Abstract
Background
The epidemiology of painful episodes in infants and younger children with SCD has not been well studied, particularly for pain managed at home.
Procedure
SCD infants identified by newborn screening were enrolled in a longitudinal observational study of pain symptoms requiring parents to report the presence or absence of pain daily. When sickle cell related-pain events occurred, pain occurrence, location, associated symptoms and the treatment provided also were reported.
Results
103 children were enrolled at a median age of 7.2 months; 50 had an SS genotype, 32 SC, 6 SB0thalassemia, and 15 SB+thalassemia. Parents/guardians reported for a median of 3.8 years (range 0.3–7.6 years) assessing pain for a total of 141,197 days, excluding any period of recurrent transfusions, with an additional 28,079 days of missing data (16%). Children had pain reported on 2,288 days (1.6%), representing 768 distinct episodes of pain, of which 108 required hospitalizations (14%). Pain locations and symptoms consistent with dactylitis were most prevalent (80%) in the 0–12 month age group, and became progressively less prevalent thereafter. Group-based trajectory modeling of pain episode or pain day frequency identified several trajectory groups with progressively older ages of peak pain frequency, which included 40–45% of SS/ SB0thalassemia and 10–12% of SC/ SB+thalassemia children.
Conclusions
Pain is relatively infrequent in SCD infants and young children and commonly managed at home. Analyses of longitudinal pain trajectories suggest several different pain trajectories, differing in their frequency, age of onset, and age at peak pain frequency with clinical implications for hydroxyurea management.
Keywords: Pain, sickle cell disease, infants
Introduction
Early studies of infants with sickle cell disease (SCD) suggested that pain can occur as early as 6–12 months of age, typically manifested as dactylitis, a painful swelling of the hands and/or feet that limited manual dexterity or ambulation[1]. The Cooperative Study of Sickle Cell Disease (CSSCD), the first large prospective longitudinal study of SCD in the US, confirmed that the occurrence of painful crisis often began in the first year of life and increased steadily with half of all SS and SC patients experiencing a painful crisis by 4.9 years and 7.1 years of age, respectively [2]. A contemporary cohort from the BABY HUG trial demonstrated a 25–30 % prevalence of pain events prior to randomization at mean age 13·6 months in 193 infants with HbSS (n=187) or Sβ°thalassaemia (n=6) [3]. Either lower levels of fetal hemoglobin at 2 or 5 years of age [4], or a combination of lower hematocrit and higher WBC at 12–18 months of age [5], or higher reticulocyte counts [5] have been associated with more frequent acute care visits or hospitalizations for SCD complications. Early onset of pain events, particularly dactylitis, has been associated with more frequent subsequent sickle-related complications in several studies [6,7].
We conducted an eight year longitudinal study to further characterize the pain experienced by young children with SCD staring in early infancy as reported by parents or guardians using daily reports [8], rather than relying on counts of hospitalizations or acute care visits, which underestimate the frequency of vaso-occlusive pain episodes in school-aged children, adolescents [9], or adults [10]. Due to the complexity of relating unpredictable and relatively infrequent episodes of pain, we also report on a statistical approach that groups individuals into similar trajectories of pain occurrence over time.
Methods
Enrollment and data collection was conducted from Jan 1999-Jan 2008 at the Marian Anderson Sickle Cell Center and funded through the Comprehensive Sickle Cell Centers program. Families in eastern Pennsylvania and southern New Jersey with SCD infants identified by newborn screening were first approached about the study, usually between 3 to 6 months of age, after completion of confirmatory diagnostic testing and initial disease-related education. After informed consent, parents or guardians were trained in pain assessment and daily reporting by a nurse researcher who was an expert in pediatric pain assessment. Medical staff also trained parents or guardians in the appropriate usage of non-opioid and opioid oral analgesics, and non-pharmacologic comfort measures, and reviewed procedures for seeking medical treatment advice or urgent care.
Every day parents recorded the presence or absence of pain during the preceding 24 hours. When sickle cell related-pain events occurred, parents/guardians reported pain occurrence, location, associated symptoms, and the treatment that they provided. Daily paper diaries were predominantly used in the first year of life to familiarize families with pain assessment and reporting as previously described [8]. Subsequently a daily pager system was used to provide a method that allowed daily reporting on the presence or absence of pain, but with reduced participant burden. The system provided automatic notification to study staff of a pain report who then called the family. A structured interview script was used to guide further detailed data collection. Families who were unable to effectively comply with providing daily reports used a system of monthly telephone contacts by study staff using a similarly structured interview script for data collection in conjunction with a daily calendar kept by the parent or guardian.
Study visits to reinforce appropriate data collection and pain management skills were conducted at the time of routine hematologic visits every three months in the first two years of life, and every 4 months thereafter. During acute care visits or hospitalizations for pain study staff verified appropriate parental pain and physical assessments, and retraining was provided if needed. Parents ceased daily reporting for any period of chronic transfusion for clinical events (splenic sequestration, recurrent acute chest syndrome, surgery), but were allowed to resume study data reporting two months after the last transfusion.
