Abstract
In vivo tests were performed to assess the influence of the protease inhibitor TL-3 on feline immunodeficiency virus (FIV)-induced central nervous system (CNS) deficits. Twenty cats were divided into four groups of five animals each. Group 1 received no treatment, group 2 received TL-3 only, group 3 received FIV strain PPR (FIV-PPR) only, and group 4 received FIV-PPR and TL-3. Animals were monitored for immunological and virological status, along with measurements of brain stem auditory evoked potential (BAEP) changes. Groups 1 and 2 remained FIV negative, and groups 3 and 4 became virus positive and seroconverted by 3 to 5 weeks postinoculation. No adverse effects were noted with TL-3 only. The average peak viral load for the virus-only group 3 animals was 1.32 × 106 RNA copies/ml, compared to 6.9 × 104 copies/ml for TL-3-treated group 4 cats. Group 3 (virus-only) cats exhibited marked progressive delays in BAEPs starting at 2 weeks post virus exposure, which is typical of infection with FIV-PPR. In contrast, TL-3-treated cats of group 4 exhibited BAEPs similar to those of control and drug-only cats. At 97 days postinfection, treatments were switched; i.e., group 4 animals were taken off TL-3 and group 3 animals were treated with TL-3. BAEPs in group 3 animals returned to control levels, while BAEPs in group 4 animals remained at control levels. After 70 days on TL-3, group 3 was removed from the drug treatment regimen. Delays in BAEPs immediately increased to levels observed prior to TL-3 treatment. The findings show that early TL-3 treatment can effectively eliminate FIV-induced changes in the CNS. Furthermore, TL-3 can counteract FIV effects on the CNS of infected cats, although continued treatment is required to maintain unimpaired CNS function.
Feline immunodeficiency virus (FIV) is a member of the lentivirus family and represents a significant pathogen in the cat population, particularly in the United States and Japan (18, 30). The virus causes an AIDS-like syndrome in the cat with disease progression similar to that observed in human immunodeficiency virus type 1 (HIV-1)-infected humans. Additionally, certain strains of FIV induce central nervous system (CNS) dysfunction in the cat reminiscent of effects ascribed to HIV-1 infections in humans (11, 16), including alteration of sleep patterns and delays in brain stem auditory evoked potential (BAEP) changes (19). FIV infection results in a marked reduction in rapid eye movement sleep and increased wakefulness in experimentally infected animals (20). In addition, signs of dementia are commonly reported in end-stage infected cats in the field (3, 4, 7, 18, 21). Unlike many other symptoms that occur early in FIV infection, changes in sleep patterns and delays in BAEPs are progressive and are not resolved by the end of the acute phase of infection (19, 22-24).
Combination drug therapies have been used successfully in the treatment of AIDS brought on by infection of individuals with HIV-1. In particular, the use of highly specific inhibitors of the viral aspartic protease (PR) in combination with inhibitors of reverse transcriptase (RT), referred to as highly active retroviral therapy, suppress HIV-1 replication to undetectable levels (2, 6, 9, 17). However, in the cat, drug treatment modalities have been limited to experimental use of RT inhibitors such as zidovudine or dideoxyinosine, since protease inhibitors that have been developed for use against HIV-1 are ineffective against FIV (5, 25). Recently, a compound has been identified, termed TL-3, that was developed as a broad-based protease inhibitor with efficacy against HIV-1, FIV, and simian immunodeficiency virus ex vivo (12, 13). In the present study, we have carried out in vivo analyses to determine if TL-3 can ameliorate or reduce the CNS deficits noted with FIV infection, as well as the viral load and secondary responses to virus infection in the periphery. The results show that treatment of cats with TL-3 in conjunction with high-dose FIV strain PPR (FIV-PPR) infection can prevent the onset and progression of functional CNS pathology. Furthermore, TL-3 treatment of infected cats showing delays in BAEPs resulted in the return of potentials to control latencies. Removal of the latter cats from the TL-3 treatment regimen resulted in reappearance of the CNS deficits noted prior to treatment. Importantly, animals protected initially by TL-3 treatment did not develop delays in BAEPs after being taken off the drug, implying that early protection may substantially limit CNS involvement during FIV infection.
MATERIALS AND METHODS
In vivo infection protocol. Four groups of five cats each were used, in the following scheme: group 1, no treatment; group 2, TL-3 treatment only; group 3, FIV-PPR infection only; group 4, FIV-PPR infection plus TL-3 treatment. Baseline CD4+ and CD8+ counts, BAEPs, blood chemistry panels, complete blood counts, weight, and general health conditions were recorded for each of the 20 cats prior to initiation of the experiment and over the course of treatment. The cats were 15 weeks old at the beginning of the experiment. The average cat weight prior to experimental treatment was 2 kg. All cats exhibited similar weight gains of ∼1 kg by the end of the first 5 weeks and an additional ∼0.4 kg by 15 weeks postinfection. Drug-treated animals (groups 2 and 4) received 2 × 20 mg of TL-3 orally per day in gelatin capsules. This dosage was shown in pilot studies to be well tolerated by both cats and mice (see Results). The compound was administered twice daily to groups 2 and 4, between 7 and 8 a.m. and between 3 and 4 p.m., for 96 days. At the end of this period, groups 2 and 4 were removed from the drug treatment regimen and animals in group 3 were placed on TL-3 under the regimen previously used with groups 2 and 4. Treatment was sustained for 70 days, after which all animals were removed from the TL-3 treatment regimen.
Animals were housed individually, kept on a 12-h light-dark cycle, and fed lab chow and water ad libitum in a temperature- and humidity-controlled environment. Animals were manipulated daily and familiarized with the recording environment to reduce stress associated with handling.
