Abstract
Natural infection with measles virus (MeV) is initiated when the virus reaches epithelial cells in the respiratory tract, oropharynx, or conjunctivae. Human epithelial cells infected with MeV frequently show growth suppression. In this study, we investigated the possible mechanisms for this suppression. The bronchiolar epithelial cell A549 showed growth arrest in G0/G1 following MeV infection or treatment with gamma interferon (IFN-γ). IFN regulatory factor-1 (IRF-1) was upregulated during MeV infection, although A549 did not produce IFN-γ. Cells of the cervical squamous cell line SiHa persistently infected with various strains of MeV displayed slower growth than uninfected SiHa cells, although the growth rates varied depending on the MeV strain. Transfection of antisense-oriented IRF-1 cDNA released the MeV-infected SiHa cells from growth suppression. Although these infected cells did not produce IFN-γ and suppressed IFN-α/β-induced Jak1 phosphorylation, Jak1 was constitutively phosphorylated. The growth rates negatively correlated with levels of both IRF-1 expression and constitutively phosphorylated Jak1. These results indicate that MeV upregulates IRF-1 in a manner that is independent of IFN but dependent on the JAK/STAT pathway. This induction of IRF-1 appears to suppress cell growth, although the extent seems to vary among MeV strains.
Measles is a highly contagious disease characterized by a prodrome of fever, cough, coryza, and conjunctivitis, followed by Koplik's spots and a generalized maculopapular rash. Despite the availability of an effective live attenuated vaccine, measles is still a severe problem with high morbidity and mortality rates, primarily in children in developing countries. The major complications of measles are encephalitides, alveobronchiolitis, and otitis media. Encephalitides and alveobronchiolitis are the major causes of death. Natural infection with the measles virus (MeV) is initiated when the virus reaches epithelial cells in the respiratory tract, oropharynx, or conjunctivae. MeV rapidly grows in epithelial cells of the respiratory tract, translocates into regional lymph nodes, and then causes viremia and general symptoms (8, 19).
Immunosuppression is considered to be a major cause of the high morbidity and mortality rates of acute measles. The complications of measles, such as alveobronchiolitis and otitis media, are caused by secondary infections. During MeV infection, lymphocytes are unresponsive to mitogens and undergo cell growth arrest in G0/G1 (3, 16, 17, 35). This is believed to be a mechanism of immunosuppression. Naniche et al. (17) reported that wild-type MeV strains suppressed alpha/beta interferon (IFN-α/β) production in human peripheral blood mononuclear cells (PBMC), but Vero cell-adapted MeV strains (laboratory strains) strongly induced IFN-α/β. Nevertheless, the wild-type strains were more sensitive to IFN effects than laboratory strains, and the lower induction of IFN-α/β by wild-type strains resulted in efficient replication. We have also found that the growth of epithelial cells is suppressed by MeV infection. However, the IFN signal transduction system is suppressed to similar extents in epithelial cells infected with wild-type strains or laboratory strains (36; T. Okabayashi, S. Yokota, N. Yokosawa, H. Saito, and N. Fujii, submitted for publication).
IFN regulatory factor-1 (IRF-1) was initially identified as a transcriptional activator of the IFN-β gene (15). However, IRF-1 has various biological activities, and a number of genes have been reported to be IRF-1 targets (13, 23, 31, 32). The IRF-1 target genes contributing to the antiviral response are those for IFN-α/β, 2′,5′-oligoadenylate synthetase, and PKR. Those contributing to major histocompatibility complex class II molecule expression are the genes for class II transactivator and TAP-1/LMP-2. IRF-1 also induces several types of caspases and p21WAF1/CIP1, which are considered to contribute to apoptosis and antiproliferation, respectively. IRF-1 is recognized not only as a regulator of IFN-α/β genes but also as a negative regulator of cell proliferation (13, 23, 25, 31). IRF-1 mRNA is generally expressed at a low level in all cell types but is upregulated by the presence of various stimuli, such as IFN, retinoic acid, prolactin, double-stranded RNA, and virus (13). The strongest inducer is IFN-γ. The IRF-1 promoter contains an IFN-γ-activated sequence (GAS) and an NF-κB binding motif (10, 18, 20). These motifs mediate transcriptional activation by the binding of IFN-γ-activated factor (GAF) and NF-κB, respectively. GAF consists of a dimer of phosphorylated STAT-1, and STAT-1 is phosphorylated by janus kinases (JAK), which are associated with IFN receptors. This system is called the JAK/STAT pathway (29). These transcription factors synergistically activate IRF-1 expression (10, 18, 20). In addition, a retinoid-responsive element has been identified in the promoter (12). In the present study, we show that IRF-1 is upregulated by MeV infection and contributes to the growth arrest of MeV-infected human epithelial cells.
MATERIALS AND METHODS
Cell lines and viruses.
The bronchiolar carcinoma cell line A549 and the cervical squamous carcinoma cell line SiHa were obtained from the American Type Culture Collection (Manassas, Va.). MeV strains Edmonston, Hälle, Schwarz, and CAM70 were described previously (4, 6). Strains AK1 (24) and SMU were clinical isolates obtained from throat swabs of measles patients, using B95a cells and Vero cells for propagation, respectively. SiHa cell lines persistently infected with MeV were established as previously described (4, 6). Virus propagation and titer determination of MeV strains, except AK1, were performed by using Vero cells as indicator cells. In the case of strain AK1, B95a cells were used, because the strain does not adapt to Vero cells. Acute-infection experiments were performed as follows. The seeded A549 cells were precultured for 24 h. The cells were infected with MeV Hälle at a multiplicity of infection (MOI) of 1 for 1 h, washed, and then cultured under normal conditions.
Cell proliferation assay.
Cell numbers were determined with a gentian violet dye-binding assay as previously described (14). The cell proliferation rate was determined by the uptake of 5-bromodeoxyuridine (5BrdU) into DNA, using a cell proliferation enzyme-linked immunosorbent assay (ELISA) BrdU (colorimetric) kit (Roche Diagnostic, Mannheim, Germany).
Cell cycle analysis by DNA histogram.
Cells were stained with propidium iodide using a CycleTEST PLUS DNA reagent kit (BD Bioscience, San Jose, Calif.) and analyzed with a FACSCalibur flow cytometer (BD Bioscience). The results were analyzed using ModFit LT software (BD Bioscience).
Western blotting.
