Abstract
There are many different types of enteric neurons. Previous studies have identified the time at which some enteric neuron subtypes are born (exit the cell cycle) in the mouse, but the birthdates of some major enteric neuron subtypes are still incompletely characterized or unknown. We combined 5-ethynynl-2’-deoxyuridine (EdU) labeling with antibody markers that identify myenteric neuron subtypes to determine when neuron subtypes are born in the mouse small intestine. We found that different neurochemical classes of enteric neuron differed in their birthdates; serotonin neurons were born first with peak cell cycle exit at E11.5, followed by neurofilament-M neurons, calcitonin gene-related peptide neurons (peak cell cycle exit for both at E12.5-E13.5), tyrosine hydroxylase neurons (E15.5), nitric oxide synthase 1 (NOS1) neurons (E15.5) and calretinin neurons (P0). The vast majority of myenteric neurons had exited the cell cycle by P10. We did not observe any EdU+/NOS1+ myenteric neurons in the small intestine of adult mice following EdU injection at E10.5 or E11.5, which was unexpected as previous studies have shown that NOS1 neurons are present in E11.5 mice. Studies using the proliferation marker, Ki67, revealed that very few NOS1 neurons in the E11.5 and E12.5 gut were proliferating. However, Cre-lox-based genetic fate-mapping revealed a small sub-population of myenteric neurons that appears to express NOS1 only transiently. Together, our results confirm a relationship between enteric neuron subtype and birthdate, and suggest that some enteric neurons exhibit neurochemical phenotypes during development that are different from their mature phenotype.
Introduction
There are many different functional types of enteric neurons (Brookes, 2001, Uyttebroek et al., 2010, Furness, 2012), but little is known about the mechanisms involved in the generation of enteric neuron subtype diversity (Hao and Young, 2009, Laranjeira and Pachnis, 2009, Gershon, 2010, Sasselli et al., 2012, Obermayr et al., 2013a). The birthdate of a neuron is the age at which a precursor undergoes its last division before differentiating into a neuron, and it can be an important determinant of neuronal subtype fate. For example, in the cerebral cortex there is a sequential production of different neuron subtypes, and a progressive restriction in the developmental potential of progenitors (Leone et al., 2008). Moreover, the age at which cell cycle exit occurs is also an important determinant in the differential response of different subtypes of enteric neurons to developmental cues and disturbances (Chalazonitis et al., 2008, Gershon, 2010, Li et al., 2010, Wang et al., 2010, Li et al., 2011). A landmark study by Pham et al (1991), who used tritiated thymidine birthdating, first showed that some enteric neuron subtypes in the mouse differ in their birthdates. A later study used BrdU to identify additional enteric neuron subtypes that exit the cell cycle from E12.5 in the mouse (Chalazonitis et al., 2008). Although myenteric neuron subtypes in the mouse have been well characterized based on neurochemistry and electrophysiology (Sang and Young, 1996, Nurgali et al., 2004, Qu et al., 2008, Neal et al., 2009, Foong et al., 2012), the peak times of cell cycle exit for some major enteric neuron subtypes are still incompletely characterized or unknown.
In the myenteric plexus of the mouse small intestine, the peak time of cell cycle exit of serotonin enteric neurons is E10, for enkephalin, neuropeptide Y and VIP neurons is E14-E15, and for CGRP neurons is E17 (Pham et al., 1991). The peak time of cell cycle exit for calbindin, NOS1, GABA and dopamine neurons was reported to be E14.5, although cell cycle exit was not examined before E12.5 in this study (Chalazonitis et al., 2008). As NOS1 neurons are present at E11.5, and are one of the first neuron sub-types to appear (Hao et al., 2010, Hao et al., 2013a), it is important to examine cell cycle exit of NOS1 neurons at earlier ages.
The neural circuitry regulating motility in the bowel consists of intrinsic sensory neurons, inhibitory and excitatory motor neurons, and ascending and descending interneurons (Furness, 2012). In this study we examined the major myenteric neuron subtypes involved in motility in the mouse. We examined the birthdates of neurons expressing NF-M and CGRP, as NF-M and CGRP are markers of putative intrinsic sensory neurons in the mouse small intestine (Grider, 2003, Qu et al., 2008). NOS1 is a marker of inhibitory motor neurons, although there is also a small population of NOS1 interneurons (Sang and Young, 1996, Qu et al., 2008), and we used calretinin as a marker of excitatory motor neurons (Sang and Young, 1996). The birthdates of serotonin neurons, which are descending interneurons, were examined as a control to compare to previous studies (Pham et al., 1991).
Materials and Methods
EdU labeling
Time plug-mated C57BL/6 mice received a single intraperitoneal injection of 5-ethynynl-2’-deoxyuridine (EdU - Invitrogen, Grand Island, USA; 50 µg/g body weight) at E10.5, E11.5, E12.5, E13.5, E15.5 and E18.0. P0 and P10 mice also received a single intraperitoneal injection of EdU (50 ug/g body weight). Experiments were approved by the Animal Ethics Committee of the Departments of Anatomy and Neuroscience, Pathology, Pharmacology and Physiology at the University of Melbourne. Except for tissue processed for CGRP immunohistochemistry, the mice were killed at 5–8 weeks of age. As CGRP is difficult to detect in the cell bodies of myenteric neurons in adult mice without pre-treatment with colchicine (Qu et al., 2008), but can be localized in the cell bodies of myenteric neurons at late embryonic and neonatal stages (Branchek and Gershon, 1989, Young et al., 1999, Turner et al., 2009), P0-P4 mice were used for examination of CGRP neurons. Mice were killed by cervical dislocation, and the intestines removed. The middle third of the small intestine was opened down the mesenteric border, pinned and fixed in 4% formaldehyde in 0.1 M phosphate buffer pH 7.3 overnight at 4°C. Tissue to be processed for serotonin immunohistochemistry was fixed as described by Chen et al. (Chen et al., 2001). After washing, wholemount preparations of longitudinal muscle/myenteric plexus were exposed to 1% Triton X-100 for 30 min and processed for immunohistochemistry using the primary antibodies shown in Table 1. They were then reacted for EdU using a Click-iT EdU Alexa Fluor 647 Kit (Invitrogen) and the manufacturer’s instructions.
Table 1.
Primary antibodies used. CGRP calcitonin gene-related peptide, GFP green fluorescent protein, NF-M neurofilament 145 kDa, NOS1 neuronal nitric oxide synthase, TH tyrosine hydroxylase
| Antigen | Cells identified by antiserum |
Immunogen | Antibody Information | Dilution |
|---|---|---|---|---|
| calretinin | excitatory motor neurons and interneurons | Human recombinant calretinin | Swant (Bellinzona, Switzerland), goat polyclonal, Cat No. CG1 | 1:1000 |
| CGRP | intrinsic sensory neurons | Synthetic rat Tyr-CGRP (23–37) | Biogenesis, goat polyclonal, Cat No. 1720-9007 | 1:1000 |
| GFP | GFP isolated from Aequorea victoria | Molecular Probes (Grand Island), rabbit polyclonal, Cat No. A11122 | 1:500 | |
| Hu | all neurons | Autoantibodies from a patient with small cell lung carcinoma | Dr Vanda Lennon, Mayo Clinic, Rochester, MN, USA, Human polyclonal | 1:5000 |
| Ki67 | proliferating cells | Synthetic peptide against the C terminus of human Ki67 | Thermo Scientific, monoclonal rabbit, clone SP6, Cat No. RM- 9106-S | 1:100 |
| NF-M | intrinsic sensory neurons | Recombinant fusion protein containing the C-terminal 168 amino acids of rat NF-M (145 kD) | Chemicon, rabbit polyclonal, Cat No. #AB1987 | 1:1000 |
| NOS1 | inhibitory motor neurons and descending interneurons | NOS1 isolated from rat brain | Dr Piers Emson, University of Cambridge, UK, sheep polyclonal | 1:2000 |
| serotonin | descending interneurons | Serotonin coupled to bovine serum albumin with paraformaldehyde | ImmunoStar, rabbit polyclonal, Cat No. #20080 | 1:2000 |
| TH | myenteric neuron of unknown function | TH isolated from rat pheochromocytoma | Chemicon, sheep polyclonal, #AB1542 | 1:160 |
Ki67 labeling
The gut caudal to the stomach was dissected from E11.5 and E12.5 mice. Following fixation, wholemount preparations and frozen sections of E11.5 and E12.5 gut were subject to antigen retrieval in which tissue was transferred to 50 mM Tris buffer and placed in a microwave oven for 1 min on high and for 10 min on low. The tissue was then washed in 0.1 M phosphate buffer and incubated with rabbit anti-Ki67 and sheep anti-NOS1 (Table 1).
Antibody characterization
The primary antisera used, their sources and dilutions are summarized in Table 1. The specificity of the goat anti-calretinin was confirmed by the absence of staining in the cerebral cortex of calretinin null mutant mice (manufacturer’s information) and in macaque cerebral cortex after preabsorption with calretinin protein (Disney and Aoki, 2008).