BAEPs and VEPs.
Sensory evoked responses of isoflurane-anesthetized cats were recorded, as described elsewhere (19), every 15 days for 34 weeks starting 17 days following TL-3 treatment and 14 days after FIV inoculation. Isoflurane-anesthetized cats were maintained at normal body temperature with a heating pad and acutely aseptically fitted with subcutaneous monopolar needle electrodes (TECA Intropak DMG-25) to differentially record BAEPs, middle-latency auditory evoked potentials (AEPs), and visual evoked potentials (VEPs). Active electrodes were implanted centrally near the vertex and on the midline over the frontal sinus. A third needle electrode was placed in the nuchal muscles and served to ground the animal to reduce signal artifacts. In order to record BAEPs and AEPs, cats were fitted with bilateral polyethylene ear tubes placed into the external auditory canal. A Y connector attached the two tubes to the central sound source (Grass Instrument Audio Amplifier). A computer program, using National Instruments software (LabView) and written for the Apple Macintosh microcomputer, was used to generate the stimuli and to collect the data. The sound stimuli consisted of a 70-dB sound pressure level condensation-produced click generated by a 100-ms input to the audio amplifier. Raw signals obtained within the first 10 ms poststimulation were amplified by a Grass P-511 amplifier, filtered between 300 and 3,000 Hz, and computer averaged. Stimuli were delivered biaurally at a rate of 10 Hz for a total of 1,024 stimuli for BAEPs and at a rate of 1 Hz for a total of 100 stimuli for AEPs.
For VEPs, a Grass clinical photostimulator was used to deliver flashes (0.1-ms duration) with the photostimulator intensity set at 16. The flash lamp was placed 30 cm from the nose of the cat. Ambient lights remained on.
Evoked events were analyzed offline by using the signal-averaging capabilities of a Macintosh microcomputer with custom-made LabView software. Averaged peak latencies and amplitudes were calculated, expressed as z scores (number of standard deviations away from the mean in the observation), and compared individually between animals and as group means. The latency of each BAEP, AEP, and VEP component was measured as the time from the stimulus arrival at the cat's ears or eyes to a wave's positive peak. Amplitude was measured as the peak-to-trough distance. In addition, the P1-to-P3 and P4-to-P6 interpeak intervals were calculated by subtracting the absolute latency values for P1 and P3 and for P4 and P6. Results were compared by a repeated-measure analysis of variance, with the Scheffé F test used for specific comparisons when indicated by the analysis of variance.
Animal care, maintenance, and experimental procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Scripps Research Institute Animal Care Committee standards.
Measurement of humoral antibody responses.
All animals were monitored for humoral antibody responses to FIV by enzyme-linked immunosorbent assay for several weeks following virus inoculation as previously described (14). Briefly, 96-well microtiter plates were coated with 5 ng of gradient-purified FIV per well and the wells were blocked for nonspecific binding with 5% nonfat dry milk (BLOTTO) (8). The wells were then incubated with serial dilutions of serum obtained from control and FIV-infected animals at weekly intervals and incubated for 1 h at 37°C. The wells were then washed and incubated with alkaline phosphatase-conjugated rabbit anti-cat antibody at 37°C for an additional hour. The wells were again washed and developed with p-nitrophenyl dye to detect relative levels of anti-FIV antibody.
Detection and quantification of TL-3 in serum.
Blood was taken at 15 and 30 min and 1, 2, 4, 8, and 24 h from two cats after TL-3 dosing. The evaluation of TL-3 bioavailability in the blood by blood draws was stopped at 24 h to avoid undue stressing of the cats. Serum samples (200 μl) were diluted with 200 μl of 0.5 M Na2CO3, extracted with ethyl acetate, dried, and dissolved in methanol. Samples were analyzed on a Perkin-Elmer Sciex API-3 nanoelectrospray ionization triple-quadrupole mass spectrometer. As an internal reference for nanoelectrospray analysis, the cat serum was spiked before extraction to 500 nM with DL-1, a protease inhibitor similar in structure to TL-3 but with a higher molecular weight. The TL-3 concentration per milliliter of blood was estimated by calculating the peak height ratios of the Na+-ionized TL-3 peak to the reference standard Na+-ionized DL-1 and then comparing these values to a standard curve of the TL-3 extracted from spiked serum.
Viral RNA extraction and first-strand cDNA transcription.
Viral RNA was extracted from infected cat plasma harvested at various time points throughout the experiment with the QiaAmp viral RNA kit (Qiagen). For extraction efficiency comparisons, 109 copies of kanamycin (KAN) RNA (Promega) were spiked into each of the plasma samples prior to RNA extraction. In vitro transcription was performed on extracted FIV-PPR and KAN RNA with Stratascript RT (Stratagene) by following the manufacturer's instructions. cDNA was generated at 42°C for 1.5 h with primers for the FIV-PPR RT d(GGGGGTTCTTCCTGTAATTTATC) and KAN d(AATGGCTGGCCTGTTGAACAA) genes.
Quantitative RT-PCR (QRT-PCR).
