Abstract
Background
Alloreactive memory T cells prevent costimulatory blockade-induced heart graft survival in mice, but whether and how preexisting autoreactive T cells affect solid organ transplants under these conditions is unknown.
Methods
We tested the impact of preexisting cardiac myosin (CM)-specific immunity on murine heart transplant recipients treated with donor specific transfusion (DST) plus anti-CD154 mAb MR1.
Results
Pre-immunization with CM but not control ovalbumin abrogated the graft prolonging effects of DST/MR1, whether administered 2 weeks or >6 weeks prior to transplantation. Adoptive transfer of spleen cells from CM-immunized mice into naïve recipients had similar effects. CM-specific immunity did not cross-react with donor antigens and pre-immunization with CM had no impact on the survival or histology of DST/MR1 treated syngeneic heart grafts, the latter indicating that persistent autoimmunity is insufficient to cause rejection in the context of costimulatory blockade. We observed that the CM-pre-immunized mice produced higher frequencies of donor-reactive T cells with higher ratios of CD8+/CD4+Foxp3+ cells, suggesting that the autoreactive memory T cells provide help for activation of alloreactive T cells despite the costimulatory blockade.
Conclusions
These mechanistic insights linking auto- and alloimmunity in a model of murine heart transplantation have clinical relevance to the known association between autoimmunity and an elevated risk of acute and chronic heart transplant injury in humans.
Keywords: autoimmunity, heart transplantation, T cells, graft survival
Introduction
Primed and memory T cells differ from naïve T cells in that the former a) are present at higher frequency, b) have lower/different costimulatory requirements, c) are capable of more rapidly engaging effector functions, d) have wider trafficking patterns, and e) are resistant to many immunosuppressant medications (1–3). These features facilitate protection against recurrent pathogen infections but are detrimental in the context of transplantation. Multiple murine studies using varied systems from a number of laboratories showed that CD4+ and/or CD8+ donor-reactive memory T cells accelerate allograft rejection and prevent costimulatory blockade-induced allograft tolerance(4–10), regardless of whether they were originally primed through exposure to donor antigens or primed in response to pathogens that cross react with donor antigens (heterologous immunity). The concepts derived from these murine studies apply to non-human primates (11, 12) and humans (13–18). Human kidney allograft recipients with the highest frequencies of pre-transplant donor-reactive T cells have an elevated risk of developing posttransplant rejection episodes and have worse posttransplant allograft function compared to those without donor reactive T cells (13, 15, 17).
Autoimmunity can contribute to primary organ failure and organ failure due to non-immune etiologies has been associated with “bystander” activation of organ specific, autoreactive T cells and production of autoantibodies (19–31). Regardless of the etiology, autoimmunity present prior to transplantation has the potential to recur following re-exposure to the autoantigen after transplantation and thereby, participates in allograft injury (32–34). Associative evidence in human transplant recipients by our group, among others, indicates strong associations between autoimmunity and posttransplant injury in human transplant recipients (22, 25, 35–37).
Posttransplant reactivation of autoreactive memory T cells has been hypothesized to impede tolerance induction to islet transplants, although mechanisms remain obscure (21, 38–40). Autoreactive memory CD4+ T cells can recognize and reactivate following exposure to graft-derived autoantigens presented by recipient APCs (analogous to CD4+ T cells responding through the “indirect” allorecognition pathway) despite costimulatory blockade or other tolerogenic therapies. Such autoreactive T cells have the potential to rapidly expand, enter the allograft and mediate autoimmune injury. Alternatively or in addition, the reactivated autoreactive T cells may cross react with donor MHC, thereby driving transplant injury through heterologous recognition of alloantigens in the graft. A third, non-mutually exclusive, hypothetical model is that reactivation of autoreactive T cells by indirectly presented donor autoantigens on recipient APCs provides helper signals (transmitted through the APC) that facilitate expansion of alloreactive CD8+ T cells that in turn, mediate rejection. Indirectly primed alloreactive CD4+ T cells can provide help for activation/expansion of donor-reactive (direct pathway) CD8+ T cells (41) via activation of a recipient APC that has acquired intact donor MHC (42–44), and/or by producing IL-2 which acts as a paracrine stimulus to support CD8+ T cell responses. Previously published work has also shown that memory alloreactive CD4+ T cells responding through the indirect pathway thwart costimulatory blockade induced tolerance induction, and that the rejection is caused in part by expansion of donor reactive CD8+ cells (45, 46).
Together, these observations support the need for studying whether and how pre-existing autoreactive T cells impact costimulatory blockade induced allograft prolongation following heart transplantation. Toward this end, we performed a series of cardiac transplant experiments in BALB/c mice that were pre-immunized with the clinically relevant cardiac autoantigen, cardiac myosin (CM). Our findings demonstrate that preexisting CM-reactive T cell immunity shortens survival of fully MHC disparate cardiac allografts despite donor specific transfusion (DST) plus anti-CD40L mAb MR1. Experiments aimed at addressing mechanisms indicate absence of significant recurrent autoimmune injury or heterologous immunity, but rather support the conclusion that the memory autoreactive T cells facilitate priming of donor MHC-reactive T cells that ultimately result in graft rejection. Because T cell autoimmunity (including T cells reactive to CM) is commonly detectable in heart transplant candidates regardless of the primary etiology of their heart disease, understanding the role of autoreactive T cells in this scenario has important clinical implications.
