Abstract
Pyrazinamide (PZA) plays the important role in shortening the tuberculosis treatment period and in treating MDR-TB. Phenotypic PZA susceptibility methods are limited because they require specialized acidified media, which increases costs and complexity. In this study we developed a genotypic high resolution melt (HRM) analysis technique to detect pncA mutations associated with PZA resistant M. tuberculosis. Seven overlapping primer pairs were designed to cover the entire pncA gene and upstream regions. Each gene segment was individually amplified by real-time PCR followed by HRM analysis. The assay was evaluated on 98 clinical M. tuberculosis isolates (41 PZA susceptible by MGIT method, 55 PZA resistant, 2 undetermined). HRM was 94% concordant to full-length sequencing results, with most discrepancies attributable to mixed populations per HRM or transversions. Sequencing and HRM yielded 82% and 84% concordance, respectively, to phenotypic PZA susceptibilities by MGIT, with most discrepancies attributable to isolates with wild-type pncA but phenotypic PZA resistance. This HRM technique is a simple and high-throughput method for screening clinical M. tuberculosis samples for PZA resistance.
Keywords: Pyrazinamide, High resolution melt, genotypic, MDR-TB
INTRODUCTION
Pyrazinamide (PZA) is a first-line drug for the treatment of tuberculosis. Its use allows shortening of the treatment period from 9 to 6 months and it is also widely included in regimens for MDR-Tb.1–4 The importance of PZA susceptibility testing has increased due to the recognition of PZA monoresistant strains of M. tuberculosis and the need for improved MDR-TB regimens, since some new TB drugs such as bedaquiline appear to benefit from PZA.5, 6 Unfortunately conventional susceptibility testing for PZA is limited by the requirement for acidic media, which inhibits the growth of M. tuberculosis.7 The radiometric BACTEC 460 system is no longer available and the newer non-radiometric BACTEC MGIT 960 system requires 8 to 12 days, has potential for cross contamination, and false resistance has been reported.8–10
PZA is a prodrug that requires activation to its active metabolite pyrazinoic acid by pyrazinamidase, which is encoded by the pncA gene.11 A correlation of approximately 85% has been observed between phenotypic PZA resistance and pncA mutation.12–14 A number of molecular methods have been developed for the detection of other TB drug resistance-associated mutations including the line probe assays MTBDRplus, MTBDRsl, INNO-LiPA Rif.TB, and Xpert MTB/RIF.15–17 These probe based assay are suitable for commonly known mutations in hotspot regions of specific genes. pncA mutations associated with PZA resistance, however, appear to be dispersed throughout the 561 base pair gene and an upstream promoter-containing region, several hundred base pairs overall, which makes the development of probe-based methods complex.12–14 A gel based PCR–single strand conformation polymorphism method has been used as has a temperature-mediated heteroduplex method.14, 18 There are also line probe assays which use multiple (e.g., 49) probes for reverse hybridization after nested PCR.19, 20 Such methods are laborious and prone to amplicon contamination. Closed systems are now feasible, including melt-based assays with sloppy molecular beacons or lights-on/lights-off probes21–23 yet such strategies for pncA would still require complex design and utilization of dozens of probes. Direct sequencing of pncA amplicon is in our view the best genotypic strategy, however this requires a costly apparatus.
High-resolution melt (HRM) curve analysis is a simple technique. After endpoint PCR with a fluorescent dye, PCR amplicons are heated and fluorescence loss is monitored in real-time. Sequence variants are detected by differing melt profiles from reference DNA without the need for specific probes. HRM has been widely utilized for a variety of applications.24–26 In the tuberculosis arena, HRM has been used for detecting rifampin, isoniazid, streptomycin, and fluoroquinolone resistant M. tuberculosis.27–31 In this study, we describe an HRM technique to detect pncA mutations and compare these data to sequencing and phenotypic PZA susceptibilities. This method, which involves simultaneous amplification of 7 overlapping fragments, detects pncA mutation within 2 hours using only a real-time PCR machine.
