Abstract
Apoptosis of uninfected bystander CD4+ T cells contributes to T-cell depletion during human immunodeficiency virus type 1 (HIV-1) pathogenesis. The viral and host mechanisms that lead to bystander apoptosis are not well understood. To investigate properties of the viral envelope glycoproteins (Env proteins) that influence the ability of HIV-1 to induce bystander apoptosis, we used molecularly cloned viruses that differ only in specific amino acids in Env. The ability of these strains to induce bystander apoptosis was tested in herpesvirus saimiri-immortalized primary CD4+ T cells (CD4/HVS), which resemble activated primary T cells. Changes in Env that increase affinity for CD4 or CCR5 or increase coreceptor binding site exposure enhanced the capacity of HIV-1 to induce bystander apoptosis following viral infection or exposure to nonreplicating virions. Apoptosis induced by HIV-1 virions was inhibited by CD4, CXCR4, and CCR5 antibodies or by the CXCR4 inhibitor AMD3100, but not the fusion inhibitor T20. HIV-1 virions with mutant Envs that bind CXCR4 but are defective for CD4 binding or membrane fusion induced apoptosis, whereas CXCR4 binding-defective mutants did not. These results demonstrate that HIV-1 virions induce apoptosis through a CXCR4- or CCR5-dependent pathway that does not require Env/CD4 signaling or membrane fusion and suggest that HIV-1 variants with increased envelope/receptor affinity or coreceptor binding site exposure may promote T-cell depletion in vivo by accelerating bystander cell death.
Human immunodeficiency virus type 1 (HIV-1) infection causes functional impairment and progressive loss of CD4+ T cells, leading to immunodeficiency and AIDS (19, 61). The mechanisms of CD4+-T-cell depletion are poorly understood. Direct lysis of infected CD4+ T cells and apoptosis of bystander cells occur during HIV-1 replication in vitro (15, 36, 39, 41, 44). The relative importance of direct cytopathic effects compared to apoptosis of uninfected bystander cells remains a subject of controversy (36, 39, 41, 44, 52, 53). Most of the apoptotic CD4+ T cells in the peripheral blood and lymph nodes of HIV-1-infected patients are uninfected (4, 17, 30, 45, 62, 64). Increased apoptosis of other types of uninfected cells (i.e., CD8+ T cells, B cells, thymocytes, and neurons) is also detected in AIDS patients (42, 71, 73, 75). These findings suggest that apoptosis of uninfected bystander cells plays an important role in AIDS pathogenesis.
HIV-1 infection can induce cell death in infected and uninfected CD4+ T cells by several different mechanisms. Several HIV-1 proteins, including Tat, Vpr, and Nef, have cytotoxic effects in tissue culture (33, 58, 74, 80). However, the major direct cytopathic effects of HIV-1 are mediated by the viral envelope glycoproteins gp120 and gp41 (Env) (15, 55, 59). The HIV-1 Env can cause direct lysis of infected cells via a mechanism that is dependent on membrane fusion (15, 52, 53). Syncytia formed by the fusion of infected cells also undergo lysis or apoptosis (55). In addition to causing direct cytopathic effects, the HIV-1 Env has been implicated in bystander cell death (28, 42, 44, 65, 78). Soluble, virion-associated, or cell-associated gp120 can prime uninfected T cells for activation-induced or Fas/FasL-mediated apoptosis (5, 21, 29, 42, 80). Alternatively, the HIV-1 envelope glycoproteins can induce proapoptotic signaling via interactions with their receptors (1, 9, 18, 28, 42, 78).
The HIV-1 envelope glycoproteins bind to CD4 and the CCR5 or CXCR4 coreceptors to mediate membrane fusion and virus entry (7, 25). CD4 binding induces exposure of the coreceptor binding site in gp120, which enables binding to a coreceptor. CCR5-tropic isolates (R5 strains) predominate in the early stages of infection, whereas isolates that use both CCR5 and CXCR4 (R5X4 strains) or CXCR4 alone (X4 strains) emerge during the later stages. The emergence of R5X4 or X4 strains frequently correlates with high levels of CD4+-T-cell depletion (20, 46), suggesting that R5X4 and X4 viruses may be more cytopathic than R5 viruses. The higher levels of T-cell depletion induced by R5X4 and X4 viruses compared to R5 viruses is at least partly due to the higher percentage of CD4+ T cells expressing CXCR4 (90 to 100%) compared to CCR5 (15 to 30%) (11, 40), since R5 viruses or soluble R5 gp120 can be highly cytopathic for CCR5-expressing cells (39, 51, 78, 82).
The interactions between the HIV-1 envelope glycoproteins and CD4, CXCR4, and/or CCR5 play an important role in direct and indirect viral cytopathicity (1, 5, 8, 9, 15, 36, 37, 42, 44, 49, 53, 78). These interactions are influenced by properties of the Env that include coreceptor tropism, fusogenicity, and envelope/receptor affinity and by host cell molecules incorporated into the viral envelope (7, 14, 25, 31). HIV-1 isolates differ in their capacities to induce cell death in uninfected and infected cells (36, 38, 44, 49, 51, 65). Determinants of HIV-1 cytopathicity are located in the env gene (15, 37, 44, 53, 65, 78), but properties of the Env that influence bystander cell death have not been defined.
In this study, we investigated properties of the HIV-1 envelope glycoproteins that influence the ability of HIV-1 to induce bystander apoptosis in CD4+ T cells and define the requirements for receptor binding and membrane fusion in the generation of a proapoptotic signal. We demonstrate that HIV-1 variants with increased envelope/receptor affinity or increased exposure of the coreceptor binding site have an enhanced capacity to induce apoptosis in uninfected CD4+ T cells. The induction of bystander apoptosis requires coreceptor binding but not CD4 binding or membrane fusion. These results suggest that HIV-1 virions induce a proapoptotic signal through a CXCR4- or CCR5-dependent pathway that does not require Env/CD4 signaling, membrane fusion, or virus entry and suggest that HIV-1 variants with increased envelope/receptor affinity or coreceptor binding site exposure may promote T-cell depletion in vivo by accelerating bystander cell death.
MATERIALS AND METHODS
Cells.
293T cells were maintained in Dulbecco's modified Eagle medium supplemented with 10% (vol/vol) bovine calf serum (Sigma Chemical, St. Louis, Mo.) and 100 μg of penicillin and streptomycin/ml. Peripheral blood mononuclear cells (PBMC) were purified from the blood of healthy HIV-1-negative donors by Ficoll-Hypaque density gradient centrifugation and cultured in lymphocyte culture medium (1:1 volume of RPMI 1640 and AIM-V medium [Sigma] supplemented with 10% [vol/vol] fetal bovine serum and 100 μg of penicillin and streptomycin/ml). PBMC were stimulated with 2 μg of phytohemagglutinin (Sigma)/ml for 3 days. The T-cell fraction was isolated by negative selection using a pan-T-cell isolation kit (Miltenyi Biotech, Auburn, Calif.) and cultured in the presence of 10 U of interleukin-2 (Roche Molecular Biochemicals, Indianapolis, Ind.)/ml.
HVS transformation of CD4+ and CD8+ T cells.
The herpesvirus saimiri (HVS) subgroup C strain 488 (HVS-C488) was kindly provided by R. Desrosiers. For HVS transformation, primary CD4+ or CD8+ T cells were positively selected using CD4 or CD8 magnetic beads (Miltenyi Biotech) and infected with HVS-C488 as described previously (10). Briefly, 2 × 106 cells were infected with 0.5 ml of HVS-C488 viral stock and incubated at 37°C for 2 h. The cells were then transferred to a T-25 flask and cultured in lymphocyte culture medium supplemented with 10 U of interleukin-2/ml.
Viruses.
