Abstract
Open reading frame 1 in the viral genome of Cymbidium ringspot virus encodes a 33-kDa protein (p33), which was previously shown to localize to the peroxisomal membrane in infected and transgenic plant cells. To determine the sequence requirements for the organelle targeting and membrane insertion, the protein was expressed in the yeast Saccharomyces cerevisiae in native form (33K) or fused to the green fluorescent protein (33KGFP). Cell organelles were identified by immunolabeling of marker proteins. In addition, peroxisomes were identified by simultaneous expression of the red fluorescent protein DsRed containing a peroxisomal targeting signal and mitochondria by using the dye MitoTracker. Fluorescence microscopy showed the 33KGFP fusion protein concentrated in a few large bodies colocalizing with peroxisomes. These bodies were shown by electron microscopy to be composed by aggregates of peroxisomes, a few mitochondria and endoplasmic reticulum (ER) strands. In immunoelectron microscopy, antibodies to p33 labeled the peroxisomal clumps. Biochemical analysis suggested that p33 is anchored to the peroxisomal membrane through a segment of ca. 7 kDa, which corresponds to the sequence comprising two hydrophobic transmembrane domains and a hydrophilic interconnecting loop. Analysis of deletion mutants confirmed these domains as essential components of the p33 peroxisomal targeting signal, together with a cluster of three basic amino acids (KRR). In yeast mutants lacking peroxisomes p33 was detected in the ER. The possible involvement of the ER as an intermediate step for the integration of p33 into the peroxisomal membrane is discussed.
Cymbidium ringspot virus (CymRSV) is a member of the genus Tombusvirus, family Tombusviridae. CymRSV genome is composed of a linear, monopartite, positive-sense RNA molecule of 4,733 nucleotides (GenBank accession number X15511) and contains five functional open reading frames (ORFs) (6, 14). The 5′-proximal ORF (ORF1) encodes a 33-kDa protein (p33). The readthrough of the amber stop codon of ORF1 results in the translation of a 92-kDa (p92) fusion protein (ORF2). The readthrough portion of p92 contains the eight conserved motifs (PI to PVIII) of RNA-dependent RNA polymerases of supergroup II of the positive-strand RNA viruses (19). Both p33 and p92 are required for viral replication, and they are translated directly from the viral genome. However, the readthrough product is synthesized in smaller amounts due to the low frequency of the amber stop codon suppression event (21). ORF3 encodes the 41-kDa coat protein which is translated from a subgenomic RNA. Two nested ORFs follow: ORF4 codes for a 21-kDa protein that is required for virus movement in infected plants, and ORF5 encodes a 19-kDa protein which plays an important role in symptom expression and in posttranscriptional gene silencing suppression (39). Both ORF4 and ORF5 are expressed via a second subgenomic RNA.
In infected plant cells CymRSV elicits the formation of vesiculated structures (multivesicular bodies [MVBs]). They consist of a main body surrounded by many spherical to ovoid vesicles 80 to 150 nm in diameter, resulting from proliferation of the limiting membrane of peroxisomes (22, 33). Viral replication may take place in the vesicles of MVBs as suggested by the association of the p33/p92 proteins with modified peroxisomes in infected cells (1, 21, 29). The information to target the CymRSV replication complex to peroxisomes is contained in the p33 protein product of ORF1. Similarly, the ORF1 product (p36) of the related Carnation Italian ringspot virus (CIRV) is responsible for the localization of CIRV replication complex to mitochondria, the site of CIRV replication (3, 29, 31, 41).
Computer analysis of p33 suggested the presence, downstream of a hydrophilic sequence, of two hydrophobic domains long enough to span the limiting membrane of peroxisomes. The two putative transmembrane domains (TMDs) are separated by a hydrophilic loop. Both the N- and C-terminal regions would be cytosolic, whereas the loop is probably protruding inside the peroxisomal matrix (29). The peroxisomal targeting signals (PTS) differ according to whether the peroxisomal proteins are destined to the matrix or to the membrane. Most peroxisomal matrix proteins contain the PTS1 signal consisting of the C-terminal tripeptide SKL or its minor variants (AKL, CKL, SRL, or SHL), whereas only a few have the N-terminal PTS2 signal which consists of the nonapeptide (R/K)(L/V/I)(X)5(H/Q)(L/A) (13, 26). CymRSV p33 contains neither PTS1 nor PTS2 signals. On the other hand, the membrane PTS (mPTS) involved in targeting peroxisomal membrane proteins (PMPs) is not defined, since a consensus sequence has not yet been identified. However, one to several TMDs and stretches of positive amino acids in their proximity have been shown to be required (see references 17 and 38 and references therein).
Peroxisomal targeting and membrane integration of CymRSV p33 were analyzed here. To facilitate the dissection and analysis of the putative peroxisomal signal, p33 was expressed in Saccharomyces cerevisiae in the presence or absence of peroxisomes, and its intracellular localization and cytological effects were investigated by fluorescence and electron microscopy. Deletion mutants were produced to evaluate the relative contribution of different regions of the viral protein in determining the association to peroxisomes. Our results indicate a predominant role for the two TMDs and the short hydrophilic regions close to them.
MATERIALS AND METHODS
Yeast strains, cell growth, and transformation.
The S. cerevisiae strains used in the present study were UTL-7A (MATa leu2-3,112 ura3-52 trp1) (10) and UTL-7Apex19Δ (same as UTL7-A but pex19Δ::LEU2) (12). Transformation with plasmids was done by the lithium acetate-polyethylene glycol method (18). Liquid culturing for peroxisome induction was performed as described previously (23). Briefly, cells were cultured in SGd medium (3% glycerol, 0.1% glucose, and 0.67% yeast nitrogen base, supplemented with appropriated amino acids and bases) for 48 h and then “boosted” with 10xYP (10% yeast extract, 20% peptone) to a 1× concentration for 4 h. Cells were harvested by centrifugation and reinoculated to a final A600 of 1 into oleate medium (0.1% [vol/vol] oleic acid, 0.2% [vol/vol] Tween 40, 0.05% yeast extract, and 0.67% yeast nitrogen base, supplemented with appropriated amino acids and bases). Yeast cultures were grown at 30°C for 16 to 20 h.