All pain reports were reviewed as they were collected, and were adjudicated by consensus of study staff and PI or Co-PI. Episodes of pain separated by only one day of absent pain were infrequent, but were aggregated into a combined episode for the purposes of episode analyses. The study PI assigned a primary diagnosis and secondary diagnoses, if appropriate, for each admission during the study period based on the medical treatment provided to the patient. Only those with a primary diagnosis of pain were included in the analyses of pain hospitalizations. The data from all three modes of data collection were entered into an encounter based database program (Medlog, Information Analysis Corporation, Crystal Bay, NV) as three separate databanks. The data was then extracted into SAS 8.2 (SAS Institute Inc., Cary, NC) and SPSS (IBM Software) for data cleaning and analysis. Analyses of pain episodes and pain days included all episodes or days irrespective of treatment location (home/hospital).
Statistical Analysis
Continuous variables were summarized using mean, median, standard deviation and ranges, while categorical variables were summarized using frequency counts and percentages. The number of subjects with SCD pain and dactylitis was summarized by diagnosis and age with comparison of incidence rates between diagnoses using Fisher’s exact test. Association between hemoglobinopathy diagnosis and age at first pain episode was assessed using the log-rank test. Subjects were censored if they had no pain at the end of follow-up or at the beginning of a 6 month or longer period of missing pain diary data.
Latent class growth analysis (LCGA) [11] was used to model pain episodes, pain days, of pain hospitalizations per age-year. To account for absent data collection from periods of transfusions within any given year, the numbers of pain episodes (or days) were annualized by multiplying the number of episodes (or days) by 365 and dividing by the number of days under observation for each age year. Pain episodes and pain hospitalizations were assumed to follow a Poisson distribution. Pain days were square-root transformed and assumed to be normally distributed. This analysis approach groups subjects into a finite number of latent classes that have a similar growth curve or outcome trajectory over time. The class-specific trajectories were assumed to be quadratic curves in time. Two-, three-, and four-class models were fit, and the best-fitting model was chosen as the one with the lowest Bayesian Information Criterion (BIC) score. LCGA was performed using Mplus software [12]. Subjects were assigned to their most likely class based on posterior probabilities of class membership. Association between pain class membership and hemoglobinopathy diagnosis was evaluated using Pearson’s chi-square test.
Results
Over the study period, 103 children were enrolled (Table 1) whose parents reported at least 60 days of observations, beginning at a median age of 7.2 months (range 1.5, 65.2 months). Seven additional children were enrolled, but their parents provided less than 30 days of pain reports and were considered inevaluable. The study participants represented about 70% of SCD children referred to the Center by local newborn screening programs during the study time period; the most common reasons for refusal were perceived inability to complete daily reporting or excessive travel times to the Center. An SS genotype was present in 50 children (48.5%), SC in 32 (31.1%), SB0thalassemia in 6 (5.8%), SB+thalassemia in 15 (14.6%). The gender distribution was 58% male and 42% female. Hydroxyurea was not used during the study as the results of the BABY HUG clinical trial [3] were not yet available.
Table I.
Sample and Data Reporting Characteristics
| Age at study entry (months) | |
| N | 103 |
| Mean | 12.44 |
| Standard deviation | 12.260 |
| Median | 7.2 |
| Min, Max | 1.5, 65.2 |
| Diagnosis [1] | |
| SC | 32 (31.1%) |
| SB+Thal | 15 (14.6%) |
| SS | 50 (48.5%) |
| SB0Thal | 6 (5.8%) |
| Gender [1] | |
| Male | 60 (58.3%) |
| Female | 43 (41.8%) |
| Length of time enrolled in study (years) | |
| N | 103 |
| Mean | 3.75 |
| Standard deviation | 2.124 |
| Median | 3.8 |
| Min, Max | 0.3, 7.6 |
| Total number of families in study using report method for >50% of reporting, N [1] | 103 |
| Diary subset | 28 (27.2%) |
| Pager subset | 35 (34.0%) |
| Phone subset | 33 (32.0%) |
| No dominant method | 7 (6.8%) |
| Total number of days children were assessed for pain in study, N [2] | 141,197 |
| Diary subset | 36,433 (25.8%) |
| Pager subset | 52,092 (36.9%) |
| Phone subset | 52,672 (37.3%) |
| Total number of days children had pain, N [3] | 2,288 (1.6%) |
| Diary subset | 745 (0.5%) |
| Pager subset | 737 (0.5%) |
| Phone subset | 806 (0.6%) |
| Pain episode length (days) | |
| N | 768 |
| Mean | 2.98 |
| Standard deviation | 2.94 |
| Median | 2 |
| Min, Max | 1, 23 |
Table includes only those participants who were in study for at least 60 days. [1] Percentages of Diagnoses are calculated from the total number of participants in the study. [2] Percentages are calculated from the total number of days assessed for pain. [3] Percentages are calculated from the total number of days assessed for pain for the corresponding data subset.
