SUMMARY
It is set in stone that Mycobacterium tuberculosis is a facultative intracellular bacterial parasite. This axiom drives our knowledge of the host response, the way we design vaccines against the organism by generating protective T cells, and to a lesser extent, the way we try to target antimicrobial drugs. The purpose of this article is to commit total heresy. I believe that M. tuberculosis can equally well be regarded as an extracellular pathogen and may in fact spend a large percentage of its human lung “life-cycle” in this environment. It is of course intracellular as well, but this may well be little more than a brief interlude after infection of a new host during which the bacterium must replicate to increase its chances of transmission and physiologically adapt prior to moving back to an extracellular phase. As a result, by focusing almost completely on just the intracellular phase, we may be making serious strategic errors in the way we try to intervene in this pathogenic process. It is my opinion that when a TB bacillus enters the lungs and starts to reside inside an alveolar macrophage, its central driving force is to switch on a process leading to lung necrosis, since it is only by this process that the local lung tissue can be destroyed and the bacillus can be exhaled and transmitted. I present here a new model of the pathogenesis of the disease that attempts to unify the pathogenic process of infection, disease, persistence [rather than latency], and reactivation.
A new model of pathology and pathogenesis
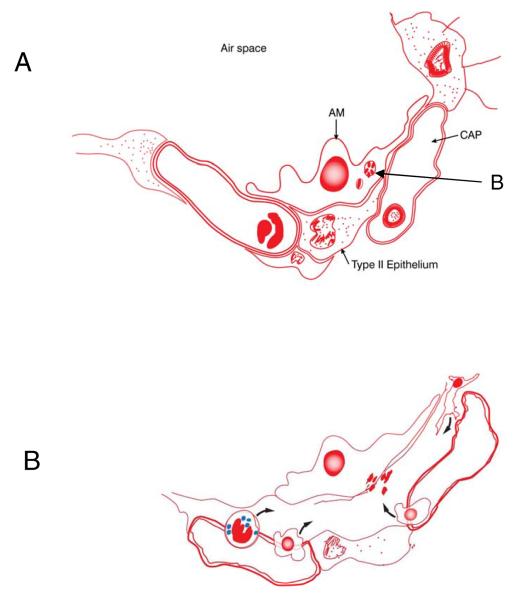
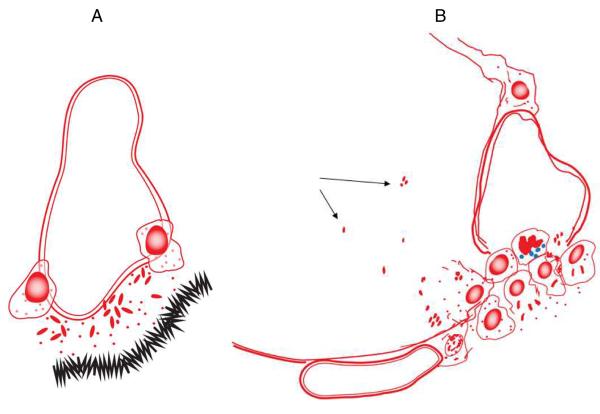
The process of infection is initiated when the bacterium [or perhaps even a cluster or clump, see below], delivered in a water droplet into an alveolus in the lung, is ingested by an alveolar macrophage. Classical electron microscopy studies suggests that at least some of these cells adhere to the alveolar epithelial surface and substantially extend and spread out their cell membranes [Fig.1A]. This is followed by a process which is critical to the bacterium because it must not only survive, but find its way into the interstitium of the lung if it is going to establish an infection. This process is still poorly understood, but it could involve or include the ESX secretion systems now being increasingly well characterized, although to date this has not been specifically shown.1–3 Once in the interstitium the bacillus [or bacilli, if there is some initial replication in the alveolar macrophage or if the ingested droplet contains a clump of bacteria] can now be taken up by monocytes leaving the local capillary bed, or by dendritic cells [which roam the lung parenchyma in large numbers] arriving from the airspace surface or from lymphatic capillaries [Fig.1B]. Indeed, there is now convincing evidence that very early carriage of bacilli out of the initial sites of implantation and into the draining lymphatics is a key event in triggering the acquired immune response.4–6
Fig.1.
[A] In the first stage of the infection process an alveolar macrophage engulfs a bacterium [“B”, or possibly a clump/cluster, see text]. The cell then extends its cytoplasm and spreads across the alveolar epithelial surface. Soon afterwards [perhaps involving the use of their ESX system peptides?] the bacilli somehow cross the basal membrane [panel B]. This creates local inflammation and swelling between the alveolar epithelium and the capillary endothelium allowing the influx of tissue fluid. This in turn allows the influx of macrophages and neutrophils from the blood, and dendritic cells from the lung parenchyma.