Goat anti-CGRP (Kalous et al., 2009) reacts with whole molecule (1–37) as well as the 23–37 C-terminal fragment (manufacturer’s information). Western blot analysis revealed that this antibody recognizes the synthetic rat α-CGRP peptide and detects a single band at 16 kDa in homogenates of rat medulla oblongata (Yasuhara et al., 2008). In addition, pre-absorption with CGRP 1–37 abolishes immunostaining in rat medulla (Gnanamanickam and Llewellyn-Smith, 2011).
Rabbit anti-GFP shows no staining in Drosophila or zebrafish retina (Zagoraiou et al., 2009, Allison et al., 2010) or mouse intestine (Hao et al., 2013b) lacking the reporter gene.
Human anti-Hu was collected from a patient suffering from small cell lung carcinoma and severe sensorimotor neuropathy. This antiserum recognizes a single band of 41 kDa in Western blots of extracts of chicken brain (Erickson et al., 2012).
Rabbit anti-Ki67 was raised against a peptide representing the C-terminus of human Ki67. In sympathetic ganglia of embryonic mice, the temporal pattern of staining with this antibody matches that of the M phase marker, phosopho histone 3, and the S phase marker, BrdU (Gonsalvez et al., 2013). Furthermore, staining is observed in the basal cell layer of the lingual epithelium of mice, where proliferation is known to occur, but not in the brainstem (Bartel, 2012). In the current study, Ki67 immunostaining was restricted to nuclei, but not every nucleus was Ki67+.
Rabbit anti-neurofilament M (NF-M) was raised against a recombinant fusion protein containing the C-terminal 168 amino acids of rat NF-M (145 kD). In Western blots, the NF-M antibody detected NF-M in mouse brain lysates (manufacturer’s datasheet). Furthermore, by immunoblotting, the NF-M antibody recognizes NF-M but not NF-H in the brain of chicken, cow and pig, and using immunohistochemistry, only stains neurons across a wide range of species (Harris et al., 1991).
Sheep anti-NOS1 was raised again rat brain NOS1; when reacted with purified baculovirus-expressed rat neuronal NOS1 protein, the antibody recognizes one main protein with a molecular mass of 155kD in Western blots (Herbison et al., 1996). In addition, NOS1 immunoreactivity in mouse enteric neurons is abolished following pre-absorption of the diluted antibody with purified NOS1 protein (Young and Ciampoli, 1998).
Rabbit anti-serotonin was raised against serotonin coupled to bovine serum albumin with paraformaldehyde, and staining is eliminated by pretreatment of the diluted antibody with 20 µg/ml serotonin/bovine serum albumin (manufacturer’s information), but serotonin labeling in rat spinal cord is not altered by BSA preabsorption (Takeoka et al., 2009).
Sheep anti-tyrosine hydroxylase (TH) was raised against TH from rat pheochromocytoma, and in immunoblotting studies, the antibody reacts with the ~60 kDa TH protein in PC12 cells stimulated with okadaic acid (manufacturer’s information).
With the exception of the anti-Ki67, all antisera have been used previously in studies of peripheral autonomic neurons of mice (Callahan et al., 2008, Qu et al., 2008, Yan and Keast, 2008, Mongardi Fantaguzzi et al., 2009, Li et al., 2010, Li et al., 2011, Erickson et al., 2012, Hao et al., 2013b). The percentages of myenteric neurons stained with each antiserum, and the morphologies of the neuron subtypes, matched previous descriptions. For example, NOS1 immunostained neurons comprised between 30–35% of all (Hu+) myenteric neurons and most possessed lamellar dendrites (Sang and Young, 1996, Qu et al., 2008, Obermayr et al., 2013b). NF-M neurons comprised around 20% of myenteric neurons and possessed large, smooth cell bodies (Dogiel II morphology), while calretinin neurons comprised 40–45% of myenteric neurons and included both neurons with lamellar dendrites and neurons with Dogiel II morphology (Sang and Young, 1996, Qu et al., 2008). The proportions of serotonin and TH neurons were not determined, but as reported previously, they occurred at low frequency; serotonin neurons possessed lamellar dendrites (Qu et al., 2008).
Secondary antisera
Secondary antiserum used for calretinin, CGRP, NOS1 and TH immunolabeling was donkey anti-sheep FITC (1:100; Jackson Immunoresearch, West Grove, PA, USA); for NF-M, serotonin, GFP and Ki67 was donkey anti -rabbit FITC (1:200, Jackson ImmunoResearch); and for Hu was donkey anti-human Texas Red (1:100, Jackson ImmunoResearch) or donkey anti-human Alexa 647 (1:200, Jackson ImmunoResearch).
Imaging
Single focal plane images were obtained on a Zeiss Pascal confocal microscope using a X20 objective lens (area of field of view = 154,900 µm2) for adult tissue, and a X40 objective lens for embryonic and postnatal tissue (area of field of view = 38,700 µm2). Fields of view were selected by starting in one corner of each preparation and then moving across the tissue in a raster fashion from side-to-side and from top-to-bottom, but avoiding areas that were folded or damaged.
Analysis of EdU retention
Images were imported into ImageJ for manual cell counting using the “Cell Counting and Marking” plugin, which ensured that every labeled neuron in an image was counted, but only once. Immunolabelled neurons that were not completely within the field of view were counted if >50% of their nucleus was visible. EdU+ neurons were defined as those cells with 3 or more particles of EdU immunostaining. TH and serotonin neurons comprise 1% or less of myenteric neurons and so only small numbers of neurons were present in each preparation. Therefore, the proportions of serotonin or TH neurons that had incorporated EdU were expressed as the proportion of the total number of (serotonin or TH) neurons observed across a minimum of 3 preparations, each from a different animal. For the other, more abundant, neuron subtypes, the percentage of neuron subtype that was EdU+ was determined for each preparation (each from a different animal). The mean and SEM of the proportion of EdU+ neurons was determined from a minimum of 3 preparations for each neuron subtype following EdU injections at each age (see Table 2).
Table 2.
Total numbers of neurons of each type examined in adult mice (or P0-P4 mice for CGRP) following injection of EDU at different developmental ages. The number of mice is shown in brackets.
| Hu | calretinin | CGRP | NF-M | NOS1 | serotonin | TH | |
|---|---|---|---|---|---|---|---|
| E10.5 | 5247 (11) | 808 (4) | 421 (4) | 283 (4) | 503 (3) | 47 (4) | 7 (3) |
| E11.5 | 3516 (13) | 615 (6) | 73 (3) | 94 (3) | 676 (7) | 33 (3) | 16 (5) |
| E12.5 | 1952 (8) | 690 (4) | 170 (3) | 46 (3) | 263 (4) | 49 (4) | 33 (4) |
| E13.5 | 1433 (7) | 264 (3) | 215 (3) | 201 (4) | 240 (4) | 48 (5) | 27 (7) |
| E15.5 | 4678 (12) | 678 (4) | 379 (4) | 247 (4) | 519 (4) | 44 (4) | 54 (6) |
| E18.5 | 1926 (9) | 307 (3) | 418 (4) | 115 (3) | 354 (4) | 29 (3) | 23 (4) |
| P0 | 3172 (10) | 332 (3) | ND | 148 (3) | 411 (3) | ND | 28 (4) |
| P10 | 1947 (6) | ND | ND | ND | ND | ND | ND |
ND: not determined
Genetic fate mapping of Nos1-expressing neurons
Nos1-Cre mice were mated to Rosa-CAG-eGFP or Rosa-CAG-TdTomato reporter mice to generate Nos1-eGFP or Nos1-TdTomato mice (Leshan et al., 2012). The procedures and care of the mice were approved by the University of Michigan Committee on the Use and Care of Animals. The middle section of small intestine of 4–5 week old mice was fixed as described above and processed for immunohistochemistry using antibodies to NOS1 and Hu (and GFP for tissue from the Nos1-eGFP mouse only) (Table 1). From each mouse (n = 3), a minimum of 14 fields of view (area of each field of view = 38,700 µm2) containing a total of 100–168 cells expressing eGFP or TdTomato were analyzed to determine the overlap between NOS1immunoreactivity and eGFP (or TdTomato) expression.
Photomicrograph production
Figures were produced using CorelDRAW. Minor adjustments were made to the brightness and contrast of some images.