PCR products were generated from the FIV-PPR and KAN cDNAs with an ABI PRISM 7700 thermal cycler (PE Applied Biosystems, Foster City, Calif.) and LUX primer technology (Invitrogen). Primer pairs were designed with the LUX Designer software available at www.invitrogen.com/LUX (15). A 5′ region of the FIV-PPR RT gene (96 bp) and an 80-bp KAN gene sequence were amplified per sample in separate wells. The following primer pairs, one of which was 3′ labeled with the reporter dye FAM (6-carboxyfluorescein), were used: FIV-PPR RT forward primer d(GGACTACCTCACCCTGCTGGA) and reverse primer d(ctactttGGATCGAGGGGAATGGTAAAGTAG, where the lowercase letters are the complementary bases for hairpin formation opposite the primer end) and KAN forward primer d(caaaccCAATCACGAATGAATAACGGTTTG) and reverse primer d(AATGGCTGGCCTGTTGAACAA). PCR mixtures (25 μl) were set up in duplicate per sample per gene (RT or KAN) with 5 μl of cDNA, the ready-to-use Platinum Quantitative PCR SuperMix-UDG (Invitrogen) mixture, and either the primer pair (10 μM each) for FIV-PPR RT or the primer pair for KAN. Reaction mixtures were first heated at 50°C for 2 min (UDG treatment) and then denatured at 95°C for 2 min. A two-step cycling protocol was used for 40 cycles of 95°C for 15 s and 60°C for 45 s.
Standard curves were generated in duplicate for each QRT-PCR run with 1-log serial dilutions (107 to 101) of either a plasmid containing the 5′ 4-kb sequence of FIV (includes the gene for RT) or the pET28a(+)vector plasmid (Novagen) containing the gene for KAN. Standard dilutions were aliquoted and maintained at −20°C for less than 2 months in order to ensure a single freeze-thaw cycle and DNA stability. The QRT-PCR standard curve slopes ranged from −3.5 to −3.0, indicating PCR efficiencies (E = 10[−1/slope]) between 93 and 115%. The correlation coefficients (R2) ranged from 0.98 to 0.99, indicating good linearity of the standard curve. Accumulated PCR products resulting in fluorescence signals were analyzed with a Sequence Detector Software program (SDS, version 1.9.1). We obtained nearly identical values for KAN quantitation in every KAN sample well, indicating minimal loss due to experimental manipulation. Background values (week 0 plasma samples) were subtracted per sample. Viral load values closely resembled previously published viral loads obtained from FIV-infected cats (10), and detection limits were in the range of 100 copies/ml of plasma. Data were statistically analyzed in a Microsoft Excel program.
RESULTS
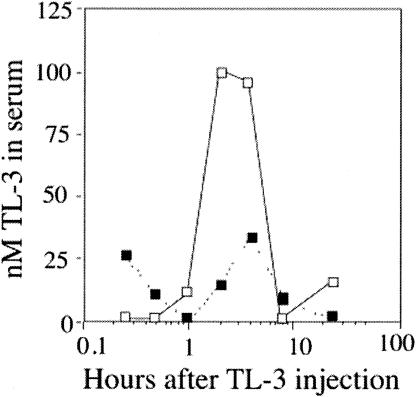
TL-3 is orally bioavailable in cats. Given that TL-3 had been previously used only in vitro, we first assessed the toxicity of the compound in mice. Mice were given 4 mg of TL-3 per mouse twice per day in 1% carboxymethyl cellulose (as a carrier) over 3 days via a stomach tube, for a total dose of 320 mg/kg per day. No adverse clinical signs, such as weight loss, ruffled fur, anemia, or lethargy, were observed during the following 3-week observation period. Next, we tested the bioavailability of TL-3 and its effects in two specific-pathogen-free male cats. Cats were administered a single gelatin capsule containing TL-3 at a dose of 10 mg/kg of body weight. Blood was drawn at 15 and 30 min and 1, 2, 4, 8, and 24 h after dosing to evaluate the presence of TL-3 in the serum. Serum samples obtained from blood were analyzed with a nanoelectrospray ionization triple-quadrupole mass spectrometer (see Materials and Methods). The results presented in Fig. 1 document TL-3 in the serum, with peak levels between 4 and 6 h after ingestion, confirming bioavailability after oral administration and the presence of the protease inhibitor over a time period typical for other protease inhibitors (26, 31). The two cats were clinically evaluated over a 2-week period, and no adverse effects were noted as a result of TL-3 treatment.
FIG. 1.
Quantitative nanospray analysis of cat serum after oral TL-3 administration. Two cats were each administered a single gelatin capsule containing TL-3 at a dose of 10 mg/kg of body weight. Blood samples were taken at 15 and 30 min and 1, 2, 4, 8, and 24 h after dosing. The TL-3 concentration per milliliter of blood was estimated by calculating the peak height ratios of the Na+-ionized TL-3 peak to the reference standard, Na+-ionized DL-1, and then comparing these values to a standard curve of TL-3 extracted from spiked serum.
TL-3 efficacy in cats.
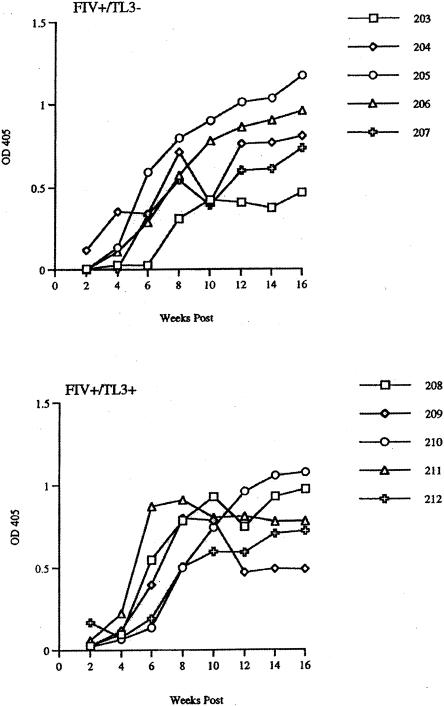
A study was initiated with 20 specific-pathogen-free female cats, divided at random into four groups of five cats each. Group 1 received no treatment, group 2 received 2 × 20 mg of TL-3 orally (gelatin capsules) per day, group 3 received an intravenous inoculation of high-dose (10,000 50% tissue culture infective doses) FIV-PPR only, and group 4 received both the drug and the virus, as described for groups 2 and 3, respectively. Animals were given 20 mg of TL-3 at 8 a.m. and again at 4:30 p.m. for 72 h prior to virus administration. All animals were continuously monitored for overall health, behavior, and relative weight gain. Blood was drawn from all animals at weekly intervals for assessment of blood chemistry, cell counts, antigenemia, humoral antibody, and drug quantification. As previously noted in the TL-3 drug efficacy pilot study, no negative drug effects were noted in drug-only group 2. All of the cats in groups 3 and 4 seroconverted (Fig. 2), indicating that all of the cats were infected with the high-dose FIV-PPR inoculum. Interestingly, cat 203 showed an approximately 2-week delay in the appearance of antiviral antibody relative to the other animals in the untreated group 3 (Fig. 2).
