Abstract
The hypothalamic neuropeptide oxytocin has drawn the attention of scientists for more than a century. The understanding of the function of oxytocin has expanded dramatically over the years from a simple peptide adept at inducing uterine contractions and milk ejection to a complex neuromodulator with a capacity to shape human social behavior. Decades of research have outlined oxytocin’s ability to enhance intricate social activities ranging from pair bonding, sexual activity, affiliative preferences, and parental behaviors. The precise neural mechanisms underlying oxytocin’s influence on such behaviors have just begun to be understood. Research suggests that oxytocin interacts closely with the neural pathways responsible for processing motivationally relevant stimuli. In particular, oxytocin appears to impact dopaminergic activity within the mesocorticolimbic dopamine system, which is crucial not only for reward and motivated behavior but also for the expression of affiliative behaviors. Though most of the work performed in this area has been done using animal models, several neuroimaging studies suggest similar relationships may be observed in humans. In order to introduce this topic further, this paper will review the recent evidence that oxytocin may exert some of its social-behavioral effects through its impact on motivational networks.
Keywords: oxytocin, motivation, dopamine, salience, valence
1. Introduction
This year marks the 60th anniversary since oxytocin was first synthesized and its structure established in the Nobel Prize winning work of Vincent du Vigneaud (du Vigneaud et al., 1953, 1954). Since that landmark work, scientists have grown to appreciate oxytocin as both a hormone contributing significantly to reproductive processes and as a complex neuromodulator capable of affecting a wide range of behaviors. While comprised of only nine amino acids, oxytocin is a highly influential neuropeptide known to significantly impact a variety of social and reproductive behaviors including pair bonding, as well as maternal, affiliative and sexual behaviors (see Burkett and Young, 2012, Campbell, 2010, Donaldson and Young, 2008, Lee et al., 2009, Meyer-Lindenberg et al., 2011).
While historically considered a prosocial hormone, research suggests oxytocin promotes both positive (i.e. maternal behaviors, pair-bonding, altruism, trust) and negative social interactions (i.e. aggression, territoriality) depending on the context. Experimental manipulations of oxytocin indicate this neuropeptide enhances the salience of social cues as evidenced by greater attention devoted toward inspecting social cues, heightened cognitive processing of social information, and increased effort devoted to engaging social entities. Oxytocin may also be involved in shifting attributions of motivational value or valence towards social stimuli, resulting in alternative appetitive and aversive value assignments to certain social objects. For instance, oxytocin appears to be responsible for the aversive to appetitive shift in the appraisal of newborn pups in female rats. How precisely oxytocinergic activity shapes such processes is still being explored; however, multiple intersecting lines of research have revealed oxytocinergic modulation of activity within motivational networks, particularly within dopaminergic mesocorticolimbic circuits, may be involved.
This review will discuss how oxytocin may facilitate social behavior by increasing the salience of social stimuli and by shifting valence assessments of social objects through its effects on motivational networks. First, major pathways responsible for processing motivationally relevant stimuli will be reviewed, placing special emphasis on the influence of oxytocin and dopamine within pathways. This will be followed by a description of how particular components of social motivation are impacted by oxytocinergic and dopaminergic activity. Finally, a brief comment will be given on oxytocin’s potential role as a mediator of salience and valence attribution for objects imbued with social value.
2. Anatomy of Motivation
Social interactions can be a source of joy or dread depending on the context. Key decisions must be made when encountering acquaintances and strangers; first, to determine whether they are friend or foe, then resolve whether any action is necessary and finally, if action is called for, settle on which action is appropriate (i.e. pursue, avoid, or attack). Such assessments are vital to survival and involve the engagement of motivational processes, which form an internal drive that pushes an organism to identify and engage motivationally relevant stimuli.
Motivation can be succinctly defined as “the set of processes through which organisms regulate the probability, proximity and availability of stimuli” (Salamone and Correa, 2012). Motivated behavior arises as a consequence of the collective activity of a network of interconnected regions that act to signal the significance of stimuli, assign motivational value and select appropriate action (Kalivas and Volkow, 2005, Figure 1). The ventral tegmental area (VTA) and its widespread projections to cortical and limbic regions form the foundation of the motivational circuitry. Dopaminergic neurons within VTA project to the nucleus accumbens, hippocampus, amygdala, ventral pallidum and various cortical areas including the prefrontal cortex to form the mesocorticolimbic pathway (Figure 1). The regions that make up this pathway share strong reciprocal connections and each respond to motivationally relevant stimuli. As there are large number of articles and reviews written regarding the roles each of these areas play in motivational behavior, this section of the review will serve to briefly summarize the overall anatomical organization of the major structures that make up the motivational system and highlight the key functions each region is generally believed to contribute to motivated behavior.
Figure 1. Simplified Schematic of Neuroanatomical Regions Contributing to Motivational Processes.
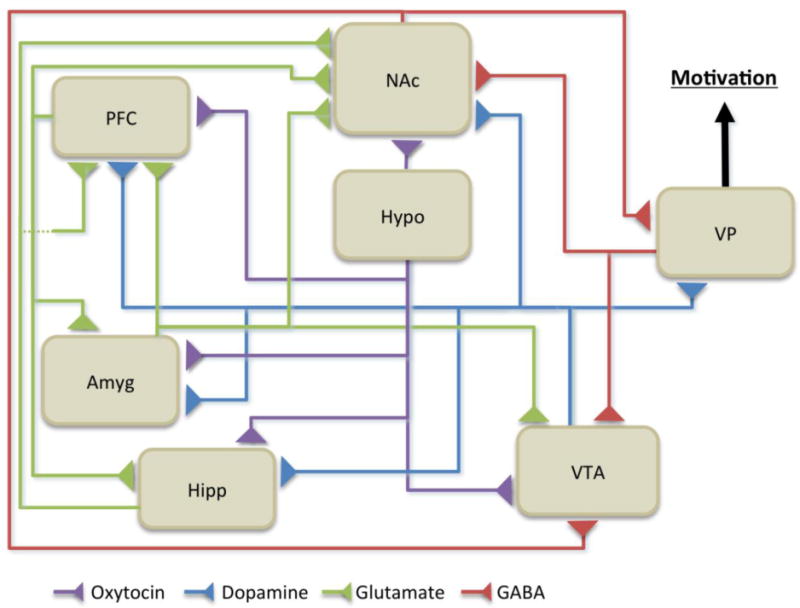
Adapted from Kalivas and Nakamura, 1999, Blum et al., 2008 and Skuse et al., 2009. This simplified diagram outlines the major connections between regions that are believed to participate in the processing of social and motivationally relevant stimuli. Dopaminergic projections (blue) arising from the ventral tegmental area form the basis of the mesocorticolimbic pathway, which plays important roles in the development of motivated behaviors. The ventral pallidum is hypothesized to act as a major output region in this pathway ultimately connecting with motor systems to initiate motivated action. Dopaminergic activity within the mesocorticolimbic pathway can be modulated by oxytocin (purple) and the interaction between these two systems can influence a variety of social behaviors. Abbreviations: Amyg, amygdala; Hipp, hippocampus; Hypo, hypothalamus; NAc, nucleus accumbens; PFC, prefrontal cortex; VP, ventral palldium; VTA; ventral tegmental area.
Prefrontal Cortex
The most anterior portion of the frontal cortex, this area consists of multiple subregions that contribute to a wide variety of activities spanning from cognition to motivation to decision making to emotion. The prefrontal cortex sends glutamatergic projections to many regions within the mesocorticolimbic network including the nucleus accumbens, amygdala, hippocampus, and ventral pallidum and through these connections may provide top-down processing control to guide motivated behavior (Figure 1). As discussed in detail in an excellent review by Rushworth and colleagues, multiple subregions within the prefrontal cortex appear to directly contribute to the processing of rewards (Rushworth et al., 2011). The medial portions of the prefrontal cortex, including the ventromedial and medial orbitofrontal cortices, seem to carry representations of reward value. Similarly, the anterior cingulate cortex appears to encode the associations between a given action and a reward, for instance linking the amount of exerted effort necessary to receive a particular reward. On the other hand, the more lateral portions of the prefrontal cortex are hypothesized to be involved in the assignment of value to alternative rewards and comparing these values among different reward options (Rushworth et al., 2011).
Amygdala
This small structure in the medial temporal lobe has proposed roles in emotion, learning, memory as well as motivation (see reviews Baxter and Murray, 2002 and Murray and Wise, 2010). The amygdala receives and responds to sensory information from auditory, gustatory, visual, and olfactory domains (Zald and Pardo, 2002). Stimulation of the amygdala elicits recruitment of attention processes that boost processing of incoming sensory information and enhance vigilance (Davis and Whalen, 2001). Indeed, given the its connections with sensory domains as well as its glutamatergic projections to the nucleus accumbens and prefrontal cortex, the amygdala is well positioned to detect salient incoming stimuli and heighten arousal to aid in response. Early work revealed the amygdala responded primarily to cues related to fear, threat and danger and played a primary role in fear conditioning (e.g. Adolphs et al., 1995, LeDoux, 2000, Prather et al., 2001); however the amygdala appears to be reactive to positive salient events as well (Belova et al., 2008, Bermudez and Schultz, 2010a, b, Garavan et al., 2001, Hamann et al., 1999, 2002, Salinas et al., 1993, Santos et al., 2011). For instance, the amygdala is recruited during stimulus-reward learning (e.g. Baxter et al., 2000, Bermudez and Schultz, 2010a, Everitt et al., 2003, Everitt et al., 1991). Neuronal recordings in non-human primates indicate amygdalar neurons respond to cues predicting future reward and encode reward value and magnitude (Belova et al., 2008, Bermudez and Schultz, 2010a, b, Salinas et al., 1993).
Hippocampus
This structure located within the medial temporal lobe is a key neuroanatomical substrate in learning and memory. This region shares reciprocal connections with the prefrontal cortex, which plays important roles in cognition and memory (Barbas and Blatt, 1995, Laroche et al., 2000). Hippocampal damage results in memory impairments in declarative memory or, rather, memory for facts and events (Eichenbaum, 2004). The hippocampus is important for processing contextual information in memory such as where the reward was previously located (Eichenbaum, 2004, Luo et al., 2011) and is hypothesized to transmit this contextual information through its connections with the VTA and the nucleus accumbens to link context with reward (Ito et al., 2008, Luo et al., 2011). The hippocampus is also involved in novelty detection and hippocampal activation is necessary for salient novel stimuli to stimulate VTA activity (Floresco and Grace, 2003, Legault and Wise, 2001, Lisman and Grace, 2005, Lodge and Grace, 2006). Dopaminergic activity within the VTA is influenced by glutamatergic afferents originating from the hippocampus and projecting to medium spiny neurons in the nucleus accumbens (Floresco et al., 2001, Groenewegen et al., 1987).
Ventral Pallidum
Located within the basal ganglia, the ventral pallidum receives projections from a variety of neural structures including the amygdala, hippocampus and nucleus accumbens and sends projections to thalamic relays or to subcortical outputs (Zhang et al., 2012). The ventral pallidum also sends inhibitory γ –aminobutyric acid (GABA) projections to the VTA and nucleus accumbens. Reductions in GABAergic activity from the ventral pallidum results in increases in activity in dopamine neurons contained within the VTA that consequently increases extracellular dopamine within the nucleus accumbens (Floresco et al., 2003). Originally conceptualized as a region primarily handling the translation of limbic activity into motor behavior, current views suggest neurons within the ventral pallidum code for incentive salience as well as hedonic value (Smith et al., 2011).