MATERIALS AND METHODS
Mycobacterial strains and culture conditions
Mycobacterial strains used in this study included clinical isolates and M. tuberculosis H37Rv (ATCC 27294). Tb isolates were cultured on Lowenstein-Jensen medium at 37°C for 2–3 weeks. Cell suspensions were prepared in Middlebrook 7H9 (M7H9) broth supplemented with Middlebrook OADC enrichment (Difco, Livonia, MI, USA) and adjusted to 0.5 McFarland for MGIT PZA susceptibility assay and DNA extraction. A total of 98 clinical isolates including 82 from Thailand (Department of Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok), 15 from Tanzania (from Kilimanjaro Clinical Research Institute, Moshi, Kilimanjaro, Tanzania), and 1 from University of Virginia were used. Isolates were chosen on the basis of having been tested for PZA susceptibility by MGIT (2 did not grow in MGIT) towards a goal of approximately 50 susceptible and resistant isolates for assay development purposes. All work was approved by the University of Virginia Institutional Biosafety Committee and Human Investigation Committees.
MGIT pyrazinamide susceptibility assay
PZA susceptibility tests were carried out in MGIT PZA medium (BD, Franklin Lakes, NJ, USA) according to manufacturer’s protocol. Briefly, a 0.5 McFarland suspension was diluted 1:5 and 1:50 in sterile distilled water and dilutions of 1:50 were inoculated into MGIT PZA medium plus supplement without drug, dilution 1:5 were inoculated into MGIT PZA medium plus supplement with 100 μg/ml PZA drug and incubated in MGIT instrument at 37°C. Results were read automatically within 14 days after inoculation of media. M. tuberculosis H37Rv, susceptible to PZA, was used for quality control. All mixed population isolates were tested for PZA susceptibility twice for confirmation.
Sequencing of the pncA gene
DNA was isolated from 2–3 weeks of Tb culture. Briefly, 200 μl of 0.5 McFarland suspension in sterile distilled water was transferred to 2 ml screw cap tube, heat inactivated at 100°C for 30 min, and DNA extracted using DNeasy Blood & Tissue kit (Qiagen Inc, Valencia, CA, USA) according to manufacturer’s protocol. The pncA gene was amplified by PCR using the forward primer 5′-GGTCATGTTCGCGATCGTCG-3′ and reverse primer 5′-ACAGTTCATCCCGGTTCGGC-3′ of Campbell et al32. Each 25-μl PCR mixture contained 12.5 μl HotStarTaq master mix (Qiagen Inc, Valencia, CA, USA), 0.15 μl of each forward and reverse 50 μM primers, 7.2 μl nuclease free water, and 5 μl of genomic DNA. PCR was performed on a MyCycler (Bio-Rad, Hercules, CA, USA) with initial denaturation at 95°C for 15 min followed by 35 cycles of denaturation at 95°C for 30 sec, annealing at 60°C for 30 sec, and extension at 72°C for 30 sec, with a final extension at 72°C for 7 min. PCR products were analyzed on 2% agarose-gels, verified PCR products were purified using MinElute® PCR Purification Kit (Qiagen Inc, Valencia, CA, USA), measured spectrophotometrically, diluted with nuclease free water, mixed with primers and submitted to GeneWiz (GeneWiz Inc; South Plainfield, NJ, USA) for DNA sequencing.
Real-time PCR and High Resolution Melt Analysis
Seven pairs of overlapping primer were designed using Primer3 to cover upstream and the entire 561 bp pncA open reading frame (Table 1). Each primer pair (0.15 μl of each forward and reverse primer, 50 μM stock) was utilized in singleplex amplifications, with 25 μl PCR mixture containing 12.5 μl Type-it HRM PCR mastermix (Qiagen Inc, Valencia, CA, USA), 7.2 μl nuclease free water, and 5 μl of genomic DNA. Real time PCR was performed on Rotor Gene Q (Qiagen Inc, Valencia, CA, USA) including an initial denaturation step at 95°C for 10 min, followed by 40 cycles of denaturation at 95°C for 10 sec, annealing at 55°C for 30 sec, and extension at 72°C for 10 sec, followed by final extension step at 72°C for 2 min. For high resolution melt fluorescence was acquired using the green channel (Excitation 470 ± 10nm, Detection 510 ± 5 nm). First, heteroduplex formation occurred for 30 sec at 50°C, followed by melt with 0.1°C increments for 2 seconds each from 84–94°C for pncA1, pncA2, and pncA7 primer pairs, from 81–91°C for pncA3, pncA5, and pncA6 primer pairs, and 79–89°C for pncA4 primer pairs. The reference M. tuberculosis H37Rv was included in each run as a wild-type positive control and nuclease free water was used for negative control.
TABLE 1.