Plasmids encoding the HIV-1 ELI1, ELI6, and SG3 proviruses have been described previously (32, 34, 67, 81). Plasmids encoding the green fluorescent protein (GFP)-expressing proviruses 89.6-GFP and NL4-3-GFP were generously provided by G. Herbein (60) and C. Aiken, respectively. NL4-3-GFP expresses GFP in place of the nef gene. 89.6-GFP expresses both GFP and a reconstituted nef gene under the control of an internal ribosomal entry site in the nef position. 293T cells were transfected by the calcium phosphate method with 20 μg of provirus plasmid DNA. The medium was replaced after 16 h, and supernatants were harvested after 72 h. Reverse transcriptase (RT) activity in the supernatants was measured using [3H]dTTP incorporation as described previously (70). To generate virus stocks for apoptosis assays, virus produced in 293T cells was used to infect HVS-immortalized CD4+ T cells (CD4/HVS) and infection was monitored by RT assays. After clarification by low-speed centrifugation (200 × g for 5 min), the virus stock supernatants were stored at −80°C. Plasmids containing the HxB, 8x, HxB(V3BaL), and 8x(V3BaL) envelopes (43) and the Hx-IIIB, 8x-IIIB, and 8xD368R-IIIB viruses were generously provided by J. Hoxie. To construct proviruses, the KpnI-BamHI fragment of the envelope plasmids was cloned into pNL4-3, which encodes the NL4-3 provirus, to generate HxB-NL4-3, 8x-NL4-3, HxB(V3BaL)-NL4-3, and 8x(V3BaL)-NL4-3. 293T cells were transfected with 20 μg of provirus plasmid DNA. The medium was replaced after 16 h, and transfected cells were cocultured with SupT1 cells for 12 h. SupT1 cells were then monitored for virus production by RT assay, and virus stock supernatants were harvested as described above. Plasmids expressing the Hx, Hx F522Y, Hx R308A, and Hx R315A envelopes were generously provided by J. Sodroski (6, 53). To generate virus stocks, 293T cells were transfected with 16 μg of the pSVCATΔBgl provirus plasmid (65), 3 μg of envelope plasmid, 4 μg of plasmid expressing HLA-DRα, and 4 μg of plasmid expressing HLA-DRβ7. The medium was replaced after 16 h, and supernatants were harvested after 72 h.
Infections.
A total of 3 × 106 cells were incubated with 5 × 104 RT cpm of virus stock for 3 h in 1 ml at 37°C. The cells were washed once and plated in a 6-well tissue culture plate in 3 ml of medium. Twice weekly, the cells were split to keep the total cell density at 106 cells/ml. At these time points, culture supernatant was removed for RT assays and cells were removed for flow cytometric staining.
Flow cytometry.
Briefly, 106 cells were washed twice in phosphate-buffered saline (PBS) and stained with 7-aminoactinomycin D (7AAD; Via-Probe; PharMingen) for 15 min at room temperature. For intracellular p24 staining, cells were washed twice in PBS and fixed and permeabilized using a Cytofix/Cytoperm kit (Pharmingen). The cells were resuspended in 50 μl of Perm/Wash buffer and incubated with a 1:200 dilution of the KC57-RD1 α-p24 monoclonal antibody or mouse IgG1-RD1 isotype control (Coulter, Fullerton, Calif.) for 20 min at 4°C. For staining of apoptotic cells, the cells were washed twice with Perm/Wash buffer and then stained using a terminal dUTP nick-end labeling (TUNEL) kit (In Situ Cell Death Detection kit; Roche) as directed by the manufacturer. The cells were then washed twice with PBS and analyzed using a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.) or an Epics XL flow cytometer (Coulter). Data were analyzed using Cell Quest (Becton Dickinson) and Expo32 (Coulter) software.
CD4 affinity.
A total of 3 × 106 CD4/HVS T cells were infected with 105 RT cpm of ELI1 and ELI6 as described above. Five days postinfection, 2 × 106 cells were washed twice with PBS containing 2% bovine serum albumin and 0.02% sodium azide. Then, 106 cells were incubated with increasing concentrations of soluble CD4-immunoglobulin (Ig) (6) for 45 min at 37°C, while the remaining 106 cells were incubated with 10 μg of a human anti-gp120 antibody (ImmunoDiagnostics [Woburn, Mass.] no. 2501)/ml for 45 min at 4°C. Both sets of cells were then washed twice and incubated with a goat anti-human Ig-phycoerythrin (PE) antibody (Jackson ImmunoResearch, West Grove, Pa.) for 45 min at 4°C. The cells were washed twice and analyzed using an Epics XL flow cytometer. Data were analyzed using Expo32 (Coulter) and Prism (GraphPad, San Diego, Calif.) software.
Apoptosis induced by nonreplicating virions.
To determine the ability of HIV-1 to induce apoptosis in the absence of virus replication, 106 CD4+ HVS T cells were preincubated in lymphocyte medium with 1 μΜ 3′-azido-3′-deoxythymidine (AZT; Sigma) for 1 h at 37°C prior to incubation with virus stocks produced in CD4/HVS T cells or SupT1 cells, unless otherwise indicated. Alternatively, virions were UV inactivated for 45 min. Some samples were also preincubated for 1 h with 10 μg of anti-CD4 antibody (QS4120 [Calbiochem, San Diego, Calif.] and RPA-T4 [Pharmingen])/ml, 10 μg of anti-CXCR4 antibody (12G5; PharMingen)/ml, 10 μg of anti-CCR5 antibody (2D7; Pharmingen)/ml, 5 μg of anti-gp120 antibody (1b12; kindly provided by J. Sodroski and D. Burton)/ml; cross-reactive anti-gp120 (ImmunoDiagnostics no. 1121), 1.2 μM AMD3100 (26, 72), or 1 μg of T20 (American Peptide Co., Sunnyvale, Calif.)/ml. One hundred thousand RT units of virus, or an equivalent volume of supernatant from uninfected control CD4+ HVS T cell or SupT1 cultures, were added to the cells. As an additional control, some samples were incubated with virus stock supernatant that had been cleared of pelletable components, including virions, by centrifugation at 15,000 × g for 1 h. The cells were incubated for 72 h, stained with 7AAD, α-p24-RD1, and TUNEL-fluorescein isothiocyanate (FITC) as described above and analyzed by flow cytometry.
RESULTS
HIV-1 infection induces bystander apoptosis in CD4/HVS cells.
To investigate the ability of HIV-1 to induce apoptosis in uninfected bystander T cells, we used HVS-immortalized CD4+- and CD8+-T-cell lines derived from the peripheral blood T lymphocytes of an HIV-negative healthy donor. These cell lines resemble activated mature human CD4+ and CD8+ T lymphocytes (10), as indicated by high expression of activation markers such as CD25, HLA-DR, and CD95/Fas (Table 1). These cell lines express high levels of CXCR4 and CCR5 (Table 1) and were derived from one donor, eliminating confounding effects of donor variability. Furthermore, HVS-immortalized T cells have been shown to be susceptible to apoptosis (48).
TABLE 1.
Characterization of immortalized CD4+ and CD8+ T cells
| Cell surface marker | % Positive cellsa
|
|
|---|---|---|
| CD4+ T cells | CD8+ T cells | |
| CD4 | 99 | 0 |
| CD8 | 0 | 98 |
| CD25 | 99 | 61 |
| HLA-DR | 99 | 99 |
| CD95 | 99 | 99 |
| CXCR4 | 60 | 42 |
| CCR5 | 72 | 51 |
Data were obtained from flow cytometric analysis of CD4/HVS and CD8/HVS cells as described in Materials and Methods.
CD4/HVS cells were infected with the highly fusogenic X4 HIV-1 isolate SG3 (34), which has been shown to induce high levels of bystander apoptosis in primary brain cultures (65). SG3 replicated to high levels, peaking on days 7 to 11 (data not shown). On days 7 (Fig. 1) and 14 (data not shown), cells were stained with 7AAD, anti-p24-PE, and TUNEL-FITC and analyzed by flow cytometry. 7AAD was used to exclude necrotic cells, including cells killed by single-cell lysis, and debris from the p24/TUNEL analysis. On day 7, approximately 8% of cells in SG3-infected cultures were 7AAD positive, compared to 3% of the cells in control cultures. Approximately 1.5% of the cells were 7AAD-p24 double positive, representing cells undergoing single-cell lysis. SG3 infection induced high levels of apoptosis (26 to 31%), which were up to 25-fold higher than those observed in uninfected control cultures. Approximately 18 to 27% of the cells in cultures infected with SG3 stained positive for p24. However, only 2 to 6% were TUNEL-p24 double positive. Thus, up to 80% of the apoptotic cells were not productively infected.
FIG. 1.
Flow cytometric analysis of apoptosis in CD4/HVS T cells infected with SG3. Cells were infected with SG3 as described in Materials and Methods and stained on day 7 with TUNEL-FITC, anti-p24-PE, and 7AAD and analyzed by flow cytometry. 7AAD-positive cells were gated out of the TUNEL/p24 analysis.
The ELI6 variant induces higher levels of bystander apoptosis in CD4/HVS T cells than the parental primary X4 isolate ELI1.
To determine whether changes in the viral Env that increase affinity for CD4 also enhance the capacity of HIV-1 to induce bystander apoptosis, we used the primary X4 HIV-1 isolate ELI1 and the variant ELI6, which was derived by adapting ELI1 to growth in T-cell lines. The resulting envelope mutations in the adapted virus were cloned back into ELI1 to create ELI6, which is isogenic to ELI1 except for the point mutations G427R, in the C4 domain of gp120 near the CD4 binding site, and M7V, in the fusion domain of gp41 (32, 67, 81). Previous studies have shown that ELI6 has an increased ability to bind to CD4, enhanced fusogenicity on cells expressing low levels of CD4, and increased susceptibility to sCD4-induced gp120 shedding compared to ELI1 (32, 47, 67, 81), suggesting an increase in viral affinity for CD4.
