Abstract
Female sexual behavior is an established model of a naturally motivated behavior which is regulated by activity within the mesolimbic dopamine system. Repeated activation of the mesolimbic circuit by female sexual behavior elevates dopamine release and produces persistent postsynaptic alterations to dopamine D1 receptor signaling within the nucleus accumbens. Here we demonstrate that sexual experience in female Syrian hamsters significantly increases spine density and alters morphology selectively in D1 receptor expressing medium spiny neurons within the nucleus accumbens core, with no corresponding change in dopamine receptor binding or protein expression. Our findings demonstrate that previous life experience with a naturally motivated behavior has the capacity to induce persistent structural alterations to the mesolimbic circuit that can increase reproductive success and are analogous to the persistent structural changes following repeated exposure to many drugs of abuse.
Keywords: Lordosis, dendrite, dopamine receptor, synaptic plasticity, motivation
Introduction
Activity of the mesolimbic dopamine circuitry is an established component of the regulation of motivated behaviors (Giuliano and Allard, 2001a, b; Ikemoto and Panksepp, 1999; Kelley, 2004). A fundamental property of the mesolimbic dopamine system is its exquisite plasticity, which is presumed to regulate adaptive changes in motivated behaviors (Humphries and Prescott, 2010). Sexual behavior has emerged as one such model of motivated behavior in which synaptic plasticity within the mesolimbic system is accompanied by behavioral changes associated with increased reproductive success (Frohmader et al., 2010; Meisel and Mullins, 2006).
It is well documented that repeated sexual experience induces long term biochemical and molecular changes within in the mesolimbic circuit, specifically within the nucleus accumbens (NAc) (Bradley and Meisel, 2001; Bradley et al., 2004; Hedges et al., 2009; Pitchers et al., 2010a; Pitchers et al., 2010b; Pitchers et al., 2012; Pitchers et al., 2013). Indeed, of the more interesting findings from this emerging literature has been the identification of increased density of dendritic spines in medium spiny neurons (MSNs), the principal neuron within the NAc, following male or female sexual experience (Meisel and Mullins, 2006; Pitchers et al., 2010a).
MSNs are generally thought to express one of two main dopamine receptor subtypes, either dopamine D1 or D2 receptors, which have opposing effects on cellular excitability (Wickens, 1990). Heightened mesolimbic dopamine release following sexual experience (Kohlert and Meisel, 1999) is accompanied by enhanced postsynaptic coupling of dopamine D1 receptors to G-proteins (Bradley et al., 2004), resulting in activation of signaling pathways and nuclear transcription factors (Meisel and Mullins, 2006) mechanistically linked to long-term modifications of dendritic structure (Muly et al., 2001; Penzes et al., 2011; Penzes et al., 2001). As alterations to a select MSN phenotype could have significant impact on the overall functional output of the NAc (Albin et al., 1989; DeLong, 1990; Ikemoto, 2007; Sesack and Grace, 2010), an important but unstudied question centers on whether the persistent structural plasticity that occurs following sexual experience is cell-type specific.
In this study we therefore evaluated whether increases in spine density of MSNs were localized to neurons containing either dopamine D1 or D2 receptors (DAD1R or DAD2R respectively). We also evaluated whether sexual experience induced any alterations in dopamine receptor protein expression or binding. Here we identify for the first time selective increases in dendritic spine density restricted to the DAD1R expressing MSN population following experience with a natural motivated behavior.
Materials and Methods
Animals
Adult female Syrian hamsters (Charles Rivers Laboratories, Wilmington, MA, USA) were individually housed in polycarbonate cages (51 cm long × 41 cm wide × 20 cm high) and kept under a 14:10 light:dark cycle, with lights out at 13:00 hours. Food and water were available to the animals ad libitum. All animal procedures were in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and approved by the University of Minnesota IACUC.
Surgery
Female hamsters were bilaterally ovariectomized under sodium pentobarbital anesthesia (Nembutal; 8.5 mg per 100 gm body weight, i.p.), given postsurgical analgesics (Torbugesic, Fort Dodge Animal Health, Fort Dodge, IA, 1.0 mg/0.2 ml/hamster, sc) and antibiotics (Baytril, Bayer Animal Health, Shawnee, KS, 10 mg/kg, sc), and allowed to recover for 10 days prior to behavioral testing.
Sexual experience
Ovariectomized female hamsters were primed for sexual experience once a week for six consecutive weeks by giving two daily subcutaneous injections of estradiol benzoate (10 μg in 0.1 ml of cottonseed oil) approximately 48 and 24 h prior to the sexual behavior test followed by a subcutaneous injection of progesterone (500 μg in 0.1 ml of cottonseed oil) 4–6 h prior to the sexual behavior test. Females that received sexual experience were presented with a sexually experienced male hamster for a 10 min session 4–6 h after the progesterone injection. Each male and female were paired only once during the duration of the sexual experience tests. Female controls received the same hormone regime, but remained in their home cages for the duration of the experiment. All animals were sacrificed one week following the final sexual experience pairing. This regimen of behavioral testing and one week sacrifice was chosen to be consistent with the timing used in our previous studies (e.g., Meisel and Mullins, 2006) and because the one week (but not one day) survival was a time point in which male sexual behavior in rats produced similar dendritic spine changes (Pitchers et al., 2010a).
DiI labeling
One week following the last pairing for sexual behavior, female animals were overdosed with an i.p. injection of 0.2 ml Sleepaway (26% sodium pentobarbital, 7.8% isopropyl alcohol, 20.7% propylene glycol, distilled water; Fort Dodge Animal Health) and transcardially perfused with 25 mM phosphate buffered saline (PBS, pH = 7.2) for 3 min at a flow rate of 25 ml/min, followed by 1.5% paraformaldehyde in 25 mM PBS for 20 min. After perfusion, brains were removed, blocked coronally just rostral to the cerebellum, and post-fixed for 1 h in 1.5% paraformaldehyde in 25 mM PBS. Brains were Vibratome (Lancer Series 1000, St. Louis, MO, USA) sectioned in either 300 μm (DiI labeling only) or 150 μm (DiI and immunofluorescence) serial, coronal sections through the nucleus accumbens. Sections were placed in 25 mM PBS until labeled with DiI.