Primer sequences used to amplify pncA gene of M. tuberculosis in this study.
| Segment | Primers | Position | Sequences | Product size (bp) |
|---|---|---|---|---|
| 1 | pncA1-F | (−80)–(−61) | 5′-GGCGTCATGGACCCTATATC-3′ | 217 |
| pncA1-R | 137-119 | 5′-GCCACGACGTGATGGTAGT-3′ | ||
| 2 | pncA2-F | 21–39 | 5′-CGACGTGCAGAACGACTTC-3′ | 179 |
| pncA2-R | 199-180 | 5′-ACGAGGAATAGTCCGGTGTG-3′ | ||
| 3 | pncA3-F | 136–155 | 5′-GCAACCAAGGACTTCCACAT-3′ | 166 |
| pncA3-R | 301-281 | 5′-CGGTGTAGGCACCCTTGTAG-3′ | ||
| 4 | pncA4-F | 236–254 | 5′-CGGACTTCCATCCCAGTCT-3′ | 103 |
| pncA4-R | 338-320 | 5′-CCGTTCTCGTCGACTCCTT-3′ | ||
| 5 | pncA5-F | 266–285 | 5′-CAATCGAGGCGGTGTTCTAC-3′ | 150 |
| pncA5-R | 415-395 | 5′-CACAATGATCGGTGGCAATA-3′ | ||
| 6 | pncA6-F | 373–392 | 5′-GTCGATGAGGTCGATGTGGT-3′ | 118 |
| pncA6-R | 490-472 | 5′-ACACACCCGCTGTCAGGT-3′ | ||
| 7 | pncA7-F | 396–415 | 5′-TATTGCCACCGATCATTGTG-3′ | 167 |
| pncA7-R | 562-543 | 5′-ATCAGGAGCTGCAAACCAAC-3′ |
High resolution melt analysis
Melt curve data were analyzed and normalized with the Rotor-Gene Q software. The software analyzes the difference in the shape and temperature shifts of the melt curve of the test sample from that of M. tuberculosis H37Rv. HRM curves were analyzed by selecting two normalization regions, one occurring prior to the melt of the amplicon and one following complete separation of the two strands. Normalization region 1 was set at 87–87.5 °C and normalization region 2 was set at 91.5–92 °C for pncA1, pncA2, and pncA7 primer pairs. For pncA3, pncA5, and pncA6, the normalization regions were set at 84–84.5 °C and 88.5–89 °C for normalization region 1 and 2 respectively, and 82–82.5°C and 86.5–87 °C for pncA4. The reference M. tuberculosis H37Rv was set as a wild-type genotype control and percent confidence was set at 70% for all amplicons where ≥ 70 % similarity to M. tuberculosis H37Rv was automatically categorized as wild-type and < 70% confidence was categorized as variation.
RESULTS
Comparison of HRM analysis with Sanger sequencing
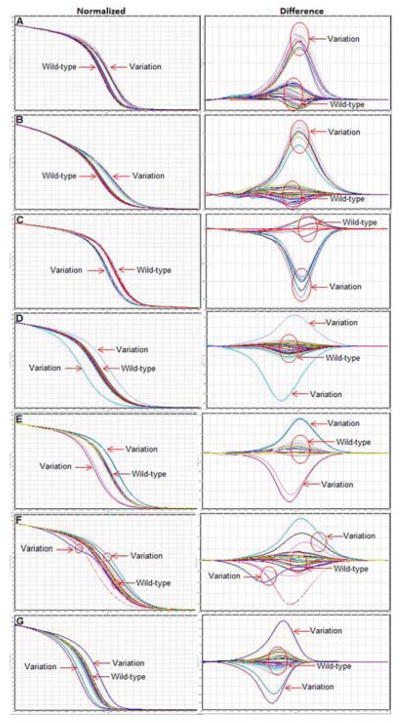
HRM analysis of the pncA gene was performed on DNA from 98 M. tuberculosis clinical isolates using 7 overlapping fragments. Representative normalized melt curves of pncA1, pncA2, pncA3, pncA4, pncA5, pncA6, and pncA7 are shown in Figure 1A, B, C, D, E, F, and G respectively. Each line indicates the melt curve profile for an individual sample. The normalized graph shows melt temperature shifts versus wild-type amplicon, with leftward variation indicating a lower melt temperature (e.g., C→A, C→T, G→A, G→T) and a rightward variation indicating a higher melt temperature (e.g., A→C, T→C, A→G, T→G). The difference graph uses the same data but plots the negative first derivative (−dF/dt) on the Y axis. We set the HRM software to define variation as <70% similarity with wild-type sequence.
FIG. 1.