Construction of plasmids.
To prepare a clone containing the p33 fused to the green fluorescent protein (GFP), a clone of the yeast codon-optimized form of GFP (5) in the vector pYES2 was used (pYES-GFP) (30). The CymRSV 5′ noncoding region and p33 coding sequence were amplified by PCR with a full-length cDNA clone of CymRSV as a template and the primers NAB3 (5′-CCCAAGCTTGGAAATCCTCCAGGACACC-3′) and NAB4 (CCCAAGCTTTCACACCCAGGGAGTCC-3′), which contained a HindIII digestion site at the 5′ end. After HindIII digestion, the PCR-generated fragment was cloned in the HindIII site of plasmid pYES-GFP to generate pYES-33KGFP. The PvuII-SmaI fragment of pYES-33KGFP, which contains the CymRSV 5′ noncoding region and p33 and GFP codons, was subcloned in the SmaI unique site of the yeast expression vector pEL26 (8) under the control of the oleate-inducible CTA1 promoter. The vector expressing free GFP (pEL26-GFP) was prepared by excising the GFP sequence from plasmid pYES-GFP by EcoRI/HindIII digestion. The fragment was blunt ended with Klenow enzyme and subcloned into the SmaI site of pEL26.
Deletion mutants of clone pEL26-33KGFP were obtained either by PCR amplification of selected regions of the p33 coding sequence and reinsertion between appropriate restriction sites (naturally present or introduced by site-directed mutagenesis) within the p33 coding sequence or by cleavage at appropriate restriction sites (present or introduced) and religation. All mutations were confirmed by sequencing.
The sequence encoding the red fluorescent protein DsRed fused to the peroxisomal targeting signal AKL was excised as a BglII fragment from plasmid pDsRed-AKL (40), blunt ended by using Klenow enzyme, and inserted into the SmaI site of vector pAL, which contains the constitutive promoter ADH1 and the selectable marker LEU2 (V. Pantaleo et al., unpublished results), yielding the plasmid pALDsRedAKL.
Protein extraction and Western blot analysis.
Cells were grown until they reached an A600 of 2, and 10 ml of culture was processed as described previously (30). Briefly, cells were spheroplasted with lyticase (10 U/ml), lysed in 1 ml of lysis buffer (50 mM Tris [pH 7.5], 15 mM MgCl2, 10 mM KCl, 0.3 M sorbitol, 0.1% β-mercaptoethanol, 5 μg of leupeptin/ml, 2 μg of aprotinin/ml), and incubated 30 min at 30°C. The cell lysate was centrifuged at 500 × g for 3 min at 4°C to remove unbroken cells and debris, and the supernatant was centrifuged at 30,000 × g for 30 min at 4°C to yield supernatant and pellet fractions. For membrane extraction analysis, the P30 fraction was incubated for 30 min on ice in the presence of one of the following reagents: 100 mM Na2CO3 (pH 11.5), 4 M urea, or 1 M KCl (35). After centrifugation at 30,000 × g for 30 min at 4°C, the pellet and supernatant were subjected to immunoblot analysis with an anti-p33 antiserum (21).
Light and electron microscopy.
Fixation of cells with formaldehyde and immunofluorescence staining were performed as described previously (27). Rabbit polyclonal antisera to S. cerevisiae peroxisomal Fox3 (9), mitochondrial YHM2 (4), endoplasmic reticulum (ER) Kar2 (28), and Golgi Emp47 (36) protein markers were used for the identification of respective organelles. The antibodies were used at a 1:100 dilution except anti-Fox3p, which was used at a 1:500 dilution. Rhodamine-conjugated goat anti-rabbit immunoglobulin G secondary antibody (2 μg/ml) was from Molecular Probes. Mitochondria were visualized in living cells with the mitochondrial specific dye MitoTracker (CMTMRos; Molecular Probes).
Fluorescence images were obtained with a Leica TCS SP2 confocal laser scanning microscope with a ×63 objective lens. A sequential scan procedure was applied during imaging acquisition with fluorescein isothiocyanate (FITC) and tetramethyl rhodamine isothiocyanate (TRITC) lasers. Multiple serial optical sections were analyzed, and single representative Z sections were shown.
For electron microscopy, cells were fixed and processed as described previously (30).
RESULTS AND DISCUSSION
Expression and localization of CymRSV p33 in yeast cells.
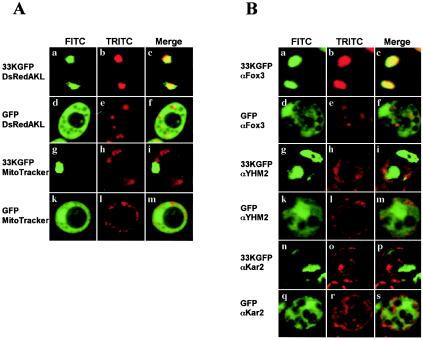
To determine the subcellular localization properties of p33, the CymRSV ORF1 cDNA was fused in frame with GFP and placed under control of the oleate-activated CTA1 promoter in the plasmid pEL26-33KGFP. Cells were either transformed only with this plasmid or cotransformed with plasmid pALDsRedAKL, which contains the coding sequence of the red fluorescing protein DsRed with a peroxisomal targeting signal (40). Control cells were transformed with plasmid pEL26-GFP expressing only the GFP sequence, together with the pALDsRedAKL plasmid. Mitochondria were visualized with the dye MitoTracker in living cells transformed with pEL26-33KGFP or pEL26-GFP alone. Both biogenesis of peroxisomes and expression of the viral protein fused or not to GFP were obtained by growing yeast cells in the presence of oleate. Fluorescence microscopy of the p33GFP-expressing cells showed a green pattern consisting of one or two large spots colocalized with the red peroxisomal marker (Fig. 1A, a to c) but not with the mitochondrial signal (Fig. 1A, g to i). In contrast, cells expressing free GFP showed green fluorescence dispersed in the cytosol and not associated with peroxisomes (Fig. 1A, d to f) nor mitochondria (Fig. 1A, k to m). Peroxisomes in GFP expressing cells appeared as several small bodies rather than the few large bodies present in the p33GFP-expressing cells (compare Fig. 1A, b and e).
