Abstract
Over the past decade, considerable research has accumulated showing that chronic pain patients experiencing high levels of negative affect (i.e., anxiety, depression) are at increased risk for prescription opioid misuse. The primary objective of the present study was to examine the factors that underlie the association between negative affect (NA) and prescription opioid misuse among patients with chronic pain. In this study, 82 patients with chronic musculoskeletal pain being prescribed opioid medication completed the Current Opioid Misuse Measure (COMM), a well-validated self-report questionnaire designed to assess prescription opioid misuse. Patients were also asked to complete self-report measures of pain intensity, NA, and opioid craving. A bootstrapped multiple mediation analysis was used to examine the mediating role of patients’ pain intensity and opioid craving in the association between NA and prescription opioid misuse. Consistent with previous research, we found a significant association between NA and prescription opioid misuse. Interestingly, results revealed that opioid craving, but not pain intensity, mediated the association between NA and opioid misuse. Discussion addresses the potential psychological and neurobiological factors that might contribute to the inter-relationships between NA, opioid craving, and prescription opioid misuse in patients with pain. The clinical implications of our findings are also discussed.
Keywords: Chronic pain, prescription opioid misuse, negative affect, opioid craving
1.0. Introduction
Over the past decade, there has been a substantial rise in the use of opioids for the treatment of chronic noncancer pain (CNCP). Despite the potential benefits of opioid therapy, the rise in the use of opioids has been accompanied by escalating rates of prescription opioid misuse and abuse.7,20,26,46,67,77 Prescription opioid misuse, which broadly refers to the use of opioids in a manner other than how they are prescribed, has become a major concern for clinicians involved in the treatment of pain.8,46,61,68,76,82 Because of these concerns, increasing efforts have been devoted to examining the factors that may be associated with prescription opioid misuse among patients with chronic pain.
One of the most consistent findings that have emerged from previous studies among patients with chronic pain is the association between psychiatric symptoms and prescription opioid misuse (for a review, see 82). For example, symptoms of negative affect, such as anxiety and depression, have emerged as robust and powerful predictors of opioid misuse. In some studies, it has been found that patients with high levels of negative affect were 2–3 times more likely to misuse prescription opioids than patients with low levels of negative affect.11,27,36,52,85,90 To date, research has yet to determine the factors that are responsible for the association between negative affect and prescription opioid misuse in patients with pain. It is possible, for example, that negative affect leads to higher levels of pain, which in turn leads to an increased risk for opioid misuse. In previous research, increased negative affect has been found to be prospectively associated with increased pain intensity.3,12,34,35,79,95 High pain intensity, in turn, has been found to be associated with an increased risk for opioid misuse.2,36,43
Findings from recent studies suggest that opioid craving might also be responsible, in part, for the association between negative affect and prescription opioid misuse. The concept of craving is commonly used in the substance use literature and refers to the need or desire to consume certain drugs or illicit substances.24,65,74,80 Among individuals with drug use problems, it has been shown that increases in negative affect may trigger drug craving.6,19,28,39,72,73 Drug craving, in turn, has been found to increase the likelihood of drug use and abuse (for reviews, see71,74,81). Interestingly, similar findings have been reported among patients with chronic pain being prescribed opioid medication, with higher levels of negative affect being associated with higher levels of opioid craving.89 In patients with pain, self-reports of opioid craving have been found to be prospectively associated with various indices of prescription opioid misuse, including physician ratings of opioid misuse and abnormal urine toxicology screens.16,86,89 Given that self-reports of opioid craving have been found to be associated both with negative affect (NA) and prescription opioid misuse, there are reasons to believe that opioid craving may be responsible, at least in part, for the increased rates of prescription opioid misuse observed among patients with high levels of NA.