High resolution melt analysis of the entire pncA gene and upstream regions. Normalized (left panel) and difference graphs (right panel) of 7 amplicons are shown: pncA1 (A), pncA2 (B), pncA3(C), pncA4 (D), pncA5 (E), pncA6 (F), and pncA7 (G). Each line indicates the melt curve profile for an individual sample. In the difference plot, the melt curve profile of M. tuberculosis H37Rv was compared with the curve profiles of all other samples. The baseline represents M. tuberculosis H37Rv and other wild-type isolates. Isolates with “Variation” or mutant profiles, as determined by HRM software as <70% similar to wild-type, are shown.
The performance of HRM analysis was compared with Sanger sequencing as shown in Table 2. Interrogation of the entire pncA1-pncA7 fragment by HRM yielded 94% (92/98) concordance with sequencing. The HRM analysis of pncA1 yielded 100% concordance with sequencing of that region, while pncA2, pncA3, pncA4, pncA5, pncA6, and pncA7 yielded 88%, 94%, 95%, 95%, 96%, and 97% respectively. The majority of discrepancies were sequence mutations that fell in the primer regions and were not detected by HRM, hence the rationale for the incorporation of overlapping fragments (e.g., mutations in the forward or reverse primer regions of pncA2 could also be detected via the pncA1 or pncA3 amplicons, respectively). Of the 6 discrepancies, 2 were mutations detected by sequencing categorized as wild-type by HRM, specifically a C to G transversion at position 169 and a G deletion at position 290. The 4 remaining discrepancies were wild-type by sequencing but considered variation by HRM analysis. Further examination these 4 isolates showed slight variation curves on HRM, localized to pncA3 segment for isolate 1 and pncA5 segment for isolate 2, 3, and 4. Sequencing revealed mixed traces within these fragments, with both wild-type and mutant sequences present (Figure 2), suggesting that HRM was in fact accurate.
TABLE 2.
Performance of High resolution melt analysis comparing to Sanger sequencing.
| pncA gene segment | HRM | Sequencing (n)
|
Accuracy (%) | |
|---|---|---|---|---|
| No mutation | Mutatione | |||
| Entire pncA1–7 |
Wild-type | 50 | 2cd | 93.9 |
| Variation | 4a | 42 | ||
| pncA1 | Wild-type | 82 | 0 | 100 |
| Variation | 0 | 16 | ||
| pncA2 | Wild-type | 76 | 12bc | 87.8 |
| Variation | 0 | 10 | ||
| pncA3 | Wild-type | 83 | 5bc | 93.9 |
| Variation | 1a | 9 | ||
| pncA4 | Wild-type | 91 | 3bd | 94.9 |
| Variation | 2a | 2 | ||
| pncA5 | Wild-type | 87 | 2bd | 94.9 |
| Variation | 3a | 6 | ||
| pncA6 | Wild-type | 87 | 3b | 95.9 |
| Variation | 1a | 7 | ||
| pncA7 | Wild-type | 86 | 3b | 96.9 |
| Variation | 0 | 9 | ||
mixed population with wild-type predominant in sequencing trace file,
mutation at HRM primer region,
transversion C to G,
G deletion,
the total number of isolates with mutations identified in the (entire) pncA amplicon (n=44) is less than the number of isolates with mutations identified in the 7 pncA amplicons because these 7 amplicons overlap and will double-count mutations in overlapping regions. For example 8 isolates contained mutation at position 92 which was detected by both pncA1 primer pair ((−80) to137) and pncA2 primer pair (21 to 199).
FIG. 2.
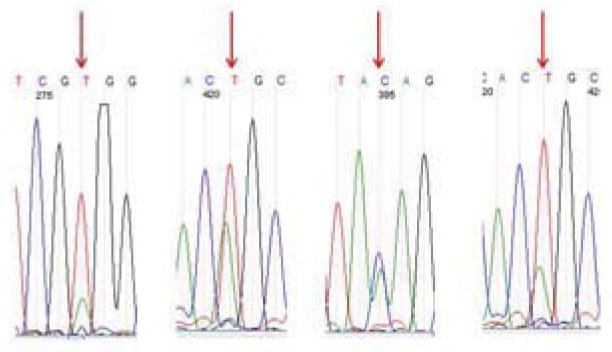
Discrepancies by sequencing and HRM. HRM showed variation for 4 samples whose sequencing revealed mixed population of sequences (arrows).