To investigate the ability of ELI1 and ELI6 to induce apoptosis during infection of CD4+ T cells, CD4/HVS cells were infected with these viruses. Cells infected with SG3 were used as a positive control. All of the viruses replicated to high levels and exhibited similar replication kinetics, with replication peaking on day 7 (data not shown). On days 7 and 11, cells were stained with 7AAD, anti-p24-PE, and TUNEL-FITC and analyzed by flow cytometry. Approximately 21 to 24% of cells in infected cultures were p24 positive on day 7, and 12 to 20% were p24 positive on day 11 (Fig. 2). The amount of p24 staining varied among cultures infected with different viruses and did not always correlate directly with RT levels. This discrepancy might reflect the measurement of RT values at discrete time points, which may not accurately reflect daily variations in replication kinetics. On days 7 and 11, high levels of apoptosis above background (29 to 54%) were seen in infected cultures (Fig. 2) and less than 10% of the cells were TUNEL-p24 double positive. Up to 47% of the cells were TUNEL positive and p24 negative, representing bystander cells that were not productively infected. On days 7 and 11, ELI6 induced apoptosis in a greater total number of cells and in more p24-negative cells than ELI1. Levels of cells that were TUNEL-7AAD double positive were similar among the infected cell cultures (not shown). Thus, an adapted variant with increased ability to bind to CD4 and increased fusogenicity had a greater capacity to induce bystander apoptosis than the parental virus.
FIG. 2.
Flow cytometric analysis of apoptosis in CD4/HVS T cells infected with X4 HIV-1 viruses. Cells were infected with equivalent amounts of SG3, ELI1, or ELI6 as described in Materials and Methods and cultured for 2 weeks. On days 7 and 11, cells were stained with TUNEL-FITC and anti-p24-PE and analyzed by flow cytometry. 7AAD-positive cells were gated out of the TUNEL/p24 analysis. Results are representative of six independent experiments.
In the preceding experiment, we could not exclude the possibility that some TUNEL-positive, p24-negative cells may have been recently infected and therefore may not produce detectable levels of p24. To verify that HIV-1 infection induces apoptosis in uninfected bystander CD4/HVS T cells, we performed additional experiments using recombinant viruses that contain a gene for GFP either in place of the nef gene (NL4-3-GFP) or under the control of an internal ribosomal entry site in tandem with nef gene expression (89.6-GFP) (41). Because GFP is expressed as an early viral gene product, GFP expression is a sensitive marker for infected cells (41). CD4/HVS cells were infected with the R5X4 89.6-GFP virus or X4 NL4-3-GFP virus and stained on days 7, 10, and 14 with 7AAD and annexin-V-PE. GFP expression was used to detect infected cells, and annexin-V was used to detect apoptotic cells. 7AAD-positive cells were excluded from the GFP and annexin-V analysis. The infection peaked on day 10, with approximately 5% of the cells in the 89.6-infected culture and 8% of the cells in the NL4-3-infected culture being positive for GFP expression (Fig. 3). Annexin-V staining increased over time in the infected cultures, from approximately 5% on day 7 to approximately 12% on day 10 and 30 to 45% on day 14. At all time points, the fraction of GFP-annexin-V double-positive cells was only a small percentage (5 to 8%) of the total annexin-V-positive fraction, suggesting that the majority of apoptotic cells were uninfected (Fig. 3). Staining with anti-p24-PE showed that all of the GFP-positive cells were p24 positive (data not shown), indicating that staining with the anti-p24 antibody labeled all of the productively infected cells. Furthermore, over 97% of syncytia in CEMx174 T-cell cultures infected with serial dilutions of the GFP-expressing viruses were GFP positive (data not shown), indicating that the viruses were capable of expressing GFP in all productively infected cells and did not delete the exogenous gene. Collectively, these results indicate that HIV-1 infection induces apoptosis in uninfected CD4/HVS T cells.
FIG. 3.
Flow cytometric analysis of apoptosis in CD4/HVS T cells infected with GFP-expressing HIV-1 viruses. Cells were infected with equivalent amounts of 89.6-GFP and NL4-3-GFP as described in Materials and Methods and stained with annexin-V-PE and 7AAD on days 7, 10, and 14. 7AAD-positive cells were gated out of the analysis of annexin-V-positive cells (white columns), GFP-positive cells (gray columns), or annexin-V-GFP double-positive cells (black columns). Results are representative of two independent experiments.
ELI6 has increased affinity for CD4 compared to ELI1.
To verify that ELI6 has a higher affinity for CD4 compared to ELI1, we measured the binding of a range of concentrations of sCD4-IgG to Env expressed on the surface of CD4/HVS T cells infected with these viruses. Five days postinfection, cells were incubated with increasing concentrations of sCD4-IgG, stained with a PE-conjugated anti-human IgG antibody, and analyzed by flow cytometry. Matched cells were incubated with a human anti-gp120 antibody and stained with PE anti-human IgG, and the sCD4-IgG binding data were normalized for relative gp120 expression on the cell surface and for background sCD4-IgG binding to uninfected control cells. Nonlinear regression analysis was used to generate a binding curve and calculate the CD4 dissociation constants for the two viruses (Fig. 4). These studies demonstrated that ELI6 has an increased affinity for sCD4-IgG compared to ELI1, with dissociation constants of 21 versus 127 nM, respectively.
FIG. 4.
ELI6 has a higher affinity for CD4 than does ELI1. CD4/HVS T cells were infected with ELI1 or ELI6. Five days postinfection, cells were incubated with sCD4-IgG and stained with an anti-human IgG-PE antibody as described in Materials and Methods. Matched cells were incubated with a human anti-gp120 antibody and stained with an anti-human IgG-PE antibody. Cells were analyzed by flow cytometry, and the sCD4-IgG binding data were normalized for relative gp120 expression on the cell surface and for background sCD4-IgG binding to uninfected control cells. MFI, mean fluorescence intensity. The quantitation represents means and standard deviations (error bars) of duplicate samples. Results are representative of two independent experiments.
Nonreplicating HIV-1 virions induce apoptosis in CD4/HVS cells.
The preceding experiments demonstrated distinct differences in the levels of bystander apoptosis induced by viruses that differ in their affinities for CD4. However, we could not control for effects that might be caused by differences in replication kinetics. Therefore, we developed assays to examine T-cell apoptosis in the absence of viral replication. CD4/HVS cells were preincubated with 1 μM AZT to inhibit reverse transcription and prevent productive virus replication and were then incubated with HIV-1 virions. Alternatively, virions were UV inactivated for 45 min prior to incubation with CD4/HVS cells. Cultures were monitored for p24-positive cells for 7 days to confirm the absence of virus replication. No p24-positive cells were detected (data not shown), indicating that virus replication did not occur. Following 72 h of incubation with SG3, ELI1, or ELI6, TUNEL staining was performed to detect apoptotic cells. HIV-1 virions induced apoptosis in approximately two- to fourfold more cells than did control supernatants (11 to 20% versus 5%) (Fig. 5A). ELI6 induced apoptosis in twofold more cells than did ELI1 (20 versus 11%). In contrast to the results observed in CD4/HVS cells, none of the viruses induced apoptosis in CD8/HVS cells (Fig. 5B). These results suggest that increased viral affinity for CD4 and/or increased fusogenicity can enhance the ability of HIV-1 virions to induce bystander apoptosis in CD4+ T cells.
FIG. 5.
HIV-1 virions induce apoptosis in CD4+ T cells in the absence of virus replication. CD4/HVS T cells (A and C) and CD8/HVS T cells (B) were preincubated with 1 μM AZT and then incubated with control supernatants or HIV-1 virions or with the nonpelletable fraction (S15) of control or viral stock supernatants (C) as described in Materials and Methods. After 72 h, cells were stained with TUNEL-FITC (black columns) and 7AAD (data not shown). 7AAD-positive cells were gated out of the TUNEL analysis. The quantitation represents means and standard deviations (error bars) of triplicate samples. *, P < 0.05; **, P < 0.005 versus control by Student's two-tailed t test. Results are representative of six independent experiments.
The virus stocks used in the preceding experiments were produced in CD4/HVS cells, raising the possibility that soluble factors in the virus stock, such as inflammatory cytokines or soluble gp120, may have contributed to the induction of apoptosis. In particular, ELI6 was shown to be highly susceptible to soluble CD4-induced gp120 shedding (47, 81), raising the possibility that soluble gp120 could have contributed to the induction of bystander apoptosis. To address these questions, virions were removed from the virus stock by centrifugation and CD4/HVS cells were incubated with the nonpelletable (S15) fraction for 72 h. In contrast to ELI6 virus stock, the S15 fraction did not induce apoptosis in CD4/HVS T cells (Fig. 5C). These results indicate that CD4/HVS T-cell apoptosis is induced primarily by HIV-1 virions rather than by soluble gp120 or other soluble factors present in the virus stocks.
