Abstract
The human herpesvirus 6 (HHV-6) glycoprotein H (gH)-glycoprotein L (gL) complex associates with glycoprotein Q (gQ) (Y. Mori, P. Akkapaiboon, X. Yang, and K. Yamanishi, J. Virol. 77:2452-2458, 2003), and the gH-gL-gQ complex interacts with human CD46 (Y. Mori, X. Yang, P. Akkapaiboon, T. Okuno, and K. Yamanishi, J. Virol. 77:4992-4999, 2003). Here, we show that the HHV-6 U47 gene, which is a positional homolog of the human cytomegalovirus glycoprotein O (gO) gene, encodes a third component of the HHV-6 gH-gL-containing envelope complex. A monoclonal antibody (MAb) against the amino terminus of HHV-6 gO reacted in immunoblots with protein species migrating at 120 to 130 kDa and 74 to 80 kDa in lysates of HHV-6-infected cells and with a 74- to 80-kDa protein species in purified virions. The 80-kDa form of gO was coimmunoprecipitated with an anti-gH MAb, but an anti-gQ MAb, which coimmunoprecipitated gH, did not coprecipitate gO. Furthermore, the gH-gL-gO complex did not bind to human CD46, indicating that the complex was not a ligand for CD46. These findings suggested that the viral envelope contains at least two kinds of tripartite complexes, gH-gL-gQ and gH-gL-gO, and that the gH-gL-gO complex may play a role different from that of gH-gL-gQ during viral infection. This is the first report of two kinds of gH-gL complexes on the viral envelope in a member of the herpesvirus family.
Human herpesvirus 6 (HHV-6) was first isolated from the peripheral blood of patients with AIDS and lymphoproliferative disorders (8, 28). HHV-6 isolates can be classified into two variants, HHV-6A and HHV-6B. HHV-6B is the causative agent of exanthem subitum (40). The classification of the two variants is based on nucleotide sequence differences, as well as their immunological and biological characteristics (1-3, 6, 37).
In the herpesvirus family, the envelope glycoproteins play critical roles in viral infection, including attachment, penetration, cell-to-cell spread, and the envelopment and maturation of nascent viral particles. In all of the human and animal herpesviruses studied to date, homologs of glycoprotein H (gH) and glycoprotein L (gL) have been found (9, 14-17, 20, 21, 26, 31-33, 38, 39, 41, 42). These two envelope glycoproteins, which associate to form a gH-gL complex, have been implicated as key participants in fusion events that are critical to herpesvirus infection. Studies of the HHV-6 gH (14, 27) and gL proteins have shown them to be representative gH and gL homologs (19, 20).
Santoro et al. showed that human CD46 is a cellular receptor for HHV-6 (30). Recently, we showed that HHV-6A, but not HHV-6B, mediates fusion from without in a variety of cells expressing human CD46 (23) and that anti-gH and anti-gB monoclonal antibodies (MAbs) inhibit the cell-cell fusion induced by HHV-6A. Furthermore, we found that the HHV-6A gH-gL complex interacts with one form of the U100 gene products, which we designated glycoprotein Q (gQ) (22), and we identified the gH-gL-gQ complex of HHV-6A as the viral ligand for human CD46 (25). Santoro et al. have also reported that HHV-6 gH associates with CD46 by a coimmunoprecipitation study (29).
In the case of Epstein-Barr virus (EBV), a third viral glycoprotein, gp42, associates with the gH-gL complex (18, 35, 36). Recently, a third viral gene product of human cytomegalovirus (HCMV), glycoprotein O (gO), was identified as a member of the gH-gL complex (11, 12, 34). The gene for HCMV gO has positional homologs in the betaherpesvirus subfamily; thus, its homolog is encoded by HHV-6.
In this study, we analyzed the products encoded by the U47 gene of HHV-6, which is a positional homolog of the HCMV gO gene, and found that gO and gQ separately form tripartite complexes with gH and gL on the viral envelope. Furthermore, the gH-gL-gO complex did not bind to human CD46, indicating that the complex was not a ligand for CD46, although the gH-gL-gQ complex binds to CD46.
MATERIALS AND METHODS
Cells and viruses.
T-cell lines HSB-2 and MT-4 were cultured in RPMI 1640 medium with 10% fetal calf serum. Umbilical cord blood mononuclear cells (CBMCs) were prepared as described previously (24). HHV-6A strains GS and U1102 and HHV-6B strain HST and clinical isolate KTY were propagated on CBMCs, and the titers of the viruses were estimated by using HSB-2 or MT-4 cells. Cell-free HHV-6 was prepared as described previously (24). When HHV-6-infected CBMCs showed evidence of more than 80% infection by immunofluorescent-antibody assay, the cells were lysed by freeze-thawing twice and spun at 1,500 × g for 10 min. The supernatant was used as cell-free virus. Partially purified virions were isolated as described previously (22). HSB-2 cells were infected with HHV-6, and at 72 h postinfection, the cells were spun at 1,500 × g for 15 min at 4°C. The supernatant from the cells was concentrated by centrifugation at 20,000 rpm for 2 h at 4°C through a 15% sucrose cushion in an SW28 rotor (Beckman). Virions were collected from the bottom. Gradient-purified virions were obtained as follows. HSB-2 or MT-4 cells were infected with strain GS or HST and cultured for 3 days. At the end of the incubation period, the culture medium was harvested by centrifugation. The virus was harvested by precipitation from the medium with 10% polyethylene glycol (molecular weight, 20,000) and 0.45% NaCl. The virus pellet was resuspended in PBS, layered over a 30-ml gradient of 15 to 60% sucrose in PBS, and spun for 2 h at 20,000 rpm in an SW28 rotor (Beckman). A fluffy white band was harvested. The harvested sample was layered onto a 10-ml 10 to 40% CsCl gradient in Tris-EDTA and spun for 48 h at 20,000 rpm in an SW40 rotor (Beckman). The virus band was harvested and used as purified virions.
