.de Jager, S. M., M. Menges, U. M. Bauer, and J. A. Murray. 2001. Arabidopsis E2F1 binds a sequence present in the promoter of S-phase-regulated gene AtCDC6 and is a member of a multigene family with differential activities. Plant Mol. Biol. 47:555-568. [DOI] [PubMed] [Google Scholar]
- 12.de Jager, S. M., and J. A. Murray. 1999. Retinoblastoma proteins in plants. Plant Mol. Biol. 41:295-299. [DOI] [PubMed] [Google Scholar]
- 13.Dellaporta, S. L., J. Wood, and J. B. Hicks. 1983. A plant DNA minipreparation: version II. Plant Mol. Bio. Rep. 1:19-21. [Google Scholar]
- 14.del Pozo, J. C., M. B. Boniotti, and C. Gutierrez. 2002. Arabidopsis E2Fc functions in cell division and is degraded by the ubiquitin-SCF(AtSKP2) pathway in response to light. Plant Cell 14:3057-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Durfee, T., H. S. Feiler, and W. Gruissem. 2000. Retinoblastoma-related proteins in plants: homologues or orthologues of their metazoan counterparts? Plant Mol. Biol. 43:635-642. [DOI] [PubMed] [Google Scholar]
- 16.Dyson, N., P. Guida, K. Munger, and E. Harlow. 1992. Homologous sequences in adenovirus E1A, and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J. Virol. 66:6893-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Egelkrout, E., L. Mariconti, R. Cella, D. Robertson, and L. Hanley-Bowdoin. 2002. The activity of the proliferating cell nuclear antigen promoter is differentially regulated by two E2F elements during plant development. Plant Cell 14:3225-3236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egelkrout, E. M., D. Robertson, and L. Hanley-Bowdoin. 2001. Proliferating cell nuclear antigen transcription is repressed through an E2F consensus element and activated by geminivirus infection in mature leaves. Plant Cell 13:1437-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elmer, J. S., L. Brand, G. Sunter, W. E. Gardiner, D. M. Bisaro, and S. G. Rogers. 1988. Genetic analysis of tomato golden mosaic virus II. Requirement for the product of the highly conserved AL1 coding sequence for replication. Nucleic Acids Res. 16:7043-7060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fontes, E. P. B., P. A. Eagle, P. A. Sipe, V. A. Luckow, and L. Hanley-Bowdoin. 1994. Interaction between a geminivirus replication protein and origin DNA is essential for viral replication. J. Biol. Chem. 269:8459-8465. [PubMed] [Google Scholar]
- 21.Fontes, E. P. B., H. J. Gladfelter, R. L. Schaffer, I. T. D. Petty, and L. Hanley-Bowdoin. 1994. Geminivirus replication origins have a modular organization. Plant Cell 6:405-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gallet, X., B. Charloteaux, A. Thomas, and R. Brasseur. 2000. A fast method to predict protein interaction sites from sequences. J. Mol. Biol. 302:917-926. [DOI] [PubMed] [Google Scholar]
- 23.Gjorup, O. V., P. E. Rose, P. S. Holman, B. J. Bockus, and B. S. Schaffhausen. 1994. Protein domains connect cell cycle stimulation directly to initiation of DNA replication. Proc. Natl. Acad. Sci. USA 91:12125-12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gorbalenya, A. E., and E. V. Koonin. 1993. Helicase: amino acid sequence comparisons and structure-function relationships. Curr. Opin. Struct. Biol. 3:419-429. [Google Scholar]
- 25.Grafi, G., R. J. Burnett, T. Helentjaris, B. A. Larkins, J. A. Decaprio, W. R. Sellers, and W. G. Kaelin. 1996. A maize cDNA encoding a member of the retinoblastoma protein family: involvement in endoreduplication. Proc. Natl. Acad. Sci. USA 93:8962-8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gutierrez, C. 2000. DNA replication and cell cycle in plants: learning from geminiviruses. EMBO J. 19:792-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gutierrez, C., E. Ramirez-Parra, M. M. Castellano, and J. C. del Pozo. 2002. G1 to S transition: more than a cell cycle engine switch. Curr. Opin. Plant Biol. 5:480-486. [DOI] [PubMed] [Google Scholar]
- 28.Hanley-Bowdoin, L., S. B. Settlage, B. M. Orozco, S. Nagar, and D. Robertson. 1999. Geminiviruses: models for plant DNA replication, transcription, and cell cycle regulation. Crit. Rev. Plant Sci. 18:71-106. [PubMed] [Google Scholar]
- 29.Harbour, J. W., and D. C. Dean. 2000. The Rb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14:2393-2409. [DOI] [PubMed] [Google Scholar]
- 30.Herwig, S., and M. Strauss. 1997. The retinoblastoma protein: a master regulator of cell cycle, differentiation and apoptosis. Eur. J. Biochem. 246:581-601. [DOI] [PubMed] [Google Scholar]