The primary purpose of the present study was to examine the potential role of pain intensity and opioid craving as mediators of the association between NA and prescription opioid misuse among patients with chronic pain. In this study, a sample of 82 patients with chronic musculoskeletal pain being prescribed opioid medication completed the Current Opioid Misuse Measure (COMM; 15), a well-validated self-report questionnaire designed to assess prescription opioid misuse. Patients also completed self-report measures of pain intensity, NA, and opioid craving. A bootstrapped multiple mediation analysis was used to examine the mediating role of pain intensity and opioid craving in the association between NA and opioid misuse.
2.0. Methods
2.1. Participants
The Human Subjects Committee of Brigham and Women’s Hospital (BWH) approved the study procedures and written informed consent was obtained from every participant. This was a cross-sectional, between-subject study performed in a single, large, urban, university-based pain management center. Patients included in the present study were part of a larger study in which patients were enrolled in a randomized clinical trial (RCT) of a behavioral intervention designed to improve prescription opioid compliance (for methods of the RCT, see45). Data included in the present study were collected at the beginning of the RCT (i.e., baseline), following patient recruitment and double blinded randomization.
The study sample consisted of 82 patients (50 men, 32 women) with a diagnosis of chronic back or neck pain, with or without radicular symptoms. All patients were prescribed opioids by a board-certified, fellowship-trained, pain medicine physician, with at least five years of consultant-level experience. All patients were evaluated by a physician and underwent a physical examination. Patients met the following inclusion criteria: (1) chronic back or neck pain for more than 6 months, (2) an average pain score of 4 or greater on a pain intensity scale of 0–10, with medication, (3) able to speak and understand English, (4) prescribed opioid medication for more than 6 months, and (5) at risk for prescription opioid misuse based on their responses on the Screener and Opioid Assessment for Patients with Pain – Revised (SOAPP-R scores ≥18;14), or based on past records of abnormal urine screens and/or physician ratings of opioid misuse (Addictions Behavior Checklist: ABC;94).
Patients were excluded from participation if they met any of the following criteria: (1) current diagnosis of cancer, (2) acute osteomyelitis or acute bone disease, (3) present or past DSM-IV diagnosis of any psychotic disorder, (4) active substance abuse or dependence of any other kind within the past year (i.e., positive on the Mini International Neuropsychiatric Interview; M.I.N.I. v.5.0.69).
2.2. Measures
2.2.1 Demographic questionnaire
Patients were asked to complete a demographic questionnaire, which included information about patients’ age, gender, ethnicity, and education level. Patients were also asked to report any history of medical, psychiatric, and/or substance use problems, and to report which opioid medication they were currently taking. Patients’ reports of medication were verified by a research assistant using the electronic medical record system, and published tables were used to convert daily opioid dosages into morphine equivalents.
2.2.2. Screening for substance use disorders
The Mini-International Neuropsychiatric Interview (M.I.N.I. v.5.0; 69) was used to screen for active opioid addiction or any other active substance use disorder. The M.I.N.I was designed as a brief structured interview for the major Axis I problems included in the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV; 5). We used section K to assess the presence of a current non-alcohol psychoactive substance use disorder (SUD). The M.I.N.I includes 3 sub-sections and a total of 12 questions designed to assess any SUD related to the use of (1) stimulants, (2) cocaine, (3) non-prescription opioids, (4) hallucinogens, (5) heroin, (6) inhalants, (7) marijuana, (8) non-prescription tranquilizers, and (9) other substances of abuse. The M.I.N.I was administered and scored by a trained research assistant. All questions on the M.I.N.I (section K) require a yes or no answer, and the scoring is performed based on a diagnostic algorithm related to DSM criteria for SUD. The M.I.N.I can be administered rapidly, but may take up to 15 minutes when administered to polysubstance users. The MINI has been shown to be a reliable and valid screening tool for substance use disorders in patients with and without chronic pain conditions.33,45,64,69
2.2.3. Current Opioid Misuse Measure (COMM)