Rabbit anti-IRF-1 (C-20), anti-IRF-3 (FL-425), anti-STAT-1 (E-23), and anti-Jak2 (C-20) antibodies and a mouse anti-cyclin E (HE12) antibody were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Rabbit anti-Jak1pYpY1022/1023 and anti-Jak2pYpY1007/1008 antibodies were purchased from BioSource (Camarillo, Calif.). Rabbit anti-phospho-STAT1 (Tyr701) and anti-Jak1 antibodies and a mouse anti-human retinoblastoma (Rb) protein antibody were purchased from Cell Signaling (Beverly, Mass.), Upstate (Lake Placid, N.Y.), and Pharmingen, respectively. The rabbit anti-MeV V protein antibody was kindly donated by Kaoru Takeuchi and Atsushi Kato.
Preparation of total cell lysates, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blotting were carried out as previously described (37, 38). Alkaline phosphatase-conjugated anti-rabbit or anti-mouse immunoglobulin antibody (BioSource) and bromochloroindolylphosphate-Nitro Blue Tetrazolium were used as a secondary antibody and an enzyme substrate for Western blotting, respectively.
RT-PCR.
Total RNA was isolated from cells by using an RNeasy Mini Kit (Qiagen, Hilden, Germany). Reverse transcription (RT)-PCR was carried out with a OneStep RT-PCR kit (Qiagen). The specificities of the detected bands were confirmed by performing PCR without an RT reaction. That the RT-PCR was quantitative was confirmed by the linearity of determination curves with various amounts of RNA. The following primer sets were used to detect each mRNA; IRF-1 sense (5′-CCAGAGAAAAGAAAGAAAGTCG-3′) and antisense (5′-CACATGGCGACAGTGCTGG-3′), IFN-α2 sense (5′-CCTGATGAAGGAGGACTCCATT-3′) and antisense (5′-AAAAAGGTGAGCTGGCATACG-3′), IFN-α4 sense (5′-GAAGAGACTCCCCTGATGAATGT-3′) and antisense (5′-GCACAGGTATACACCAAGCTTCTTC-3′), IFN-β sense (5′-GTCTCCTCCAAATTGCTCTC-3′) and antisense (5′-ACAGGAGCTTCTGACACTGA-3′), IFN-γ sense (5′-ATGAAATATACAAGTTATATCTTGGCTTT-3′) and antisense (5′-GATGCTCTTCGACCTCGAAACAGCAT-3′), and IL-8 sense (5′-ACTTAGATGTCAGTGCATAAAGAC-3′) and antisense (5′-TTATGAATTCTCAGCCCTCTTCAA-3′). The primer sets for MeV hemagglutinin and GAPDH (glyceraldehyde-3-phosphodehydrogenase) mRNAs were described previously (36).
ELISA.
The amounts of IFN-γ and interleukin-8 (IL-8) in the culture supernatant were determined with an ELISA development kit for human IFN-γ and human IL-8 (Genzyme-Techne, Minneapolis, Minn.), respectively.
ELDIA.
Nuclear extracts were prepared using NE-PER nuclear and cytoplasmic extraction reagents (Pierce, Rockford, Ill.). The DNA binding activities of transcription factors in cells were determined by an enzyme-linked DNA-protein interaction assay (ELDIA) essentially according to a previously described method (2). Briefly, a biotin-labeled double-stranded GAS oligonucleotide (5′-GCCTGATTTCCCCGAAATGACGGC-3′) derived from the IRF-1 promoter was immobilized on a streptavidin-coated microtiter plate. After the wells were blocked, nuclear extract was dispensed into the wells, and then a mouse anti-STAT-1 antibody (BD Bioscience) and a horseradish peroxidase-conjugated anti-mouse antibody were incubated. The specific binding was detected by developing the plate with 3,3′,5,5′-tetramethylbenzidine solution (BioSource). Binding activity was expressed as absorbance at 450 nm (A450). DNA binding of NF-κB p65 and p50 was determined using a BD Mercury Transfactor NF-κB p65 kit and an NF-κB p50 kit (BD Bioscience), respectively. The specificities of binding to various DNA motifs were confirmed by inhibition experiments using unlabeled double-stranded competitor oligonucleotides at a concentration of 200 ng/well. The following oligonucleotides were used as competitors (wild) and their controls (mutant). IRF-1 wild (5′-GCCTGATTTCCCCGAAATGACGGC-3′) and mutant (5′-GCCTGATTTCCCCGCCATGACGGC-3′), NF-κB p65 wild (5′-AGCTTGGGGTATTTCCAGCCG-3′), and NF-κB p50 wild (5′-GCCATGGGGGGATCCCCGGGC-3′) and mutant (5′-GCCATGGGCCGATCCCCGGGC-3′).
Luciferase reporter gene assay.
The reporter gene assay was carried out as described previously (36, 38). The pGAS-Luc vector, which contains a GAS enhancer element and a firefly luciferase gene (Stratagene, La Jolla, Calif.), and the pRL-TK vector (Promega, Madison, Wis.), which was used as an internal control for transfection efficacy, were cotransfected into SiHa cells. After cultivation for 24 h, MeV Hälle was infected at an MOI of 1 for 24 h or IFN-γ was treated for 12 h. The cells were lysed, and luciferase activities were determined using the Dual-Luciferase Reporter Assay System (Promega).
IRF-1 expression plasmids.
IRF-1 cDNA was obtained by RT-PCR from the RNA from IFN-γ-treated SiHa cells. The primer set for preparing full-length IRF-1 cDNA was sense, 5′-GAATTCGCTCCTGCAGCAGAGCCAACA-3′, and antisense, 5′-GGATCCAGAGGAATAAGAGGGGCCCAG-3′. The resulting PCR fragment was inserted into a pTargeT vector (Promega). The plasmids carrying sense- and antisense-oriented IRF1 cDNAs were cloned and used for further study.
Transient transfection of IRF-1 and antisense IRF-1 plasmids.
The plasmid carrying sense- or antisense-oriented IRF1 cDNA was transfected into uninfected or MeV-infected SiHa cells by using a SuperFect reagent (Qiagen) according to the manufacturer's instruction manual unless otherwise specified. Twenty-four hours after transfection, the cell proliferation rate and the level of IRF-1 protein expression were determined by a 5BrdU uptake assay and Western blotting, respectively.
Neutralization of IFN during cell culture.