FIG. 1.
Fusion protein p33GFP localizes to peroxisomes in S. cerevisiae. (A) Yeast cells were transformed with a plasmid expressing fused p33GFP (a to c and g to i) or free GFP (d to f and k to m). The cells in panels a to c and d to f were cotransformed with a plasmid expressing the protein DsRedAKL localizing on peroxisomes. The cells in panels g to i and k to m were treated with the mitochondrial stain MitoTracker. Living cells were analyzed by fluorescence microscopy, and representative images for the FITC (left column, green), TRITC (middle column, red) channels, and merged signals (right column) are shown. (B) Cells of the same transformants as in panel A expressing fused p33GFP (a to c, g to i, and n to p) or free GFP (d to f, k to m, and q to s) were fixed and immunostained with antibodies to proteins localizing to peroxisomes (Fox3), mitochondria (YHM2), or the ER (Kar2). Representative images for GFP (left column, FITC), rhodamine (middle column, TRITC), and merged signals (right column) are shown.
Immunofluorescence experiments on cells expressing GFP alone or p33GFP were performed by using the peroxisomal Fox3, or the mitochondrial YHM2, or the ER Kar2 protein markers. In the diffuse green fluorescing GFP-expressing cells, the Fox3, YHM2, and Kar2 markers were distributed over several, bright punctate or elongated structures identified as peroxisomes, mitochondria, or ER strands, respectively (Fig. 1B, d to f, k to m, and q to s). In the p33GFP-expressing cells, antibodies to Fox3 reacted strongly with one or two green large bodies (Fig. 1B, a to c), which were not detected with antibodies to YHM2 (Fig. 1B, g to i) and Kar2 (Fig. 1B, n to p), thus confirming that peroxisomes are the only target of the p33GFP.
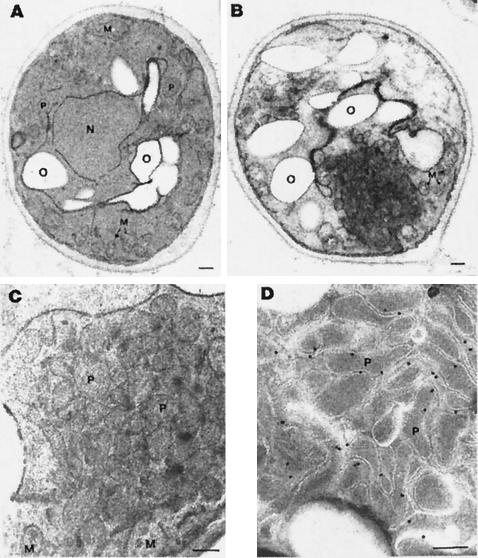
Electron microscopic analysis was carried out to investigate in more detail the nature of these structures. A number of scattered small peroxisomal profiles were detected in sectioned GFP-expressing cells (Fig. 2A), whereas clumps of organelles mixed with membranous structures were detected in p33GFP-expressing cells (Fig. 2B). The compact texture of these clumps made the identification of single organelles difficult, except for mitochondria. Many of them were localized at the periphery of the aggregates, whereas the central mass appeared to be made up of membranous elements and peroxisome-resembling structures. Several cells contained clumps of organelles and fewer membranous elements, which were more readily identified as peroxisomes (Fig. 2C). These findings suggested that the large bodies targeted by the p33GFP seen by fluorescence microscopy consisted of aggregates of normal-sized peroxisomes sometimes mixed with membranes and mitochondria. Immunoelectron microscopy with a p33-specific antiserum showed the labeling of peroxisomal clumps, particularly at the limiting membrane of the organelles (Fig. 2D). Virtually, no labeling of mitochondria and other cytoplasmic areas was observed. Labeling was also absent in samples treated with preimmune rabbit antiserum and in sections treated with GAR-gold only (results not shown).
FIG. 2.
Electron micrographs of transformed yeasts. Cells were transformed with pEL26-GFP (A) and pEL26-33KGFP (B to D). The cells in panels A, B, and C were fixed with formaldehyde-glutaraldehyde and KMnO4 and then embedded in Spurr's resin. (B and C) Note the aggregation of peroxisomes with (B) or without (C) membranes in pEL26-33KGFP-transformed cells. (D) Cells fixed with formaldehyde-glutaraldehyde and embedded in LR White resin. Sections were treated sequentially with anti-p33 antibodies and anti-rabbit antibodies conjugated with gold. Gold label is mainly at the organelle membrane. O, oleate droplets; N, nucleus; M, mitochondria; P, peroxisomes. Scale bar, 200 nm.
p33 is an integral PMP.
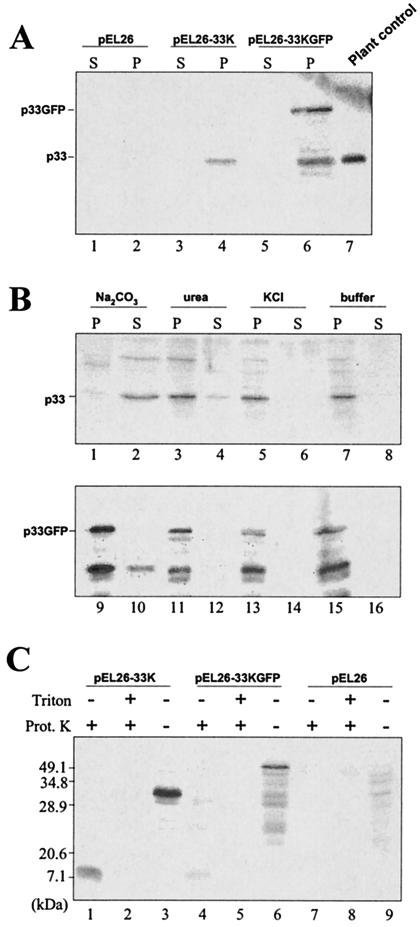
To examine the subcellular membrane association of p33 in yeast, cells expressing p33 alone or fused to GFP and cells transformed with the vector pEL26 were spheroplasted and lysed, and the lysates were submitted to differential centrifugation, first at 500 × g to eliminate nuclei and residual intact cells and then at 30,000 × g to separate a pellet and supernatant fractions. These fractions were analyzed by immunoblotting with the p33 antiserum. As shown in Fig. 3A, no signal was detected in lysates from control cells transformed with the vector, whereas a p33-specific band was present in the pellet, but not in the supernatant fraction, from cells expressing p33 alone or fused to GFP. The two bands, consistently detected in the pellet of p33GFP, corresponded to p33 alone and to the GFP fusion protein. Whether the form corresponding to p33 originated from premature termination of translation or hydrolysis in a specific point of the fusion protein has not been ascertained. Nevertheless, the occurrence of fused and nonfused p33 in the 30,000 × g pellet suggests that p33 is intimately associated with membranes, likely deriving from peroxisomes, as shown by the electron microscopic analysis.