The COMM15 is a 17-item self-report questionnaire designed to identify patients who are currently misusing their prescribed opioid medication. COMM items are designed to assess a variety of behaviors that are indicative of opioid misuse (e.g., In the past 30 days, how often have you taken your medications differently from how they are prescribed?) or indicative of more general aberrant/nonadherence behaviors (e.g., how often have you had to show up at the clinic without an appointment?). Some items also assess potential emotional/psychiatric problems that may be associated with prescription opioid misuse. Items are rated from 0 (never) to 4 (very often). The COMM has been shown to have good predictive validity, with significant correlations between COMM scores and other indices of prescription opioid misuse, such as the Prescription Drug Use Questionnaire (PDUQ;22), physician ratings of opioid misuse (Prescription Opioid Therapy Questionnaire: POTQ;55), and urine toxicology screens.13,15 The overall accuracy of the COMM for identifying opioid misuse behaviors, as measured by the area under the curve ratio, was 0.81 (95% CI, 0.74–0.86; p < 0.001; coefficient α = .86), suggesting adequate reliability and predictive validity.13,15
2.2.4. Self-report measures of pain intensity, negative affect (NA), and craving
Patients were asked to provide self-reports of pain intensity, NA, and opioid craving through an electronic diary questionnaire (Hewlett Packard © IPAQ) using a personal digital assistant (PDA).53 Diary data were downloaded and saved as part of each patient’s study file. Ratings of pain intensity, NA, and craving were all provided using visual analogue scales (VAS) based on symptoms experienced over the past 24 hours. The use of VAS enabled to keep the timing of assessment (i.e., past 24 hours) constant across all outcome domains, and to examine concurrent associations between pain intensity, NA, and opioid craving. Previous studies have supported the reliability and validity of PDA methods for the assessment of pain intensity, NA, and craving among patients with chronic pain.43,44,45,53,89
2.2.4.1. Self-reports of pain intensity
Using the electronic diary questionnaire, patients were asked to rate the average level of pain they experienced over the past 24 hours on a 0–10 visual analog scale (VAS) with the endpoint 0 (no pain) and 10 (worst pain possible). Ratings of pain were automatically converted and stored on a 0–100 scale.
2.2.4.2. Self-reports of opioid craving
Using the electronic diary questionnaire, patients were asked to rate the level of craving they experienced over the past 24 hours. Craving was assessed using three different items: 1) How much have you craved your opioid medication? 2) How often have you found yourself thinking about the next opioid dose? 3) How strong was your urge to take more opioid medication than prescribed? These items were rated on a 0–100 visual analog scale (VAS) and were based on the Cocaine Craving Scale validated by Weiss et al.92
2.2.4.2. Self-reports of negative affect (NA)
Using the electronic diary questionnaire, patients were asked to rate the level of anxiety (“how tense and anxious have you been?”) and depression (“how depressed and discouraged have you been?”) they experienced over the past 24 hours on a 0–10 visual analog scale (VAS) with the endpoint 0 (not much) and 10 (very much). Ratings of anxiety and depression were automatically converted and stored on a 0–100 scale.
2.3 Data reduction and analysis
All data were analyzed using SPSS v.20 (SPSS Inc, Chicago, IL, USA). Descriptive data for continuous variables were presented as means and standard deviations (SDs), and were analyzed using Independent samples t-tests. Descriptive data for categorical variables were presented as percentages, and were analyzed using chi-square tests.
Consistent with previous research,42,87,88 an index of negative affect (NA) was computed by averaging patients’ ratings of anxiety and depression. In our study, the use of a composite index of NA stemmed primarily from the considerable shared variance between measures of anxiety and depression. The 3 craving items from the electronic diary questionnaire were also averaged to create a composite index of craving, which ranged from 0–100.89
For purposes of the present study, only the COMM items that were designed to directly assess prescription opioid misuse were included in the analyses (see Appendix 1). The COMM items that assessed emotional/psychiatric issues associated with opioid misuse were excluded from the analyses given the potential overlap between these items and measures of NA. Removing these items allowed us to ensure that the association between NA and opioid misuse (i.e., COMM) was not artificially inflated due to overlapping item content. The COMM items that were designed to assess broader aberrant/nonadherence behaviors (e.g., how often have you shown up at the clinic without an appointment) were also excluded from the analyses. These items were excluded so that COMM scores reflected a more precise and reliable assessment of self-reported prescription opioid misuse. After removing COMM items related to emotional/psychiatric issues and more general aberrant behaviors, the Cronbach’s alpha remained greater than 0.70 (α = .74), supporting the internal reliability of this subset of COMM items (COMM-s).
