Abstract
Water may affect the mechanical behavior of bone by interacting with the mineral and organic phases through two major pathways: i.e. hydrogen bonding and polar interactions. In this study, dehydrated bone was soaked in several solvents (i.e. water, heavy water (D2O), ethylene glycol (EG), dimethylformamide (DMF), and carbon tetrachloride(CCl4)) that are chemically harmless to bone and different in polarity, hydrogen bonding capability and molecular size. The objective was to examine how replacing the original matrix water with the solvents would affect the mechanical behavior of bone. The mechanical properties of bone specimens soaked in these solvents were measured in tension in a progressive loading scheme. In addition, bone specimens without any treatments were tested as the baseline control whereas the dehydrated bone specimens served as the negative control. The experimental results indicated that 22.3±5.17vol% of original matrix water in bone could be replaced by CCl4, 71.8±3.77vol% by DMF, 85.5±5.15vol% by EG, and nearly 100% by D2O and H2O, respectively. CCl4 soaked specimens showed similar mechanical properties with the dehydrated ones. Despite of great differences in replacing water, only slight differences were observed in the mechanical behavior of EG and DMF soaked specimens compared with dehydrated bone samples. In contrast, D2O preserved the mechanical properties of bone comparable to water. The results of this study suggest that a limited portion of water (<15vol% of the original matrix water) plays a pivotal role in the mechanical behavior of bone and it most likely resides in small matrix spaces, into which the solvent molecules larger than 4.0Å cannot infiltrate.
Keywords: Water, bone, toughness, solvents, ultrastructural spaces
INTRODUCTION
Bone is a natural composite material with a highly hierarchical structure and consists of three major constituents: i.e. mineral, organic matrix, and water, respectively. It has been known for decades that removal of water (dehydration) may lead to a marked decrease in the toughness and an increase in the stiffness of bone, suggesting that water plays an important role in both pre-and post-yield behavior of bone [1, 2].
Previous NMR studies reveal that water in bone is present in three different conformations: namely freely mobile water in pores, such as Haversian canals, canaliculi, and lacunae spaces; bound water at surfaces and/or within the mineral and collagen phases; and structural water as part of collagen and mineral molecules [3–5]. Water may reside in the gap between the mineral-collagen interface in an order of several angstroms [6]. On the other hand, such matrix water may be replaced by minerals during continuous mineralization process [7]. Moreover, removal of water was speculated to alter the behavior of the collagen phase, thus reducing its capacity to dissipate energy in bone [8]. Furthermore, dynamic mechanical analyses indicate that bone viscoelastic behavior is most likely related to water in bone rather than the collagen phase itself [9, 10]. However, the respective contribution of these three types of matrix water to the mechanical properties of bone is still poorly understood.
In this study, we hypothesized that the bulk mechanical properties of bone are significantly related to the water molecules that reside in extremely small (i.e. angstrom level) spaces of bone matrix, into which only water or a solvent akin to water can infiltrate. To test the hypothesis, we proposed to replace water in bone matrix with several solvents that are harmless to the structural integrity of bone constituents (i.e. mineral and collagen) and have different molecular size (i.e. kinetic diameter) and/or chemical characteristics (i.e. polarity and hydrogen bonding ability). Then, the correlation of molecular size, polarity, and hydrogen bonding ability with the soaking ability of the solvents into bone matrix and its effect on the mechanical behavior of bone were investigated.
MATERIALS & METHODS
Specimen preparation
Six human cadaveric tibiae of male donors (N=6) were procured from a Willed Body Program (UT Southwestern Medical Center at Dallas, TX) with the stipulation that the donors had no known bone diseases. The donor ages were 51, 52, 54, 56, 58 and 76 years, respectively. Seven (7) dog-bone-shaped tensile test specimens were prepared from the mid diaphysis of each tibia using a CNC machine and randomly divided into seven (7) groups, including four (4) test groups (Table 1) in addition to a control (dehydrated and rehydrated), a baseline control (wet bone without any treatment), and a negative control (dehydrated) group. The specimens had a gauge length of 10mm and a gauge cross-section of 2.0mm×2.0mm. The prepared specimens were preserved in a phosphate buffered saline (PBS) solution and stored in a freezer at −20° C prior to the treatments.
Table 1.
| Solvents | Molecular structure |
Kinetic diameter dk (Å) |
Molecular Weight M (g/mol) |
Density @25°C ρ(g/cm3) |
Relative permittivity εr (−) |
Dipole Moment p (D) |
H-H bond energy (kJ/mol) |
Viscosity @20°C η (cP) |
|---|---|---|---|---|---|---|---|---|
| H2O |  |
2.4–2.6 | 18.015 | 0.997 | 78.30 | 1.85 | 20.5 | ~1.000 |
| D2O |  |
2.6 | 20.04 | 1.104 | 77.94 | 1.85 | 8% higher than H2O | 1.251 |
| EG HO(CH2)2OH[28] |  |
~ 4.0 | 62.07 | 1.11 | 42.0 | 2.33 | 25.1 | 16.10 |
| DMF (CH3)2NC(O)H |  |
5.5 | 73.09 | 0.944 | 36.7 | 3.80 | No donors, but acceptors | 0.920 |
| CCl4 |  |
4.65–5.9 | 153.8 | 1.584 | 2.238 | N/A | N/A | 0.84–0.95 |
H2O: Water; D2O: Heavy water; EG: Ethylene Glycol; DMF: Dimethylformamide; CCl4: Carbon Tetrachloride.
Selection of solvents
Since water molecules interact with the mineral and organic phases in bone through two major pathways: i.e. hydrogen bonding and polar interactions, the following solvents were selected from a pool of potential solvents that have different polarity, hydrogen bonding capability and molecular size (Table 1).
Water (H2O)
Water is a good polar solvent [11, 12] and has an estimated intermolecular hydrogen bond energy of 20.5kJ/mol [13]. Water has the smallest molecular size (2.4–2.6Å) compared with the other solvents [14, 15].
Heavy water (D2O)
Heavy water molecules contain two deuterium (hydrogen isotope) atoms in lieu of the hydrogen atoms in water molecules. It has similar polarity, chemical structure and molecular size (2.6Å) [16] compared to water, with a slightly higher hydrogen bond energy (~8%).
Ethylene glycol (EG)
Ethylene glycol molecules have two hydrogen atoms attached to two separate oxygen atoms, thus allowing it to form strong hydrogen bonds readily with other molecules and high polarity akin to water. In addition, EG is the smallest organic molecule that can form hydrogen bond network like H2O [17]. However, it has a much larger molecular size (4.0Å) [18] compared with H2O and is the greatest among all other solvents in viscosity. Since it is harmless to biological systems, the polymerized ethylene glycol (polyethylene glycol) have been used as cell culture scaffolds in tissue engineering studies [19, 20].