FIG. 3.
Western blot analysis of protein extracts from pEL26-GFP, pEL26-33K, and pEL26-33KGFP yeast cells fractionated in a 30,000 × g pellet (P) and supernatant (S). (A) Accumulation of nonfused and GFP-fused p33 in the pellet. The sizes of the protein expressed in yeast were determined by comparison with p33 extracted from a CymRSV-infected control plant (lane 7). Note the occurrence of both p33 and p33GFP in the same extract (lane 6). (B) Immunoblot analysis of membrane extracts from cells expressing p33 (upper blot) or p33GFP (lower blot) treated with carbonate, urea, or KCl and then separated by centrifugation into supernatant and pellet fractions. The resistance to carbonate extraction was weak with unfused p33 and higher with fused p33GFP. Conversely, resistance to urea and high-salt extraction was compatible with the properties of integral proteins. (C) Protein extracts from pEL26-33K, pEL26-33KGFP, and pEL26 transformed yeasts were treated with proteinase K in the presence or absence of Triton X-100. A protected fragment of equal size was obtained with unfused and fused p33.
To further discriminate between a peripheral association of p33 with membranes and a true integration in the membrane, the pellet obtained at 30,000 × g was subjected to treatments known to remove proteins weakly bound to membranes. As shown in Fig. 3B, p33 and p33GFP were resistant to urea and high-salt extraction, whereas carbonate treatment resulted in a bimodal distribution of p33 or p33GFP in the pellet and supernatant fractions, findings similar to what has been observed with some PMPs (8). These results are a further confirmation of the membrane integration of p33.
As reported above, computer analysis predicted that p33 is anchored to the peroxisomal membrane via the two hydrophobic domains. The domains, each composed of 18 amino acids (aa), and the connecting loop of 22 aa form a stretch of at least 68 aa that should be resistant to protease treatment, whereas both the N- and the C-terminal regions would be digested. To prove this, the membrane pellet fraction was digested with proteinase K with or without treatment with the nonionic detergent Triton X-100. As shown in Fig. 3C, one band of ∼7 kDa was detected upon protease treatment in the absence of Triton X-100. In contrast, when membranes were treated with the detergent, the 7-kDa fragment was no longer detected. These results confirmed our hypothesis, indicating that a large part of p33 is exposed to the cytoplasm and that only a region approximately corresponding to the two transmembrane segments and the connecting hydrophilic sequence is protected from proteolysis.
Mislocalization of p33 in absence of peroxisomes.
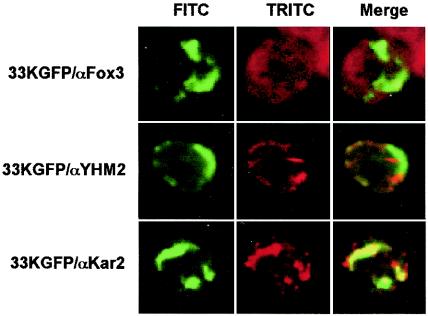
A number of cellular PMPs are sorted to peroxisomal membrane after binding to the peroxisomal protein Pex19p, which acts as an mPTS receptor, shuttling between the cytosol and the peroxisomal membrane (16). To examine whether p33 also requires interactions with Pex19p for insertion into the peroxisomal membrane, the yeast two-hybrid assay system was used. Although no apparent interaction of p33 and Pex19p was observed (not shown), its occurrence cannot be excluded, since the negative result could depend on other factors, such as improper folding or insufficient targeting to the nucleus (7). Therefore, whether the membrane insertion of p33 requires the presence of Pex19p was further investigated by using the yeast mutant pex19Δ, which does not synthesize Pex19p and lacks peroxisomes. In this mutant most cellular PMPs do not associate with membranes and are dispersed in the cytosol or are rapidly degraded (15). In contrast, extracts of pex19Δ cells transformed with pEL26-33KGFP contained membrane-associated p33GFP since it was detected in the pellet fraction and was resistant to extraction with 4 M urea or high salt concentrations (not shown). In addition, microscopic observations of the yeast mutant expressing p33GFP revealed that the green fluorescence was not diffused in the cytosol but was associated with elongated structures, which were identified as strands of ER by immunofluorescence staining with an antibody anti-Kar2 (Fig. 4, lower row). Mitochondria were not affected by the expression of p33GFP (Fig. 4, middle row), and the absence of peroxisomes was confirmed by the presence of Fox3 diffused in the cytosol (Fig. 4, upper row). These results suggest that the ER membranes could be an intermediate step to the integration of p33 into the peroxisomal membrane. However, it cannot be excluded that the association of p33 with the ER membrane in the yeast mutant pex19Δ is the consequence of a takeover by the ER of hydrophobic proteins unable to reach the normal acceptor membranes, as observed for some normal PMPs (15, 37).
FIG. 4.
Expression of the 33KGFP in the yeast strain pex19Δ lacking peroxisomes. Yeast cells expressing p33GFP were immunostained with antibodies to the protein markers of peroxisomes (Fox3, upper row), mitochondria (YHM2, middle row), or ER (Kar2, lower row). Representative images for GFP (left column, FITC), rhodamine (middle column, TRITC), and merged signals (right column) are shown.