Nucleus Accumbens
The nucleus accumbens, situated within the striatum, receives glutamatergic projections from the amygdala, hippocampus and prefrontal cortex and in turn sends GABAergic projections to the VTA, substantia nigra and ventral pallidum (Haber and Knutson, 2009, Haber et al., 1990). This region is proposed to act in an integrative capacity assimilating goal directed information from the prefrontal cortex, environmental context from the hippocampus, and emotional significance from the amygdala to ultimately influence motor planning and action execution through its connections with the ventral pallidum and midbrain (Kalivas and Nakamura, 1999, Luo et al., 2011, Mogenson and Yang, 1991). Through these extensive reciprocal connections, the nucleus accumbens is in an ideal position to influence “the translation of will into action” (Goto and Grace, 2005, Kalivas and Nakamura, 1999, Mogenson and Yang, 1991). As will be discussed below, dopaminergic activity within the nucleus accumbens has been identified as a key neurochemical substrate in the attribution of salience.
Summary
Activity within and the interactions between the various brain regions that make up the mesocorticolimbic pathway contribute to the formation of motivated behavior. Innervated by ascending projections originating from the VTA, each region within the mesocorticolimbic pathway is hypothesized to play essential roles in the processing of motivationally relevant stimuli including salient social stimuli. As will be discussed, two neurochemicals acting in this pathway, dopamine and oxytocin, serve to shape social motivation and influence the expression of affiliative behaviors.
3. Role of Dopamine in Motivation
Dopamine was first implicated in “reward” processes in the 1950s in a series of experiments that explored electrical stimulation of the brain in rats. Olds and Milner noted that a rat that had been implanted with an electrode, within what was later identified to be the nucleus accumbens, preferentially spent time in the area where it had received electrical stimulation through the electrode the day prior (Olds and Milner, 1954). Further, when rats were trained to press a lever to electrically stimulate the electrodes themselves, they would press the lever repeatedly at astonishing rates reaching thousands of times per hour (Milner, 1989, Olds and Milner, 1954). Similar observations were then made in humans a short time later (Bishop et al., 1963). Shortly after Olds and Milner’s observations, the presence of dopamine containing neurons within the VTA which projected to the nucleus accumbens would be established (Falck and Hillarp, 1959) and subsequent research would demonstrate that self-electrical stimulation could be dramatically reduced by applying dopaminergic antagonists or lesions to this area (Fibiger et al., 1987, Rolls et al., 1974). This work indicated a primary role for mesocorticolimbic dopamine in reward.
Dopamine acts on a family of related G protein-coupled receptors usually divided into two major classes: D1-like, consisting of D1 and D5 receptor subtypes, and D2-like, consisting of D2, D3 and D4 receptor subtypes (for a thorough review of dopaminergic signaling see Beaulieu and Gainetdinov, 2011). D1 receptors are enriched along the striatonigral ‘direct’ pathway whereas D2 receptors are observed along the striatopallidal ‘indirect’ pathway (Gerfen et al., 1990). D1 receptors are differentiated from D2 receptors based on their ability to either stimulate (D1) or inhibit (D2) adenylyl cyclase activity. Activation of adenylyl cyclase results in cyclic adenosine monophosphate (cAMP) production, which can have significant effects on intracellular signaling. As such, activation of either D1 or D2 receptors can have opposing effects on neuronal activity. For instance, while D1 and D2 receptor subtypes are both involved in reward learning, they appear to have slightly different roles. D1 receptor activation in the direct pathway appears to be crucial in the acquisition phases of reward learning whereas D2 receptor deactivation in the indirect pathway seems to be necessary to switch to a new reward-seeking strategy (Yawata et al., 2012).
Dopamine was originally posited to code for the pleasure derived from reward based on the results from electrical stimulation experiments and observations that when animals encountered either natural rewards (e.g. food, sex) or artificial rewards (e.g. drugs) dopamine release was stimulated and the firing rate of dopamine neurons in the mesocorticolimbic pathway increased (e.g. Corrigall et al., 1992, Di Chiara and Imperato, 1988, Matsumoto and Hikosaka, 2009, Wise and Rompre, 1989). However, landmark work done by Wolfram Schultz and colleagues demonstrated that while dopamine neurons will indeed fire in response to unexpected rewards, they only show weak responses to fully anticipated rewards (Hollerman and Schultz, 1998, Schultz, 1998, Schultz et al., 1998). Furthermore, large reductions or exaggerations in dopamine levels induced within the mesocorticolimbic system do not seem to alter hedonic reactions to natural rewards (Berridge et al., 1989, Kaczmarek and Kiefer, 2000, Pecina et al., 1997, Wyvell and Berridge, 2000). Instead, dopamine antagonists applied to the nucleus accumbens result in a reduction in the effort animals are willing to exert to obtain such rewards while leaving hedonic reactions to those rewards intact; or rather, a reduction in “wanting” as opposed to “liking” (Berridge and Robinson, 1998, Robinson et al., 2005). Hence, dopaminergic manipulations appear to influence assignment of motivational value or salience, impacting the drive toward such rewards, rather than the pleasure derived from the reward itself (Berridge and Robinson, 1998, Bromberg-Martin et al., 2010, Salamone, 2007). A further complication of the hedonic hypothesis of dopamine is that mesocorticolimbic dopaminergic fluctuations do not selectively interfere with behavioral responses towards appetitive stimuli but also affect aversive motivational processes as well. Dopamine neurons become engaged in response to negative, aversive and stressful stimuli (Abercrombie et al., 1989, D’Angio et al., 1987, Horvitz, 2000, Pezze and Feldon, 2004, Scott et al., 2006) and dopamine depletions and antagonists applied within the mesocorticolimbic system impair active avoidance responses and block the development of place aversion (Salamone, 1994).
So, what does dopamine code for precisely? No consensus has emerged as of yet, however, current evidence suggests dopamine signaling carries multiple fragments of information. As recently reviewed by Bromberg-Martin and colleagues, it appears that while dopaminergic neurons generally encode an alerting signal for unexpected cues, distinct populations of dopamine neurons encode: 1) motivational value, whereby certain dopamine neurons are excited by appetitive stimuli, which promote approach, and inhibited by aversive stimuli, which promote withdrawal and 2) motivational salience, as other dopamine neurons show weak responses to neutral events but are excited by salient events (i.e. regardless of whether the event was appetitive or aversive in nature (Bromberg-Martin et al., 2010). As such, modulation of dopamine neurotransmission in these circuits could lead to changes in either of these processes. As several lines of evidence suggest oxytocin modulates activity in dopamine circuits, experimental data linking mesocorticolimbic dopamine activity and oxytocinergic functioning will be reviewed next.
4. Oxytocin interactions with motivational circuitry
4.1 Neurobiology of Oxytocin
Oxytocin is a circular nonapeptide synthesized primarily within paraventricular nucleus (PVN) and the supraoptic nucleus (SON) of the hypothalamus. The posterior lobe of the pituitary contains axonal projections originating from the hypothalamus, which secrete oxytocin for release into circulation. Peripherally, oxytocin acts on reproductive physiologic processes to induce uterine contraction and stimulate milk ejection (Dale, 1906). Centrally, oxytocin neurons originating in the PVN also send projections to extrahypothalamic regions including the amygdala, hippocampus, nucleus accumbens and VTA (Figure 1) (Ross et al. 2009, Brinton et al., 1984, Caffé et al., 1989, Fliers et al., 1986, Krémarik et al., 1993, Loup et al., 1991, Van Leeuwen et al., 1985).
Oxytocin is released in response to psychological and physical stressors as well as in reaction to a variety of social stimuli (e.g. Jezova et al., 1995, Lang et al., 1983, Onaka, 2004, Robinson et al., 2002, Wigger and Neumann, 2002). In addition to stimulation through direct projections, oxytocin can also be released somatodendritically to regulate its own activity (Moos et al., 1984) and may travel through diffusion to activate extrahypothalamic areas containing oxytocin receptors (see review Stoop, 2012). Oxytocin binds primarily to its only oxytocin receptor (OXTR) although shows weak affinity towards vasopressin receptors. The OXTR belongs to the G-protein coupled seven transmembrane receptor (GPCR) superfamily and is centrally expressed in regions throughout the brain and spinal cord including within the hypothalamus, nucleus accumbens, amygdala and VTA (see review Stoop, 2012). OXTR are found in key areas within the mesocorticolimbic system and stimulation of OXTR within these regions, particularly within the VTA and nucleus accumbens, has been noted to influence motivated behavior, as will later be discussed. The distribution of oxytocin receptors in the brain is highly species dependent and has been shown to correspond to expression of social behaviors such as pair bonding (Insel and Young, 2001).
4.2 Oxytocin and Dopamine
Recent studies show activation of oxytocin neurons thought to target the VTA stimulates mesocorticolimbic dopaminergic neurons (Melis et al., 2007, Melis et al., 2009, Succu et al., 2011, Succu et al., 2008). Oxytocin receptors and corresponding messenger RNA have been localized within the VTA (Freund-Mercier et al., 1987, Sofroniew, 1983, Vaccari et al., 1998). Extensive work done by Melis, Argiolas and colleagues indicates that stimulation of dopamine receptors located on the cell bodies of oxytocinergic neurons within the PVN lead to penile erection in rats (e.g. Argiolas and Melis, 2004, Melis and Argiolas, 2003). Oxytocin injected into the VTA induces penile erections in rats and stimulates extracellular dopamine increases within the nucleus accumbens, which receives dopaminergic projections from the VTA (Melis et al., 2007, Succu et al., 2008). Application of an oxytocin receptor antagonist via intracerebroventricular (ICV) injection significantly diminishes dopamine agonist stimulated dopaminergic release in the nucleus accumbens and the pro-erectile effect of dopamine (Succu et al., 2007).
Oxytocin stimulation of the amygdala and hippocampus can also lead to penile erection and increases in extracellular dopamine within the nucleus accumbens in rats (Melis et al., 2009). This is suggested to occur due to activation of glutamatergic projections from the amygdala and hippocampus that act to stimulate mesolimbic dopamine neurons within the VTA (Melis et al., 2009, Succu et al., 2011). More recent work has also demonstrated that oxytocin can also induce yawning when injected into the amygdala, hippocampus and VTA and this behavior occurs concurrently with increases in extracellular dopamine within the nucleus accumbens as well as the medial prefrontal cortex, which is blocked by ICV injections of oxytocin receptor antagonists (see review (Sanna et al., 2012). While oxytocin regulates dopamine release in extrahypothalamic regions, oxytocinergic neurons are susceptible to modulation by dopamine themselves as they also express dopamine receptors (Baskerville et al., 2009). Indeed, dopamine release in the nucleus accumbens has been demonstrated to produce stimulation of dopamine neurons within the PVN that can ultimately activate oxytocinergic neurons and induce penile erection (Melis et al., 2007, Melis et al., 2009, Succu et al., 2011, Succu et al., 2007).
5. Oxytocin, Dopamine and Social Salience
The location of oxytocin’s receptors throughout the mesocorticolimbic dopamine system places oxytocin in an ideal position to influence a wide range of motivated behaviors. Several groups of researchers have posited that oxytocin’s ability to influence behavioral responses to social stimuli is at least partially mediated by its capacity to increase the salience of social cues (Averbeck, 2010, Bartz et al., 2011, Burkett and Young, 2012, Gordon et al., 2011, Shamay-Tsoory et al., 2009). This fits well with the notion that dopamine plays primary roles in salience attribution and oxytocin is capable of affecting this activity.