Dimethylformamide (DMF)
DMF has a polarity comparable to EG, but lacks the ability to serve as a donor of hydrogen bonding like EG. DMF has a similar viscosity, but a larger molecular size (5.50Å) compared to EG [15]. DMF also has been safely used as a co-solvent in cell culture [21, 22] and does not cause denaturation of collagen molecules [17].
Carbon tetrachloride (CCl4)
CCl4 possesses neither the hydrogen bonding ability nor the polarity due to its tetrahedral symmetry and has a molecular size (4.65–5.90Å) comparable to that of MDF [14]. Its viscosity (0.84–0.95cP at 20°C) is slightly lower than that of H2O (1.00cP at 20°C) even though its density is much higher. CCl4 has also been used as co-solvent in cell culture studies [23].
In this study, the kinetic diameter was used to estimate the molecular size of the solvents. In addition, the hydrogen bonding energy was used as the measure of the ability of solvent molecules to form hydrogen bonds with others. Moreover, the dielectric constant and dipole moment were used to define the polarity of the solvents. Finally, the viscosity of the solvents was also listed in the table in comparison between the solvents for viscous flow in bone.
Although it is hard, if not impossible, to adjust only one variable (i.e. polarity, hydrogen bonding ability, and molecular size) while keeping all others exactly the same, it is still possible to markedly vary one parameter while keeping the others relatively similar between two solvents. In this study, we managed to select the solvents that could be compared in this manner. For instance, water (H2O) is very similar to Heavy water (D2O) except for a slight difference in hydrogen bonding energy. In addition, the major difference between water (H2O) and ethylene glycols (EG) is the molecular size (2.6Å vs. 4.0Å) while the other chemical characteristics are very similar. Comparing EG and DMF, their major difference is reflected in the hydrogen bonding ability. Comparing DMF and CCl4, their major difference is in polarity.
In theory, these solvents are considered to be chemically inert to the mineral (mainly hydroxylapatite) phase of bone. Also unlikely is the negative effect of the selected solvents on the structural integrity of collagen as they are often used as co-solvents in biological studies [19, 20, 23–26]. To further verify this, a pilot study was performed by treating demineralized bone samples (N=2) in each of the selected solvents for three days at ambient temperature and then having them tested in tension. During the entire soaking process, we did not observe any visual damage and dissolved residues in the solvents. The mechanical tests indicated that the failure strain of all samples was between 0.21–0.25 irrespective of the solvents, which is very consistent with that (0.21–0.22) of controls (soaked only in PBS). By ruling out the negative effect of the selected solvents on the structural integrity of bone constituents, the possible effect of the solvents on bone would be inflicted mainly through intermolecular interactions, such as polarity and hydrogen bonding ability.
Replacement of water with the selected solvents
Before soaking, the matrix water was first removed from all bone specimens using a simple dehydration protocol [2]. Briefly, the specimens were dried in 1.0mm Hg vacuum at 70°C till the weight loss leveled off to reach the equilibrium. This dehydration condition was chosen because our previous study showed that drying bone samples at this temperature for 4 hours would remove all mobile and most bound water without inflicting structural damage to the mineral and collagen phases in bone [2, 27]. Afterwards, the weight change of the dehydrated specimens was measured and converted to the volume of water removed from the specimens. Then, the dehydrated specimens were soaked in these solvents until no changes in the soaking weight were observed. Then, the weight after soaking was measured for the specimens. The volume percent of water replaced by each solvent was determined using the following formula:
| 1 |
The soaking history was plotted as the volume of water replaced by the solvent as a function of time.
Mechanical Testing
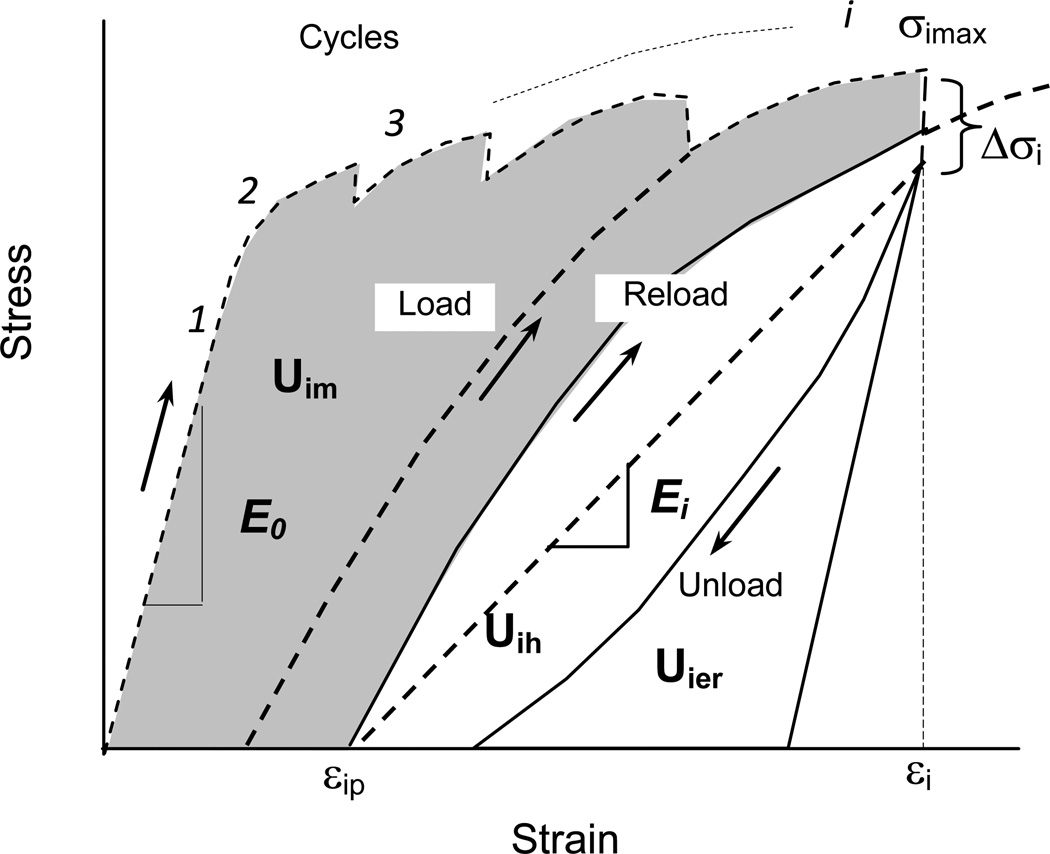
Tensile tests of all bone specimens were performed on a Bose ElectroForce 3330 mechanical test machine. To fully capture the evolution of the mechanical behavior of bone in the loading process with increasing strain, a well-defined progressive loading protocol (Fig. 1) with multiple diagnostic loading cycles was employed following the procedure described elsewhere [28]. The loading scheme consists of a series of diagnostic loading-unloading cycles to capture the mechanical properties of bone at each incremental loading level (strain). Each cycle included four steps: (a) Loading at constant rate of 0.05mm/s to an incremental displacement level; (b) Dwelling for 150 seconds at a specified displacement level; (c) Unloading at a constant rate of 0.05mm/s to a preset load level of 25N; and (d) Dwelling again for 150 seconds at the preset load of 25N prior to the next loading cycle. The test specimens were kept wet by constantly dripping the corresponding soaking media to the specimens during the entire test. The following parameters were measured to quantify the mechanical behavior of bone specimens (Fig. 1):
Applied stress and strain in each cycle (σi and εi): These stress and strain values were measured at the end of the loading step in each cycle to determine the stress-strain relationship over the loading process.