FIG. 2.
Seroconversion of FIV-infected cats as a function of TL-3 treatment and time postinfection. Enzyme-linked immunosorbent assay analyses were performed as described in Materials and Methods. All uninfected animals remained seronegative (data not shown). Seroconversion rates remained within a similar range for all FIV-infected animals, except cat 203 (top panel, empty box), which showed a delayed response relative to those of identically treated cats. Symbols represent data points for cats 203 to 207 (upper panel) and 208 to 212 (lower panel). OD 405, optical density at 405 nm.
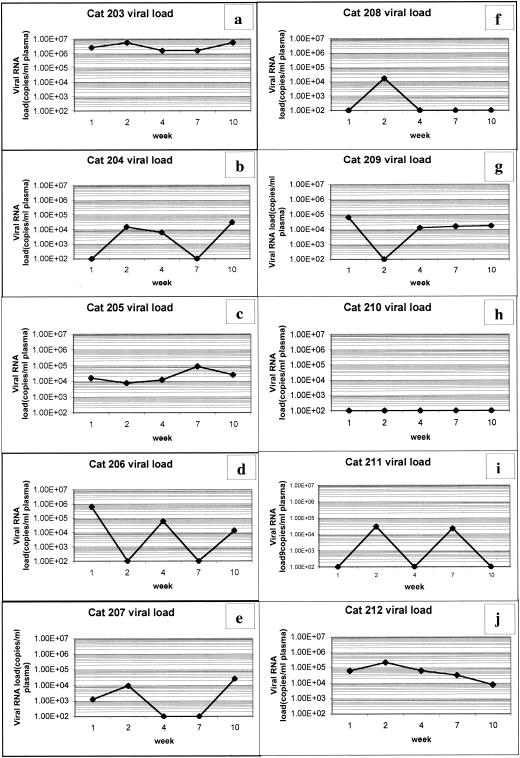
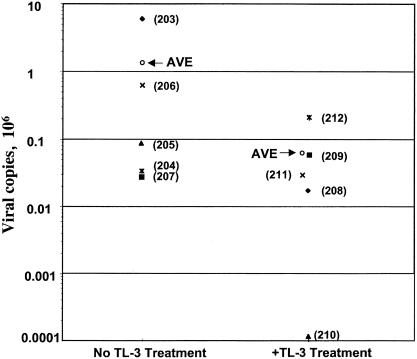
QRT-PCR was conducted on RNA isolated from plasma samples taken at different intervals during the study (Fig. 3). The viral load varied between the different group 3 (Fig. 3a to e) and group 4 (Fig. 3f to j) cats, with values ranging as high as 5.8 × 106 copies/ml (cat 203) to levels below the detection limit of the assay (fewer than 102 copies/ml [cat 210]). Cat 203 (Fig. 3a) consistently showed a higher viral load over time compared to the other cats. Six of the 10 cats showed an increase in the viral load during the second week postinfection (three from each treatment group). Four of five cats in non-TL-3-treated group 3 showed a renewed viral load increase after the seventh week (Fig. 3a, b, d, and e), which did not occur in TL-3-treated group 4 (Fig. 3f to j). An increase in the average peak viral load was observed in the absence of TL-3 treatment compared to the presence of TL-3 treatment (Fig. 4, AVE). The average peak viral load over the first 10 weeks of the experiment was 1.32 × 106 for the untreated group and 6.9 × 104 for the TL-3-treated group. After 10 weeks, overall viral load values for all cats were no longer measurable by QRT-PCR (i.e., values were below 102 copies/ml; data not shown).
FIG. 3.
Viral RNA load measurements. Cat plasma viral loads of groups 3 (a to e) and 4 (f to j) were measured at various times postinfection. Group 3, FIV infection minus TL-3 treatment; group 4, FIV infection plus TL-3 treatment. Viral load measurements at 1, 2, 4, 7 and 10 weeks postinfection are shown. Most cats exhibited a viral load increase at 2 weeks postinfection. Cat 203 (a) consistently maintained higher viral loads compared to those of identically treated cats in group 3. Cat 210 (h) had a high baseline value.
FIG. 4.
Peak viral load as a function of TL-3 treatment. Comparison of the peak viral loads of non-TL-3-treated group 3 animals with those of TL-3-treated group 4 animals. The average peak viral load (AVE) was 1.32 × 106 for group 3 and 6.9 × 104 for group 4. Individual cat numbers are shown in parentheses.
Effects of TL-3 on sensory evoked responses.