Apoptosis induced by nonreplicating HIV-1 virions is inhibited by anti-CD4, -CXCR4, and -gp120 monoclonal antibodies and by the CXCR4 inhibitor AMD3100, but not by the fusion inhibitor T20.
To determine whether CD4/HVS T-cell apoptosis induced by HIV-1 virions can be inhibited by blocking the interaction between gp120 and its receptors or preventing virus-cell fusion, we used monoclonal antibodies against CD4, CXCR4, or gp120, the CXCR4 inhibitor AMD3100, and T20, an HIV-1 fusion inhibitor (56). The CD4 antibodies QS4120 and RPA-T4 bind to domain 1 of CD4 and prevent binding to gp120. The CXCR4 antibody 12G5 binds to the first and second extracellular loops of CXCR4 and blocks infection by X4 HIV-1 strains. The gp120 antibodies 1b12 and ImmunoDiagnostics no. 1121 neutralize HIV-1 infection and syncytium formation. CD4/HVS cells were incubated for 1 h with 1 μM AZT and an antibody or inhibitor and then with ELI6 virions for 72 h. Apoptosis induced by HIV-1 virions was significantly reduced by preincubation with CD4, CXCR4, or gp120 antibodies (Fig. 6A) or AMD3100 (Fig. 6B) (P < 0.05). QS4120 and RPA-T4 inhibited apoptosis induced by ELI6 by 40 to 50%, whereas 12G5 and the gp120 antibodies inhibited apoptosis by 60 to 65%. AMD3100 had the most potent inhibitory effect, inhibiting apoptosis by approximately 80%. In contrast, concentrations of the fusion inhibitor T20 that completely blocked virus infection (data not shown) did not inhibit apoptosis induced by ELI6. Thus, virions induce a proapoptotic signal through a CD4- or CXCR4-dependent pathway that does not require membrane fusion or virus entry.
FIG. 6.
Inhibition of apoptosis by CD4, CXCR4, and gp120 antibodies and AMD3100. CD4/HVS T cells (A and B) or primary T cells (C) were preincubated for 1 h with 1 μM AZT and antibodies against CD4 (QS4120 and RPA-T4), CXCR4 (12G5), or gp120 (1B12 or Immunodx [ImmunoDiagnostics no. 1121]), the CXCR4 antagonist AMD3100, or the fusion inhibitor T20 as indicated and then incubated with ELI6 virions as described for Fig. 5. After 72 h, cells were stained with TUNEL-FITC (black columns) and 7AAD (data not shown). 7AAD-positive cells were gated out of the TUNEL analysis. The quantitation represents means and standard deviations (error bars) of triplicate samples. *, P < 0.05 versus ELI6 alone; **, P < 0.05 versus control by Student's two-tailed t test. Results are representative of two independent experiments.
We then examined the ability of ELI6 virions to induce apoptosis in primary T cells. Primary CD4+ and CD8+ T cells were negatively selected from phytohemagglutinin-stimulated PBMC and incubated with 105 RT counts of UV-inactivated ELI6 for 72 h. Cultures were then stained to detect apoptotic cells. ELI6 virions induced twofold higher levels of apoptosis over background (Fig. 6C). The absence of viral replication was confirmed by p24 staining and by RT assay of the culture supernatant. The monoclonal antibody 12G5 and CXCR4-inhibitor AMD3100 inhibited apoptosis induced by ELI6 by up to 77% (P < 0.05). Thus, binding of the Env to CXCR4 is required for apoptosis induced by nonreplicating X4 HIV-1 virions in primary T cells.
Apoptosis induced by nonreplicating HIV-1 virions requires CXCR4 binding but not CD4 binding or membrane fusion.
Previous studies have suggested that X4 HIV-1 virions induce a proapoptotic signal through CD4 and/or CXCR4 (13, 44, 69). However, whether CD4 binding and membrane fusion are required for the generation of the proapoptotic signal are unclear. To address this issue, we used mutant Envs that are defective for binding to CD4 or CXCR4 or for fusion with target cells. Virions were pseudotyped with the wild-type HxBc2 Env (Hx), an Hx Env with a mutation in the fusion peptide, F522Y, which abolishes the ability of the Env to fuse (53), or an Hx Env with a mutation in the V3 loop, either R308A or R315A, which abolishes CXCR4 binding (6).
During initial experiments, we found that pseudotyped virions produced by transient transfection of 293T cells did not induce apoptosis in CD4/HVS T cells (data not shown). One possible explanation for this unexpected result is that 293T cells do not express certain proteins such as major histocompatibility complex (MHC) class II and ICAM-1, which are expressed on T cells, become incorporated into the HIV-1 envelope, and enhance the ability of virions to induce apoptosis (2, 12, 14, 27, 28). Consistent with the results of previous studies (27, 28), we found that virions produced from 293T cells cotransfected with MHC class II isoforms HLA-DRα/β1 or HLA-DRα/β7 had the capacity to induce apoptosis in CD4/HVS T cells at levels similar to those induced by virions produced in T cells (data not shown). Therefore, virions for subsequent experiments were produced in 293T cells by cotransfecting an env− provirus plasmid with a plasmid expressing an HIV-1 envelope and plasmids expressing MHC class II DRα/β7.
UV-inactivated virions pseudotyped with wild-type or mutant Hx Envs were incubated with CD4/HVS T cells for 72 h. Virions pseudotyped with the wild-type Hx Env induced significantly higher levels of apoptosis compared to control supernatants (P < 0.05) (Fig. 7A). Virions pseudotyped with a fusion-defective Env containing the F522Y mutation induced similar levels of apoptosis compared to virions pseudotyped with the Hx Env, as did virions in cultures incubated with T20 (Fig. 7A). In contrast, virions pseudotyped with Envs defective for binding to CXCR4 (R308A or R315A) did not induce significant levels of apoptosis (Fig. 7A). These data suggest that CXCR4 binding, but not membrane fusion or virus entry, is required for HIV-1 virions to induce apoptosis in CD4/HVS T cells.
FIG. 7.
CXCR4 binding, but not CD4 binding or membrane fusion, is required for induction of apoptosis by HIV-1 virions. CD4/HVS T cells were incubated with equivalent amounts of UV-inactivated HIV-1 virions pseudotyped with wild-type or mutant (A and C) Env or were preincubated with 1 μM AZT for 1 h and then incubated with equivalent amounts of ELI6 virions or NL4-3 virions containing the HxB, 8x, HxB(V3BaL), or 8x(V3BaL) envelopes (B). CD4/HVS T cells were preincubated with AMD3100 for 1 h prior to incubation with virions where indicated (C). After 72 h, cells were stained with TUNEL-FITC and 7AAD (data not shown). 7AAD-positive cells were gated out of the analysis. The quantitation represents means and standard deviations (error bars) of triplicate samples. *, P < 0.05 versus control by Student's two-tailed t test. Results are representative of two independent experiments.
To further explore the relationship between CD4 binding, coreceptor binding, and apoptosis, we used viruses with envelope glycoproteins that interact with coreceptors independently of CD4. 8x and 8x(V3BaL) are CD4-independent HIV-1 viruses that can utilize CXCR4 or CCR5, respectively, in the absence of CD4 to mediate fusion and virus entry (43, 54). However, these viruses retain the ability to bind to CD4 and the efficiency of these strains is enhanced when CD4 is present (43). The 8x virus, derived by passage on cells lacking CD4, contains point mutations in the Env that increase exposure of the coreceptor binding site and allow coreceptor binding in the absence of CD4. These conformational changes are also associated with increased fusogenicity (43, 54). The 8x(V3BaL) virus was derived by replacing the V3 loop of 8x with the V3 loop from BaL, conferring R5 tropism to the virus. To determine whether increased exposure of the coreceptor binding site is associated with an increased ability of these variants to induce bystander cell death compared to the parental viruses, we cloned the 8x and 8x(V3BaL) envelopes and their parental envelopes, HxB and HxB(V3BaL), respectively, into the NL4-3 provirus and then tested the ability of these viruses to induce apoptosis in CD4/HVS cells in the absence of virus replication. 8x-NL4-3 and 8x(V3BaL)-NL4-3 virions induced twofold higher levels of apoptosis compared with the HxB-NL4-3 and HxB(V3BaL)-NL4-3 parental controls (P < 0.05) (Fig. 7B). These results suggest that increased exposure of the coreceptor binding site is associated with an enhanced capacity of X4 and R5 HIV-1 virions to induce bystander apoptosis.