There is a massive amount of information regarding survival mechanisms employed by the bacillus inside macrophages, which will not be recounted here. The general concept is that there is a counter-balance between the macrophage attempting to kill the bacillus, and the bacillus taking counter-measures. Most of these mechanisms have been observed in vitro, but whether these all occur in the lungs is very much less clear. One example is host cell apoptosis; this is readily observed in cell cultures, but macrophages infected with virulent strains rapidly become necrotic not apoptotic7, as do neutrophils,8 and for that matter apoptotic cells in the lungs of infected animals that can be seen under the microscope in vivo are relatively rare.7, 9
The next stage is equally critical, because now the bacterium needs to replicate and drive the initial infection into an active disease state. Here, one can argue that the organism has evolved to drive a single event. It is not, as a recent review10 tries to argue, to come to some sort of balance with the host response. Instead, the bacillus drives the mechanism essential to its transmission – necrosis. A bacillus in a macrophage in a granuloma cannot infect the next victim; only an extracellular bacillus in a necrotic cavity can achieve this. In hindsight, one can argue that our concepts of bacterial survival followed by chronic disease and a state of dormancy or latency have primarily arisen from our studies in mice – an animal species in which the bacillus has difficulty in driving any degree of necrosis at all.11
In most models to date the generation of necrosis is regarded as an endpoint; in the popular Lurie/Dannenberg model T cell-mediated “excessive DTH” drives necrosis and the subsequent florid replication of bacilli in cavities – despite the fact that DTH T cells could never survive in necrosis, nor can florid bacterial replication even be seen. In fact, in complete contrast, I would argue that the process of necrosis is the starting point rather than the endpoint. In studies in my laboratory, local foci of tissue damage occur very rapidly -- in the guinea pig model these are evident in 5–10 days, and we think they coalesce to form the central necrosis characteristic of both guinea pigs and humans.9, 11 Such damage creates inflammation and this attracts neutrophils, a cell we feel is a key player in the overall process yet mostly ignored to date [an attitude now hopefully changing]12, 13
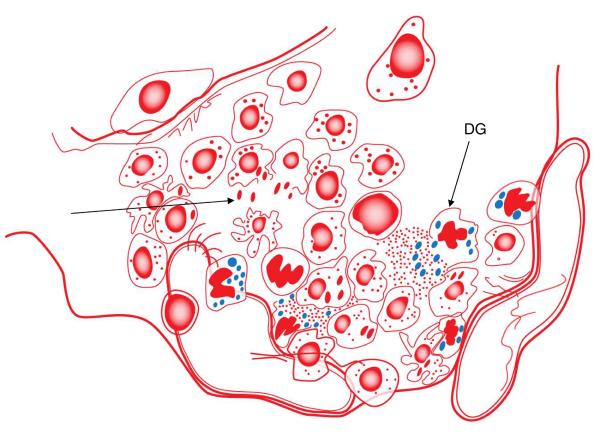
Under the microscope evidence of interstitial inflammation becomes evident as the infectious focus becomes established [Fig.2]. Because the interstitium becomes inflamed and swells with tissue fluid, this allows macrophages and neutrophils to begin to accumulate. Some neutrophils may be able to kill bacilli14 at this point, but these cells are short lived and some will almost certainly degranulate, releasing enzymes that may damage the basement membrane and the vascular endothelium; this, we suspect, is the very beginning of the necrosis that is the hallmark of the disease. Unfortunately, moreover, one of the primary anti-microbial mechanisms of neutrophils is the production of toxic oxygen radicals. The cell wall of the bacillus is adept at scavenging these radicals15, but the local capillary endothelium is not, and as a result the local microvasculature rapidly undergoes severe oxidative damage,16 as well as compression by the developing granuloma. These processes, I would propose, sets in motion a necrotic process which is irreversible, at least in the sense that host mechanisms, such as wound healing and the onset of dystrophic calcification, cannot fully prevent it. As the local capillary bed collapses as a result of this cumulative damage, the ability of T cells and macrophages to infiltrate the central areas of the lesion becomes increasingly compromised. The tissue fluid can still deliver oxygen to some extent, but the outcome is a region becoming increasingly hypoxic.17, 18 The surviving bacilli must adapt to this, but [I would propose] not to nutrient starvation. While the latter is a popular idea [especially in models to screen drugs for “latent” disease], the reality is that the lesion probably contains a simply vast amount of host cell membranes and cholesterol left behind by dying [short lived] neutrophils. This may be a key event in ensuring the persistence of bacilli within necrosis, and indeed could provide a 2-carbon source that could sustain these bacilli for many years.19
Fig.2.
Initial infection now triggers a considerable influx of cells which creates an initial lesion or infectious focus. In most cases the incoming macrophages kill the bacteria but when this fails the bacteria multiply and these macrophages are themselves killed, releasing bacteria [arrow]. Released bacilli are probably at this stage phagocytosed by newly arriving cells, amplifying the process. At this point the influx is predominantly macrophages, with some neutrophils and a smaller number of lymphocytes. The neutrophils produce and secrete oxygen radicals and while this probably has little impact on the mycobacteria these radicals cause oxidative damage to the capillary endothelium, and in addition these structures [and probably adjacent lymphatic capillaries as well] are compressed and collapsed by the continuing cellular influx. These events, probably coupled with local neutrophil death and degranulation [DG], create tiny foci of initial necrosis [visible in the lungs of guinea pigs by 5–7 days].
Such mechanisms also threaten the bacillus itself, and so it quickly adapts into a stress response, primarily controlled by the DosR regulon, a process that has been very well characterized.20–22 This is generally interpreted as a response to full blown host immunity in which the surviving bacteria switch on a large number of stress-related or “latency” genes that enables them to “hide” in a state of latency or dormancy from which they may be able to safely reappear at a later time. But are we completely misinterpreting this information? In my alternative model, the bacilli are sensing these events and adapting accordingly. True, some bacteria will be killed off, but the generation of the host response indicates necrosis will soon be emerging, and with it cavitation, escape, and transmission. The survivors will not have it easy, at least for a while, so DosR and other genes need to be turned on. In other words, the DosR response is not one of preparing for latency and “switching off”, it is quite the reverse – it is a “switching on and adapting” response which is preparing for escape and transmission [however long that may take].