A sheep anti-human leukocyte IFN-α antibody and a rabbit anti-human IFN-β antibody were purchased from Chemicon (Temecula, Calif.) and Pepro Tech EC (London, United Kingdom), respectively. For the IFN-neutralizing culture, 10 μl of anti-IFN-α antibody/ml and 5 μg of IFN-β antibody/ml were added to the cell culture medium. For the control experiment, the same amounts of an unrelated immunoglobulin G derived from unimmunized sheep and rabbit sera were added to the culture media.
Inhibition of NF-κB activation.
Ammonium pyrrolidinedithiocarbamate (PDTC), an NF-κB inhibitor, was purchased from Sigma-Aldrich (St. Louis, Mo.). A549 cells were infected with strain Hälle at an MOI of 1 and cultured for 1 or 2 days with or without 50 μM PDTC. The protein levels of IRF-1 and IL-8 were determined by Western blotting of the cell lysate and ELISA of the culture supernatant, respectively. mRNA levels were determined by RT-PCR.
RESULTS
MeV causes growth arrest in A549 cells and upregulates IRF-1.
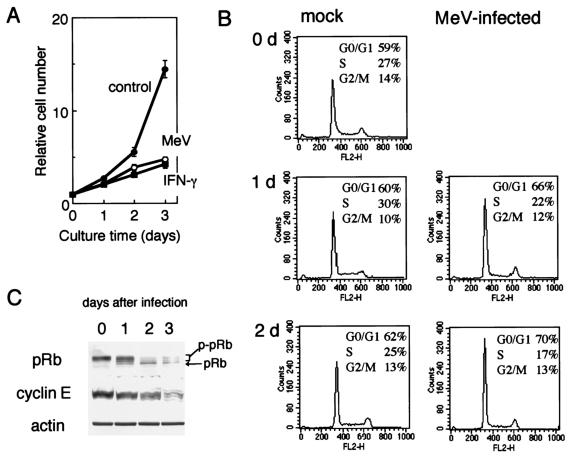
We first tested the Hälle strain of MeV in A549 cells; however, similar results were obtained with other MeV strains, such as Edmonston and SMU (data not shown). A549 cells showed severe growth arrest during infection with MeV strain Hälle (Fig. 1A). Similar growth arrest was observed after IFN-γ treatment of the cells. During these periods, no significant cytopathic effect was observed; cytopathic effect appeared after 4 or 5 days of infection. The cells were arrested in G0/G1 phase, as deduced from the DNA histogram derived from a flow cytometer (Fig. 1B), and the phosphorylated form of Rb protein and the total protein level of cyclin E dramatically decreased (Fig. 1C). Unphosphorylated Rb protein acts as a negative regulator of cell proliferation and is primarily found in resting cells. The Rb protein is phosphorylated in late G1, and thus, phosphorylated Rb protein is found in proliferating cells (22). Cyclin E is a G1 cyclin involved in G1/S phase transition in association with cyclin-dependent kinase 2 (27).
FIG. 1.
Growth arrest of A549 cells during infection with MeV strain Hälle. (A) Growth of A549 cells under normal conditions (control), infected with MeV (MOI, 1) (MeV), or treated with IFN-γ (1,000 U/ml) (IFN-γ), as determined by cell numbers. Cell numbers were determined by the gentian violet dye binding assay. The error bars represent standard deviations. (B) Cell cycle analysis by flow cytometry of A549 cells infected with MeV after propidium iodide staining. mock, uninfected control; d, day(s). (C) Western blotting analysis of Rb protein and cyclin E in A549 cells infected with MeV. pRb and p-pRb indicate unphosphorylated and phosphorylated forms of Rb protein, respectively. Actin was used as a control for protein loading.
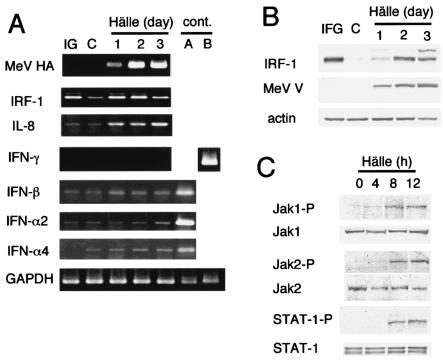
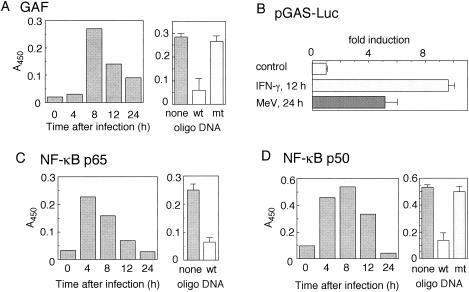
We examined changes in the levels of several cytokines and their related factors during MeV infection in A549 cells (Fig. 2). We found that IRF-1 was upregulated by MeV infection at both the mRNA (Fig. 2A) and protein (Fig. 2B) levels. However, IFN-γ, which is the most potent IRF-1 inducer, was not found in an ELISA of the culture supernatant of MeV-infected A549 cells (the detectable level is <10 pg/ml) (data not shown), and IFN-γ mRNA was also undetectable. The mRNAs of IFN-α/βs, namely, IFN-β, IFN-α2, and IFN-α4, were slightly elevated. We next examined the activation status of the JAK-STAT pathway. Phosphorylation of Jak1, Jak2, and STAT-1, which contribute to the IFN-γ signaling pathway (29), was observed 8 h after MeV infection (Fig. 2C), whereas phosphorylation of Tyk2 and STAT-2, which contribute to IFN-α/β signaling in addition to Jak1 and STAT-1 (29), was not observed (data not shown). In agreement with this result, GAF, a dimer of phosphorylated STAT-1, was formed and bound to GAS, an oligonucleotide derived from the IRF-1 promoter, as determined by an ELDIA (Fig. 3A). The binding was inhibited by a competitor oligonucleotide but not by its irrelevant mutant. The results from a reporter gene assay using luciferase as a reporter indicated that GAS-dependent transcription was activated by MeV infection (Fig. 3B). The reporter activity induced by MeV infection was ∼50% of IFN-γ-induced activity. This indicated that the activated GAF induced by MeV was functional. Binding of NF-κB p50 and NF-κB p65 to their respective binding consensus motifs was also detected, and the bindings were inhibited by respective competitor oligonucleotides (Fig. 3C and D). The result suggested that NF-κB was activated in A549 cells during MeV infection. This is consistent with the induction of IL-8 mRNA during MeV infection (Fig. 2A). In conclusion, both GAF and NF-κB were activated in A549 cells by MeV infection, and the GAF was formed and activated in an IFN-γ-independent manner.