Correlation of genotypic and phenotypic pyrazinamide resistance
The genotypic sequencing and HRM analysis were compared with phenotypic MGIT PZA susceptibility results as shown in Table 3. Two isolates did not grow in the MGIT control media thus phenotypic susceptibility results were not available. Sequencing of pncA was concordant to phenotypic PZA susceptibility testing for 79/96 isolates (82%). Among discrepancies, 15/17 were PZA resistant while sequencing detected no mutation, and 2 were PZA susceptible with sequencing mutations, both of which were silent mutations at Ser65Ser.
TABLE 3.
Assay performance of Sequencing and HRM compared to MGIT PZA susceptibility test.
| Sequencing | MGIT PZA susceptibility test (n)
|
Accuracy (%) | HRM | MGIT PZA susceptibility test (n)
|
Accuracy (%) | ||
|---|---|---|---|---|---|---|---|
| Susceptible | Resistant | Susceptible | Resistant | ||||
| No mutation | 39 | 15a | 82.3 | Wild-type | 39 | 13b | 84.4 |
| Mutation | 2c | 40 | Variation | 2c | 42 | ||
11 isolates had no mutation and remaining isolates had mixed populations but were wild-type predominant
11 isolates were no-mutation, 1 isolate was a C to G transversion, 1 isolate was a 290 G deletion,
2 isolates had silent mutation Ser65Ser
HRM analysis was concordant with phenotypic PZA susceptibility for 81/96 isolates (84%). Among discrepancies, similar to sequencing, 13/15 were PZA resistant while HRM detected no variation. The 2 PZA susceptible isolates with the silent mutation Ser65Ser were falsely categorized as variation by HRM. Overall, therefore, there were 2 fewer discrepancies with HRM than sequencing. This owed to detection of PZA resistant isolates that had mixed wild-type/variation pncA by HRM but were purely wild-type by sequencing.
The pncA mutation profiles
The 31 different pncA mutations found in this study are shown in Supplemental Table 1. Nine are new mutations not reported in previous studies.33 The mutations found in M. tuberculosis isolates obtained from Thailand, Tanzania, and Virginia were different from each other. pncA mutations were observed in 35/82 (43%) Thailand isolates, the most frequent being Ile31Thr (T92C; n=8). Asp12Asn, Tyr103Stop, Val139Gly, and Thr142Met were found in 2 isolates each, and another 19 mutation profiles were found in 1 isolate of each. For Tanzanian isolates, 12/15 (80%) revealed pncA mutation: 5 were silent mutations at Ser65Ser, 2 with Glu111Stop, 2 with Ser65Ser/Val128Gly, and 1 each with Asp49Gly, Val169Ala, Ser179Ile. The one isolate from Virginia had a position 290 G deletion.
DISCUSSION
The current liquid-based culture systems for PZA susceptibility are complicated by their requirement for acidified media and cost not to mention turnaround time. These are particular problems for resource-limited areas, which may not have adequate susceptibility testing facilities yet may have a high burden of MDR-TB. The WHO currently recommends universal inclusion of PZA in MDR-TB regimens, however the quality of evidence for this recommendation is low, and is largely driven by the poor reproducibility of the conventional PZA method.1, 34 In such places, a rapid genotypic-based method would be highly desirable. Unfortunately, whole length sequencing requires costly sequencing equipment. Outsourcing the sequencing to a commercial vendor is a less costly option (around 8–12 $/sample in the US) but these services are generally located in richer countries, thus would require expensive shipping from resource-limited settings. By contrast, real-time PCR platforms with HRM capabilities are becoming fairly widespread and the reagent cost of this assay is around 0.8 $/reaction or 6 $/sample. As such, this HRM method may be useful and provide substantial clinical value.
This HRM yielded 94% concordance to standard genotypic sequencing. Discrepancies were often mixed populations detected by HRM but not sequencing. The 82–84% correlation with phenotypic PZA susceptibility was expected, as this degree of correlation has been observed in larger studies.32 The major discrepancies between HRM (or sequencing) and phenotypic susceptibility results were due to 11 PZA-resistant isolates that were wild-type by pncA sequencing or HRM. We repeated the MGIT method on these isolates and only 2 remained resistant and 9 were susceptible on re-assay. Such potential false resistance by MGIT method has been reported.8–10 The two other isolates presumably have resistance mechanisms beyond pncA such as efflux or mutations in other genes such as rpsA.35, 36 We did note limitations of our HRM assay, specifically the inability to detect transversions, silent mutations, or a small deletions.