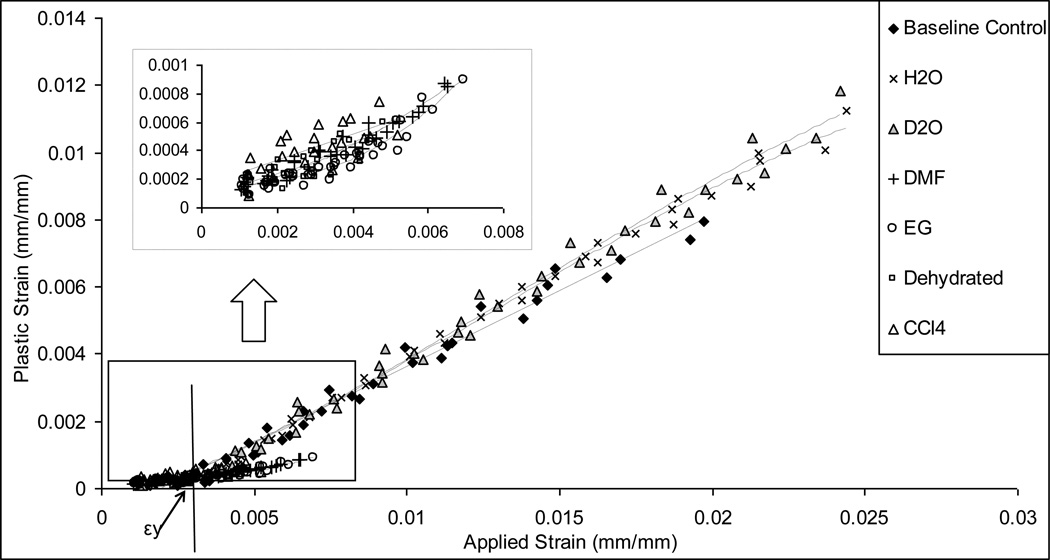
Plastic strain (εp): The plastic strain was measured as the residual strain at the end of each cycle.
Yield strain (εy) and plastic flow coefficient (k): εy was measured as the onset strain of plastic deformation, which was determined by fitting the linear part of the plastic strain (εp) vs. applied strain (εi) curve to the equation: εp=kεi − εy, where εy is the intercept with the axis of applied strain and k is the slope of the curve, indicating the intensity of plastic flow with the bulk deformation.
Ultimate stress (σu): σu was defined as the maximum stress measured during the entire loading process.
Initial elastic modulus (E0): E0 was calculated as the slope of the linear part of the stress-strain curve in the loading step of the first cycle.
Progressive elastic modulus (Ei): Ei was calculated in each loading cycle as the slope of the line joining the equilibrium points at the end of stress relaxation dwell and the end of creep dwell in the cycle (Fig. 1). Since the modulus loss can be defined as an exponential decay with the applied strain (εi) as described in previous studies [29]: Ei = E0e-mεi, where m was defined as the modulus decay factor, reflecting the sensitivity of bone to modulus loss.
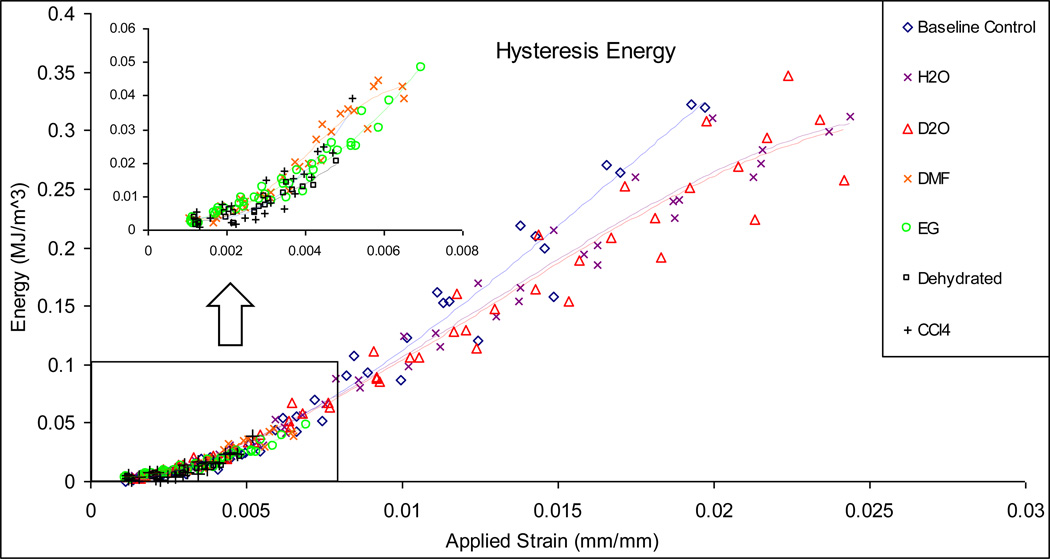
Hysteresis energy (Uh): Uh was determined as the area between the load-unload regions of the stress-strain curve in each loading cycle, which is a measure of the energy dissipation due to the viscous response of bone.
Plastic strain energy (Up): Up was determined as the area between the load and reload regions in every two consecutive cycles, which is related to the energy dissipation in bone due to plastic deformation in the loading cycle.
Released elastic strain energy (Uer): Uer was determined as the area between the unload curve and the line from the beginning point of unloading with the slope of the initial elastic modulus (E0), which is related to the elastic strain energy released due to damage accumulation in bone.
Figure 1.
Schematic illustration of the progressive loading scheme and determination of instantaneous mechanical properties of bone in each load cycle.