Results
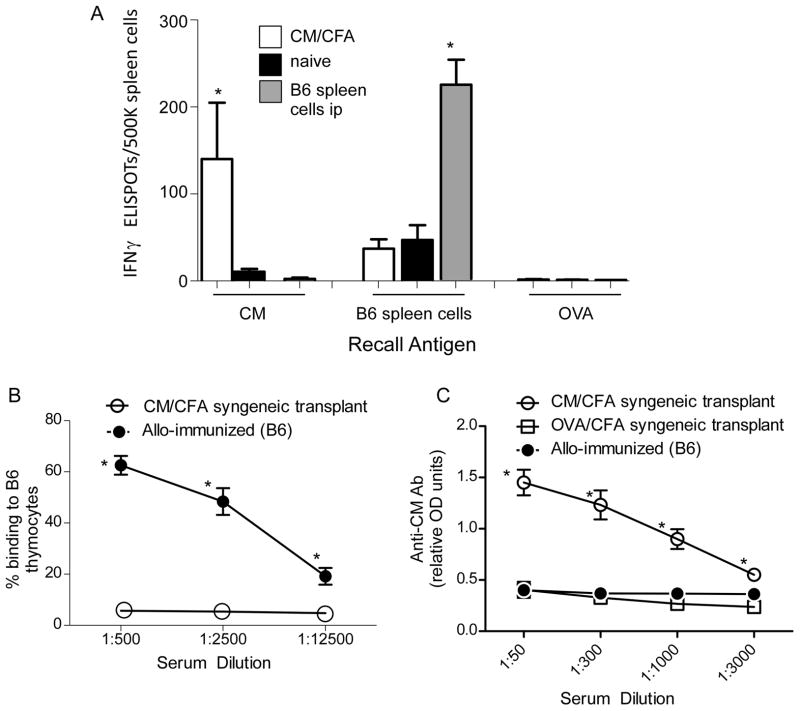
We immunized BALB/c mice with CM mixed in CFA (CM/CFA) or control ovalbumin in CFA (OVA/CFA) and 2 weeks later analyzed spleen cells for reactivity to CM and cross-reactivity to B6 alloantigens (Figure 1). Spleen cells from CM/CFA-immunized mice contained CM-specific IFNγ producers (Figure 1A) and a low frequency of H-2b-reactive immune cells, the latter indistinguishable from that found in naïve mice. In converse experiments we immunized BALB/c mice with B6 (H-2b) spleen cells and host splenocytes for reactivity to CM. These assays revealed undetectable cellular responses to CM but strong H-2b-reactive IFNγ-producing cellular immunity (Figure 1A). When we tested serum CM/CFA immunized mice (2 weeks after immunization) for anti-CM antibodies by ELISA we detected weak responses, indistinguishable from naïve animals (data not shown). In an attempt to boost the humoral responses to CM, we immunized BALB/c mice with CM/CFA (or control OVA/CFA) and 2 weeks later performed syngeneic BALB/c heart transplants. Two weeks after transplantation (hearts beating strongly) we tested sera for reactivity to CM and for cross reactivity to H-2b alloantigens (Figure 1B–C). These assays showed a high titer of anti-CM IgG in CM/CFA immunized mice without reactivity to H-2b thymocytes. Sera from BALB/c mice injected i.p. with B6 spleen cells contained anti-H-2b IgG without detectable anti-CM IgG. Together the findings indicate that BALB/c mice immunized with CM/CFA generate specific anti-CM immunity that does not cross react with H-2b alloantigens.
Figure 1.
CM-reactive immunity and anti-H-2b immunity do not cross react. A. Spleen cells from naïve BALB/c mice (black), from BALB/c mice 2 weeks after immunization with CM/CFA (white), or from BALB/c mice 2 weeks after i.p. injection of 15×106 B6 spleen cells (grey) were tested in IFNγ ELISPOT assays against CM (10 μg/mL), B6 spleens cells or equimolar, control, ovalbumin (OVA). n=4/group, *p<0.05. B–C. Sera was obtained from BALB/c mice that were immunized with CM/CFA or OVA/CFA and 2 weeks later transplanted with syngeneic BALB/c hearts (sera obtained 2 weeks after transplant) or from mice 2 weeks after i.p. injection of 15×106 B6 (H-2b) spleen cells. The sera were tested for binding to B6 thymocytes by flow cytometry (B) or for reactivity to CM by ELISA, serum diluted at the dilutions shown (C). n=4 per group, *p<0.05 versus same dilution in other groups. No reactivity was detected using sera from naïve mice (data not shown).
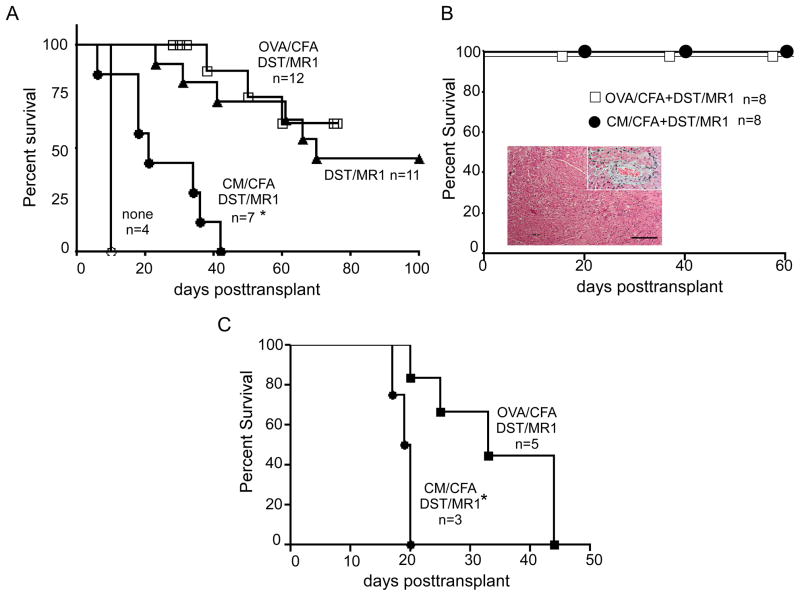
We tested the impact of preexisting anti-CM immunity on heart allograft survival using a commonly employed treatment strategy, donor specific transfusion (DST) plus anti-CD40L mAb MR1. We immunized BALB/c mice with CM/CFA or control OVA/CFA and 14 d later transplanted the animals with B6 heart allografts plus DST/MR1 (Fig 2A). Naïve BALB/c mice transplanted with B6 hearts ± DST/MR1 served as controls. While DST/MR1 prolonged heart graft survival in non-pre-immunized mice (DST/MR1 alone) and in control OVA/CFA treated mice (from 8 d to > 50 d), hearts transplanted into mice pre-immunized with CM/CFA rejected with a MST of 30 d (Fig 2A). Syngeneic BALB/c hearts transplanted into CM/CFA immunized recipients beat for greater than 70 d (Fig 2B) without significant histological evidence of injury. Native hearts in these animals (treated with DST/MR1) examined up to 2 months posttransplant showed no evidence of inflammation (data not shown).
Figure 2.