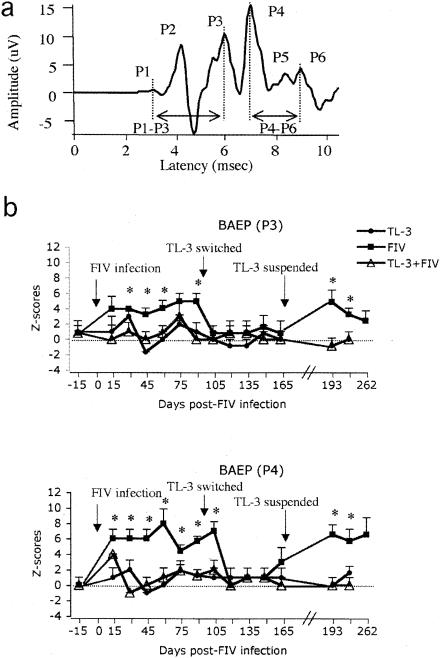
Six waves were identified in the short BAEP complex by visual inspection, P1 to P6 (Fig. 5a). The BAEPs of the TL-3-treated group (group 2) showed no latency changes over the course of the study in any of the six waves (Fig. 5b, filled circles) compared to controls. On the other hand, BAEP waves from FIV-infected cats (group 3) showed significant delays in the latency of the P3 and P4 waves starting at 30 and 15 days post FIV infection, respectively (P < 0.01), compared to the control group (Fig. 5b, filled squares). In contrast, BAEPs of TL-3-treated, FIV-infected animals (group 4) did not show significant changes (Fig. 5b, empty triangles) compared to controls.
FIG. 5.
Measurement of BAEP changes. (a) Representative BAEP recorded from an anesthetized cat. Its principal positive peaks (P) and P1-to-P3 and P4-to-P6 interpeak intervals are indicated. Latencies were measured from the onset of audio stimulation. (b) Averaged waveform latencies of P3 (upper) and P4 (bottom) BAEPs expressed as z scores. Increases in z scores indicate delays in peak latency from the control values (dotted line). Significant delays in the FIV-only group 3 cats were observed on P3 and P4 starting at 30 and 15 days, respectively, post FIV infection (*, P < 0.01 compared to control group). Note that when FIV group 3 cats were treated with TL-3, an improvement in the latency of these peaks was observed. However, when TL-3 treatment was suspended, the significant delays in P3 and P4 reappeared. There were five cats in each group.
Interestingly, after treatments were switched and the group 3 animals started to receive TL-3, the delays observed in the latency of P3 and P4 returned to control levels, while BAEP waves in the group 4 cats did not change with TL-3 withdrawal (Fig. 5b). After 70 days on the switched treatment, TL-3 treatment was suspended for all cats. The P3 and P4 latencies increased significantly in the group 3 cats (P < 0.01) 22 days post TL-3 withdrawal compared to those of controls (Fig. 5b).
In addition to the absolute values of the BAEP latency peaks, we calculated both the P1-to-P3 and P4-to-P6 interpeak intervals (regions denoted in Fig. 5a), as a measure of the transmission time of stimuli along the lower and upper brain stem portions, respectively. The P1-to-P3 interpeak interval was significantly longer in group 3 (FIV-infected cats) (2.70 ± 0.01 ms; P < 0.05) compared to control values (group 1) (2.66 ± 0.01 ms), whereas the P4-to-P6 interpeak intervals were significantly shorter in group 3 (1.67 ± 0.02 ms; P < 0.05) compared to control values (1.72 ± 0.01 ms). These disruptions were observed at 30 and 45 days postinfection, respectively. As with the absolute BAEP wave latency values, the interpeak intervals in the FIV-infected group returned to control levels after TL-3 treatment commenced. However, withdrawal of TL-3 treatment induced a renewed increase in the P1-to-P3 interpeak interval (2.74 ± 0.01 ms) and a renewed decrease in the P4-to-P6 interpeak interval (1.61 ± 0.01 ms) in FIV-infected cats (group 3) (P < 0.01) compared to control cats (2.67 ± 0.01 and 1.70 ± 0.01 ms, respectively). These alterations were observed 22 days after TL-3 withdrawal.
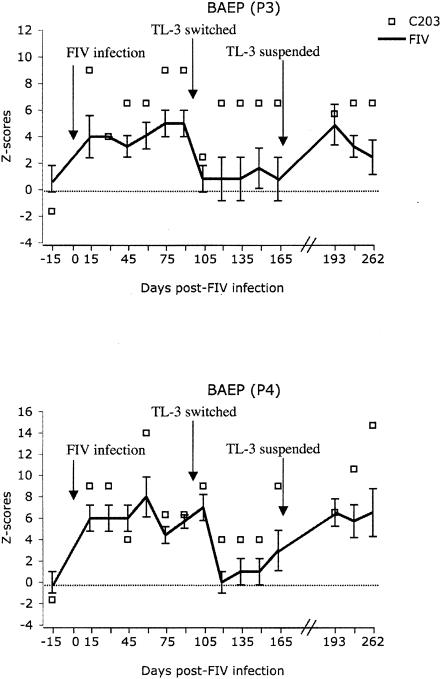
AEPs and VEPs did not show significant differences in the latency of their waves compared to those of the control group. None of the amplitudes of the BAEP, AEP, and VEP complexes reached the level of statistical significance compared to the control values (data not shown). Note that the BAEP values of cat 203, in both P3 and P4, were markedly exacerbated (Fig. 6, boxes) compared to the average values for all group 3 (virus-only) cats (solid lines). Cat 203 initially responded to TL-3 treatment started on day 97, but increased latencies returned to near untreated levels despite drug treatment, which is particularly apparent in P3 (Fig. 6, upper panel).