Antibodies.
As described previously (5), hybridoma clones producing MAbs (designated AgO-B and AgO-N-1) were established from the splenocytes of mice immunized with purified recombinant protein AgO-N. To obtain purified AgO-N, the following procedure was used. A primer pair (AgObamF [5′-ggatccTACACGTCTGATCCCTTAGAAGC-3′, where the lowercase letters represent a restriction enzyme site], and AgOsalR [5′-gtcgacTTAGTTTGCAGTCGTCGAGGATAG-3′]) was used to amplify inserts from HHV-6A cDNA (strain U1102) for the amino terminus of the U1102-encoded gO gene (corresponding to the codons for amino acids 59 to 147). The PCR products were inserted into prokaryotic expression vector pQE30 (Qiagen) via the BamHI and SalI restriction sites. The resulting expression plasmid encoded the gO gene products with an N-terminal tag containing six histidine residues (MRGSHHHHHHGS), AgO-N. The recombinant proteins were expressed in Escherichia coli and purified under denaturing conditions in accordance with the manufacturer's (Qiagen) instructions. An MAb against HHV-6A gH, 1D3, was generously provided by G. Campadelli-Fiume, University of Bologna, Bologna, Italy.
Mouse antisera specific for HHV-6A gH and gL and the MAb for HHV-6A gQ, U100-119, were previously generated as described elsewhere (22).
Endoglycosidase digestion.
For endoglycosidase digestion, endoglycosidase H (endo H) and peptide N-glycosidase F (PNGase F) were purchased from New England Biolabs. Material immunologically precipitated from cells or lysed cells was resuspended in digestion buffer and digested with endo H or PNGase F in accordance with the manufacturer's instructions.
Immunoblotting.
HHV-6- and mock-infected cells were lysed in radioimmunoprecipitation assay (RIPA) buffer (0.01 M Tris-HCl [pH 7.4], 0.15 M NaCl, 1% sodium deoxycholate, 1% Nonidet P-40, 0.1% sodium dodecyl sulfate [SDS], 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride). The lysed proteins were resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and electrotransferred onto a polyvinylidene difluoride (PVDF) membrane for immunoblotting. Standard prestained molecular weight markers (Bio-Rad) were included in parallel lanes. After blocking, the membranes were incubated for 1 h with blocking buffer (10 mM Tris-HCl [pH 7.2], 0.15 M NaCl, 3% skim milk, 0.1% Tween 20) containing the MAbs or antisera. The reactive bands were visualized with a horseradish peroxidase-conjugated second antibody and enhanced chemiluminescence (ECL) detection reagents (Amersham Pharmacia Biotech).
Immunoelectron microscopy.
MT-4 or HSB-2 cells were infected with HST or GS, and 72 h later they were washed with phosphate-buffered saline (PBS) and fixed in 0.1% glutaraldehyde and 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.4) for 10 min, after which they were fixed for an additional 12 h in 4% paraformaldehyde. To determine the exact location of the antigen, we used the cryo-thin-section immunogold method. The specimens were immersed in 1.89 M sucrose with 20% polyvinylpyrrolidone and frozen with liquid nitrogen. Thin frozen sections were then cut with an ultramicrotome (ULTRACUT S; Reichert-Nissei, Tokyo, Japan) and mounted on Formvar carbon-coated nickel grids. The sections were rinsed with PBS and then with PBS containing 1% bovine serum albumin and incubated overnight with MAbs in PBS. On the following day, the cells were incubated for 1 h with anti-mouse immunoglobulin G conjugated with 5-nm colloidal gold particles, fixed again in 2% glutaraldehyde in PBS, postfixed with 1% OsO4, embedded in LR white (London resin), and observed with a Hitachi H-7100 electron microscope. For control experiments, ultrathin sections were directly incubated with the second antibody without pretreatment with the first antibodies.
RESULTS
Predicted features of the HHV-6 U47 gene products.
The HHV-6A strain U1102 U47 gene encoded a 651-amino-acid protein with a predicted polypeptide backbone of 73 kDa, and the HHV-6B strain HST U47 gene encoded a 738-amino-acid protein with a predicted polypeptide backbone of 83 kDa (Fig. 1) (7, 10, 13). SOSUI hydropathy analysis revealed a hydrophobic sequence near the N terminus (amino acids 26 to 48 for variant A and 1 to 26 for variant B) that may serve as a signal sequence. Another hydrophobic sequence that could potentially act as a membrane-spanning domain was also detected (amino acids 148 to 170 for variant A and 117 to 139 for variant B). The primary amino acid sequence contained 18 potential N-linked glycosylation sites within the variant A U47 primary sequence and 23 within the variant B U47 primary sequence. HHV-6 U47 is a positional homolog of the HCMV gO gene; therefore, we designated the U47 gene products HHV-6 gO.
FIG. 1.
Amino acid sequence alignment of the predicted products of the HHV-6A and HHV-6B U47 genes. Amino acids conserved between the two variants are in shaded boxes, and the hydrophobic domain is underlined. The numbers on the right denote amino acid residues. U1102, HHV-6A variant strain; HST, HHV-6B variant strain.
Characterization of U47 gene products, gO, in HHV-6-infected cells.
To analyze the U47 gene, we first produced MAbs against the U47 gene products with a prokaryotic recombinant form of the U47 protein N terminus, AgO-N. These MAbs, named AgO-B and AgO-N-1, recognized the recombinant protein against which it was produced (data not shown).