Anti-CM immunity abrogates effects of DST/MR1 heart allograft survival. Survival curves of allogeneic B6 hearts (A) or syngeneic BALB/c hearts (B) in DST/MR1 treated recipients transplanted 2 weeks after immunization with CM/CFA, OVA/CFA or no pre-immunization as noted. Inset in B shows representative H&E stained graft tissue and high power view of representative artery from syngeneic transplants of CM/CFA pre-immunized and DST/MR1 treated mice, obtained 12 weeks after transplantation. Scale bar=100 μm. C. Survival curves of allogeneic B6 hearts in DST/MR1 treated recipients transplanted 45 days after immunization with CM/CFA or OVA/CFA. *p<0.05 versus other groups on the same graph.
To assess the contribution of acute inflammation from the CFA immunization to outcome we immunized BALB/c mice with CM/CFA or control OVA/CFA but waited 45 days before transplanting cardiac allografts under the cover of DST/MR1 (Fig 2C). Under these conditions, pre-existing anti-CM immunity also shortened transplant survival compared to the OVA/CFA pre-treated mice although allografts in both groups ultimately rejected.
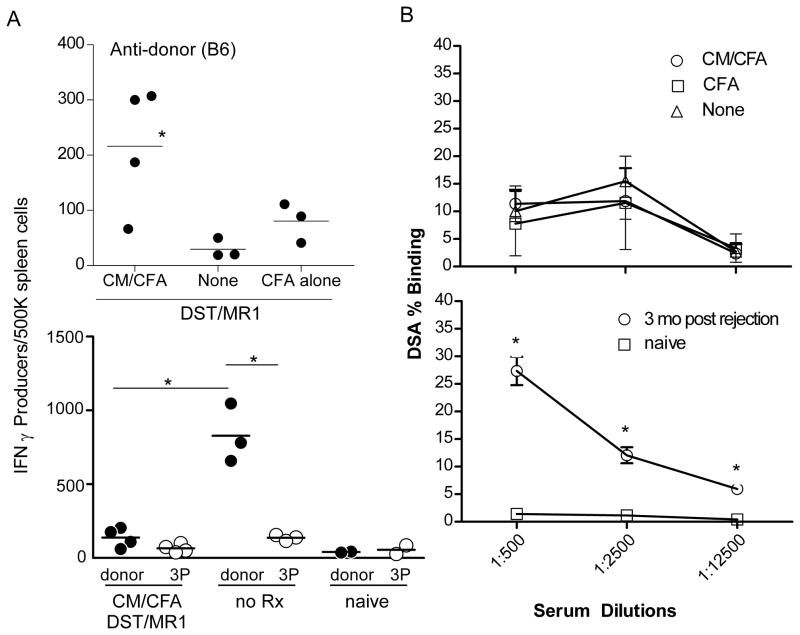
To begin to decipher mechanisms underlying the effects, we immunized BALB/c mice with CM/CFA (or control OVA/CFA) and 14 days later transplanted them with B6 heart grafts plus DST/MR1. We sacrificed the animals 2 weeks after transplantation and tested spleen cells for reactivity to B6 alloantigens by cytokine ELISPOT (Fig 3A). Spleen cells from transplanted mice pre-immunized with CM/CFA contained ~200/500,000, B6-reactive IFNγ-producers despite DST/MR1, while spleen cells from recipients pre-immunized with CFA plus DST/MR1 responded to donor antigens at low frequencies that were similar to those from naïve mice (<50/500,000, Fig 3A, top). In control experiments (Fig 3A, bottom) we observed that spleen cells from untreated BALB/c mice that rejected B6 hearts (d 8 posttransplant) contained >700/500,000 donor-reactive, IFNγ producers, with a low frequency (<100/500,000) of third-party reactive IFNγ producers.
Figure 3.
Pre-existing anti-CM immunity enhances anti-donor T cell responses despite DST/MR1. A. Top: Spleen cells were obtained 2 weeks after B6 heart transplantation and DST/MR1 treatment in groups of animals pre-immunized with CM/CFA, CFA alone or not pre-immunized, and tested for frequency of donor (B6) reactive IFNγ producers by ELISPOT. *p<0.05 vs. none and CFA alone. Bottom: In a separate set of control experiments spleen cells were obtained from a) BALB/c mice 2 weeks after B6 heart transplantation and DST/MR1 treatment that were pre-immunized with CM/CFA, b) untreated BALB/c recipients of B6 hearts at rejection (day 8 posttransplant) and c) naïve BALB/c mice. The spleen cells were tested in IFNγ ELISPOT assays for reactivity to B6 and third party (3P) C3H spleen cells (*p<0.05). B. Top: Sera were obtained 2 weeks after transplant from DST/MR1 treated B6 allograft recipients that were either unimmunized mice (none), pre-immunized with CM/CFA, or pre-immunized with CFA alone, and tested by flow cytometry for binding to B6 thymocytes. Bottom: In control experiments, sera were obtained from naïve mice and from untreated mice 3 months after rejecting a B6 allograft, and tested by flow cytometry for binding to B6 thymocytes. None of the sera bound to syngeneic BALB/c thymocytes (not shown). *p<0.05 versus other groups. *p<0.05 versus other groups on the same graph.
When we tested for serum anti-donor MHC antibodies (obtained 2 weeks after transplant) we observed similar low titers in mice treated with DST/MR1 whether or not they were pre-immunized with CM/CFA or OVA/CFA (Fig 3B top). Control experiments showed higher titers of anti-donor antibodies in untreated allograft recipients that rejected their grafts and absence of serum anti-donor antibodies in naïve mice (Fig 3B, bottom).