Results
EdU is an analogue of thymidine and is incorporated into DNA during active DNA synthesis. A single pulse of EdU was delivered to E10.5, E11.5, E12.5, E13.5, E15.5, E18.0, P0 or P10 mice. The mice were killed at 5–8 weeks of age, except for mice to be processed for CGRP immunostaining following EdU injection at prenatal stages, which were killed at P0-P4. We then determined the proportion of each neuron subtype in the myenteric plexus of the jejunum that was EdU+. The pulse of EdU will label cells in S phase at the time of delivery. Cells that undergo their final S phase at the time of exposure to EdU will retain a strong EdU signal, while cells that undergo further rounds of cell division will dilute the EdU. Cells that undergo one or a small number of subsequent cell divisions are also likely to retain some EdU, but we did not attempt to discriminate between different levels of EdU staining, other than to arbitrarily define cells with 3 or more particles of EdU immunostaining as being EdU+. The numbers of neurons of each type examined in the jejunum following injection of EdU at different ages are shown in Table 2. We did not observe any cell that expressed a neuron sub-type marker (calretinin, CGRP, NF-M, NOS1, serotonin or TH) that did not express the pan-neuronal marker, Hu. The data are summarized in figures 1 and 2: Figure 1 shows the percentage of each cell type that was labeled following EdU injections at different ages, and figure 2 shows cumulative birthdates in which the cumulative percentage of EdU-labeled cells of each neuron type born by a given age was calculated from the data shown in figure 1.
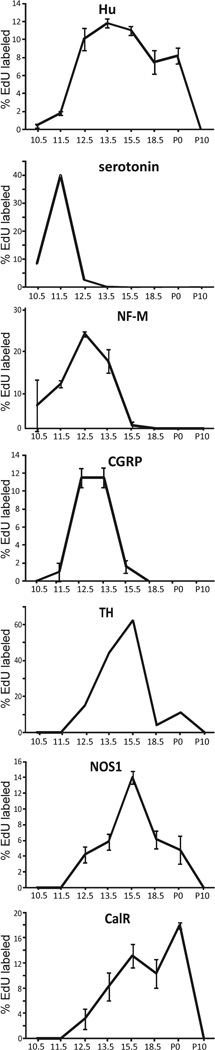
Figure 1.
Percentages of different myenteric neuron types in the mouse small intestine to retain EdU following EdU injections at different ages. For Hu, NF-M, CGRP, NOS1 and calretinin neurons, the data are shown as mean ∀ SEM from a minimum of 3 mice. Serotonin and TH neurons comprise only a very small proportion of myenteric neurons and so data from different animals were pooled following EdU injections at each age. The birthdates of the myenteric neuron population as a whole (Hu+ neurons) vary from E10.5 (or before) to post-natal ages, but the vast majority are born prior to P10. Different myenteric neuron subtypes exit the cell cycle at different, but overlapping, ages. The peak time of cell exit for serotonin neurons is E11.5, for NF-M and CGRP neurons is E12.5-E13.5, for TH and NOS1 neurons is E15.5 and for calretinin neurons is P0.
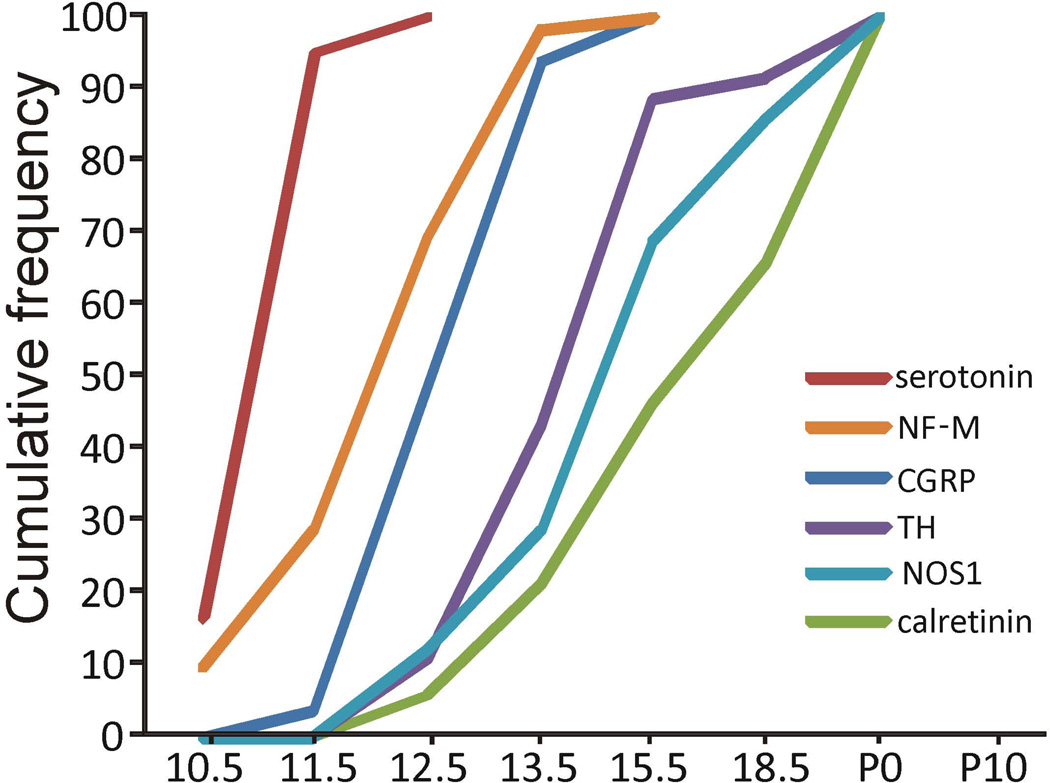
Figure 2.
Cumulative birthdate curves for different subtypes of myenteric neurons in the mouse small intestine calculated from the data shown in figure 1, showing sequential generation of different myenteric neuron subtypes. The curve for each neuron subtype was generated by determining the total number of EDU+ cells of that neuron subtype observed following EDU injection at all ages, calculating the proportion of the total that was EDU+ following injection at E10.5, and then adding the proportion of EDU+ neurons observed following injections at each subsequent age (cumulative frequencies). For example, 95% of the total number of EDU+ serotonin neurons observed were observed following EDU injection at E11.5 or E10.5. In contrast, the number of EDU+ calretinin neurons observed after injection of EDU at E10.5, E11.5, E12.5, E15.5 and E18.5 combined accounted for only 66% of the total number of EDU+ calretinin neurons observed.
All (Hu+) myenteric neurons
Following injection of EdU at E10.5, around 0.5% of all (Hu+) myenteric neurons in the jejunum was EdU+ (Figs. 1, 3A,C), and around 2% of Hu+ neurons was EdU+ after EdU at E11.5 (Fig. 1). 8–12% of Hu+ neurons had incorporated EdU following EdU injection at E12.5, E13.5, E15.5 and P0 (Fig. 1). We did not observe any Hu+ cells that had incorporated EdU following a pulse of EdU at P10, although EdU+/Hu-negative cells, presumably glia, were common in ganglia (Fig. 4A,B). These data show that the peak time of cell cycle exit for the myenteric neuron population as a whole is between E12.5 and P0, and by P10, the vast majority of neuron precursors appear to have exited the cell cycle.
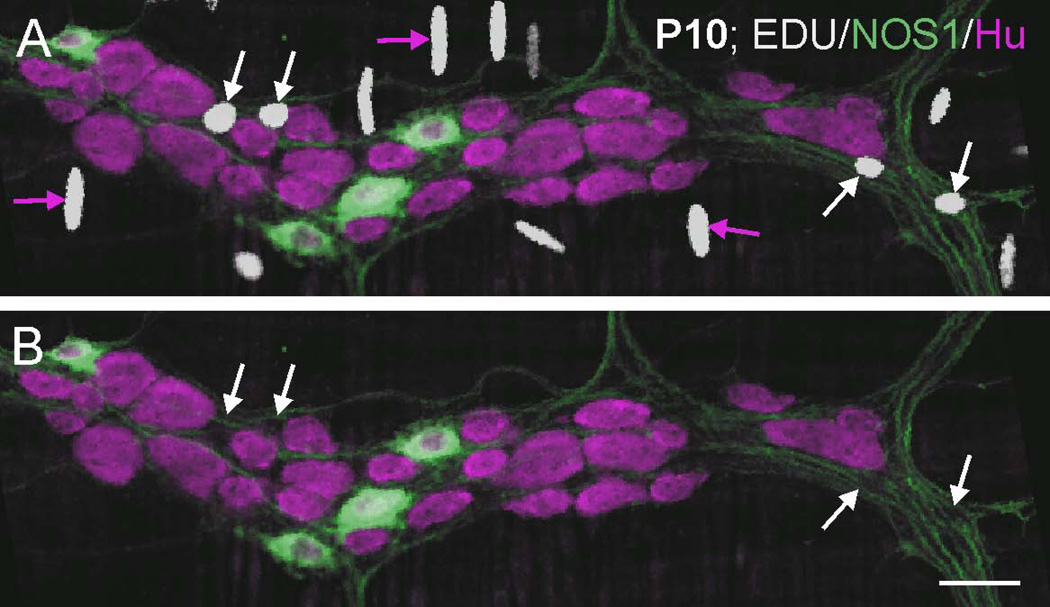
Figure 4.
Myenteric ganglion from the small intestine of an adult mouse following EdU injection at P10 and immunostaining for EdU (white), NOS1 (green, to reveal the intermodal strands), and the pan-neuronal marker, Hu (magenta). EdU+ cells are present in the ganglia and intermodal strands (A, white arrows), but do not show Hu immunoreactivity (B) and hence are likely to be glial cells. Numerous EdU+ nuclei are also present in the circular muscle layer (magenta arrows). Scale bar: 25 µm.