Parents/guardians reported on SCD pain occurrence, behaviors, and home management in their children for a median of 3.8 years (range 0.3–7.6 years), excluding any period of recurrent transfusions (Table 1). Most families predominantly used one of the reporting formats during their study participation. The total number of reported days in each format (diary, pager, and phone reporting) were similar ranging from 36,433 to 52,672 days. The total number of days children were assessed for pain and reported with any one of the three reporting systems was 141,197 days with an additional 28,079 days (16%) where data was not reported. Families exited the study when they no longer wanted to continue daily reporting, or when their child reached 8 years of age.
Hospitalizations
As expected in this age group, the most common primary admission diagnosis for hospitalization during the study period was fever requiring antibiotic therapy (61.6%). Other frequent diagnoses included painful episodes (18%) or acute chest syndrome (11.7%), while admissions for surgery (4%) or splenic sequestration (3.5%) were less common. All other sickle cell complications represented less than 1% of all admissions. Nine subjects had a total of 24 hospitalizations for Reactive Airway Disease (RAD) symptoms.
Pain Occurrence
Parents or guardians reported pain in their children on a total of 2,288 days (1.6%). This represented 768 distinct episodes of pain typically lasting several days (3 ± 2.9 days, range 1–23 days), of which 108 required hospitalizations (14%) (Table I). Over 80% of children with reported pain in the 0–12 month age interval had pain locations (hands or feet) and signs and symptoms (swelling or tenderness) consistent with dactylitis, which became progressively less prevalent in older age intervals reaching a level of about 10% by age 48–60 months (Table II). There was a trend toward a higher frequency of sickle pain occurrence in the children with SS or SB0thalassemia genotypes compared to those with SC or SB+thalassemia genotypes at all age groups, which reached statistical significance only for the 12–24 month and 24–36 month age groups (Table II). All sickle cell pain episodes or only dactylitis episodes were of longer median duration in the children with SS or SB0thalassemia genotypes compared to those with SC or SB+thalassemia genotypes at all age groups, but this difference rarely reached statistical significance.
Table II.
Pain Occurrence by Age, Location, and Genotype
| Diagnosis | ||||
|---|---|---|---|---|
| SC/SB+Thalassemia n (%) |
SS/SB0Thalassemia n (%) |
Total | P-value | |
| 0–12 months of age, N | 32 | 36 | 68 | |
| All SCD pain, n (%) | 7 (21.9) | 14 (38.9) | 21 (30.9) | 0.1890 |
| Any dactylitis, n (%) | 5 (15.6) | 12 (33.3) | 17 (25.0) | 0.1596 |
| 12–24 months of age, N | 43 | 39 | 82 | |
| All SCD pain, n (%) | 11 (25.6) | 20 (51.3) | 31 (37.8) | 0.0227 |
| Any dactylitis, n (%) | 7 (16.3) | 13 (33.3) | 20 (24.4) | 0.1212 |
| 24–36 months of age, N | 42 | 41 | 83 | |
| All SCD pain, n (%) | 12 (28.6) | 24 (58.5) | 36 (43.4) | 0.0079 |
| Any dactylitis, n (%) | 8 (19.0) | 15 (36.6) | 23 (27.7) | 0.0898 |
| 36–48 months of age, N | 34 | 39 | 73 | |
| All SCD pain, n (%) | 15 (44.1) | 26 (66.7) | 41 (56.2) | 0.0624 |
| Any dactylitis, n (%) | 4 (11.8) | 11 (28.2) | 15 (20.6) | 0.1451 |
| 48–60 months of age, N | 28 | 34 | 62 | |
| All SCD pain, n (%) | 12 (42.9) | 23 (67.7) | 35 (56.5) | 0.0721 |
| Anydactylitis, n (%) | 2 (7.1) | 5 (14.7) | 7 (11.3) | 0.4419 |
| 60–72 months of age, N | 22 | 29 | 51 | |
| All SCD pain, n (%) | 12 (54.5) | 22 (75.9) | 34 (66.7) | 0.1401 |
| Any dactylitis, n (%) | 1 (4.5) | 4 (13.8) | 5 (9.8) | 0.3745 |
| 72–84 months of age, N | 17 | 20 | 37 | |
| All SCD pain, n (%) | 8 (47.1) | 13 (65.0) | 21 (56.8) | 0.3309 |
| Any dactylitis, n (%) | 2 (11.8) | 1 (5.0) | 3 (8.1) | 0.5843 |
| 84–96 months of age, N | 13 | 12 | 25 | |
| All SCD pain, n (%) | 5 (38.5) | 9 (75.0) | 14 (56.0) | 0.1107 |
| Any dactylitis, n (%) | 0 | 3 (25.0) | 3 (12.0) | 0.0957 |
Percentages are calculated from the total number of children assessed during the specified age range. P values were obtained from Fisher’s exact test and Kruskal-Wallis test for categorical and continuous variables, respectively.