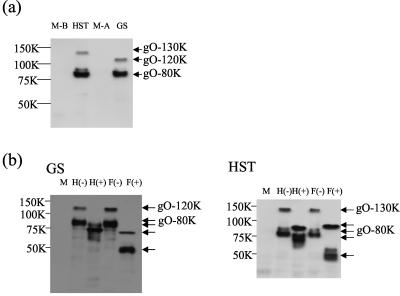
To characterize the U47 gene products in HHV-6-infected cells with the MAbs, lysates from HHV-6- or mock-infected cells were immunoblotted with AgO-N-1. AgO-N-1 specifically reacted with broadly migrating protein species. The staining was between 120 and 130 kDa (gO-130K) and between 75 and 80 kDa (gO-80K) in MT-4 cells infected with either strain HST (Fig. 2a, HST) or clinical isolate KTY (data not shown) of HHV-6B. In the case of HHV-6A, broad staining was seen between 110 and 120 kDa (gO-120K) and between 75 and 80 kDa (gO-80K) in HSB-2 cells infected with strain GS (Fig. 2a, GS) or U1102 (data not shown).
FIG. 2.
Immunoblotting of HHV-6-infected cells. (a) MT-4 and HSB-2 cells infected with HHV-6B strain HST and HHV-6A strain GS, respectively, at a multiplicity of infection of 0.1 and mock-infected MT-4(M-B) and HSB-2 (M-A) cells. The cells were lysed with RIPA buffer at 72 h postinfection, resolved by SDS-8% PAGE under reducing conditions, and electrotransferred onto a PVDF membrane, and the blots were reacted with the anti-gO MAb AgO-N-1. (b) Lysates from GS- or HST-infected cells were digested with endo H [H(+)] and PNGase F [F(+)], resolved by SDS-8% PAGE under reducing conditions, and electrotransferred onto a PVDF membrane, and the blots were reacted with anti-gO MAb AgO-N-1. M, mock infection. The values beside the panels are molecular masses in kilodaltons.
Because the HHV-6 U47 gene products are predicted to contain potential N-linked glycosylation sites, we next examined whether the U47 gene products were glycosylated by treating them with endo H, which removes immature, high-mannose asparagine (N)-linked oligosaccharides but not the mature, complex oligosaccharides, and PNGase F, which removes both the high-mannose and complex N-linked oligosaccharides. The lysates from HST-infected MT-4 cells were digested with endo H or PNGase F. Figure 2b shows the digested proteins analyzed by SDS-PAGE under reducing conditions. The 75- to 80-kDa proteins shifted in electrophoretic mobility to approximately 70 to 75 kDa after endo H treatment and to 50 to 55 kDa after PNGase F digestion in both GS- and HST-infected cells, while the 120- to 130-kDa proteins of HST shifted to 85 to 95 kDa and the 110- to 120-kDa proteins of GS shifted to 72 to 74 kDa after either endo H treatment or PNGase F digestion. Thus, the results indicate that the 75- to 80-kDa protein (gO-80K) of both variants contained complex N-linked oligosaccharides and the 120- to 130-kDa protein (gO-130K) of HST and the 110- to 120-kDa protein (gO-120K) of GS contained immature, high-mannose, N-linked oligosaccharides. One possibility is that gO-80K is a product of proteolytic cleavage during virus infection, and another possibility is that it is a product of alternative RNA splicing. Further experiments are required to determine the relationships between the various forms of these proteins.
HHV-6 gO is present in virions.
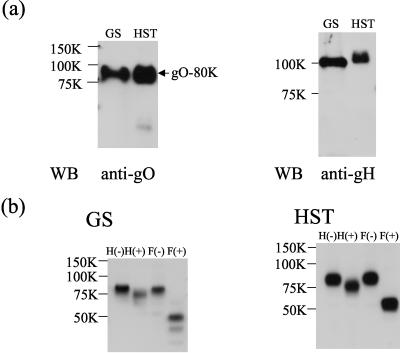
HCMV gO has been reported to be packaged into the viral particle and to form a gH-gL-gO complex in virions. Our finding that the 80-kDa form of HHV-6 gO was sensitive to PNGase-F but not to endo H raised the possibility that the 80-kDa gO protein is packaged into HHV-6 particles. To test this possibility, gradient-purified virions were lysed with sample buffer. The presence of gO in the mature viral particle was verified by immunoblotting of the lysate from purified GS and HST virions with the AgO-N-1 MAb. Only the 80-kDa protein was detected in virions with the AgO-N-1 MAb (Fig. 3a, WB [Western blotting], anti-gO), and its expression in virions was abundant. HHV-6 gH was also detected in the purified virions as a positive control (Fig. 3a, WB, anti-gH). We next examined whether the gO products expressed on viral particles were glycosylated. The lysates of purified HST or GS virions were digested with endo H or PNGase F. Figure 3b shows that the 75- to 80-kDa proteins of GS and HST shifted in electrophoretic mobility to approximately 50 to 55 kDa after PNGase F digestion.
FIG. 3.
HHV-6 gO is expressed in virions. (a) Gradient-purified virions of strains GS and HST were lysed in reducing SDS-PAGE sample buffer, resolved by SDS-PAGE, and electrotransferred to a PVDF membrane. The blots were probed with anti-gO MAb AgO-N-1 (anti-gO) or anti-gH antiserum (anti-gH). WB, Western blotting. (b) Purified virions of GS or HST were lysed with RIPA buffer, digested with endo H (H+) and PNGase F (F+), and resolved by SDS-PAGE, and the gels were electrotransferred to PVDF membranes. The blots were probed with AgO-N-1. The values beside the panels are molecular masses in kilodaltons.
Furthermore, to confirm that HHV-6 gO was present in virions, immunoelectron micrographs were prepared. As shown in Fig. 4, the gO protein of GS and HST was abundant in virions.
FIG. 4.