FIG. 2.
Changes in mRNA and protein levels of IRF-1, IL-8, and IFNs and activation state of the JAK/STAT pathway in A549 cells during MeV strain Hälle infection. (A) mRNA levels of MeV hemagglutinin (HA), IRF-1, IL-8, IFN-α2, IFN-α4, IFN-β, and IFN-γ determined by semiquantitative RT-PCR. C, untreated control. IG, treated with IFN-γ (1,000 IU/ml; 24 h); cont., positive control for RT-PCR experiments; A, RNA from TPA-treated (20 nM; 24 h) U937 cells persistently infected with Hälle; B, RNA from SMT-1 cells (a human T-cell line persistently infected with human T-lymphotropic virus type 1 [5]). GAPDH was used as a control. (B) Protein levels of MeV V protein and IRF-1 by Western blotting. Actin was used as a control. (C) Phosphorylation status of Jak1, Jak2, and STAT-1. Phosphorylation of each protein was examined by Western blotting using antibodies specific for the respective phosphorylated forms. Their total protein levels in cells were determined as a control.
FIG. 3.
Activation of GAF and NF-κB in MeV-infected A549 cells determined by ELDIA and luciferase reporter gene assay. (A, C, and D) ELDIA. Nuclear extracts were prepared from A549 cells infected with MeV strain Hälle for various times. The extracts (30 μg of protein) were applied to a microplate coated with a double-stranded oligonucleotide containing the GAF (A), NF-κB p65 (C), or NF-κB p50 (D) binding motif. The GAS-GAF binding motif is derived from the promoter of the IRF-1 gene. The specific binding of each transcription factor was determined by subsequent incubation with a specific primary antibody, a horseradish peroxidase-labeled secondary antibody, and tetramethylbenzidine solution. The binding activity was expressed as the A450. The specificity of the binding was confirmed by inhibition experiments with the respective double-stranded competitor oligonucleotides (wild type [wt]) or irrelevant mutant oligonucleotides (mt) (right). (B) GAS activation determined by a reporter gene assay in A549 cells. The reporter plasmids pGAS-Luc and pRL-TK, as internal controls for transfection efficacy, were transfected into A549 cells. The transiently expressed transfectants were infected with MeV at an MOI of 1 for 24 h or treated with 1,000 IU of IFN-γ/ml for 12 h. The cell lysates were assayed for luciferase (Luc) activity. Experiments were performed in quadruplicate. The results are expressed as induction (n-fold) relative to the value obtained from experiments without treatment. The error bars represent standard deviations.
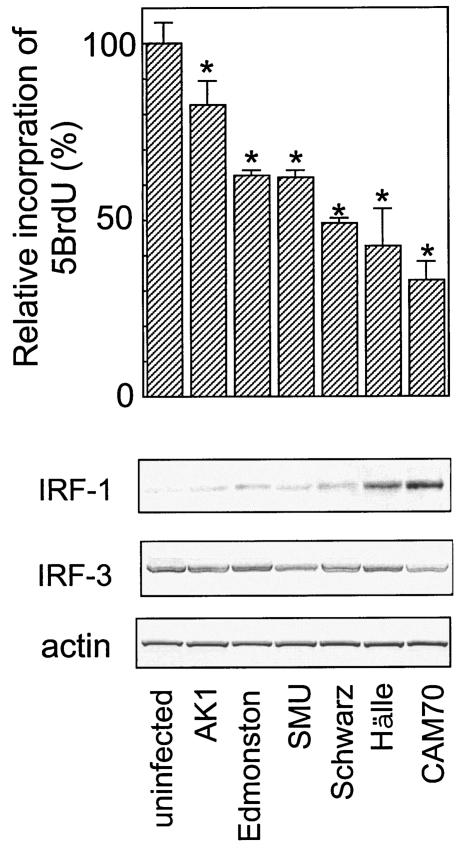
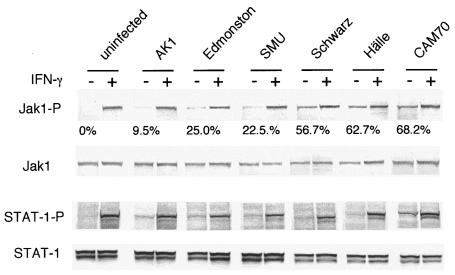
Upregulation of IRF-1 and constitutive activation of Jak1 in epithelial cells persistently infected with MeV.
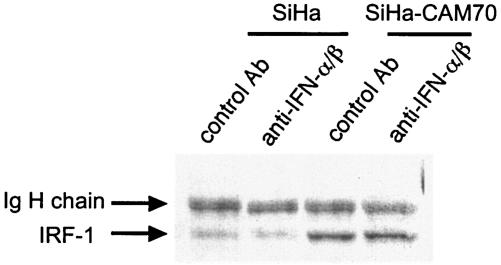
We established SiHa cells persistently infected with six different MeV strains as described previously (4, 36). We examined the growth rates of these infected SiHa cells with a 5BrdU uptake assay. The cells showed various growth rates (Fig. 4), which were highly negatively correlated with the basal levels of IRF-1 protein expression but not with the expression level and phosphorylation status of IRF-3, which is detected as slower-migrating bands. Jak1 and STAT-1 were constitutively phosphorylated to various extents in the MeV-infected SiHa cells (Fig. 5). The degree of constitutive phosphorylation of Jak1 was significantly correlated with the expression of IRF-1 and with the growth suppression of the cells (Fig. 4 and 5). We also examined the expression of IFNs. Neither uninfected nor infected SiHa cells produced IFN-γ, as determined from mRNA by RT-PCR or from IFN-γ secreted into the supernatant by ELISA (data not shown). However, IFN-β mRNA was detected in both uninfected and infected SiHa cells, and IFN-α2 and IFN-α4 mRNAs were detected in MeV-infected cells, with the exception of cells infected with the Schwarz strain (data not shown). IFN-α/β can also upregulate IRF-1, although larger amounts of IFN-α/β are required for a response comparable to that induced by IFN-γ (28). To examine whether IFN-α and IFN-β produced by infected and uninfected cells contribute to the upregulation of IRF-1 by an autocrine mechanism, we neutralized IFN-α/β during cell culture using anti-IFN-α and anti-IFN-β antibodies (Fig. 6). Under these conditions, induction of 2′,5′-oligoadenylate synthetase mRNA by treatment with IFN-α and IFN-β (100 IU/ml each) was almost completely suppressed (data not shown). The expression levels of IRF-1 in both SiHa and SiHa-CAM70 cells were not significantly altered by the addition of anti-IFN-α/β antibodies (Fig. 6). The results indicated that SiHa cells persistently infected with MeV showed upregulation of IRF-1 in an IFN-α/β- as well as IFN-γ-independent manner.