Time to First Pain Event
A total of 75 of the 103 participants were available for analysis of time to first pain episode as the parents or guardians reported no pain occurrence prior to enrollment at 6.3 ± 3.1 months (median 5.8 months). The time to first reported occurrence of pain was influenced by sickle genotype, and was significantly shorter in the SS genotype (median, 13.9 months, p=0.002, log rank test) compared to those with SC (43.6 months), SB+thalassemia (21.6 months), or SB0thalassemia (35.8 months) genotypes. Female and male infants had similar times to first pain episode (p=0.89).
Trajectories of Pain
Latent Class Growth Analyses were performed on the longitudinal measurements of number of pain episode, pain days, or pain hospitalizations per age-year, excluding any periods of clinically-indicated transfusion therapy.
Pain Episodes
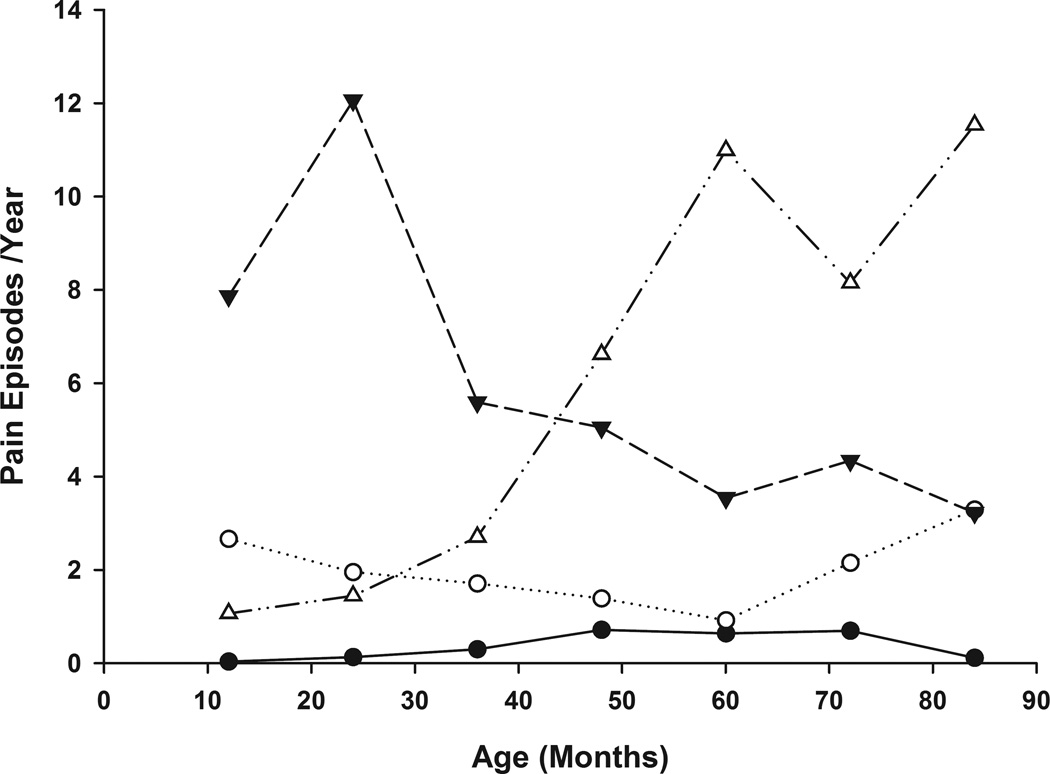
Longitudinal trajectories results for the pain episode data (Figure 1) suggested a four-class (trajectory) model: 1) Class 1A – No/Few Pain (n=53, 49%) with little to no pain over the entire observation period; 2) Class 1B-Low Pain (n=21, 23%) characterized by relatively constant incidence of pain episodes at a low to moderate level (2 episodes / year); 3) Class 1C-Early/Intermediate High Pain (n=12, 12%) characterized by an initial higher pain rate with a decrease in incidence over time to an intermediate level (4 episodes / year); and 4) Class 1D-Increasing High Pain (n=17, 16 %) characterized by low incidence of pain episodes at age 12 months followed by increasing pain over time (8 to 12 episodes / year).
Figure 1. Age-Related Trajectories of Pain Episode Frequency.
Latent class trajectories of yearly participant pain episode frequency are plotted over time. The four latent classes are represented as follows: Class 1A (No/Few Pain)  , Class 1B (Low Pain)
, Class 1B (Low Pain)  , Class 1C (Early/Intermediate High Pain)
, Class 1C (Early/Intermediate High Pain)  , Class 1D (Increasing High Pain)
, Class 1D (Increasing High Pain)  .
.