Electron micrographs demonstrating the presence of HHV-6 gO in virions. In HSB-2 and MT-4 cells infected with GS (A) and HST (B), respectively, immunogold particles indicating HHV-6 gO are abundantly detected in virions. In the sections directly incubated with the second antibody, no gold particles are discernible in virions in either HSB-2 (C) or MT-4 (D) cells. n, nucleus; bars, 300 nm (150 nm in insets).
HHV-6 gO associates with the gH-gL complex.
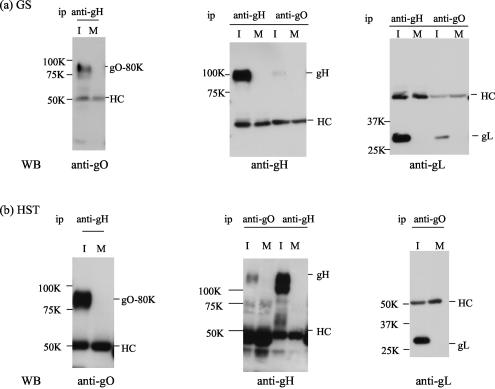
HCMV gO has been reported to form a gH-gL-gO complex. To investigate whether HHV-6 gO also forms a gH-gL-gO complex, the immunoprecipitates of GS- or HST-infected cells obtained with anti-gH MAb 1D3 were immunoblotted with anti-HHV-6 gO MAb AgO-N-1. Interestingly, AgO-N-1 reacted only with the 80-kDa gO protein (Fig. 5a and b), which was sensitive to PNGase F, but not to endo H, and was expressed in virions, supporting the idea that HHV-6 gO associates with the gH-gL complex. To further elucidate the nature of the complex, lysates from HHV-6-infected cells were immunoprecipitated with AgO-N-1. These precipitates were then immunoblotted with anti-gH or -gL antiserum. The gH and gL proteins were detected with the anti-gH and -gL antisera, respectively (Fig. 5, WB, anti-gH and -gL), but not with preimmune serum (data not shown). However, in GS-infected cell lysates, the gH and gL bands coimmunoprecipitated by the anti-gO MAb were faint. These findings indicated that only the 80-kDa form of gO interacts with the gH-gL complex. Accordingly, it was clear that the 80-kDa gO could be coprecipitated with gH (Fig. 5) and that the association among these glycoproteins was stable, even in the presence of 0.1% SDS.
FIG. 5.
Detection of a gH-gL-gQ complex in HHV-6-infected cells by immunoblotting. Lysates from mock-infected (M) and GS (a)- or HST (b)-infected (I) cell lysates were immunoprecipitated (ip) with an MAb against HHV-6 gH (1D3) (anti-gH) or HHV-6 gO (AgO-N-1) (anti-gO), and the precipitated proteins were subjected to SDS-8 or 10% PAGE under reducing conditions. The gels were electrotransferred to PVDF membranes and probed with anti-gO antiserum, anti-gH monospecific antiserum, or anti-gL monospecific antiserum. HC, immunoglobulin heavy chain of the immunoprecipitating antibody. The values beside the panels are molecular masses in kilodaltons. WB, Western blotting.
gH-gL-gO complexes are present in purified HHV-6 virions.
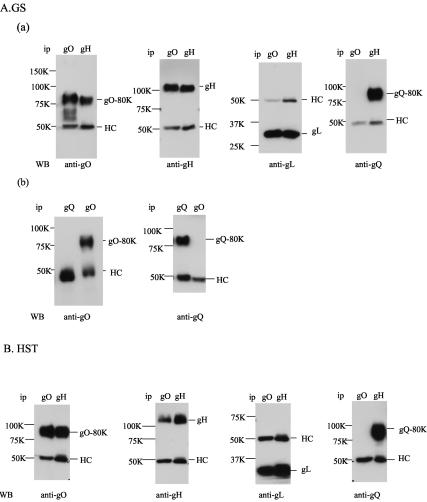
Next, to determine whether the gH-gL-gO complex is formed in virions as it is in HCMV, immunoprecipitation of partially purified GS or HST virions was performed with anti-HHV-6 gO MAb AgO-N-1 or an MAb against HHV-6 gH, ID3 (Fig. 6A and B). The precipitated proteins were then immunoblotted with AgO-N-1 or with anti-gH or anti-gL serum. Figure 6 shows that the 110-kDa HST protein, the 105-kDa gH protein (Fig. 6A, part a, and B, WB, anti-gH), and the 30-kDa gL protein (Fig. 6A, part a, and B, WB, anti-gL) derived from the immunoprecipitates obtained with MAb AgO-N-1 were detected with the anti-gH and anti-gL sera, respectively, and the 80-kDa gO proteins of GS and HST derived from the immunoprecipitates obtained with MAb 1D3 were detected with the anti-gO MAb (Fig. 6A, part a, and B, WB, anti-gO). On the basis of these data, the 80-kDa form of gO was abundant in virions, where it was associated with gH-gL. Previously, we showed that HHV-6 gQ also makes a tripartite complex with gH-gL on the virion envelope. To examine whether gH-gL-gQ and gH-gL-gO complexes exist in virions independently or not, the lysates from purified GS virions were immunoprecipitated with anti-gQ MAb AU100-119 and anti-gO MAb AgO-N-1 and then immunoblotted with the MAbs. The gO and gQ proteins were each detected by their own MAb but not by the other MAb (Fig. 6A, part b, WB, anti-gO and -gQ), while gQ-80K of GS was coprecipitated by the anti-gH MAb (Fig. 6A, part a, WB, anti-gQ). In the case of HST, as well as GS, gQ was not coimmunoprecipitated by the anti-gO MAb (Fig. 6B, WB, anti-gQ) although gH and gL were coimmunoprecipitated by the MAb (Fig. 6B, WB, anti-gH and -gL). The MAb for gH, 1D3, coprecipitated gL, gQ, and gO of HST virion lysates (Fig. 6B). The anti-HHV-6B gQ MAbs we produced can be used for WB of gQ-80K but not for immunoprecipitation of gQ-80K. These data show that both complexes, gH-gL-gO and gH-gL-gQ, in virions do not interact and exist in GS and HST.