Suitably adapted, the bacillus can now move to the next stage, in which it will survive as an extracellular organism within the necrosis its presence has driven the host response to create [an emerging concept19 is that it hyperconserves immunodominant epitopes23 to maximize the host response for this very reason]. Rather than becoming latent or dormant, as the popular concept holds, it now must take steps to allow it to persist. It must keep some of its enzyme systems going, and so it accumulates iron24 and copper25 to achieve this. Nutrient starvation is a popular component of latency models, but in fact this is the very least of its worries, since it is in a swimming pool of accumulating and then dying neutrophils [not that obvious by microscopy but now revealed directly by flow cytometry in the guinea pig model26] providing it with a quite massive carbon source, especially cholesterol.27, 28 Nevertheless it must keep its energy expending processes to a minimum, including any initial attempt at further replication. Even to keep these minimal processes active it still needs some energy, and this may explain the excellent activity29 at this time of the drug TMC207 [bedaquiline], which specifically targets bacterial ATP synthesis.30
Now extracellular, the bacilli persisting in the remaining necrosis sit and wait patiently for cavitation and escape [Fig.3]. In humans, this could be a rapid event or it could occupy much of the lifespan of the host. In our guinea pig models of chemotherapy, bacilli first find themselves in necrosis after about 40–50 days but we start to observe spontaneous reactivation29 or can force it31 in these animals nearly a year later. In the latter study these animals were approximately two-thirds of the way into their natural life-span when we tried to reactivate disease. They were completely healthy, but still retained small regions of residual necrosis that could still be sufficient to harbor individual bacteria or clumps. What this emphasizes is that if bacilli find themselves in necrosis maybe just 30–40 days after low dose aerosol in the guinea pig model, they are spending >90% of their lifespan in the animal in an extracellular phase.
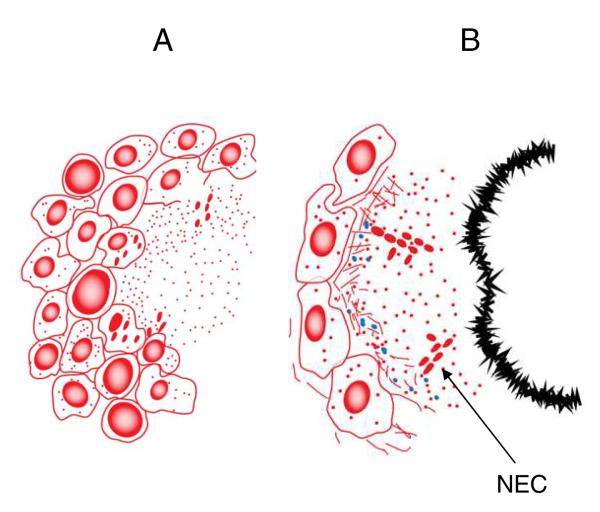
Fig.3.
As cells die in the initial focus of infection, the lesion now is starting to take on the appearance of the classical granuloma [A] with a necrotic core and a circular rim of leukocytes. The small areas of necrosis have now coalesced forming a central structure around which macrophages and the first incoming sensitized lymphocytes now accumulate and surround in large numbers. Some bacteria are probably killed at this point, but others are released by dying macrophages and thus become extracellular. The bacilli physiologically adapt to survive in the necrosis by forming biofilm-like clusters or communities [NECs]. In humans it is thought these necrotic lesions erode into larger airways creating cavities; this does not happen often in the guinea pig model but it can be observed when the isolate is highly virulent [as, unfortunately, our recent studies looking at Beijing and other strains suggest many of them are]. At this time the surviving persisting bacilli abandon planktonic growth [so they cannot be detected by CFU determinations] and undergo major adaptations, which probably includes cell wall modifications making them very hard or impossible to see by acid fast staining. Also at this time the lesion shows substantial dystrophic calcification [the leading edge of which is depicted here as a black bar], physically isolating the remaining NECs in the residual necrosis between this structure and the intact cellular layer of the granuloma [panel B].
The current model of pathogenesis has the latent bacteria living in macrophages, from within which they reawaken, replicate, and burst out triggering reactivation disease. In the model proposed here, in complete contrast, the persisting bacilli are extracellular and within some form of cluster or biofilm-like community within the necrosis. Here, they are using the substantial 2-carbon sources discussed above, and in fact they may be using nuclear material from the dead neutrophils as an initial attachment or scaffold to develop into these biofilms [R. Basaraba, personal communication]. These issues first became evident in studies involving drug testing in the guinea pig model17, in which we observed clusters of bacteria in an acellular rim surrounding primary lesions. As chemotherapy continued these became harder to see by AFB staining, but when more advanced staining techniques were applied the actual numbers of bacilli present were very considerable [and certainly not representative of the CFU values obtained]32, and for that matter seem to represent a variety of phenotypically distinct subpopulations.33 Many of these could be dead of course, but there is a real possibility we have been dreadfully undercounting the actual numbers of bacilli present [making us think a drug regimen is highly effective in such models when in fact it is not].
I have proposed the term “Necrosis-associated Extracellular Clusters” or NECs to describe these structures19, and I further propose here that the existence of these NECs, if they exist in humans [where [possibly extracellular] bacilli on the rims/lesion interfaces have been observed34, 35], then they represent the absolute heart of the problem, both in terms of the pathogenesis of persistence and reactivation, but also the practical consequences for interventions.
Does size matter?
In most laboratories specializing in animal modeling, considerable care is taken in preparing single cell suspensions of bacteria used for low dose aerosol infections. But is this actually realistic? In the guinea pig model bacilli that persist within necrotic lesions appear visually to be a combination of single bacterial rods and small clumps of bacteria ranging in size from a few to as many as 20–30 within the NECs. Do such clumps favor the establishment of active disease and necrotizing cavities in the human lung?