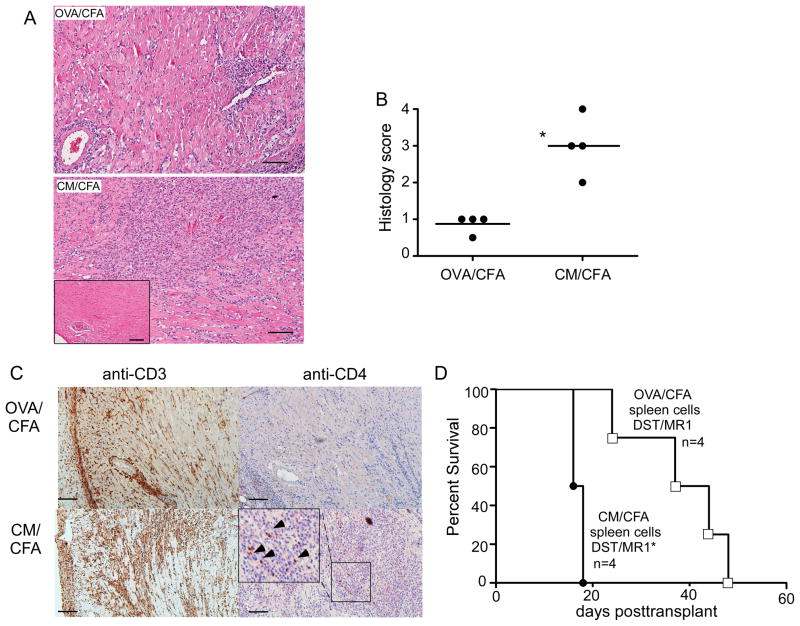
Histological examination of 14 d posttransplant allografts revealed significantly more mononuclear cell infiltration in the CM/CFA immunized mice (Fig 4A–B), and the infiltrates stained strongly positive for CD3 (Fig 4C). Staining the graft tissue for CD4 expression (Fig 4C) revealed rare CD4+ cells without differences between groups of animals pre-immunized with OVA/CFA or CM/CFA (p=non-significant, n=3–4, not shown).
Figure 4.
Pre-existing anti-CM T cell immunity augments intragraft mononuclear cell infiltrates and precipitates rejection. A–B. Representative H&E stained tissue sections (A, scale bar=100 μm) and quantitative histological scores (B) of d 14 posttransplant allografts in DST/MR1 treated mice pre-immunized with OVA/CFA or CM/CFA. *p<0.05. C. Representative immunohistochemical staining of serial tissue sections of the same grafts with anti-CD3 (left) or anti-CD4 (right) of d 14 posttransplant allografts in DST/MR1 treated mice pre-immunized with OVA/CFA (top) or CM/CFA (bottom). Scale bar 100 μm. Inset: magnified area demonstrating rare CD4+ cells (arrowheads). D. Survival curves of allogeneic B6 hearts in DST/MR1 treated recipients transplanted 24 h after adoptive transfer of 5×107 in vitro activated spleen cells from CM/CFA immunized or control OVA/CFA immunized mice. *p<0.05.
We next tested whether immune cells from CM/CFA-immunized mice are sufficient to accelerate allograft rejection despite DST/MR1. We pooled spleen cells from CM/CFA immunized BALB/c mice and from OVA/CFA immunized controls, re-stimulated them in vitro for 72 h and adoptively transferred them into naïve BALB/c recipients of B6 heart transplants under the cover of DST/MR1 (Fig 4D). The hearts rejected significantly faster in adoptive hosts given spleen cells from CM-immunized mice as compared to the OVA-reactive spleen cell control recipients. CD4+ T cells appeared to be sufficient to mediate the effect as 2 of 4 DST/MR1-treated allograft recipients adoptively transferred with purified splenic CD4+ T cells from CM/CFA immunized mice rejected their grafts (days 21 and 36 posttransplant) while all 4 of the controls adoptively transferred with CD4+ T cells from OVA/CFA immunized mice were beating on day 60 (data not shown).
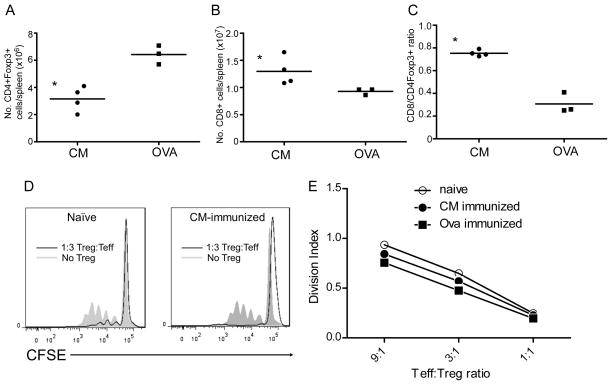
When we quantified the numbers of CD4+CD25+Foxp3+ T cells and CD8+ T cells in the spleens of each animal, we observed fewer CD4+CD25+Foxp3+ (Fig 5A) and more CD8+ T cells (Fig 5B) in the CM/CFA immunized mice. Based on observations by others indicating that CD8+/Treg ratios provide an indirect measure of in vivo Treg function (47) in which higher CD8+/Treg ratios imply increased CD8+ T cell expansion and less in vivo suppression, we calculated CD8+/Treg ratios in each animal (Fig 5C). These analyses revealed significantly higher CD8+/Treg ratios in the CM/CFA immunized mice. In vitro suppression assays using enriched splenic CD4+CD25+Foxp3+ T cells from each group of animals (>94% pure) confirmed their ability to suppress donor reactive T cell immunity without differences in per-cell suppressive capacities among the groups (Fig 5D–E).
Figure 5.
CM specific immunity results in lower Treg/CD8 ratios despite DST/MR1. AB. Absolute numbers of Foxp3+ CD4+ T cells (A) and CD8+ T cells (B) in spleens of mice obtained 2 weeks after heart transplantation plus DST/MR1 as determined by flow cytometry. C. Calculated ratios of CD8+ cells to Treg for each mouse. D–E. Representative results (D) and quantitative suppression indices (E) of in vitro suppression assays using purified splenic CD25+CD4+Foxp3+ T cells obtained from naïve, CM/CFA immunized or control, OVA/CFA immunized mice 2 weeks after B6 heart transplantation under the cover of DST/MR1. *p<0.05
Discussion
Our data support the conclusion that pre-existing autoreactive T cells specific for CM are important functional barriers to DST/MR1-induced prolongation of cardiac allograft survival in mice. The observed acceleration of graft rejection occurred when CM/CFA immunization was performed remotely (>45 d earlier) and occurred following adoptive transfer of spleen cells (or CD4+ cells) from CM-immunized animals into naïve hosts (Fig 2). Allografts transplanted into OVA/CFA immunized mice (or mice adoptively transferred with spleen cells from OVA/CFA immunize mice) did ultimately reject (Figs 2C and 4D) consistent with some nonspecific effects of CFA immunization on preventing tolerance induction as shown by others (48–50). Importantly, however, the allografts in CM immunized (or adoptively transferred) mice rejected with faster kinetics than their OVA immunized counterparts demonstrating a specific effect of CM immunity on overcoming the protective effects of DST/MR1. The absence of detectable cellular and humoral cross reactivity between CM and B6 antigens (Fig 1) does not support a role for heterologous immunity as a mechanism.