FIG. 6.
Formation of a gH-gL-gO complex in purified HHV-6 virions. (A, part a) Lysates of partially purified GS virions with RIPA buffer were immunoprecipitated (ip) with anti-gH MAb 1D3 (gH) or anti-gO MAb AgO-N-1 (gO), electroblotted, and probed with the anti-gO MAb, anti-gH antiserum, anti-gL antiserum, or anti-gQ MAb. (A, part b) Lysates of partially purified GS virions with RIPA buffer were immunoprecipitated with the anti-gO MAb (gO) or anti-gQ MAb (gQ), electroblotted, and probed with the anti-gO MAb or the anti-gQ MAb. HC, immunoglobulin heavy chain of the immunoprecipitating antibody; LC, immunoglobulin light chain of the immunoprecipitating antibody. (B) Lysates of partially purified HST virions with RIPA buffer were immunoprecipitated with anti-gH MAb 1D3 (gH) or anti-gO MAb AgO-N-1 (gO), electroblotted, and probed with the anti-gO MAb, anti-gH antiserum, anti-gL antiserum, or anti-gQ MAb. HC, immunoglobulin heavy chain of the immunoprecipitating antibody. The values beside the panels are molecular masses in kilodaltons. WB, Western blotting.
The HHV-6A gH-gL-gQ complex binds to human CD46, but the gH-gL-gO complex does not.
Previously, it was shown that the HHV-6A gH-gL-gQ complex binds to human CD46. The tripartite complex might be important for this interaction, because gH-gL or gQ alone did not bind to CD46 (25). Therefore, we investigated whether the gH-gL-gO complex also associates with human CD46.
To investigate if the gH-gL-gO complex associates with human CD46, the culture supernatants from baculovirus-infected cells containing one of the secreted proteins, CD46(309his) or CD4(387his), were concentrated 10-fold by Centricon (Millipore) as described previously (25). The concentrated supernatant (300 μl) containing CD46(309his) or CD4(387his) was then incubated with Immobilized Cobalt Chelate (ProFound Pull-Down PolyHis protein, Protein Interaction Kit; Pierce). After the resin was washed, it was incubated with the lysates from the purified virions of GS and HST. After extensive washing, the proteins were eluted in elution buffer (washing buffer with 290 mM imidazole), and the eluted proteins were detected by immunoblotting with the MAbs for HHV-6 gQ and gO or antisera for HHV-6 gH and gL. In the case of GS, none of the proteins eluted from the CD4(387his)-bound resin were immunoblotted with any of the MAbs or antisera. Of the proteins eluted from the CD46(309his)-bound resin, as expected, the 80-kDa gQ-80K, gH, and gL proteins were detected on the blot membrane with the anti-gQ MAb and the anti-gH and anti-gL antisera, respectively, but the gO-80K protein was not (Fig. 7, GS), supporting the idea that both complexes exist in GS virions and only gH-gL-gQ interacts with CD46. To eliminate the possibility of a lower gO-80K protein signal level, the blot membrane was exposed for 1 h; however, the signal was not detected. Surprisingly, in the case of HST, no proteins eluted from the CD4(387his)- or CD46(309his)-bound resin were immunoblotted with any of the MAbs or antisera, indicating that that the HHV-6B gH-gL-gQ complex does not associate with CD46 (Fig. 7, HST).
FIG. 7.
The HHV-6A gH-gL-gO complex is not a ligand of human CD46. The culture supernatants from baculovirus-infected cells, containing secreted protein CD46(309his) or CD4(387his), were concentrated 10-fold by Centricon (Millipore) as described previously (25). The concentrated supernatant (300 μl) containing CD46(309his) or CD4(387his) was then incubated with Immobilized Cobalt Chelate (ProFound Pull-Down PolyHis protein, Protein Interaction Kit; Pierce) at 4°C for 2 h. After the resin was washed, it was incubated with the lysates from the GS or HST virions at 4°C for 4 h. After extensive washing with washing buffer (ProFound Pull-Down PolyHis protein, Protein Interaction Kit; Pierce), the proteins were eluted in elution buffer (washing buffer with 290 mM imidazole), and the eluted proteins were detected by immunoblotting with anti-gH antiserum, anti-gL antiserum, anti-gQ MAb, anti-gO MAb, or anti-His antibody. (Top panels) Although HHV-6A (GS) gQ-80K, gH, and gL were detected in the samples eluted from the CD46(309his)-bound resin, but not from the CD4(387his)-bound resin, gO-80K was not found in the samples eluted from either the CD46(309his)-bound or the CD4(387his)-bound resin. (Bottom panels) Neither HHV-6B (HST) gQ-80K, gH, gL, nor gO-80K was detected in the samples eluted from either the CD46-bound or the CD4-bound resin. V, input of virion lysates; WB, Western blotting. The values beside the panels are molecular masses in kilodaltons.
DISCUSSION
Our results have demonstrated that HHV-6 U47 encodes the gO homolog of HHV-6 and the 80-kDa HHV-6A and HHV-6B envelope glycoproteins gO-80K and gQ-80K separately form tripartite complexes with HHV-6 gH and gL. These results are consistent with previous descriptions of HCMV gO, which associates with the HCMV gH-gL complex (11). The gO positional homologs have been reported to be present only in other betaherpesviruses, indicating that they represent betaherpesvirus-specific envelope glycoprotein homologs (11).
The endo H-sensitive precursor form of gO-120K for GS or gO-130K for HST was abundant in the lysates of HHV-6-infected cells, and the gO-80K protein, which associates with gH-gL, was expressed in both virions and virus-infected cells, but the virion-associated gH-gL-gO complex was not abundant in infected cells, suggesting that the mature complexes might arise late in the secretory pathway.
