Abstract
Glial cell-line derived neurotrophic factor (GDNF) supports and maintains the neuromuscular system during development and through adulthood by promoting neuroplasticity. The aim of this study was to determine if different modes of exercise can promote changes in GDNF expression and neuromuscular junction (NMJ) morphology in slow and fast twitch muscles. Rats were randomly assigned to a run training (run group), swim training (swim group), or sedentary control group. GDNF protein content was determined by enzyme-linked immunosorbant assay. GDNF protein content increased significantly in soleus (SOL) following both training protocols (P < 0.05). Although not significant, an increase of 60% in the extensor digitorum longus (EDL) followed swim-training (NS; P < 0.06). NMJ morphology was analyzed by measuring α-bungarotoxin labeled post-synaptic end plates. GDNF content and total end plate area were positively correlated. End plate area decreased in EDL of run group and increased in SOL of swim group. The results indicate that GDNF expression and NMJ morphological changes are activity dependent and that different changes may be observed by varying the exercise intensity in slow and fast twitch fibers.
Keywords: Neuromuscular junction, fast-twitch muscle, slow-twitch muscle, exercise physiology, skeletal muscle, neuroplasticity
Maintaining a healthy neuromuscular system requires a continual supply of neurotrophic factors (NFs) to support developing and mature motor neurons. NFs support motor neurons throughout the lifespan by supporting maturation during embryonic life, maintenance through adulthood, and regeneration after injury (Keller-Peck et al., 1997; Keller Peck et al., 2001; Oppenheim et al., 1995; Trupp et al., 1995; Wang et al., 2002; Yan et al., 1995; Zwick et al., 2001). NFs have been shown to act alone and synergistically in their role to promote proper development and plasticity of adult and aged neurons. Of the many NFs identified to date, glial cell-line derived neurotrophic factor (GDNF) has been shown to be the most potent trophic factor for motor neuron survival in vitro (Henderson et al., 1994; Lin et al., 1993) and in vivo (Henderson et al., 1994). GDNF has been classified as belonging to the transforming growth factor superfamily and has found to be a muscle-derived factor that regulates pre- and post-synaptic plasticity (Lin et al., 1993).
GDNF abides by the neurotrophic theory by being expressed in target skeletal muscle tissue, by being retrogradely transported to axonal cell bodies (Yan et al., 1995), and by providing survival support for motor neurons throughout their lifespan (Keller Peck et al., 2001). When compared to other NFs, GDNF has been shown to be up to 2500-fold more potent, being able to save nearly 100% of axotomized motor neurons and was the only factor to prevent axotomy-induced motor neuron atrophy (Henderson et al., 1994).
Although GDNF was first identified in midbrain dopaminergic neurons (Lin et al., 1993), it has since been found in numerous sites such as skeletal muscle, Schwann cells, motor neuron axons and cell bodies (Henderson et al., 1994; Nosrat et al., 1996; Springer et al., 1994; Springer et al., 1995; Trupp et al., 1995). GDNF exerts its effects through the GDNF-GFR- 1-GPI complex, which interacts with Ret tyrosine kinase receptors that can be found localized presynaptically in the neuromuscular junction (Airaksinen and Saarma, 2002). The loss of Ret receptors decreases motor neuron survival embryonically, reduces adult motor neuron end plate numbers by half and results in a marked deficiency of maturing axon terminals within the adult muscle (Baudet et al., 2008). The overexpression of GDNF or treatment with GDNF has been shown to increase nerve terminal sprouting, slow the process of synapse elimination, and increase the number and size of end plates (Keller Peck et al., 2001; Nguyen et al., 1998; Zwick et al., 2001).
Neurotrophic factor secretion has been shown to increase following synaptic activity and may act to enhance transmission (Schinder and Poo, 2000). In the central nervous system (CNS), NFs have been shown to exhibit enhanced or reduced expression and secretion following increased or decreased synaptic activity, respectively (Lauterborn et al., 2000; Zafra et al., 1991). The amount of NF secreted has also been shown to increase in proportion to the level of synaptic activity. The presence of secreted NFs, in turn, has been shown to induce a potentiation of transmitter release from presynaptic nerve terminals (Wang and Poo, 1997). Similar findings have been shown in vitro where NFs increase following chronic depolarization in culture (Lohof et al., 1993; Vianney and Spitsbergen, 2011) and in vivo, where NFs increase following exercise (Gómez Pinilla et al., 2002; McCullough et al., 2011; Wehrwein et al., 2002).