5.1 Maternal Behavior
To put it awfully inadequately, infants are highly salient to their mothers. Over 80 years of rodent research has indicated that pups have high inherent value to parturient females. As this has been reviewed extensively elsewhere (e.g. Lonstein and Morrell, 2007, Trezza et al., 2011, Numan and Stolzenberg, 2009), this manuscript will highlight some of the observations that indicate dams are highly driven to maintain contact with pups and how this behavior can be modified through challenges to dopaminergic and oxytocinergic systems.
Lactating and recently parturient rodents will exert intense effort to gain access to pups while virgin female rats will tend to avoid or show aggression towards pups when first exposed to them (Fleming and Anderson, 1987, Fleming and Rosenblatt, 1974, Jakubowski and Terkel, 1985a, b, but note differences in laboratory mouse strains, Lonstein and De Vries, 2000, Noirot, 1972). Rats that have recently given birth and have been trained to press a bar in order to retrieve pups will press the bar continuously, hundreds of times over multiple hours in order to receive both their own pups as well as foster pups (see review Trezza et al., 2011). Dams also prefer to spend time in areas previously associated with pups (i.e. conditioned place preference) and exhibit preferences for cues associated with pups over those associated with drugs of abuse such as cocaine (Ferris et al., 2005, Mattson et al., 2001, Mattson et al., 2003). The neurobiological systems underlying such maternal drive are still being explored, however, a growing body of evidence suggests both dopaminergic and oxytocinergic systems play key roles in maternal behavior.
Maternal motivation, or the responsiveness of a mother towards her young, is influenced by activity within the mesocorticolimbic system (see Numan and Stolzenberg, 2009 for a comprehensive review). Lactating dams exhibit enhanced activity within the nucleus accumbens, striatum, prefrontal cortex, thalamus and VTA in response to suckling by pups (Febo et al., 2005, Ferris et al., 2005). Dopamine is released within the nucleus accumbens when a mother rat grooms or licks her offspring or after being reunited with her offspring after forced separation (Champagne et al., 2004, Hansen et al., 1993). Maternal rats exhibiting high levels of pup licking and grooming behavior tend to exhibit larger increases in dopaminergic activity in the nucleus accumbens as a result of grooming their pups relative to their low licking and grooming counterparts (Shahrokh et al., 2010, Champagne et al., 2004). Such increases in extracellular dopamine activity observed in these mothers are positively associated with the time spent tending to their pups (Champagne et al., 2004). Artificially increasing extracellular dopamine levels within the nucleus accumbens through use of a dopamine reuptake inhibitor can enhance mother and pup interactions (Champagne et al., 2004) whereas applying dopaminergic antagonists or dopaminergic neurotoxins to the region interrupts such interactions (Hansen et al., 1993, Hansen et al., 1991a, Keer and Stern, 1999, Numan et al., 2005). Modulation of VTA activity can also influence maternal behaviors. For instance, pharmacological inactivation of the VTA abolishes the conditioned place preference for pups in postpartum females (Seip and Morrell, 2009). Inactivating the VTA by bupivacaine or by selectively destroying the dopamine neurons within the VTA can also reduce maternal behaviors such as pup retrieval and grooming (Seip and Morrell, 2009, Hansen et al., 1991b).
Oxytocinergic activity within the motivational system also serves as an important neurobiological substrate underlying maternal behavior. Mother rats displaying high levels of pup grooming behavior show an increased number oxytocinergic projections from the PVN to the VTA relative to rats exhibiting low levels of pup grooming behavior (Shahrokh et al., 2010). Similarly, oxytocin receptor levels within areas such as the medial preoptic area, nucleus accumbens, VTA and amygdala differ between rats displaying varying levels of maternal behavior, with increased maternal behavior being associated with higher oxytocin receptor concentrations (Francis et al., 2000, Shahrokh et al., 2010). Oxytocin has been theorized to play crucial roles both in the initiation of maternal behaviors and the modification of such behaviors once established (see Numan and Stolzenberg, 2009). Maternal behavior can be elicited through administration of oxytocin in virgin rats (Pedersen et al., 1982); whereas oxytocin antagonists applied within the VTA impair both pup retrieval and time spent in nursing postures over pups in parturient dams (Pedersen et al., 1994). Further, oxytocin receptor knockout postpartum mice show higher latencies to retrieve pups placed at the corners of the cage and spend less time in nursing postures (Takayanagi et al., 2005, but also see Nishimori et al., 1996). Interestingly, oxytocin also appears to influence an infant’s behavior towards their mother. As mothers actively seek out their offspring, so do offspring seek out their mothers. Infant rats not only seek out their mothers but also show preferences with areas associated with maternal odors; preferences can be blocked through central administration of oxytocin antagonists (Nelson and Panksepp, 1996). Furthermore, oxytocin knockout pups also fail to show a preference for their mother over a novel lactating female and emit fewer ultrasonic vocalizations when separated from their mother (Winslow et al., 2000, Ross and Young, 2009).
It is of note that the actions of oxytocin and dopamine on maternal behavior do not appear to be entirely separate. For example, utilizing in vivo voltammetry, Shahrokh and colleagues demonstrated that oxytocin infusions into the VTA could enhance dopamine levels in the nucleus accumbens (Shahrokh et al., 2010). Furthermore, they observed that increases in dopaminergic concentrations in the nucleus accumbens induced by pup grooming among the maternal rats that demonstrate the highest levels of this behavior could be inhibited through administration of an oxytocin receptor antagonist into the VTA (Shahrokh et al., 2010).
Although less well studied in humans, bonding between mothers and children has also been associated with activity within mesocorticolimbic circuits. Functional magnetic resonance imaging (fMRI) paradigms have shown recruitment of the amygdala, ventral striatum, VTA/substantia nigra as well as areas within the frontal cortex and insula in response to mothers seeing photographs of their own children versus other peoples children (Bartels and Zeki, 2004, Leibenluft et al., 2004, Strathearn et al., 2009, Strathearn et al., 2008, but see Wittfoth-Schardt et al., 2012). Strathearn and colleagues demonstrated that new mothers classified as having a secure attachment style exhibited heightened BOLD activation within the orbitofrontal cortex, medial prefrontal cortex, ventral striatum and hypothalamus while viewing pictures of their own infants relative to mothers displaying insecure/dismissing attachment styles (Strathearn et al., 2009). Neural activity within hypothalamus was engaged in response to mothers viewing their own child’s face versus an unknown child’s face and this activity was significantly correlated with plasma oxytocin responses induced by physical interaction with their baby (Strathearn et al., 2009). Furthermore, oxytocin levels have been shown to predict the presentation of affectionate parental behaviors in humans (see Gordon et al., 2011). Plasma levels of oxytocin are positively related to the attention and affection mothers and fathers show their infant children (see Gordon et al., 2011). Other lines of research indicate mothers hearing crying sounds from an infant exhibit enhanced BOLD activation within the temporal lobe and amygdala (Riem et al., 2011). This amygdala activation is significantly attenuated following treatment by intranasal oxytocin and is suggested to reflect the stress-reducing effects of oxytocin that may help promote responsiveness to crying infants (Riem et al., 2011). Indeed, oxytocin, which is released during breast-feeding, has been demonstrated to lower reactivity of the hypothalamic pituitary adrenal (HPA) axis reducing levels of stress hormones including adrenocorticotropic hormone (ACTH) and cortisol, which may promote maternal care (see reviews by Heinrichs et al., 2009, Neumann and Landgraf, 2012, Uvnas-Moberg, 1997).
In sum, these data indicate infants are highly salient and mothers will devote a great deal of time and energy obtaining access to their offspring and show preferences for stimuli associated with them. These activities are associated with activity within multiple regions in the mesocorticolimbic pathway known to be critical in the processing of rewarding and motivationally salient stimuli. Interfering with oxytocinergic activity in these regions significantly modifies these maternal behaviors. In addition to promoting increased salience attribution towards offspring, oxytocin may also encourage a shift in valence assignment. For instance, for wild virgin rodents, pups can be treated as aversive stimuli, prompting attack. However, following parturition or treatment with oxytocin, pups are no longer regarded with disdain but rather as appetitive stimuli worth expending effort to obtain and care for.
5.2 Pair Bonding
Humans, unlike most mammals, are capable of forming long-lasting monogamous pair bonds. One other mammalian species that has been shown to form pair bonds are prairie voles (Insel and Young, 2001). These rodents have served as the primary animal model for studying the formation of pair bonds (Insel and Young, 2001). Unlike their montane counterparts, this species’ male-female pairs will share a nest, defend their shared territory and both participate in parental care (Young et al., 1998). Males will display partner preferences for specific females following prolonged mating and exhibit aggression towards female strangers after extended cohabitation (Aragona et al., 2006). This review will highlight some of the major findings that relate to the involvement of dopamine and oxytocin in pair-bonding, but interested readers are encourage to consult one of the many excellent reviews available on this topic (e.g. Young et al., 2011).
Pair bond development is influenced by dopaminergic activity within the nucleus accumbens (Liu and Wang, 2003, Young et al., 1998). Dopamine neurotransmission is increased within the nucleus accumbens of prairie voles following mating and these increases are necessary for the formation of partner preference formation in males (Aragona et al., 2003). Pharmacologically blocking dopamine receptors by haloperidol prior to mating results in an elimination in partner preference by males (Aragona et al., 2003). In situations where partner preferences would not necessarily be established, such as following short term cohabitation without mating, D2-receptor agonists applied to the nucleus accumbens shell can induce partner preferences (Aragona et al., 2006). Activation of D1-like receptors on the other hand can block the formation of such pair bonds (Aragona et al., 2006). The differential impact of D1 and D2 activation on partner preference formation appears to be due to the opposing intracellular changes induced by D1 and D2 receptor activation noted earlier (Aragona and Wang, 2007). Interestingly, D1-like receptor levels within the nucleus accumbens undergo changes following pair bonding in prairie vole males. Specifically, it appears that following the formation of a pair bond, D1-like receptor expression is increased in the nucleus accumbens in male prairie voles. Pair bonded males will then exhibit affiliative behavior towards their mates while expressing aggressive behavior towards strangers that can be blocked through antagonism of D1-like receptors within the nucleus accumbens (Aragona and Wang, 2009).
In addition to the nucleus accumbens, dopaminergic activity within other regions within the mesocorticolimbic network including the prefrontal cortex appear to be involved in the development of partner preferences. For instance, monogamous prairie voles exhibit lower concentrations of D1-like receptors and higher concentrations of D2-like receptors within the medial prefrontal cortex compared to their more promiscuous counterparts (Smeltzer et al., 2006). Dopaminergic activity within the VTA may also contribute to pair bond formation as application of either GABAergic or glutamatergic antagonists within the VTA, both which presumably enhance dopamine release within the nucleus accumbens, can induce partner preferences in male prairie voles (Curtis and Wang, 2005). Altogether these data indicate dopaminergic activity plays an influential role in the formation of pair bonds.
Multiple lines of evidence indicate oxytocin is also key neurobiological substrate underlying the development of partner preferences (see review Young and Wang, 2004). The distribution of oxytocin receptors varies substantially between monogamous prairie voles and polygamous montane voles (Insel and Shapiro, 1992). Monogamous prairie voles show substantially higher oxytocin receptor concentrations within multiple areas including within the nucleus accumbens, amygdala, bed nucleus of the stria terminalis, and prefrontal cortex compared to the nonmonogamous montane voles (Insel and Shapiro, 1992). Indeed, the concentration of oxytocin receptors within the nucleus accumbens in female prairie voles is positively related to the speed at which they form partner preferences (Ross et al., 2009). Altering oxytocin receptor expression in the nucleus accumbens using viral vector-mediated gene transfer can also affect partner preference formation in female prairie voles (Ross et al., 2009). Partner preferences can also be shaped via pharmacological manipulations of oxytocin. For instance, ICV administration of oxytocin or injection of oxytocin directly into the nucleus accumbens can induce partner preferences in absence of mating (Cho et al., 1999). Correspondingly, application of oxytocin receptor antagonists into either the nucleus accumbens or prefrontal cortex can block mating-induced partner preferences in female prairie voles (Young et al., 2001).