FIG. 6.
Averaged waveform latencies of P3 (upper) and P4 (bottom) BAEPs expressed as z scores for cat 203 compared to those of identically treated cats. Significantly greater delays were observed in the P3 and P4 measurements of cat 203 (C203, empty box) starting at 30 and 15 days, respectively, post FIV infection (P < 0.01) compared to those of identically treated cats in group 3 (FIV).
DISCUSSION
Previous studies have shown that the protease inhibitor TL-3 exhibits broad efficacy in tissue culture against FIV, HIV-1, and simian immunodeficiency virus (12), as well as several drug-resistant HIV-1 isolates recovered from patients undergoing therapy with currently approved antiprotease drugs (1). In the present study, we wished to perform initial tests to determine the efficacy of the compound in cats. Initial tests showed that high-dose treatment of mice with TL-3 had no ill effects. Studies were then performed with cats that showed the appearance of the compound in serum, with peaks between 4 and 6 h postadministration (Fig. 1). The level of TL-3 in these two animals varied considerably, consistent with the genetic variability of these outbred animals. Also, a high drug dosage was not maintained over a 24-h period. Nevertheless, the findings indicate that treatment with TL-3 resulted in lower peak viral loads in the first 10 weeks of the experiment, with average values of 6.9 × 104 copies/ml for the TL-3-treated animals in group 4, as opposed to 1.32 × 106 copies/ml for the virus-only group 3 animals (Fig. 4). Considering that average peak viral loads for a heterogeneous animal pool may not be ideal, we simultaneously present individual peak viral loads for the same time period (Fig. 3). After 10 weeks postinfection, viral loads in all infected animals decreased below the detection limit (data not shown). The findings suggest that strong humoral and cell-mediated immunity is able to substantially control the viral load in the periphery after the initial acute phase of infection. However, given the observed influence of TL-3 treatment on BAEP measurements in infected cats at later times, the results indicate that viral loads in the CNS are still sufficient to cause delays in BAEPs after viral loads in the periphery have fallen to levels below the detection limit of the assay (<102 copies/ml). Similar to what is thought to occur in HIV infections, FIV may also avoid immune surveillance in privileged cell environments such as the CNS.
Sensitive tests were performed to monitor changes in BAEPs as a function of FIV infection, a criterion that has consistently shown delays in FIV-PPR-infected cats (20, 22). The neurophysiologic dysfunction associated with FIV infection that we have observed in previous studies resembles changes in brain stem conduction potentials seen in humans with HIV-1 infection, Alzheimer's disease, and Parkinson's disease with dementia (27-29). In both HIV-infected patients and FIV-infected cats, the predominant delays in potential (BAEP latencies occur in the late waveforms [IV and V in humans, P4 and P5 in cats]), which represent conduction through the upper level of the brain stem. In addition, the alterations in the latency of BAEP components correlate with progression of neurologic disease. In the TL-3 study, FIV-only group 3 cats showed marked delays in the latency of P4 compared to the other three groups, consistent with altered upper-level brain stem conduction mechanisms. Our results also indicated delays in the latency of the P3 wave, providing evidence that the lower brain stem was also compromised in the FIV-infected cats (group 3) (Fig. 5b). These results confirm previous studies that demonstrate that infection of cats with certain FIVs induces disruptions in the components of the BAEPs (20, 22). On the other hand, the FIV-infected, TL-3-treated cats (group 4) did not have a significant change compared to either uninfected cats (group 1) or cats receiving TL-3 alone (group 2), suggesting that the maintenance of a low viral load early on afforded by TL-3 treatment limited the influence of the virus on CNS function. The results imply that there may be a threshold viral load that is required for successful early crossing of the blood-brain barrier and productive infection of the CNS that was not obtained in the presence of TL-3. The fact that animals receiving early TL-3 treatment did not develop CNS lesions when removed from the drug treatment regimen at 97 days postinfection suggests that treatment with TL-3 at the beginning of FIV infection has a protective effect against virus establishment in vivo, especially in the CNS. The protective effect results in inhibition of the delays in the latency of the component of the BAEPs, and this threshold may be raised in older animals. The findings may relate to the degree of development of the blood-brain barrier in these young animals. Treatment that reduces the early viral load may afford a degree of protection until the animals mature.
The protective effect of TL-3 was also observed in the FIV-infected cats (group 3), where a normalization in the latency of the P3 and P4 waves was observed after the cats received the TL-3 treatment as of 97 days postinfection (Fig. 5b). These results suggest that the early neurological alterations observed in infected animals could be reversed by opportune treatment with this protease inhibitor. However, the reappearance of alterations in the latency of BAEPs after the withdrawal of TL-3 in FIV-infected cats (group 3), despite undetectable viral load fluctuations in the plasma, supports the notion that the viral load is variable, depending on the compartment analyzed. It also suggests that the protective effect of TL-3 requires continued drug treatment once the virus has crossed the blood-brain barrier and the CNS has been compromised. In summary, the findings here are consistent with the notion that an increased viral load in the CNS (i.e., in the absence of drug treatment) results in exacerbated delays in BAEP and that TL-3, by virtue of limiting the viral load in the CNS, resolves these delays. It is likely that there exists a viral load threshold for infection of the CNS in immature cats that is not reached in TL-3-treated animals (group 4) but is reached in infected animals not receiving TL-3 (group 3). As the animals mature, the CNS becomes more resistant to infection, and this, coupled with a strong immune response, would explain why we did not see BAEP delays in TL-3-treated group 4 when it was taken off drug treatment at 97 days. The CNS of infected animals that did not receive TL-3 treatment initially (group 3) were infected early, so that BAEP delays (ameliorated by TL-3 treatment at day 97) returned when drug treatment was terminated at 167 days.