The 8x envelope glycoprotein does not require CD4 expression on target cells in order to bind CXCR4 and trigger membrane fusion but retains the ability to bind to CD4 when it is present on the target cell surface. To determine the requirement for CD4 binding in the generation of a proapoptotic signal by HIV-1 virions, we used a mutant of 8x, 8xD368R, that cannot bind to CD4 while retaining the ability to bind CXCR4 directly. Virions pseudotyped with either the 8x or 8xD368R envelope induced significantly higher levels of apoptosis in CD4/HVS T cells than control supernatants (P < 0.05) (Fig. 7C). 8x virions induced slightly higher levels of apoptosis than did 8xD368R, but this difference was not statistically significant, suggesting that CD4 binding is not required for HIV-1 virions to induce apoptosis. Blocking the interaction between Env and CXCR4 with AMD3100 inhibited the induction of apoptosis by 8x and 8xD368R virions (Fig. 7C). Thus, HIV-1 virions induce a proapoptotic signal through CXCR4. In contrast, Env signaling through CD4 is not required for virion-induced apoptosis. SDF-1, the natural ligand of CXCR4, can induce both survival and apoptotic signaling in primary CD4+ T cells (79). At concentration ranges of 1 to 10 μg/ml, we found that SDF-1 treatment for 72 h induced low levels of apoptosis over background in CD4/HVS T cells (data not shown). Thus, CXCR4-mediated signaling induced by the physiological ligand at high concentrations can induce apoptotic signaling in CD4/HVS T cells.
Affinity for CCR5 influences the ability of nonreplicating R5 virions to induce apoptosis.
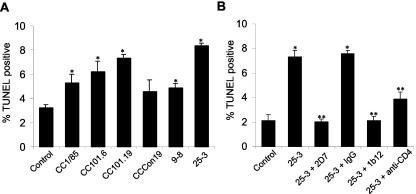
To investigate whether the ability of HIV-1 virions to induce apoptosis is influenced by changes in Env that increase affinity for a coreceptor, we used viruses adapted to growth in the presence of the small-molecule CCR5 inhibitor AD101 (77). These viruses were derived by serial passage of the parental primary R5 virus CC1/85 under the selection of AD101 to generate CC101.6 and CC101.19, selected after 6 and 19 weeks of passage, respectively. CCCon19 is a control virus passaged for 19 weeks without selection. UV-inactivated CC101.19, and to a lesser extent CC101.6, virions induced higher levels of apoptosis in CD4/HVS T cells than the parental virus or the passaged control virus (Fig. 8A). 25-3, which contains the CC101.19 envelope gene cloned into NL4-3, induced significantly higher levels of apoptosis than 9-8, which contains the cloned CC1/85 parental envelope (50) (P < 0.05) (Fig. 8A). Apoptosis induced by 25-3 was inhibited by the CCR5 antibody 2D7, a gp120 antibody, and the small-molecule CCR5 inhibitor TAK-779 and was partially blocked by a CD4 antibody (Fig. 8B and data not shown). These findings suggest that changes in the Env that increase viral affinity for CCR5 can also enhance the capacity of HIV-1 virions to induce bystander apoptosis in CD4/HVS T cells.
FIG. 8.
Apoptosis induced by HIV-1 virions with increased affinity for CCR5. CD4/HVS T cells were incubated with equivalent amounts of UV-inactivated CC1/85, CC101.6, CC101.19, CCCon19, 9-8, or 25-3 HIV-1 virions (A) or were preincubated with antibodies against CCR5 (2D7), gp120 (1b12), or CD4 (QS4120) or control IgG for 1 h and then incubated with equivalent amounts of 25-3 (B). After 72 h, cells were stained with TUNEL-FITC and 7AAD (data not shown). 7AAD-positive cells were gated out of the analysis. The quantitation represents means and standard deviations (error bars) of triplicate samples. *, P < 0.05 versus control (A and B); **, P < 0.05 versus 25-3 alone (B) by Student's two-tailed t test. Results are representative of two independent experiments.
DISCUSSION
The immunologic and virologic mechanisms that lead to bystander CD4+-T-cell death during HIV-1 infection remain poorly understood. In this study, we investigated HIV-1-induced bystander apoptosis in HVS-immortalized CD4+ T cells, which resemble activated mature primary CD4+ T cells (10). We found that infection of CD4/HVS T cells with X4 or R5X4 strains caused high levels of bystander apoptosis. Furthermore, exposure of CD4/HVS T cells to HIV-1 virions induced apoptosis in the absence of productive infection. Changes in the Env that increase envelope/receptor affinity or coreceptor binding site exposure enhanced the capacity of HIV-1 virions to induce bystander apoptosis. These results suggest that HIV-1 variants with increased envelope/receptor affinity and/or enhanced coreceptor binding site exposure may promote T-cell depletion in vivo by accelerating bystander CD4+-T-cell death
The relative importance of direct cytopathicity versus bystander apoptosis during HIV-1 infection in vitro and in vivo has been the subject of much debate (15, 16, 28, 37, 40, 42, 44, 52, 53). Direct cytopathic effects are the predominant cause of CD4+-T-cell death in T-cell lines infected with lab-adapted HIV-1 strains (15, 52, 53, 55, 57), whereas apoptosis of both infected and uninfected CD4+ T cells occurs in lymphoid tissue explants infected with lab-adapted or primary strains (37, 44). Several groups have reported that HIV-1-induced bystander apoptosis in primary T cells is highly dependent on the presence of monocytes/macrophages (3, 21, 41, 42, 66), which can prime T cells to undergo apoptosis induced by Fas, tumor necrosis factor alpha, or gp120 (3, 21, 42, 66) and are present in human lymphoid tissue explants (35, 37, 44). Remarkably, we found that high levels of bystander apoptosis are induced by infection of CD4/HVS T cells with primary X4 HIV-1 isolates (SG3 and ELI1), or GFP-expressing X4 and R5X4 recombinant strains (NL4-3-GFP and 89.6-GFP), in the absence of monocytes/macrophages and other antigen-presenting cells. CD4/HVS T cells are highly activated (10) and therefore may not require priming by antigen-presenting cells to undergo bystander apoptosis. Thus, apparent discrepancies in the literature on HIV-1-mediated killing of infected versus uninfected cells may in part reflect differences between experimental systems, such as the level of T-cell activation and the cell culture microenvironment.
The fusion inhibitor T20 prevented virus entry but did not inhibit the induction of apoptosis by HIV-1 virions. Moreover, the levels of apoptosis induced by HIV-1 virions pseudotyped with a fusion-defective mutant Env were similar to those induced by virions with wild-type Env. These results imply that membrane fusion is not required for HIV-1 to induce bystander apoptosis and argue against a “fusion from without” mechanism in which HIV-1 particles induce massive cell membrane fusion leading to membrane permeability and cell death. We used flow cytometric analysis to detect p24 antigen or GFP as a marker for productively infected cells. One concern is whether all of the presumed bystander cells are truly uninfected, since this method may not detect infected cells in the early stages of the replication cycle. A similar concern applies to studies using cultures treated with AZT where viruses enter cells but replication is blocked during reverse transcription. However, our experiments using T20 and a fusion-defective mutant indicate that virus entry and the early steps of infection are not required for HIV-1-induced bystander apoptosis and suggest a critical role for events prior to membrane fusion.
HIV-1 virions with Envs defective for CXCR4 binding were also defective for inducing apoptosis in CD4/HVS T cells. Furthermore, AMD3100 and an anti-CXCR4 monoclonal antibody inhibited apoptosis induced by X4 HIV-1 virions. Likewise, TAK-779 and an anti-CCR5 monoclonal antibody inhibited apoptosis induced by R5 HIV-1 virions. These findings suggest that binding of the Env to CXCR4 or CCR5 is required for the induction of bystander apoptosis. CXCR4- and CCR5-dependent signal transduction pathways include activation of G proteins and the focal adhesion tyrosine kinases Pyk2 and FAK along with the proapoptotic p38 mitogen-activated protein kinase (22, 23, 63, 69). To test whether the proapoptotic signal is Gαi-dependent, we incubated CD4/HVS T cells with pertussis toxin (1 μg/ml) prior to the addition of HIV-1 virions (G.H.H. and D.G., unpublished observation). However, the results could not be interpreted because pertussis toxin treatment caused high background levels of apoptosis. Further studies are required to determine the role of specific signaling pathways in bystander apoptosis induced by HIV-1 virions.
In contrast to the requirement for coreceptor binding, CD4 binding was not intrinsically required for HIV-1-induced bystander apoptosis. In particular, HIV-1 virions with envelope glycoproteins that interact directly with CXCR4 but are defective for CD4 binding retained the ability to induce apoptosis. Furthermore, bystander apoptosis was inhibited by AMD3100 and an anti-CXCR4 monoclonal antibody, which do not interfere with the binding of gp120 to CD4. These results contrast with previous studies, which suggested that the HIV-1 Env induces proapoptotic signals through CD4 (1, 5, 8). However, Env binding to CD4 leads to subsequent Env-coreceptor binding by triggering exposure of the coreceptor binding site (25). Thus, Env binding to CD4 indirectly leads to coreceptor engagement. CD4-mediated signaling can prime T cells for activation-induced apoptosis and/or enhance coreceptor-mediated signaling pathways (5, 21, 66, 76). However, our studies suggest that Env-CD4 binding is not sufficient to induce apoptosis in the absence of Env-coreceptor binding.