To our knowledge, this is the first English language report of HRM for analyzing pncA mutations. One previous article has been published in the Chinese literature which utilized the Roche LightCycler 480.37 Of note we evaluated this assay on other cyclers (Bio-Rad CFX and ABI Viia7) but found the Rotor-gene curves most easy to interpret (data not shown). Some HRM assays for Isoniazid or Rifampin include spiking of specimens with a susceptible strain such as H37Rv – we considered this but decided against it because this obliterates the ability to detect heteroresistance, a feature that was seen in some of our isolates and that we feel is relevant.
Ultimately, the utility of any pncA-based assay will depend on whether ~85% accuracy versus phenotypic susceptibility is acceptable. This answer will require extensive clinical study, but for the time being we think it likely is. We report the exact pncA mutations in the Supplemental table because growing the genotypic-phenotypic database is important for this effort of understanding the extent that genotypic information can inform phenotypic results.
CONCLUSIONS
We report a high resolution melt curve pncA genotypic susceptibility method to determine PZA susceptibility. The method can be used at any laboratory that has the Rotor Gene real-time PCR platform to rapidly screen PZA susceptibility.
Supplementary Material
Acknowledgments
FUNDING
This work was supported by National Institutes of Health grant R01 AI093358 (to E.H.).
Footnotes
ETHICAL APPROVAL
All work was approved by the University of Virginia Institutional Biosafety Committee and Human Investigation Committees and was conducted in compliance with the Declaration of Helsinki.
COMPETING INTERESTS: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Heifets L, Lindholm-Levy P. Pyrazinamide sterilizing activity in vitro against semidormant Mycobacterium tuberculosis bacterial populations. The American review of respiratory disease. 1992;145:1223–1225. doi: 10.1164/ajrccm/145.5.1223. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez-Albisua I, Vidal ML, Joya-Verde G, del Castillo F, de Jose MI, Garcia-Hortelano J. Tolerance of pyrazinamide in short course chemotherapy for pulmonary tuberculosis in children. The Pediatric infectious disease journal. 1997;16:760–763. doi: 10.1097/00006454-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Clinical trial of six-month and four-month regimens of chemotherapy in the treatment of pulmonary tuberculosis: the results up to 30 months. Tubercle. 1981;62:95–102. doi: 10.1016/0041-3879(81)90016-7. [DOI] [PubMed] [Google Scholar]
- 4.Snider DE, Jr, Rogowski J, Zierski M, Bek E, Long MW. Successful intermittent treatment of smear-positive pulmonary tuberculosis in six months: a cooperative study in Poland. The American review of respiratory disease. 1982;125:265–267. doi: 10.1164/arrd.1982.125.2.265. [DOI] [PubMed] [Google Scholar]
- 5.Hannan MM, Desmond EP, Morlock GP, Mazurek GH, Crawford JT. Pyrazinamide-monoresistant Mycobacterium tuberculosis in the United States. Journal of clinical microbiology. 2001;39:647–650. doi: 10.1128/Jcm.39.2.647-650.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tasneen R, Li SY, Peloquin CA, Taylor D, Williams KN, Andries K, Mdluli KE, Nuermberger EL. Sterilizing activity of novel TMC207- and PA-824-containing regimens in a murine model of tuberculosis. Antimicrob Agents Chemother. 2011;55:5485–5492. doi: 10.1128/AAC.05293-11. AAC.05293-11 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stottmeier KD, Beam RE, Kubica GP. Determination of drug susceptibility of mycobacteria to pyrazinamide in 7H10 agar. The American review of respiratory disease. 1967;96:1072–1075. doi: 10.1164/arrd.1967.96.5.1072. [DOI] [PubMed] [Google Scholar]
- 8.Scarparo C, Ricordi P, Ruggiero G, Piccoli P. Evaluation of the fully automated BACTEC MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide, streptomycin, isoniazid, rifampin, and ethambutol and comparison with the radiometric BACTEC 460TB method. Journal of clinical microbiology. 2004;42:1109–1114. doi: 10.1128/Jcm.42.3.1109-1114.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chedore P, Bertucci L, Wolfe J, Sharma M, Jamieson F. Potential for Erroneous Results Indicating Resistance When Using the Bactec MGIT 960 System for Testing Susceptibility of Mycobacterium tuberculosis to Pyrazinamide. Journal of clinical microbiology. 2010;48:300–301. doi: 10.1128/Jcm.01775-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Piersimoni C, Mustazzolu A, Giannoni F, Bornigia S, Gherardi G, Fattorini L. Prevention of False Resistance Results Obtained in Testing the Susceptibility of Mycobacterium tuberculosis to Pyrazinamide with the Bactec MGIT 960 System Using a Reduced Inoculum. Journal of clinical microbiology. 2013;51:291–294. doi: 10.1128/Jcm.01838-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung D. 2003;7:6–21. [PubMed] [Google Scholar]