The long term survival of syngeneic heart grafts in CM-immunized recipients, associated with minimal graft inflammation (Fig 2), indicates that the expansion of preexisting autoreactive T cells following exposure to the graft derived self-antigen is insufficient to cause significant graft injury under the tested conditions, which include DST/MR1 therapy. While previous work by Benichou and colleagues showed that CM/CFA immunization could precipitate rejection of a syngeneic heart (31), their experiments were performed in the absence of immunosuppression, providing a potential explanation to account for our disparate observations. BALB/c mice are susceptible to CM/CFA-initiated autoimmune myocarditis in their native hearts using a protocol that requires 2 sequential immunizations (51), but we did not observe histological evidence of myocarditis in the native hearts of any of our test animals suggesting that any primed autoreactive T cells preferentially traffic toward the inflamed allograft rather than to the un-inflamed native heart.
Our experimental results indicate that the pre-existing anti-CM immunity facilitates induction of potent donor-reactive T cell responses without significant effects on alloantibody production (Fig 3). Treg isolated from CM-immunized recipients of allografts were capable of in vitro suppression but the observed increase in splenic CD8+/Treg ratios suggests an inability of the Treg to restrain CD8+ T cell expansion in vivo (Fig 5), a hypothesis that needs additional study. Based on experimental evidence from other model systems, we speculate that following transplantation, the donor-derived CM is likely processed and presented by recipient APCs (analogous to indirect antigen presentation) which interact with the primed CM-reactive T cells in the host. Bidirectional T cell/APC activation likely ensues despite costimulatory blockade as documented for memory T cells responding via the indirect pathway (45). Because recipient APCs can obtain and express donor MHC on their surfaces (42–44), the activated APCs could prime donor-reactive T cell immunity resulting expansion of the CD8+ T cell repertoire, raising the CD8+/Treg ratio and resulting in earlier graft rejection. Alternatively or in addition, IL-2 produced by activated autoreactive CD4+ T cells could act as a local paracrine stimulus to expand alloreactive CD8+ T cells that cause allograft injury.
The finding that preexisting autoreactive T cell immunity negatively impacts graft survival has clinically important implications. Multiple research groups have documented a high prevalence of heart specific autoreactivity in cardiac transplant candidates, regardless of the etiology of their end stage heart disease (26, 28, 52, 53). Others and we have observed an association between such autoreactivity and an elevated posttransplant risk of acute and or chronic heart graft injury in humans (35, 36). Thus, our results provide an important, clinically relevant model system for further evaluation of mechanisms leading to graft injury and for testing novel therapies targeting autoreactive T cells that together could positively impact outcomes in human heart transplant recipients.
Material and Methods
Mice
C57BL/6 (H-2b) and BALB/c (H-2d) mice were purchased from The Jackson Laboratory (Bar Harbor, ME), and housed in the Center for Comparative Medicine and Surgery at the Mount Sinai School of Medicine, following IACUC guidelines.
Antibodies and Reagents
Anti-CD40L mAb MR1 was purchased from Bio X Cell (West Lebanon, NH). Antibodies against CD4, CD8 (BD Pharmingen, San Diego, CA), and mouse IgG (eBioscience, San Diego, CA) were used for flow cytometry. Intracellular FoxP3 staining (eBioscience) was performed according to kit manufacturer’s instructions. Chicken egg ovalbumin was purchased from Sigma-Aldrich (St. Louis, MO).
Cardiac Myosin Purification
Murine cardiac myosin was purified as described (54). Briefly, hearts were isolated from naïve C57BL/6 mice and homogenized using a motor-driven glass-Teflon homogenizer (PowerGen 1000, Fisher Scientific, Pittsburgh, PA). CM was purified from the homogenate after 3 cycles of centrifugation, precipitation, and re-dissolution, and ultimately aliquotted and stored at −80 °C in a solution of 50 mM sodium pyrophosphate. Identification of the isolate was performed by SDS-PAGE and Western blot using anti-CM heavy chain antibody (Novus Biologicals, Littleton, CO), and concentration was determined by Lowry assay using the DC Protein Assay kit (Bio-Rad, Hercules, CA). CM was mixed with complete Freund’s adjuvant (55, 56) and administered as a subcutaneous injection above the thigh.
Heart Transplantation
Heterotopic murine heart transplants were performed by the microsurgical shared resource facility at Mount Sinai School of Medicine in accordance with the guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International. Donor specific transfusion (DST) of 1 × 107 splenocytes in single-cell suspension in PBS, plus 500 μg MR1, were administered 24 h prior to transplant via retro-orbital intravenous injections. Grafts were considered rejected when their heartbeats could no longer be detected by palpation. Grafts were formalin- fixed and embedded in paraffin, and sections were stained with H&E for evaluation. Histological scoring was performed blindly by analyzing a minimum of 3 different slides per graft by PSH: 0=no mononuclear cell infiltration, 1=mononuclear cell infiltration of <10% of the cross sectional area, 2= mononuclear cell infiltration of >10% and <50% of the cross sectional area. 3= mononuclear cell infiltration of >10% and <50% of the cross sectional area, 4= mononuclear cell infiltration of >10% and <50% of the cross sectional area plus intra parenchymal hemorrhage. Immunohistochemical staining of graft tissue for CD3 and CD4 expression was performed as described (57).
Adoptive Transfer
Spleen cells from immunized mice were cultured in RPMI 1640 with 10% FCS and L-Glutamine, sodium pyruvate, non-essential amino acids, penicillin/streptomycin, β-mercaptoethanol in T75 flasks with 1 μg/mL Concanavalin A (MP Biomedicals, Santa Ana, CA) for 72 hours, then washed and counted. 5 × 107 cells were injected retro-orbitally into adoptive hosts at the time of the transplant, 24 h after DST/MR1.