Statistical Analysis
Simple Student t-tests were performed to detect the differences in all measured parameters between the test groups. The statistical significance was considered only if p < 0.05.
RESULTS
Water replacement by the selected solvents (Table 2)
Table 2.
Volume of water replaced by the selected solvents (N=6)
| D2O | H2O | EG | DMF | CCl4 | |
|---|---|---|---|---|---|
| Weight fraction of original water (wt%) | 11.8±0.56 | 11.2±0.67 | 11.7±0.83 | 11.6±0.68 | 11.5±0.90 |
| Water volume replaced (vol%) | 96.1±1.38 | 98.6±2.96 | 85.5±5.15 | 71.8±3.77 | 22.3±5.17 |
| Soaking time to equilibrium (days) | ~ 1 | ~ 1 | > 12 | > 10 | > 15 |
The weight fraction of original water in the bone specimens was very consistent across all test groups. The soaking results indicated that H2O (98.6±2.96%) and D2O (96.1±1.38%) could almost completely replace the original volume of water removed by dehydration. EG and DMF replaced 85.5±5.15% and 71.8±3.77% of the original volume of water, respectively. In contrast, bone soaking in CCl4 reached saturation at only 22.3±5.17% of the original volume of water. In addition, the soaking rate of bone in EG, DMF and CCl4 was much slower than that in H2O and D2O.
Stress-strain relationship
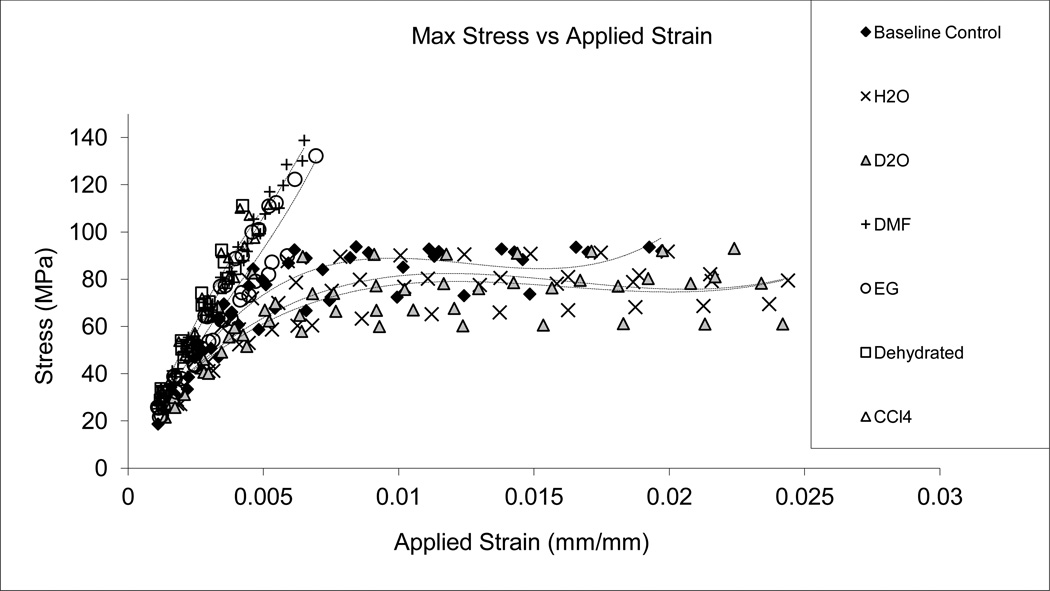
Similar strain-stress curves were observed among the baseline control, H2O and D2O soaked specimens, demonstrating an initial linear portion, yielding, and considerable post-yielding deformation (Fig. 2). In contrast, negative control (Dehydrated), CCl4, DMF, and EG soaked specimens all exhibited a linear stress-strain behavior without appreciable post-yield deformation. Among them, however, DMF and EG groups allowed for a higher maximum stress and slightly greater failure strain compared to dehydrated and CCl4 groups. Below are more details about the pre-yield, post-yield, and viscous responses of bone in different test groups.
Figure 2.
Stress-strain relationship obtained using the progressive loading protocol. The H2O, D2O and base line control specimens indicated the initial elastic, yielding, and post-yield deformation, whereas EG, DMF, CCl4, and Dehydrated specimens exhibited a brittle behavior, with an increased stiffness, strength, and no yielding.
Initial elastic modulus, modulus loss and associated energy dissipation
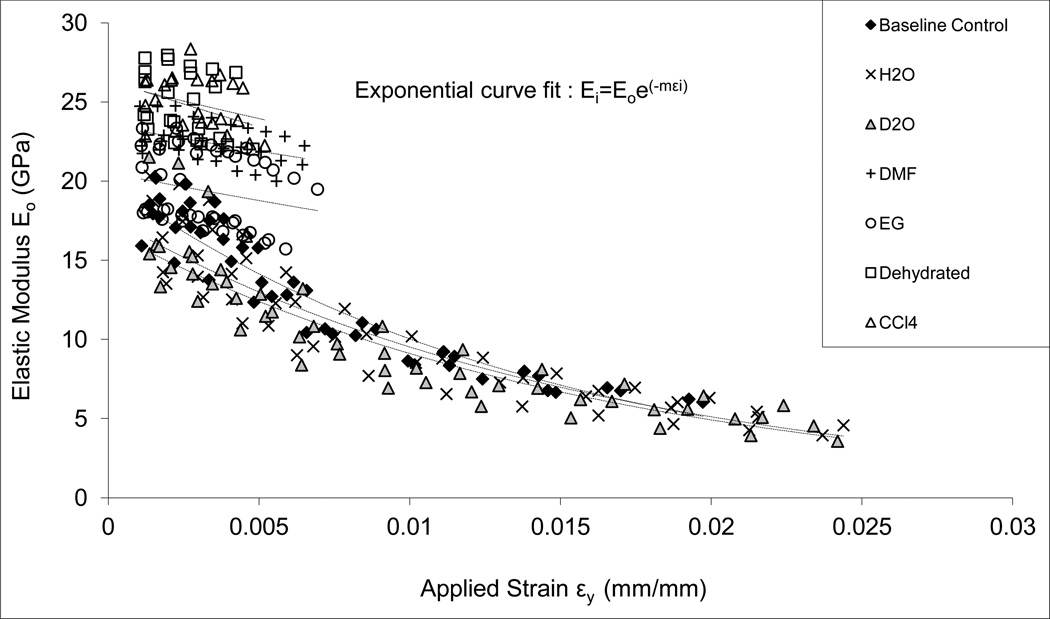
Soaking in different solvents had significant effects on the initial elastic modulus (E0) of bone (Table 3). The dehydrated and CCl4 groups had the highest initial elastic modulus, followed by EG and DMF groups, and H2O, D2O, with baseline control groups exhibiting the lowest initial elastic modulus.