- 31.Hill, J. E., J. O. Strandberg, E. Hiebert, and S. G. Lazarowitz. 1998. Asymmetric infectivity of pseudorecombinants of cabbage leaf curl virus and squash leaf curl virus: implications for bipartite geminivirus evolution and movement. Virology 250:283-292. [DOI] [PubMed] [Google Scholar]
- 32.Horvath, G. V., A. Pettko-Szandtner, K. Nikovics, M. Bilgin, M. Boulton, J. W. Davies, C. Gutierrez, and D. Dudits. 1998. Prediction of functional regions of the maize streak virus replication-associated proteins by protein-protein interaction analysis. Plant Mol. Biol. 38:699-712. [DOI] [PubMed] [Google Scholar]
- 33.Huntley, R., S. Healy, D. Freeman, P. Lavender, S. deJager, J. Greenwood, J. Makker, E. Walker, M. Jackman, Q. Xie, A. J. Bannister, T. Kouzarides, C. Gutierrez, J. H. Doonan, and J. A. H. Murray. 1998. The maize retinoblastoma protein homologue ZmRb-1 is regulated during leaf development and displays conserved interactions with G1/S regulators and plant cyclin D (CycD) proteins. Plant Mol. Biol. 37:155-169. [DOI] [PubMed] [Google Scholar]
- 34.Jansen-Durr, P. 1996. How viral oncogenes make the cell cycle. Trends Genet. 12:270-275. [DOI] [PubMed] [Google Scholar]
- 35.Kaelin, W. G. 1999. Functions of the retinoblastoma protein. Bioessays 21:950-958. [DOI] [PubMed] [Google Scholar]
- 36.Kim, H. Y., B. Y. Ahn, and Y. Cho. 2001. Structural basis for the inactivation of retinoblastoma tumor suppressor by SV40 large T antigen. EMBO J. 20:295-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong, L., and L. Hanley-Bowdoin. 2002. A geminivirus replication protein interacts with a protein kinase and a motor protein that display different expression patterns during plant development and infection. Plant Cell 14:1817-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kong, L. J., B. M. Orozco, J. L. Roe, S. Nagar, S. Ou, H. S. Feiler, T. Durfee, A. B. Miller, W. Gruissem, D. Robertson, and L. Hanley-Bowdoin. 2000. A geminivirus replication protein interacts with the retinoblastoma protein through a novel domain to determine symptoms and tissue specificity of infection in plants. EMBO J. 19:3485-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kosugi, S., and Y. Ohashi. 2002. E2F sites that can interact with E2F proteins cloned from rice are required for meristematic tissue-specific expression of rice and tobacco proliferating cell nuclear antigen promoters. Plant J. 29:45-59. [DOI] [PubMed] [Google Scholar]
- 40.Laufs, J., W. Traut, F. Heyraud, V. Matzeit, S. G. Rogers, J. Schell, and B. Gronenborn. 1995. In vitro cleavage and joining at the viral origin of replication by the replication initiator protein of tomato yellow leaf curl virus. Proc. Natl. Acad. Sci. USA 92:3879-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lavia, P., and P. Jansen-Durr. 1999. E2F target genes and cell-cycle checkpoint control. Bioessays 21:221-230. [DOI] [PubMed] [Google Scholar]
- 42.Lee, J. O., A. A. Russo, and N. P. Pavletich. 1998. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature 391:859-865. [DOI] [PubMed] [Google Scholar]
- 43.Liu, L., K. Saunders, C. L. Thomas, J. W. Davies, and J. Stanley. 1999. Bean yellow dwarf virus RepA, but not Rep, binds to maize retinoblastoma protein, and the virus tolerates mutations in the consensus binding motif. Virology 256:270-279. [DOI] [PubMed] [Google Scholar]
- 44.Lucy, A. P., M. I. Boulton, J. W. Davies, and A. J. Maule. 1996. Tissue specificity of Zea mays infection by maize streak virus. Mol. Plant-Microbe Interact. 9:22-31. [Google Scholar]
- 45.Luque, A., A. P. Sanz-Burgos, E. Ramirez-Parra, M. M. Castellano, and C. Gutierrez. 2002. Interaction of geminivirus Rep protein with replication factor C and its potential role during geminivirus DNA replication. Virology 302:83-94. [DOI] [PubMed] [Google Scholar]
- 46.Murray, J. A. H. 1997. The retinoblastoma protein is in plants! Trends Plant Sci. 2:82-84. [Google Scholar]
- 47.Nagar, S., T. J. Pedersen, K. Carrick, L. Hanley-Bowdoin, and D. Robertson. 1995. A geminivirus induces expression of a host DNA synthesis protein in terminally differentiated plant cells. Plant Cell. 7:705-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nakagami, H., M. Sekine, H. Murakami, and A. Shinmyo. 1999. Tobacco retinoblastoma-related protein phosphorylated by a distinct cyclin-dependent kinase complex with Cdc2/cyclin D in vitro. Plant J. 18:243-252. [DOI] [PubMed] [Google Scholar]