Exercise and GDNF independently have been shown to induce similar changes in the neuromuscular system, such as enhancing maintenance of synapses, inducing axonal sprouting (Andonian and Fahim, 1987; Keller Peck et al., 2001; Zwick et al., 2001), and increasing end plate complexity and size (Deschenes et al., 1993; Keller Peck et al., 2001). Exercise has been shown to induce synaptic activity and alter GDNF expression in skeletal muscle and spinal cord (McCullough et al., 2011; McCullough et al., 2013; Wehrwein et al., 2002). Previous studies have shown that GDNF expression is regulated in an activity dependent manner and its expression may depend on the recruitment of muscle fibers (McCullough et al., 2011; Wehrwein et al., 2002). For example, following low intensity exercise, an increase of GDNF expression was observed in the SOL, a predominantly slow twitch muscle, but decreased in the EDL, a predominantly fast twitch muscle (McCullough et al., 2011). This may indicate that the low speed selected for the run training (10m/min) was not of high enough intensity to recruit fast twitch muscle fibers and consequently decreased GDNF protein content. Swim training has been shown to demand the recruitment of fast twitch dorsiflexors more than run training, due to its higher cycling rate and to overcome the added resistance of the water medium during the recovery phase (Gruner and Altman, 1980; Roy et al., 1991). Swim training has shown similar recruitment patterns as run training in fast twitch muscle fibers only when the treadmill reached higher speeds of ~67m/min (Roy et al., 1991).
It is, therefore, our broad aim to examine GDNF expression and NMJ morphology in predominantly slow and fast twitch muscle fibers, following run and swim training. It is our hypothesis that the fast twitch muscle fibers will be recruited and subsequently display an increase in GDNF protein content following swim training when compared to run training (10m/min).
1. EXPERIMENTAL PROCEDURES
1.1 Subjects
Six-month old Sprague Dawley rats (Charles River, Kalamazoo, MI) were housed in rooms lighted from 7 AM-7 PM and given free access to standard rat chow and water ad libitum. Approval for this work was obtained from the Institutional Animal Care and Use Committee at Western Michigan University. Rats were randomly assigned to a swim group (n=6), run group (n=5) or control group (n=6). The control group was housed in individual standard living chambers and remained sedentary throughout the study.
1.2 Training Protocols
The training protocol duration was based on previous studies that observed changes in GDNF expression following two weeks of exercise (McCullough et al., 2011; McCullough et al., 2013).
Rats in the swim-train group (swim group) swam in a barrel (3/barrel) for 2 hours/day for 2 weeks. Each swim consisted of five 24-min bouts separated by 10-min rest times. The water was filled to a depth of 100 cm and maintained at 35°C, which was large enough for each rat to swim freely as previously published (Li et al., 2012; McCullough et al., 2013).
Rats in the run train group (run group) were placed in forced running wheels (Lafayette Instruments, Lafayette, IN) for 2 hours/day for 2 weeks as previously published (McCullough et al., 2013). The wheels were set at 10m/min and each run consisted of five 24-min bouts separated by 10-min rest times to match the swim exercise.
1.3 Tissue Preparation and Storage
At the conclusion of the 2-week training period, rats from all groups were weighed and then euthanized via CO2 asphyxiation and thoracotomy. Immediately following death, the soleus (SOL) and the extensor digitorum longus (EDL) muscles were removed. The muscles from the left side of the body were used for immunohistochemical analysis. Muscles were washed with phosphate-buffered saline (PBS: 0.225 M NaCl, 0.02 M NaH2PO4, and 0.08 M Na2HPO4), frozen at resting length in isopentane and stored at −80°C. The muscles from the right side of the body were used to determine GDNF protein content using enzyme-linked immunosorbent assay (ELISA), as previously published (McCullough et al., 2011). Briefly, muscles were dipped in liquid nitrogen, smashed into fine powder, and homogenized in sample processing buffer solution (0.55 M NaCl, 0.02 M NaH2PO4, 0.08 M Na2HPO4, 2 mM EDTA, 0.1 mM benzethonium chloride, 2 mM benzamidine, 20 KIU/ml aprotinin, 0.5% BSA, and 0.05% Tween-20). Homogenates were centrifuged at 4°C for 30 min and the resultant supernatant was decanted and stored at −80°C.
1.4 Immunohistochemistry
To analyze GDNF localization and NMJ structure, the middle sections of the SOL and EDL were removed from connective tissue, embedded in optimum cutting temperature compound (Sakura Finetek, Torrance, CA, USA) and cut on a Leica microtome-cryostat. Tissues were cut horizontally at 60μm for end plate and GDNF visualization and cut transversely at 10μm for fiber type analysis. All sections were thaw mounted on HistoBond Microscope Slides (VWR, 195 International, Bridgeport, NJ, USA). To help sections adhere, slides were vacuum sealed and left overnight at 4°C. Slides were then fixed using 4% paraformaldehyde diluted in PBS at room temperature for 1h. After fixing, the tissue was incubated in buffer containing 5% normal serum from the host species for the secondary antibody. Primary antibodies were incubated on sections overnight in a humidified chamber. To identify GDNF protein, polyclonal rabbit anti-GDNF antibody was used (Santa Cruz Biotechnologies; 1:200). End plates were identified using α-bungarotoxin (1:200) directly conjugated to AlexaFluor 488®. All fiber type specific primary antibodies were purchased from Developmental Studies Hybridoma Bank, University of Iowa. The following day, slides were washed in PBS and secondary antibodies were applied at a concentration of 1:500. Secondary antibodies were conjugated to AlexaFluor 488®, AlexaFluor 568®, or AlexaFluor 647®. Slides were washed a final time followed by the application of 1:1 glycerol:PBS and a glass coverslip for microscopy.