Univariate associations between measures of pain intensity, negative affect (NA), craving, and prescription opioid misuse (COMM-s) were assessed using Pearson correlations. Then, a multiple mediation analysis was conducted to assess whether pain intensity or opioid craving mediated the association between negative affect (NA) and prescription opioid misuse. In a multiple mediation model, it is possible to test the ‘overall’ mediation effect for all mediators included in the model (i.e., total indirect effect), and to test the effects of each mediator independently (i.e., specific indirect effects). Specific indirect effects are interpreted as the indirect (i.e., mediation) effect of the IV on the DV through a given mediator controlling for all other mediators included in the model.63,70 The multiple mediation analysis was conducted using the bootstrapping procedure described by Preacher and Hayes.62,63 Bootstrapping is a nonparametric procedure that is increasingly being used to test mediation (i.e., indirect) effects. The mediation effect is commonly referred to as the ‘indirect’ effect because it is assumed to reflect the influence of an independent variable (IV) on a dependent variable (DV) through the influence of one (or more) mediator variable(s).9,50,62 Mediator variables are different from moderator variables because they are expected to ‘explain’ the association between the IV and DV, whereas moderator variables are expected to only ‘influence’ the strength of the association between the IV and DV.9,50,51
The bootstrap mediation analysis was performed using an SPSS macro (syntax).62 The bootstrapping procedure treats the sample as a population and is accomplished by taking a new sample of size n (where n = original sample size) from the available data, sampling with replacement, and computing the indirect effect (i.e., path a × b) for each sample. This process is repeated over and over for a total of k times, preferably at least 1000 times. The distribution of the k values of a × b serves as an empirical, nonparametric approximation of the sampling distribution of ab. The mean of the k estimates of ab is used as a point estimate of the indirect (i.e., mediation) effect, and the standard deviation functions as the standard error of the sampling distribution of ab. Bootstrapping provides a way of circumventing power deficiencies of normal theory tests (e.g., Sobel) typically introduced by the non-normality in the sampling distribution of ab.37,51,70 Once completed, the bootstrapped sampling distribution is used to generate confidence intervals (CI) around point estimates in the mediation model. In the present study, bias-corrected 95% confidence interval (CI) were produced for each potential mediator, and were used to test the significance of total and specific indirect (i.e., mediation) effects. As recommended, estimates of indirect effects were considered significant in the case zero was not included within the confidence intervals.62,63 For each indirect effect (a × b), results from the normal theory (i.e., Sobel) test were also provided.
Bootstrap analyses were first conducted using patient sex and age as covariates (i.e, adjusted model), and then re-conducted without the inclusion of these covariates (i.e., unadjusted model). Given that these covariates did not exert any significant partial effects and that adjusted and unadjusted bootstrapped mediation models yielded similar patterns of findings, results of unadjusted mediation models were presented.
3.0. Results
3.1. Descriptive statistics
Descriptive statistics for all study measures are presented in Table 1, separately for men and women. Analyses revealed no significant sex differences in age, pain intensity, negative affect, opioid craving, opioid misuse, or average daily opioid dose (all p’s > .05). Analyses revealed that 73.2% (60/82) of the sample reported at least one prescription opioid misuse behavior within the past month.
Table 1.