- 12.Morlock GP, Crawford JT, Butler WR, Brim SE, Sikes D, Mazurek GH, Woodley CL, Cooksey RC. Phenotypic characterization of pncA mutants of Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 2000;44:2291–2295. doi: 10.1128/Aac.44.9.2291-2295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sreevatsan S, Pan X, Zhang Y, Kreiswirth BN, Musser JM. Mutations associated with pyrazinamide resistance in pncA of Mycobacterium tuberculosis complex organisms. Antimicrobial agents and chemotherapy. 1997;41:636–640. doi: 10.1128/aac.41.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scorpio A, LindholmLevy P, Heifets L, Gilman R, Siddiqi S, Cynamon M, Zhang Y. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 1997;41:540–543. doi: 10.1128/aac.41.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brossier F, Veziris N, Jarlier V, Sougakoff W. Performance of MTBDR plus for detecting high/low levels of Mycobacterium tuberculosis resistance to isoniazid. The international journal of tuberculosis and lung disease: the official journal of the International Union against Tuberculosis and Lung Disease. 2009;13:260–265. [PubMed] [Google Scholar]
- 16.Ajbani K, Nikam C, Kazi M, Gray C, Boehme C, Balan K, Shetty A, Rodrigues C. Evaluation of Genotype MTBDRsl Assay to Detect Drug Resistance Associated with Fluoroquinolones, Aminoglycosides and Ethambutol on Clinical Sediments. PloS one. 2012;7 doi: 10.1371/journal.pone.0049433. ARTN e49433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jureen P, Werngren J, Hoffner SE. Evaluation of the line probe assay (LiPA) for rapid detection of rifampicin resistance in Mycobacterium tuberculosis. Tuberculosis. 2004;84:311–316. doi: 10.1016/j.tube.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Mohamed AM, Bastola DR, Morlock GP, Cooksey RC, Hinrichs SH. Temperature-mediated Heteroduplex analysis for detection of pncA mutations associated with pyrazinamide resistance and differentiation between mycobacterium tuberculosis and mycobacterium bovis by denaturing high-performance liquid chromatography. Journal of clinical microbiology. 2004;42:1016–1023. doi: 10.1128/Jcm.42.3.1016-1023.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sekiguchi J, Nakamura T, Miyoshi-Akiyama T, Kirikae F, Kobayashi I, Augustynowicz-Kopec E, Zwolska Z, Morita K, Suetake T, Yoshida H, Kato S, Mori T, Kirikae T. Development and evaluation of a line probe assay for rapid identification of pncA mutations in pyrazinamide-resistant mycobacterium tuberculosis strains. Journal of clinical microbiology. 2007;45:2802–2807. doi: 10.1128/JCM.00352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitarai S, Kato S, Ogata H, Aono A, Chikamatsu K, Mizuno K, Toyota E, Sejimo A, Suzuki K, Yoshida S, Saito T, Moriya A, Fujita A, Sato S, Matsumoto T, Ano H, Suetake T, Kondo Y, Kirikae T, Mori T. Comprehensive multicenter evaluation of a new line probe assay kit for identification of Mycobacterium species and detection of drug-resistant Mycobacterium tuberculosis. Journal of clinical microbiology. 2012;50:884–890. doi: 10.1128/JCM.05638-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chakravorty S, Kothari H, Aladegbami B, Cho EJ, Lee JS, Roh SS, Kim H, Kwak H, Lee EG, Hwang SH, Banada PP, Safi H, Via LE, Cho SN, Barry CE, Alland D. Rapid, High-Throughput Detection of Rifampin Resistance and Heteroresistance in Mycobacterium tuberculosis by Use of Sloppy Molecular Beacon Melting Temperature Coding. Journal of clinical microbiology. 2012;50:2194–2202. doi: 10.1128/Jcm.00143-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chakravorty S, Aladegbami B, Thoms K, Lee JS, Lee EG, Rajan V, Cho EJ, Kim H, Kwak H, Kurepina N, Cho SN, Kreiswirth B, Via LE, Barry CE, Alland D. Rapid Detection of Fluoroquinolone-Resistant and Heteroresistant Mycobacterium tuberculosis by Use of Sloppy Molecular Beacons and Dual Melting-Temperature Codes in a Real-Time PCR Assay. Journal of clinical microbiology. 2011;49:932–940. doi: 10.1128/Jcm.02271-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice JE, Reis AH, Rice LM, Carver-Brown RK, Wangh LJ. Fluorescent signatures for variable DNA sequences. Nucleic Acids Res. 2012;40 doi: 10.1093/nar/gks731. ARTN e164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slany M, Vanerkova M, Nemcova E, Zaloudikova B, Ruzicka F, Freiberger T. Differentiation of Staphylococcus spp. by high-resolution melting analysis. Can J Microbiol. 2010;56:1040–1049. doi: 10.1139/W10-091. [DOI] [PubMed] [Google Scholar]