1.5 Quantification: End Plate Size
A confocal microscope (Zeiss LSM 510) was used to visualize and collect end plate images. Once it was determined that the end plate was within the longitudinal border of the myofiber, a z-series of scans constructed a detailed picture using a C-Apochromat 63x/1.2 water correction objective (Zeiss, LLC). Fifty random endplates were captured from the EDL and SOL muscles for morphological analysis from at least three animals per group. Each end plate was measured for total area, stained area, total perimeter and stained perimeter using ImageJ software as previously described (Deschenes et al., 2006). In brief, a box was drawn around each end plate with lines touching each side of the stained area to determine total area and total perimeter. Total area included the stained regions and non-stained regions interspersed within those clusters residing inside the drawn box. Total Perimeter included the length of the box drawn around the end plate clusters that encompassed the stained and nonstained regions. Threshold was used to draw a line around only the stained area and stained perimeter of the individual end plate clusters.
1.6 Quantification: Muscle Fiber Cross Sectional Area (CSA)
Frozen transverse cross-sections (10μm) were stained against myosin heavy chain for determination of muscle fiber cross-sectional area (CSA) in the SOL and EDL muscles. Widefield fluorescence microscopy was used to analyze random samples of muscle fibers from the EDL and SOL muscles as previously published (Legerlotz et al., 2008). 125–150 muscle fibers were analyzed from three different animals per group to determine average myofiber CSA.
1.7 Statistics
All statistical analysis was performed using SPSS statistical software. Descriptive statistics were calculated to define means and standard errors for all variables. The results were reported as the mean ± standard error of the mean (SEM). A one-way ANOVA and Tukey post hoc test were used to assess the statistical significance among the different groups. Linear regression analysis was performed on the individual samples to evaluate the association between variables. Statistical differences were considered significant at P<0.05.
2. RESULTS
2.1 Training Alters GDNF Protein Content
An ELISA was used in order to detect GDNF protein changes following exercise protocols in the SOL and EDL muscle fibers when compared to sedentary controls. An increase in GDNF protein content was observed (P < 0.05) following both run and swim training in the SOL muscles when compared to controls (Table 1). GDNF protein content in the EDL muscle was mostly affected following swim training. Although the increase in the swim group was not significant, it was increased by 60% (P < 0.06) when compared to the control group and was significantly different (P < 0.05) when compared to the run group.
TABLE 1.
Training Alters GDNF Protein Content Values are means ± S.E.M., Protein content (pg/mg/tissue weight)
| Soleus | EDL | |
|---|---|---|
| Control | 2.5 ± 2.2 | 4.5 ± 2.4 |
| Run | 7.6 ±6.4* | 4.0 ± 1.4 |
| Swim | 8.6 ± 4.5* | 7.2 ± 3.0# |
2.2 Training Induces Changes in Weight and CSA of Muscle
The animal's body weight, relative muscle weight and muscle fiber CSA were measured in order to observe any changes following the two weeks of training when compared to sedentary controls.
The animals showed a decrease in their body weight following two weeks of run training (P < 0.05). Following swim training both the body weight and the relative muscle weight of the EDL muscle was increased when compared to the run and control groups (P < 0.05; Table 2).
TABLE 2.
Animal Body and Muscle Weights (Values are means ± S.E.M.)
| Body Wt (g) | Tissue Wt (mg) | Relative Muscle Wt (mg/g) | ||
|---|---|---|---|---|
| SOL | Control | 459 ± 10 | 298 ± 19 | 0.65 ± 0.04 |
| Run | 408 ± 11*# | 243 ± 31 | 0.65 ± 0.06 | |
| Swim | 453 ± 10 | 267 ± 23 | 0.59 ± 0.05 | |
| EDL | Control | 459 ± 10 | 238 ± 18 | 0.52 ± 0.04 |
| Run | 408 ± 11*# | 247 ± 21 | 0.62± 0.03 | |
| Swim | 453 ± 10 | 302 ± 20* | 0.67 ± 0.04* |
The changes observed in the CSA of the muscle fibers were similar to GDNF trends in that both training protocols induced changes in the SOL muscle (Table 3). The CSA of the SOL muscle fibers decreased significantly following both training protocols (P < 0.05).
TABLE 3.
Training Alters Muscle Fiber CSA Values are means ± S.E.M.
| Soleus | EDL | |
|---|---|---|
| Control | 3831 ± 180 | 2574 ± 98 |
| Run | 2109 ± 65*# | 2352 ± 103 |
| Swim | 3243 ± 101* | 2679 ± 104# |
Neither training protocol significantly altered the CSA of the EDL muscle fibers when compared to controls (Table 3). Although the CSA of the EDL muscle showed an increased trend, it was only significantly different from the run group (P < 0.05).
2.3 Training Alters End Plate Morphology in SOL Muscle Fibers
The end plates of the SOL and EDL muscle fibers were measured to determine any changes in morphology following two weeks of training when compared to sedentary controls.