Descriptive data for study measures
| Men | Women | p | |
|---|---|---|---|
| Opioid status | 100 % | 100 % | ns |
| Daily opioid dose (ME) | 246.1 (238.6) | 160.7 (182.1) | ns |
| Age | 49.4 (7.9) | 48.0 (8.0) | ns |
| Pain intensity | 57.3 (23.1) | 64.4 (15.6) | ns |
| Negative affect (NA) | 44.4 (27.7) | 37.4 (32.2) | ns |
| Opioid craving | 13.7 (17.8) | 13.4 (20.9) | ns |
| Prescription opioid misuse (COMM-s) | 3.1 (2.9) | 2.4 (3.0) | ns |
Note. Opioid status refers to the % of patients currently taking opioids. ME, morphine equivalent (mg/d); COMM-s refers to the subset of COMM items assessing prescription opioid misuse (see Appendix 1).
p < .05
p < .01
3.2. Correlations among measures
Demographic variables (e.g., age, education) and average daily opioid doses were not significantly associated with any of the study variables (all p’s > .05).
Results of correlational analyses revealed that anxiety (r = .31, p < .01) and depression (r = .25, p < .05) were both significantly associated with prescription opioid misuse (COMM-s). Results of a Steiger’s Z-test revealed that the magnitude of correlations between these variables and prescription opioid misuse (COMM-s) was not statistically different, Z = .41, ns. Similarly, results of correlational analyses revealed that anxiety (r = .30 p < .01) and depression (r = .21, p < .05) were both significantly associated with opioid craving. Results of a Steiger’s Z-test revealed that the magnitude of correlations between these variables and opioid craving was not statistically different, Z = .61, ns. Finally, results of correlational analyses revealed a significant correlation between measures of anxiety and depression, r = 0.79, p < .01. Given the considerable shared variance between measures of anxiety and depression, a composite index of NA (i.e., anxiety, depression) was used in subsequent analyses.
Table 2 shows the correlations between self-report measures of pain, NA, opioid craving, and opioid misuse (COMM-s). A significant correlation was found between pain intensity and NA (r = .34, p < .01), and a marginally significant correlation was found between pain intensity and COMM-s scores (r = .21, p = .05). Pain intensity was not significantly associated with craving. Significant positive correlations were found between NA and COMM scores (r = .29, p < .01), and between NA and craving (r = .27, p < .05). A significant positive correlation was also found between craving and COMM-s scores (r = .43, p < .01). Given that significant inter-correlations were found between NA, potential mediators (i.e., pain intensity, opioid craving) and COMM-s scores, preconditions for mediation testing were met.
Table 2.
Correlations among study measures
| 1 | 2 | 3 | 4 | |
|---|---|---|---|---|
| 1. Pain intensity | - | .34** | .04 | .21 |
| 2. Negative affect (NA) | - | .27* | .29** | |
| 3. Opioid craving | - | .43** | ||
| 4. Prescription opioid misuse (COMM-s) | - |
Note. COMM-s refers to the subset of COMM items assessing prescription opioid misuse (see Appendix 1).