Localization of truncated p33.
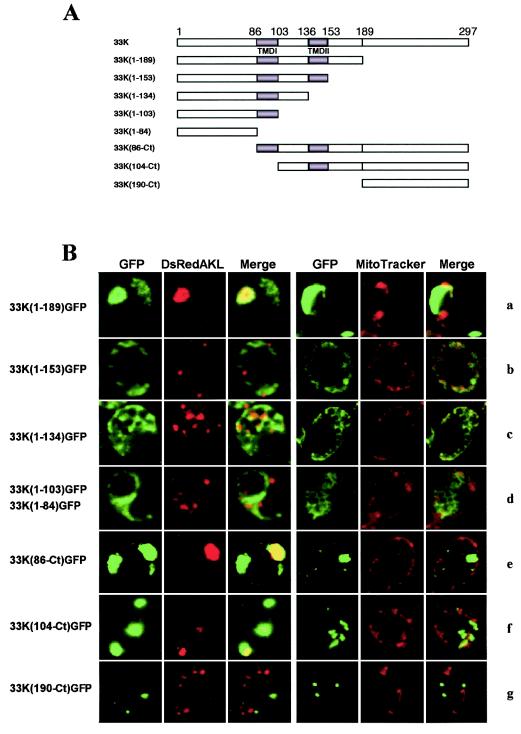
To evaluate the contribution of p33 domains to peroxisomal targeting in yeast cells, a series of deletion mutants were constructed. The truncated p33 coding sequence was maintained in frame with GFP under the control of the oleate-activated CTA1 promoter (Fig. 5A). The deletion mutant 33K(1-189) contained the ORF1 sequence up to aa 189, including the N-terminal hydrophilic region (aa 1 to 85), the two putative TMDs, TMD1 (aa 86 to 103) and TMD2 (aa 136 to 153), and the connecting loop (aa 104 to 135) (Fig. 5A). Yeast cells expressing this mutant did not differ from cells expressing the entire p33 sequence, i.e., the GFP was targeted to peroxisomes, as shown by the colocalization with the DsRedAKL protein (Fig. 5B, row a). This result was consistent with the notion that the signal for peroxisomal localization of p33 is contained in the coding sequence upstream of a SacI restriction site (position 565 in the CymRSV ORF1 sequence [3]). In fact, an infectious CymRSV/CIRV RNA hybrid containing the sequence described above from CymRSV, replacing to the corresponding region of CIRV, induced the cytopathological features of CymRSV, i.e., peroxisome-derived MVBs (3).
FIG. 5.
Expression of GFP in yeast cells transformed with different mutants of p33 obtained by progressive deletions from the N and C termini. (A) Diagram of each mutant indicating the amino acid positions of the TMDs and the relevant restriction site SacI (position 189); (B) selected images of each mutant for GFP, DsRed targeted to peroxisomes (DsRedAKL) or the mitochondrial stain (MitoTracker), and merged signals. GFP was targeted to peroxisomes when fused to aa 1 to 189 (row a) or aa 86 to 297 (row e) of p33; to peroxisomes and mitochondria when fused to aa 104 to 297 (row f); or to neither these organelles appearing as aggregates, when fused to aa 1 to 153 (row b) or 190 to 297 (row g), or completely diffused in the cytoplasm, as unfused GFP, when fused to aa 1 to 134 (row c), aa 1 to 103, or aa 1 to 84 (row d).
When p33 was progressively deleted further from the C-terminal side up to aa 84, thus removing the short hydrophilic tail after TMD2 [clone 33K(1-153)], the TMD2 [33K(1-134)], the interconnecting loop [33K(1-103)], and TMD1 [33K(1-84)], the fluorescence was dispersed into the cytosol in the form of larger [33K(1-153)] or smaller [33K(1-134)] irregular aggregates (Fig. 5B, rows b and c, respectively) or was distributed diffusely in the cytosol resembling the expression of free GFP [mutants 33K(1-103) and 33K(1-84); Fig. 5B, row d].These results suggested that the N-terminal hydrophilic region, the TMDs, the interconnecting loop, and the C-terminal hydrophilic region up to aa 189 contained the necessary signals for targeting p33 to peroxisomes.
To evaluate the relative contributions of the different domains in the C-terminal part of p33, a set of mutants was prepared by progressive deletions from the N terminus up to aa 190. The 33K(86-Ct)GFP clone lacked the entire N-terminal hydrophilic region, while containing the TMDs and the entire C-terminal region (Fig. 5A). The protein expressed from this mutant localized to peroxisomes, which appeared as large bodies, similar to those induced by the full-length clone (Fig. 5B, row e). By converse, the truncated protein expressed from the mutant 33K(104-Ct) missing the N-terminal hydrophilic region and the first TMD (Fig. 5A) formed aggregates that colocalized only partially with peroxisomes and mitochondria (Fig. 5B, row f). The exclusion of both TMDs in clone 33K(190-Ct) (Fig. 5A) resulted in the expression of small regular spherical aggregates of fluorescing protein not associated with mitochondria or peroxisomes (Fig. 5B, row g) or with ER or the Golgi apparatus (results not shown). This confirmed the strict requirement of the TMDs for the p33 association with cell membranes.
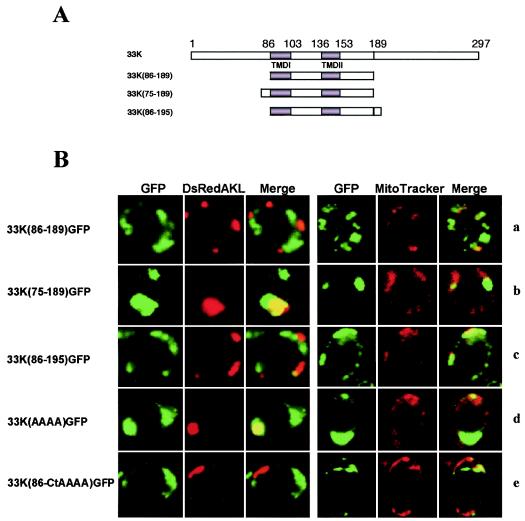
Proteins from both 33K(1-189)GFP and 33K(86-Ct)GFP clones contain the complete signal for correct targeting to peroxisomes, which should be located in the common sequence 86 to 189. Therefore, a clone [33K(86-189)GFP] was made containing only these amino acids fused to GFP (Fig. 6A). However, the protein expressed was not targeted to peroxisomes (Fig. 6B, row a), suggesting that a component of the peroxisomal signal resides upstream of aa 86 or downstream of aa 189.