The majority of people who are exposed to M.tuberculosis do not develop active disease, and this has been explained for many decades as reflecting the ability of innate immunity to destroy the bacterium at the alveolar macrophage level. However, what the actual basis of this innate “resistance” is has never been adequately explained. The concept has always been that the bacterium manages to escape destruction after ingestion, and finds its way into the interstitium where it establishes a site of infection. This certainly happens in susceptible guinea pigs, but does it happen the same way in human beings [who we generally agree are far more resistant]? Our concept is that the bacterium survives and divides, and then may be picked up by other cells entering the site attracted by molecules driving local inflammation. Then, at some point some bacteria have to be carried away, probably by motile dendritic cells, to the regional lymph nodes where acquired immunity is then triggered.5, 6
The assumption is that all this begins with a single, or perhaps a few bacilli. But in our animal models we stack the deck by giving higher numbers [~15–20 to guinea pigs, ~25 to macaques, ~100 to mice] and even then, due to the slow doubling time of the organism, it still takes a matter of weeks for the bacterial load to peak and for protective T cells to appear. An alternative possibility therefore is that the risk of developing disease is actually related less to the inhaled dose, but instead more specifically to the nature of the inhaled particle. If a single bacterium makes it through the tidal volume into the alveolus, one might think the outcome was heavily stacked in favor of the macrophage, and this might be indeed the actual reason why 75% or more of exposed individuals never get disease. But what might happen if the individual breathes in a small clump or fragment of a NEC described above? This would be far harder for an engulfing macrophage to deal with, especially if these clumps have a shell of toxic mycolic acids as others have suggested.36 [This is in fact by no means a new idea – the idea that droplets could contain large numbers of bacilli was discussed as long ago as 1944 by Arnold Rich in his classic textbook37 on this topic, and it rather questions the strategy used by animal modelists such as myself who strive to make aerosol doses as low as possible]. This could potentially make it much more likely that a productive site of infection would be set up, plus increase the possibility that the initial clumped particle could then disperse allowing some of these bacilli to soon reach the lymph nodes, resulting in host sensitization. If this model is correct then it suggests that the reason only a minority of exposed individuals get active disease, is simply because these people breathe in clumps/NECs of bacilli, whereas the majority are lucky enough to inhale single cells which they can easily destroy. This also explains the “cannot be in two places at once” enigma – either one or a few bacilli have to divide to set up a site of infection and be carried to the lymph nodes, or there are actually several deposited initially, allowing both events to happen.
An extension of this hypothesis is that not only could they be exhaled as clusters or clumps, but in this state they might be harder to detect in sputum smears. In this regard, we recently found that those in the reactivating guinea pig model were very weakly acid fast to the point we could barely find them [despite recording over a million bacilli by CFU].31 In fact, there are several well-documented studies38–40 describing transmission of tuberculosis from smear-negative individuals, estimated in two studies38, 39 to be the cause of 15–20% of cases. This has been explained in terms of the numbers of AFB bacilli needed for detection, thought to be about 5,000 to 10,00041, but I suggest here that it could equally be due to failure to visualize these due to poor AFB staining, potentially a direct consequence of adaptation/biofilming, as indeed newer fluorescence based microscopy studies are revealing.32, 33 This would help explain the obvious paradox above between smear-negativity being thought to be just “low numbers of bacilli” and yet causing >15% of the transmission of new infections.
The “in vivo pellicle” as the basis of disease reactivation
In the classic model of pathogenesis proposed by Smith and his colleagues,42 reactivation disease is a consequence of an “immunosuppressive event”. While this is obviously true in the case of HIV infection, or immunosenescence, it does not explain why relapse is frequently seen in people after getting drug treatment. Although still dismissed by many in our field, especially those wed to the latent/dormant idea, we feel that there now is no doubt that M.tuberculosis can form biofilms, and this can very easily explain the apparent “drug tolerance” of persisting bacteria.19
Recently, it was clearly demonstrated36 that M.tuberculosis can form biofilms in vitro, and that this process is quite distinct from planktonic growth. Moreover, these biofilms seem to be creating a type of extracellular matrix by somehow shedding free mycolic acids. This may well explain why these biofilms, perhaps as a direct result, become highly resistant to antibiotics. Key genetic elements in this process appear to include the psk locus. Mutants in which psk1 is interrupted fail to make biofilms43 and some more recent preliminary data also possibly indicates the involvement of the psk16 gene [unpublished data]. Moreover, such mutants have an additional in vitro marker – they prevent bacilli in in vitro colonies from forming pellicles –now itself regarded as a type of biofilm. These are colonies that grow out as domes when cultures are grown on a liquid/air interface,44 and have long been a standard way of increasing the virulence of mycobacterial stocks.
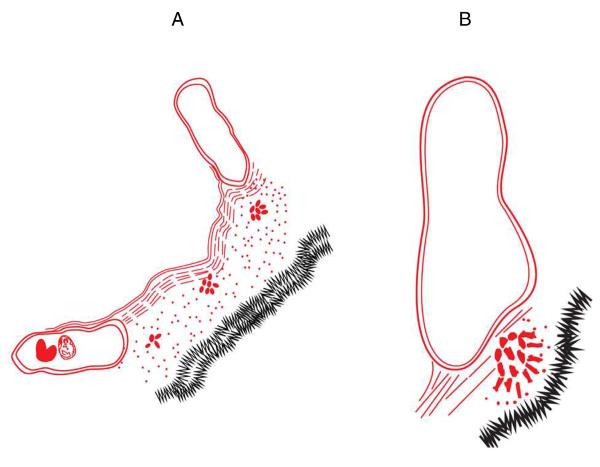
Is something similar happening in vivo? If the new model proposed here is correct, can this explain disease reactivation, or relapse after the cessation of drug therapy? Reactivation occurs from the vicinity of the residual primary lesions,13 and we know that even if this residual necrosis is minimal it can still harbor live bacilli capable of retriggering disease.45 In the putative model shown here [Fig.4] as the primary lesions heal and calcify, the residual necrosis containing the surviving NECs become highly compressed by the calcification process and these get pushed towards the edge of the lesion. This gradually brings them close to open intact airspaces [Fig.4B] and, obviously, a steep rise in oxygen tension. In essence, what has now evolved is a liquid [necrosis]/air interface similar to pellicles in vitro, as indeed Hunter and colleagues have previously suggested.46 If the “dome formation” seen in vitro is not a random occurrence, but an actual structural adaptation by the bacterial colony, is this the first step in reactivation as the bacill in the NECs begin to sense the local pO2, begin to replicate, perhaps disperse, and create a pellicle-like dome which pushes out of the rim of the necrosis [perhaps following the oxygen tension gradient]? Once into or on the edge of the normoxic tissue [Fig.4B] the presence of the NEC will cause local inflammation and attract the attention of macrophages [Fig.5A]. These host cells will usually probably destroy them, but if not a focus of reactivation disease has the potential for becoming established [Fig.5B]. In fact, this could be happening on a continuous basis, but controlled by the host memory immune response, and only subverted by secondary factors [HIV, old age, other lung infections, therapy for rheumatoid arthritis involving TNF blocking47, etc]. If indeed this process happens periodically, the outcome of this would be the continuation of T cell sensitization, thus rather nicely explaining why people thought to have “Latent TB” are IGRA-positive.