p < .05
p < .01
3.3. Potential mediating role of pain intensity and opioid craving in the association between negative affect and prescription opioid misuse
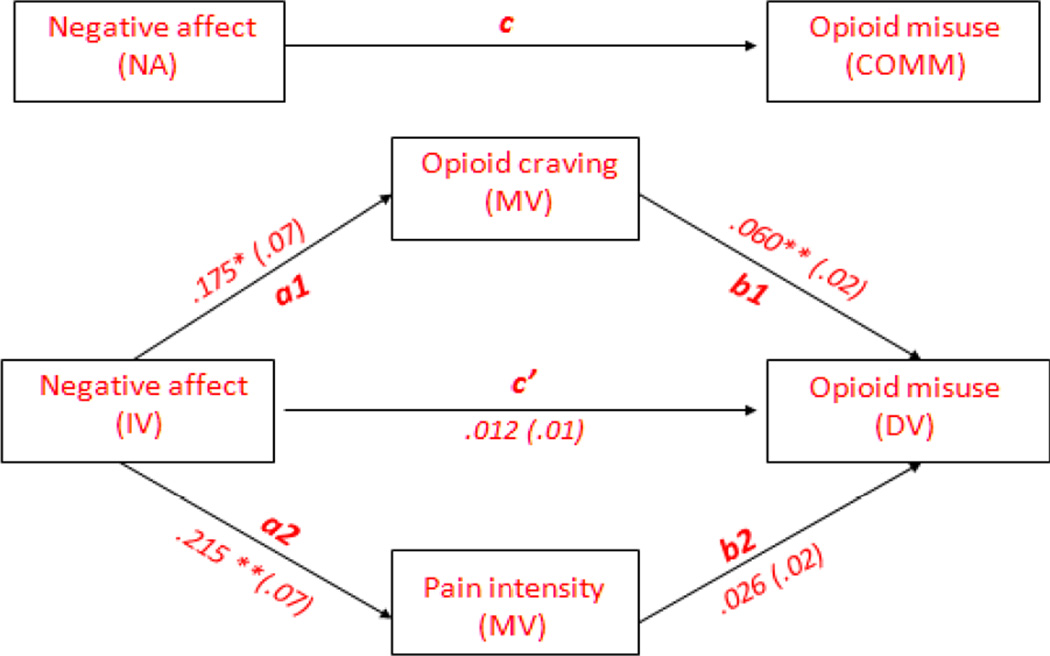
The potential mediating role of pain intensity and opioid craving in the association between negative affect (NA) and opioid misuse was examined using a bias-corrected (BC) bootstrapped multiple mediation analysis (with n = 1000 bootstrap re-samples). As shown in Figure 1 and Table 3, results of the multiple mediation analysis revealed that the direct effect of negative affect on opioid misuse (path c’) was not significant, suggesting potential mediation. The total (i.e., overall) indirect effect of pain intensity and opioid craving in the association between negative affect and opioid misuse was significant as the BC bootstrapped confidence interval (95% BC CI) did not include zero. For this total indirect effect, the Sobel Z-test was significant (Z = 2.6, p < .05).
Figure 1.
Figure depicting the mediating effect of pain intensity and opioid craving in the association between negative affect (NA) and prescription opioid misuse.
Table 3.
Bootstrapped multiple mediation analysis testing the indirect effect of negative affect (NA) on prescription opioid misuse through pain intensity and opioid craving
| Path coefficienta | Bootstrap SE | Tb | BC 95% CI | |
|---|---|---|---|---|
| Path c | .028 | .010 | 2.7** | |
| Path c’ | .012 | .010 | 1.2 | |
| Path a1 | .175 | .069 | 2.5* | |
| Path a2 | .215 | .066 | 3.2** | |
| Path b1 | .060 | .015 | 3.8** | |
| Path b2 | .026 | .016 | 1.6 | |
|
Specific indirect effects |
||||
| a1 × b1 | .010 | .006 | LL= .001; UL= .027 | |
| a2 × b2 | .005 | .003 | LL= −.001; UL= .015 | |
|
Total/overall indirect effect |
||||
| a1b1 + a2b2 | .016 | .007 | LL= .003; UL= .033 |
Note: Table shows unstandardized path coefficients for the total and specific indirect effects. Path c, total effect of NA on opioid misuse; Path c’, direct effect of NA on opioid misuse; Path a1, effect of NA on craving; Path a2, effect of NA on pain intensity; Path b1, direct effect of craving on opioid misuse; Path b2, direct effect of pain intensity on opioid misuse; Path a1 × b1, indirect effect of NA on opioid misuse through craving; Path a2 × b2, indirect effect of NA on opioid misuse through pain intensity. BC, Bias corrected; CI, Confidence interval; LL, lower limit; UL, upper limit.