Pain Days
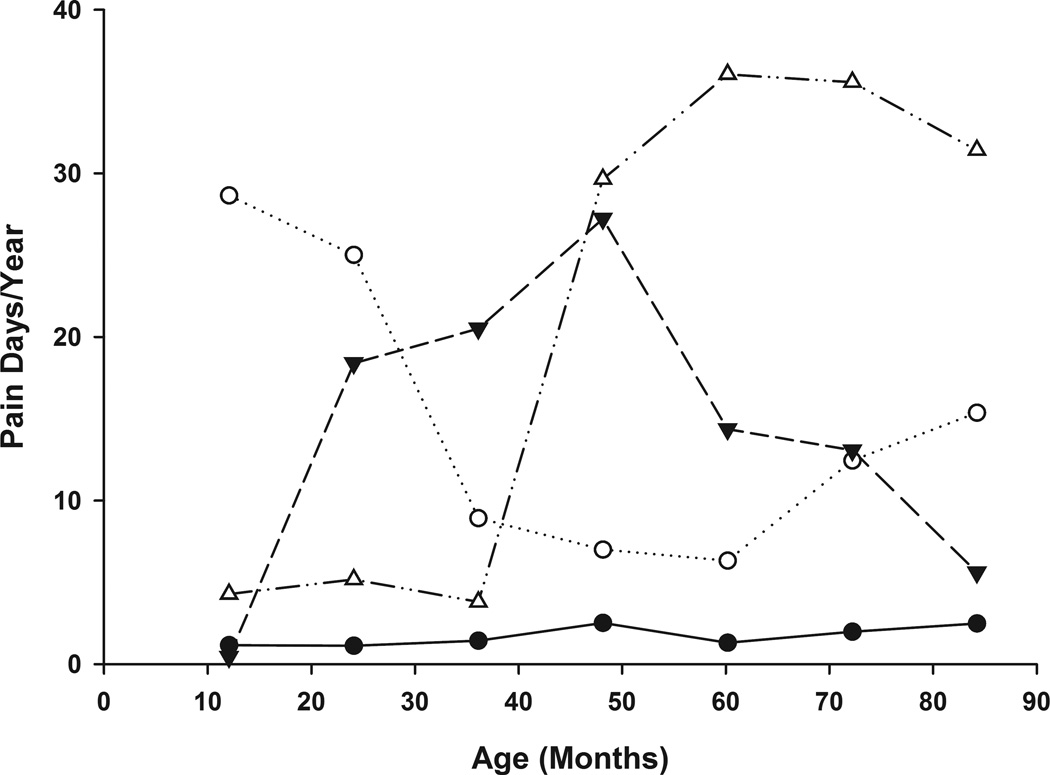
Longitudinal trajectories results of the pain day data (Figure 2) also suggested a four-class (trajectory) model: 1) Class 2A-No/Few Pain (n=73, 71%); 2) Class 2B-Early High Pain with highest pain in the first 3 years of life (n=11, 11%); 3) Class 2C-Intermediate High Pain (n=12, 11%) with peak pain frequency in the third to sixth year of life; and 4) Class 2D-Late High Pain (n=7, 7%) with peak pain starting in the 4th year of life and continuing at a high frequency thereafter. There were statistically significant relationships between class membership in the two models (p< 0.004), with all 7 individuals in the Late High pain group (Class 2D) also being members of the increasing High Pain group (Class 1D). The 53 individuals of the No/Few pain days or pain episode classes (Class 2A and Class 1A) also were the same in both trajectory models.
Figure 2. Age-Related Trajectories of Pain Day Frequency.
Latent class trajectories of yearly participant pain day frequency are plotted over time. The four latent classes are represented as follows: Class 2A (No/Few Pain)  , Class 2B (Early High Pain)
, Class 2B (Early High Pain)  , Class 2C (Intermediate High Pain)
, Class 2C (Intermediate High Pain)  , Class 2D (Late High Pain)
, Class 2D (Late High Pain)  .
.