Interestingly, cat 203 (group 3, FIV-PPR infection plus TL-3 treatment) stood out throughout the experiment. It not only exhibited significantly increased viral loads over time (Fig. 3a) compared to the other animals but also showed the latest seroconversion (Fig. 2, top panel). Furthermore, cat 203 showed the greatest increase in BAEP delays (Fig. 6) compared to other animals treated identically. Although the conclusions that may be drawn from the observation of one animal are limited, the findings regarding the exacerbated delays in BAEPs in cat 203 are consistent with the high level of virus infection noted in the periphery. In particular, the humoral antibody responses to viral infection were delayed by approximately 2 weeks in this animal, and throughout the acute phase, this cat showed viral loads significantly higher than the average of the other infected animals (Fig. 3). Apparently, this higher viral load in the periphery, coupled with delays in the immune response, resulted in substantially more infection of the CNS as well. Taken together, these results suggest that individual animal variance is an important factor in viral infection and underscore the need to examine a cohort study allowing for animal-to-animal differences.
We conclude that TL-3 may prevent early alterations in the mechanisms of brain stem conduction in FIV-infected cats. Further studies are needed to determine the mechanisms involved in this protective effect.
Acknowledgments
We thank the AIDS Resources Branch for facilitating the large-scale synthesis and purification of TL-3 required to carry out these studies. Dennis Sheeter is acknowledged for advice regarding viral load quantitation. Chi-Hey Wong and Arthur J. Olson are acknowledged for valuable discussions.
This work was supported in part by grants R01AI40882 (J.H.E.), P01GM44870 (J.H.E. and B.E.T.), F00-SRI-036 (B.B.), P30MH62261 (H.S.F.), and P01DA12444 (S.H.) from the National Institutes of Health.
REFERENCES
- 1.Buhler, B., Y. C. Lin, G. Morris, A. J. Olson, C. H. Wong, D. D. Richman, J. H. Elder, and B. E. Torbett. 2001. Viral evolution in response to the broad-based retroviral protease inhibitor TL-3. J. Virol. 75:9502-9508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collier, A. C., R. W. Coombs, D. A. Schoenfeld, R. L. Bassett, J. Timpone, A. Baruch, M. Jones, K. Facey, C. Whitacre, V. J. McAuliffe, H. M. Friedman, T. C. Merigan, R. C. Reichman, C. Hooper, and L. Corey. 1996. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N. Engl. J. Med. 334:1011-1017. [DOI] [PubMed] [Google Scholar]
- 3.Dow, S. W., M. L. Poss, and E. A. Hoover. 1990. Feline immunodeficiency virus: a neurotropic lentivirus. J. Acquir. Immune Defic. Syndr. 3:658-668. [PubMed] [Google Scholar]
- 4.Egberink, H., J. Ederveen, M. Koolen, H. Lutz, and M. C. Horzinek. 1988. Infections with feline T-lymphotropic lentivirus. Tijdschr. Diergeneeskd. 113:937-943. [PubMed] [Google Scholar]
- 5.Elder, J. H., M. Schnolzer, C. S. Hasselkus-Light, M. Henson, D. A. Lerner, T. R. Phillips, P. C. Wagaman, and S. B. Kent. 1993. Identification of proteolytic processing sites within the Gag and Pol polyproteins of feline immunodeficiency virus. J. Virol. 67:1869-1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, D. D. Richman, F. T. Valentine, L. Jonas, A. Meibohm, E. A. Emini, and J. A. Chodakewitz. 1997. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337:734-739. [DOI] [PubMed] [Google Scholar]
- 7.Hurtrel, M., J. P. Ganiere, J. F. Guelfi, L. Chakrabarti, M. A. Maire, F. Gray, L. Montagnier, and B. Hurtrel. 1992. Comparison of early and late feline immunodeficiency virus encephalopathies. AIDS 6:399-406. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, D. A., J. W. Gautsch, R. Sportsman, and J. H. Elder. 1984. Improved technique utilizing non-fat dry milk for analysis of protein and nucleic acids transferred to nitrocellulose. Gene Anal. Tech. 1:3-8. [Google Scholar]
- 9.Kirk, O., T. L. Katzenstein, J. Gerstoft, L. Mathiesen, H. Nielsen, C. Pedersen, and J. D. Lundgren. 1999. Combination therapy containing ritonavir plus saquinavir has superior short-term antiretroviral efficacy: a randomized trial. AIDS 13:F9-F16. [DOI] [PubMed] [Google Scholar]
- 10.Klein, D., C. M. Leutenegger, C. Bahula, P. Gold, R. Hofmann-Lehmann, B. Salmons, H. Lutz, and W. H. Gunzburg. 2001. Influence of preassay and sequence variations on viral load determination by a multiplex real-time reverse transcriptase-polymerase chain reaction for feline immunodeficiency virus. J. Acquir. Immune Defic. Syndr. 26:8-20. [DOI] [PubMed] [Google Scholar]
- 11.Kubicki, S., K. Henkes, K. Terstegge, and B. Ruf. 1988. HIV and the nervous system. Fischer, New York, N.Y.