Path coefficients are based on 1000 bootstraps for the indirect effect
The t-test statistic (and p-value) for the indirect effect (path a × b) is not provided because this value depends upon a normal distribution of the indirect effect. Given that indirect effects (paths ab) are positively skewed, interpretation of these p-values can be misleading and should not be used as determinants of statistical mediation. LL and UL confidence intervals were used to determine statistical significance of indirect effects.
p < .05
p < .01
Specific indirect effect (craving)
Results of the multiple mediation analysis revealed a significant effect of negative affect on craving (path a1; p < .05), and a significant direct effect of craving on opioid misuse (path b1; p < .001). Results revealed that the indirect effect of craving (path a1 × b1) was significant as the BC bootstrapped confidence interval (95% BC CI) did not include zero. For this specific indirect effect, the Sobel Z-test was significant (Z = 2.1, p < .05). Taken together, these results suggest that craving mediated the association between negative affect and opioid misuse.
Specific indirect effect (pain intensity)
Results of the multiple mediation analysis revealed a significant effect of negative affect on pain intensity (path a2; p < .005). However, the direct effect of pain intensity on opioid misuse (path b2) was not significant. Moreover, the indirect effect of pain intensity (path a2 × b2) was not significant, as the BC bootstrapped confidence interval (95% BC CI) contained zero. For this specific indirect effect, the Sobel Z-test was not significant (Z = 1.5, ns). Taken together, these results suggest that pain intensity did not mediate the association between negative affect and opioid misuse.
3.4. Alternate mediation model
In order to further evaluate the validity of the mediation model reported in our study, we conducted an additional multiple mediation analysis in which study variables (i.e., craving, negative affect, pain intensity) were interchanged within the model. This was done to examine whether an alternate mediation model could be ruled out, empirically, based on our data. Showing that an alternate mediation model can be ruled out would provide further support for the mediation model reported in our study. Results of this additional multiple mediation analysis are presented in Table 1 of supplementary materials.
In this analysis, we used negative affect (initially used as IV) as one of the mediator variables, and craving (initially used as MV) as the independent variable. In this analysis, pain intensity was also used as a mediator variable. The potential indirect (i.e., mediating) roles of negative affect and pain intensity in the association between craving and opioid misuse was examined using a bias-corrected 95% confidence interval (CI), and 1000 bootstrap re-samples. Results of this analysis revealed that the indirect/mediation effect of negative affect (path a1 × b1) was not significant, as the BC bootstrapped confidence interval (95% BC CI) contained zero. The indirect/mediation effect of pain intensity (path a2 × b2) was also not significant. Finally, the total (i.e., overall) indirect effect of negative affect and pain intensity in the association between craving and opioid misuse was not significant, as the BC bootstrapped confidence interval (95% BC CI) included zero. Taken together, results of this analysis suggest that the association between craving and opioid misuse was not mediated by either negative affect, pain intensity, or a combination of both.
4.0 Discussion
The purpose of our study was to examine the potential mediating role of pain intensity and opioid craving in the association between negative affect (NA) and prescription opioid misuse among a sample of chronic pain patients. In our study, higher levels of NA were associated with higher scores on the COMM, a self-report measure designed to assess prescription opioid misuse. This finding corroborates those of previous studies that have examined the association between measures of NA and prescription opioid misuse.11,27,36,52,66,85,90
A significant positive correlation was also found between NA and self-reports of opioid craving. Patients with high levels of NA reported higher levels of opioid craving, which is consistent with the results of a recent study showing that higher levels of anxiety and depressive symptoms were associated with higher levels of opioid craving.89 The association between NA and opioid craving is also consistent with findings from the substance use literature showing that higher levels of negative affect are associated with higher levels of craving in patients with substance use problems.6,19,28,39,71,72,73,78
Of interest in the present study was the potential mediating role of patients’ pain intensity and opioid craving in the association between NA and prescription opioid misuse (i.e., COMM-s). We found that higher levels of pain intensity were associated with increased rates of opioid misuse, which is consistent with the results of some,2,36,43 but not all 18,40,55,66 studies conducted among patients with pain. It has been suggested that patients who report high levels of pain may, in an attempt to seek pain relief, exhibit behaviors that fall within the spectrum of medication misuse or abuse.8,46,60 This phenomenon, also known as pseudoaddiction (i.e., under-treatment of pain symptoms), might have contributed to the association between self-reports of pain intensity and prescription opioid misuse observed in our study. A follow-up mediation analysis, however, revealed that patients’ pain intensity did not mediate the association between NA and prescription opioid misuse.