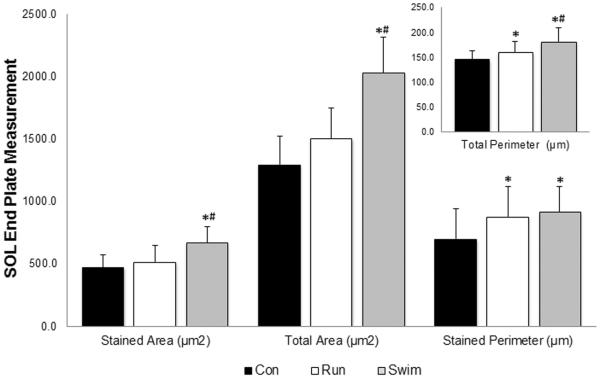
After two weeks of run training, no changes were observed for end plate area and size in the SOL muscle fibers. Run training did have an effect on measured perimeters as an increase was observed following the two weeks of training when compared to sedentary controls (P < 0.05; Figure 1). End plate plasticity was observed following two weeks of swim training in the SOL muscle in all selected measurements (P < 0.05; Figure 1). Increases in the end plate area and total perimeter were found following two weeks of swim training when compared to the run and control groups as well as an increase in the stained perimeter when compared to the control group.
Figure 1. Effects of exercise on post-synaptic endplate morphology in SOL muscle.
Cross sections (60μm) of SOL were stained with α-bungarotoxin for visualization of motor endplates. Stained and total area (μm2) increased significantly in the swim group (gray) when compared to the run group (white) and control group (black). Stained and total perimeter (μm) increased significantly in both exercise groups when compared to control. Values are displayed as the mean ±SEM. Asterisk (*) indicates a significant (P≤0.05) difference from the control group. Number symbol (#) indicates a significant (P≤0.05) difference from the run group.
2.4 Training Alters End Plate Morphology in EDL Muscle Fibers
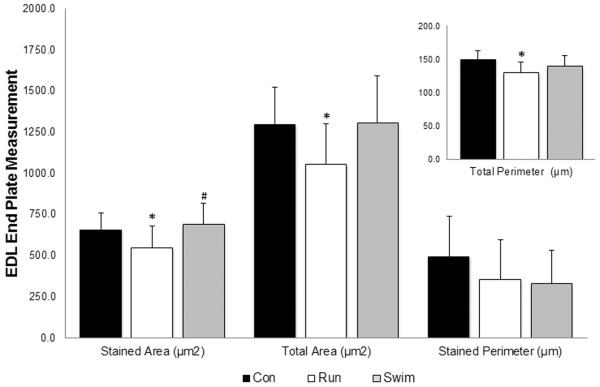
Two weeks of run training altered the end plate morphology of the EDL end plates by significantly reducing the stained area, total area, and total perimeter measurements when compared to sedentary controls (P < 0.05; Figure 2). There were no differences observed in the end plates of the swim group when compared to controls following two weeks of swim training. Although end plate measurements were not significantly different from controls, this may indicate a resistance to atrophy that was not observed following run training.
Figure 2. Effects of exercise on post-synaptic endplate morphology in EDL muscle.
Cross sections (60μm) of EDL were stained with α-bungarotoxin for visualization of motor endplates. Stained and total area (μm2) and total perimeter (μm) increased significantly in the run group (white) when compared to the control group (black). Stained area (μm2) for the swim group (grey) increased significantly from the run group. Values are displayed as the mean ±SEM. Asterisk (*) indicates a significant (P≤0.05) difference from the control group. Number symbol (#) indicates a significant (P≤0.05) difference from the run group.
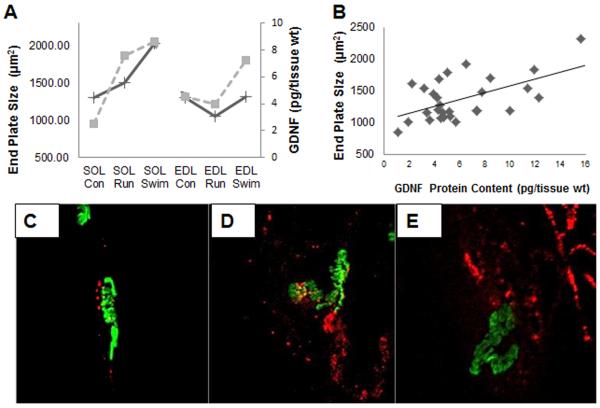
2.5 GDNF Protein Content Correlates with End Plate Total Area
Correlation statistics were ran to determine if a relationship exists between GDNF protein content and end plate measurements. GDNF protein content and end plate total area averages for all groups follow similar patterns of change (Figure 3A). A relationship exists between GDNF protein content and end plate total area for all groups (r=0.611, P<0.01, n=30; Figure 3B). GDNF was observed in and around the end plates as well as separate from the end plates (Figure 3C–E). This suggests that GDNF is expressed at the NMJ and in the muscle fibers as supported by the ELISA (Table 1). Increases in GDNF protein content and end plate area were observed in the SOL muscle following training (Figure 3C–E).