Preparation of DiI coated “bullets”
Coating of tungsten particles with lipophilic dye DiI was adapted from methods described elsewhere (Gan et al., 2009; Gan et al., 2000; Shen et al., 2009; Staffend et al., 2011; Staffend and Meisel, 2011a,b, 2012). Briefly, 2 mg of the carbocyanine fluorescent dyes, DiI or CM-DiI (Molecular Probes, Carlsbad, CA, USA), was dissolved in 75 μl methylene chloride and applied to 90 mg of 1.3 μm tungsten particles (Bio-Rad) spread evenly on a glass slide. CM-DiI was used when combined with immunochemistry for dopamine receptors (Staffend and Meisel, 2011a, b). Following application, tungsten particles were allowed to dry, then scraped from the slide and collected into 10 ml of 10 mg/ml polyvinylpyrrolidone (PVP; Sigma-Aldrich, St. Louis, MO, USA) dissolved in deionized water. The suspension was sonicated for 10 min with intermittent vortexing. Tefzel tubing (Bio-Rad) was pre-coated with 10 mg/ml PVP and dried under 0.4 liters per minute (LPM) nitrogen gas flow. The DiI/PVP suspension was quickly drawn into the Tefzel tubing and allowed to settle for 3 min. The PVP solution was withdrawn slowly from the tubing making certain not to disturb the tungsten. The Tefzel tubing was slowly rotated 360° and dried for 20 min under 0.4 LPM nitrogen gas flow. After drying, the tubing was cut into 1.3 mm segments (bullets) and stored with desiccant at 4°C in the dark until use.
Delivery of DiI-coated tungsten particles
A Helios Gene Gun (Bio-Rad) with a modified barrel (O’Brien et al., 2001) was used for delivery of DiI-coated tungsten particles. A 40 mm spacer was attached to the modified barrel to establish a consistent distance between the Gene Gun and brain section. A 70-μm nylon mesh filter (Plastok Associates Ltd., Birkenhead, Merseyside, UK) was secured at the head of the barrel to prevent large clusters of tungsten particles from reaching the tissue. The Gene Gun was loaded with DiI “bullets”. Immediately prior to labeling, PBS was removed from the well containing the sections. One bullet was shot per brain section at a distance of 40 mm at 100 pounds per square inch (PSI) for delivery of DiI coated tungsten particles. Labeled sections were re- suspended in PBS and dye was allowed to diffuse through neuronal membranes for 24 h in the dark at room temperature. Slices were post-fixed for 1 h in 4% paraformaldehyde in 25 mM PBS, and then placed in 25 mM PBS until mounted on Superfrost slides (Brain Research Laboratories, Newton Highlands, MA, USA) using 5% n-propyl-gallate in glycerin. Coverslips were sealed to prevent dehydration of tissue.
Immunofluorescence for dopamine D1 and D2 receptors
Following CM-DiI labeling, tissue sections were permeablized in 25 mM PBS with 0.1% Triton-X100 for 15 min. Following permeablization, sections were blocked in 10% BSA in 25 mM PBS for 1 h. Alternate sections were placed in 25 mm PBS plus 0.1% bovine serum albumin (BSA; wash buffer) with primary antibody to either anti-dopamine D1 (1:200, ABN20, Millipore, Billerica, MA) or anti-dopamine D2 (1:200, AB1558, Millipore) receptor, and incubated for 60 h at 4 °C. Following incubation in primary antibody the sections were rinsed three times for 10 min in wash buffer, and then incubated in biotinylated-secondary antibody (1:200, Jackson ImmunoReasearch Laboratories, Inc., West Grove, PA) for 1 h at room temperature. The sections were then washed three times for 10 min in wash buffer before being incubated in streptavidin DTAF conjugate (1:200, Jackson ImmunoReasearch Laboratories, Inc.) for 1 h at room temperature. Following this incubation, the sections were washed three times for 10 min in wash buffer then mounted on slides and coverslipped while still wet with 5% n-propyl gallate in glycerin.
Confocal imaging of dendritic spines
A Leica TCS SPE confocal microscope (Leica, Mannheim, Germany) was used to image DiI impregnated cells. DiI was imaged with excitation and emission specified to the manufacturer’s spectral characteristics (Molecular Probes, Carlsbad, CA, USA). The complete dendritic profile of each DiI impregnated neuron was captured using a 20Χ lens and XY pixel distribution of 512 Χ 512 at a frequency of 400 Hz. The neuron was scanned at 1.0 μm increments along the Z-axis and reconstructed using Leica LAS AF software to determine distance from the soma to the branch level of target dendrites prior to dendrite/spine imaging. Imaged dendritic segments of medium spiny neurons from the nucleus accumbens core (NAcCore), shell (NAcShell), and caudate/putamen (CPu) were 70–200 μm from the soma (Shen et al., 2009). After distance from soma was determined, magnification was increased to 63Χ oil immersion. Frame size was maintained at 512 Χ 512 and an optical zoom of 5.61 was utilized to allow for maximum distribution of pixel size (60 nm) to tissue dimensions (60.91 nm) without over sampling and to minimize photobleaching of the sample during collection of the Z stacks. Z-stacks of dendritic segments were taken at 0.12 μm steps, with a maximum of 200 steps. Images of three DiI impregnated cells were captured per brain region (NAcShell, NAcCore, CPu) per animal, as well as three high power dendritic segments from each cell, yielding a total of nine dendritic segments per brain region per animal. Imaging began immediately following tissue impregnation/mounting.
For tissue dual labeled with either DAD1R or DAD2R, DTAF was imaged with excitation and emission specified to the manufacturer’s spectral characteristics (Jackson ImmunoResearch Inc.) and this channel was overlaid with the DiI channel to establish the morphology of either DAD1 or DAD2 positive cells. Only cells that were positive for either DAD1 or DAD2 and colocalized with DiI were imaged per the methods above.
Quantification and analysis of dendritic spine density and spine morphology
Dendritic Z-stacks were reconstructed using the Surpass module of the Imaris software package (Version 7.1.1, Bitplane Inc., St. Paul, MN, USA). Dendritic shafts and spines were manually traced in the XY plane using the Auto Depth function of the Filament module. After tracing, accurate reconstruction of the diameter of the dendritic shaft, spine neck, and head was made possible using the diameter function with a contrast threshold appropriate to the individual image, generally between 0.1–0.5. Spine head classifications of stubby, filopodial, long thin, and mushroom were completed through the Classify Spines Wizard in the Imaris software package. Criteria for spine head classifications have been described elsewhere (Harris et al., 1992; McKinney et al., 1999).