ELISPOT assays
IFNγ ELISPOT assays were performed as described (55–58) with CM or OVA used at 1–10 μg/ml, and counted using an Immunospot image analyzer (CTL, Shaker Heights, OH)
Flow Cytometry
Samples were collected using a FACS Canto II (BD) flow cytometer and analyzed using FlowJo software (Tree Star, Ashland OR).
Alloantibody detection
Serum samples from recipient mice were diluted in PBS as indicated and incubated for 30 minutes at room temp with syngeneic, donor or third-party thymocytes as target cells. Following a wash step with PBS 1% albumin, the bound antibody was detected by incubation with fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse IgG (eBioscience) and quantified by flow cytometry.
Anti-CM antibody ELISA
ELISA plates (Fisher Scientific) were coated with 1μg/ml CM or equimolar OVA in bicarbonate buffer overnight, washed with PBS, and then blocked with 2% bovine serum albumin (BSA, Sigma-Aldrich) in PBS for 3 h at room temperature. After washing with PBS, mouse sera were diluted in 2% BSA, added to the wells and incubated overnight at 4° C. Following another PBS wash, streptavidin-HRP (R&D Systems, Minneapolis, MN) was added for 3 h and the ELISA was developed using 3,3′,5,5′-tetramethylbenzidine (TMB, Thermo Scientific, Rockford IL) and read at 450 nm.
In vitro Treg suppression assay
In vitro suppression assays were performed as published (59). Briefly polyclonal suppression assays were performed by co-culturing 5×104 CFSE-labeled (Life Technologies, Carlsbad CA) CD45.1+ naïve CD4+ T cells with 5×104 CD90.2-depleted splenocytes plus purified Treg at various dilutions in the presence of anti-CD3ε for 3 days.
Acknowledgments
The authors thank Gilles Benichou (Massachusetts General Hospital, Boston MA) for his guidance in preparing murine cardiac myosin, Praeophayom Wauhop, Paolo Cravedi, and Joon-Hyuk Sheen for their technical assistance, the microsurgery shared resource facility at the Icahn School of Medicine at Mount Sinai for performing the heart transplant procedures, and the histology core at the Icahn School of Medicine at Mount Sinai for assistance with the H&E and immunohistochemical staining of graft tissue. The work was funded through National Institutes of Health grant AI43578 awarded to PSH.
Abbreviations
- CFA
complete Freund’s adjuvant
- CM
cardiac myosin
- DST
donor specific transfusion
- ELISPOT assay
enzyme linked immunosorbent spot assay
- FITC
fluorescein isothiocyanate
- HRP
horseradish peroxidase
- IFNγ
interferon gamma
- mAb
monoclonal antibody
- MHC
major histocompatibility
- OVA
ovalbumin
- Treg
regulatory T cell
References
- 1.Valujskikh A, Lakkis FG. In remembrance of things past: memory T cells and transplant rejection. Immunol Rev. 2003;196:65–74. doi: 10.1046/j.1600-065x.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 2.Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, et al. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465–474. doi: 10.1111/j.1600-6143.2005.00759.x. [DOI] [PubMed] [Google Scholar]
- 3.Lakkis FG, Arakelov A, Konieczny BT, Inoue Y. Immunologic ‘ignorance’ of vascularized organ transplants in the absence of secondary lymphoid tissue. Nat Med. 2000;6:686–688. doi: 10.1038/76267. [DOI] [PubMed] [Google Scholar]
- 4.Pantenburg B, Heinzel F, Das L, Heeger PS, Valujskikh A. T cells primed by Leishmania major infection cross-react with alloantigens and alter the course of allograft rejection. J Immunol. 2002;169:3686–3693. doi: 10.4049/jimmunol.169.7.3686. [DOI] [PubMed] [Google Scholar]
- 5.Valujskikh A, Pantenburg B, Heeger PS. Primed allospecific T cells prevent the effects of costimulatory blockade on prolonged cardiac allograft survival in mice. Am J Transplant. 2002;2:501–509. doi: 10.1034/j.1600-6143.2002.20603.x. [DOI] [PubMed] [Google Scholar]
- 6.Kitchens WH, Haridas D, Wagener ME, Song M, Kirk AD, Larsen CP, et al. Integrin antagonists prevent costimulatory blockade-resistant transplant rejection by CD8(+) memory T cells. Am J Transplant. 2012;12:69–80. doi: 10.1111/j.1600-6143.2011.03762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo DJ, Anderson DJ, Weaver TA, Leopardi F, Song M, Farris AB, et al. Belatacept and sirolimus prolong nonhuman primate renal allograft survival without a requirement for memory T cell depletion. Am J Transplant. 2013;13:320–328. doi: 10.1111/j.1600-6143.2012.04342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh K, Kozyr N, Stempora L, Kirk AD, Larsen CP, Blazar BR, et al. Regulatory T cells exhibit decreased proliferation but enhanced suppression after pulsing with sirolimus. Am J Transplant. 2012;12:1441–1457. doi: 10.1111/j.1600-6143.2011.03963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adams AB, Williams MA, Jones TR, Shirasugi N, Durham MM, Kaech SM, et al. Heterologous immunity provides a potent barrier to transplantation tolerance. J Clin Invest. 2003;111:1887–1895. doi: 10.1172/JCI17477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weaver TA, Charafeddine AH, Agarwal A, Turner AP, Russell M, Leopardi FV, et al. Alefacept promotes co-stimulation blockade based allograft survival in nonhuman primates. Nat Med. 2009;15:746–749. doi: 10.1038/nm.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, et al. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3:86ra51. doi: 10.1126/scitranslmed.3002093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Augustine JJ, Siu DS, Clemente MJ, Schulak JA, Heeger PS, Hricik DE. Pre-transplant IFN-gamma ELISPOTs are associated with post-transplant renal function in African American renal transplant recipients. Am J Transplant. 2005;5:1971–1975. doi: 10.1111/j.1600-6143.2005.00958.x. [DOI] [PubMed] [Google Scholar]
- 14.Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, et al. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267–2275. [PubMed] [Google Scholar]