Serotonin neurons
Serotonin neurons are descending interneurons and they comprise only ~1% of myenteric neurons (Sang et al., 1997, Qu et al., 2008, Neal et al., 2009). EdU+/serotonin neurons were observed following injection of EdU at E10.5 (Fig. 3C,D), E11.5 and E12.5 only, with the peak birthdate being E11.5 (Figs. 1,2). The only other class of neuron we examined that incorporated EdU following injection of EdU at E10.5 was the NF-M neurons.
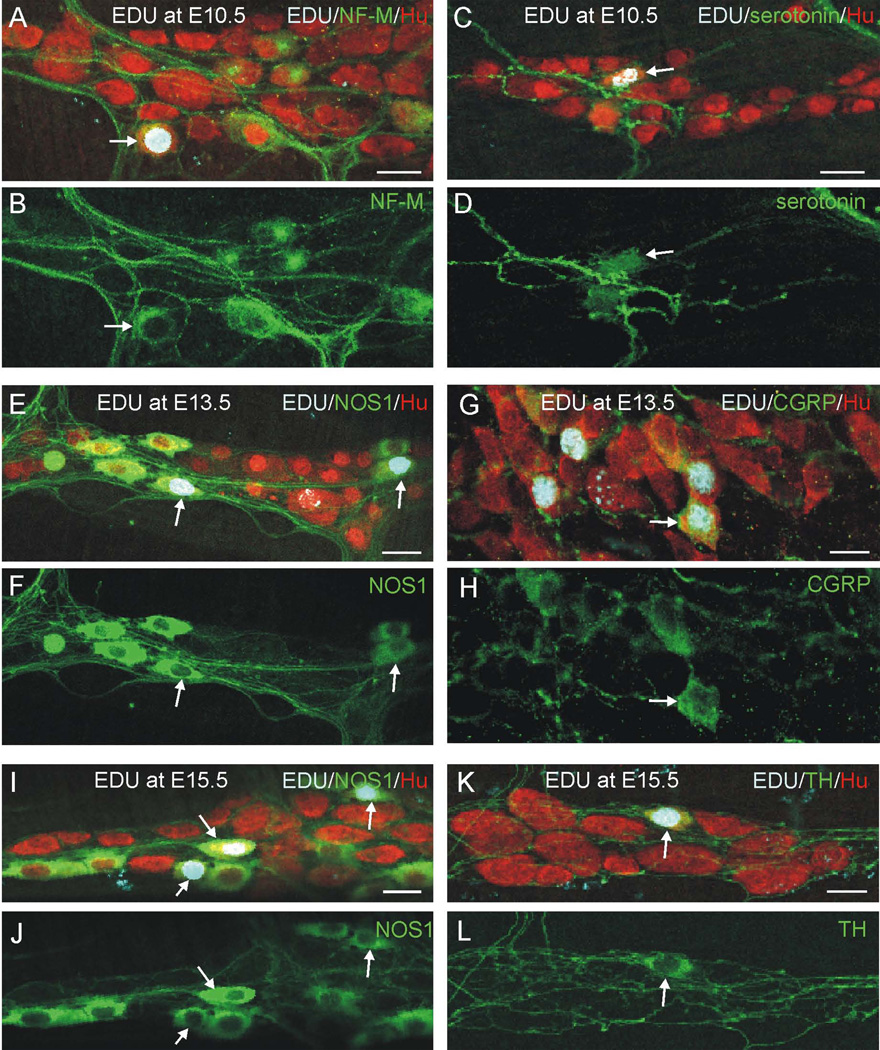
Figure 3.
Confocal microscope images of myenteric ganglia in wholemount preparations of myenteric plexus/longitudinal muscle of small intestine following injection of EdU at E10.5 (A–D), E13.5 (E–H) or E15.5 (I–L) and immunostaining for EdU (pale blue), different myenteric neuron subtype markers (green), and the pan-neuronal marker, Hu (red). All preparations are from adults, except for panels G and H, which are from P0 mice. A–D: Following EdU injection at E10.5, sub-populations of NF-M (A,B) and serotonin (C,D) neurons are EdU+ (arrows). E–H: Sub-populations of NOS1 (E,F) and CGRP (G,H) neurons are EDU+ (arrows) following EdU injection at E13.5. I–L: Following EdU injection at E15.5, sub-populations of NOS1 (I,J) and TH (K,L) neurons are EdU+ (arrows). Scale bars: 25 µm
NF-M neurons
Although all neurons in the embryonic mouse gut that express pan-neuronal markers also show NF-M immunostaining (Hao et al., 2009), in the small intestine of adult mice, only neurons with Dogiel type II morphology show NF-M staining; these neurons are thought to be intrinsic sensory neurons (Qu et al., 2008). EdU+/NF-M+ neurons were observed following EdU injections at E10.5 (Fig. 3A,B), E11.5, E12.5, E13.5 and E15.5, although only very rare EdU+ neurons were observed following injection at E15.5 (Fig. 1). The peak birthdate for NF-M neurons was E12.5 (Figs. 1,2).
CGRP neurons
Pharmacological studies in the mouse colon suggest that the sensory neurons involved in the peristaltic reflex release CGRP (Grider, 2003). Furthermore, most myenteric CGRP neurons in the ileum and colon of mice are Dogiel type II neurons, and thus like NF-M, CGRP is likely to be a marker of intrinsic sensory neurons (Nurgali et al., 2004, Qu et al., 2008). The incorporation of EdU by CGRP neurons was examined in the small intestine from P0-P4 mice because, unlike adult mice, the cell bodies of CGRP neurons in neonatal mice can be detected without the use of colchicine (Young et al., 1999, Qu et al., 2008). EdU+/CGRP+ neurons were not observed following EdU injection at E10.5, but a small number of EdU+/CGRP+ neurons were observed following EdU injection at E11.5 (Fig. 3G,H). The peak birthdate for CGRP neurons was E12.5-E13.5 (Figs. 1,2). Only a small number of EdU+/CGRP+ neurons were observed following EdU injection at E15.5, and none following EdU at E18.5 (Fig. 1).
TH neurons
TH neurons comprise <0.5% of myenteric neurons in the mouse small intestine (Qu et al., 2008). The peak birthdate of TH neurons was E13.5-E15.5 (Fig. 3K,L, but small numbers of EdU+/TH+ cells were also observed following EdU injections at E12.5, E18.5 and P0 (Fig. 1).
NOS1 neurons
NOS1 neurons in the myenteric plexus of the mouse small intestine comprise multiple sub-types of neurons, but the majority are inhibitory motor neurons to the circular muscle (Qu et al., 2008). During development, NOS1 neurons are first observed at E11.5, and there is a large increase in the number of NOS1 neurons between E11.5 and E12.5 (Hao et al., 2010). We therefore expected that some NOS1 neuron precursors would exit the cell cycle at E10.5 and E11.5 prior to the first expression of NOS1. However, following EdU injection at E10.5 or E11.5, EdU+/NOS1+ neurons were not observed (Fig. 1). EdU+/NOS1+ neurons were observed following injection of EdU at E12.5, E13.5 (Fig. 3E,F), E15.5 (Fig. 3 I,J), E18.5 and P0, with the peak birthdate being E15.5 (Figs. 1,2).
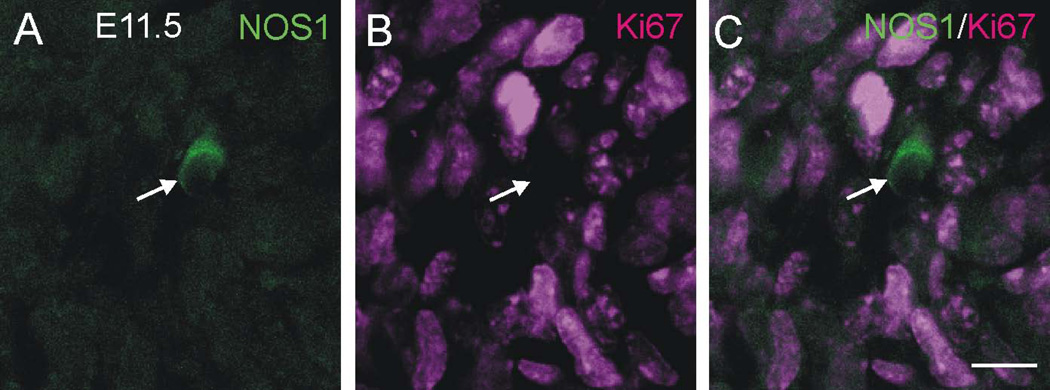
The absence of EdU+/NOS1+ neurons following EdU injection at E10.5 and E11.5 raised the possibility that the first NOS1 neurons to appear during development are still proliferating. We therefore examined whether NOS1 neurons in the gut of E11.5 and E12.5 mice show Ki67 staining. Ki67 is a marker of proliferating cells and is present during G1, S, G2 and M phases, but absent during G0 (resting) phase. No Ki67+/NOS1+ neurons were observed in the E11.5 gut (n = 12 NOS cells from 4 preparations) (Fig. 5A–C), and only 3% of NOS1+ neurons in the E12.5 gut showed any Ki67 staining (2 Ki67+ cells out of 67 NOS1 cells examined from 3 embryos).