Figure 3. Correlation between GDNF levels in skeletal muscle and total end plate area.
End plates were visualized with α-bungarotoxin and measurements were taken using ImageJ software. Tissues were processed for GDNF protein content using ELISA and visualized by staining with anti-GDNF antibodies (blue). End plate total area (μm2) was measured by drawing a box around the entire end plate, including the stained ACh receptor clusters and the area in between. (A) Average measurements showing the overall trend for all groups, comparing GDNF protein content (dashed line) and end plate total area (solid line). (B) Levels of GDNF (pg/mg tissue wt.) were positively correlated with end plate measurements for total area (r=.611, P<0.01, n=30). (C–E) Visualization of GDNF was labeled with rabbit anti-GDNF (blue) in and around SOL end plates labeled with α-bungarotoxin (green) in control (C), run (D), and swim groups (E).
3. DISCUSSION
3.1 GDNF Expression & NMJ Plasticity in Soleus
One of the main findings of this study was the ability of the soleus muscle to increase GDNF protein content and exhibit significant plasticity at the NMJ following two weeks of run and swim training. Both training protocols were sufficient to induce an increase in GDNF protein content, further supporting an activity-dependent mechanism for neurotrophic support (McCullough et al., 2011). The observation that SOL muscle fiber CSA decreases with these exercise plans suggests that these muscles were recruited with these activities (Deschenes et al., 1993; Deschenes et al., 2006; Waerhaug et al., 1992). The training-induced reduction in fiber diameter is an indication of recruitment and is thought to be a positive adaptation to more readily exchange oxygen, carbon dioxide and waste products prolonging time to fatigue (Hoppeler et al., 2003).
The changes in the CSA of the SOL muscle fibers were most significant following the run training when compared to the swim and control groups possibly due to the differences in recruitment patterns of the two exercises selected. The two protocols place different demands on the recruitment patterns (Ariano et al., 1973) of the homogeneous slow twitch SOL muscle (Armstrong and Phelps, 1984) and the fast twitch EDL muscle (Ariano et al., 1973). The low intensity speeds selected for the run-training protocol have been shown to nearly maximize the recruitment of the slow twitch SOL muscle fibers while higher cycling rates, such as swimming (more steps per unit time) may decrease that activation (Roy et al., 1991).
Both training protocols were able to alter the post-synaptic apparatus of the SOL muscle, consistent with previous findings supporting exercise as a means to alter end plate morphology in fully mature rats (Deschenes et al., 1993; McCullough et al., 2011; Waerhaug et al., 1992). GDNF protein content and end plate total area in the SOL were found to be positively correlated following run training consistent with previously findings (McCullough et al., 2011). The total end plate area may increase following presynaptic changes, in order to avoid fatigue (Hill et al., 1991). This activity-dependent adaptive mechanism may increase neurotransmitter stores (Stephens and Taylor, 1972) and quantal storage and release (Dorlöchter et al., 1991). This may subsequently demand the enlargement of its counterpart, the acetylcholine (ACh) receptor field. An increase in post-synaptic ACh receptor field has been previously shown following overexpression of GDNF (Keller Peck et al., 2001; Wang et al., 2002; Zwick et al., 2001).
3.2 GDNF Expression & NMJ Plasticity in EDL
The second substantial finding in this study was the ability of swim-training to increase GDNF protein content in fast-twitch muscle fibers by 60% compared to controls. Although this was not found to be statistically significant, it may be physiologically relevant and clinically important. These findings may provide encouragement for future studies to fine tune exercise prescription in order to recruit and support fast twitch muscle fibers and their innervating neurons through expression of neurotrophic factors.
The EDL was presumably recruited as evidence of hypertrophy of the EDL muscle fibers based on the mean absolute and relative muscle weight gain following swim training. It has been previously shown the increased cycling rates in swimming escalate the demands for additional power via recruitment of larger fast-twitch fibers (Henneman, 1981) and may be responsible for the trend towards an increase in GDNF content. Given the additional demand to meet the higher intensity swim-training and the resistance to dorsiflexion offered by the water medium, the fast twitch EDL muscle has been shown to be more heavily recruited than the slow SOL muscle during swim-training. These results suggest that with further increases in intensity, such as resistance training, a statistically significant increase in GDNF protein content in fast-twitch fibers may result.
This finding may be clinically significant given that sarcopenia, the age associated decline in skeletal muscle mass and strength, is closely associated with increased frailty in the elderly, with decreased functional mobility and independence leading to a diminished quality of life, morbidity and mortality (Aniansson et al., 1984; Nevitt et al., 1989; Roubenoff, 2001). Sarcopenia appears to be fiber-type specific as large motor units that innervate fast twitch skeletal muscle present as the most vulnerable to denervation, followed by intermediate and finally the most resilient small motor units (Edström et al., 2007; Doherty, 2003; Frey et al., 2000). The denervation of large motor units causes terminal sprouting of adjacent surviving motor units to muscle fibers left without innervation (Rich and Lichtman, 1989). This increases the size of the remaining motor units and may cause overburdening and exhaustion of that neuron, progressively leading to further denervation and eventual disability and mortality.