Table 3.
Mechanical properties of bone treated by different solvents (N=6)
| Properties Solvents |
Initial Elastic Modulus E0 (GPa) |
Modulus Decay Constant m (−) |
Yield Strain εy (%) |
Plastic Flow Coefficient k (−) |
Failure Strain εf (%) |
Ultimate Stress σu (MPa) |
|---|---|---|---|---|---|---|
| Wet Bone (Baseline Control) | 18.7±1.70b | 78.9±9.23 | 0.260±0.050 | 0.50±0.03 | 1.99±0.77b | 84.8±11.9 |
| H2O (Control) | 16.9±3.00b | 66.5±11.5 | 0.240±0.022 | 0.50±0.021 | 2.86±1.41b | 76.7±12.3 |
| D2O (Heavy Water) | 17.4±2.60b | 65.3±9.87 | 0.220±0.042 | 0.50±0.018 | 3.10±0.68b | 84.1±16.4 |
| EG (Ethylene glycol) | 20.6±2.50a,b | – | – | – | 0.59±0.13a | 112±12.6a |
| DMF (Dimethylformamide) | 23.3±1.40a | – | – | – | 0.70±0.13a | 131±25.0a |
| CCl4 (Carbon Tetrachloride) | 26.0±1.60a | – | – | – | 0.50±0.05a | 97.6±22.7 |
| Dehydrated (Negative Control) | 25.5±1.70a | – | – | – | 0.43±0.06a | 98.1±16.7 |
Statistically different from control and
Statistically different from negative control (p < 0.05).
In addition, a significant modulus loss (exponential decay) was observed in bone specimens of H2O, D2O, and baseline control groups. However, no significant modulus loss (only a slight trend of decrease) was exhibited in the specimens of EG, DMF, CCl4, and Dehydrated groups (Fig. 3).
Figure 3.
Elastic modulus as a function of applied strain obtained using the progressive loading protocol. The H2O, D2O and base line control specimens indicated an exponential decay (Ei=E0e-mεi); EG and DMF soaked specimens showed a slight linear decrease; and CCl4, and Dehydrated specimens exhibited little changes in the elastic modulus with the increasing applied strain.
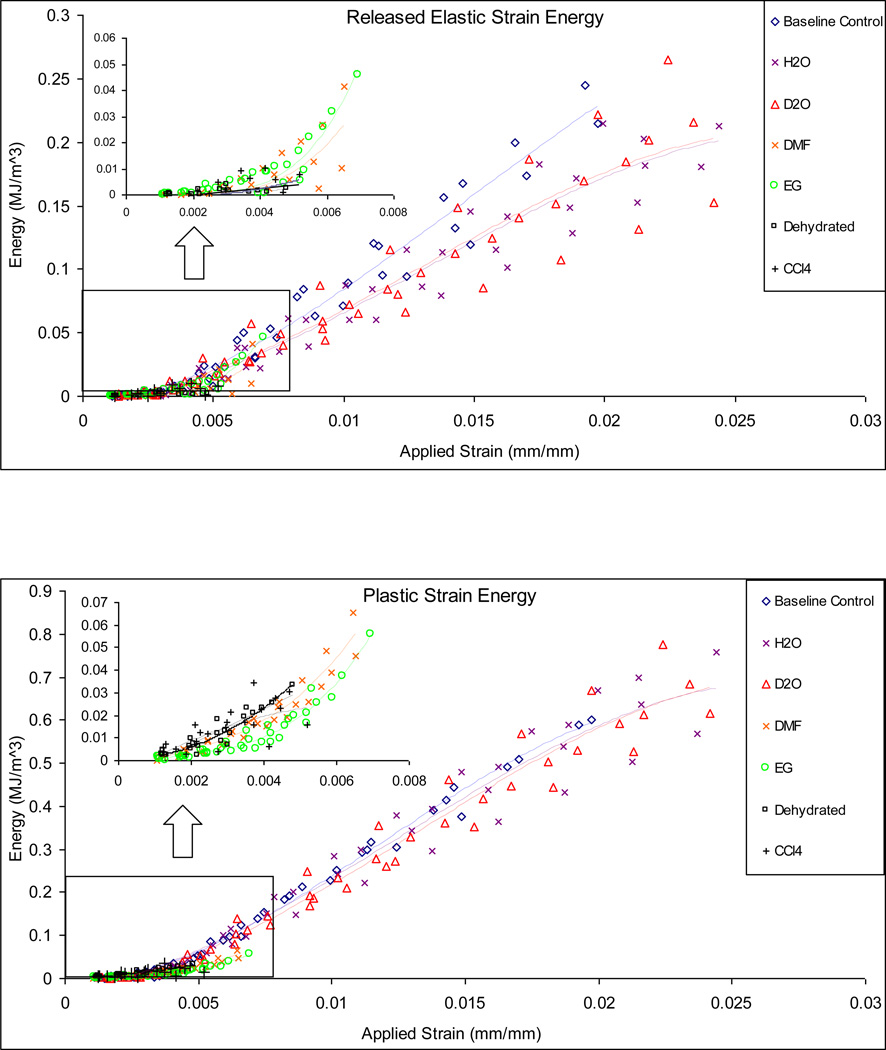
Correspondingly, H2O, D2O and baseline control specimens exhibited a much higher capacity in dissipating the released elastic strain energy (Uer). It was also noted that the baseline control showed a slightly steeper slope in dissipation of released elastic strain energy with the applied strain compared to H2O and D2O groups (Fig. 6a). In contrast, no released elastic strain energy dissipation was observed in the CCl4 soaked and dehydrated specimens, whereas the DMF and EG soaked specimens only exhibited an onset of released elastic strain energy dissipation (Fig. 6a).
Figure 6.
Released elastic strain energy (a), plastic flow (b), and hysteresis (c) energy dissipation in bone specimens soaked in different solvents. The H2O, D2O and base line control specimens show similar capacity of dissipating energy in all three mechanisms aforementioned, while EG and DMF, CCl4, and Dehydrated specimens exhibit very limited capacity in energy dissipation.
Yielding, post-yield deformation and associated energy dissipation
Significant differences were observed in the yield strain (εy) and the post-yield deformation of bone among the control and test groups (Table 3). First, the DMF, EG, CCl4 and dehydrated groups hardly showed any appreciable yielding and a trivial plastic deformation, whereas the H2O, D2O and baseline control groups exhibited the transition (yielding) from the elastic deformation to the post-yield plastic deformation with a relatively consistent yield strain (εy) and plastic flow coefficient (k).