FIG. 4.
Cell growth rates and IRF-1 expression in SiHa cells persistently infected with various strains of MeV. (Top) The growth rate was determined by incorporation of 5BrdU. The value of uninfected SiHa cells was set at 100%. Experiments were performed in quadruplicate, and the mean value plus standard deviation was calculated. *, significant (P < 0.01) difference from the value in the uninfected cells. (Bottom) Western blot analyses of IRF-1, IRF-3, and actin. Actin was a control for protein loading.
FIG. 5.
Phosphorylation status of Jak1 and STAT-1 in SiHa cells persistently infected with various strains of MeV. Cell lysates from SiHa cells and SiHa cells infected with various MeV strains were applied to Western blotting using antibodies specific for the phosphorylated form of Jak1 or STAT-1. Each cell line was treated with IFN-γ (1,000 U/ml for 15 min) (+) as a positive control for phosphorylation of Jak1 and STAT-1. The protein levels of total Jak1 and STAT-1 were determined as a control for protein loading. Constitutive Jak1 phosphorylation in MeV-infected cells was determined and expressed as a percentage relative to the value obtained from the same cell line treated with IFN-γ.
FIG. 6.
Effects of neutralizing antibodies against IFN-α and IFN-β on IRF-1 expression in SiHa cells and SiHa cells persistently infected with MeV CAM70. SiHa and SiHa-CAM70 cells were cultured for 2 days in the presence of a mixture of sheep anti-human IFN-α antibody and rabbit anti-human IFN-β antibody or the same amounts of unrelated sheep and rabbit antibodies (control Ab). Cell lysates derived from the treated cells were analyzed by Western blotting using an anti-IRF-1 antibody. Immunoglobulin (Ig) H chain indicates the immunoglobulin heavy-chain band derived from antibodies added to the culture medium.
IRF-1 plays a large role in the growth arrest of epithelial cells.
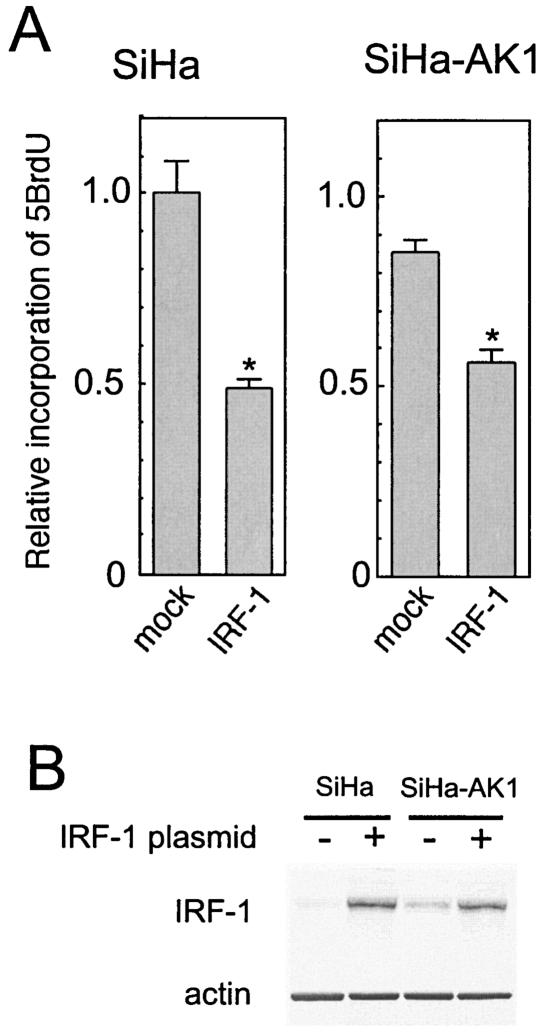
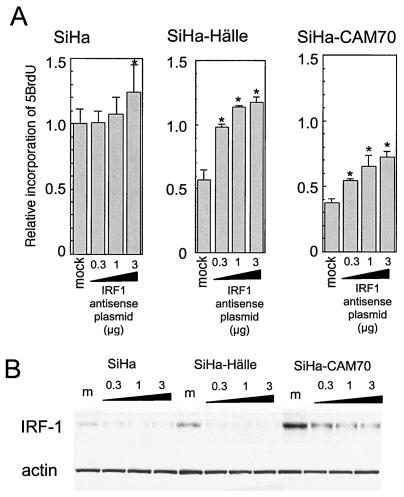
To confirm the role of the MeV-upregulated IRF-1 in the arrest of cell growth, IRF-1 was overexpressed or downregulated by transfection with expression plasmids carrying IRF-1 cDNA in the sense or antisense orientation, respectively. Antisense-oriented IRF-1 cDNA has been reported to suppress IRF-1 expression (21, 26). Overexpression of IRF-1 significantly suppressed the growth rates of uninfected and AK1-infected SiHa cells (Fig. 7). Transfection of the plasmid carrying the antisense-oriented cDNA into SiHa cells infected with MeV Hälle and CAM70 reduced the levels of IRF-1 and released the cells from growth arrest in a dose-dependent manner (Fig. 8).
FIG. 7.
Effects of overexpression of IRF-1 on the cell growth rate in SiHa cells and SiHa cells infected with MeV strain AK1. (A) The growth rate was determined by incorporation of 5BrdU. The value of uninfected SiHa cells transfected with control plasmid was set at 1. Experiments were performed in quadruplicate, and the mean value plus standard deviation was calculated. *, significant (P < 0.01) difference from mock-transfected control. (B) Western blot analysis of IRF-1 in SiHa and AK1-transfected SiHa cells transfected with IRF-1 expression plasmid (+) or control plasmid (−).
FIG. 8.