Fig.4.
At this point the surviving bacteria are in residual necrosis not that distant from airspaces and capillaries. As the lesions “heal” and calcify and fibrotic tissue is reabsorbed the residual necrosis becomes substantially compressed by the central calcification [A]. Somehow, the NECs are able to sense this -- perhaps the increasing local oxygen tension -- and are pushed towards the liquid [necrosis] air [airspace or capillary] interface, perhaps forming some type of “in vivo pellicle” [B].
Fig.5.
Initiation of attempted reactivation. Here, compression of NECs towards airway epithelium generates inflammatory signals, and macrophages arrive [A]. One outcome here could be that the bacilli are killed [in fact this could be the usual outcome -- this could be happening over and over again given the large number of NECs we can detect, but quickly controlled by T cell immunity], but if the dispersing NEC bacilli establish a foothold and survive then a new infectious lesion is potentially created [panel B], and freed bacilli [singly or in clumps; arrow] are pulled up the airway by exhalation. In fact, this is a likely outcome if the T cell response is destroyed by HIV infection.
I would note that the current model of disease relapse after chemotherapy is conventionally explained by the idea that the reactivating bacilli have previously undergone some sort of adaptation rendering them tolerant to drugs. This makes two assumptions: [1] that the drugs are still efficiently getting to the bacteria despite vascular collapse and necrosis but now cannot kill them, and [2] that the bacteria have a way to efficiently reverse this drug tolerance once the chemotherapy has ceased and hence can reactivate. The first point can easily be debated, and as for the second, my model proposed here is both far less complicated and much more likely based on our in vivo observations. Above all, it provides a new [and very simple] explanation for the famous “Cornell model” in that biofiming bacteria would appear “non-culturable”, but could then eventually reactivate if the immune response is depressed.
Implications for interventions
If the key elements of the above model are correct, then they have serious implications for interventions, be they vaccines or drugs. The stages described in Figs 2&3 are key points in the pathogenesis of the disease, and offer multiple drug targets. As I discuss elsewhere19 however, the field has locked into the concept that the “latent bacteria” we need to target to ensure sterilizing regimens are intracellular inside viable macrophages, have minimal oxygen, and are carbon-starved, and apparently many hundreds of thousands of compounds are being tested in assays in vitro that create these conditions. In my opinion this seems to be a serious mistake. Similarly, for a vaccine based on T cell-macrophage interactions to be fully effective, it has to act before the bacteria escape into the necrosis, a site inimical to host immune cells, and preferably earlier. As I have argued elsewhere,48 the effector memory immune response induced by the BCG vaccine49 is just too slow to achieve this. It can certainly slow down the disease process, and significantly inhibit necrosis, but unfortunately virulent strains can still produce enough inflammation to trigger the emergence of regulatory T cells50–52, a further very serious complication that to date has received little attention.
As noted above the prevailing feeling in the field is that host immunity soon forces infecting bacteria into a state of latency, where the organism is sequestered in macrophages for long periods of time, coupled with rather complicated theories as to why the host cell simply just does not then kill it in this unguarded state. If so, then sensitizing T cells to “latency antigens” would have a chance at success. However, if the model presented here is correct then these persisting bacteria are by this point extracellular and hence beyond the reach of both the T cell response and [to a degree at least] chemotherapeutic agents.53
If the key to all of this is the development of necrosis then efficacy needs to be tested in animal models that reflect this. Despite this, a model that has drawn attention lately has been developed in the mouse, where necrosis does not occur at all to any extent. In that model54 mice were given a brief, incomplete, course of chemotherapy [based on the current axiom that bacteria that initial survive drug treatment are “latent”] and then vaccinated [with fusion H56]. Since necrosis is not a factor here the surviving bacteria almost certainly remain intracellular, the vaccine stimulates the re-expansion of T cells that can detect the infected macrophages, and the regrowth of the infection after chemotherapy is withdrawn is thus reduced somewhat. However, the actual protection values reported were minimal, only showing slowing of the degree of regrowth, and if one were uncharitable one might ask why the “latent” bacteria were able to regrow at all? One can further argue that the kinetics of resistance are very different and far smaller than that seen in a model of complete chemotherapy and reinfection from our laboratory55 where resistance was almost instantaneously expressed. [One explanation is that H56 merely re-expands effector memory CD4 cells, whereas we clearly showed in our model that both [very rapid] central memory as well as effector memory cells were triggered]. In contrast, the “incomplete chemotherapy” model makes the assumption that bacilli that are still alive when the chemotherapy is suddenly withdrawn are already in some sort of latency, that they are making “latency antigens”, and that they are in macrophages [either in the lesion or in the draining lymph node] that can present these antigens to T cells. The problem here is whether any of these assumptions are true?