Next, the failure strain (εf) was significantly higher for the H2O, D2O and baseline control groups compared with the DMF and EG, CCl4 and Dehydrated groups, which all failed at very limited strains (< 0.8%).
A similar trend of plastic strain energy dissipation (Up) was observed among the bone specimens from H2O, D2O and baseline control groups, whereas trivial plastic strain energy dissipation existed for the DMF, EG, CCl4 and dehydrated groups. However, it is notable that the EG and DMF groups exhibited an onset of plastic strain energy dissipation, whereas the CCl4 and dehydrated groups exhibited no plastic energy dissipation at all (Fig. 6b).
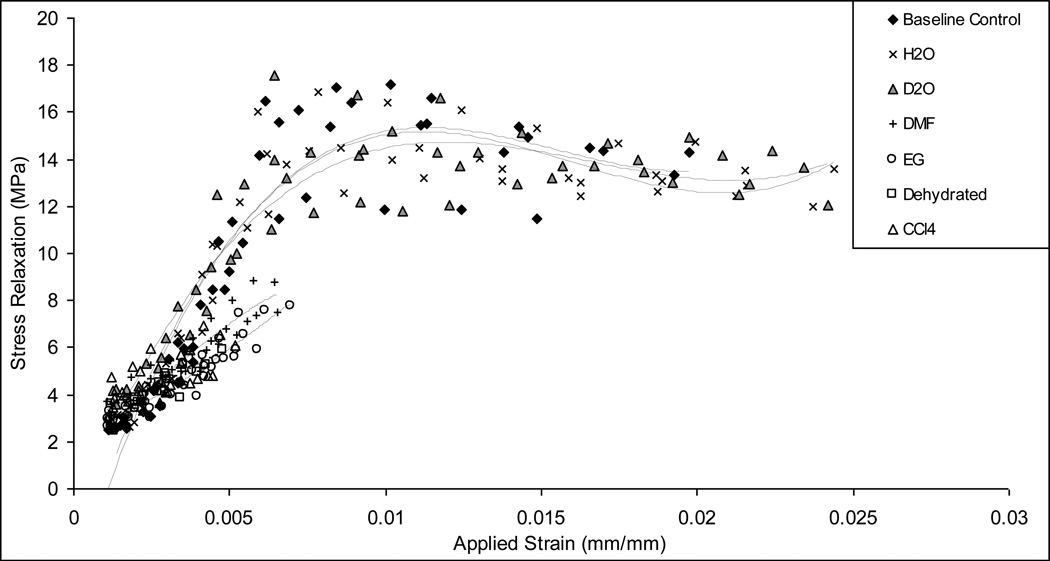
Viscous response and hysteresis energy dissipation
H2O, D2O and baseline control specimens exhibited similar increases in the stress relaxation with increasing bone deformation up to a transition point close to 0.6% strain (Fig. 5). Beyond the transition point, the stress relaxation was leveled off irrespective of increasing strain, suggesting that bone reached to a steady state of viscous response. In contrast, the stress relaxation was more limited for DMF, EG, CCl4 and Dehydrated groups, counting less than 50% of stress relaxation shown in H2O, D2O and baseline control groups.
Figure 5.
Stress relaxation as a function of applied strain obtained using the progressive loading protocol. The H2O, D2O and base line control specimens behave similarly, indicating a linear increase prior to yielding and leveled off with a slight decrease in stress relaxation with increasing strain; EG and DMF, CCl4, and Dehydrated specimens show a slight increase with the increasing applied strain.
A comparison of the hysteresis energy dissipation in bone for all experimental groups revealed that the total viscous energy dissipation in the DMF and EG specimens were considerably lower than that of the baseline control, H2O and D2O groups, with CCl4 and Dehydrated groups showing almost no hysteresis energy dissipation at all (Fig. 6c). Also observed was that the baseline control specimens showed a slightly steeper slope in dissipating viscous energy compared to the D2O, H2O specimens.
DISCUSSIONS
This study was performed to determine how the mechanical behavior of bone would be affected by replacing matrix water in bone with several selected solvents that have different polarity, hydrogen bonding ability, and molecular size. The results demonstrate that the soaking ability of the solvents into bone is remarkably different (Table 2), ranging from 22.3±5.17vol% to 98.6±2.96vol% of the original water content in bone. Accordingly, significant changes in the mechanical behavior of bone were detected.
First, it is noted that the maximum volume in bone that a solvent could infiltrate into is related to the solvent’s polarity, hydrogen bonding ability, and molecular size (Table 1 and 2). Amongst the selected solvents, D2O has a similar molecular size, polarity and hydrogen bonding ability compared with H2O, thus making it capable of infiltrating into the spaces originally occupied by water in bone. However, it is interesting to note that EG could replace only 85.5±5.15vol% of the original water in bone although its polarity and hydrogen bonding ability are relatively comparable to H2O. This disparity is most likely due to the molecular size of EG (~4.0Å), which is almost as twice large as those of H2O and D2O (2.4–2.6Å). Next, DMF has a comparable polarity, but significantly impaired hydrogen-bond ability and a slightly larger molecular size (5.5Å) than that of EG. Accordingly, It exhibits a lesser soaking capability (around 71.8±3.77vol%) compared to EG. It is known that unlike EG and H2O, which can serve as both hydrogen-bond donors and acceptors, DMF lacks ability to serve as a hydrogen-bond donor, but only a hydrogen-bond acceptor [30]. Thus, the ability of DMF to form hydrogen bonds with bone constituents (i.e. type I collagen and hydroxyapatite crystals) becomes extremely lower than that of EG and H2O [17]. However, such significant differences only trigger very limited differences (~13vol%) in soaking capacity between DMF and EG. This implies that hydrogen-bond ability may play a minor role in helping solvents infiltrate into bone matrix. Finally, CCl4 molecules have no polarity and hydrogen bonding ability, but comparable molecular size (5.9Å) and hydrogen bonding ability to DMF. However, the soaking ability (22.3±5.17%) of CCl4 is much poorer than that of DME (71.8±3.77vol%). Thus, it is presumable that polarity plays a considerable role in soaking of solvents into bone matrix.
In line with the aforementioned differences in water replacement, significant changes in the mechanical behavior of bone are manifested in three distinct clusters among the selected solvents (Table 3). Cluster 1 includes the H2O (control) and D2O soaked specimens, demonstrating a progressive modulus loss, appreciable plastic flow, and asymptotic viscous response with increasing strain, which are similar to the baseline controls. Cluster 2 contains the DMF and EG soaked specimens that have a higher stiffness and fail immediately after the onset of yielding. Cluster 3 consists of the dehydrated (negative control) and CCl4 soaked specimens, showing the highest stiffness and no plastic and viscous responses (i.e. brittle mode).