Spine density was calculated by summing the total number of spines per dendritic segment length and calculating average number of spines/10 μm. These values were then averaged to yield the number of spines/10 μm for each animal. Student’s t-test was used to evaluate statistical differences between treatment groups. Total spine population and counts of each spine class (stubby, filopodial, long thin, and mushroom) were summed for each treatment group. A χ2 test was used to determine significant differences in spine morphology. Spine branch number was calculated by summing the total number of spine branch points per dendritic segment length and averaging those values across all segments per brain region per animal. Student’s t-test was used to evaluate statistical differences between treatment groups.
Radioligand receptor binding estimation of D1 and D2 receptors
Animals were anesthetized using 0.2 ml Sleepaway (26% sodium pentobarbital, 7.8% isopropyl alcohol, 20.7% propylene glycol, distilled water; Fort Dodge Animal Health, Fort Dodge, IA, USA) and rapidly decapitated. Brains were removed and a 2 mm coronal section was taken at the level of the nucleus accumbens. One mm diameter bilateral tissue punches were collected from the nucleus accumbens (combined shell and core) and caudate nucleus. Tissue collected from one punch was processed for later Western blotting and tissue collected from the punch from the other side of the section was processed for radioligand receptor binding. Tissue was stored at −80 °C until further processing.
Point binding experiments were performed as previously described, with minor modifications (Vidi et al., 2008). Levels of D1 and D2 receptor binding were estimated using saturating concentrations of [3H]SCH-23390 (60 Ci/mmol, Amersham Biosciences, Piscataway, NJ) and [3H]spiperone (98 Ci/mmol, Perkin Elmer Life and Analytical Sciences, Waltham, MA), respectively. Tissue was resuspended in 1 ml of binding buffer (50 mM HEPES, 4 mM MgCl2, pH 7.4) and homogenized via polytron for 10 sec. 100 μl aliquots of these homogenates were added to assay tubes to determine total and non-specific binding (defined by 5 μM +/− butaclamol HCl, Sigma-Aldrich, St. Lewis, MO). D1 binding conditions were performed in the presence of 6 nM [3H]SCH-23390. D2 binding was performed in the presence of 50 nM ketanserin tartrate (to prevent labeling of non D2-like receptors, Sigma-Aldrich, St. Lewis, MO) and 1 nM [3H]spiperone. All conditions were performed in duplicate. Assays were floated in a 37 °C water bath for 30 min before harvesting via filtration onto FB glass fiber plates with ice cold wash buffer (10 mM Tris HCl, 0.9% NaCl) using a Packard Filtermate cell harvester (PerkinElmer). After air-drying overnight, Microscint-O scintillation fluid was added to the plates and radioactivity was determined with a Packard TopCount scintillation counter (PerkinElmer). Specific binding for each sample was determined as the difference between the average counts for total versus nonspecific binding. The specific binding values were normalized to the amount of protein added per well, as determined by a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL).
Western blot analysis of D1 or D2 receptors
Tissue punches from the contralateral accumbal section were homogenized in 1% SDS processing buffer (1% SDS, 50 mM NaF, 3.3 mM EGTA, 1% Halt protease inhibitor Cocktail and 1% Halt phosphatase inhibitor Cocktail; Thermo Scientific, Rockford, IL). Following homogenization, protein was quantified using the BioRad protein DC assay per manufacturer’s instructions (BioRad, Hercules, CA, USA). Laemmli Sample buffer (BioRad) and β-mercaptoethanol (Sigma-Aldrich) were added to the homogenate (50 and 10% of the total homogenate volume, respectively) before the mixture was heated for 10 min to 95°C. 50 μg of total protein was loaded into each lane and separated on a 10% polyacylamide gel (BioRad) and transferred to a nitrocellulose membrane. The membrane was blocked at room temperature for 1 h in a Tris buffered saline (TBS) solution containing 5% non-fat dried milk. The membrane was incubated overnight at 4°C with an antibody for dopamine D1 receptor (1:1000, Abcam Inc., Cambridge, MA), dopamine D2 receptor (1:200, Santa Cruz biotechnology, Inc., Santa Cruz, CA) and GAPDH (1:20,000, Millipore, MAB374), diluted in TBS containing 5% milk and 0.1% Tween 20. The next day, the membrane was washed with 0.1% Tween 20-TBS and incubated for 1 h at room temperature with a secondary antibodies IRDye680 (1:10,000) and IRDye 800 (1:10,000). Blots were scanned using the Odyssey imaging system (Li-Cor Biosciences, Lincoln, NE).
Results
Sexual experience alters spine density and morphology within the nucleus accumbens core
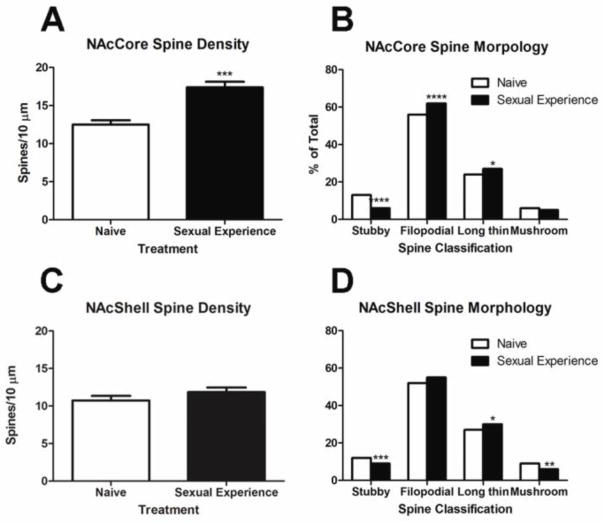
Figure 1 shows representative dendritic segments from MSNs of the NAcCore from naïve females (Figure 1A) or females who received sexual experience once per week for 6 consecutive weeks (Figure 1B). A significant increase in spine density following repeated female sexual behavior was observed in the MSNs of the NAcCore (t(14) =5.23, p<0.001, two tailed) (Figure 2A). No significant changes in spine density were observed in the NAcShell (Figure 2C) or CPu (data not shown) following the 6 week sexual experience paradigm.
Figure 1.
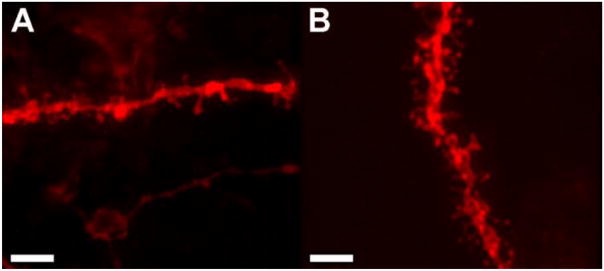
Confocal images of dendritic segments of medium spiny neurons from the nucleus accumbens core from (A) sexually naïve or (B) sexually experienced females. Scale bar = 5μm.