This is of course all very interesting, and certainly deserving debate, but is it the correct strategy to deal with supposedly “latent” bacilli? Firstly, as I have argued before19, 56, these remaining bacilli are almost certainly not latent, since a truly latent bacterium would probably not sense cavity formation and the opportunity to escape; i.e. it would completely miss the bus. An extension of this is to ask how bacilli safely hidden inside macrophages would even know a cavity was forming? Secondly, as our animal modeling now increasingly demonstrates, the truly “persisting” bacteria, even after chemotherapy, are sitting in areas of necrosis as extracellular organisms against which T cells will be impotent.
Concluding remarks
I have presented here a new model which attempts to unify our current concepts regarding the pathogenesis of tuberculosis with the capacity of bacilli to persist and subsequently give rise to reactivation disease. I should clearly stress that this model is based on a series of observations made in the guinea pig model, so there is no guarantee such events also occur in humans [although I would also note that many in our field generally agree that this is one of the better animal models of the disease process]. It is clear however, at this point in time at least, that virtually everything we know about M.tuberculosis relates to its intracellular phase. In contrast, we know nothing to date regarding its survival as an extracellular organism surviving and persisting in residual necrosis, and until we do, both new vaccination and drug discovery efforts could be futile.
Acknowledgement
I am grateful to my many colleagues in the MRL at CSU who have helped me formulate these ideas, and to David McMurray and Ann Rawkins for helping me understand the guinea pig model of tuberculosis [even though they may not agree entirely with my positions here]. I am equally grateful to Dr William Jacobs for explaining to me that the TB bacillus is indeed capable of biofilming. I thank Carl Kichinko for his assistance in producing the illustrations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Abdallah AM, Gey van Pittius NC, Champion PA, Cox J, Luirink J, Vandenbroucke-Grauls CM, Appelmelk BJ, Bitter W. Type VII secretion--mycobacteria show the way. Nat Rev. 2007;5:883–891. doi: 10.1038/nrmicro1773. [DOI] [PubMed] [Google Scholar]
- 2.Bitter W, Houben EN, Bottai D, Brodin P, Brown EJ, Cox JS, Derbyshire K, Fortune SM, Gao LY, Liu J, Gey van Pittius NC, Pym AS, Rubin EJ, Sherman DR, Cole ST, Brosch R. Systematic genetic nomenclature for type VII secretion systems. PLoS Pathog. 2009;5:e1000507. doi: 10.1371/journal.ppat.1000507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simeone R, Bottai D, Brosch R. ESX/type VII secretion systems and their role in host-pathogen interaction. Current Opin Microbiol. 2009;12:4–10. doi: 10.1016/j.mib.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 4.Chackerian AA, Alt JM, Perera TV, Dascher CC, Behar SM. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect Immun. 2002;70:4501–4509. doi: 10.1128/IAI.70.8.4501-4509.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J Exp Med. 2008;205:105–115. doi: 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Urdahl KB, Shafiani S, Ernst JD. Initiation and regulation of T-cell responses in tuberculosis. Mucosal Immunol. 2011;4:288–293. doi: 10.1038/mi.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park JS, Tamayo MH, Gonzalez-Juarrero M, Orme IM, Ordway DJ. Virulent clinical isolates of Mycobacterium tuberculosis grow rapidly and induce cellular necrosis but minimal apoptosis in murine macrophages. J Leukoc Biol. 2006;79:80–86. doi: 10.1189/jlb.0505250. [DOI] [PubMed] [Google Scholar]
- 8.Corleis B, Korbel D, Wilson R, Bylund J, Chee R, Schaible UE. Escape of Mycobacterium tuberculosis from oxidative killing by neutrophils. Cell Microbiol. 2012;14:1109–1121. doi: 10.1111/j.1462-5822.2012.01783.x. [DOI] [PubMed] [Google Scholar]
- 9.Turner OC, Basaraba RJ, Orme IM. Immunopathogenesis of pulmonary granulomas in the guinea pig after infection with Mycobacterium tuberculosis. Infect Immun. 2003;71:864–871. doi: 10.1128/IAI.71.2.864-871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Russell DG, Barry CE, 3rd, Flynn JL. Tuberculosis: what we don't know can, and does, hurt us. Science. 2010;328:852–856. doi: 10.1126/science.1184784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner OC, Basaraba RJ, Frank AA, Orme IM. Granuloma formation in mouse and guinea pig models of experimental tuberculosis. In: Boros DL, editor. Granulomatous Infections and Inflammation: Cellular and Molecular Mechanisms. ASM Press; Washington, D.C.: 2003. pp. 65–84. [Google Scholar]
- 12.Lowe DM, Redford PS, Wilkinson RJ, O'Garra A, Martineau AR. Neutrophils in tuberculosis: friend or foe? Trends Immunol. 2012;33:14–25. doi: 10.1016/j.it.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 13.Basaraba RJ. Experimental tuberculosis: the role of comparative pathology in the discovery of improved tuberculosis treatment strategies. Tuberculosis (Edinb) 2008;88(Suppl 1):S35–47. doi: 10.1016/S1472-9792(08)70035-0. [DOI] [PubMed] [Google Scholar]