Recently, we demonstrated that the gH-gL complex of HHV-6 forms a gH-gL-gQ complex. Interestingly, in immunoprecipitation assays, HHV-6 gQ and gO did not coprecipitate with each other; nevertheless, they both interacted with the gH-gL complex, indicating that gH-gL-gQ and gH-gL-gO complexes in virions do not interact.
Recently, we found that HHV-6A induces fusion from without in target cells through human CD46 (23) and furthermore that anti-gH and anti-gB MAbs inhibit the cell-cell fusion induced by HHV-6A (23). Considering these data, the HHV-6A gH-gL complex may play a critical role in the life cycle of HHV-6, most likely by facilitating the viral fusion machinery. The role of gH in fusion may be facilitated by the engagement of cellular receptors. In fact, we recently found that the HHV-6A gH-gL-gQ complex associates with human CD46, which is a cellular receptor of HHV-6 (25). Recently, Santoro et al. showed that a MAb against HHV-6 gH coimmunoprecipitated CD46 and identified gH as the CD46-binding component of HHV-6 (29). Their study raises the question of whether our recent results reflect direct binding of gH to CD46 or indirect coimmunoprecipitation of gH due to the interaction with gQ and gL. To confirm this, they reported the immunoprecipitation with an anti-gH MAb of [35S]methionine metabolic labeling and lack of a gQ-80K band. However, in our recent results, while gQ-74K was detected as a thick band, gQ-80K was detected as a faint band in a [35S]methionine metabolic labeling experiment (22). Therefore, we confirmed the 80-kDa band as gQ-80K by immunoblotting. Furthermore, they reported that the coimmunoprecipitation of CD46 and viral ligand was anti-gH MAb specific and not anti-gQ MAb specific. However, in our recent study, CD46 and viral ligand were coimmunoprecipitated with anti-gQ MAb AgQ-119 (25). One possible explanation for the discrepancy is that the anti-gQ MAb they used in their study did not precipitate gQ-80K, which interacts with gH-gL, because the 100- to 105-kDa bands that correspond to gH were not present in the figure in their report (29). In our case, we could produce MAbs against HHV-6B gQ that precipitated gQ-74K but we have not obtained MAbs that precipitate HHV-6B gQ-80K. Therefore, it is not clear whether the anti-gQ MAb they used can recognize gQ-80K for immunoprecipitation. Furthermore, they reported that CD46 could be coimmunoprecipitated by recombinant epitope-tagged gH, but only when it was expressed in HHV-6-infected cells, suggesting that the requirement of the other molecule(s) for proper folding is a likely contributor to the result (29).
In this study, we showed that the HHV-6A gH-gL-gQ complex, but not the gH-gL-gO complex, interacted with soluble CD46, indicating that the gH-gL-gO complex in virions may play a different role in HHV-6 infection from that of the gH-gL-gQ complex (Fig. 8).
FIG. 8.
Model of gH-gL-gO and gH-gL-gQ complexes of HHV-6A on the viral envelope and CD46 binding. Different tripartite complexes (gH-gL, gH-gL-gO, and gH-gL-gQ) exist on the viral envelope independently. The gH-gL-gQ complex of HHV-6A binds CD46, but the gH-gL-gO complex of HHV-6A does not. The gH-gL-gO complex may bind a cellular molecule (X) that works as a cellular receptor.
Accordingly, what role(s) does the HHV-6 gH-gL-gO complex play in HHV-6 infection? To answer this question, it is crucial to have a thorough knowledge of the intracellular processing pathways that these three proteins undergo to produce the mature functional complex.
Outside of primary T lymphocytes, the two viral variants HHV-6A and HHV-6B display different host ranges. Typically, variant A viruses replicate in HSB-2 and SupT1 cells, whereas variant B viruses grow in Molt-3 T cells (1, 4) and MT-4 cells. Interestingly, here we showed that gH, gL, and gQ of HHV-6A were pulled down with immobilized CD46 but those of HHV-6B were not, indicating that the complex of HHV-6A, but not that of HHV-6B, associates with recombinant CD46. Moreover, recombinant CD46 inhibited HHV-6A entry, but not HHV-6B entry (unpublished data). Santoro et al. (30) have reported that human CD46 is a cellular receptor of both variants, but in our data, the HHV-6B gH-gL-gQ complex did not come down with CD46. Therefore, because human CD46 may not work as a receptor of HHV-6B or the affinity of the CD46 for HHV-6B may be weaker than that of CD46 for HHV-6A, HHV-6B gH-gL-gQ does not bind to recombinant CD46. Further experiments are required to confirm this phenomenon.
There are some divergent areas between the two variants, and these regions are likely to be important in defining the biological differences between them. The predicted HHV-6A and HHV-6B gO gene products have 76.8% amino acid identity, which is much lower than the similarity between other glycoproteins, such as gB, gH, gL, and gM, suggesting that the gH-gL-gO complex may confer different biological properties on the variants that cause them to target different cells. Thus, the expression of gO in virions may be one of the important factors that determine the cell tropism differences between HHV-6A and HHV-6B.
Glycoproteins on the viral envelope are important determinants of the specificity of the initial physical interaction between the virus and the host cell factors. The HHV-6 gH-gL-gQ and gH-gL-gO complexes might confer different biological properties on the two variants that cause them to induce a different tropism, because of their low amino acid identity. It is interesting that different tripartite complexes of gH-gL exist on the viral envelope independently and may play distinct roles in virus entry.