Pain Hospitalizations
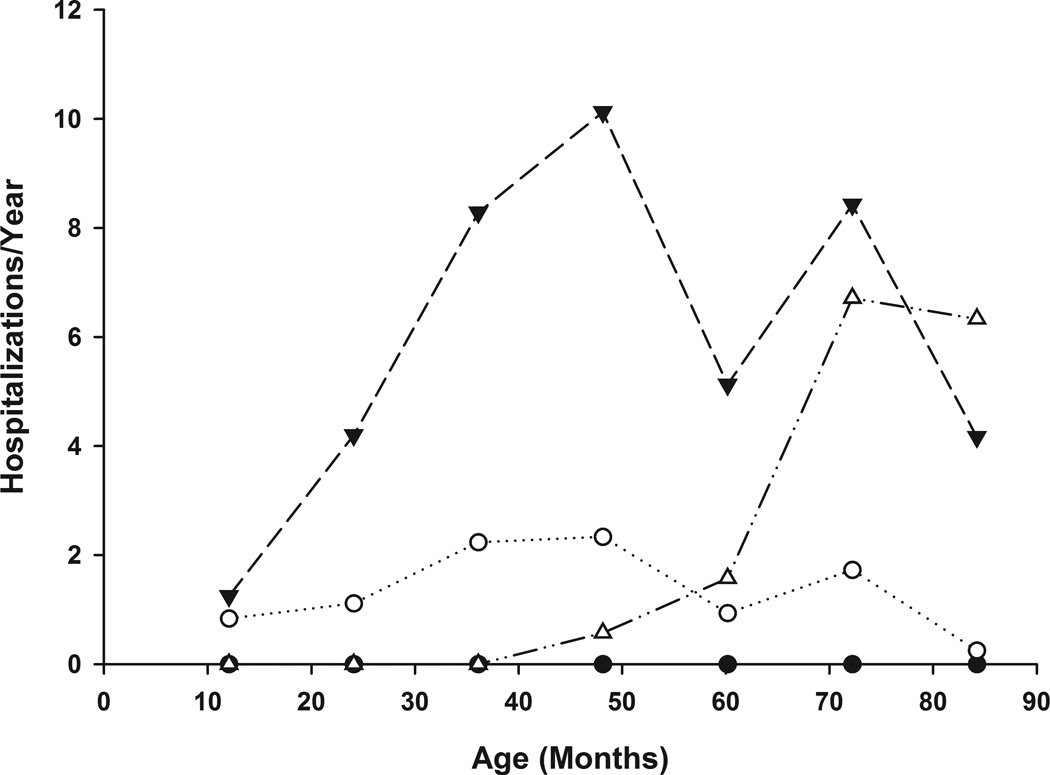
Longitudinal trajectories also were determined restricting the analyses to pain episodes that resulted in hospitalizations (Figure 3). A four trajectory was also suggested: 1) Class 3A- a pattern of no hospitalizations across the study period (n=64, 62%); 2) Class 3B- Low Pain (n=24, 23%); 3) Class 3C Early/Intermediate High Pain (n=8, 8%); and 4) Class 3D Late Increasing High Pain (n=7, 7%). There were statistically significant relationships between hospital trajectory class membership and membership in the pain episode trajectories (p< 0.009) and pain day trajectories (p<0.001), with 80% of the No/Few pain classes (Class 1A, 2A) being members of the Not Hospitalized group (Class 3A). Members of the more symptomatic pain episode (Classes 1B, 1C, 1D),or pain day classes (2B, 2C, 2D) were dispersed over the other hospitalization trajectories reflecting only a modest relationship between pain frequency and hospitalization frequency.
Figure 3. Age-Related Trajectories of Pain Hospitalization Frequency.
Latent class trajectories of yearly participant pain hospitalization frequency are plotted over time. The four latent classes are represented as follows: Class 3A (No Pain)  , Class 3B (Low Pain)
, Class 3B (Low Pain)  , Class 3C (Early/Intermediate High Pain)
, Class 3C (Early/Intermediate High Pain)  , Class 3D (Late Increasing High Pain)
, Class 3D (Late Increasing High Pain) 
Pain episode class membership was significantly associated with genotype, as the SS, Sβ°thalassemia genotypes were over-represented compared to the SC, Sβ+thalassemia genotypes in the most symptomatic pain episode trajectory, Class 1D (71% vs 29%), and underrepresented in the least symptomatic pain episode trajectory, Class 1A (43% vs 57%) (p=0.04). Restricting the trajectory analyses to only the SS, Sβ°thalassemia genotypes provided a three class solution similar to class 1C and class 1D for the symptomatic pain episode trajectories with the less symptomatic SS, Sβ°thalassemia genotypes combined in a low frequency group similar to class 1B.
For the pain day trajectories, the SS, Sβ°thalassemia genotypes represented 71–91% of the symptomatic Class 2B, 2C, and 2D members, but only 42.5% of the less symptomatic Class 2A (p=0.019). A similar pattern of genotypes was seen for class membership in the pain hospitalization trajectories with the SS and Sβ°thalassemia genotypes representing 71–87% of the members of frequent hospitalization groups, Class 3C Early/Intermediate High Pain and Class 3D-Late Increasing High Pain, but only 40% of the no hospitalization Class 3A (p=0.019). Similar trajectories for both frequency of pain days or pain hospitalizations were obtained when the trajectory analyses were restricted to SS and Sβ°thalassemia genotypes.
Discussion
Pain from vaso-occlusion represents a significant complication contributing to reduced quality of life in many children with SCD [13], and early pain from SCD could potentially lead to altered pain experiences in later childhood or as adults, similar to children and adults who experienced pain in the neonatal period [14,15]. However, there is limited data on the occurrence of sickle pain in infants with young children, particularly from longitudinal studies, so we recruited a longitudinal cohort of infants with sickle hemoglobinopathies identified by newborn screening, which allowed the enrollment of infants by about 6 months of age prior to the onset of clinical symptomatology, except for our initial participants used to develop and validate our infant pain diary [8]. During subsequent clinic visits research staff taught and supported parents as they became more knowledgeable in the care of their child with SCD and provided positive feedback as their pain management skills evolved. The resulting relationships built over time among children, parents, and study staff likely contributed to their continued participation, especially in a population often thought to be wary of participating in research studies [16]. The success of this approach is reflected in the low percentage of missing data (16%), and the substantial length of study participation (median 3.8 years).