- 14.Pedrosa J, Saunders BM, Appelberg R, Orme IM, Silva MT, Cooper AM. Neutrophils play a protective nonphagocytic role in systemic Mycobacterium tuberculosis infection of mice. Infect Immun. 2000;68:577–583. doi: 10.1128/iai.68.2.577-583.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan J, Fujiwara T, Brennan P, McNeil M, Turco SJ, Sibille JC, Snapper M, Aisen P, Bloom BR. Microbial glycolipids: possible virulence factors that scavenge oxygen radicals. PNAS. 1989;86:2453–2457. doi: 10.1073/pnas.86.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palanisamy GS, Kirk NM, Ackart DF, Shanley CA, Orme IM, Basaraba RJ. Evidence for oxidative stress and defective antioxidant response in guinea pigs with tuberculosis. PloS One. 2011;6:e26254. doi: 10.1371/journal.pone.0026254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lenaerts AJ, Hoff D, Aly S, Ehlers S, Andries K, Cantarero L, Orme IM, Basaraba RJ. Location of persisting mycobacteria in a Guinea pig model of tuberculosis revealed by r207910. Antimicrob Agents Chemother. 2007;51:3338–3345. doi: 10.1128/AAC.00276-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., 3rd Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–2340. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orme IM. Development of new vaccines and drugs for TB: limitations and potential strategic errors. Future Microbiol. 2011;6:161–177. doi: 10.2217/fmb.10.168. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boon C, Dick T. How Mycobacterium tuberculosis goes to sleep: the dormancy survival regulator DosR a decade later. Future Microbiol. 2011;7:513–518. doi: 10.2217/fmb.12.14. [DOI] [PubMed] [Google Scholar]
- 21.Chao MC, Rubin EJ. Letting sleeping dos lie: does dormancy play a role in tuberculosis? Annu Rev Microbiol. 2010;64:293–311. doi: 10.1146/annurev.micro.112408.134043. [DOI] [PubMed] [Google Scholar]
- 22.Voskuil MI, Schnappinger D, Visconti KC, Harrell MI, Dolganov GM, Sherman DR, Schoolnik GK. Inhibition of respiration by nitric oxide induces a Mycobacterium tuberculosis dormancy program. J Exp Med. 2003;198:705–713. doi: 10.1084/jem.20030205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Comas I, Chakravartti J, Small PM, Galagan J, Niemann S, Kremer K, Ernst JD, Gagneux S. Human T cell epitopes of Mycobacterium tuberculosis are evolutionarily hyperconserved. Nat Genet. 2010;42:498–503. doi: 10.1038/ng.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Basaraba RJ, Bielefeldt-Ohmann H, Eschelbach EK, Reisenhauer C, Tolnay AE, Taraba LC, Shanley CA, Smith EA, Bedwell CL, Chlipala EA, Orme IM. Increased expression of host iron-binding proteins precedes iron accumulation and calcification of primary lung lesions in experimental tuberculosis in the guinea pig. Tuberculosis (Edinb) 2008;88:69–79. doi: 10.1016/j.tube.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Samanovic MI, Ding C, Thiele DJ, Darwin KH. Copper in microbial pathogenesis: meddling with the metal. Cell Host Microbe. 2010;11:106–115. doi: 10.1016/j.chom.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ordway D, Palanisamy G, Henao-Tamayo M, Smith EE, Shanley C, Orme IM, Basaraba RJ. The cellular immune response to Mycobacterium tuberculosis infection in the guinea pig. J Immunol. 2007;179:2532–2541. doi: 10.4049/jimmunol.179.4.2532. [DOI] [PubMed] [Google Scholar]
- 27.Pandey AK, Sassetti CM. Mycobacterial persistence requires the utilization of host cholesterol. PNAS. 2008;105:4376–4380. doi: 10.1073/pnas.0711159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van der Geize R, Yam K, Heuser T, Wilbrink MH, Hara H, Anderton MC, Sim E, Dijkhuizen L, Davies JE, Mohn WW, Eltis LD. A gene cluster encoding cholesterol catabolism in a soil actinomycete provides insight into Mycobacterium tuberculosis survival in macrophages. PNAS. 2007;104:1947–1952. doi: 10.1073/pnas.0605728104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shang S, Shanley CA, Caraway ML, Orme EA, Henao-Tamayo M, Hascall-Dove L, Ackart D, Lenaerts AJ, Basaraba RJ, Orme IM, Ordway DJ. Activities of TMC207, Rifampin, and Pyrazinamide against Mycobacterium tuberculosis Infection in Guinea Pigs. Antimicrob Agents Chemother. 2011;55:124–131. doi: 10.1128/AAC.00978-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 31.Obregon-Henao A, Shanley CA, Shang S, Caraway M, Basaraba RJ, Duncan CG, Ordway DJ, Orme IM. Cortisone-forced reactivation of weakly acid fast positive Mycobacterium tuberculosis in guinea pigs previously treated with chemotherapy. Mycobac Dis. 2012;2:116–122. [Google Scholar]
- 32.Hoff DR, Ryan GJ, Driver ER, Ssemakulu CC, De Groote MA, Basaraba RJ, Lenaerts AJ. Location of intra- and extracellular M. tuberculosis populations in lungs of mice and guinea pigs during disease progression and after drug treatment. PloS One. 2011;6:e17550. doi: 10.1371/journal.pone.0017550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryan GJ, Hoff DR, Driver ER, Voskuil MI, Gonzalez-Juarrero M, Basaraba RJ, Crick DC, Spencer JS, Lenaerts AJ. Multiple M. tuberculosis phenotypes in mouse and guinea pig lung tissue revealed by a dual-staining approach. PloS One. 2010;5:e11108. doi: 10.1371/journal.pone.0011108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan G, Post FA, Moreira AL, Wainwright H, Kreiswirth BN, Tanverdi M, Mathema B, Ramaswamy SV, Walther G, Steyn LM, Barry CE, 3rd, Bekker LG. Mycobacterium tuberculosis growth at the cavity surface: a microenvironment with failed immunity. Infect Immun. 2003;71:7099–7108. doi: 10.1128/IAI.71.12.7099-7108.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peyron P, Vaubourgeix J, Poquet Y, Levillain F, Botanch C, Bardou F, Daffe M, Emile JF, Marchou B, Cardona PJ, de Chastellier C, Altare F. Foamy macrophages from tuberculous patients' granulomas constitute a nutrient-rich reservoir for M. tuberculosis persistence. PLoS Pathog. 2008;4:e1000204. doi: 10.1371/journal.ppat.1000204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ojha AK, Baughn AD, Sambandan D, Hsu T, Trivelli X, Guerardel Y, Alahari A, Kremer L, Jacobs WR, Jr., Hatfull GF. Growth of Mycobacterium tuberculosis biofilms containing free mycolic acids and harbouring drug-tolerant bacteria. Mol Microbiol. 2008;69:164–174. doi: 10.1111/j.1365-2958.2008.06274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rich A. The pathogenesis of tuberculosis. Charles C. Thomas; Baltimore: 1944. [Google Scholar]