As previous reported in the literature, the mechanical behavior of dehydrated bone could be recovered after H2O rehydration [1, 31]. It is not surprising because water molecules can be fully soaked back into the ultrastructural. However, it is interesting to note that the mechanical properties are fully recovered by soaking in D2O, which could soak into almost all spaces originally occupied by water in bone. Although D2O exhibits slightly stronger hydrogen-bond ability (~10%) and a little higher viscosity compared to water (Table 1), no differences in mechanical properties of bone were observed in this study. This suggests that solvents akin to water may also preserve the mechanical behavior of bone.
One important finding of this study is that bone becomes brittle even if 85.5±5.15vol% of the matrix spaces that are originally occupied by water in bone is refilled with EG. In this case, the EG soaked specimens lost most of its ductility (or plasticity) and ability in energy dissipation compared with the controls (H2O soaked). Since EG has relatively comparable polarity and hydrogen bonding ability with those of H2O, the reason for such a disparity is more likely due to the size difference between EG (~4.0Å) and H2O (2.4–2.6Å) molecules [14, 15, 18]. In fact, previous experimental evidence has shown that there are small ultrastructural spaces in bone that EG molecules cannot infiltrate into simply due to its molecular size. For example, previous NMR studies show that water may reside in the gap at mineral-collagen interfaces in an order of 3.0Å [5], which is larger than the estimated molecular size of water (2.4–2.6Å), but smaller than that of EG (~4.0Å). Thus, it could be postulated that the ductility of bone may be mainly dependent on the water molecules that reside in very small ultrastructural spaces (<4.0 Å).
Comparing DMF and EG soaked specimens, no significant differences in their mechanical properties and a limited change in soaking ability (about 13% differences) were observed in this study. Since the major difference exists in the hydrogen bonding ability between the two solvents, this result suggests that hydrogen bonding ability of solvents may be a minor contributor to bone mechanical properties.
Comparing CCl4 and DMF soaked specimens; significant differences exist in both mechanical properties (e.g. E0 and σu) and soaking capabilities (Table 3). Since polarity is the major difference between the two solvents, the results suggest that polarity does play a significant role in helping the solvent soaking into bone matrix. However, its effect on the mechanical behavior of bone is very limited in this case.
There are some limitations to this study. First, the bone specimens were collected only from middle aged groups in this study, which may not be representative of other age groups. Further tests across different age groups may help elucidate the effect of water on mechanical behavior with aging. Next, the wettability of the mineral and collagen surfaces were not investigated with respect to the chemical characteristics of the selected solvents in this study. This information may facilitate understanding how the selected solvents infiltrate into the ultrastructural spaces of bone. Moreover, the selection of solvents that are suitable for our purpose was limited due to the potential damage that a solvent could cause to bone during the soaking process. Finally, the progressive loading protocol employed in this study may not provide the mechanical behavior of bone under the monotonic loading conditions.
CONCLUSIONS
By soaking bone in different solvents that have different molecular size, polarity and hydrogen-bond ability, we investigated the effect of water on the mechanical properties of bone at ultrastructural levels. Based on the results of this study, it is postulated that the water molecules that reside in the ultrastructural spaces that cannot be infiltrated by solvents whose molecular size is greater than 4.0Å plays a pivotal role in mechanical properties of bone.
Figure 4.
Plastic strain εp vs. applied strain εi. The H2O, D2O and base line control specimens indicated a linear increase of plastic strain; EG and DMF soaked specimens show an onset of yielding; and CCl4, and Dehydrated specimens exhibited no plastic deformation with the increasing applied strain.
Highlights.
Matrix water was replaced by solvents with different size and properties.
The capability of the solvents to infiltrate into bone matrix varies.
Water replacement by the solvents affects the mechanical behavior of bone.
Water in the ultrastructural spaces (<4Å) dominates the tissue behavior of bone.
ACKNOWLEDGEMENTS
Research reported in this study was supported by NIAMS of the National Institutes of Health under award number of R21AR57907. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Jameson MW, Hood JA, Tidmarsh BG. The effects of dehydration and rehydration on some mechanical properties of human dentine. Journal of biomechanics. 1993;26(9):1055–1065. doi: 10.1016/s0021-9290(05)80005-3. [DOI] [PubMed] [Google Scholar]
- 2.Nyman JS, et al. The influence of water removal on the strength and toughness of cortical bone. Journal of biomechanics. 2006;39(5):931–938. doi: 10.1016/j.jbiomech.2005.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horch RA, et al. Clinically compatible MRI strategies for discriminating bound and pore water in cortical bone. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2012;68(6):1774–1784. doi: 10.1002/mrm.24186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horch RA, et al. Characterization of 1H NMR signal in human cortical bone for magnetic resonance imaging. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2010;64(3):680–687. doi: 10.1002/mrm.22459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilson EE, et al. Three structural roles for water in bone observed by solid-state NMR. Biophys J. 2006;90(10):3722–3731. doi: 10.1529/biophysj.105.070243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wilson EE, et al. Highly ordered interstitial water observed in bone by nuclear magnetic resonance. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20(4):625–634. doi: 10.1359/JBMR.041217. [DOI] [PubMed] [Google Scholar]
- 7.Wehrli FW, Fernandez-Seara MA. Nuclear magnetic resonance studies of bone water. Annals of biomedical engineering. 2005;33(1):79–86. doi: 10.1007/s10439-005-8965-8. [DOI] [PubMed] [Google Scholar]
- 8.Bella J, Brodsky B, Berman HM. Hydration structure of a collagen peptide. Structure (London, England: 1993) 1995;3(9):893–906. doi: 10.1016/S0969-2126(01)00224-6. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita J, et al. The use of dynamic mechanical analysis to assess the viscoelastic properties of human cortical bone. Journal of biomedical materials research. 2001;58(1):47–53. doi: 10.1002/1097-4636(2001)58:1<47::aid-jbm70>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita J, et al. Collagen and bone viscoelasticity: a dynamic mechanical analysis. J Biomed Mater Res. 2002;63(1):31–36. doi: 10.1002/jbm.10086. [DOI] [PubMed] [Google Scholar]