As was noted during the previous section, oxytocin and dopamine do not act alone but rather appear to interact with one another to regulate the formation of pair bonds. For instance, it appears that both D2 receptors and oxytocin receptors must be stimulated in order to facilitate the formation of partner preferences (Liu and Wang, 2003). Injection of a D2-receptor antagonist into the nucleus accumbens of female prairie voles can prevent partner preference formation induced by oxytocin and oxytocin antagonists can block partner preference formation induced by D2-receptor agonist administration (Liu and Wang, 2003). In sum, these data suggest that both oxytocin and dopamine work together to influence partner preference formation in animals.
Although little work has been done in this area in humans, some neuroimaging data suggests motivational networks are engaged in response to romantic love. Aron and colleagues performed fMRI scanning on participants who described themselves as being intensely in love while they viewed pictures of their romantic partner or a familiar person (Aron et al., 2005). This study observed heightened activation within the VTA and caudate in response to viewing a romantic partner over viewing a familiar person (Aron et al., 2005). Similar studies have shown comparable networks are recruited in response to romantic love as they are recruited in response to maternal love including the prefrontal cortex and striatum (Bartels and Zeki, 2004). In regards to the influence of oxytocin on such attachments, Schneiderman and colleagues observed heightened plasma levels of oxytocin in new couples (Schneiderman et al., 2012). Higher plasma oxytocin levels were associated with higher levels of interaction between the couples and predicted which couples would remain together 6 months later (Schneiderman et al., 2012). One recent study explored whether oxytocin could induce partner preferences in humans (Liu et al., 2012). Female and male participants received oxytocin or placebo and were show video clips of a man and a woman. A day later the participant was shown pictures of an additional man and woman and was asked to decide which of the individuals they would like to learn more about and spend time with. Participants displayed an increased preference for people they had been introduced to immediately following administration of oxytocin, however, the choices made were not directed toward persons of the opposite gender. Since this study did not look exclusively at individuals who were single and was not framed in a strictly romantic context, more work is needed to determine the role of oxytocin in human partner preference formation. In short, these data and data obtained from animal work indicate systems normally engaged for the processing of motivationally relevant appetitive stimuli are also involved in the formation of pair bonds and can be regulated by oxytocin.
5.3 Aggression
5.3.1 Maternal Defensive Aggression
Intruders and other conspecifics are potentially dangerous threats to mothers and their pups and as such are highly salient social entities that require immediate attention in order to ensure safety. Mothers will aggressively attack intruders approaching their offspring (Rosenblatt et al., 1994). Maternal aggression peaks during lactation, suggesting oxytocin may regulate this behavior (see review Bosch and Neumann, 2012). The precise effect of oxytocin on maternal aggressive behavior still remains unclear. Oxytocin release occurs during maternal defensive aggression and the volume of release within the hypothalamus and amygdala is related to the amount of aggressive behavior displayed (Bosch et al., 2005). Higher concentration of oxytocin receptors within the lateral septum is associated with higher levels of aggression in rats (Caughey et al., 2011). Chronic ICV administration of oxytocin receptor antagonists can reduce maternal aggression in strains of rats exhibiting high anxiety and higher levels of defensive aggression (Bosch et al., 2005 but see Lubin et al., 2003). While these studies give the impression that increases in oxytocin activity are associated with higher levels of maternal aggression in these studies, others have reported the opposite (e.g. Giovenardi et al., 1998, Nephew et al., 2009). These differences in results appear to be due to differences between strains and differences in oxytocin administration protocols but further study is certainly warranted (Bosch and Neumann, 2012).
5.3.2 Selective Aggression
Pair bonding in prairie voles is characterized by the display of increased preferences of one’s mate and aggressive behavior towards unfamiliar conspecifics (Aragona and Wang, 2009). Prior to pair bonding, male prairie voles show little aggression toward strangers, however, following successful pair bond formation, male prairie voles (and sometimes females) will actively attack strangers including sexually receptive females (Aragona et al., 2006, Gobrogge et al., 2007, Insel et al., 1995, Wang et al., 1997, Winslow et al., 1993). Thus, while pair bonding is associated with increased appetitive value being assigned to mates it is also characterized by aversive value assignment to once neutral conspecific strangers. This behavior is mediated by increased expression of D1 receptors within the nucleus accumbens, which occurs following extended exposure to the pair-bonded partner and is hypothesized to antagonize the formation of a second pair bond (Aragona et al., 2006). Blockade of D1 receptors in these pair-bonded voles was observed to reduce aggressive behaviors towards unfamiliar females (Aragona et al., 2006).
A human parallel to such mate selective behavior has been recently reported. Scheele and colleagues observed when heterosexual males in a relationship are treated with intranasal oxytocin, they preferred to keep attractive unfamiliar females at a greater distance relative to single men or men in relationships which had been treated with placebo (Scheele et al., 2012). However, treatment with oxytocin had no effect on the social distance preferred when encountering unfamiliar males (Scheele et al., 2012). So while there is significant evidence that oxytocin serves to enhance the salience of social cues, oxytocin, possibly through its influence on dopaminergic processes, also appears to influence shifts in valence attribution as well; resulting in the repelling of some social entities while drawing others closer.
5.4 Sexual Behavior
Engaging in sexual behavior is arguably the most fundamental form of primary reward. Sexual behavior can be divided into appetitive (the drive to perform) and consummatory (actual performance) components. This review will focus on the appetitive aspects of sexual behavior including those behaviors that serve to arouse action, bring mates together and prepare for a sexual encounter (see Everitt, 1990). Sexually receptive female rats are salient social entities to male rats. Male rats show preference for environments associated with the presence of females (Everitt, 1990). Studies examining extracellular dopamine within the nucleus accumbens observe enhanced dopamine concentrations when shown a sexually receptive female behind a screen (Damsma et al., 1992, Pfaus et al., 1990). Further, male rats will bar press to gain access to sexually receptive female rats (Everitt, 1990). Lesions to the amygdala reduce bar pressing behavior by males but do not affect copulatory behaviors (Everitt, 1990). This affect can be reversed through stimulation of dopaminergic activity within the nucleus accumbens (Everitt, 1990). D2 receptor antagonists infused into the nucleus accumbens increase the amount of time between mounts and intromissions, but not the rate of copulation; as such D2 antagonism is interpreted as reducing the appetitive but not consummatory aspects of sexual behavior (Everitt, 1990). Though establishing neurochemical distinctions between the appetitive and consummatory aspects of sexual behavior in females is more difficult, there is evidence to suggest that dopaminergic activity within the nucleus accumbens contributes to the experience of reward in females during mating (see Becker, 2009). Females display a preference for the pace at which copulation takes place during mating which appears to optimize the chances of pregnancy by engaging certain neuroendocrine processes (Adler, 1974, Erskine et al., 1989, Gilman et al., 1979, Jenkins and Becker, 2003b). Dopaminergic release within the nucleus accumbens only appears to occur when females copulate according to their pacing preferences (Becker et al., 2001, Jenkins and Becker, 2003a, Mermelstein and Becker, 1995).
Oxytocin can enhance both male and female sexual behavior. Oxytocin is hypothesized to facilitate the appetitive value of sexual encounters and, as described in detail above, plays an important role in facilitating penile erection and is released in response to genital and breast stimulation (Carter, 1992, Melis and Argiolas, 2011). Oxytocin release and subsequent penile erection can be induced by stimulation of dopaminergic activity within the mesocorticolimbic system (Melis and Argiolas, 2011). Oxytocin administration reduces the latency between intromission and ejaculation in male rats (Carter, 1992). In females, oxytocin influences the rodents display of lordosis, or an arching of the back and hindleg, in expectation of sexual behavior, which is a measure of female receptivity to a sexual encounter. Injections of oxytocin ICV increase lordosis duration and lordosis quotient (ratio of lordosis responses to male mounts, with increases in this ratio reflecting increases in female receptivity; Benelli et al., 1994). Reductions in both lordosis duration and quotient are noted following injection of an oxytocin antagonist (Benelli et al., 1994).
In humans, sexual arousal has been associated with increased neural activity within a number of regions within the motivational pathway including in the hypothalamus, striatum, amygdala insula, frontal cortex and cingulate (Arnow et al., 2002, Hamann et al., 2004, Karama et al., 2002, Park et al., 2001, Rauch et al., 1999, Redoute et al., 2000, Stoleru et al., 1999). Within the striatum, middle temporal cortex, cingulate, claustrum and in sensorimotor and pre motor areas, brain activity elicited by erotic visual stimulation has been shown to relate to sexual arousal as measured by penile turgidity (Arnow et al., 2002). Sexual arousal has also been shown to elicit slightly different responses in men and women, where men seem to recruit the amygdala and hypothalamus during the processing of sexually arousing stimuli compared to women (Hamann et al., 2004). However, both men and women exhibited significant activation within ventral striatal regions in response to sexually arousing images (Hamann et al., 2004).
Oxytocin has also been shown to play an important role in sexual behavior in humans. Oxytocin is released during sexual arousal and orgasm (Blaicher et al., 1999, Burri et al., 2008, Carmichael et al., 1987, but see Kruger et al., 2003, Murphy et al., 1987). Enhancement of oxytocin levels by intranasal administration prior to orgasm by masturbation results in increased catecholamine levels in the blood, heightened heart rate and increased perception of arousability (Burri et al., 2008). In sum, the data obtained from preclinical and human research studies indicate oxytocin, dopamine and regions within the motivational circuitry contribute to the appetitive aspects of sexual behavior and may regulate the degree of reward experienced during sexual encounters.
5.5 Other Social Interactions
The role of oxytocin mediating social interactions beyond those behaviors that occur between romantic partners or between parents and children has been explored by a number of groups (see reviews Bartz et al., 2011, Kemp and Guastella, 2011, McCall and Singer, 2012, Meyer-Lindenberg et al., 2011, Zink and Meyer-Lindenberg, 2012). Oxytocin can also influence social interactions between peers and strangers and appears to play essential roles in the identification and interpretation of social information. For instance, preclinical models have observed that oxytocin can affect social recognition (see Choleris et al., 2009, and Ross and Young, 2009 for review). Social recognition in rodents can be measured by noting the amount of time an individual spends investigating an unfamiliar entity over repeated exposures. Unfamiliar rodents, for instance, will spend a great deal of time sniffing one another at first but as time goes on the duration of these investigations will decrease as they become familiar with (i.e. recognize) one another. While oxytocin administration into the hippocampus or medial preoptic area of the hypothalamus enhances social recognition (i.e. reduced investigation time of a fellow rat encountered for the second time), ICV administration of oxytocin antagonists can result in social recognition deficits (Popik and van Ree, 1991, van Wimersma Greidanus and Maigret, 1996). Oxytocin knockout mice also exhibit social recognition impairments, however, these impairments can be reversed by oxytocin infusion into medial amygdala, indicating special involvement of the amygdala in this process (Choleris et al., 2003, Ferguson et al., 2001, Ferguson et al., 2000).