FIG. 6.
Expression of GFP in yeast cells transformed with different mutants of the p33 gene obtained by deletions in the N- and C-terminal hydrophilic regions. (A) Diagram of each mutant with the indication of the positions of the TMDs and the relevant restriction site SacI; (B) selected images of each mutant for GFP, DsRed targeted to peroxisomes or mitochondria (DsRedAKL and MitoTracker, respectively), and merged signals. In addition, images for clones 33K(AAAA)GFP and 33K(86-CtAAAA)GFP are shown; these are the same as 33KGFP and 33K(86-Ct)GFP, respectively, except that amino acids RRRR at positions 213 to 216 were mutated to AAAA. The clone 33K(75-189)GFP (row b) expresses the shortest protein containing the complete signal for peroxisomal targeting. Conversely, proteins expressed from clones 33K(86-189) and 33K(86-195) (rows a and c, respectively) are essentially cytoplasmic, although protein p33(86-195) shows a weak mitochondrial targeting. Peroxisomal targeting of the clone 33K(86-Ct)GFP (see Fig. 5) was abolished if the tetrapeptide RRRR was mutated to AAAA [clone 33K(86-CtAAAA)GFP] (row e). These same amino acids are irrelevant in the full-length context of p33 [clone 33K(AAAA)GFP] (row d).
As reported in the introduction, clusters of basic amino acids flanking hydrophobic sequences are a common feature of mPTS (see references 17 and 38 and references therein). A basic triplet KRR is located seven amino acids upstream of the TMDs at positions 76 to 78 in the p33 sequence. To evaluate the importance of this triplet, the clone 33K(75-189)GFP was prepared (Fig. 6A). The truncated protein expressed by this clone targeted to peroxisomes (Fig. 6B, row b) which appeared as large structures similar to those induced by the full-length clone 33KGFP. This indicates that the triplet KRR could complement the TMDs in forming the peroxisomal targeting signal. However, the clone 33K(86-Ct)GFP contains a completely functional peroxisomal signal in the absence of the triplet KRR; thus, an alternative component of the peroxisomal signal could reside downstream of aa 189. In particular, since the truncated protein from 33K(86-195)GFP clone (Fig. 6A) did not target peroxisomes anymore, showing only occasionally a weak mitochondrial signal (Fig. 6B, row c), an equivalent of the triplet KRR should reside downstream of aa 195. A possible candidate is the tetrapeptide RRRR at positions 213 to 216. These amino acids were shown to be dispensable for peroxisomal targeting in the context of the full-length clone 33K(AAAA)GFP in which the stretch RRRR was mutated to AAAA (Fig. 6B, row d). However, when the same mutation was done in the truncated clone 33K(86-Ct)GFP, the protein did not localize to peroxisomes anymore (Fig. 6B, row e), suggesting that the RRRR motif could be alternative to the triplet KRR in forming the peroxisomal sorting signal. Interestingly, the proteins expressed by clones 33K(104-Ct)GFP, 33K(86-189)GFP, 33K(86-195)GFP, and 33K(104-CtAAAA)GFP formed similar untargeted cytoplasmic large aggregates. All of these clones have in common the sequence from aa 104 to 189. However, since mutant 33K(1-153)GFP, which forms small aggregates, lacks the sequence from aa 153 to 189, it is likely that this sequence is responsible for the formation of the large aggregates.
In the present study mutagenesis of the nonstructural, replication-associated p33 protein of CymRSV was used to map the region of the molecule containing the signal directing p33 to the peroxisomal membrane. Wild-type and truncated proteins were fused to the N terminus of GFP to obtain a rapid means for analyzing the subcellular compartmentalization of the viral protein. Yeast cells were used for such analyses, given the easy experimental tractability of this eukaryotic system (2), which was already successfully utilized in studies of tombusvirus biology (24, 25, 30, 41). Sites of accumulation of p33 were identified by means of the red fluorescing protein DsRed, modified to direct to peroxisomes; MitoTracker staining; or immunolabeling with antibodies to proteins characteristic of these and other organelles of yeast cells. The p33 is targeted to peroxisomes where it is inserted in the limiting membrane with a fragment of ∼7 kDa likely corresponding to the sequence comprising the two TMDs and the interconnecting loop. The accessibility to proteinase K digestion of the N- and C-terminal hydrophilic parts indicates that these regions are in the cytosolic side of the peroxisomal membrane. In analogy with the mitochondrial-directed p36 and complete replicase of CIRV (41), the complete replicase of CymRSV (p92) also probably has the topology Ncyto-Ccyto, being accessible to viral replication in the interior of the cytoplasm-connected vesicles developing from the peroxisomal membrane during plant cell infection (33). Electron microscopic analysis showed no vesiculation of peroxisomes where p33 accumulates, suggesting that other factors may contribute to the formation of MVBs. Likewise, no MVBs were detected when p33 was constitutively expressed in transgenic Nicotiana benthamiana plants (1), further supporting the notion that the formation of MVBs is a complex phenomenon that is not only triggered by the integration of the viral protein in the peroxisomal membrane. Similar observations were made in yeast cells expressing the p36 of CIRV (30). In both cases, an increase in the number of the affected organelles accompanied by the proliferation of ER strands was observed. It is conceivable that ER strands constitute the primary, transient site of accumulation of CymRSV p33, from which it is transferred in a membrane-flow mode to preexisting or newly formed peroxisomes. Peroxisome-based MVBs in CymRSV-infected plant cells are frequently connected to ER strands that were originally suggested to be involved in the genesis of MVBs (32).