- 25.Merchant-Patel S, Blackall PJ, Templeton J, Price EP, Tong SYC, Huygens F, Giffard PM. Campylobacter jejuni and Campylobacter coli Genotyping by High-Resolution Melting Analysis of a flaA Fragment. Appl Environ Microb. 2010;76:493–499. doi: 10.1128/Aem.01164-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovanda A, Poljak M. Real-time polymerase chain reaction assay based on high-resolution melting analysis for the determination of the rs12979860 polymorphism involved in hepatitis C treatment response. Journal of virological methods. 2011;175:125–128. doi: 10.1016/j.jviromet.2011.04.018. [DOI] [PubMed] [Google Scholar]
- 27.Choi GE, Lee SM, Yi J, Hwang SH, Kim HH, Lee EY, Cho EH, Kim JH, Kim HJ, Chang CL. High-resolution melting curve analysis for rapid detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis clinical isolates. Journal of clinical microbiology. 2010;48:3893–3898. doi: 10.1128/JCM.00396-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramirez MV, Cowart KC, Campbell PJ, Morlock GP, Sikes D, Winchell JM, Posey JE. Rapid detection of multidrug-resistant Mycobacterium tuberculosis by use of real-time PCR and high-resolution melt analysis. Journal of clinical microbiology. 2010;48:4003–4009. doi: 10.1128/JCM.00812-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Kong F, Wang Q, Li C, Zhang J, Gilbert GL. Rapid detection of isoniazid, rifampin, and ofloxacin resistance in Mycobacterium tuberculosis clinical isolates using high-resolution melting analysis. Journal of clinical microbiology. 2011;49:3450–3457. doi: 10.1128/JCM.01068-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee AS, Ong DC, Wong JC, Siu GK, Yam WC. High-resolution melting analysis for the rapid detection of fluoroquinolone and streptomycin resistance in Mycobacterium tuberculosis. PloS one. 2012;7:e31934. doi: 10.1371/journal.pone.0031934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang F, Shen H, Guan M, Wang Y, Feng Y, Weng X, Wang H, Zhang W. High-resolution melting facilitates mutation screening of rpsL gene associated with streptomycin resistance in Mycobacterium tuberculosis. Microbiological research. 2011;166:121–128. doi: 10.1016/j.micres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Campbell PJ, Morlock GP, Sikes RD, Dalton TL, Metchock B, Starks AM, Hooks DP, Cowan LS, Plikaytis BB, Posey JE. Molecular detection of mutations associated with first- and second-line drug resistance compared with conventional drug susceptibility testing of Mycobacterium tuberculosis. Antimicrobial agents and chemotherapy. 2011;55:2032–2041. doi: 10.1128/AAC.01550-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gicquel CP-AaB. The contribution of molecular biology in diagnosing tuberculosis and detecting antibiotic resistance. www.chlaep.org.uy/descargas/curso_tb.../molecular_biology_tb%20.pdf.
- 34.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis - 2011 update. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 35.Zimic M, Fuentes P, Gilman RH, Gutierrez AH, Kirwan D, Sheen P. Pyrazinoic acid efflux rate in Mycobacterium tuberculosis is a better proxy of pyrazinamide resistance. Tuberculosis. 2012;92:84–91. doi: 10.1016/j.tube.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi W, Zhang X, Jiang X, Yuan H, Lee JS, Barry CE, 3rd, Wang H, Zhang W, Zhang Y. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. Science. 2011;333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong CY, Wang F, Liu XL. Detection of pncA mutation associated with pyrazinamide resistance in Mycobacterium tuberculosis by high-resolution melting cure analysis. Zhonghua jie he he hu xi za zhi = Zhonghua jiehe he huxi zazhi = Chinese journal of tuberculosis and respiratory diseases. 2013;36:198–201. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.