In humans, in addition playing a role in social recognition (see Kemp and Guastella 2011), oxytocin can also enhance behaviors that lead to increases in the collection of social information. For instance, oxytocin administration has been shown to orient attention toward faces, particularly the eye region (see Bartz et al., 2011, Kemp and Guastella, 2011). As such, increased attention to the eye region can increase awareness of social intent and can provide important information necessary to making decisions when social context is relevant. Concentrating on cues from the eye region of faces may lead to better inferences of mental state and increases emotional recognition; indeed, both skills are increased following oxytocin administration though valence appears to be an important mediator in determining oxytocin’s effects (see Kemp and Guastella, 2011). Oxytocin also seems to bias perception in favor of attending to social objects like those displaying biological motion (Keri and Benedek, 2009). Furthermore, oxytocin administration has been shown to enhance memory for social but not non-social stimuli (Rimmele et al., 2009).
As mentioned above, the effect of oxytocin may be dependent on the valence of the social stimuli being processed. For instance, oxytocin improves the detection and classification of positive social and emotional stimuli but not for negative stimuli (see Kemp and Guastella, 2011). Memory and recognition for positive faces is improved relative to negative faces following oxytocin administration (see Kemp and Guastella, 2011, but see Savaskan et al., 2008). Domes and colleagues have recently reported oxytocin enhances eye gaze in happy and neutral stimuli but decreases in angry faces (Domes et al., 2012). Some research indicates oxytocin can improve an individual’s ability to recognize fear but not necessarily other emotions (Fischer-Shofty et al., 2010) whereas other research seems to indicate oxytocin improves the recognition of happy faces over others (Marsh et al., 2010). Neuroimaging studies using an oxytocin intervention have found similarly mixed results (Zink and Meyer-Lindenberg, 2012). One possibility, which may explain some of the inconsistent results obtained in studies utilizing oxytocin interventions, may be due to interindividual differences in the effect of oxytocin, which could be related to intrinsic or genotypic differences (Bartz et al., 2011, Meyer-Lindenberg et al., 2011). Indeed, complex relationships between oxytocin genotype and sex have recently been reported with oxytocin gene polymorphisms predicting differential dopaminergic responses to a pain-stress challenge in a sex specific manner (Love et al., 2012).
Oxytocin effects also seem to be highly dependent on context. Oxytocin has also been shown to enhance a myriad of prosocial behaviors including promoting altruism, generosity, trust, and empathy (Bartz et al., 2011, Israel et al., 2012). However, such increases in positive social behavior are greatly dependent on the identity of the person whom this behavior is directed. De Dreu and colleagues reported that oxytocin administration was associated with increases in altruistic responses to members within the ‘ingroup’ but increases in aggressive behavior towards members within the ‘outgroup’ (De Dreu et al., 2012). These data seem to mirror some of the effects of oxytocin in animals. In rodents, for example, oxytocin increases positive social behaviors towards offspring and mates (i.e. in-group) but also increases aggression towards strangers and conspecifics (i.e. out-group).
Final Comment
Oxytocin clearly plays a central role in social behavior; however, researchers are still trying to grasp the main mechanism behind oxytocin’s effects. First considered simply as a prosocial hormone, with recent data showing oxytocin can also increase elicited aggressive behaviors it now appears the effect of oxytocin is much more complex. Some have proposed oxytocin may act as a mediator of social salience, enhancing the processing of both positive and negative social stimuli thus promoting a wide range of positive and social behaviors. Support for this theory comes from the myriad of studies showing oxytocin administration leads to increased attention and orientation towards social stimuli, enhanced memory for social stimuli over non-social stimuli, and improved social recognition. Oxytocin encourages social interactions between parents and children, partners, and peers. Indeed, animals treated with oxytocin will increase the amount of effort they are willing to exert to gain access to appetitive social agents, even at the expense of obtaining other forms of reward like food or drugs. Furthermore, the notion that oxytocin influences the attribution of motivational salience fits well with the demonstration of significant neurobiological interactions between oxytocinergic and dopaminergic systems.
However, some have pointed out this theory has difficulty explaining the occasional valence specific effects of oxytocin (Kemp and Guastella, 2011). For instance, oxytocin has been shown to enhance memory for happy faces relative to angry and neutral faces (Guastella et al., 2008). Oxytocin administration has also been observed to improve recognition of positive facial expressions but not negative (Marsh et al., 2010, but see Fischer-Shofty et al., 2010). In addition, oxytocin suppresses amygdala activation for fearful faces but enhances activity for happy faces (Gamer et al., 2010). As such, some researchers have taken to distinguishing oxytocin effects along a slightly different dimension, viewing oxytocin as an agent capable of encouraging approach related behaviors and discouraging withdrawal (Kemp and Guastella, 2011).
Another possibility is that through its interactions with the mesocorticolimbic dopamine system, oxytocin both enhances motivational salience attributions towards social stimuli and provokes shifts in motivational value. This theory arises out of observations of population specific motivational signals within dopaminergic neurons within the VTA (Bromberg-Martin et al., 2010). As discussed above, signals within certain subpopulations of dopamine neurons are hypothesized to encode motivational salience while others carry signal relating to the motivational value (appetitive versus aversive; Bromberg-Martin et al., 2010). Thus by modulating activity within this dopaminergic network, oxytocin may encourage alterations in both salience and valence assignment. If the salience of social cues increases as a result of oxytocinergic manipulation, one would expect an increase in the attention afforded towards social stimuli and a shift between inaction and action; however if processes involved in assignment of valence are also modified, one might expect more nuanced shifts in behavior, with increases in positive behavior towards certain entities and increases in negative behavior towards others. Behaviorally, oxytocin has been shown to facilitate shifts in valence attribution. For instance, in rodents, virgin rats’ behavior towards pups can switch from aggressive or apathetic to affiliative through oxytocin administration (Pedersen et al., 1982). Or in the case of pair-bonding, an appetitive to aversive shift is seen in male voles behavior towards unfamiliar sexually receptive females following successful pair bonding (Aragona and Wang, 2009). Recent studies in humans suggest oxytocin can have salience and valence independent effects. Gamer and colleagues found that oxytocin’s effects on amygdala activity elicited by emotional faces was dependent on the valence of stimuli but the effects of oxytocin on enhancement of gaze shifts towards the eyes was valence independent (Gamer et al., 2010).
Elucidating the role oxytocin plays in the processing of motivationally relevant information obtained from social stimuli is of substantial interest. Social interactions are exceedingly complex and require the evaluation of a variety of significant signals. More research is needed to ascertain how and if oxytocin modulates the degree to which social stimuli are considered relevant, determine whether these stimuli are aversive or appetitive, and what action should be taken (i.e. approach or withdrawal). Protocols that allow the differentiation between valence specific and salience specific effects may aid in our understanding of oxytocin effects on these processes.
Highlights.
Recent evidence suggests oxytocin acts to increase the salience of social stimuli.
Oxytocin may affect social behaviors through its interaction with dopamine.
Potential for oxytocin to shift assessments of value and salience via dopamine.
Interactions between oxytocin and dopamine will be reviewed
Abbreviations
- ICV
intracerebroventricular
- VTA
ventral tegmental area
- HPA axis
hypothalamic pituitary adrenal axis
- ACTH
adrenocorticotropic hormone
- cAMP
cyclic adenosine monophosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
For submission to Pharmacology, Biochemistry and Behavior.
References
- Abercrombie ED, Keefe KA, DiFrischia DS, Zigmond MJ. Differential effect of stress on in vivo dopamine release in striatum, nucleus accumbens, and medial frontal cortex. Journal of neurochemistry. 1989;52:1655–8. doi: 10.1111/j.1471-4159.1989.tb09224.x. [DOI] [PubMed] [Google Scholar]
- Adler NT. The behavioral control of reproductive physiology. Advances in behavioral biology. 1974;11:259–86. doi: 10.1007/978-1-4684-3069-1_12. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio AR. Fear and the human amygdala. J Neurosci. 1995;15:5879–91. doi: 10.1523/JNEUROSCI.15-09-05879.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Curtis JT, Stephan FK, Wang Z. A critical role for nucleus accumbens dopamine in partner-preference formation in male prairie voles. J Neurosci. 2003;23:3483–90. doi: 10.1523/JNEUROSCI.23-08-03483.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, et al. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds. Nat Neurosci. 2006;9:133–9. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Opposing regulation of pair bond formation by cAMP signaling within the nucleus accumbens shell. J Neurosci. 2007;27:13352–6. doi: 10.1523/JNEUROSCI.3216-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragona BJ, Wang Z. Dopamine regulation of social choice in a monogamous rodent species. Frontiers in behavioral neuroscience. 2009;3:15. doi: 10.3389/neuro.08.015.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argiolas A, Melis MR. The role of oxytocin and the paraventricular nucleus in the sexual behaviour of male mammals. Physiol Behav. 2004;83:309–17. doi: 10.1016/j.physbeh.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Arnow BA, Desmond JE, Banner LL, Glover GH, Solomon A, Polan ML, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain : a journal of neurology. 2002;125:1014–23. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. Journal of neurophysiology. 2005;94:327–37. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Averbeck BB. Oxytocin and the salience of social cues. Proc Natl Acad Sci USA. 2010;107:9033–4. doi: 10.1073/pnas.1004892107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Blatt GJ. Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus. 1995;5:511–33. doi: 10.1002/hipo.450050604. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–66. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in cognitive sciences. 2011;15:301–9. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Baskerville TA, Allard J, Wayman C, Douglas AJ. Dopamine-oxytocin interactions in penile erection. Eur J Neurosci. 2009;30:2151–64. doi: 10.1111/j.1460-9568.2009.06999.x. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–9. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- Becker JB. Sexual differentiation of motivation: A novel mechanism? Hormones and behavior. 2009;55:646–54. doi: 10.1016/j.yhbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–41. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belova MA, Paton JJ, Salzman CD. Moment-to-moment tracking of state value in the amygdala. J Neurosci. 2008;28:10023–30. doi: 10.1523/JNEUROSCI.1400-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benelli A, Poggioli R, Luppi P, Ruini L, Bertolini A, Arletti R. Oxytocin enhances, and oxytocin antagonism decreases, sexual receptivity in intact female rats. Neuropeptides. 1994;27:245–50. doi: 10.1016/0143-4179(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bermudez MA, Schultz W. Responses of amygdala neurons to positive reward-predicting stimuli depend on background reward (contingency) rather than stimulus-reward pairing (contiguity) Journal of neurophysiology. 2010a;103:1158–70. doi: 10.1152/jn.00933.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez MA, Schultz W. Reward magnitude coding in primate amygdala neurons. Journal of neurophysiology. 2010b;104:3424–32. doi: 10.1152/jn.00540.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain research Brain research reviews. 1998;28:309–69. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Venier IL, Robinson TE. Taste reactivity analysis of 6-hydroxydopamine-induced aphagia: implications for arousal and anhedonia hypotheses of dopamine function. Behav Neurosci. 1989;103:36–45. doi: 10.1037//0735-7044.103.1.36. [DOI] [PubMed] [Google Scholar]