The clone 33K(75-189)GFP encoded the shortest protein still capable of peroxisome targeting. Thus, the essential elements for peroxisome targeting are the tripeptide KRR, the two TMDs, and possibly some unrecognized elements between aa 154 and 189 [see the different behavior of clones 33K(1-153) and 33K(1-189)GFP]. The essential role of the tripeptide KRR in the formation of mPTS is in line with the general requirement of a cluster of basic amino acids in the proximity of transmembrane segments in cellular peroxisomal membrane proteins (see references 17 and 38 and references therein). In the absence of the tripeptide KRR, the targeting of p33 to peroxisomes can be supported by the tetrapeptide RRRR located downstream of TMD2. However, we tend to favor the tripeptide KRR as the normal constituent of p33 mPTS based on the strict conservation of KRR in all tombusviruses that induce peroxisome-derived MVBs (11, 20, 33, 34). In contrast, the cluster RRRR is not strictly conserved. Moreover, it was shown that the C-terminal hydrophilic region further away from TMDs is not involved in the recognition of the organelle source of MVBs (3).
In addition to elucidating the properties of the p33, the present study showed the possibility of using the yeast system to investigate also the replication of CymRSV as it was successfully done with other tombusviruses by using defective interfering RNAs as the replication template (24, 25).
Acknowledgments
We thank B. Distel, L. Palmieri, M. D. Rose, and H. D. Schmitt for the antibodies to Fox3, YHM2, Kar2, and Emp47, respectively; B. Distel and J. Goodman for plasmids pEL26 and pDsRedAKL, respectively; C. Capobianco for skillful technical assistance with confocal microscopy; and G. P. Martelli and L. Stavolone for advice and critical reading of the manuscript.
B.N. was a recipient of a Postdoctoral Fellowship of the Spanish Ministerio de Educación, Cultura y Deporte during the initial part of this study and is now a recipient of a Marie Curie Fellowship of the European Commission Programme Human Potential under contract number HPMF-CT-2001-01109. This research was partially supported by MIUR, Project Cluster CO3, Legge 488/92, Studi di geni di interesse biomedico e agroalimentare.
REFERENCES
- 1.Bleve-Zacheo, T., L. Rubino, M. T. Melillo, and M. Russo. 1997. The 33K protein encoded by cymbidium ringspot virus localizes to peroxisomes of infected cells and of uninfected transgenic plants. J. Plant Pathol. 79:197-202. [Google Scholar]
- 2.Botstein, D., and G. R. Fink. 1988. Yeast: an experimental organism for modern biology. Science 240:1439-1443. [DOI] [PubMed] [Google Scholar]
- 3.Burgyan, J., L. Rubino, and M. Russo. 1996. The 5′-terminal region of a tombusvirus genome determines the origin of multivesicular bodies. J. Gen. Virol. 77: 1967-1974. [DOI] [PubMed] [Google Scholar]
- 4.Cho, J. H., S. J. Ha, L. R. Kao, T. L. Megraw, and C. B. Chae. 1998. A novel DNA-binding protein bound to the mitochondrial inner membrane restores the null mutation of mitochondrial histone Abf2p in Saccharomyces cerevisiae. Mol. Cell. Biol. 18: 5712-5723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cormack, P. B., G. Bertram, M. Egerton, N. A. R. Row, S. Falkow, and A. J. Brown. 1997. Yeast-enhanced green fluorescent protein (yEGFP), a reporter of gene expression in Candida albicans. Microbiology 143:303-311. [DOI] [PubMed] [Google Scholar]
- 6.Dalmay, T., M. Russo, and J. Burgyan. 1993. Repair in vivo of altered 3′ terminus of Cymbidium ringspot tombusvirus RNA. Virology 192:551-555. [DOI] [PubMed] [Google Scholar]
- 7.Drees, B. L. 1999. Progress and variations in two-hybrid and three-hybrid technologies. Curr. Opin. Chem. Biol. 3:64-70. [DOI] [PubMed] [Google Scholar]
- 8.Elgersma, Y., A. Vos, M. Van den Berg, C. W. T. Van Roermund, P. Van der Slujids, B. Distel, and H. F. Tabak. 1996. Analysis of the carboxyl-terminal peroxisomal targeting signal 1 in a homologous context in Saccharomyces cerevisiae. J. Biol. Chem. 271:26375-26382. [DOI] [PubMed] [Google Scholar]
- 9.Erdmann, R., and W. H. Kunau. 1994. Purification and immunolocalization of the peroxisomal 3-oxoacyl-CoA-thiolase from Saccharomyces cerevisiae. Yeast 10:1173-1182. [DOI] [PubMed] [Google Scholar]
- 10.Erdmann, R., M. Veenhuis, D. Mertens, and W. H. Kunau. 1989. Isolation of peroxisome-deficient mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 86:5419-5423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Galetzka, D., M. Russo, L. Rubino, and G. Krczal. 2000. Molecular characterization of a tombusvirus associated with a disease of statice, Goniolimon tataricum (L.) Boiss. J. Plant Pathol. 82:151-155. [Google Scholar]
- 12.Götte, K., W. Girzalsky, M. Linkert, E. Baumgart, S. Kammerer, W. H. Kunau, and R. Erdmann. 1998. Pex19p, a farnesylated protein essential for peroxisome biogenesis. Mol. Cell. Biol. 18:616-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gould, S. J., G. A. Keller, M. Schneider, S. H. Howell, L. J. Garrard, J. M. Goodman, B. Distel, H. Tabak, and S. Subramani. 1990. Peroxisomal protein import is conserved between yeast, plants, insects and mammals. EMBO J. 9:85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grieco, F., J. Burgyan, and M. Russo. 1989. The nucleotide sequence of cymbidium ringspot virus RNA. Nucleic Acids Res. 17:6383.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hettema, E. H., W. Girzalsky, M. van Den Berg, R. Erdmann, and B. Distel. 2000. Saccharomyces cerevisiae Pex3p and Pex19p are required for proper localization and stability of peroxisomal membrane proteins. EMBO J. 19:223-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holroyd, C., and R. Erdmann. 2001. Protein translocation machineries of peroxisomes. FEBS Lett. 501:6-10. [DOI] [PubMed] [Google Scholar]
- 17.Honsho, M., T. Hiroshige, and Y. Fujiki. 2002. The membrane biogenesis peroxin Pex16p: topogenesis and functional roles in peroxisomal membrane assembly. J. Biol. Chem. 177:44513-44524. [DOI] [PubMed] [Google Scholar]
- 18.Ito, H., Y. Fulkuda, K. Murata, and A. Kimura. 1983. Transformation of intact yeast cells treated with alkali cations. J. Bacteriol. 153:163-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koonin, E. V. 1991. The phylogeny of RNA-dependent RNA polymerases of positive strand RNA viruses. J. Gen. Virol. 72:2197-2206. [DOI] [PubMed] [Google Scholar]
- 20.D. Kostova, D., V. Lisa, L. Rubino, C. Marzachì, P. Roggero, and M. Russo. 2004. Properties of Cucumber Bulgarian virus, a new genus in the genus Tombusvirus. J. Plant Pathol. 85:27-33.