Figure 5.
Cryostat section through the small intestine of an E11.5 mouse after immunostaining for NOS1 (A) and the proliferation marker, Ki67 (B). Many Ki67+ nuclei are present, but the nucleus of the NOS1+ neuron (arrow) does not show any Ki67 immunostaining (C). Scale bar: 10 µm.
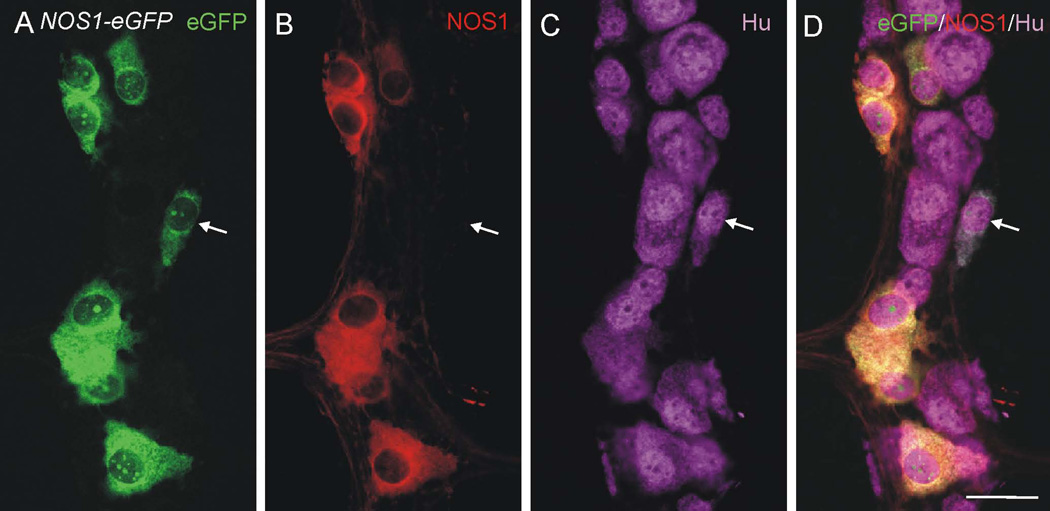
We then used in vivo Cre-lox-based genetic fate mapping to determine if there are myenteric neurons that transiently express NOS1. Nos1-Cre mice (Leshan et al., 2012) were mated to Rosa-CAG-eGFP or Rosa-CAG-TdTomato reporter mice to generate Nos1;eGFP (n = 1) or Nos1;TdTomato mice (n = 2), in which eGFP or TdTomato is permanently expressed in any cell that expressed NOS1, including cells that expressed NOS1 only transiently. Neurons that were eGFP+ or TdTomato+, but did not show NOS1 immunoreactivity, were likely to have expressed NOS1 transiently during development. 7.5 ± 1.6% (mean ± SEM) of eGFP+ or TdTomato+ neurons did not show detectable NOS1 immunoreactivity (Fig. 6; n = 401 eGFP+ or TdTomato+ neurons examined from 3 mice). We also checked the efficiency of Cre expression and recombination. In two mice (1 X Nos1;eGFP and 1 X Nos1;TdTomato), 100% of NOS1-immunoreactive neurons were eGFP+ or TdTomato+, and in the third mouse, 95% of NOS1-immunoreactive neurons were TdTomato+.
Figure 6.
Myenteric ganglion in a wholemount preparation from the small intestine of a Nos1;eGFP mouse that had been immunolabeled using antibodies to GFP, NOS1 and Hu. A GFP+/Hu+ neuron does not show detectable NOS1 immunostaining (arrow). Scale bar: 25 µm
Calretinin neurons
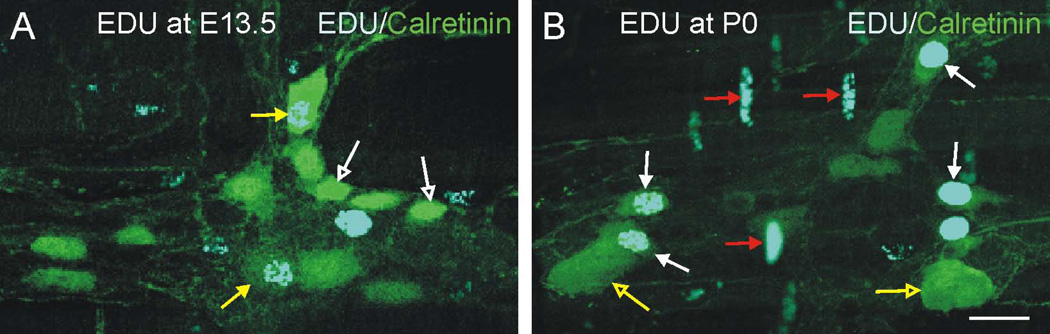
Most calretinin neurons in the myenteric plexus of the mouse small intestine are excitatory motor neurons to the circular and longitudinal muscle layers, but some putative sensory neurons also express calretinin (Qu et al., 2008). Calretinin immunoreactivity is first detected in the ENS around birth (Young et al., 1998). Like NOS1 neurons, no EdU+/calretinin+ neurons were observed following EdU injection at E10.5 or E11.5. The peak birthdate of calretinin neurons was P0 (Figs. 1,2). Although calretinin neurons comprised around 40% of total myenteric neurons, 77.0% ± 0.4 (mean ± SEM, n = 3 mice) of EdU+ neurons was calretinin+ following injection of EdU at P0. The EdU+/calretinin+ neurons we observed following injection of EdU at P0 appeared to be mainly small calretinin+ neurons Fig. 7A,B). We therefore compared the area of the cell body of individual EdU+/calretinin+ neurons following EdU injections at E13.5 and P0. Calretinin neurons that had incorporated EdU following injection of EdU at P0 were significantly smaller than calretinin neurons that incorporated EdU at E13.5 (mean ± SEM of area of EdU+ calretinin neurons, P0: 175 ± 23 µm; E13.5: 455 ± 73 µm; 2 tailed t test, p<0.05).
Figure 7.
Myenteric ganglia from the small intestine of adult mice following EdU injection at E13.5 (A) or P0 (B), and immunostaining for EdU (blue) and calretinin (red). A. Following EdU injection at E13.5, the calretinin+/EdU+ cells are large cells (yellow arrows) and the small calretinin+ cells are not EdU+ (white open arrows). B. At P0, many small calretinin+ cells are EdU+ (white arrows) but the large calretinin+ cells are not EdU+ (yellow open arrows). Numerous EdU+ nuclei are present in the circular muscle layer following EdU injection at P10 (B, red arrows), but not following EdU injection at E13.5. Scale bar: 25 µm
Discussion
Our study confirms and extends previous studies that demonstrated that different neurochemical classes of myenteric neurons are born during distinct, but overlapping, intervals in the mouse intestine (Pham et al., 1991, Chalazonitis et al., 2008). The first expression of different enteric neuron subtype markers occurs at different developmental ages (Hao and Young, 2009), and for an individual enteric neuron, the interval between exit from the cell cycle and the expression of subtype-specific markers is not currently known. However, our data suggest that the interval between cell cycle exit and first detectable expression of subtype markers varies between neuron subtypes and subtype markers from 2 days or less to around a week (see below). The relationship between enteric neuron birthday and enteric neuron subtype shown by earlier studies (Pham et al., 1991, Chalazonitis et al., 2008) and the current study suggests that it is likely that birthday influences enteric neuron subtype identity. For example, different enteric neuron subtypes might be specified by extrinsic determinative factors, which change with time and act around the time the precursors exit the cell cycle (Chalazonitis et al., 2008, Gershon, 2010, Li et al., 2010, Wang et al., 2010, Li et al., 2011, Lake and Heuckeroth, 2013), and/or the ability of enteric neuron progenitors or precursors to respond to extrinsic factors might change over time.
Enteric neurons are born over a protracted period
We found that around 0.5% of myenteric neurons are born at E10.5, which was the earliest age that we introduced EdU. However, an earlier study using tritiated thymidine birthdating showed that some enteric neurons are born as early as E8 (Pham et al., 1991). Perinatally (E18.5 and P0), we found that there were still significant numbers of myenteric neurons being born, but by P10 the vast majority of neurons appear to be postmitotic as none of nearly 2000 Hu+ neurons examined after injection of EdU at P10 had incorporated and retained EdU. A stage-specific lineage tracing study reported that 1.6% of myenteric neurons are generated from Sox10+ progenitors at P30 (Laranjeira et al., 2011); these data are not necessarily inconsistent with the current study as it is possible that enteric neurogenesis can occur without cell division (Joseph et al., 2011, Laranjeira et al., 2011). It is also likely that to detect low numbers of dividing enteric neuron precursors at postnatal stages, thymidine analogues would need to be introduced over an extended period of time (Liu et al., 2009), rather than as a single injection as was used in the current study.