- 38.Behr MA, Warren SA, Salamon H, Hopewell PC, Ponce de Leon A, Daley CL, Small PM. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999;353:444–449. doi: 10.1016/s0140-6736(98)03406-0. [DOI] [PubMed] [Google Scholar]
- 39.Hernandez-Garduno E, Cook V, Kunimoto D, Elwood RK, Black WA, FitzGerald JM. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax. 2004;59:286–290. doi: 10.1136/thx.2003.011759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tostmann A, Kik SV, Kalisvaart NA, Sebek MM, Verver S, Boeree MJ, van Soolingen D. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis. 2008;47:1135–1142. doi: 10.1086/591974. [DOI] [PubMed] [Google Scholar]
- 41.Hobby GL, Holman AP, Iseman MD, Jones JM. Enumeration of tubercle bacilli in sputum of patients with pulmonary tuberculosis. Antimicrob Agents Chemother. 1973;4:94–104. doi: 10.1128/aac.4.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith DW, Wiegeshaus EH. What animal models can teach us about the pathogenesis of tuberculosis in humans. Rev Infect Dis. 1989;11(Suppl 2):S385–393. doi: 10.1093/clinids/11.supplement_2.s385. [DOI] [PubMed] [Google Scholar]
- 43.Pang JM, Layre E, Sweet L, Sherrid A, Moody DB, Ojha A, Sherman DR. The polyketide Pks1 contributes to biofilm formation in Mycobacterium tuberculosis. J Bacteriol. 2012;194:715–721. doi: 10.1128/JB.06304-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim KS, Salton MR, Barksdale L. Ultrastructure of superficial mycosidic integuments of Mycobacterium sp. J Bacteriol. 1976;125:739–743. doi: 10.1128/jb.125.2.739-743.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shang S, Shanley CA, Caraway ML, Orme EA, Henao-Tamayo M, Hascall-Dove L, Ackart D, Orme IM, Ordway DJ, Basaraba RJ. Drug treatment combined with BCG vaccination reduces disease reactivation in guinea pigs infected with Mycobacterium tuberculosis. Vaccine. 2012;30:1572–1582. doi: 10.1016/j.vaccine.2011.12.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hunter RL, Olsen MR, Jagannath C, Actor JK. Multiple roles of cord factor in the pathogenesis of primary, secondary, and cavitary tuberculosis, including a revised description of the pathology of secondary disease. Ann Clin Lab Sci. 2006;36:371–386. [PubMed] [Google Scholar]
- 47.Plessner HL, Lin PL, Kohno T, Louie JS, Kirschner D, Chan J, Flynn JL. Neutralization of tumor necrosis factor (TNF) by antibody but not TNF receptor fusion molecule exacerbates chronic murine tuberculosis. J Infect Dis. 2007;195:1643–1650. doi: 10.1086/517519. [DOI] [PubMed] [Google Scholar]
- 48.Orme IM. The Achilles heel of BCG. Tuberculosis (Edinb) 2010;90:329–332. doi: 10.1016/j.tube.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Henao-Tamayo MI, Ordway DJ, Irwin SM, Shang S, Shanley C, Orme IM. Phenotypic definition of effector and memory T-lymphocyte subsets in mice chronically infected with Mycobacterium tuberculosis. Clin Vaccine Immunol. 2010;17:618–625. doi: 10.1128/CVI.00368-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ordway DJ, Shang S, Henao-Tamayo M, Obregon-Henao A, Nold L, Caraway M, Shanley CA, Basaraba RJ, Duncan CG, Orme IM. Mycobacterium bovis BCG-mediated protection against W-Beijing strains of Mycobacterium tuberculosis is diminished concomitant with the emergence of regulatory T cells. Clin Vaccine Immunol. 2011;18:1527–1535. doi: 10.1128/CVI.05127-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shang S, Harton M, Tamayo MH, Shanley C, Palanisamy GS, Caraway M, Chan ED, Basaraba RJ, Orme IM, Ordway DJ. Increased Foxp3 expression in guinea pigs infected with W-Beijing strains of M. tuberculosis. Tuberculosis (Edinb) 2011;91:378–385. doi: 10.1016/j.tube.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ordway D, Henao-Tamayo M, Harton M, Palanisamy G, Troudt J, Shanley C, Basaraba RJ, Orme IM. The hypervirulent Mycobacterium tuberculosis strain HN878 induces a potent TH1 response followed by rapid down-regulation. J Immunol. 2007;179:522–531. doi: 10.4049/jimmunol.179.1.522. [DOI] [PubMed] [Google Scholar]
- 53.Grosset J. Mycobacterium tuberculosis in the extracellular compartment: an underestimated adversary. Antimicrob Agents Chemother. 2003;47:833–836. doi: 10.1128/AAC.47.3.833-836.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Schoolnik GK, Cassidy JP, Billeskov R, Andersen P. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat Med. 2011;17:189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- 55.Henao-Tamayo M, Obregon-Henao A, Ordway DJ, Shang S, Duncan CG, Orme IM. A mouse model of tuberculosis reinfection. Tuberculosis (Edinb) 92:211–217. doi: 10.1016/j.tube.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orme M. The latent tuberculosis bacillus (I'll let you know if I ever meet one) Int J Tuberc Lung Dis. 2001;5:589–593. [PubMed] [Google Scholar]