Parents reported that their children experienced pain on 2,288 days, including those managed at home and in the hospital setting. This represented 1.6% of days observed, which was considerably less frequent then that observed (14%) for a similar study of older children and adolescents [17]. Of the 768 distinct episodes of pain reported by parents, 108 required hospitalizations (14%), which was considerably more frequent than that seen in our previous study (5%) of older children and adolescents [18].
All reporting formats required parents to report the affected body location, presence of swelling, and any change in use of extremities. Episodes associated with reports of pain locations in hands or feet, swelling, and limitations in movement were categorized as dactylitis. Consistent with reports derived from acute care visits [1,2], parents reported that most pain events in the first year of life (>80%), including those managed exclusively at home, displayed signs and symptoms consistent with dactylitis. Similarly, consistent with previous studies [1,2,7], parents reported a smaller percentage of these events in the second and third years of life, and very few dactylitis events after 4 years of age. Similar to the CSSCD, Jamaican, or Guadelope cohorts an earlier pain onset was seen in individuals with the SS genotype, but the absolute time to first event varied depending on the definition of the pain event and its method of ascertainment.
We sought to take advantage of the longitudinal nature of our data collection by applying latent-class growth analysis (LCGA), a group-based trajectory modeling strategy, which is based on the assumption that data arise from observing a mixture of individuals from a limited number of latent classes, each with their own developmental time path (trajectory class). This approach allowed the identification of a small number of groups, in which the time course for evolution of pain across time defined the groups (Figures 1, 2, 3). Examination of the pain day data revealed one group (Class 2A), and the pain episode data two groups (Classes 1A, 1B) of infants and young children with a persistently low frequency of pain across all ages, representing about 70% of the overall sample and containing almost all of the milder sickling genotypes, SC and Sβ+thalassemia, and a much smaller proportion of the SS or Sβ0thalassemia genotypes. Examination of the pain episode data identified two groups of symptomatic individuals, infants with early onset of high pain frequency (Class 1C), and those with later onset of frequent pain (Class 1D), both of which largely contained individuals with more severe sickling genotypes, SS and Sβ0thalassemia. Analysis of the pain day data, which captures both frequency and duration of pain, segregated these two most symptomatic groups into three groups characterized by peaks of high pain frequency in early infancy (Class 2B), middle childhood (Class 2C), or later childhood (Class 2D). Both trajectory analyses identify 40–45% of SS/Sβ0thalassemia and 10–12% of SC/Sβ+thalassemia individuals as symptomatic with pain during childhood, which is broadly consistent with clinical experience, and was identical to the size of the frequent pain hospitalization group of the placebo arm of the BABY HUG clinical trial [19].
There are a number of limitations to this study. It is possible that our pain frequency or severity results may be influenced by the population, geographical or analgesic practices [20,21] characteristic our center. The percentage of episodes managed at home also may have been higher than in other studies as parents had substantial on-call support from medical professionals who were part of the research team. Proxy reporting by parents may provide less certainty for exact times of pain onset and/or resolution, particularly for very young children or for relatively inexperienced parents.
Conclusion
Our study demonstrates the feasibility of initial recruitment and subsequent daily reporting of clinical events by families of infants and young children with SCD over many years, particularly when careful consideration is given to enhance family support and minimizing respondent burden. The qualitatively similar trajectories of pain episodes and pain days suggest that either may be used as a measure of pain frequency. The choice of pain metric to use should reflect planned ascertainment procedures and statistical analysis strategies. While quantitatively different, the qualitative patterns of pain frequencies were similar for episodes managed in the hospital and those managed at home suggesting that either could be used as measures of pain frequency in very young children with SCD. While hospitalized episodes are more easily ascertained from electronic medical records, they are relatively infrequent and intervention studies using such endpoints would likely require a larger sample size then those studies using all episodes. Studies limited to hospitalized episodes also may not be able to definitively determine whether pain is truly reduced in frequency rather than reduced in intensity with a resulting shift in care location.
The observation that about 15% of SCD infants and young children have patterns of frequent hospitalizations for pain quantitatively similar to that seen in older SCD children and adolescents suggests the possibility that very early pain experiences may influence or predict subsequent pain experience in later childhood and adolescence. The occurrence of frequent pain in almost half of young children with the SS or Sβ0thalassemia genotypes, much of which occurred after 1–2 years of age, reinforces the potential benefit of early intervention with oral hydroxyurea to reduce an escalating burden of pain.
Acknowledgements
We wish to acknowledge Patricia O’Neal for her many outstanding contributions to this study. We also wish to thank the families and nursing staff at St Christopher’s Hospital for Children whose dedication to relieving pain in children made this study possible. This study was funded by NIH Comprehensive Sickle Cell Center grant # U54HL070585.