Denervation has been shown to occur before myofiber atrophy and is a necessary prerequisite for muscle mass loss and fiber type conversion (Deschenes et al., 2010). Therefore, it has been suggested that by resisting the initial steps of denervation of the large motor units, the aging process of sarcopenia may be delayed in the neuromuscular system (Deschenes et al., 2010). In addition, significant changes have been observed in fast-twitch muscle fibers after disuse (Grimby et al., 1980) suggesting that exercise may play a key role in delaying the onset of aging in recruited fibers and in the neuromuscular system.
Both GDNF and exercise independently have been shown to protect large motor neurons from degeneration. GDNF-treated mice increased the large labeled motor neurons by 18-fold compared to β-Gal-treated mice and 3-fold more than untreated mice in a transgenic amyotrophic lateral sclerosis model (Mohajeri et al., 1999). Further studies are needed to determine if manipulation of exercise prescription can significantly increase GDNF protein content in fast twitch muscle fibers, leading to protection of large motor neurons.
3.4 Conclusion
These findings support that GDNF is activity dependent and that it may be possible to increase GDNF's protein content in slow and fast twitch muscle fibers following different modes and intensities of exercise. It also supports the idea that GDNF plays a role in remodeling the NMJ in slow and fast twitch muscle fibers. This continues to be encouraging for the use of exercise as a mechanism to increase GDNF protein content in skeletal muscle, leading to enhanced plasticity of the NMJ and enhanced neuromuscular health. With further research, guidelines can carefully inform exercise prescription that maximizes the neuromuscular benefits in both slow and fast twitch muscle fibers.
Highlights
Swim and run training recruit soleus muscle (type-1) and increase GDNF protein content and end plate area
Running does not recruit extensor digitorum longus muscle (type II) and decreases GDNF protein content and end plate area
Swimming recruits extensor digitorum longus muscle (type II) and increases GDNF protein content and end plate area
Acknowledgements and Grants
This work was supported by NIH grant 1 R15 AG022908-01A2, NSF grant DBI 0552517 and Western Michigan University.
The BA-F8, SC-71 and BF-F3 antibodies developed by Stefano Schiaffino and the 6H1 antibody developed by Christine Lucas was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242.
Abbreviations
- GDNF
Glial cell line-derived neurotrophic factor
- NMJ
Neuromuscular Junction
- SOL
Soleus
- EDL
Extensor Digitorum Longus
- NF
Neurotrophic Factor
- ELISA
Enzyme-Linked Immunosorbant Assay
- CSA
Cross Sectional Area
- SEM
Standard Error of the Mean
- ACh
Acetylcholine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Airaksinen MS, Saarma M. THE GDNF FAMILY: SIGNALLING, BIOLOGICAL FUNCTIONS AND THERAPEUTIC VALUE. Nature Reviews. Neuroscience. 2002;3:383–94. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- Andonian M, Fahim M. Effects of endurance exercise on the morphology of mouse neuromuscular junctions during ageing. J Neurocytol. 1987;16:589–599. doi: 10.1007/BF01637652. [DOI] [PubMed] [Google Scholar]
- Aniansson A, Zetterberg C, Hedberg M, Henriksson KG. Impaired muscle function with aging. A background factor in the incidence of fractures of the proximal end of the femur. Clin Orthop Relat Res. 1984;(191):193–201. [PubMed] [Google Scholar]
- Ariano MA, Edgerton VR, Armstrong RB. Hindlimb muscle fiber populations of five mammals. J Histochem Cytochem. 1973;21:51–55. doi: 10.1177/21.1.51. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Phelps RO. Muscle fiber type composition of the rat hindlimb. Am J Anat. 1984;171:259–272. doi: 10.1002/aja.1001710303. [DOI] [PubMed] [Google Scholar]
- Baudet C, Pozas E, Adameyko I, Andersson E, Ericson J, Ernfors P. Retrograde signaling onto Ret during motor nerve terminal maturation. J Neurosci. 2008;28:963–975. doi: 10.1523/JNEUROSCI.4489-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR, Maresh CM, Crivello JF, Armstrong LE, Kraemer WJ, Covault J. The effects of exercise training of different intensities on neuromuscular junction morphology. J Neurocytol. 1993;22:603–615. doi: 10.1007/BF01181487. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Tenny KA, Wilson MH. Increased and decreased activity elicits specific morphological adaptations of the neuromuscular junction. Neuroscience. 2006;137:1277–1283. doi: 10.1016/j.neuroscience.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty TJ. Invited review: aging and sarcopenia. J Appl Physiol. 2003;95:1717–1726. doi: 10.1152/japplphysiol.00347.2003. [DOI] [PubMed] [Google Scholar]