Figure 2.
Spine density and morphology of medium spiny neurons from the nucleus accumbens core or shell. (A) Sexual experience significantly increased spine density of medium spiny neurons within the nucleus accumbens core (***p<0.001) and (B) significantly shifted spine morphology, reducing stubby spines (***p<0.001) with higher proportions of filapodial (****p<0.001) and long thin spines (*p<0.05). (C) Sexual experience had no significant effect on spine density of medium spiny neurons within the nucleus accumbens shell, however (D) significant increases in long thin spines (*p<0.05) with concurrent decreases in mushroom (**p<0.01) and stubby spines (***p<0.001) were identified. (N = 8 per treatment group)
Spines were classified into one of four categories in order of maturity: stubby (least mature), filapodial, long thin, or mushroom (most mature). Summation of all four spine categories for a given treatment group provided normative values for the total spine number per treatment group, population counts for each spine subtype in a given brain region, as well as the percentage of total spine population.
Following repeated sexual behavior, significant shifts in spine morphology were observed in all three brain regions that were analyzed (Figures 2B, 2D). Specifically, within the NAcCore, repeated sexual experience resulted in a significant reduction of stubby spines (χ2(1) = 77.45, p<0.001, two tailed), with concurrent significant increases in both filapodial (χ2(1) = 15.99, p<0.001, two tailed) and long thin spines (χ2(1) = 4.08, p<0.05, two tailed). In the NAcShell, as similar pattern of change was observed with significant decreases of stubby spines (χ2(1) = 11.48, p<0.001, two tailed) and significant increases of long thin spines (χ2(1) = 3.89, p<0.05, two tailed). In addition to these changes, significant decreases in mushroom spines were also observed in the NAcShell following repeated sexual behavior (χ2(1) = 7.10, p<0.01, two tailed). In MSNs of the CPu of females who had received repeated sexual experience (data not shown), significant decreases in the proportion of stubby spines were observed (χ2(1) = 10.10, p<0.01, two-sided), with no significant changes observed in any other spine subtype.
Increases in dendritic spines are restricted to D1 receptor expressing medium spiny neurons
In a parallel group of animals, spine density changes in MSNs labeled for either DAD1R or DAD2R were evaluated (Figure 3). A considerable number of medium spiny neurons were labeled with each antibody, though it was our impression that only a subset of each cell type was visibly labeled. Consequently, we only analyzed medium spiny neurons clearly labeled for either DAD1R or DAD2R ignoring neurons with no immunolabeling.
Figure 3.
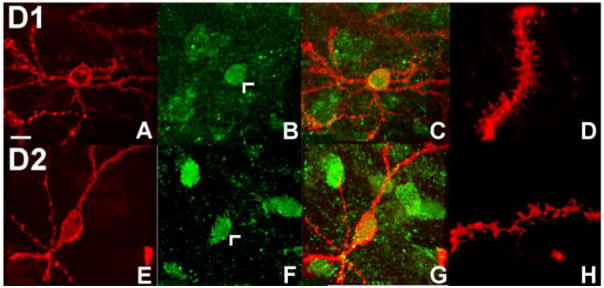
Colocalization of either dopamine D1 or D2 immunolabeled medium spiny neurons with DiI. (A) DiI labeled medium spiny neuron from the nucleus accumbens core. (B) The same medium spiny neuron labeled for D1 receptors (arrowhead). (C) Overlay of images from (A) and (B) showing co-localization of DiI and D1 label. (D) Confocal image of a dendritic segment from the DiI/D1-positive medium spiny neuron shown in (C). (E) DiI labeled medium spiny neuron from the nucleus accumbens core, (F) labeled for D2 receptors (arrowhead). (G) Co-localization of DiI (E) and D2-positive (F) cell used to determine phenotype specific spine density. (H) Confocal image of a dendritic segment from the DiI/D1-positive medium spiny neuron shown in (G).
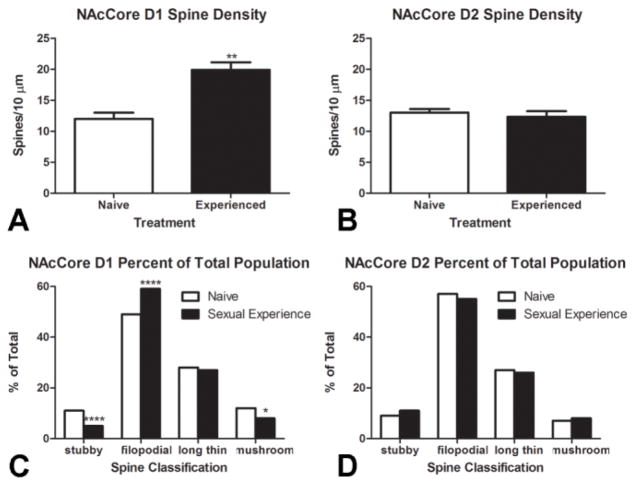
Following sexual experience (Figure 4A), significant increases in spine density were observed (t(6) =5.23, p<0.001, two tailed) in the DAD1R expressing population of medium spiny neurons within the NAcCore with no significant differences in spine density observed in DAD2R expressing MSNs. No significant differences in spine density in either DAD1R or DAD2R expressing MSNs were observed in the NAcShell (Figure 4B). No significant differences in spine density were observed in DAD1R expressing MSNs within the CPu (data not shown), however, a small but significant increase in spine density of DAD2R expressing MSNs was observed following sexual experience (t(6) =2.67, p <0.05, two tailed).
Figure 4.
Alterations to dendritic spine structure following sexual experience were isolated to dopamine D1 neurons within the nucleus accumbens core. (A) Significant increases in spine density resulting from sexual experience were restricted to the dopamine D1 population (**p<0.001), (B) with no significant differences detected in the dopamine D2 population (p>0.05). (N = 4 per treatment group) (C) Shifts in spine morphology depicted in Figure 5B were isolated to the dopamine D1 population with significantly fewer stubby (****p<0.001) and mushroom (*p<0.05) spines with greater proportions of filapodial (****p<0.001) spines. (D) No significant differences in spine morphology were observed in the dopamine D2 population.