Recent findings have shown that the EBV gH-gL complex associates with a third viral gene product, gp42 (18, 35, 36), and the gH-gL-gp42 complex of EBV is needed for entry of the virus into B cells but not for its entry into epithelial cells (36). Although it is unknown whether there is any cell specificity for the gH-gL-gO complex and HHV-6, the complex might interact with a cellular factor(s) other than CD46, possibly to accelerate virus entry as shown Fig. 8. Efforts are under way to determine the specific molecular functions of gO and of the gH-gL-gO complex in HHV-6 infection.
Acknowledgments
This study was supported in part by a grant-in-aid for scientific research (C) from the Japan Society for the Promotion of Science (JSPS) of Japan and a grant-in-aid for specially promoted research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan.
We thank Misa Kinoshita and Yun Bao Jiang (Osaka University Medical School) for help in producing the MAbs.
REFERENCES
- 1.Ablashi, D. V., N. Balachandran, S. F. Josephs, C. L. Hung, G. R. Krueger, B. Kramarsky, S. Z. Salahuddin, and R. C. Gallo. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184:545-552. [DOI] [PubMed] [Google Scholar]
- 2.Aubin, J. T., H. Collandre, D. Candotti, D. Ingrand, C. Rouzioux, M. Burgard, S. Richard, J. M. Huraux, and H. Agut. 1991. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J. Clin. Microbiol. 29:367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun, D. K., G. Dominguez, and P. E. Pellett. 1997. Human herpesvirus 6. Clin. Microbiol. Rev. 10:521-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campadelli-Fiume, G., P. Mirandola, and L. Menotti. 1999. Human herpesvirus 6: an emerging pathogen. Emerg. Infect. Dis. 5:353-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhepakson, P., Y. Mori, Y. B. Jiang, H. L. Huang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2002. Human herpesvirus-6 rep/U94 gene product has single-stranded DNA-binding activity. J. Gen. Virol. 83:847-854. [DOI] [PubMed] [Google Scholar]
- 6.Dockrell, D. H. 2003. Human herpesvirus 6: molecular biology and clinical features. J. Med. Microbiol. 52:5-18. [DOI] [PubMed] [Google Scholar]
- 7.Dominguez, G., T. R. Dambaugh, F. R. Stamey, S. Dewhurst, N. Inoue, and P. E. Pellett. 1999. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J. Virol. 73:8040-8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Downing, R. G., N. Sewankambo, D. Serwadda, R. Honess, D. Crawford, R. Jarrett, and B. E. Griffin. 1987. Isolation of human lymphotropic herpesviruses from Uganda. Lancet ii:390. [DOI] [PubMed] [Google Scholar]
- 9.Gompels, U. A., M. A. Craxton, and R. W. Honess. 1988. Conservation of glycoprotein H (gH) in herpesviruses: nucleotide sequence of the gH gene from herpesvirus saimiri. J. Gen. Virol. 69:2819-2829. [DOI] [PubMed] [Google Scholar]
- 10.Gompels, U. A., J. Nicholas, G. Lawrence, M. Jones, B. J. Thomson, M. E. Martin, S. Efstathiou, M. Craxton, and H. A. Macaulay. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29-51. [DOI] [PubMed] [Google Scholar]
- 11.Huber, M. T., and T. Compton. 1998. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 72:8191-8197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huber, M. T., and T. Compton. 1999. Intracellular formation and processing of the heterotrimeric gH-gL-gO (gCIII) glycoprotein envelope complex of human cytomegalovirus. J. Virol. 73:3886-3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Isegawa, Y., T. Mukai, K. Nakano, M. Kagawa, J. Chen, Y. Mori, T. Sunagawa, K. Kawanishi, J. Sashihara, A. Hata, P. Zou, H. Kosuge, and K. Yamanishi. 1999. Comparison of the complete DNA sequences of human herpesvirus 6 variants A and B. J. Virol. 73:8053-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Josephs, S. F., D. V. Ablashi, S. Z. Salahuddin, L. L. Jagodzinski, F. Wong-Staal, and R. C. Gallo. 1991. Identification of the human herpesvirus 6 glycoprotein H and putative large tegument protein genes. J. Virol. 65:5597-5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye, J. F., U. A. Gompels, and A. C. Minson. 1992. Glycoprotein H of human cytomegalovirus (HCMV) forms a stable complex with the HCMV UL115 gene product. J. Gen. Virol. 73:2693-2698. [DOI] [PubMed] [Google Scholar]
- 16.Khattar, S. K., S. van Drunen Littel-van den Harke, S. K. Attah-Poku, L. A. Babiuk, and S. K. Tikoo. 1996. Identification and characterization of a bovine herpesvirus-1 (BHV-1) glycoprotein gL which is required for proper antigenicity, processing, and transport of BHV-1 glycoprotein gH. Virology 219:66-76. [DOI] [PubMed] [Google Scholar]
- 17.Klupp, B. G., J. Baumeister, A. Karger, N. Visser, and T. C. Mettenleiter. 1994. Identification and characterization of a novel structural glycoprotein in pseudorabies virus, gL. J. Virol. 68:3868-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li, Q., S. M. Turk, and L. M. Hutt-Fletcher. 1995. The Epstein-Barr virus (EBV) BZLF2 gene product associates with the gH and gL homologs of EBV and carries an epitope critical to infection of B cells but not of epithelial cells. J. Virol. 69:3987-3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu, D. X., U. A. Gompels, L. Foa-Tomasi, and G. Campadelli-Fiume. 1993. Human herpesvirus-6 glycoprotein H and L homologs are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology 197:12-22. [DOI] [PubMed] [Google Scholar]