- Dorlöchter M, Irintchev A, Brinkers M, Wernig A. Effects of enhanced activity on synaptic transmission in mouse extensor digitorum longus muscle. J Physiol (Lond) 1991;436:283–292. doi: 10.1113/jphysiol.1991.sp018550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edström E, Altun M, Bergman E, Johnson H, Kullberg S, Ramírez-León V, Ulfhake B. Factors contributing to neuromuscular impairment and sarcopenia during aging. Physiol Behav. 2007;92:129–135. doi: 10.1016/j.physbeh.2007.05.040. [DOI] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez Pinilla F, Ying Z, Roy R, Molteni R, Edgerton VR. Voluntary exercise induces a BDNF-mediated mechanism that promotes neuroplasticity. J Neurophysiol. 2002;88:2187–2195. doi: 10.1152/jn.00152.2002. [DOI] [PubMed] [Google Scholar]
- Grimby G, Gustafsson E, Peterson L, Renstram P. Quadriceps function and training after knee ligament surgery. Med Sci Sports Exerc. 1980;12:70–75. [PubMed] [Google Scholar]
- Gruner JA, Altman J. Swimming in the rat: Analysis of locomotor performance in comparison to stepping. Experimental Brain Research. 1980;40:374–382. doi: 10.1007/BF00236146. [DOI] [PubMed] [Google Scholar]
- Henderson CE, Phillips HS, Pollock RA, Davies AM, Lemeulle C, Armanini M, Simpson LC, Moffet B, Vandlen RA, Koliatsos VE, Rosenthal A. Gdnf - a Potent Survival Factor for Motoneurons Present in Peripheral-Nerve and Muscle. Science. 1994;266:1062–1064. doi: 10.1126/science.7973664. [DOI] [PubMed] [Google Scholar]
- Henneman E, Mendell L. Handbook of Physiology. The Nervous System. Motor Control. Vol. II. American Physiological Society; Bethesda, MD: 1981. Functional organization of the motoneuronal pool and its input; pp. 423–507. [Google Scholar]
- Hill RR, Robbins N, Fang ZP. Plasticity of presynaptic and postsynaptic elements of neuromuscular junctions repeatedly observed in living adult mice. J Neurocytol. 1991;20:165–182. doi: 10.1007/BF01186990. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Vogt M, Weibel ER, Fluck M. Response of skeletal muscle mitochondria to hypoxia. Exp Physiol. 2003;88:109–119. doi: 10.1113/eph8802513. [DOI] [PubMed] [Google Scholar]
- Keller Peck CR, Feng G, Sanes JR, Yan Q, Lichtman JW, Snider WD. Glial cell line-derived neurotrophic factor administration in postnatal life results in motor unit enlargement and continuous synaptic remodeling at the neuromuscular junction. J Neurosci. 2001;21:6136–6146. doi: 10.1523/JNEUROSCI.21-16-06136.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller-Peck CR, Parsadanian AS, Zhou L, Snider WD. Glial overexpression of GNDF protects facial motoneurons against axotomy-induced cell death. Society for Neuroscience Abstracts. 1997;23:620. [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive Modulation of AMPA Receptors Increases Neurotrophin Expression by Hippocampal and Cortical Neurons. The Journal of Neuroscience. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legerlotz K, Elliott B, Guillemin B, Smith HK. Voluntary resistance running wheel activity pattern and skeletal muscle growth in rats. Exp Physiol. 2008;93:754–762. doi: 10.1113/expphysiol.2007.041244. [DOI] [PubMed] [Google Scholar]
- Li H, Shen Z, Lu Y, Lin F, Wu Y, Jiang Z. Muscle NT-3 levels increased by exercise training contribute to the improvement in caudal nerve conduction velocity in diabetic rats. Molecular Medicine Reports. 2012;6:69–74. doi: 10.3892/mmr.2012.897. [DOI] [PubMed] [Google Scholar]
- Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260:1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- Lohof AM, Ip NY, Poo M. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- McCullough M, Gyorkos A, Spitsbergen J. Short-term exercise increases GDNF protein levels in the spinal cord of young and old rats. Neuroscience. 2013;240:258–268. doi: 10.1016/j.neuroscience.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCullough MJ, Peplinski NG, Kinnell KR, Spitsbergen JM. Glial cell line-derived neurotrophic factor protein content in rat skeletal muscle is altered by increased physical activity in vivo and in vitro. Neuroscience. 2011;174:234–244. doi: 10.1016/j.neuroscience.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohajeri MH, Figlewicz DA, Bohn MC. Intramuscular grafts of myoblasts genetically modified to secrete glial cell line-derived neurotrophic factor prevent motoneuron loss and disease progression in a mouse model of familial amyotrophic lateral sclerosis. Hum Gene Ther. 1999;10:1853–1866. doi: 10.1089/10430349950017536. [DOI] [PubMed] [Google Scholar]