- Bishop MP, Elder ST, Heath RG. Intracranial self-stimulation in man. Science. 1963;140:394–6. doi: 10.1126/science.140.3565.394. [DOI] [PubMed] [Google Scholar]
- Blaicher W, Gruber D, Bieglmayer C, Blaicher AM, Knogler W, Huber JC. The role of oxytocin in relation to female sexual arousal. Gynecologic and obstetric investigation. 1999;47:125–6. doi: 10.1159/000010075. [DOI] [PubMed] [Google Scholar]
- Blum K, Gardner E, Oscar-Berman M, Gold M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Current pharmaceutical design. 2012;18:113–8. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25:6807–15. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Brinton RE, Wamsley JK, Gee KW, Wan YP, Yamamura HI. [3H]oxytocin binding sites in the rat brain demonstrated by quantitative light microscopic autoradiography. European Journal of Pharmacology. 1984;102:365–7. doi: 10.1016/0014-2999(84)90270-x. [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68:815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkett JP, Young LJ. The behavioral, anatomical and pharmacological parallels between social attachment, love and addiction. Psychopharmacology (Berl) 2012;224:1–26. doi: 10.1007/s00213-012-2794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burri A, Heinrichs M, Schedlowski M, Kruger TH. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology. 2008;33:591–600. doi: 10.1016/j.psyneuen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Caffé AR, Van Ryen PC, Van der Woude TP, Van Leeuwen FW. Vasopressin and oxytocin systems in the brain and upper spinal cord of Macaca fascicularis. The Journal of Comparative Neurology. 1989;287:302–25. doi: 10.1002/cne.902870304. [DOI] [PubMed] [Google Scholar]
- Campbell A. Oxytocin and human social behavior. Personality and social psychology review: an official journal of the Society for Personality and Social Psychology, Inc. 2010;14:281–95. doi: 10.1177/1088868310363594. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. The Journal of clinical endocrinology and metabolism. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carter CS. Oxytocin and sexual behavior. Neurosci Biobehav Rev. 1992;16:131–44. doi: 10.1016/s0149-7634(05)80176-9. [DOI] [PubMed] [Google Scholar]
- Caughey SD, Klampfl SM, Bishop VR, Pfoertsch J, Neumann ID, Bosch OJ, et al. Changes in the intensity of maternal aggression and central oxytocin and vasopressin V1a receptors across the peripartum period in the rat. J Neuroendocrinol. 2011;23:1113–24. doi: 10.1111/j.1365-2826.2011.02224.x. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–23. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–9. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Choleris E, Clipperton-Allen AE, Phan A, Kavaliers M. Neuroendocrinology of social information processing in rats and mice. Front Neuroendocrinol. 2009;30:442–59. doi: 10.1016/j.yfrne.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Choleris E, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW, Ogawa S. An estrogen-dependent four-gene micronet regulating social recognition: a study with oxytocin and estrogen receptor-alpha and -beta knockout mice. Proc Natl Acad Sci USA. 2003;100:6192–7. doi: 10.1073/pnas.0631699100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall WA, Franklin KB, Coen KM, Clarke PB. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology (Berl) 1992;107:285–9. doi: 10.1007/BF02245149. [DOI] [PubMed] [Google Scholar]
- Curtis JT, Wang Z. Ventral tegmental area involvement in pair bonding in male prairie voles. Physiol Behav. 2005;86:338–46. doi: 10.1016/j.physbeh.2005.08.022. [DOI] [PubMed] [Google Scholar]
- D’Angio M, Serrano A, Rivy JP, Scatton B. Tail-pinch stress increases extracellular DOPAC levels (as measured by in vivo voltammetry) in the rat nucleus accumbens but not frontal cortex: antagonism by diazepam and zolpidem. Brain Res. 1987;409:169–74. doi: 10.1016/0006-8993(87)90755-4. [DOI] [PubMed] [Google Scholar]
- Dale HH. On some physiological actions of ergot. The Journal of Physiology. 1906;34:163–206. doi: 10.1113/jphysiol.1906.sp001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damsma G, Pfaus JG, Wenkstern D, Phillips AG, Fibiger HC. Sexual behavior increases dopamine transmission in the nucleus accumbens and striatum of male rats: comparison with novelty and locomotion. Behav Neurosci. 1992;106:181–91. doi: 10.1037//0735-7044.106.1.181. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–80. [PubMed] [Google Scholar]
- Domes G, Steiner A, Porges SW, Heinrichs M. Oxytocin differentially modulates eye gaze to naturalistic social signals of happiness and anger. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–4. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- du Vigneaud V, Ressler C, Swan J, Roberts C, Katsoyannis P, Gordon S. The synthesis of an octapeptide amide with the hormonal activity of oxytocin. Journal of the American Chemical Society. 1953;75:4879–4880. [Google Scholar]
- du Vigneaud V, Ressler C, Swan J, Roberts C, Katsoyannis P. The Synthesis of Oxytocin. Journal of the American Chemical Society. 1954;76:3115–21. [Google Scholar]
- Eichenbaum H. Hippocampus: cognitive processes and neural representations that underlie declarative memory. Neuron. 2004;44:109–20. doi: 10.1016/j.neuron.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Erskine MS, Kornberg E, Cherry JA. Paced copulation in rats: effects of intromission frequency and duration on luteal activation and estrus length. Physiol Behav. 1989;45:33–9. doi: 10.1016/0031-9384(89)90163-7. [DOI] [PubMed] [Google Scholar]
- Everitt BJ. Sexual motivation: a neural and behavioural analysis of the mechanisms underlying appetitive and copulatory responses of male rats. Neurosci Biobehav Rev. 1990;14:217–32. doi: 10.1016/s0149-7634(05)80222-2. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Cardinal RN, Parkinson JA, Robbins TW. Appetitive behavior: impact of amygdala-dependent mechanisms of emotional learning. Ann N Y Acad Sci. 2003;985:233–50. [PubMed] [Google Scholar]
- Everitt BJ, Morris KA, O’Brien A, Robbins TW. The basolateral amygdala-ventral striatal system and conditioned place preference: further evidence of limbic-striatal interactions underlying reward-related processes. Neuroscience. 1991;42:1–18. doi: 10.1016/0306-4522(91)90145-e. [DOI] [PubMed] [Google Scholar]
- Falck B, Hillarp NA. On the cellular localization of catechol amines in the brain. Acta Anat (Basel) 1959;38:277–9. doi: 10.1159/000141530. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–44. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21:8278–85. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Hearn EF, Matzuk MM, Insel TR, Winslow JT. Social amnesia in mice lacking the oxytocin gene. Nature genetics. 2000;25:284–8. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25:149–56. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibiger HC, LePiane FG, Jakubovic A, Phillips AG. The role of dopamine in intracranial self-stimulation of the ventral tegmental area. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1987;7:3888–96. doi: 10.1523/JNEUROSCI.07-12-03888.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer-Shofty M, Shamay-Tsoory SG, Harari H, Levkovitz Y. The effect of intranasal administration of oxytocin on fear recognition. Neuropsychologia. 2010;48:179–84. doi: 10.1016/j.neuropsychologia.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Anderson V. Affect and nurturance: mechanisms mediating maternal behavior in two female mammals. Progress in neuro-psychopharmacology & biological psychiatry. 1987;11:121–7. doi: 10.1016/0278-5846(87)90049-2. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Rosenblatt JS. Maternal behavior in the virgin and lactating rat. Journal of comparative and physiological psychology. 1974;86:957–72. doi: 10.1037/h0036414. [DOI] [PubMed] [Google Scholar]
- Fliers E, Guldenaar SE, van de Wal N, Swaab DF. Extrahypothalamic vasopressin and oxytocin in the human brain; presence of vasopressin cells in the bed nucleus of the stria terminalis. Brain Research. 1986;375:363–7. doi: 10.1016/0006-8993(86)90759-6. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. J Neurosci. 2003;23:3930–43. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Todd CL, Grace AA. Glutamatergic afferents from the hippocampus to the nucleus accumbens regulate activity of ventral tegmental area dopamine neurons. J Neurosci. 2001;21:4915–22. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci. 2003;6:968–73. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- Francis DD, Champagne FC, Meaney MJ. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J Neuroendocrinol. 2000;12:1145–8. doi: 10.1046/j.1365-2826.2000.00599.x. [DOI] [PubMed] [Google Scholar]
- Freund-Mercier MJ, Stoeckel ME, Palacios JM, Pazos A, Reichhart JM, Porte A, et al. Pharmacological characteristics and anatomical distribution of [3H]oxytocin-binding sites in the Wistar rat brain studied by autoradiography. Neuroscience. 1987;20:599–614. doi: 10.1016/0306-4522(87)90113-8. [DOI] [PubMed] [Google Scholar]
- Gamer M, Zurowski B, Buchel C. Different amygdala subregions mediate valence-related and attentional effects of oxytocin in humans. Proc Natl Acad Sci USA. 2010;107:9400–5. doi: 10.1073/pnas.1000985107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12:2779–83. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Mahan LC, Susel Z, Chase TN, Monsma FJ, Jr, et al. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science. 1990;250:1429–32. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Gilman DP, Mercer LF, Hitt JC. Influence of female copulatory behavior on the induction of pseudopregnancy in the female rat. Physiol Behav. 1979;22:675–8. doi: 10.1016/0031-9384(79)90229-4. [DOI] [PubMed] [Google Scholar]
- Giovenardi M, Padoin MJ, Cadore LP, Lucion AB. Hypothalamic paraventricular nucleus modulates maternal aggression in rats: effects of ibotenic acid lesion and oxytocin antisense. Physiol Behav. 1998;63:351–9. doi: 10.1016/s0031-9384(97)00434-4. [DOI] [PubMed] [Google Scholar]
- Gobrogge KL, Liu Y, Jia X, Wang Z. Anterior hypothalamic neural activation and neurochemical associations with aggression in pair-bonded male prairie voles. J Comp Neurol. 2007;502:1109–22. doi: 10.1002/cne.21364. [DOI] [PubMed] [Google Scholar]
- Gordon I, Martin C, Feldman R, Leckman JF. Oxytocin and social motivation. Developmental cognitive neuroscience. 2011;1:471–93. doi: 10.1016/j.dcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–12. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, Vermeulen-Van der Zee E, te Kortschot A, Witter MP. Organization of the projections from the subiculum to the ventral striatum in the rat. A study using anterograde transport of Phaseolus vulgaris leucoagglutinin. Neuroscience. 1987;23:103–20. doi: 10.1016/0306-4522(87)90275-2. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biological psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The Reward Circuit: Linking Primate Anatomy and Human Imaging. Neuropsychopharmacology. 2009:1–23. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Lynd E, Klein C, Groenewegen HJ. Topographic organization of the ventral striatal efferent projections in the rhesus monkey: an anterograde tracing study. J Comp Neurol. 1990;293:282–98. doi: 10.1002/cne.902930210. [DOI] [PubMed] [Google Scholar]
- Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13:15–9. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–93. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hamann SS, Herman RAR, Nolan CLC, Wallen KK. Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience. 2004;7:411–6. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacology, biochemistry, and behavior. 1993;45:673–6. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacology, biochemistry, and behavior. 1991a;39:71–7. doi: 10.1016/0091-3057(91)90399-m. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Lofberg L, Svensson K. Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci. 1991b;105:588–98. doi: 10.1037//0735-7044.105.4.588. [DOI] [PubMed] [Google Scholar]