Changes in the envelope glycoproteins that increase envelope/receptor affinity or coreceptor binding site exposure enhanced the capacity of HIV-1 to induce bystander apoptosis. These strain-dependent differences were independent of effects on replication efficiency, as they were also observed in experiments using nonreplicating virions. Mechanistically, these properties of the envelope glycoproteins can impact bystander apoptosis by enhancing Env-coreceptor interactions and thereby increasing the intensity of coreceptor-mediated proapoptotic signaling. To this end, increased viral affinity for CD4 could indirectly enhance coreceptor-mediated signaling by increasing virion attachment and coreceptor binding site exposure (25). Alternatively, HIV-1 virions with increased affinity for CD4 may have a greater capacity to induce apoptosis in cells that express CD4 at low levels (25, 38, 47). Bystander apoptosis can also be influenced by certain host cell proteins, such as MHC class II and ICAM-1, which are incorporated into HIV-1 virions (12, 27, 28; G.H.H. and D.G., unpublished results). Consistent with these findings, we found that virions produced in 293T cells did not induce apoptosis in CD4/HVS T cells unless provirus plasmids were cotransfected with MHC class II isoforms. Incorporation of MHC class II molecules into HIV-1 virions may enhance binding to target cells via interactions with CD4 and/or induce signaling events that prime T cells to undergo apoptosis.
In summary, our experiments suggest that HIV-1 virions induce a proapoptotic signal in CD4+ T cells through a CXCR4- or CCR5-mediated pathway that does not require CD4 signaling or membrane fusion. The ability of HIV-1 to induce bystander apoptosis is influenced by properties of the Env that enhance envelope/coreceptor interactions, including envelope/receptor affinity, exposure of the coreceptor binding site, and host cell membrane proteins on the virion surface. Previous studies have estimated that only 0.00001 to 0.01% of HIV-1 virions are infectious in vitro and in vivo (24, 68). Thus, noninfectious virions may contribute to HIV-1 pathogenicity in vivo by inducing bystander apoptosis mediated by Env-coreceptor interactions. Therapeutic strategies to block these interactions may reduce CD4+-cell depletion in AIDS patients.
Acknowledgments
We thank J. Sodroski, J. LaBonte, J. Hoxie, and J. Wang for helpful discussions. We are also grateful to J. Sodroski for providing the Hx, Hx F522Y, Hx R308A, and Hx R315A envelopes, J. Sodroski and D. Burton for providing the 1b12 antibody, J. Hoxie for providing the HxB, HxB(V3BaL), 8x, 8x(V3BaL), and 8xD368R envelopes, J. Moore and S. Kuhmann for providing the CC1/85, CC101.6, CC101.19, CCCon.19, 25-3, and 9-8 viruses, R. Desrosiers for providing owl monkey kidney cells and HVS-C488, G. Herbein for providing 89.6-GFP, C. Aiken for providing NL4-3-GFP, and ImmunoDiagnostics, Inc., for providing the cross-reactive anti-gp120.
This work was supported by NIH NS35734 to D.G. Core facilities were supported by Center for AIDS Research grants (AI28691) and a DFCI/Harvard Center for Cancer Research grant. D.G. is an Elizabeth Glaser Scientist who was supported by the Pediatric AIDS Foundation.
REFERENCES
- 1.Arthos, J., C. Cicala, S. M. Selig, A. A. White, H. M. Ravidranath, D. Van Ryk, T. D. Steenbeke, E. Machado, P. Khazanie, M. S. Hanback, D. B. Hanback, R. L. Rabin, and A. S. Fauci. 2002. The role of the CD4 receptor versus HIV coreceptors in envelope-mediated apoptosis in peripheral blood mononuclear cells. Virology 292:98-106. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, L. O., J. W. Bess, Jr., R. C. Sowder II, R. E. Benveniste, L. E. Henderson, and J. D. Lifson. 1992. Cellular proteins bound to immunodeficiency viruses: implications for pathogenesis and vaccines. Science 258:1935-1938. [DOI] [PubMed] [Google Scholar]
- 3.Badley, A. D., D. Dockrell, M. Simpson, R. Schut, D. H. Lynch, P. Leibson, and C. V. Paya. 1997. Macrophage-dependent apoptosis of CD4+ T lymphocytes from HIV-1 infected individuals is mediated by FasL and tumor necrosis factor. J Exp. Med. 185:55-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Badley, A. D., D. H. Dockrell, A. Algeciras, S. Ziesmer, A. Landay, M. M. Lederman, E. Connick, H. Kessler, D. Kuritzkes, D. H. Lynch, P. Roche, H. Yagita, and C. V. Paya. 1998. In vivo analysis of Fas/FasL interactions in HIV-1 infected patients. J. Clin. Investig. 102:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banda, N. K., J. Bernier, D. K. Kurahara, R. Kurrle, N. Haigwood, R. P. Sekaly, and T. H. Finkel. 1992. Crosslinking CD4 by human immunodeficiency virus gp120 primes T cells for activation-induced apoptosis. J. Exp. Med. 176:1099-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basmaciogullari, S., G. J. Babcock, D. Van Ryk, W. Wojtowicz, and J. Sodroski. 2002. Identification of conserved and variable structures in the human immunodeficiency virus gp120 glycoprotein of importance for CXCR4 binding. J. Virol. 76:10791-10800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 8.Berndt, C., B. Mopps, S. Angermuller, P. Gierschik, and P. H. Krammer. 1998. CXCR4 and CD4 mediate a rapid CD95-independent cell death in CD4+ T cells. Proc. Natl. Acad. Sci. USA 95:12556-12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biard-Piechaczyk, M., V. Robert-Hebmann, V. Richard, J. Roland, R. A. Hipskind, and C. Devaux. 2000. Caspase-dependent apoptosis of cells expressing the chemokine receptor CXCR4 is induced by cell membrane-associated human immunodeficiency virus type 1 envelope glycoprotein (gp120). Virology 268:329-344. [DOI] [PubMed] [Google Scholar]
- 10.Birsinger, B., I. Muller-Fleckenstein, D. Simmer, G. Lang, S. Wittman, E. Platzer, R. C. Desrosiers, and B. Fleckenstein. 1992. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc. Natl. Acad. Sci. USA 89:3116-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bleul, C. C., L. Wu, J. A. Hoxie, T. A. Springer, and C. R. Mackay. 1997. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc. Natl. Acad. Sci. USA 94:1925-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bounou, S., J. E. Leclerc, and M. J. Tremblay. 2002. Presence of host ICAM-1 in laboratory and clinical strains of human immunodeficiency virus type 1 increases virus infectivity and CD4+-T-cell depletion in human lymphoid tissue, a major site of replication in vivo. J. Virol. 76:1004-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briant, L., V. Robert-Hebmann, V. Sivan, A. Brunet, J. Pouyssegur, and C. Devaux. 1998. Involvement of extracellular signal-regulated kinase module in HIV-mediated CD4 signals controlling activation of nuclear factor-kB and AP-1 transcription factors. J. Immunol. 160:1875-1885. [PubMed] [Google Scholar]
- 14.Cantin, R., J. F. Fortin, G. Lamontagne, and M. Tremblay. 1997. The presence of host-derived HLA-DR1 on human immunodeficiency virus type 1 increases viral infectivity. J. Virol. 71:1922-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao, J., I. Park, A. Cooper, and J. Sodroski. 1996. Molecular determinants of acute single-cell lysis by human immunodeficiency virus type 1. J. Virol. 70:1340-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carbonari, M., A. M. Pesce, M. Cibati, A. Modica, L. Dell'Anna, G. D'Offizi, A. Angelici, S. Uccini, A. Modesti, and M. Fiorilli. 1997. Death of bystander cells by a novel pathway involving early mitochondrial damage in human immunodeficiency virus-related lymphadenopathy. Blood 90:209-216. [PubMed] [Google Scholar]
- 17.Carbonari, M., M. Cibati, A. M. Pesce, D. Sbarigia, P. Grossi, G. D'Offizi, G. Luzi, and M. Fiorilli. 1995. Frequency of provirus-bearing CD4+ cells in HIV type 1 infection correlates with extent of in vitro apoptosis of CD8+ but not of CD4+ cells. AIDS Res. Hum. Retrovir. 11:789-794. [DOI] [PubMed] [Google Scholar]