- 20.Liu, D. X., U. A. Gompels, J. Nicholas, and C. Lelliott. 1993. Identification and expression of the human herpesvirus 6 glycoprotein H and interaction with an accessory 40K glycoprotein. J. Gen. Virol. 74:1847-1857. [DOI] [PubMed] [Google Scholar]
- 21.McGeoch, D. J., and A. J. Davison. 1986. DNA sequence of the herpes simplex virus type 1 gene encoding glycoprotein gH, and identification of homologues in the genomes of varicella-zoster virus and Epstein-Barr virus. Nucleic Acids Res. 14:4281-4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori, Y., P. Akkapaiboon, X. Yang, and K. Yamanishi. 2003. The human herpesvirus 6 U100 gene product is the third component of the gH-gL glycoprotein complex on the viral envelope. J. Virol. 77:2452-2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mori, Y., T. Seya, H. L. Huang, P. Akkapaiboon, P. Dhepakson, and K. Yamanishi. 2002. Human herpesvirus 6 variant A but not variant B induces fusion from without in a variety of human cells through a human herpesvirus 6 entry receptor, CD46. J. Virol. 76:6750-6761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori, Y., H. Yagi, T. Shimamoto, Y. Isegawa, T. Sunagawa, R. Inagi, K. Kondo, Y. Tano, and K. Yamanishi. 1998. Analysis of human herpesvirus 6 U3 gene, which is a positional homolog of human cytomegalovirus UL 24 gene. Virology 249:129-139. [DOI] [PubMed] [Google Scholar]
- 25.Mori, Y., X. Yang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2003. Human herpesvirus 6 variant A glycoprotein H-glycoprotein L-glycoprotein Q complex associates with human CD46. J. Virol. 77:4992-4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pachl, C., W. S. Probert, K. M. Hermsen, F. R. Masiarz, L. Rasmussen, T. C. Merigan, and R. R. Spaete. 1989. The human cytomegalovirus strain Towne glycoprotein H gene encodes glycoprotein p86. Virology 169:418-426. [DOI] [PubMed] [Google Scholar]
- 27.Qian, G., C. Wood, and B. Chandran. 1993. Identification and characterization of glycoprotein gH of human herpesvirus-6. Virology 194:380-386. [DOI] [PubMed] [Google Scholar]
- 28.Salahuddin, S. Z., D. V. Ablashi, P. D. Markham, S. F. Josephs, S. Sturzenegger, M. Kaplan, G. Halligan, P. Biberfeld, F. Wong-Staal, B. Kramarsky, et al. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596-601. [DOI] [PubMed] [Google Scholar]
- 29.Santoro, F., H. L. Greenstone, A. Insinga, M. K. Liszewski, J. P. Atkinson, P. Lusso, and E. A. Berger. 2003. Interaction of the gH glycoprotein of human herpesvirus 6 with the cellular receptor CD46. J. Biol. Chem. 279: 25964-25969. [DOI] [PubMed] [Google Scholar]
- 30.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 31.Scott, S. D., G. D. Smith, N. L. Ross, and M. M. Binns. 1993. Identification and sequence analysis of the homologues of the herpes simplex virus type 1 glycoprotein H in Marek's disease virus and the herpesvirus of turkeys. J. Gen. Virol. 74:1185-1190. [DOI] [PubMed] [Google Scholar]
- 32.Spaete, R. R., K. Perot, P. I. Scott, J. A. Nelson, M. F. Stinski, and C. Pachl. 1993. Coexpression of truncated human cytomegalovirus gH with the UL115 gene product or the truncated human fibroblast growth factor receptor results in transport of gH to the cell surface. Virology 193:853-861. [DOI] [PubMed] [Google Scholar]
- 33.Stokes, A., D. G. Alber, J. Greensill, B. Amellal, R. Carvalho, L. A. Taylor, T. R. Doel, R. A. Killington, I. W. Halliburton, and D. M. Meredith. 1996. The expression of the proteins of equine herpesvirus 1 which share homology with herpes simplex virus 1 glycoproteins H and L. Virus Res. 40:91-107. [DOI] [PubMed] [Google Scholar]
- 34.Theiler, R. N., and T. Compton. 2002. Distinct glycoprotein O complexes arise in a post-Golgi compartment of cytomegalovirus-infected cells. J. Virol. 76:2890-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, X., and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus lacking glycoprotein gp42 can bind to B cells but is not able to infect. J. Virol. 72:158-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang, X., W. J. Kenyon, Q. Li, J. Mullberg, and L. M. Hutt-Fletcher. 1998. Epstein-Barr virus uses different complexes of glycoproteins gH and gL to infect B lymphocytes and epithelial cells. J. Virol. 72:5552-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wyatt, L. S., N. Balachandran, and N. Frenkel. 1990. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J. Infect. Dis. 162:852-857. [DOI] [PubMed] [Google Scholar]
- 38.Xu, J., P. B. Dallas, P. A. Lyons, G. R. Shellam, and A. A. Scalzo. 1992. Identification of the glycoprotein H gene of murine cytomegalovirus. J. Gen. Virol. 73:1849-1854. [DOI] [PubMed] [Google Scholar]
- 39.Xu, J., A. A. Scalzo, P. A. Lyons, H. E. Farrell, W. D. Rawlinson, and G. R. Shellam. 1994. Identification, sequencing and expression of the glycoprotein L gene of murine cytomegalovirus. J. Gen. Virol. 75:3235-3240. [DOI] [PubMed] [Google Scholar]
- 40.Yamanishi, K., T. Okuno, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065-1067. [DOI] [PubMed] [Google Scholar]
- 41.Yaswen, L. R., E. B. Stephens, L. C. Davenport, and L. M. Hutt-Fletcher. 1993. Epstein-Barr virus glycoprotein gp85 associates with the BKRF2 gene product and is incompletely processed as a recombinant protein. Virology 195:387-396. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida, S., L. F. Lee, N. Yanagida, and K. Nazerian. 1994. Identification and characterization of a Marek's disease virus gene homologous to glycoprotein L of herpes simplex virus. Virology 204:414-419. [DOI] [PubMed] [Google Scholar]