- 15.Hricik DE, Rodriguez V, Riley J, Bryan K, Tary-Lehmann M, Greenspan N, et al. Enzyme linked immunosorbent spot (ELISPOT) assay for interferon-gamma independently predicts renal function in kidney transplant recipients. Am J Transplant. 2003;3:878–884. doi: 10.1034/j.1600-6143.2003.00132.x. [DOI] [PubMed] [Google Scholar]
- 16.Nickel P, Bestard O, Volk HD, Reinke P. Diagnostic value of T-cell monitoring assays in kidney transplantation. Curr Opin Organ Transplant. 2009;14:426–431. doi: 10.1097/MOT.0b013e32832c5999. [DOI] [PubMed] [Google Scholar]
- 17.Bestard O, Nickel P, Cruzado JM, Schoenemann C, Boenisch O, Sefrin A, et al. Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J Am Soc Nephrol. 2008;19:1419–1429. doi: 10.1681/ASN.2007050539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nather BJ, Nickel P, Bold G, Presber F, Schonemann C, Pratschke J, et al. Modified ELISPOT technique--highly significant inverse correlation of post-Tx donor-reactive IFNgamma-producing cell frequencies with 6 and 12 months graft function in kidney transplant recipients. Transpl Immunol. 2006;16:232–237. doi: 10.1016/j.trim.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Weber DJ, Wilkes DS. The Role of Autoimmunity in Obliterative Bronchiolitis Post Lung Transplantation. Am J Physiol Lung Cell Mol Physiol. 2012 doi: 10.1152/ajplung.00378.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarma NJ, Tiriveedhi V, Angaswamy N, Mohanakumar T. Role of antibodies to self-antigens in chronic allograft rejection: potential mechanism and therapeutic implications. Hum Immunol. 2012;73:1275–1281. doi: 10.1016/j.humimm.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Q, Wang D, Hadley GA, Bingaman AW, Bartlett ST, Farber DL. Long-term islet graft survival in NOD mice by abrogation of recurrent autoimmunity. Diabetes. 2004;53:2338–2345. doi: 10.2337/diabetes.53.9.2338. [DOI] [PubMed] [Google Scholar]
- 22.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. IL-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–3506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sumpter TL, Wilkes DS. Role of autoimmunity in organ allograft rejection: a focus on immunity to type V collagen in the pathogenesis of lung transplant rejection. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1129–1139. doi: 10.1152/ajplung.00330.2003. [DOI] [PubMed] [Google Scholar]
- 24.Mares DC, Heidler KM, Smith GN, Cummings OW, Harris ER, Foresman B, et al. Type V collagen modulates alloantigen-induced pathology and immunology in the lung. Am J Respir Cell Mol Biol. 2000;23:62–70. doi: 10.1165/ajrcmb.23.1.3924. [DOI] [PubMed] [Google Scholar]
- 25.Dinavahi R, George A, Tretin A, Akalin E, Ames S, Bromberg JS, et al. Antibodies reactive to non-HLA antigens in transplant glomerulopathy. J Am Soc Nephrol. 2011;22:1168–1178. doi: 10.1681/ASN.2010111183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caforio AL, Mahon NJ, McKenna WJ. Cardiac autoantibodies to myosin and other heart-specific autoantigens in myocarditis and dilated cardiomyopathy. Autoimmunity. 2001;34:199–204. doi: 10.3109/08916930109007385. [DOI] [PubMed] [Google Scholar]
- 27.Caforio AL, Mahon NJ, Tona F, McKenna WJ. Circulating cardiac autoantibodies in dilated cardiomyopathy and myocarditis: pathogenetic and clinical significance. Eur J Heart Fail. 2002;4:411–417. doi: 10.1016/s1388-9842(02)00010-7. [DOI] [PubMed] [Google Scholar]
- 28.Caforio AL, Tona F, Bottaro S, Vinci A, Dequal G, Daliento L, et al. Clinical implications of anti-heart autoantibodies in myocarditis and dilated cardiomyopathy. Autoimmunity. 2008;41:35–45. doi: 10.1080/08916930701619235. [DOI] [PubMed] [Google Scholar]
- 29.Dubel L, Farges O, Sato Y, Bismuth H. Development of anti-tissue antibodies in the rat liver transplant model. Transplantation. 1998;65:1135–1137. doi: 10.1097/00007890-199804270-00022. [DOI] [PubMed] [Google Scholar]
- 30.Dubel L, Farges O, Johanet C, Sebagh M, Bismuth H. High incidence of antitissue antibodies in patients experiencing chronic liver allograft rejection. Transplantation. 1998;65:1072–1075. doi: 10.1097/00007890-199804270-00011. [DOI] [PubMed] [Google Scholar]
- 31.Fedoseyeva EV, Zhang F, Orr PL, Levin D, Buncke HJ, Benichou G. De novo autoimmunity to cardiac myosin after heart transplantation and its contribution to the rejection process. J Immunol. 1999;162:6836–6842. [PubMed] [Google Scholar]
- 32.Rolls HK, Kishimoto K, Dong VM, Illigens BM, Sho M, Sayegh MH, et al. T-cell response to cardiac myosin persists in the absence of an alloimmune response in recipients with chronic cardiac allograft rejection. Transplantation. 2002;74:1053–1057. doi: 10.1097/00007890-200210150-00028. [DOI] [PubMed] [Google Scholar]
- 33.Fedoseyeva EV, Kishimoto K, Rolls HK, Illigens BM, Dong VM, Valujskikh A, et al. Modulation of tissue-specific immune response to cardiac myosin can prolong survival of allogeneic heart transplants. J Immunol. 2002;169:1168–1174. doi: 10.4049/jimmunol.169.3.1168. [DOI] [PubMed] [Google Scholar]
- 34.Veillette GR, Sahara H, Meltzer AJ, Weiss MJ, Iwamoto Y, Kim KM, et al. Autoimmune sensitization to cardiac myosin leads to acute rejection of cardiac allografts in miniature swine. Transplantation. 2011;91:1187–1191. doi: 10.1097/TP.0b013e318218415d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalache S, Dinavahi R, Pinney S, Mehrotra A, Cunningham MW, Heeger PS. Anticardiac myosin immunity and chronic allograft vasculopathy in heart transplant recipients. J Immunol. 2011;187:1023–1030. doi: 10.4049/jimmunol.1004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jurcevic S, Ainsworth ME, Pomerance A, Smith JD, Robinson DR, Dunn MJ, et al. Antivimentin antibodies are an independent predictor of transplant-associated coronary artery disease after cardiac transplantation. Transplantation. 2001;71:886–892. doi: 10.1097/00007890-200104150-00011. [DOI] [PubMed] [Google Scholar]