Effects of transfection with antisense IRF-1 cDNA on cell growth rates in SiHa cells and SiHa cells infected with MeV strain Hälle or CAM70. (A) The growth rates of SiHa and virus-infected SiHa cells transfected with various amounts of plasmid carrying antisense-oriented IRF-1 cDNA were determined by incorporation of 5BrdU. The value of uninfected SiHa cells transfected with control plasmid was set at 1. Experiments were performed in quadruplicate, and the mean value plus standard deviation was calculated. *, significant (P < 0.01) difference from mock-transfected control. (B) Western blotting analysis of IRF-1 and actin (as a control). m, mock.
IRF-1 induction is not mediated by NF-κB.
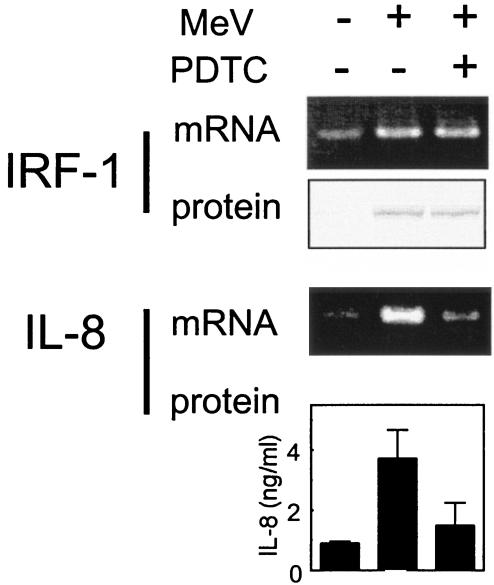
The IRF-1 promoter contains binding sequences for GAF and NF-κB (10, 18, 20), and MeV induced both GAF and NF-κB (Fig. 3). To clarify whether NF-κB contributes to the induction of IRF-1, we examined the effect of an NF-κB inhibitor, PDTC. A549 cells were infected with MeV strain Hälle with or without 50 μM PDTC. The mRNA and protein levels of IRF-1 and IL-8 were determined after 1 or 2 days of culture (Fig. 9). The IL-8 gene, a typical NF-κB-dependent gene, was used as a control. Both IL-8 protein secreted into the culture supernatant and IL-8 mRNA were markedly decreased by the addition of PDTC. In contrast, neither the mRNA nor the protein level of IRF-1 was significantly changed by PDTC. The results suggested that activated NF-κB induced by MeV infection contributed less to the induction of IRF-1 than GAF.
FIG. 9.
Effects of an NF-κB inhibitor, PDTC, on IRF-1 expression in A549 cells infected with MeV. A549 cells were infected with MeV Hälle at an MOI of 1 with (+) or without (−) 50 μM PDTC. After 1 day of infection, the mRNA levels of IRF-1 and IL-8 were determined by RT-PCR. After 2 days of infection, the protein levels of IRF-1 were determined by Western blotting, and IL-8 in the culture supernatant was measured by ELISA. The error bars represent standard deviations.
DISCUSSION
In this study, we found that MeV infection caused epithelial cells to be arrested in G0/G1. This growth arrest was suggested to occur via the induction of IRF-1. tenOever et al. showed that MeV infection induces IRF-1 and -7 and activates IRF-3 (33). However, they did not discuss the biological significance of IRF-1 induction. IRF-1 is known as a tumor suppresser and a negative regulator of cell proliferation (9, 13, 23, 25, 31, 32). Several reports mention that SiHa cells showed growth inhibition in response to increasing IRF-1 expression after IFN-α/β or retinoic acid treatment (1, 7). In our study, MeV upregulated IRF-1, but not IRF-3. IRF-1 was not significantly induced in cells infected with a strain of mumps virus or a strain of human parainfluenza virus type 2, which also belong to the Paramyxovirinae, either during acute infection in A549 cells or persistent infection in SiHa cells. The cells infected with these viruses also did not show cell growth suppression (data not shown). Furthermore, the abilities of MeV strains to induce IRF-1 varied, and the levels of IRF-1 correlated well with the degree of growth arrest in SiHa cells persistently infected with various MeV strains (Fig. 4). In addition, exogenous overexpression of IRF-1 inhibited cell growth (Fig. 7), and transfection of IRF-1 antisense cDNA released MeV-infected SiHa cells from growth arrest (Fig. 8). These lines of evidence suggest that upregulation of IRF-1 due to MeV infection is a prominent contributor to cell growth arrest. IRF-1 is induced by various stimuli (13, 23, 32). NF-κB and GAF are known to be transactivators of the IRF-1 gene (10, 18, 20). We examined which of these might be the mechanism of IRF-1 expression and MeV infection. Both GAF and NF-κB were activated by MeV infection in A549 cells, and GAF was formed and activated in an IFN-γ-independent manner. However, NF-κB is not likely to contribute to IRF-1 expression in MeV-infected epithelial cells, because an NF-κB inhibitor, PDTC, did not affect the induction (Fig. 9). In contrast, we observed that phosphorylation of Jak1, Jak2, and STAT-1 and the binding of GAF to the GAS element derived from the IRF-1 promoter occurred following MeV infection. Constitutive phosphorylation of Jak1 was observed in persistently infected SiHa cells, and the degree of phosphorylation varied among MeV strains and was highly correlated with the level of IRF-1 expression. These results indicate that GAF is a major transactivator of MeV-induced IRF-1 expression. However, the activation mechanism of GAF seems to be unusual in that SiHa cells persistently infected with MeV showed upregulation of IRF-1 independently of IFN-γ and IFN-α/β. IFN-γ was produced by neither uninfected nor infected cells. Although IFN-α/β was slightly upregulated by MeV infection (Fig. 2), the JAK/STAT signaling pathway through the IFN-α/β receptor is shut off in MeV-infected SiHa cells by the action of the viral V protein (36) (Okabayashi et al., submitted). All SiHa cells infected with various MeV strains show the inhibition of IFN-α/β-induced Jak1 phosphorylation to similar extents (Okabayashi et al., submitted). Furthermore, we confirmed that neutralizing anti-IFN-α/β antibodies did not affect IRF-1 induction by MeV (Fig. 6). These lines of evidence indicate that the activation of the JAK/STAT pathway, which ordinarily occurs through IFN-γ stimuli, occurs in an IFN-independent manner during MeV infection, and then the resulting GAF induces IRF-1. MeV should suppress IFN-α/β signal-related Jak1 phosphorylation, and conversely, activate IFN-γ signal-related Jak1 phosphorylation. Consistent with this, a previous study showed that the IFN-γ response is slightly enhanced in MeV-infected cells compared with uninfected cells in some cases (36). The mechanism of these opposing effects of MeV on Jak1 is still unknown and might depend on the environment of the Jak1 molecules in the cells. For example, the mode of association of Jak1 and various cytokine receptors, including the IFN-α receptor β chain and IFN-γ receptor α chain, are variable ways and might affect the downstream effects of Jak1 (34).