- Hauser HH, Gandelman RR. Lever pressing for pups: evidence for hormonal influence upon maternal behavior of mice. Hormones and behavior. 1985;19:454–68. doi: 10.1016/0018-506x(85)90041-8. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers in neuroendocrinology. 2009;30:548–57. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–9. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Horvitz JC. Mesolimbocortical and nigrostriatal dopamine responses to salient non-reward events. Neuroscience. 2000;96:651–6. doi: 10.1016/s0306-4522(00)00019-1. [DOI] [PubMed] [Google Scholar]
- Insel TR, Preston S, Winslow JT. Mating in the monogamous male: behavioral consequences. Physiol Behav. 1995;57:615–27. doi: 10.1016/0031-9384(94)00362-9. [DOI] [PubMed] [Google Scholar]
- Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci USA. 1992;89:5981–5. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nature Reviews Neuroscience. 2001;2:129–36. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Israel S, Weisel O, Ebstein RP, Bornstein G. Oxytocin, but not vasopressin, increases both parochial and universal altruism. Psychoneuroendocrinology. 2012;37:1341–4. doi: 10.1016/j.psyneuen.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ito R, Robbins TW, Pennartz CM, Everitt BJ. Functional interaction between the hippocampus and nucleus accumbens shell is necessary for the acquisition of appetitive spatial context conditioning. Journal of Neuroscience. 2008;28:6950–9. doi: 10.1523/JNEUROSCI.1615-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubowski M, Terkel J. Incidence of pup killing and parental behavior in virgin female and male rats (Rattus norvegicus): differences between Wistar and Sprague-Dawley stocks. Journal of comparative psychology (Washington, DC: 1983) 1985a;99:93–7. [PubMed] [Google Scholar]
- Jakubowski M, Terkel J. Transition from pup killing to parental behavior in male and virgin female albino rats. Physiol Behav. 1985b;34:683–6. doi: 10.1016/0031-9384(85)90364-6. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. Eur J Neurosci. 2003a;18:1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Female rats develop conditioned place preferences for sex at their preferred interval. Horm Behav. 2003b;43:503–7. doi: 10.1016/s0018-506x(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Jezova D, Skultetyova I, Tokarev DI, Bakos P, Vigas M. Vasopressin and oxytocin in stress. Annals of the New York Academy of Sciences. 1995;771:192–203. doi: 10.1111/j.1749-6632.1995.tb44681.x. [DOI] [PubMed] [Google Scholar]
- Kaczmarek HJ, Kiefer SW. Microinjections of dopaminergic agents in the nucleus accumbens affect ethanol consumption but not palatability. Pharmacology, biochemistry, and behavior. 2000;66:307–12. doi: 10.1016/s0091-3057(00)00182-9. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nakamura M. Neural systems for behavioral activation and reward. Curr Opin Neurobiol. 1999;9:223–7. doi: 10.1016/s0959-4388(99)80031-2. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–13. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Karama S, Lecours AR, Leroux J-M, Bourgouin P, Beaudoin G, Joubert S, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiology & Behavior. 1999;67:659–69. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Kemp AH, Guastella AJ. The Role of Oxytocin in Human Affect: A Novel Hypothesis. Current Directions in Psychological Science. 2011;20:222–31. [Google Scholar]
- Keri S, Benedek G. Oxytocin enhances the perception of biological motion in humans. Cognitive, affective & behavioral neuroscience. 2009;9:237–41. doi: 10.3758/CABN.9.3.237. [DOI] [PubMed] [Google Scholar]
- Krémarik P, Freund-Mercier MJ, Stoeckel ME. Histoautoradiographic detection of oxytocin- and vasopressin-binding sites in the telencephalon of the rat. The Journal of Comparative Neurology. 1993;333:343–59. doi: 10.1002/cne.903330304. [DOI] [PubMed] [Google Scholar]
- Kruger TH, Haake P, Chereath D, Knapp W, Janssen OE, Exton MS, et al. Specificity of the neuroendocrine response to orgasm during sexual arousal in men. The Journal of endocrinology. 2003;177:57–64. doi: 10.1677/joe.0.1770057. [DOI] [PubMed] [Google Scholar]
- Lang RE, Heil JW, Ganten D, Hermann K, Unger T, Rascher W. Oxytocin unlike vasopressin is a stress hormone in the rat. Neuroendocrinology. 1983;37:314–6. doi: 10.1159/000123566. [DOI] [PubMed] [Google Scholar]
- Laroche S, Davis S, Jay TM. Plasticity at hippocampal to prefrontal cortex synapses: dual roles in working memory and consolidation. Hippocampus. 2000;10:438–46. doi: 10.1002/1098-1063(2000)10:4<438::AID-HIPO10>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual review of neuroscience. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee H-J, Macbeth AH, Pagani JH, Young WS. Oxytocin: the great facilitator of life. Progress in Neurobiology. 2009;88:127–51. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legault M, Wise RA. Novelty-evoked elevations of nucleus accumbens dopamine: dependence on impulse flow from the ventral subiculum and glutamatergic neurotransmission in the ventral tegmental area. Eur J Neurosci. 2001;13:819–28. doi: 10.1046/j.0953-816x.2000.01448.x. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini M, Harrison T, Haxby JV. Mothers’s neural activation in response to pictures of their children and other children. Biological psychiatry. 2004;56:225–32. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lisman JE, Grace AA. The hippocampal-VTA loop: controlling the entry of information into long-term memory. Neuron. 2005;46:703–13. doi: 10.1016/j.neuron.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Liu JC, Guastella AJ, Dadds MR. Exploring the role of intra-nasal oxytocin on the partner preference effect in humans. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.07.009. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang ZX. Nucleus accumbens oxytocin and dopamine interact to regulate pair bond formation in female prairie voles. Neuroscience. 2003;121:537–44. doi: 10.1016/s0306-4522(03)00555-4. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Grace AA. The hippocampus modulates dopamine neuron responsivity by regulating the intensity of phasic neuron activation. Neuropsychopharmacology. 2006;31:1356–61. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Sex differences in the parental behavior of rodents. Neurosci Biobehav Rev. 2000;24:669–86. doi: 10.1016/s0149-7634(00)00036-1. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Morrell JI. Neuroendocrinology and neurochemistry of maternal motivation and behavior. In: Lajtha A, Blaustein J, editors. Handbook of Neurochemistry and Molecular Neurobiology: Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology. Berlin: Springer-Verlag; 2007. pp. 197–229. [Google Scholar]
- Loup F, Tribollet E, Dubois-Dauphin M, Dreifuss JJ. Localization of high-affinity binding sites for oxytocin and vasopressin in the human brain. An autoradiographic study. Brain Research. 1991;555:220–32. doi: 10.1016/0006-8993(91)90345-v. [DOI] [PubMed] [Google Scholar]
- Love TM, Enoch MA, Hodgkinson CA, Pecina M, Mickey B, Koeppe RA, et al. Oxytocin gene polymorphisms influence human dopaminergic function in a sex-dependent manner. Biol Psychiatry. 2012;72:198–206. doi: 10.1016/j.biopsych.2012.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubin DA, Elliott JC, Black MC, Johns JM. An oxytocin antagonist infused into the central nucleus of the amygdala increases maternal aggressive behavior. Behavioral Neuroscience. 2003;117:195–201. doi: 10.1037/0735-7044.117.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo AH, Tahsili-Fahadan P, Wise RA, Lupica CR, Aston-Jones G. Linking context with reward: a functional circuit from hippocampal CA3 to ventral tegmental area. Science. 2011;333:353–7. doi: 10.1126/science.1204622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh AA, Yu HH, Pine DS, Blair RJ. Oxytocin improves specific recognition of positive facial expressions. Psychopharmacology (Berl) 2010;209:225–32. doi: 10.1007/s00213-010-1780-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature. 2009;459:837–41. doi: 10.1038/nature08028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115:683–94. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology (Berl) 2003;167:1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15:681–8. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Central oxytocinergic neurotransmission: a drug target for the therapy of psychogenic erectile dysfunction. Current drug targets. 2003;4:55–66. doi: 10.2174/1389450033347190. [DOI] [PubMed] [Google Scholar]
- Melis MR, Argiolas A. Central control of penile erection: A re-visitation of the role of oxytocin and its interaction with dopamine and glutamic acid in male rats. Neuroscience and biobehavioral reviews. 2011;35:939–55. doi: 10.1016/j.neubiorev.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Melis MR, Melis T, Cocco C, Succu S, Sanna F, Pillolla G, et al. Oxytocin injected into the ventral tegmental area induces penile erection and increases extracellular dopamine in the nucleus accumbens and paraventricular nucleus of the hypothalamus of male rats. European Journal of Neuroscience. 2007;26:1026–35. doi: 10.1111/j.1460-9568.2007.05721.x. [DOI] [PubMed] [Google Scholar]
- Melis MR, Succu S, Sanna F, Boi A, Argiolas A. Oxytocin injected into the ventral subiculum or the posteromedial cortical nucleus of the amygdala induces penile erection and increases extracellular dopamine levels in the nucleus accumbens of male rats. Eur J Neurosci. 2009;30:1349–57. doi: 10.1111/j.1460-9568.2009.06912.x. [DOI] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109:354–65. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Domes G, Kirsch P, Heinrichs M. Oxytocin and vasopressin in the human brain: social neuropeptides for translational medicine. Nat Rev Neurosci. 2011;12:524–38. doi: 10.1038/nrn3044. [DOI] [PubMed] [Google Scholar]
- Milner PM. The discovery of self-stimulation and other stories. Neuroscience and biobehavioral reviews. 1989;13:61–7. doi: 10.1016/s0149-7634(89)80013-2. [DOI] [PubMed] [Google Scholar]
- Mogenson G, Yang C. The contribution of basal forebrain to limbic-motor integration and the mediation of motivation to action. Adv Exp Med Biol. 1991;295:267–90. doi: 10.1007/978-1-4757-0145-6_14. [DOI] [PubMed] [Google Scholar]
- Moos F, Freund-Mercier MJ, Guerne Y, Guerne JM, Stoeckel ME, Richard P. Release of oxytocin and vasopressin by magnocellular nuclei in vitro: specific facilitatory effect of oxytocin on its own release. The Journal of endocrinology. 1984;102:63–72. doi: 10.1677/joe.0.1020063. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Seckl JR, Burton S, Checkley SA, Lightman SL. Changes in oxytocin and vasopressin secretion during sexual activity in men. The Journal of clinical endocrinology and metabolism. 1987;65:738–41. doi: 10.1210/jcem-65-4-738. [DOI] [PubMed] [Google Scholar]
- Murray EA, Wise SP. Interactions between orbital prefrontal cortex and amygdala: advanced cognition, learned responses and instinctive behaviors. Curr Opin Neurobiol. 2010;20:212–20. doi: 10.1016/j.conb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E, Panksepp J. Oxytocin mediates acquisition of maternally associated odor preferences in preweanling rat pups. Behav Neurosci. 1996;110:583–92. doi: 10.1037//0735-7044.110.3.583. [DOI] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behav Neurosci. 2009;123:949–57. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends in neurosciences. 2012;35:649–59. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci USA. 1996;93:11699–704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noirot E. Ultrasounds and maternal behavior in small rodents. Dev Psychobiol. 1972;5:371–87. doi: 10.1002/dev.420050410. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, et al. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci. 2005;119:1588–604. doi: 10.1037/0735-7044.119.6.1588. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front Neuroendocrinol. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Olds J, Milner P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. Journal of comparative and physiological psychology. 1954;47:419–27. doi: 10.1037/h0058775. [DOI] [PubMed] [Google Scholar]
- Onaka T. Neural pathways controlling central and peripheral oxytocin release during stress. J Neuroendocrinol. 2004;16:308–12. doi: 10.1111/j.0953-8194.2004.01186.x. [DOI] [PubMed] [Google Scholar]