Relationship between enteric neuron subtype and birthdate
Our study focused on markers that identify different functional subtypes of myenteric neurons (Sang and Young, 1996, Sang et al., 1997, Qu et al., 2008), and therefore have relevance for the development of the functional circuits controlling motility.
Consistent with an earlier study (Pham et al., 1991), we found that serotonin neurons, which are descending interneurons, have a peak birthdate at E11.5. Most serotonin neurons were born between E10.5 and E12.5, and it is also possible that some are born prior to E10; serotonin neurons were the first neurons of those we examined to be born. Uptake of 5-[3H]HT can be first detected by neurites in the mouse small intestine at E12–13 (Rothman and Gershon, 1982, Rothman et al., 1984), and thus the interval between cell cycle exit and first expression of serotonergic properties appears to be only around 2 days. Importantly, spontaneous release of serotonin from enteric neurons generated early during ENS development appears to influence the development of neuronal subtypes that are generated later in ENS development (Li et al., 2011).
NF-M and CGRP have been reported to be markers of intrinsic sensory neurons in the mouse (Grider, 2003, Qu et al., 2008), although mechanosensitive myenteric neurons in the mouse ileum and colon also include other neuronal phenotypes (Mazzuoli and Schemann, 2012). We found that the peak birthdates of NF-M and CGRP neurons were E12.5-E13.5. Thus it appears that some intrinsic sensory neurons are born relatively early in the development of the ENS. Although NF-M and CGRP are thought to identify the same population of myenteric neurons, there were some small differences between our birthdate data for NF-M and CGRP neurons; most importantly, following EdU injection at E10.5, we found some NF-M+/EdU+ neurons, but did not observe any CGRP neurons that had incorporated and retained EdU. This suggests that CGRP and NF-M expression do not overlap 100% in adult myenteric neurons. It is very difficult to examine directly the overlap between NF-M and CGRP in myenteric neurons as in our hands CGRP immunoreactivity can only be detected in the cell bodies of myenteric neurons from around E17.5 until P4/P5. However, NF-M is not an exclusive marker of Dogiel type II neurons during embryonic and early postnatal development, and so the overlap between NF-M and CGRP cannot be examined in early postnatal tissue. Although CGRP can be detected in the cell bodies of adult myenteric neurons if preparations are cultured in the presence of cochicine, phenotypic changes can occur during the culturing. Our data for the birthdate of CGRP neurons (range of E11.5-E15.5, with the peak at E12.5-E13.4) show some differences from a previous study that used tritiated thymidine incorporation and retention to identify enteric neuron birthdates and found that myenteric CGRP neurons exit the cell cycle between E10 and P3, with a peak birthdate at E17 (Pham et al., 1991). The reasons for the differences between studies are unclear. As CGRP immunoreactivity in myenteric neurons is first detected around 17 (Branchek and Gershon, 1989), our data suggest that for CGRP neurons, the interval between cell cycle exit and detectable CGRP immunoreactivity is around 6 days.
There is a small population of enteric dopaminergic (TH+) neurons in the myenteric plexus of adult mice, which do not arise from the transiently catecholaminergic cells that are present during mid-embryonic stages (Li et al., 2004). We found that myenteric TH neurons exited the cell cycle between E12.5 and P0, with a peak birthdate at E15.5. These data are consistent with an earlier study that found that peak cell cycle exit of TH neurons occurs at E14.5 (Chalazonitis et al., 2008). As TH immunoreactivity by dopaminergic neurons is first detected perinatally (Li et al., 2004), it appears that the interval between cell cycle exit and detectable TH immunoreactivity in dopaminergic neurons is around one week.
NOS1 immunoreactivity can first be detected in a subpopulation of enteric neurons at E11.5 (Hao and Young, 2009, Hao et al., 2010) and there is a large increase in the number of NOS1 neurons thereafter. We therefore anticipated that some NOS1 neuron precursors would become postmitotic at E10.5 and E11.5. Surprisingly, we did not observe any EdU+/NOS1+ neurons following injection of EdU at E10.5 or E11.5. There are several possible explanations for this unexpected result. First, the earliest NOS1 neurons to exit the cell cycle comprise only a very small proportion of the NOS1 neurons in the adult ENS, and we did not sample them in our study. We think this is unlikely because we examined a total of 1179 NOS1 neurons following EdU injections at E10.5 or E11.5 and did not encounter a single neuron that had retained EdU. The second possibility is that NOS1 expression commences prior to cell cycle exit, and hence the first NOS1 neurons are still proliferative. However, <3% of the NOS1 neurons we examined in the E11.5 and E12.5 gut showed any staining for the proliferation marker, Ki67. Moreover, in a previous study, we examined over 800 NOS1 neurons in the E12.5 gut using a nucleic acid stain, and none was observed to be undergoing mitosis at the time of fixation (Young et al., 2005). Together the data strongly suggest that the vast majority of neurons showing NOS1 immunoreactivity in the E11.5-E12.5 gut are postmitotic. The third possibility is that the first myenteric neurons to express NOS1 only express NOS1 transiently. Previous studies have shown that NOS1 is transiently expressed in a variety of CNS regions during development (Wetts and Vaughn, 1993, Bredt and Snyder, 1994, Ward et al., 1994, Judas et al., 1999). Furthermore, we have previously shown that NOS1 is transiently expressed perinatally by some submucosal neurons in the mouse; this was easily detected as there are very few NOS1+ neurons in submucosal ganglia of the small intestine of adult mice (Young and Ciampoli, 1998, Mongardi Fantaguzzi et al., 2009). Lineage tracing using 4–5 week old Nos1-Cre;eGFP (or TdTomato) mice revealed around 7% of neurons that expressed the reporter gene but did not show NOS1 immunoreactivity, suggesting that they had expressed NOS1 transiently sometime during development. Future studies using stage-specific lineage tracing are required to examine whether the neurons that express NOS1 around E11.5 are the myenteric neurons that show transient expression of NOS1. It would be interesting to know whether transiently NOS1 neurons function as nitrergic neurons. Electrical field stimulation-induced relaxations are already present in the intestine of E16 mice (Gershon and Thompson, 1973), but experiments have not yet been performed in younger mice to determine at what age(s) the inhibitory innervation of the muscle is first functional and when nitric oxide is first released from neurons. As NOS1 appears to be expressed transiently by some cells, it is not possible to estimate the interval between exit from the cell cycle and first expression of NOS1 with any accuracy; nonetheless, because some NOS1 neurons exit the cell cycle at E12.5, and NOS1 neurons are abundant from E12.5 (Branchek and Gershon, 1989), it is possible that the interval between cell cycle exit and NOS1 expression is very short.
Calretinin is expressed by multiple subtypes of enteric neurons in the mouse including excitatory motor neurons to the circular muscle and putative sensory neurons (Sang and Young, 1996, Qu et al., 2008); most calretinin neurons are cholinergic neurons (Sang and Young, 1998, Young et al., 1998). We showed that the peak time of cell cycle exit for calretinin neurons is at P0, but they exit the cell cycle over a protracted period of time commencing at E12.5. Calretinin immunoreactivity is first detected in enteric neurons around birth (Hao and Young, 2009), and therefore the interval between cell cycle exit and detectable calretinin immunoreactivity for at least some neurons is probably around one week. We also showed that the calretinin neurons that exit the cell cycle at E13.5 are significantly larger than the calretinin neurons that exit the cell cycle at P0. The large calretinin neurons that exit the cell cycle at E13.5 are likely to be the NF-M/CGRP/Dogiel type II neurons, whereas the small calretinin neurons that exit the cell cycle around birth are likely to include excitatory muscle motor neurons. However, some excitatory motor neurons are functional prior to birth as excitatory junction potentials (Ward et al., 1997) and electrical field stimulation-induced contractions (Gershon and Thompson, 1973) have been observed in the small intestine of mice at E16–17; our data suggest that there is an increase in the number of excitatory motor neurons after birth. Consistent with this idea, we recently showed a large increase in the number of cholinergic neurons between P0 and P10 in the mouse small intestine (Hao et al., 2013b).
Conclusions
Different classes of myenteric neuron exit the cell cycle at variable, but overlapping, times during development. Our data also suggest that NOS1 is expressed transiently by some myenteric neurons during development. It is already well established that TH is expressed transiently by some enteric neurons or their precursors during embryonic development (Gershon et al., 1984, Baetge and Gershon, 1989, Baetge et al., 1990a, Baetge et al., 1990b, Li et al., 2010, Obermayr et al., 2013b). Little is known about the control of neurotransmitter phenotype in the ENS (Hao and Young, 2009, Laranjeira and Pachnis, 2009, Gershon, 2010, Sasselli et al., 2012), and changes in expression of neurotransmitter synthetic enzymes by enteric neurons during development is likely to make the transcriptional regulation of different enteric neuron subtypes challenging to decipher. Finally, our data suggest that for some neuron subtypes (calretinin, CGRP and dopaminergic neurons), the interval between cell cycle exit and detectable neuronal subtype marker expression is around one week, but for other neuron subtypes (serotonin and NOS1), the interval might be only 2 days or less.