- 12.Lee, T., G. S. Laco, B. E. Torbett, H. S. Fox, D. L. Lerner, J. H. Elder, and C. H. Wong. 1998. Analysis of the S3 and S3′ subsite specificities of feline immunodeficiency virus (FIV) protease: development of a broad-based protease inhibitor efficacious against FIV, SIV, and HIV in vitro and ex vivo. Proc. Natl. Acad. Sci. USA 95:939-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, T., V.-D. Le, Y.-C. Lin, A. L. Wong, J. H. Elder, and C.-H. Wong. 1999. Development of a new type of protease inhibitors with small P3 residue efficacious against FIV and HIV variants. J. Am. Chem. Soc. 121:1145-1155. [Google Scholar]
- 14.Lerner, D. L., P. C. Wagaman, T. R. Phillips, O. Prospero-Garcia, S. J. Henriksen, H. S. Fox, F. E. Bloom, and J. H. Elder. 1995. Increased mutation frequency of feline immunodeficiency virus lacking functional deoxyuridine-triphosphatase. Proc. Natl. Acad. Sci. USA 92:7480-7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nazarenko, I., R. Pires, B. Lowe, M. Obaidy, and A. Rashtchian. 2002. Effect of primary and secondary structure of oligodeoxyribonucleotides on the fluorescent properties of conjugated dyes. Nucleic Acids Res. 30:2089-2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman, S. E., A. D. Chediak, M. Kiel, and M. A. Cohn. 1990. Sleep disturbances in HIV-infected homosexual men. AIDS 4:775-781. [DOI] [PubMed] [Google Scholar]
- 17.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, and S. D. Holmberg. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen, N. C., E. H. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 235:790-793. [DOI] [PubMed] [Google Scholar]
- 19.Phillips, T. R., O. Prospero-Garcia, D. L. Puaoi, D. L. Lerner, H. S. Fox, R. A. Olmsted, F. E. Bloom, S. J. Henriksen, and J. H. Elder. 1994. Neurological abnormalities associated with feline immunodeficiency virus infection. J. Gen. Virol. 75(Pt. 5):979-987. [DOI] [PubMed] [Google Scholar]
- 20.Phillips, T. R., O. Prospero-Garcia, D. W. Wheeler, P. C. Wagaman, D. L. Lerner, H. S. Fox, L. R. Whalen, F. E. Bloom, J. H. Elder, and S. J. Henriksen. 1996. Neurologic dysfunctions caused by a molecular clone of feline immunodeficiency virus, FIV-PPR. J. Neurovirol. 2:388-396. [DOI] [PubMed] [Google Scholar]
- 21.Podell, M., M. Oglesbee, L. Mathes, S. Krakowka, R. Olmstead, and L. Lafrado. 1993. AIDS-associated encephalopathy with experimental feline immunodeficiency virus infection. J. Acquir. Immune Defic. Syndr. 6:758-771. [PubMed] [Google Scholar]
- 22.Prospero-Garcia, O., N. Herold, T. R. Phillips, J. H. Elder, F. E. Bloom, and S. J. Henriksen. 1994. Sleep patterns are disturbed in cats infected with feline immunodeficiency virus. Proc. Natl. Acad. Sci. USA 91:12947-12951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prospero-Garcia, O., N. Herold, A. K. Waters, T. R. Phillips, J. H. Elder, and S. J. Henriksen. 1994. Intraventricular administration of a FIV-envelope protein induces sleep architecture changes in rats. Brain Res. 659:254-258. [DOI] [PubMed] [Google Scholar]
- 24.Prospero-Garcia, O., S. Huitron-Resendiz, S. C. Casalman, M. Sanchez-Alavez, O. Diaz-Ruiz, L. Navarro, D. L. Lerner, T. R. Phillips, J. H. Elder, and S. J. Henriksen. 1999. Feline immunodeficiency virus envelope protein (FIVgp120) causes electrophysiological alterations in rats. Brain Res. 836:203-209. [DOI] [PubMed] [Google Scholar]
- 25.Schnolzer, M., H. R. Rackwitz, A. Gustchina, G. S. Laco, A. Wlodawer, J. H. Elder, and S. B. Kent. 1996. Comparative properties of feline immunodeficiency virus (FIV) and human immunodeficiency virus type 1 (HIV-1) proteinases prepared by total chemical synthesis. Virology 224:268-275. [DOI] [PubMed] [Google Scholar]
- 26.Shetty, B. V., M. B. Kosa, D. A. Khalil, and S. Webber. 1996. Preclinical pharmacokinetics and distribution to tissue of AG1343, an inhibitor of human immunodeficiency virus type 1 protease. Antimicrob. Agents Chemother. 40:110-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tachibana, H., K. Kawabata, M. Takeda, and M. Sugita. 1993. Multimodal evoked potentials in Binswanger's disease and Alzheimer's disease. Int. J. Neurosci. 69:197-206. [DOI] [PubMed] [Google Scholar]
- 28.Tachibana, H., M. Takeda, and M. Sugita. 1989. Brainstem auditory evoked potentials in patients with multi-infarct dementia and dementia of the Alzheimer type. Int. J. Neurosci. 48:325-331. [DOI] [PubMed] [Google Scholar]
- 29.Tachibana, H., M. Takeda, and M. Sugita. 1989. Short-latency somatosensory and brainstem auditory evoked potentials in patients with Parkinson's disease. Int. J. Neurosci. 44:321-326. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto, J. K., H. Hansen, E. Ho, T. Morishita, T. Okuda, T. Sawa, R. Nakamura, and N. Pedersen. 1989. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J. Am. Vet. Med. Assoc. 194:213-220. [PubMed] [Google Scholar]
- 31.Yuan, J. H., J. C. Stolzenbach, C. M. Salamon, S. S. Snook, and G. L. Schoenhard. 1997. Improvement of bioavailability of the HIV protease inhibitor SC-52151 in the beagle dog by coadministration of the CYP3A4 inhibitor, ketoconazole. Xenobiotica 27:489-497. [DOI] [PubMed] [Google Scholar]