References
- 1.Stevens MC, Padwick M, Serjeant GR. Observations on the natural history of dactylitis in homozygous sickle cell disease. Clin Pediatr (Phila) 1981;20(5):311–317. doi: 10.1177/000992288102000501. [DOI] [PubMed] [Google Scholar]
- 2.Gill FM, Sleeper LA, Weiner SJ, et al. Clinical events in the first decade in a cohort of infants with sickle cell disease. Cooperative Study of Sickle Cell Disease. Blood. 1995;86(2):776–783. [PubMed] [Google Scholar]
- 3.Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: a multicentre, randomised, controlled trial (BABY HUG) Lancet. 2011;377(9778):1663–1672. doi: 10.1016/S0140-6736(11)60355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bailey K, Morris JS, Thomas P, et al. Fetal haemoglobin and early manifestations of homozygous sickle cell disease. Arch Dis Child. 1992;67(4):517–520. doi: 10.1136/adc.67.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342(2):83–89. doi: 10.1056/NEJM200001133420203. [DOI] [PubMed] [Google Scholar]
- 6.Quinn CT, Shull EP, Ahmad N, et al. Prognostic significance of early vaso-occlusive complications in children with sickle cell anemia. Blood. 2007;109(1):40–45. doi: 10.1182/blood-2006-02-005082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foucan L, Ekouevi D, Etienne-Julan M, et al. Early onset dactylitis associated with the occurrence of severe events in children with sickle cell anaemia. The Paediatric Cohort of Guadeloupe (1984–99) Paediatr Perinat Epidemiol. 2006;20(1):59–66. doi: 10.1111/j.1365-3016.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 8.Ely B, Dampier C, Gilday M, et al. Caregiver report of pain in infants and toddlers with sickle cell disease: reliability and validity of a daily diary. J Pain. 2002;3(1):50–57. doi: 10.1054/jpai.2002.xb30064. [DOI] [PubMed] [Google Scholar]
- 9.Dampier C, Ely E, Brodecki D, et al. Home management of pain in sickle cell disease: a daily diary study in children and adolescents. J Pediatr Hematol Oncol. 2002;24(8):643–647. doi: 10.1097/00043426-200211000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148(2):94–101. doi: 10.7326/0003-4819-148-2-200801150-00004. [DOI] [PubMed] [Google Scholar]
- 11.Roeder K, Lynch K, Nagin D. Modeling Uncertainty in Latent Class Membership: A Case Study in Criminology. Journal of the American Statistical Association. 1999;94(447):766–767. [Google Scholar]
- 12.Muthén LKaM BO. Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén; 1998–2012. [Google Scholar]
- 13.Dampier C, Lieff S, LeBeau P, et al. Health-related quality of life in children with sickle cell disease: a report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatr Blood Cancer. 2010;55(3):485–494. doi: 10.1002/pbc.22497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peters JW, Schouw R, Anand KJ, et al. Does neonatal surgery lead to increased pain sensitivity in later childhood? Pain. 2005;114(3):444–454. doi: 10.1016/j.pain.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Hermann C, Hohmeister J, Demirakca S, et al. Long-term alteration of pain sensitivity in school-aged children with early pain experiences. Pain. 2006;125(3):278–285. doi: 10.1016/j.pain.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Ely B, Coleman C. Recruitment and retention of children in longitudinal research. J Spec Pediatr Nurs. 2007;12(3):199–202. doi: 10.1111/j.1744-6155.2007.00115.x. [DOI] [PubMed] [Google Scholar]
- 17.Dampier C, Ely B, Brodecki D, et al. Characteristics of pain managed at home in children and adolescents with sickle cell disease by using diary self-reports. J Pain. 2002;3(6):461–470. doi: 10.1054/jpai.2002.128064. [DOI] [PubMed] [Google Scholar]
- 18.Dampier C, Setty BN, Eggleston B, et al. Vaso-occlusion in children with sickle cell disease: clinical characteristics and biologic correlates. J Pediatr Hematol Oncol. 2004;26(12):785–790. [PubMed] [Google Scholar]
- 19.Thornburg CD, Files BA, Luo Z, et al. Impact of hydroxyurea on clinical events in the BABY HUG trial. Blood. 2012;120(22):4304–4310. doi: 10.1182/blood-2012-03-419879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballas SK, Bauserman RL, McCarthy WF, et al. Utilization of analgesics in the multicenter study of hydroxyurea in sickle cell anemia: effect of sex, age, and geographical location. American journal of hematology. 2010;85(8):613–616. doi: 10.1002/ajh.21750. [DOI] [PubMed] [Google Scholar]
- 21.Smith WR, Ballas SK, McCarthy WF, et al. The association between hydroxyurea treatment and pain intensity, analgesic use, and utilization in ambulatory sickle cell anemia patients. Pain medicine. 2011;12(5):697–705. doi: 10.1111/j.1526-4637.2011.01096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]