- 18.Cicala, C., J. Arthos, A. Rubbert, S. Selig, K. Wildt, O. J. Cohen, and A. S. Fauci. 2000. HIV-1 envelope induces activation of caspase-3 and cleavage of focal adhesion kinase in primary human CD4+ T cells. Proc. Natl. Acad. Sci. USA 97:1178-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clerici, M., N. I. Stocks, R. A. Zajac, R. N. Boswell, D. R. Lucey, C. S. Via, and G. M. Shearer. 1989. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J. Clin. Investig. 84:1892-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Connor, R. I., H. Mohri, Y. Cao, and D. D. Ho. 1993. Increased viral burden and cytopathicity correlate temporally with CD4+ T-lymphocyte decline and clinical progression in human immunodeficiency virus type 1-infected individuals. J. Virol. 67:1772-1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cottrez, F., F. Manca, A. G. Dalgleish, F. Arenzana-Seisdedos, A. Capron, and H. Groux. 1997. Priming of human CD4+ antigen-specific T cells to undergo apoptosis by HIV-infected monocytes. J. Clin. Investig. 99:257-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis, C. B., I. Dikic, D. Unutmaz, C. M. Hill, J. Arthos, M. A. Siani, D. A. Thompson, J. Schlessinger, and D. R. Littman. 1997. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J. Exp. Med. 186:1793-1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Del Corno, M., Q. Liu, D. Schols, E. De Clercq, S. Gessani, B. D. Freedman, and R. G. Collman. 2001. HIV-1 gp120 and chemokine activation of Pyk2 and mitogen-activated protein kinases in primary macrophages mediated by calcium-dependent, pertussis toxin-insensitive chemokine receptor signaling. Blood 98:2909-2916. [DOI] [PubMed] [Google Scholar]
- 24.Dimitrov, D. S., R. S. Willey, H. Sato, L. J. Chang, R. Blumenthal, and M. A. Martin. 1993. Quantitation of human immunodeficiency virus type 1 infection kinetics. J. Virol. 67:2182-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doms, R. W., and D. Trono. 2000. The plasma membrane as a combat zone in the HIV battlefield. Genes Dev. 14:2677-2688. [DOI] [PubMed] [Google Scholar]
- 26.Donzella, G. A., D. Schols, S. W. Lin, J. A Este, K. A. Nagashima, P. J. Maddon, G. P Allaway, T. P Sakmar, G. Henson, E. De Clercq, and J. P. Moore. 1998. AMD3100, a small molecule inhibitor of HIV-1 entry via the CXCR4 co-receptor. Nat. Med. 4:72-77. [DOI] [PubMed] [Google Scholar]
- 27.Esser, M. T., D. R. Graham, L. V. Coren, C. M. Trubey, J. W. Bess, Jr., L. O. Arthur, D. E. Ott, and J. D. Lifson. 2001. Differential incorporation of CD45, CD80 (B7-1), CD86 (B7-2), and major histocompatibility complex class I and II molecules into human immunodeficiency virus type 1 virions and microvesicles: implications for viral pathogenesis and immune regulation. J. Virol. 75:6173-6182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esser, M. T., J. W. Bess, Jr., K. Suryanarayana, E. Chertova, D. Marti, M. Carrington, L. O. Arthur, and J. D. Lifson. 2001. Partial activation and induction of apoptosis in CD4+ and CD8+ T lymphocytes by conformationally authentic noninfectious human immunodeficiency virus type 1. J. Virol. 75:1152-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estaquier, J., J.-D. Lelievre, F. Petit, T. Brunner, L. Moutouh-de Parseval, D. D. Richman, J. C. Ameisen, and J. Corbeil. 2002. Effects of antiretroviral drugs on human immunodeficiency virus type 1-induced CD4+-T-cell death. J. Virol. 76:5966-5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 31.Fortin, J.-F., R. Cantin, G. Lamontagne, and M. Tremblay. 1997. Host-derived ICAM-1 glycoproteins incorporated on human immunodeficiency virus type 1 are biologically active and enhance viral infectivity. J. Virol. 71:3588-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fujita, K., J. Silver, and K. Peden. 1992. Changes in both gp120 and gp41 can account for increased growth potential and expanded host range of human immunodeficiency virus type 1. J. Virol. 66:4445-4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Geleziunas, R., W. Xu, K. Takeda, H. Ichijo, and W. C. Greene. 2001. HIV-1 Nef inhibits ASK1-dependent death signalling providing a potential mechanism for protecting the infected host cell. Nature 410:834-838. [DOI] [PubMed] [Google Scholar]
- 34.Ghosh, S. K., P. N. Fultz, E. Keddie, M. S. Saag, P. M. Sharp, B. H. Hahn, and G. M. Shaw. 1993. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology 194:858-864. [DOI] [PubMed] [Google Scholar]
- 35.Glushakova, S., B. Baibakov, L. B. Margolis, and J. Zimmerberg. 1995. Infection of human tonsil histocultures: a model for HIV pathogenesis. Nat. Med. 1:1320-1322. [DOI] [PubMed] [Google Scholar]
- 36.Glushakova, S., J. C. Grivel, W. Fitzgerald, A. Sylwester, J. Zimmerberg, and L. B. Margolis. 1998. Evidence for the HIV-1 phenotype switch as a causal factor in acquired immunodeficiency. Nat. Med. 4:346-349. [DOI] [PubMed] [Google Scholar]
- 37.Glushakova, S., Y. Yi, J. C. Grivel, A. Singh, D. Schols, E. De Clercq, R. G. Collman, and L. Margolis. 1999. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J. Clin. Investig. 104:R7-R11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gorry, P. R., J. Taylor, G. Holm, A. Mehle, T. Morgan, M. Cayabyab, M. Farzan, H. Wang, J. E. Bell, K. Kunstman, J. P. Moore, S. M. Wolinsky, and D. Gabuzda. 2002. Increased CCR5 affinity and reduced CCR5/CD4 dependence of a neurovirulent primary human immunodeficiency virus type 1 isolate. J. Virol. 76:6277-6292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grivel, J. C., and L. B. Margolis. 1999. CCR5- and CXCR4-tropic HIV-1 are equally cytopathic for their T-cell targets in human lymphoid tissue. Nat. Med. 5:592-593. [DOI] [PubMed] [Google Scholar]
- 40.Grivel, J. C., N. Malkevitch, and L. Margolis. 2000. Human immunodeficiency virus type 1 induces apoptosis in CD4+ but not in CD8+ T cells in ex vivo-infected human lymphoid tissue. J. Virol. 74:8077-8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herbein, G., C. Van Lint, J. L. Lovett, and E. Verdin. 1998. Distinct mechanisms trigger apoptosis in human immunodeficiency virus type 1-infected and in uninfected bystander T lymphocytes. J. Virol. 72:660-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herbein, G., U. Mahlknecht, F. Batliwalla, P. Gregersen, T. Pappas, J. Butler, W. A. O'Brien, and E. Verdin. 1998. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature 395:189-194. [DOI] [PubMed] [Google Scholar]
- 43.Hoffman, T. L., C. C. LaBranche, W. Zhang, G. Canziani, J. Robinson, I. Chaiken, J. A. Hoxie, and R. W. Doms. 1999. Stable exposure of the coreceptor-binding site in a CD4-independent HIV-1 envelope protein. Proc. Natl. Acad. Sci. USA 96:6359-6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jekle, A., O. T. Keppler, E. De Clercq, D. Schols, M. Weinstein, and M. A. Goldsmith. 2003. In vivo evolution of human immunodeficiency virus type 1 toward increased pathogenicity through CXCR4-mediated killing of uninfected CD4 T cells. J. Virol. 77:5846-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katsikis, P. D., E. S. Wunderlich, C. A. Smith, L. A. Herzenberg, and L. A. Herzenberg. 1995. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J. Exp. Med. 181:2029-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koot, M., I. P. Keet, A. H. Vos, R. E. de Goede, M. T. Roos, R. A. Coutinho, F. Miedema, P. T. Schellekens, and M. Tersmette. 1993. Prognostic value of HIV-1 syncytium-inducing phenotype for rate of CD4+ cell depletion and progression to AIDS. Ann. Intern. Med. 118:681-688. [DOI] [PubMed] [Google Scholar]