- 11.Clough SA, et al. Dipole moment of water from Stark measurements of H[sub 2]O, HDO, and D[sub 2]O. The Journal of Chemical Physics. 1973;59(5):2254–2259. [Google Scholar]
- 12.Srinivasan KR, Kay RL. Pressure dependence of the dielectric constant of H[sub 2]O and D[sub 2]O. The Journal of Chemical Physics. 1974;60(9):3645–3648. [Google Scholar]
- 13.Feyereisen MW, Feller D, Dixon DA. Hydrogen Bond Energy of the Water Dimer. The Journal of Physical Chemistry. 1996;100(8):2993–2997. [Google Scholar]
- 14.Breck DW. Zeolite Molecular Sieves: Structure, Chemistry and Use. New York: Wiley; 1974. [Google Scholar]
- 15.Elshof Jt, et al. Transport mechanisms of water and organic solvents through microporous silica in the pervaporation of binary liquids. Microporous and mesoporous materials. 2003;(65):197–208. [Google Scholar]
- 16.Drisdell WS, et al. Determination of the evaporation coefficient of D2O. Atmospheric Chemistry and Physics. 2008;(8):6699–6706. [Google Scholar]
- 17.Kuznetsova N, et al. Solvent hydrogen-bond network in protein self-assembly: solvation of collagen triple helices in nonaqueous solvents. Biophysical journal. 1997;72(1):353–362. doi: 10.1016/S0006-3495(97)78674-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sekulic J, Elshof JEt, Blank DHA. Selective Pervaporation of Water through a Nonselective Microporous Titania Membrane by a Dynamically Induced Molecular Sieving Mechanism. Langmuir : the ACS journal of surfaces and colloids. 2005;(21):508–510. doi: 10.1021/la047458p. [DOI] [PubMed] [Google Scholar]
- 19.Steinmetz NJ, Bryant SJ. The effects of intermittent dynamic loading on chondrogenic and osteogenic differentiation of human marrow stromal cells encapsulated in RGD-modified poly(ethylene glycol) hydrogels. Acta biomaterialia. 2011;7(11):3829–3840. doi: 10.1016/j.actbio.2011.06.031. [DOI] [PubMed] [Google Scholar]
- 20.Benoit DSW, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28(1):66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 21.Principles of cryopreservation in Cryopreservation and freeze-drying protocols. In: Pegg D, editor; Day J, Stacey G, editors. Methods in Molecular Biology. Second ed. 2007. pp. 39–57. [Google Scholar]
- 22.Walicka MA, Adelstein SJ, Kassis AI. Chemical modification of 5-[125I]iodo-2'-deoxyuridine toxicity in mammalian cells in vitro. International journal of radiation biology. 2001;77(5):625–630. doi: 10.1080/09553000110034630. [DOI] [PubMed] [Google Scholar]
- 23.Farkas D, Tannenbaum SR. Characterization of chemically induced hepatotoxicity in collagen sandwiches of rat hepatocytes. Toxicological sciences : an official journal of the Society of Toxicology. 2005;85(2):927–934. doi: 10.1093/toxsci/kfi145. [DOI] [PubMed] [Google Scholar]
- 24.Song T-Y, Yen G-C. Protective effects of fermented filtrate from Antrodia camphorata in submerged culture against CCl4-induced hepatic toxicity in rats. Journal of agricultural and food chemistry. 2003;51(6):1571–1577. doi: 10.1021/jf0209701. [DOI] [PubMed] [Google Scholar]
- 25.Bozzini S, et al. Poly(ethylene glycol) and hydroxy functionalized alkane phosphate selfassembled monolayers reduce bacterial adhesion and support osteoblast proliferation. The International journal of artificial organs. 2011;34(9):898–907. doi: 10.5301/ijao.5000047. [DOI] [PubMed] [Google Scholar]
- 26.Reimer P, Bader A, Weissleder R. Application of a stable cell culture assay for the functional assessment of novel MR contrast agents. European radiology. 1997;7(4):527–531. doi: 10.1007/s003300050197. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, et al. The role of collagen in determining bone mechanical properties. J. Orthop. Res. 2001 doi: 10.1016/S0736-0266(01)00047-X. [DOI] [PubMed] [Google Scholar]
- 28.Nyman JS, et al. Differences in the mechanical behavior of cortical bone between compression and tension when subjected to progressive loading. Journal of the mechanical behavior of biomedical materials. 2009;2(6):613–619. doi: 10.1016/j.jmbbm.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leng H, Dong XN, Wang X. Progressive post-yield behavior of human cortical bone in compression for middle-aged and elderly groups. Journal of biomechanics. 2009;42(4):491–497. doi: 10.1016/j.jbiomech.2008.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anslyn E, Dougherty D. Modern Physical Organic Chemistry. USA: University Science Books; 2006. [Google Scholar]
- 31.Broz JJ, et al. Effects of rehydration state on the flexural properties of whole mouse long bones. Journal of biomechanical engineering. 1993;115(4A):447–449. doi: 10.1115/1.2895510. [DOI] [PubMed] [Google Scholar]
- 32.Sengers JV, et al. Viscosity of H2O in the Critical Region. International Journal of Thermophysics. 2009;30(2):374–384. [Google Scholar]
- 33.Hardy R, Cottington R. Viscosity of Deuterium Oxide and Water in the Range 5° to 125° C. The Journal of Research of the National Bureau of Standards. 1949;42:573–578. [Google Scholar]
- 34.Schott H. Direct comparison of the strength of hydrogen bonds formed by H2O and D2O. Journal of Macromolecular Science, Part B. 1988;27(1):119–123. [Google Scholar]
- 35.Nakamura M, Tamura K, Murakami S. Isotope effects on thermodynamic properties: mixtures of x(D2O or H2O) + (1 - x)CH3CN at 298.15 K. Thermochim. Acta. 1995;(253):127–136. [Google Scholar]
- 36.Greenwood N, Earnshaw A. Chemistry of the elements. 2nd ed. Oxford: Butterworth Heinemann; 1997. [Google Scholar]
- 37.Vértes A, et al., editors. Handbook of Nuclear Chemistry. New York: Springer Dordrecht Heidelberg; 2011. [Google Scholar]
- 38.Tourky AR, Rizk HA, Elanwar IM. The Dipolar Character of Ethylene Glycol. Zeitschrift für Physikalische Chemie. 1962;31(3–4):161–168. [Google Scholar]
- 39.ZAHN M, et al. Dielectric Properties of Water and Water/Ethylene Glycol Mixtures for Use in Pulsed Power System Design. P R O C E E D I N G S OF THE IEEE. 1986;74(9):1182–1221. [Google Scholar]
- 40.Batista ER, Xantheas SS, Jonsson H. Multipole moments of water molecules in clusters and ice Ih from first principles calculations. The Journal of Chemical Physics. 1999;111(13):6011–6015. [Google Scholar]
- 41.Gauden PA, et al. Estimating the pore size distribution of activated carbons from adsorption data of different adsorbates by various methods. Journal of colloid and interface science. 2004;273(1):39–63. doi: 10.1016/j.jcis.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 42.Wohlfarth C, Lechne MD, editors. Viscosity of Pure Organic Liquids and Binary Liquid Mixtures. New York: Springer; 2009. [Google Scholar]