- Park K, Kang HK, Seo JJ, Kim HJ, Ryu SB, Jeong GW. Blood-oxygenation-level-dependent functional magnetic resonance imaging for evaluating cerebral regions of female sexual arousal response. Urology. 2001;57:1189–94. doi: 10.1016/s0090-4295(01)00992-x. [DOI] [PubMed] [Google Scholar]
- Pecina S, Berridge KC, Parker LA. Pimozide does not shift palatability: separation of anhedonia from sensorimotor suppression by taste reactivity. Pharmacology, biochemistry, and behavior. 1997;58:801–11. doi: 10.1016/s0091-3057(97)00044-0. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–50. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108:1163–71. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Prog Neurobiol. 2004;74:301–20. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Damsma G, Nomikos GG, Wenkstern DG, Blaha CD, Phillips AG, et al. Sexual behavior enhances central dopamine transmission in the male rat. Brain Res. 1990;530:345–8. doi: 10.1016/0006-8993(90)91309-5. [DOI] [PubMed] [Google Scholar]
- Popik P, van Ree JM. Oxytocin but not vasopressin facilitates social recognition following injection into the medial preoptic area of the rat brain. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 1991;1:555–60. doi: 10.1016/0924-977x(91)90010-r. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–8. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Dougherty DD, Alpert NM, Orr SP, Lasko M, et al. Neural activation during sexual and competitive arousal in healthy men. Psychiatry research. 1999;91:1–10. doi: 10.1016/s0925-4927(99)00020-7. [DOI] [PubMed] [Google Scholar]
- Redoute J, Stoleru S, Gregoire MC, Costes N, Cinotti L, Lavenne F, et al. Brain processing of visual sexual stimuli in human males. Hum Brain Mapp. 2000;11:162–77. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riem MM, Bakermans-Kranenburg MJ, Pieper S, Tops M, Boksem MA, Vermeiren RR, et al. Oxytocin modulates amygdala, insula, and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biol Psychiatry. 2011;70:291–7. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson DA, Wei F, Wang GD, Li P, Kim SJ, Vogt SK, et al. Oxytocin mediates stress-induced analgesia in adult mice. The Journal of Physiology. 2002;540:593–606. doi: 10.1113/jphysiol.2001.013492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Sandstrom SM, Denenberg VH, Palmiter RD. Distinguishing whether dopamine regulates liking, wanting, and/or learning about rewards. Behav Neurosci. 2005;119:5–15. doi: 10.1037/0735-7044.119.1.5. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Rolls BJ, Kelly PH, Shaw SG, Wood RJ, Dale R. The relative attenuation of self-stimulation, eating and drinking produced by dopamine-receptor blockade. Psychopharmacology (Berl) 1974;38:219–30. doi: 10.1007/BF00421374. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Factor EM, Mayer AD. Relationship between maternal aggression and maternal care in the rat. Aggressive Behavior. 1994;20:243–55. [Google Scholar]
- Ross HE, Freeman SM, Spiegel LL, Ren X, Terwilliger EF, Young LJ. Variation in oxytocin receptor density in the nucleus accumbens has differential effects on affiliative behaviors in monogamous and polygamous voles. J Neurosci. 2009;29:1312–8. doi: 10.1523/JNEUROSCI.5039-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–47. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70:1054–69. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- Salamone JD. The involvement of nucleus accumbens dopamine in appetitive and aversive motivation. Behav Brain Res. 1994;61:117–33. doi: 10.1016/0166-4328(94)90153-8. [DOI] [PubMed] [Google Scholar]
- Salamone JD. Functions of mesolimbic dopamine: changing concepts and shifting paradigms. Psychopharmacology (Berl) 2007;191:389. doi: 10.1007/s00213-006-0623-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–85. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salinas JA, Packard MG, McGaugh JL. Amygdala modulates memory for changes in reward magnitude: reversible post-training inactivation with lidocaine attenuates the response to a reduction in reward. Behav Brain Res. 1993;59:153–9. doi: 10.1016/0166-4328(93)90162-j. [DOI] [PubMed] [Google Scholar]
- Sanna F, Argiolas A, Melis MR. Oxytocin-induced yawning: sites of action in the brain and interaction with mesolimbic/mesocortical and incertohypothalamic dopaminergic neurons in male rats. Horm Behav. 2012;62:505–14. doi: 10.1016/j.yhbeh.2012.08.010. [DOI] [PubMed] [Google Scholar]
- Santos A, Mier D, Kirsch P, Meyer-Lindenberg A. Evidence for a general face salience signal in human amygdala. Neuroimage. 2011;54:3111–6. doi: 10.1016/j.neuroimage.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Savaskan E, Ehrhardt R, Schulz A, Walter M, Schachinger H. Post-learning intranasal oxytocin modulates human memory for facial identity. Psychoneuroendocrinology. 2008;33:368–74. doi: 10.1016/j.psyneuen.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Gunturkun O, Deutschlander S, Maier W, Kendrick KM, et al. Oxytocin modulates social distance between males and females. J Neurosci. 2012;32:16074–9. doi: 10.1523/JNEUROSCI.2755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin during the initial stages of romantic attachment: relations to couples’ interactive reciprocity. Psychoneuroendocrinology. 2012;37:1277–85. doi: 10.1016/j.psyneuen.2011.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, Tremblay L, Hollerman JR. Reward prediction in primate basal ganglia and frontal cortex. Neuropharmacology. 1998;37:421–9. doi: 10.1016/s0028-3908(98)00071-9. [DOI] [PubMed] [Google Scholar]
- Scott D, Heitzeg M, Koeppe R, Stohler C, Zubieta J. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. J Neurosci. 2006;26:10789–95. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Morrell JI. Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup- but not cocaine-paired contexts. Behav Neurosci. 2009;123:1325–38. doi: 10.1037/a0017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang T-Y, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–86. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and schadenfreude (gloating) Biol Psychiatry. 2009;66:864–70. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Skuse DH, Gallagher L. Dopaminergic-neuropeptide interactions in the social brain. Trends Cogn Sci (Regul Ed) 2009;13:27–35. doi: 10.1016/j.tics.2008.09.007. [DOI] [PubMed] [Google Scholar]
- Smeltzer MD, Curtis JT, Aragona BJ, Wang Z. Dopamine, oxytocin, and vasopressin receptor binding in the medial prefrontal cortex of monogamous and promiscuous voles. Neurosci Lett. 2006;394:146–51. doi: 10.1016/j.neulet.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Smith KS, Berridge KC, Aldridge JW. Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA. 2011;108:E255–64. doi: 10.1073/pnas.1101920108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–14. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- Stoleru S, Gregoire MC, Gerard D, Decety J, Lafarge E, Cinotti L, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Archives of sexual behavior. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–59. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–66. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Succu S, Sanna F, Argiolas A, Melis MR. Oxytocin injected into the hippocampal ventral subiculum induces penile erection in male rats by increasing glutamatergic neurotransmission in the ventral tegmental area. Neuropharmacology. 2011;61:181–8. doi: 10.1016/j.neuropharm.2011.03.026. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Cocco C, Melis T, Boi A, Ferri GL, et al. Oxytocin induces penile erection when injected into the ventral tegmental area of male rats: role of nitric oxide and cyclic GMP. Eur J Neurosci. 2008;28:813–21. doi: 10.1111/j.1460-9568.2008.06385.x. [DOI] [PubMed] [Google Scholar]
- Succu S, Sanna F, Melis T, Boi A, Argiolas A, Melis MR. Stimulation of dopamine receptors in the paraventricular nucleus of the hypothalamus of male rats induces penile erection and increases extracellular dopamine in the nucleus accumbens: Involvement of central oxytocin. Neuropharmacology. 2007;52:1034–43. doi: 10.1016/j.neuropharm.2006.10.019. [DOI] [PubMed] [Google Scholar]
- Takayanagi Y, Yoshida M, Bielsky IF, Ross HE, Kawamata M, Onaka T, et al. Pervasive social deficits, but normal parturition, in oxytocin receptor-deficient mice. Proc Natl Acad Sci USA. 2005;102:16096–101. doi: 10.1073/pnas.0505312102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trezza V, Campolongo P, Vanderschuren LJ. Evaluating the rewarding nature of social interactions in laboratory animals. Developmental cognitive neuroscience. 2011;1:444–58. doi: 10.1016/j.dcn.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvnas-Moberg K. Physiological and endocrine effects of social contact. Ann N Y Acad Sci. 1997;807:146–63. doi: 10.1111/j.1749-6632.1997.tb51917.x. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139:5015–33. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen FW, van Heerikhuize J, van der Meulen G, Wolters P. Light microscopic autoradiographic localization of [3H]oxytocin binding sites in the rat brain, pituitary and mammary gland. Brain Research. 1985;359:320–5. doi: 10.1016/0006-8993(85)91443-x. [DOI] [PubMed] [Google Scholar]
- van Wimersma Greidanus TB, Maigret C. The role of limbic vasopressin and oxytocin in social recognition. Brain Res. 1996;713:153–9. doi: 10.1016/0006-8993(95)01505-1. [DOI] [PubMed] [Google Scholar]
- Wang Z, Hulihan TJ, Insel TR. Sexual and social experience is associated with different patterns of behavior and neural activation in male prairie voles. Brain Res. 1997;767:321–32. doi: 10.1016/s0006-8993(97)00617-3. [DOI] [PubMed] [Google Scholar]
- Wigger A, Neumann ID. Endogenous opioid regulation of stress-induced oxytocin release within the hypothalamic paraventricular nucleus is reversed in late pregnancy: a microdialysis study. Neuroscience. 2002;112:121–9. doi: 10.1016/s0306-4522(02)00068-4. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature. 1993;365:545–8. doi: 10.1038/365545a0. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hearn EF, Ferguson J, Young LJ, Matzuk MM, Insel TR. Infant vocalization, adult aggression, and fear behavior of an oxytocin null mutant mouse. Horm Behav. 2000;37:145–55. doi: 10.1006/hbeh.1999.1566. [DOI] [PubMed] [Google Scholar]
- Wise RA, Rompre PP. Brain dopamine and reward. Annual review of psychology. 1989;40:191–225. doi: 10.1146/annurev.ps.40.020189.001203. [DOI] [PubMed] [Google Scholar]
- Wittfoth-Schardt D, Grunding J, Wittfoth M, Lanfermann H, Heinrichs M, Domes G, et al. Oxytocin modulates neural reactivity to children’s faces as a function of social salience. Neuropsychopharmacology. 2012;37:1799–807. doi: 10.1038/npp.2012.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyvell CL, Berridge KC. Intra-accumbens amphetamine increases the conditioned incentive salience of sucrose reward: enhancement of reward “wanting” without enhanced “liking” or response reinforcement. J Neurosci. 2000;20:8122–30. doi: 10.1523/JNEUROSCI.20-21-08122.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawata S, Yamaguchi T, Danjo T, Hikida T, Nakanishi S. Pathway-specific control of reward learning and its flexibility via selective dopamine receptors in the nucleus accumbens. Proc Natl Acad Sci USA. 2012;109:12764–9. doi: 10.1073/pnas.1210797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KA, Gobrogge KL, Liu Y, Wang Z. The neurobiology of pair bonding: insights from a socially monogamous rodent. Front Neuroendocrinol. 2011;32:53–69. doi: 10.1016/j.yfrne.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young LJ, Lim MM, Gingrich B, Insel TR. Cellular mechanisms of social attachment. Horm Behav. 2001;40:133–8. doi: 10.1006/hbeh.2001.1691. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat Neurosci. 2004;7:1048–54. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z, Insel TR. Neuroendocrine bases of monogamy. Trends in neurosciences. 1998;21:71–5. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
- Zald DH, Pardo JV. The neural correlates of aversive auditory stimulation. Neuroimage. 2002;16:746–53. doi: 10.1006/nimg.2002.1115. [DOI] [PubMed] [Google Scholar]
- Zhang J, Berridge KC, Aldridge JW. Computational Models of Incentive-Sensitization in Addiciton: Dynamic Limbic Transformation of Learning into Motivation. In: Ahmed BGSH, editor. Computational Neuroscience of Drug Addiction. Springer; 2012. pp. 189–204. [Google Scholar]
- Zink CF, Meyer-Lindenberg A. Human neuroimaging of oxytocin and vasopressin in social cognition. Horm Behav. 2012;61:400–9. doi: 10.1016/j.yhbeh.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]