Acknowledgements
We thank Drs Piers Emson and Vanda Lennon for the NOS1 and Hu antisera respectively.
Funding information: NHMRC Project Grants #628349 and #1047953 (HMY), NIH DK57768 (MGM)
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest.
Role of authors: Study concept and design: HMY, CRA. Acquisition of data and analysis: AJB, LAS, DGG, MBA, HMY. Provision of material: MBA, DPO and MGM. Manuscript preparation: HMY with AJB, LAS, DGG, CRA. All authors commented on and approved the final manuscript.
References
- 1.Allison WT, Barthel LK, Skebo KM, Takechi M, Kawamura S, Raymond PA. Ontogeny of cone photoreceptor mosaics in zebrafish. J Comp Neurol. 2010;518:4182–4195. doi: 10.1002/cne.22447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baetge G, Gershon MD. Transient catecholaminergic (TC) cells in the vagus nerves and bowel of fetal mice: relationship to the development of enteric neurons. Dev Biol. 1989;132:189–211. doi: 10.1016/0012-1606(89)90217-0. [DOI] [PubMed] [Google Scholar]
- 3.Baetge G, Pintar JE, Gershon MD. Transiently catecholaminergic (TC) cells in the bowel of the fetal rat: precursors of noncatecholaminergic enteric neurons. Dev Biol. 1990a;141:353–380. doi: 10.1016/0012-1606(90)90391-u. [DOI] [PubMed] [Google Scholar]
- 4.Baetge G, Schneider KA, Gershon MD. Development and persistence of catecholaminergic neurons in cultured explants of fetal murine vagus nerves and bowel. Development. 1990b;110:689–701. doi: 10.1242/dev.110.3.689. [DOI] [PubMed] [Google Scholar]
- 5.Bartel DL. Glial responses after chorda tympani nerve injury. J Comp Neurol. 2012;520:2712–2729. doi: 10.1002/cne.23069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Branchek TA, Gershon MD. Time course of expression of neuropeptide Y, calcitonin gene-related peptide, and NADPH diaphorase activity in neurons of the developing murine bowel and the appearance of 5-hydroxytryptamine in mucosal enterochromaffin cells. J Comp Neurol. 1989;285:262–273. doi: 10.1002/cne.902850208. [DOI] [PubMed] [Google Scholar]
- 7.Bredt DS, Snyder SH. Transient nitric oxide synthase neurons in embryonic cerebral cortical plate, sensory ganglia, and olfactory epithelium. Neuron. 1994;13:301–313. doi: 10.1016/0896-6273(94)90348-4. [DOI] [PubMed] [Google Scholar]
- 8.Brookes SJ. Classes of enteric nerve cells in the guinea-pig small intestine. Anat Rec. 2001;262:58–70. doi: 10.1002/1097-0185(20010101)262:1<58::AID-AR1011>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 9.Callahan T, Young HM, Anderson RB, Enomoto H, Anderson CR. Development of satellite glia in mouse sympathetic ganglia: GDNF and GFR alpha 1 are not essential. Glia. 2008;56:1428–1437. doi: 10.1002/glia.20709. [DOI] [PubMed] [Google Scholar]
- 10.Chalazonitis A, Pham TD, Li Z, Roman D, Guha U, Gomes W, Kan L, Kessler JA, Gershon MD. Bone morphogenetic protein regulation of enteric neuronal phenotypic diversity: relationship to timing of cell cycle exit. J Comp Neurol. 2008;509:474–492. doi: 10.1002/cne.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen JJ, Li Z, Pan H, Murphy DL, Tamir H, Koepsell H, Gershon MD. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001;21:6348–6361. doi: 10.1523/JNEUROSCI.21-16-06348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Disney AA, Aoki C. Muscarinic acetylcholine receptors in macaque V1 are most frequently expressed by parvalbumin-immunoreactive neurons. J Comp Neurol. 2008;507:1748–1762. doi: 10.1002/cne.21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson CS, Zaitoun I, Haberman KM, Gosain A, Druckenbrod NR, Epstein ML. Sacral neural crest-derived cells enter the aganglionic colon of Ednrb−/− mice along extrinsic nerve fibers. J Comp Neurol. 2012;520:620–632. doi: 10.1002/cne.22755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foong JP, Nguyen TV, Furness JB, Bornstein JC, Young HM. Myenteric neurons of the mouse small intestine undergo significant electrophysiological and morphological changes during postnatal development. J Physiol. 2012;590:2375–2390. doi: 10.1113/jphysiol.2011.225938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 16.Gershon MD. Developmental determinants of the independence and complexity of the enteric nervous system. Trends Neurosci. 2010;33:446–456. doi: 10.1016/j.tins.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Gershon MD, Rothman TP, Joh TH, Teitelman GN. Transient and differential expression of aspects of the catecholaminergic phenotype during development of the fetal bowel of rats and mice. J Neurosci. 1984;4:2269–2280. doi: 10.1523/JNEUROSCI.04-09-02269.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gershon MD, Thompson EB. The maturation of neuromuscular function in a multiply innervated structure: development of the longitudinal smooth muscle of the foetal mammalian gut and its cholinergic excitatory, adrenergic inhibitory, and non-adrenergic inhibitory innervation. J Physiol (Lond) 1973;234:257–277. doi: 10.1113/jphysiol.1973.sp010345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gnanamanickam GJ, Llewellyn-Smith IJ. Innervation of the rat uterus at estrus: a study in full-thickness, immunoperoxidase-stained whole-mount preparations. J Comp Neurol. 2011;519:621–643. doi: 10.1002/cne.22515. [DOI] [PubMed] [Google Scholar]
- 20.Gonsalvez DG, Cane KN, Landman KA, Enomoto H, Young HM, Anderson CR. Proliferation and cell cycle dynamics in the developing stellate ganglion. J Neurosci. 2013;33:5969–5979. doi: 10.1523/JNEUROSCI.4350-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grider JR. Neurotransmitters mediating the intestinal peristaltic reflex in the mouse. J Pharmacol Exp Ther. 2003;307:460–467. doi: 10.1124/jpet.103.053512. [DOI] [PubMed] [Google Scholar]
- 22.Hao MM, Anderson RB, Kobayashi K, Whitington PM, Young HM. The migratory behavior of immature enteric neurons. Dev Neurobiol. 2009;69:22–35. doi: 10.1002/dneu.20683. [DOI] [PubMed] [Google Scholar]
- 23.Hao MM, Bornstein JC, Vanden Berghe P, Lomax AE, Young HM, Foong JPP. The emergence of neural activity and its role in the development of the enteric nervous system. Dev Biol. 2013a doi: 10.1016/j.ydbio.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 24.Hao MM, Bornstein JC, Young HM. Development of myenteric cholinergic neurons in ChATCre;R26R-YFP mice. J Comp Neurol. 2013b doi: 10.1002/cne.23354. in press. [DOI] [PubMed] [Google Scholar]
- 25.Hao MM, Moore RE, Roberts RR, Nguyen T, Furness JB, Anderson RB, Young HM. The role of neural activity in the migration and differentiation of enteric neuron precursors. Neurogastroenterol Motil. 2010;22:e127–e137. doi: 10.1111/j.1365-2982.2009.01462.x. [DOI] [PubMed] [Google Scholar]
- 26.Hao MM, Young HM. Development of enteric neuron diversity. J Cell Mol Med. 2009;13:1193–1210. doi: 10.1111/j.1582-4934.2009.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris J, Ayyub C, Shaw G. A molecular dissection of the carboxyterminal tails of the major neurofilament subunits NF-M and NF-H. J Neurosci Res. 1991;30:47–62. doi: 10.1002/jnr.490300107. [DOI] [PubMed] [Google Scholar]
- 28.Herbison AE, Simonian SX, Norris PJ, Emson PC. Relationship of neuronal nitric oxide synthase immunoreactivity to GnRH neurons in the ovariectomized and intact female rat. J Neuroendocrinol. 1996;8:73–82. doi: 10.1111/j.1365-2826.1996.tb00688.x. [DOI] [PubMed] [Google Scholar]
- 29.Joseph NM, He S, Quintana E, Kim YG, Nunez G, Morrison SJ. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J Clin Invest. 2011;121:3398–3411. doi: 10.1172/JCI58186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Judas M, Sestan N, Kostovic I. Nitrinergic neurons in the developing and adult human telencephalon: transient and permanent patterns of expression in comparison to other mammals. Microsc Res Tech. 1999;45:401–419. doi: 10.1002/(SICI)1097-0029(19990615)45:6<401::AID-JEMT7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 31.Kalous A, Osborne PB, Keast JR. Spinal cord compression injury in adult rats initiates changes in dorsal horn remodeling that may correlate with development of neuropathic pain. J Comp Neurol. 2009;513:668–684. doi: 10.1002/cne.21986. [DOI] [PubMed] [Google Scholar]