As with spine density, animals were examined to determine if structural plasticity within the accumbens was isolated to a particular phenotype of MSNs (Figure 4C, 4D). Repeated sexual behavior significantly decreased stubby spines (χ2(1) = 24.03, p<0.001, two tailed) and mushroom spines (χ2(1) = 5.65, p<0.05, two tailed), with concurrent increases in filapodial spines (χ2(1) = 20.78, p<0.001, two tailed) within the DAD1R population of MSNs in the NAcCore (Figure 4C). No significant shifts in spine morphology were observed in the DAD2R expressing population of the NAcCore. Within the NAcShell (Figure 4D), a significant increase in filapodial spines (χ2(1) = 8.86, p<0.01, two-sided) was observed in the D1 population, with a concurrent decrease in long thin spines (χ2(1) = 7.53, p<0.01, two-sided) and no significant changes in either stubby or mushroom spines (data not shown). A small but significant increase in mushroom spines was observed (χ2(1) = 5.02, p<0.05, two-sided) in the DAD2R population of the NAcShell, with no significant changes in stubby, filapodial or long thin spine subtypes (data not shown). The only effect of sexual experience on spine morphology that was observed in the DAD1R expression phenotype of the CPu was a significant increase in stubby spine (χ2(1) = 5.57, p<0.05, two-sided) DAD1R phenotype, with no significant differences in DAD1R filapodial, long thin or mushroom spines or D2 stubby, filapodial, long thin or mushroom spines (data not shown).
Sexual experience does not affect dopamine receptor binding
Repeated sexual experience had no significant effect on dopamine receptor binding for either DAD1R or DAD2R in tissue homogenates from either the NAc (Figure 5A) or CPu (Figure 5B).
Figure 5.
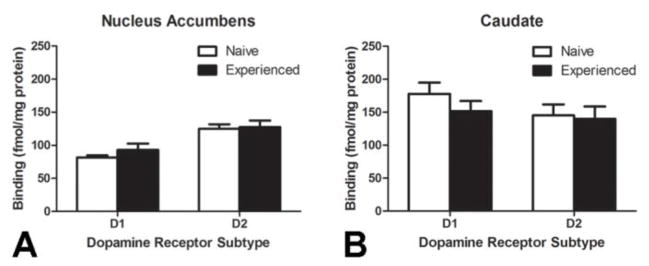
No significant differences in binding for either dopamine D1 ([3H]SCH-23390) or D2 ([3H]spiperone) receptor sub-types in the (A) nucleus accumbens or (B) caudate were observed following repeated sexual experience in female hamsters. (N = 6 per treatment group)
Sexual experience does not affect dopamine receptor protein expression
Western blots of either DAD1R or DAD2R labeling were normalized to GAPDH. Statistical analysis revealed no significant differences in DAD1R or DAD2R expression following sexual experience in either the NAc (Figure 6A) or CPu (Figure 6B).
Figure 6.
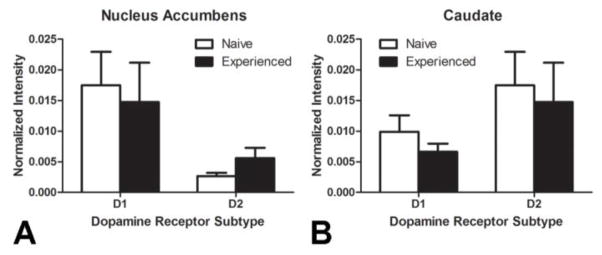
No significant differences in total protein for either the dopamine D1 or D2 receptor was detected in either the (A) nucleus accumbens or (B) caudate following repeated sexual experience in female hamsters. (N = 6 per treatment group)
Discussion
Synaptic plasticity in the nucleus accumbens is a process through which the ongoing expression of motivated behaviors can be modified as a result of prior experience (Bradley et al., 2005; Goto and Grace, 2005; Kelley et al., 1997; Meisel and Mullins, 2006). We have developed a model of such experience based plasticity in which we discovered that repeated sexual experience produces augmented responsiveness, both pre- and postsynaptically, in the nucleus accumbens of female Syrian hamsters (Hedges et al., 2010; Meisel and Mullins, 2006). Besides female sexual behavior, other motivated behaviors such as male sexual behavior, aggression, and salt appetite induce similar patterns of plasticity in the nucleus accumbens (Pitchers et al., 2010a; Roitman et al., 2002; Staffend and Meisel, 2012), supporting a common neurobiological process across motivated behaviors. Here we add to that knowledge base with a detailed analysis of regional and phenotype specific structural plasticity following female sexual behavior experience.
During sexual interactions with males, there is an elevation in extracellular dopamine in the nucleus accumbens of female hamsters and rats (Becker et al., 2001; Jenkins and Becker, 2003; Kohlert and Meisel, 1999; Kohlert et al., 1997; Meisel et al., 1993; Mermelstein and Becker, 1995). With repeated sexual experience, females exhibit a sensitized elevation in dopamine release during these sexual interactions (Becker et al., 2001; Fiorino and Phillips, 1999; Jenkins and Becker, 2003; Kohlert and Meisel, 1999). The presynaptic changes in synaptic dopamine are mirrored in a cascade of postsynaptic modifications including enhanced cAMP accumulation, ERK signaling, and Fos (particularly ΔFosB) accumulation (Bradley and Meisel, 2001; Bradley et al., 2004; Meisel and Mullins, 2006; Hedges et al., 2009; Pitchers KK et al., 2010b). This plasticity, driven by dopaminergic neurotransmission, appears to culminate in structural plasticity as evidenced by increases in dendritic spines.
Medium spiny neurons in the nucleus accumbens exhibit a typical hyperpolarized resting potential, termed the down state, though they can also maintain a resting potential that is close to the firing potential, termed the up state (O’Donnell and Grace, 1995; Yim and Mogenson, 1988). The up state of medium spiny neurons is gated by glutamatergic input from hippocampal afferents, and in this state, action potentials are often released in bursts (O’Donnell and Grace, 1995). Phasic dopamine release from the ventral tagmental area, generally coincident to glutamatergic drive from the hippocampus, is associated with biologically relevant stimuli (Gerfen and Surmeier, 2011; Schultz, 1998, 2013; Wickens et al., 2003; Zweifel et al., 2008) and modulates the integration of glutamatergic input into the circuitry of the nucleus accumbens (reviewed in (Sesack and Grace, 2010)). What is interesting in this regard is that the coincidence of glutamatergic and phasic dopaminergic release within the nucleus accumbens preferentially activates D1 expressing medium spiny neurons, and is thought to be necessary for the long term plastic changes in this brain region (Floresco et al., 2001a, b; Smith-Roe and Kelley, 2000; West and Grace, 2002). This parallels our discovery that the increases in dendritic spine density following sexual experience are restricted to medium spiny neurons expressing D1 receptors. Further, sexual experience produces a sensitized cAMP accumulation to D1 receptor stimulation (Bradley et al., 2004), in the absence of changes in D1 receptor protein or binding, as reported in this study. In principle, D2 receptor activation, which inhibits intracellular signaling and puts medium spiny neurons in the hyperpolarized down state (Floresco et al., 2001b; Planert et al., 2013), could have led to a corresponding decrease in dendritic spine density. This possibility was not borne out as there were no spine density changes in nucleus accumbens neurons expressing D2 receptors.