- Nevitt M, Cummings S, Kidd S, Black D. Risk factors for recurrent nonsyncopal falls: A prospective study. JAMA. 1989;261:2663–2668. [PubMed] [Google Scholar]
- Nguyen QT, Parsadanian AS, Snider WD, Lichtman JW. Hyperinnervation of neuromuscular junctions caused by GDNF overexpression in muscle. Science. 1998;279:1725–1729. doi: 10.1126/science.279.5357.1725. [DOI] [PubMed] [Google Scholar]
- Nosrat CA, Tomac A, Lindquist E, Lindskog S, Humpei C, Stromberg I, Ebendal T, Hoffer BJ, Olson L. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res. 1996;286:191–207. doi: 10.1007/s004410050688. [DOI] [PubMed] [Google Scholar]
- Oppenheim RW, Houenou LJ, Johnson JE, Lin LF, Li L, Lo AC, Newsome AL, Prevette DM, Wang S. Developing motor neurons rescued from programmed and axotomy-induced cell death by GDNF. Nature. 1995;373:344–346. doi: 10.1038/373344a0. [DOI] [PubMed] [Google Scholar]
- Rich M, Lichtman JW. Motor nerve terminal loss from degenerating muscle fibers. Neuron. 1989;3:677–688. doi: 10.1016/0896-6273(89)90236-5. [DOI] [PubMed] [Google Scholar]
- Roubenoff R. Origins and clinical relevance of sarcopenia. Canadian journal of applied physiology. 2001;26:78–89. doi: 10.1139/h01-006. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hutchinson DL, Pierotti DJ, Hodgson JA, Edgerton VR. EMG patterns of rat ankle extensors and flexors during treadmill locomotion and swimming. J Appl Physiol. 1991;70:2522–2529. doi: 10.1152/jappl.1991.70.6.2522. [DOI] [PubMed] [Google Scholar]
- Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- Springer JE, Mu X, Bergmann LW, Trojanowski JQ. Expression of Gdnf Messenger-Rna in Rat and Human Nervous-Tissue. Exp Neurol. 1994;127:167–170. doi: 10.1006/exnr.1994.1091. [DOI] [PubMed] [Google Scholar]
- Springer JE, Seeburger JL, Jin HE, Gabrea A, Blankenhorn EP, Bergman LW. cDNA sequence and differential mRNA regulation of two forms of glial cell line-derived neurotrophic factor in Schwann cells and rat skeletal muscle. Exp Neurol. 1995;131:47–52. doi: 10.1016/0014-4886(95)90006-3. [DOI] [PubMed] [Google Scholar]
- Stephens JA, Taylor A. Fatigue of maintained voluntary muscle contraction in man. J Physiol (Lond) 1972;220:1–18. doi: 10.1113/jphysiol.1972.sp009691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trupp M, Ryden M, Jornvall H, Funakoshi H, Timmusk T, Arenas E, Ibanez CF. Peripheral Expression and Biological-Activities of Gdnf, a New Neurotrophic Factor for Avian and Mammalian Peripheral Neurons. J Cell Biol. 1995;130:137–148. doi: 10.1083/jcb.130.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vianney J, Spitsbergen JM. Cholinergic neurons regulate secretion of glial cell line-derived neurotrophic factor by skeletal muscle cells in culture. Brain Res. 2011;1390:1–9. doi: 10.1016/j.brainres.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waerhaug O, Dahl HA, Kardel K. Different effects of physical training on the morphology of motor nerve terminals in the rat extensor digitorum longus and soleus muscles. Anat Embryol. 1992;186:125–128. doi: 10.1007/BF00174949. [DOI] [PubMed] [Google Scholar]
- Wang C, Yang F, He X, Je H, Zhou J, Eckermann K, Kawamura D, Feng L, Shen L, Lu B. Regulation of neuromuscular synapse development by glial cell line-derived neurotrophic factor and neurturin. The Journal of biological chemistry. 2002;277:10614–10625. doi: 10.1074/jbc.M106116200. [DOI] [PubMed] [Google Scholar]
- Wang X, Poo M. Potentiation of Developing Synapses by Postsynaptic Release of Neurotrophin-4. Neuron. 1997;19:825–835. doi: 10.1016/s0896-6273(00)80964-2. [DOI] [PubMed] [Google Scholar]
- Wehrwein EA, Roskelley EM, Spitsbergen JM. GDNF is regulated in an activity-dependent manner in rat skeletal muscle. Muscle Nerve. 2002;26:206–211. doi: 10.1002/mus.10179. [DOI] [PubMed] [Google Scholar]
- Yan Q, Matheson C, Lopez OT. In vivo neurotrophic effects of GDNF on neonatal and adult facial motor neurons. Nature. 1995;373:341–344. doi: 10.1038/373341a0. [DOI] [PubMed] [Google Scholar]
- Zafra F, Castrén E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proceedings of the National Academy of Sciences. 1991;88:10037–10041. doi: 10.1073/pnas.88.22.10037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwick M, Teng L, Mu X, Springer JE, Davis BM. Overexpression of GDNF induces and maintains hyperinnervation of muscle fibers and multiple end-plate formation. Exp Neurol. 2001;171:342–350. doi: 10.1006/exnr.2001.7753. [DOI] [PubMed] [Google Scholar]