- 21.Lupo, R., L. Rubino, and M. Russo. 1994. Immunodetection of the 33K/92K polymerase proteins in cymbidium ringspot virus-infected and in transgenic plant tissue extracts. Arch. Virol. 138:135-142. [DOI] [PubMed] [Google Scholar]
- 22.Martelli, G. P., D. Gallitelli, and M. Russo. 1988. Tombusviruses, p. 13-72. In R. Koenig (ed.), The plant viruses: polyhedral virions with monopartite RNA genomes. Plenum Publishing Corp., New York, N.Y.
- 23.McNew, J. A., and J. M. Goodman. 1994. An oligomeric protein is imported into peroxisomes in vivo. J. Cell Biol. 127:1245-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Panavas, T., and P. D. Nagy. 2003. Yeast as a model host to study replication and recombination of defective interfering RNA of Tomato bushy stunt virus. Virology 314:315-325. [DOI] [PubMed] [Google Scholar]
- 25.Pantaleo, V., L. Rubino, and M. Russo. 2003. Replication of Carnation Italian ringspot virus defective interfering RNA in Saccharomyces cerevisiae. J. Virol. 77:2116-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rachubinski, R. A., and S. Subramani. 1995. How proteins penetrate peroxisomes. Cell 83:525-528. [DOI] [PubMed] [Google Scholar]
- 27.Restrepo-Hartwig, M., and P. Ahlquist. 1999. Brome mosaic virus RNA replication proteins 1a and 2a colocalize and 1a independently localizes on the yeast endoplasmic reticulum. J. Virol. 73:10303-10309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose, M. D., L. M. Misra, and J. P. Vogel. 1989. KAR2, a karyogamy gene, is the yeast homolog of the mammalian BiP/GRP78 gene. Cell 57:1211-1221. [DOI] [PubMed] [Google Scholar]
- 29.Rubino, L., and M. Russo. 1998. Membrane targeting sequences in tombusvirus infections. Virology 252:431-437. [DOI] [PubMed] [Google Scholar]
- 30.Rubino, L., A. Di Franco, and M. Russo. 2000. Expression of a plant virus nonstructural protein in Saccharomyces cerevisiae causes membrane proliferation and altered mitochondrial morphology. J. Gen. Virol. 81:279-286. [DOI] [PubMed] [Google Scholar]
- 31.Rubino, L., F. Weber-Lotfi, A. Dietrich, C. Stussi-Garaud, and M. Russo. 2001. The open reading frame-encoded (36K) protein of Carnation Italian ringspot virus localizes to mitochondria. J. Gen. Virol. 82:29-36. [DOI] [PubMed] [Google Scholar]
- 32.Russo, M., A. Di Franco, and G. P. Martelli. 1983. The fine structure of Cymbidium ringspot virus infections in host tissues. III. Role of peroxisomes in the genesis of multivesicular bodies. J. Ultrastruct. Res. 82:52-63. [DOI] [PubMed] [Google Scholar]
- 33.Russo, M., A. Di Franco, and G. P. Martelli. 1987. Cytopathology in the identification and classification of tombusviruses. Intervirology 28:134-143. [DOI] [PubMed] [Google Scholar]
- 34.Russo, M., C. Vovlas, L. Rubino, F. Grieco, and G. P. Martelli. 2002. Molecular characterization of a tombusvirus isolated from diseased pear trees in southern Italy. J. Plant Pathol. 84:161-166. [Google Scholar]
- 35.Schaad, M. C., P. E. Jensen, and J. C. Carrington. 1997. Formation of plant RNA virus replication complexes on membranes: role of an endoplasmic reticulum-targeted viral protein. EMBO J. 16:4049-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schröder, S., F. Schimmoller, B. Singer-Kruger, and H. Riezman. 1995. The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1-1 mutation in α-COP. J. Cell Biol. 131:895-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stroobants, A. K., E. H. Hettema, M. van den Berg, and H. F. Tabak. 1999. Enlargement of the endoplasmic reticulum membrane in Saccharomyces cerevisiae is not necessarily linked to the unfolded protein response via Ire1p. FEBS Lett. 453:210-214. [DOI] [PubMed] [Google Scholar]
- 38.Subramani, S., A. Koller, and W. B. Snyder. 2000. Import of peroxisomal matrix and membrane proteins. Annu. Rev. Biochem. 69:399-418. [DOI] [PubMed] [Google Scholar]
- 39.Szittya, G., A. Molnár, D. Silhavy, C. Hornyik, and J. Burgyan. 2002. Short defective interfering RNAs of tombusviruses are not targeted but trigger posttranscriptional gene silencing against their helper virus. Plant Cell 14:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang, X., M. J. Unruh, and J. M. Goodman. 2001. Discrete targeting signals direct Pmp47 to oleate-induced peroxisomes in Saccharomyces cerevisiae. J. Biol. Chem. 276:10897-10905. [DOI] [PubMed] [Google Scholar]
- 41.Weber-Lotfi, F., A. Dietrich, M. Russo, and L. Rubino. 2002. Mitochondrial targeting and membrane anchoring of a viral replicase in plant and yeast cells. J. Virol. 76:10485-10496. [DOI] [PMC free article] [PubMed] [Google Scholar]