Besides the increase in dendritic spine density, we also observed a shift in the distribution of spines classified according to categories of geometric shape. Within the core of the nucleus accumbens this shift in spine geometry favored a filopodial spine type. The filopodial spines are thought to represent a highly plastic spine state (Kasai et al., 2003). Glutamatergic afferents onto these spines make what are termed silent synapses (Matsuzaki et al., 2001). Though not truly silent, these synapses contain a smaller number of AMPA receptors, with an accompanying increase in NMDA receptors (Matsuzaki et al., 2001; Noguchi et al., 2011)). As AMPA activation is needed to remove the magnesium block that limits NMDA currents, these synapses show little intrinsic excitability yet they are more susceptible to long term plasticity such as the induction of LTP (Matsuzaki et al., 2004; Noguchi et al., 2005; Noguchi et al., 2011).
In our previous histological studies, sexual experience produced changes in endpoints such as pERK, c-Fos, or ΔFosB that were restricted to the core subregion of the nucleus accumbens, with no changes observed in the shell subregion (Bradley and Meisel, 2001; Hedges et al., 2009; Meisel and Mullins, 2006). Further, we used the caudate as a control for regional specificity and have typically found the caudate to be unaffected by sexual experience (Bradley and Meisel, 2001; Hedges et al., 2009; Meisel and Mullins, 2006). Consequently it was surprising to us that the shell of the nucleus accumbens had changes in distribution of dendritic spine type (though in the absence of changes in spine density) restricted to medium spiny neurons expressing D1 receptors that mimicked those seen in the core of the accumbens. Even more surprising were the changes in spine morphology in the caudate that were restricted to the D2-expressing medium spiny neurons. At the same time that we note these findings, we must admit that we are unable to incorporate them into our mechanistic model at this time.
The mechanism that has been emerging from studies of both female hamsters and male rats is that sexual experience increases dopaminergic signaling through D1 receptors, altering intracellular signaling pathways in a way that promotes the accumulation of ΔFosB (Hedges et al., 2009; Meisel and Mullins, 2006; Pitchers et al., 2010b; Pitchers et al., 2013; Wallace et al., 2008). This elevated expression of ΔFosB then promotes structural plasticity that underlies behavioral changes (e.g., sexual reward and increased copulatory efficiency) following sexual experience (Hedges et al., 2009; Pitchers et al., 2010b; Pitchers et al., 2013; Wallace et al., 2008). Consistent with these structural changes are electrophysiological studies following both simple overexpression of ΔFosB or repeated male sexual behavior indicating that AMPA:NMDA ratios in nucleus accumbens medium spiny neurons decrease with corresponding increases in silent synapses (Grueter et al., 2013; Pitchers et al., 2013). It is a priority of ours to determine the physiological changes that accompany the dendritic spine changes in our hamster model.
The results of our study highlight the cell-type specific structural alterations in the mesolimbic circuit following sexual experience and perhaps more broadly with motivated behaviors. This mesolimbic circuitry is better known to be conscripted by drugs of abuse, with the resultant plasticity the basis of drug addiction (Grueter et al., 2012; Luscher and Malenka, 2011; Nestler, 2012). Drugs of abuse also produce changes in spine density within the nucleus accumbens (Dobi et al., 2011; Kim et al., 2011; Lee et al., 2006; Li et al., 2004; Shen et al., 2009), which are isolated to the dopamine D1 population of medium spiny neurons (Dobi et al., 2011; Kim et al., 2011; Lee et al., 2006). It is in this context that a guiding principle for our work is to test the proposition that high frequency sexual behavior experience impacts the nucleus accumbens creating a means through which certain life events can create a vulnerability for drug addiction (Hedges et al., 2010).
Acknowledgments
This work was supported by NIH grants DA013680 (RLM), T32 DA07234 (V. Seybold, PI), and MH60397 (VJW). Nancy Staffend is now at Michigan State University, Neuroscience Department, 293 Farm Lane, Rm 108, East Lansing, MI 48824.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–375. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- Becker JB, Rudick CN, Jenkins WJ. The role of dopamine in the nucleus accumbens and striatum during sexual behavior in the female rat. J Neurosci. 2001;21:3236–3241. doi: 10.1523/JNEUROSCI.21-09-03236.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KC, Haas AR, Meisel RL. 6-Hydroxydopamine lesions in female hamsters (Mesocricetus auratus) abolish the sensitized effects of sexual experience on copulatory interactions with males. Behav Neurosci. 2005;119:224–232. doi: 10.1037/0735-7044.119.1.224. [DOI] [PubMed] [Google Scholar]
- Bradley KC, Meisel RL. Sexual behavior induction of c-Fos in the nucleus accumbens and amphetamine-stimulated locomotor activity are sensitized by previous sexual experience in female Syrian hamsters. J Neurosci. 2001;21:2123–2130. doi: 10.1523/JNEUROSCI.21-06-02123.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley KC, Mullins AJ, Meisel RL, Watts VJ. Sexual experience alters D1 receptor-mediated cyclic AMP production in the nucleus accumbens of female Syrian hamsters. Synapse. 2004;53:20–27. doi: 10.1002/syn.20030. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dobi A, Seabold GK, Christensen CH, Bock R, Alvarez VA. Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J Neurosci. 2011;31:1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorino DF, Phillips AG. Facilitation of sexual behavior and enhanced dopamine efflux in the nucleus accumbens of male rats after D-amphetamine-induced behavioral sensitization. J Neurosci. 1999;19:456–463. doi: 10.1523/JNEUROSCI.19-01-00456.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Dopamine D1 and NMDA receptors mediate potentiation of basolateral amygdala-evoked firing of nucleus accumbens neurons. J Neurosci. 2001a;21:6370–6376. doi: 10.1523/JNEUROSCI.21-16-06370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Blaha CD, Yang CR, Phillips AG. Modulation of hippocampal and amygdalar-evoked activity of nucleus accumbens neurons by dopamine: cellular mechanisms of input selection. J Neurosci. 2001b;21:2851–2860. doi: 10.1523/JNEUROSCI.21-08-02851.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohmader KS, Pitchers KK, Balfour ME, Coolen LM. Mixing pleasures: review of the effects of drugs on sex behavior in humans and animal models. Horm Behav. 2010;58:149–162. doi: 10.1016/j.yhbeh.2009.11.009. [DOI] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong RO, Lichtman JW. Ballistic delivery of dyes for structural and functional studies of the nervous system. Cold Spring Harbor Protoc. 2009;4:1–6. doi: 10.1101/pdb.prot5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan WB, Grutzendler J, Wong WT, Wong RO, Lichtman JW. Multicolor “DiOlistic” labeling of the nervous system using lipophilic dye combinations. Neuron. 2000;27:219–225. doi: 10.1016/s0896-6273(00)00031-3. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Ann Rev Neurosci. 2011;34:441–466. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliano F, Allard J. Dopamine and male sexual function. Eur Urol. 2001a;40:601–608. doi: 10.1159/000049844. [DOI] [PubMed] [Google Scholar]