- 32.Lake JI, Heuckeroth RO. Enteric Nervous System Development: Migration, Differentiation, and Disease. Am J Physiol Gastrointest Liver Physiol. 2013 doi: 10.1152/ajpgi.00452.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laranjeira C, Pachnis V. Enteric nervous system development: Recent progress and future challenges. Auton Neurosci. 2009;151:61–69. doi: 10.1016/j.autneu.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Laranjeira C, Sandgren K, Kessaris N, Richardson W, Potocnik A, Vanden Berghe P, Pachnis V. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121:3412–3424. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leone DP, Srinivasan K, Chen B, Alcamo E, McConnell SK. The determination of projection neuron identity in the developing cerebral cortex. Curr Opin Neurobiol. 2008;18:28–35. doi: 10.1016/j.conb.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leshan RL, Greenwald-Yarnell M, Patterson CM, Gonzalez IE, Myers MG., Jr Leptin action through hypothalamic nitric oxide synthase-1-expressing neurons controls energy balance. Nat Med. 2012;18:820–823. doi: 10.1038/nm.2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Z, Caron MG, Blakely RD, Margolis KG, Gershon MD. Dependence of serotonergic and other nonadrenergic enteric neurons on norepinephrine transporter expression. J Neurosci. 2010;30:16730–16740. doi: 10.1523/JNEUROSCI.2276-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Z, Chalazonitis A, Huang YY, Mann JJ, Margolis KG, Yang QM, Kim DO, Cote F, Mallet J, Gershon MD. Essential roles of enteric neuronal serotonin in gastrointestinal motility and the development/survival of enteric dopaminergic neurons. J Neurosci. 2011;31:8998–9009. doi: 10.1523/JNEUROSCI.6684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci. 2004;24:1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu MT, Kuan YH, Wang J, Hen R, Gershon MD. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J Neurosci. 2009;29:9683–9699. doi: 10.1523/JNEUROSCI.1145-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazzuoli G, Schemann M. Mechanosensitive enteric neurons in the myenteric plexus of the mouse intestine. PLoS One. 2012;7:e39887. doi: 10.1371/journal.pone.0039887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mongardi Fantaguzzi C, Thacker M, Chiocchetti R, Furness JB. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009;336:179–189. doi: 10.1007/s00441-009-0773-2. [DOI] [PubMed] [Google Scholar]
- 43.Neal KB, Parry LJ, Bornstein JC. Strain-specific genetics, anatomy and function of enteric neural serotonergic pathways in inbred mice. J Physiol. 2009;587:567–586. doi: 10.1113/jphysiol.2008.160416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Norris PJ, Charles IG, Scorer CA, Emson PC. Studies on the localization and expression of nitric oxide synthase using histochemical techniques. Histochem J. 1995;27:745–756. [PubMed] [Google Scholar]
- 45.Nurgali K, Stebbing MJ, Furness JB. Correlation of electrophysiological and morphological characteristics of enteric neurons in the mouse colon. J Comp Neurol. 2004;468:112–124. doi: 10.1002/cne.10948. [DOI] [PubMed] [Google Scholar]
- 46.Obermayr F, Hotta R, Enomoto H, Young HM. Development and developmental disorders of the enteric nervous system. Nat Rev Gastroenterol Hepatol. 2013a;10:43–57. doi: 10.1038/nrgastro.2012.234. [DOI] [PubMed] [Google Scholar]
- 47.Obermayr F, Stamp LA, Anderson CR, Young HM. Genetic fate-mapping of tyrosine hydroxylase-expressing cells in the enteric nervous system. Neurogastroenterol Motil. 2013b;25:e283–e291. doi: 10.1111/nmo.12105. [DOI] [PubMed] [Google Scholar]
- 48.Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J Comp Neurol. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- 49.Qu ZD, Thacker M, Castelucci P, Bagyanszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008;334:147–161. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 50.Rothman TP, Gershon MD. Phenotypic expression in the developing murine enteric nervous system. J Neurosci. 1982;2:381–393. doi: 10.1523/JNEUROSCI.02-03-00381.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rothman TP, Nilaver G, Gershon MD. Colonization of the developing murine enteric nervous system and subsequent phenotypic expression by the precursors of peptidergic neruons. J Comp Neurol. 1984;225:13–23. doi: 10.1002/cne.902250103. [DOI] [PubMed] [Google Scholar]
- 52.Sang Q, Williamson S, Young HM. Projections of chemically identified myenteric neurons of the small and large intestine of the mouse. J Anat. 1997;190:209–222. doi: 10.1046/j.1469-7580.1997.19020209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sang Q, Young HM. Chemical coding of neurons in the myenteric plexus and external muscle of the small and large intestine of the mouse. Cell Tissue Res. 1996;284:39–53. doi: 10.1007/s004410050565. [DOI] [PubMed] [Google Scholar]
- 54.Sang Q, Young HM. The identification and chemical coding of cholinergic neurons in the small and large intestine of the mouse. Anat Rec. 1998;251:185–199. doi: 10.1002/(SICI)1097-0185(199806)251:2<185::AID-AR6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 55.Sasselli V, Pachnis V, Burns AJ. The enteric nervous system. Dev Biol. 2012;366:64–73. doi: 10.1016/j.ydbio.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 56.Takeoka A, Kubasak MD, Zhong H, Roy RR, Phelps PE. Serotonergic innervation of the caudal spinal stump in rats after complete spinal transection: effect of olfactory ensheathing glia. J Comp Neurol. 2009;515:664–676. doi: 10.1002/cne.22080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Turner KN, Schachner M, Anderson RB. Cell adhesion molecule L1 affects the rate of differentiation of enteric neurons in the developing gut. Dev Dyn. 2009;238:708–715. doi: 10.1002/dvdy.21861. [DOI] [PubMed] [Google Scholar]
- 58.Uyttebroek L, Shepherd IT, Harrisson F, Hubens G, Blust R, Timmermans JP, Van Nassauw L. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio) J Comp Neurol. 2010;518:4419–4438. doi: 10.1002/cne.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang H, Hughes I, Planer W, Parsadanian A, Grider JR, Vohra BP, Keller-Peck C, Heuckeroth RO. The timing and location of glial cell line-derived neurotrophic factor expression determine enteric nervous system structure and function. J Neurosci. 2010;30:1523–1538. doi: 10.1523/JNEUROSCI.3861-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward SM, Harney SC, Bayguinov JR, McLaren GJ, Sanders KM. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J Physiol (Lond) 1997;505:241–258. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ward SM, Shuttleworth CW, Kenyon JL. Dorsal root ganglion neurons of embryonic chicks contain nitric oxide synthase and respond to nitric oxide. Brain Res. 1994;648:249–258. doi: 10.1016/0006-8993(94)91124-x. [DOI] [PubMed] [Google Scholar]
- 62.Wetts R, Vaughn JE. Transient expression of beta-NADPH diaphorase in developing rat dorsal root ganglia neurons. Brain Res Dev Brain Res. 1993;76:278–282. doi: 10.1016/0165-3806(93)90219-z. [DOI] [PubMed] [Google Scholar]
- 63.Yan H, Keast JR. Neurturin regulates postnatal differentiation of parasympathetic pelvic ganglion neurons, initial axonal projections, and maintenance of terminal fields in male urogenital organs. J Comp Neurol. 2008;507:1169–1183. doi: 10.1002/cne.21593. [DOI] [PubMed] [Google Scholar]
- 64.Yasuhara O, Aimi Y, Matsuo A, Kimura H. Distribution of a splice variant of choline acetyltransferase in the trigeminal ganglion and brainstem of the rat: comparison with calcitonin gene-related peptide and substance P. J Comp Neurol. 2008;509:436–448. doi: 10.1002/cne.21754. [DOI] [PubMed] [Google Scholar]
- 65.Young HM, Ciampoli D. Transient expression of neuronal nitric oxide synthase by neurons of the submucous plexus of the mouse small intestine. Cell Tissue Res. 1998;291:395–401. doi: 10.1007/s004410051009. [DOI] [PubMed] [Google Scholar]
- 66.Young HM, Ciampoli D, Hsuan J, Canty AJ. Expression of ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase-immunoreactivity by undifferentiated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev Dyn. 1999;216:137–152. doi: 10.1002/(SICI)1097-0177(199910)216:2<137::AID-DVDY5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 67.Young HM, Torihashi S, Ciampoli D, Sanders KM. Identification of neurons that express stem cell factor in the mouse small intestine. Gastroenterology. 1998;115:898–908. doi: 10.1016/s0016-5085(98)70262-8. [DOI] [PubMed] [Google Scholar]
- 68.Young HM, Turner KN, Bergner AJ. The location and phenotype of proliferating neural-crest-derived cells in the developing mouse gut. Cell Tissue Res. 2005;320:1–9. doi: 10.1007/s00441-004-1057-5. [DOI] [PubMed] [Google Scholar]
- 69.Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron. 2009;64:645–662. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]