The degree of IRF-1-mediated growth arrest varied depending on the virus strains. Interestingly, the wild-type strain AK1, which was isolated and passsaged in B95a cells and did not adapt to Vero cells (24), induced the least growth arrest among the MeV strains tested. The wild-type strains are considered to be highly pathogenic because they can induce clinical symptoms resembling human measles in experimental cynomolgus and squirrel monkey infection models (11, 30). On the other hand, SiHa cells infected with CAM70, which is a vaccine strain, showed the most severe growth arrest and produced extremely small amounts of virus (5.2 × 101 PFU/ml) in the culture supernatant compared to cells infected with strains other than CAM70 and AK1 (2.4 × 103 to 8.8 × 103 PFU/ml) (data not shown). Naniche et al. (17) reported that wild-type strains replicated more efficiently in human PBMC than laboratory strains, including Edmonston. They indicated that PBMC produced IFN-α/β by infection with laboratory strains but not with wild-type strains, and the induced IFN caused the lower efficacy of viral replication. In our study, the IFN signaling pathway was suppressed in epithelial cells by MeV, including wild-type strains and laboratory strains (36) (Okabayashi et al., submitted). Furthermore, suppression of proliferation in the infected cells is IFN independent. In natural MeV infection, the initial targets of the virus are host epithelial cells, followed by dendritic cells in the respiratory tract. After passing through these initial barriers, MeV reaches regional lymph nodes, where the virus rapidly replicates. Finally, a large amount of virus spreads hematogenically throughout the body. IRF-1-mediated growth suppression of epithelial cells may lead to the virus being confined to the initial barrier by suppression of virus replication. Thus, it is considered to be a host strategy to contain MeV at the initial stages of infection.
Acknowledgments
We thank Hiroyuki Saito for providing MeV strain AK1 and Kaoru Takeuchi and Atsushi Kato for providing the anti-MeV V protein antibody.
REFERENCES
- 1.Arany, I., W. E. Whitehead, K. J. Grattendick, I. A. Ember, and S. K. Tyring. 2002. Suppression of growth by all-trans retinoic acid requires prolonged induction of interferon regulatory factor 1 in cervical squamous carcinoma (SiHa) cells. Clin. Diagn. Lab. Immunol. 9:1102-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benotmane, A. M., M. F. Hoylaerts, D. Collen, and A. Belayew. 1997. Nonisotopic quantitative analysis of protein-DNA interactions at equilibrium. Anal. Biochem. 250:181-185. [DOI] [PubMed] [Google Scholar]
- 3.Engelking, O., L. M. Fedorov, R. Lilischkis, V. ter Meulen, and S. Schneider-Schaulies. 1999. Measles virus-induced immunosuppression in vitro is associated with deregulation of G1 cell cycle control proteins. J. Gen. Virol. 80:1599-1608. [DOI] [PubMed] [Google Scholar]
- 4.Fujii, N., K. Kimura, T. Murakami, T. Indoh, S. Ishida, K. Fujinaga, and K. Oguma. 1990. Suppression of interferon-induced oligo-2′,5′-adenylate synthetase induction in persistent infection. J. Gen. Virol. 71:3071-3074. [DOI] [PubMed] [Google Scholar]
- 5.Fujii, N., K. W. Kwon, T. Yashiki, K. Kimura, E. Isogai, H. Isogai, S. Sekiguchi, and K. Oguma. 1992. Oligo-2′,5′-adenylate synthetase activity in cells persistently infected with human T-lymphotropic virus type I (HTLV-I). Microbiol. Immunol. 36:425-429. [DOI] [PubMed] [Google Scholar]
- 6.Fujii, N., K. Oguma, K. Kimura, T. Yamashita, S. Ishida, K. Fujinaga, and T. Yashiki. 1988. Oligo-2′,5′-adenylate synthetase activity in K562 cell lines persistently infected with measles or mumps virus. J. Gen. Virol. 69:2085-2091. [DOI] [PubMed] [Google Scholar]
- 7.Giandomenico, V., F. Lancillotti, G. Fiorucci, Z. A. Percario, R. Rivabene, W. Malorni, E. Affabris, and G. Romeo. 1997. Retinoic acid and IFN inhibition of cell proliferation is associated with apoptosis in squamous carcinoma cell lines: role of IRF-1 and TGase II-dependent pathways. Cell Growth Differ. 8:91-100. [PubMed] [Google Scholar]
- 8.Griffin, D. E. 2001. Measles virus, p. 1401-1441. In D. M. Knipe and P. M. Howley (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, Pa.