- Giuliano F, Allard J. Dopamine and sexual function. Int J Impot Res. 2001b;13:S18–28. doi: 10.1038/sj.ijir.3900719. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopaminergic modulation of limbic and cortical drive of nucleus accumbens in goal-directed behavior. Nat Neurosci. 2005;8:805–812. doi: 10.1038/nn1471. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Robison AJ, Neve RL, Nestler EJ, Malenka RC. FosB differentially modulates nucleus accumbens direct and indirect pathway function. Proc Natl Acad Sci USA. 2013;110:1923–1928. doi: 10.1073/pnas.1221742110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Jensen FE, Tsao B. Three-dimensional structure of dendritic spines and synapses in rat hippocampus (CA1) at postnatal day 15 and adult ages: implications for the maturation of synaptic physiology and long-term potentiation. J Neurosci. 1992;12:2685–2705. doi: 10.1523/JNEUROSCI.12-07-02685.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Chakravarty S, Nestler EJ, Meisel RL. Delta FosB overexpression in the nucleus accumbens enhances sexual reward in female Syrian hamsters. Genes Brain Behav. 2009;8:442–449. doi: 10.1111/j.1601-183X.2009.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges VL, Staffend NA, Meisel RL. Neural mechanisms of reproduction in females as a predisposing factor for drug addiction. Front Neuroendocrinol. 2010;31:217–231. doi: 10.1016/j.yfrne.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries MD, Prescott TJ. The ventral basal ganglia, a selection mechanism at the crossroads of space, strategy, and reward. Prog Neurobiol. 2010;90:385–417. doi: 10.1016/j.pneurobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. doi: 10.1016/j.brainresrev.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Panksepp J. The role of nucleus accumbens dopamine in motivated behavior: a unifying interpretation with special reference to reward-seeking. Brain Res Rev. 1999;31:6–41. doi: 10.1016/s0165-0173(99)00023-5. [DOI] [PubMed] [Google Scholar]
- Jenkins WJ, Becker JB. Dynamic increases in dopamine during paced copulation in the female rat. Eur J Neurosci. 2003;18:1997–2001. doi: 10.1046/j.1460-9568.2003.02923.x. [DOI] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, Nakahara H. Structure-stability-function relationships of dendritic spines. Trends Neurosci. 2003;26:360–368. doi: 10.1016/S0166-2236(03)00162-0. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Ventral striatal control of appetitive motivation: role in ingestive behavior and reward-related learning. Neurosci Biobehav Rev. 2004;27:765–776. doi: 10.1016/j.neubiorev.2003.11.015. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Smith-Roe SL, Holahan MR. Response-reinforcement learning is dependent on N-methyl-D-aspartate receptor activation in the nucleus accumbens core. Proc Natl Acad Sci USA. 1997;94:12174–12179. doi: 10.1073/pnas.94.22.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Park BH, Lee JH, Park SK, Kim JH. Cell type-specific alterations in the nucleus accumbens by repeated exposures to cocaine. Biol Psychiatry. 2011;69:1026–1034. doi: 10.1016/j.biopsych.2011.01.013. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Meisel RL. Sexual experience sensitizes mating-related nucleus accumbens dopamine responses of female Syrian hamsters. Behav Brain Res. 1999;99:45–52. doi: 10.1016/s0166-4328(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Kohlert JG, Rowe RK, Meisel RL. Intromissive stimulation from the male increases extracellular dopamine release from fluoro-gold-identified neurons within the midbrain of female hamsters. Horm Behav. 1997;32:143–154. doi: 10.1006/hbeh.1997.1415. [DOI] [PubMed] [Google Scholar]
- Lee K, Kim Y, Kim AM, Helmin K, Nairn AC, Greengard P. Cocaine-induced dendritic spine formation in D1 and D2 dopamine receptor-containing medium spiny neurons in nucleus accumbens. Proc Natl Acad Sci USA. 2006;103:3399–3404. doi: 10.1073/pnas.0511244103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Acerbo MJ, Robinson TE. The induction of behavioural sensitization is associated with cocaine-induced structural plasticity in the core (but not shell) of the nucleus accumbens. Eur J Neurosci. 2004;20:1647–1654. doi: 10.1111/j.1460-9568.2004.03612.x. [DOI] [PubMed] [Google Scholar]
- Luscher C, Malenka RC. Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron. 2011;69:650–663. doi: 10.1016/j.neuron.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, Kasai H. Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nat Neurosci. 2001;4:1086–1092. doi: 10.1038/nn736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Honkura N, Ellis-Davies GC, Kasai H. Structural basis of long-term potentiation in single dendritic spines. Nature. 2004;429:761–766. doi: 10.1038/nature02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinney RA, Capogna M, Durr R, Gahwiler BH, Thompson SM. Miniature synaptic events maintain dendritic spines via AMPA receptor activation. Nat Neurosci. 1999;2:44–49. doi: 10.1038/4548. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Camp DM, Robinson TE. A microdialysis study of ventral striatal dopamine during sexual behavior in female Syrian hamsters. Behav Brain Res. 1993;55:151–157. doi: 10.1016/0166-4328(93)90111-3. [DOI] [PubMed] [Google Scholar]
- Meisel RL, Mullins AJ. Sexual experience in female rodents: cellular mechanisms and functional consequences. Brain Res. 2006;1126:56–65. doi: 10.1016/j.brainres.2006.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein PG, Becker JB. Increased extracellular dopamine in the nucleus accumbens and striatum of the female rat during paced copulatory behavior. Behav Neurosci. 1995;109:354–365. doi: 10.1037//0735-7044.109.2.354. [DOI] [PubMed] [Google Scholar]