- 47.Kozak, S. L., E. J. Platt, N. Madani, F. E. Ferro, Jr., K. Peden, and D. Kabat. 1997. CD4, CXCR-4, and CCR-5 dependencies for infections by primary patient and laboratory-adapted isolates of human immunodeficiency virus type 1. J. Virol. 71:873-882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kraft, M. S., G. Henning, H. Fickenscher, D. Lengenfelder, J. Tschopp, B. Fleckenstein, and E. Meinl. 1998. Herpesvirus saimiri transforms human T-cell clones to stable growth without inducing resistance to apoptosis. J. Virol. 72:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kreisberg, J. F., D. Kwa, B. Schramm, V. Trautner, R. Connor, H. Schuitemaker, J. I. Mullins, A. B. van't Wout, and M. A. Goldsmith. 2001. Cytopathicity of human immunodeficiency virus type 1 primary isolates depends on coreceptor usage and not patient disease status. J. Virol. 75:8842-8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kuhmann, S. E., P. Pugach, K. J. Kunstman, J. Taylor, R. L. Stanfield, A. Snyder, J. M. Strizki, J. Riley, B. M. Baroudy, I. A. Wilson, B. T. Korber, S. M. Wolinsky, and J. P. Moore. 2003. Genetic and phenotypic analyses of human immunodeficiency virus type 1 escape from a small-molecule CCR5 inhibitor. J. Virol. 78:2790-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kwa, D., J. Vingerhoed, B. Boeser-Nunnink, S. Broersen, and H. Schuitemaker. 2001. Cytopathic effects of non-syncytium-inducing and syncytium-inducing human immunodeficiency virus type 1 variants on different CD4+-T-cell subsets are determined only by coreceptor expression. J. Virol. 75:10455-10459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.LaBonte, J. A., N. Madani, and J. Sodroski. 2003. Cytolysis by CCR5-using human immunodeficiency virus type 1 envelope glycoproteins is dependent on membrane fusion and can be inhibited by high levels of CD4 expression. J. Virol. 77:6645-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.LaBonte, J. A., T. Patel, W. Hofmann, and J. Sodroski. 2000. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J. Virol. 74:10690-10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LaBranche, C. C., T. L. Hoffman, J. Romano, B. S. Haggarty, T. G. Edwards, T. J. Matthews, R. W. Doms, and J. A. Hoxie. 1999. Determinants of CD4 independence for a human immunodeficiency virus type 1 variant map outside regions required for coreceptor specificity. J. Virol. 73:10310-10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Laurent-Crawford, A. G., B. Krust, Y. Riviere, C. Desgranges, S. Muller, M. Paule Kieny, C. Dauguet, and A. G. Hovanessian. 1993. Membrane expression of HIV envelope glycoproteins triggers apoptosis in CD4 cells. AIDS Res. Hum. Retrovir. 9:761-773. [DOI] [PubMed] [Google Scholar]
- 56.Lawless, M. K., S. Barney, K. I. Guthrie, T. B. Bucy, S. R. Petteway, Jr., and G. Merutka. 1996. HIV-1 membrane fusion mechanism: structural studies of the interactions between biologically-active peptides from gp41. Biochemistry 35:13697-13708. [DOI] [PubMed] [Google Scholar]
- 57.Lenardo, M. J., S. B. Angleman, V. Bounkeua, J. Dimas, M. G. Duvall, M. B. Graubard, F. Hornung, M. C. Selkirk, C. K. Speirs, C. Trageser, J. O. Orenstein, and D. L. Bolton. 2002. Cytopathic killing of peripheral blood CD4+ T lymphocytes by human immunodeficiency virus type 1 appears necrotic rather than apoptotic and does not require env. J. Virol. 76:5082-5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li, C. J., D. J. Friedman, C. Wang, V. Metelev, and A. B. Pardee. 1995. Induction of apoptosis in uninfected lymphocytes by HIV-1 Tat protein. Science 268:429-431. [DOI] [PubMed] [Google Scholar]
- 59.Lu, Y., Y. Koga, K. Tanaka, M. Sasaki, G. Kimura, and K. Nomoto. 1994. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J. Virol. 68:390-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mahlknecht, U., C. Deng, M. C. Lu, T. C. Greenough, J. L. Sullivan, W. A. O'Brien, and G. Herbein. 2000. Resistance to apoptosis in HIV-infected CD4+ T lymphocytes is mediated by macrophages: role for Nef and immune activation in viral persistence. J. Immunol. 165:6437-6446. [DOI] [PubMed] [Google Scholar]
- 61.McCune, J. M. 2001. The dynamics of CD4+ T-cell depletion in HIV disease. Nature 410:974-979. [DOI] [PubMed] [Google Scholar]
- 62.Meyaard, L., S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. Keet, and F. Miedema. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217-219. [DOI] [PubMed] [Google Scholar]
- 63.Misse, D., M. Cerutti, N. Noraz, P. Jourdan, J. Favero, G. Devauchelle, H. Yssel, N. Taylor, and F. Veas. 1999. A CD4-independent interaction of human immunodeficiency virus-1 gp120 with CXCR4 induces their cointernalization, cell signaling, and T-cell chemotaxis. Blood 93:2454-2462. [PubMed] [Google Scholar]
- 64.Muro-Cacho, C. A., G. Pantaleo, and A. S. Fauci. 1995. Analysis of apoptosis in lymph nodes of HIV-infected persons. J. Immunol. 154:5555-5566. [PubMed] [Google Scholar]
- 65.Ohagen, A., S. Ghosh, J. He, K. Huang, Y. Chen, M. Yuan, R. Osathanondh, S. Gartner, G. Shaw, and D. Gabuzda. 1999. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J. Virol. 73:897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Oyaizu, N., Y. Adachi, F. Hashimoto, T. W. McCloskey, N. Hosaka, N. Kayagaki, H. Yagita, and S. Pahwa. 1997. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cells apoptosis. J. Immunol. 158:2456-2463. [PubMed] [Google Scholar]
- 67.Peden, K., M. Emerman, and L. Montagnier. 1991. Changes in growth properties on passage in tissue culture of viruses derived from infectious molecular clones of HIV-1LAI, HIV-1MAL, and HIV-1ELI. Virology 185:661-672. [DOI] [PubMed] [Google Scholar]
- 68.Piatak, M., Jr., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 69.Popik, W., J. E. Hesselgesser, and P. M. Pitha. 1998. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J. Virol. 72:6406-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rho, H., B. Poiesz, F. Ruscetti, and R. C. Gallo. 1981. Characterization of the reverse transcriptase from a new retrovirus (HTLV) produced by a human cutaneous T-cell lymphoma cell line. Virology 112:355-360. [DOI] [PubMed] [Google Scholar]
- 71.Samuelsson, A., C. Brostrom, N. van Dijk, A. Sonnerborg, and F. Chiodi. 1997. Apoptosis of CD4+ and CD19+ cells during human immunodeficiency virus type 1 infection—correlation with clinical progression, viral load, and loss of humoral immunity. Virology 238:180-188. [DOI] [PubMed] [Google Scholar]
- 72.Schols, D., S. Struyf, J. Van Damme, J. A. Este, G. Henson, and E. De Clercq. 1997. Inhibition of T-tropic HIV strains by selective antagonization of the chemokine receptor CXCR4. J. Exp. Med. 186:1383-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shi, B., U. De Girolami, J. He, S. Wang, A. Lorenzo, J. Busciglio, and D. Gabuzda. 1996. Apoptosis induced by HIV-1 infection of the central nervous system. J. Clin. Investig. 98:1979-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stewart, S. A., B. Poon, J. Y. Song, and I. S. Y. Chen. 2000. Human immunodeficiency virus type 1 Vpr induces apoptosis through caspase activation. J. Virol. 74:3105-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su, L., H. Kaneshima, M. Bonyhadi, S. Salimi, D. Kraft, L. Rabin, and J. M. McCune. 1995. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity 2:25-36. [DOI] [PubMed] [Google Scholar]
- 76.Tateyama, M., N. Oyaizu, T. W. McCloskey, S. Than, and S. Pahwa. 2000. CD4 T lymphocytes are primed to express Fas ligand by CD4 cross-linking and to contribute to CD8 T-cell apoptosis via Fas/FasL death signaling pathway. Blood 96:195-202. [PubMed] [Google Scholar]
- 77.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vlahakis, S. R., A. Algeciras-Schimnich, G. Bou, C. J. Heppelmann, A. Villasis-Keever, R. G. Collman, and C. V. Paya. 2001. Chemokine-receptor activation by env determines the mechanism of death in HIV-infected and uninfected T lymphocytes. J. Clin. Investig. 107:207-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vlahakis, S. R., A. Villasis-Keever, T. Gomez, M. Vanegas, N. Vlahakis, C. V. Paya. 2002. G protein-coupled chemokine receptors induce both survival and apoptotic signaling pathways. J. Immunol. 169:5546-5554. [DOI] [PubMed] [Google Scholar]
- 80.Westendorp, M. O., R. Frank, C. Ochsenbauer, K. Stricker, J. Dhein, H. Walczak, K. M. Debatin, and P. H. Krammer. 1995. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature 375:497-500. [DOI] [PubMed] [Google Scholar]
- 81.Willey, R. L., M. A. Martin, and K. W. Peden. 1994. Increase in soluble CD4 binding to and CD4-induced dissociation of gp120 from virions correlates with infectivity of human immunodeficiency virus type 1. J. Virol. 68:1029-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yao, Q., R. W. Compans, and C. Chen. 2001. HIV envelope proteins differentially utilize CXCR4 and CCR5 coreceptors for induction of apoptosis. Virology 285:128-137. [DOI] [PubMed] [Google Scholar]