- 49.Oakenfull, E. A., C. Riou-Khamlichi, and J. A. Murray. 2002. Plant D-type cyclins and the control of G1 progression. Philos. Trans. R. Soc. Lond. B 357:749-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Neil, K. T., and W. F. DeGrado. 1990. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science 250:646-651. [DOI] [PubMed] [Google Scholar]
- 51.Orozco, B. M., and L. Hanley-Bowdoin. 1998. Conserved sequence and structural motifs contribute to the DNA binding and cleavage activities of a geminivirus replication protein. J. Biol. Chem. 273:24448-24456. [DOI] [PubMed] [Google Scholar]
- 52.Orozco, B. M., and L. Hanley-Bowdoin. 1996. A DNA structure is required for geminivirus origin function. J. Virol. 270:148-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orozco, B. M., L. J. Kong, L. A. Batts, S. Elledge, and L. Hanley-Bowdoin. 2000. The multifunctional character of a geminivirus replication protein is reflected by its complex oligomerization properties. J. Biol. Chem. 275:6114-6122. [DOI] [PubMed] [Google Scholar]
- 54.Pant, V., D. Gupta, N. R. Choudhury, V. G. Malathi, A. Varma, and S. K. Mukherjee. 2001. Molecular characterization of the Rep protein of the blackgram isolate of Indian mungbean yellow mosaic virus. J. Gen. Virol. 82:2559-2567. [DOI] [PubMed] [Google Scholar]
- 55.Ramirez-Parra, E., C. Frundt, and C. Gutierrez. 2003. A genome-wide identification of E2F-regulated genes in Arabidopsis. Plant J. 33:801-811. [DOI] [PubMed] [Google Scholar]
- 56.Rossi, V., S. Locatelli, C. Lanzanova, M. B. Boniotti, S. Varotto, A. Pipal, M. Goralik-Schramel, A. Lusser, C. Gatz, C. Gutierrez, and M. Motto. 2003. A maize histone deacetylase and retinoblastoma-related protein physically interact and cooperate in repressing gene transcription. Plant Mol. Biol. 51:401-413. [DOI] [PubMed] [Google Scholar]
- 57.Rossi, V., S. Varotto, S. Locatelli, C. Lanzanova, M. Lauria, E. Zanotti, H. Hartings, and M. Motto. 2001. The maize WD-repeat gene ZmRbAp1 encodes a member of the MSI/RbAp sub-family and is differentially expressed during endosperm development. Mol. Genet. Genomics 265:576-584. [DOI] [PubMed] [Google Scholar]
- 58.Rybicki, E. P. 1994. A phylogenetic and evolutionary justification for three genera of geminiviridae. Arch. Virol. 139:49-77. [DOI] [PubMed] [Google Scholar]
- 59.Salati, R., M. K. Nakhla, D. P. Maxwell, M. G. Carvalho, Y. M. Hou, and R. L. Gilbertson. 1997. Complete nucleotide sequence of an infectious clone of tomato yellow leaf curl geminivirus from the Dominican Republic. Phytopathology 87:S84. [Google Scholar]
- 60.Settlage, S. B., B. Miller, and L. Hanley-Bowdoin. 1996. Interactions between geminivirus replication proteins. J. Virol. 70:6790-6795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sidle, A., C. Palaty, P. Dirks, O. Wiggan, M. Kiess, R. M. Gill, A. K. Wong, and P. A. Hamel. 1996. Activity of the retinoblastoma family proteins, pRB, p107, and p130, during cellular proliferation and differentiation. Crit. Rev. Biochem. Mol. Biol. 31:237-271. [DOI] [PubMed] [Google Scholar]
- 62.Stevens, R., L. Mariconti, P. Rossignol, C. Perennes, R. Cella, and C. Bergounioux. 2002. Two E2F sites in the Arabidopsis MCM3 promoter have different roles in cell cycle activation and meristematic expression. J. Biol. Chem. 277:32978-32984. [DOI] [PubMed] [Google Scholar]
- 63.Taya, Y. 1997. RB kinases and RB-binding proteins: new points of view. Trends Biochem. Sci. 22:14-17. [DOI] [PubMed] [Google Scholar]
- 64.Turnage, M. A., N. Muangsan, C. G. Peele, and D. Robertson. 2002. Geminivirus-based vectors for gene silencing in Arabidopsis. Plant J. 30:107-114. [DOI] [PubMed] [Google Scholar]
- 65.Xiao, B., J. Spencer, A. Clements, N. Ali-Khan, S. Mittnacht, C. Broceno, M. Burghammer, A. Perrakis, R. Marmorstein, and S. J. Gamblin. 2003. Crystal structure of the retinoblastoma tumor suppressor protein bound to E2F and the molecular basis of its regulation. Proc. Natl. Acad. Sci. USA 100:2363-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Xie, Q., P. Sanz-Burgos, G. J. Hannon, and C. Gutierrez. 1996. Plant cells contain a novel member of the retinoblastoma family of growth regulatory proteins. EMBO J. 15:4900-4908. [PMC free article] [PubMed] [Google Scholar]
- 67.Xie, Q., P. Suarez-Lopez, and C. Gutierrez. 1995. Identification and analysis of a retinoblastoma binding motif in the replication protein of a plant DNA virus: requirement for efficient viral DNA replication. EMBO J. 14:4073-4082. [DOI] [PMC free article] [PubMed] [Google Scholar]