- 37.Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–631. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pearson T, Markees TG, Serreze DV, Pierce MA, Wicker LS, Peterson LB, et al. Islet cell autoimmunity and transplantation tolerance: two distinct mechanisms? Ann N Y Acad Sci. 2003;1005:148–156. doi: 10.1196/annals.1288.016. [DOI] [PubMed] [Google Scholar]
- 39.Seung E, Mordes JP, Greiner DL, Rossini AA. Induction of tolerance for islet transplantation for type 1 diabetes. Curr Diab Rep. 2003;3:329–335. doi: 10.1007/s11892-003-0026-9. [DOI] [PubMed] [Google Scholar]
- 40.Mojtahedi Z, Farjadian S, Ghaderi A. Does autoantigen administration inhibit recurrence of type 1 diabetes in transplanted islets? Med Hypotheses. 2005;64:986–988. doi: 10.1016/j.mehy.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 41.Lee RS, Grusby MJ, Glimcher LH, Winn HJ, Auchincloss H., Jr Indirect recognition by helper cells can induce donor-specific cytotoxic T lymphocytes in vivo. J Exp Med. 1994;179:865–872. doi: 10.1084/jem.179.3.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Afzali B, Lombardi G, Lechler RI. Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transplant. 2008;13:438–444. doi: 10.1097/MOT.0b013e328309ee31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smyth LA, Afzali B, Tsang J, Lombardi G, Lechler RI. Intercellular transfer of MHC and immunological molecules: molecular mechanisms and biological significance. Am J Transplant. 2007;7:1442–1449. doi: 10.1111/j.1600-6143.2007.01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tsang JY, Chai JG, Lechler R. Antigen presentation by mouse CD4+ T cells involving acquired MHC class II:peptide complexes: another mechanism to limit clonal expansion? Blood. 2003;101:2704–2710. doi: 10.1182/blood-2002-04-1230. [DOI] [PubMed] [Google Scholar]
- 45.Chen Y, Heeger PS, Valujskikh A. In vivo helper functions of alloreactive memory CD4+ T cells remain intact despite donor-specific transfusion and anti-CD40 ligand therapy. J Immunol. 2004;172:5456–5466. doi: 10.4049/jimmunol.172.9.5456. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Q, Chen Y, Fairchild RL, Heeger PS, Valujskikh A. Lymphoid sequestration of alloreactive memory CD4 T cells promotes cardiac allograft survival. J Immunol. 2006;176:770–777. doi: 10.4049/jimmunol.176.2.770. [DOI] [PubMed] [Google Scholar]
- 47.Boenisch O, D’Addio F, Watanabe T, Elyaman W, Magee CN, Yeung MY, et al. TIM-3: a novel regulatory molecule of alloimmune activation. J Immunol. 2010;185:5806–5819. doi: 10.4049/jimmunol.0903435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen L, Ahmed E, Wang T, Wang Y, Ochando J, Chong AS, et al. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182:6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Wang T, Zhou P, Ma L, Yin D, Shen J, et al. TLR engagement prevents transplantation tolerance. Am J Transplant. 2006;6:2282–2291. doi: 10.1111/j.1600-6143.2006.01489.x. [DOI] [PubMed] [Google Scholar]
- 50.Porrett PM, Yuan X, LaRosa DF, Walsh PT, Yang J, Gao W, et al. Mechanisms underlying blockade of allograft acceptance by TLR ligands. J Immunol. 2008;181:1692–1699. doi: 10.4049/jimmunol.181.3.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pummerer CL, Luze K, Grassl G, Bachmaier K, Offner F, Burrell SK, et al. Identification of cardiac myosin peptides capable of inducing autoimmune myocarditis in BALB/c mice. J Clin Invest. 1996;97:2057–2062. doi: 10.1172/JCI118642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morgun A, Shulzhenko N, Unterkircher CS, Diniz RV, Pereira AB, Silva MS, et al. Pre- and post-transplant anti-myosin and anti-heat shock protein antibodies and cardiac transplant outcome. J Heart Lung Transplant. 2004;23:204–209. doi: 10.1016/S1053-2498(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 53.Warraich RS, Pomerance A, Stanley A, Banner NR, Dunn MJ, Yacoub MH. Cardiac myosin autoantibodies and acute rejection after heart transplantation in patients with dilated cardiomyopathy. Transplantation. 2000;69:1609–1617. doi: 10.1097/00007890-200004270-00015. [DOI] [PubMed] [Google Scholar]
- 54.Shiverick KT, Thomas LL, Alpert NR. Purification of cardiac myosin. Application to hypertrophied myocardium. Biochim Biophys Acta. 1975;393:124–133. doi: 10.1016/0005-2795(75)90222-6. [DOI] [PubMed] [Google Scholar]
- 55.Heeger PS, Forsthuber T, Shive C, Biekert E, Genain C, Hofstetter HH, et al. Revisiting tolerance induced by autoantigen in incomplete Freund’s adjuvant. J Immunol. 2000;164:5771–5781. doi: 10.4049/jimmunol.164.11.5771. [DOI] [PubMed] [Google Scholar]
- 56.Heeger PS, Valujskikh A, Lehmann PV. Comprehensive assessment of determinant specificity, frequency, and cytokine signature of the primed CD8 cell repertoire induced by a minor transplantation antigen. J Immunol. 2000;165:1278–1284. doi: 10.4049/jimmunol.165.3.1278. [DOI] [PubMed] [Google Scholar]
- 57.Vieyra M, Leisman S, Raedler H, Kwan WH, Yang M, Strainic MG, et al. Complement regulates CD4 T-cell help to CD8 T cells required for murine allograft rejection. Am J Pathol. 2011;179:766–774. doi: 10.1016/j.ajpath.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raedler H, Vieyra MB, Leisman S, Lakhani P, Kwan W, Yang M, et al. Anti-complement component C5 mAb synergizes with CTLA4Ig to inhibit alloreactive T cells and prolong cardiac allograft survival in mice. Am J Transplant. 2011;11:1397–1406. doi: 10.1111/j.1600-6143.2011.03561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257–268. doi: 10.1084/jem.20121525. [DOI] [PMC free article] [PubMed] [Google Scholar]