- 9.Harada, H., T. Taniguchi, and N. Tanaka. 1998. The role of interferon regulatory factors in the interferon system and cell growth control. Biochimie 80:641-650. [DOI] [PubMed] [Google Scholar]
- 10.Imanishi, D., K. Yamamoto, H. Tsushima, Y. Miyazaki, K. Kuriyama, M. Tomonaga, and T. Matsuyama. 2000. Identification of a novel cytokine response element in the human IFN regulatory factor-1 gene promoter. J. Immunol. 165:3907-3916. [DOI] [PubMed] [Google Scholar]
- 11.Kobune, F., H. Takahashi, K. Terao, T. Ohkawa, Y. Ami, Y. Suzaki, N. Nagata, H. Sakata, K. Yamanouchi, and C. Kai. 1996. Nonhuman primate models of measles. Lab. Anim. Sci. 46:315-320. [PubMed] [Google Scholar]
- 12.Kolla, V., X. Weihua, and D. V. Kalvakolanu. 1997. Modulation of interferon action by retinoids. Induction of murine STAT1 gene expression by retinoic acid. J. Biol. Chem. 272:9742-9748. [DOI] [PubMed] [Google Scholar]
- 13.Kröger, A., M. Köster, K. Schroeder, H. Hauser, and P. P. Mueller. 2002. Activities of IRF-1. J. Interferon Cytokine Res. 22:5-14. [DOI] [PubMed] [Google Scholar]
- 14.Kubota, T., N. Yokosawa, S. Yokota, and N. Fujii. 2001. C terminal CYS-RICH region of mumps virus structural V protein correlates with block of interferon α and γ signal transduction pathway through decrease of STAT 1-α. Biochem. Biophys. Res. Commun. 283:255-259. [DOI] [PubMed] [Google Scholar]
- 15.Miyamoto, M., T. Fujita, Y. Kimura, M. Maruyama, H. Harada, Y. Sudo, T. Miyata, and T. Taniguchi. 1988. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-β gene regulatory elements. Cell 54:903-913. [DOI] [PubMed] [Google Scholar]
- 16.Naniche, D., S. I. Reed, and M. B. Oldstone. 1999. Cell cycle arrest during measles virus infection: a G0-like block leads to suppression of retinoblastoma protein expression. J. Virol. 73:1894-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naniche, D., A. Yeh, D. Eto, M. Manchester, R. M. Friedman, and M. B. Oldstone. 2000. Evasion of host defenses by measles virus: wild-type measles virus infection interferes with induction of alpha/beta interferon production. J. Virol. 74:7478-7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ohmori, Y., R. D. Schreiber, and T. A. Hamilton. 1997. Synergy between interferon-gamma and tumor necrosis factor-alpha in transcriptional activation is mediated by cooperation between signal transducer and activator of transcription 1 and nuclear factor κB. J. Biol. Chem. 272:14899-14907. [DOI] [PubMed] [Google Scholar]
- 19.Oxman, M. N. 2002. Measles virus, p. 791-828. In D. D. Richman, R. J. Whitley, and F. G. Hayden (ed.), Clinical virology, 2nd ed. ASM Press, Washington, D.C.
- 20.Pine, R. 1997. Convergence of TNFα and IFNγ signalling pathways through synergistic induction of IRF-1/ISGF-2 is mediated by a composite GAS/κB promoter element. Nucleic Acids Res. 25:4346-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reis, L. F., H. Harada, J. D. Wolchok, T. Taniguchi, and J. Vilcek. 1992. Critical role of a common transcription factor, IRF-1, in the regulation of IFN-β and IFN-inducible genes. EMBO J. 11:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riley, D. J., E. Y. Lee, and W. H. Lee. 1994. The retinoblastoma protein: more than a tumor suppressor. Annu. Rev. Cell Biol. 10:1-29. [DOI] [PubMed] [Google Scholar]
- 23.Romeo, G., G. Fiorucci, M. V. Chiantore, Z. A. Percario, S. Vannucchi, and E. Affabris. 2002. IRF-1 as a negative regulator of cell proliferation. J. Interferon Cytokine Res. 22:39-47. [DOI] [PubMed] [Google Scholar]
- 24.Saito, H., H. Sato, M. Abe, S. Harata, K. Amano, T. Suto, and M. Morita. 1992. Isolation and characterization of the measles virus strains with low hemagglutination activity. Intervirology 33:57-60. [DOI] [PubMed] [Google Scholar]
- 25.Sato, M., T. Taniguchi, and N. Tanaka. 2001. The interferon system and interferon regulatory factor transcription factors—studies from gene knockout mice. Cytokine Growth Factor Rev. 12:133-142. [DOI] [PubMed] [Google Scholar]
- 26.Sato, T., C. Selleri, N. S. Young, and J. P. Maciejewski. 1997. Inhibition of interferon regulatory factor-1 expression results in predominance of cell growth stimulatory effects of interferon-γ due to phosphorylation of Stat1 and Stat3. Blood 90:4749-4758. [PubMed] [Google Scholar]
- 27.Sherr, C. J. 1994. G1 phase progression: cycling on cue. Cell 79:551-555. [DOI] [PubMed] [Google Scholar]
- 28.Sims, S. H., Y. Cha, M. F. Romine, P. Q. Gao, K. Gottlieb, and A. B. Deisseroth. 1993. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol. Cell. Biol. 13:690-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 30.Takeuchi, K., M. Takeda, and N. Miyajima. 2002. Toward understanding the pathogenicity of wild-type measles virus by reverse genetics. Jpn. J. Infect. Dis. 55:143-149. [PubMed] [Google Scholar]
- 31.Taniguchi, T., M. S. Lamphier, and N. Tanaka. 1997. IRF-1: the transcription factor linking the interferon response and oncogenesis. Biochim. Biophys. Acta 1333:M9-M17. [DOI] [PubMed] [Google Scholar]
- 32.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623-655. [DOI] [PubMed] [Google Scholar]
- 33.tenOever, B. R., M. J. Servant, N. Grandvaux, R. Lin, and J. Hiscott. 2002. Recognition of the measles virus nucleocapsid as a mechanism of IRF-3 activation. J. Virol. 76:3659-3669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Usacheva, A., S. Kotenko, M. M. Witte, and O. R. Colamonici. 2002. Two distinct domains within the N-terminal region of Janus kinase 1 interact with cytokine receptors. J. Immunol. 169:1302-1308. [DOI] [PubMed] [Google Scholar]
- 35.Wang, M., J. E. Libbey, I. Tsunoda, and R. S. Fujinami. 2003. Modulation of immune system function by measles virus infection. II. Infection of B cells leads to the production of a soluble factor that arrests uninfected B cells in G0/G1. Viral Immunol. 16:45-55. [DOI] [PubMed] [Google Scholar]
- 36.Yokota, S., H. Saito, T. Kubota, N. Yokosawa, K. Amano, and N. Fujii. 2003. Measles virus suppresses interferon-α signaling pathway: suppression of Jak1 phosphorylation and association of viral accessory proteins, C and V, with interferon-α receptor complex. Virology 306:135-146. [DOI] [PubMed] [Google Scholar]
- 37.Yokota, S., H. Yanagi, T. Yura, and H. Kubota. 2000. Upregulation of cytosolic chaperonin CCT subunits during recovery from chemical stress that causes accumulation of unfolded proteins. Eur. J. Biochem. 267:1658-1664. [DOI] [PubMed] [Google Scholar]
- 38.Yokota, S., N. Yokosawa, T. Kubota, T. Suzutani, I. Yoshida, S. Miura, K. Jimbow, and N. Fujii. 2001. Herpes simplex virus type 1 suppresses the interferon signaling pathway by inhibiting phosphorylation of STATs and janus kinases during an early infection stage. Virology 286:119-124. [DOI] [PubMed] [Google Scholar]