- Muly EC, Greengard P, Goldman-Rakic PS. Distribution of protein phosphatases-1 alpha and -1 gamma 1 and the D(1) dopamine receptor in primate prefrontal cortex: Evidence for discrete populations of spines. J Comp Neurol. 2001;440:261–270. doi: 10.1002/cne.1384. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Transcriptional mechanisms of drug addiction. Clin Psychopharmacol Neurosci. 2012;10:136–143. doi: 10.9758/cpn.2012.10.3.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi J, Matsuzaki M, Ellis-Davies GC, Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–622. doi: 10.1016/j.neuron.2005.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GC, Kitamura K, Kano M, Matsuzaki M, Kasai H. In vivo two-photon uncaging of glutamate revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J Physiol. 2011;589:2447–2457. doi: 10.1113/jphysiol.2011.207100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien JA, Holt M, Whiteside G, Lummis SC, Hastings MH. Modifications to the hand-held Gene Gun: improvements for in vitro biolistic transfection of organotypic neuronal tissue. J Neurosci Methods. 2001;112:57–64. doi: 10.1016/s0165-0270(01)00457-5. [DOI] [PubMed] [Google Scholar]
- O’Donnell P, Grace AA. Synaptic interactions among excitatory afferents to nucleus accumbens neurons: hippocampal gating of prefrontal cortical input. J Neurosci. 1995;15:3622–3639. doi: 10.1523/JNEUROSCI.15-05-03622.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penzes P, Johnson RC, Sattler R, Zhang X, Huganir RL, Kambampati V, Mains RE, Eipper BA. The neuronal Rho-GEF Kalirin-7 interacts with PDZ domain-containing proteins and regulates dendritic morphogenesis. Neuron. 2001;29:229–242. doi: 10.1016/s0896-6273(01)00193-3. [DOI] [PubMed] [Google Scholar]
- Pitchers KK, Balfour ME, Lehman MN, Richtand NM, Yu L, Coolen LM. Neuroplasticity in the mesolimbic system induced by natural reward and subsequent reward abstinence. Biol Psychiat. 2010a;67:872–879. doi: 10.1016/j.biopsych.2009.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Frohmader KS, Vialou V, Mouzon E, Nestler EJ, Lehman MN, Coolen LM. ΔFosB in the nucleus accumbens is critical for reinforcing effects of sexual reward. Genes Brain Behav. 2010b;9:831–840. doi: 10.1111/j.1601-183X.2010.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Schmid S, Di Sebastiano AR, Wang X, Laviolette SR, Lehman MN, Coolen LM. Natural reward experience alters AMPA and NMDA receptor distribution and function in the nucleus accumbens. PLoS One. 2012;7:e34700. doi: 10.1371/journal.pone.0034700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchers KK, Vialou V, Nestler EJ, Laviolette SR, Lehman MN, Coolen LM. Natural and drug rewards act on common neural plasticity mechanisms with ΔFosB as a key mediator. J Neurosci. 2013;33:3434–3442. doi: 10.1523/JNEUROSCI.4881-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planert H, Berger TK, Silberberg G. Membrane properties of striatal direct and indirect pathway neurons in mouse and rat slices and their modulation by dopamine. PLoS One. 2013;8:e57054. doi: 10.1371/journal.pone.0057054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci. 2002;22:RC225. doi: 10.1523/JNEUROSCI.22-11-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. The phasic reward signal of primate dopamine neurons. Adv Pharmacol. 1998;42:686–690. doi: 10.1016/s1054-3589(08)60841-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Updating dopamine reward signals. Curr Opin Neurobiol. 2013;23:229–238. doi: 10.1016/j.conb.2012.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-Basal Ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HW, Toda S, Moussawi K, Bouknight A, Zahm DS, Kalivas PW. Altered dendritic spine plasticity in cocaine-withdrawn rats. J Neurosci. 2009;29:2876–2884. doi: 10.1523/JNEUROSCI.5638-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Loftus CM, Meisel RL. Estradiol reduces dendritic spine density in the ventral striatum of female Syrian hamsters. Brain Struct Funct. 2011;215:187–194. doi: 10.1007/s00429-010-0284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. DiOlistic labeling in fixed brain slices: phenotype, morphology, and dendritic spines. Current Protoc Neurosci. 2011a;Chapter 2(Unit 2.13) doi: 10.1002/0471142301.ns0213s55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. DiOlistic Labeling of neurons in tissue slices: A qualitative and quantitative analysis of methodological variations. Front Neuroanat. 2011b;5:14. doi: 10.3389/fnana.2011.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staffend NA, Meisel RL. Aggressive experience increases dendritic spine density within the nucleus accumbens core in female Syrian hamsters. Neuroscience. 2012;227:163–169. doi: 10.1016/j.neuroscience.2012.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidi PA, Chemel BR, Hu CD, Watts VJ. Ligand-dependent oligomerization of dopamine D(2) and adenosine A(2A) receptors in living neuronal cells. Molec Pharmacol. 2008;74:544–551. doi: 10.1124/mol.108.047472. [DOI] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iñiguez SD, Perrotti LI, Barrot M, DiLeone RJ, Nestler EJ, Bolaños-Guzmán CA. The influence of ΔFosB in the nucleus accumbens on natural reward-related behavior. J Neurosci. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AR, Grace AA. Opposite influences of endogenous dopamine D1 and D2 receptor activation on activity states and electrophysiological properties of striatal neurons: studies combining in vivo intracellular recordings and reverse microdialysis. J Neurosci. 2002;22:294–304. doi: 10.1523/JNEUROSCI.22-01-00294.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens J. Striatal dopamine in motor activation and reward-mediated learning: steps towards a unifying model. J Neural Transm Gen Sect. 1990;80:9–31. doi: 10.1007/BF01245020. [DOI] [PubMed] [Google Scholar]
- Wickens JR, Reynolds JN, Hyland BI. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–690. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Neuromodulatory action of dopamine in the nucleus accumbens: an in vivo intracellular study. Neuroscience. 1988;26:403–415. doi: 10.1016/0306-4522(88)90158-3. [DOI] [PubMed] [Google Scholar]
- Zweifel LS, Argilli E, Bonci A, Palmiter RD. Role of NMDA receptors in dopamine neurons for plasticity and addictive behaviors. Neuron. 2008;59:486–496. doi: 10.1016/j.neuron.2008.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]