In our study, we found that higher levels of opioid craving were associated with increased rates of opioid misuse. Similar findings have recently been reported by Wasan et al.,86 who found that opioid craving among patients with chronic pain was associated with various indices of prescription opioid misuse. Importantly, in our study, results of the mediation analysis revealed that self-reports of opioid craving mediated the association between negative affect and prescription opioid misuse. Proceeding from a mediational perspective, our results suggest that higher levels of negative affect may enhance opioid craving, which in turn may lead to an increased likelihood of prescription opioid misuse.
There are a number of possible explanations for the mediating role of craving in the association between negative affect (NA) and opioid misuse. First, it is worth noting that self-reports of craving were not associated with patients’ pain intensity, suggesting that reports of craving among patients with high levels of NA were not likely to reflect drug withdrawal in between opioid medication doses. It is also unlikely that craving reflected the presence of an opioid addiction problem given that all patients were screened for the presence of an active substance use disorder (SUD). It is important to point out that patients may experience opioid craving and exhibit prescription opioid misuse behaviors without necessarily having an opioid addiction problem.4,8 In other words, craving is a necessary but not sufficient criterion for prescription opioid addiction. This is consistent with the new conceptualization of opioid-use disorder in the DSM-V.57
A number of psychological explanations may be invoked to account for the mediating role of opioid craving in the association between NA and prescription opioid misuse. For example, it is possible that patients with high levels of NA have difficulty coping with pain without the use of medication, which in turn may enhance the craving experience, the perceived need to use opioid medication, and the tendency to misuse prescription opioids. Among patients with chronic pain, patients with high levels of NA have been found to have low self-efficacy beliefs and poor pain coping skills (for a review, see 47), two variables that have been shown to be associated with reduced medication compliance among patients with other health-related conditions.17,25,38,75 Another possibility is that patients high in NA hold pre-existing personality traits that increase susceptibility to drug craving and prescription drug misuse. For example, it has been shown that individuals high in NA tend to be more impulsive, a personality trait that has been found to be associated with higher levels of drug craving,56,83 and with an increased likelihood of developing drug use problems.23,30,93
Another possible explanation for the mediating role of craving in the association between negative affect (NA) and opioid misuse is the interaction between the neural mechanisms that are involved in the regulation of NA, craving, and drug use. The neural pathways involved in the regulation of craving and drug use are primarily located within the mesolimbic areas, and involve brain regions such as the nucleus accumbens, the amygdala, the prefrontal cortex, and the anterior cingulate cortex (for reviews, see8,91). The mesolimbic system receives direct projections from cortical areas involved in the regulation of negative affect (NA),48,49,73 providing a neural basis for the influence of NA on craving and patterns of drug use. For example, previous studies have found that NA may enhance drug craving and the likelihood of drug abuse through increased noradrenergic and dopaminergic activity in cortical and subcortical mesolimbic areas.48,71,72 Interestingly, NA has also been found to be associated with decreased central serotonergic (5-HT) activity,1,29,31,32,41 one of the main neurotransmitter systems involved in the regulation of craving and drug use. In previous studies, decreased 5-HT activity has been found to be associated with enhanced self-reports of craving and with an increased likelihood of drug abuse in patients with various forms of drug problem.21,84 In the context of our study, it is thus possible that patients with high levels of NA were characterized by dysfunctions in noradrenergic, dopaminergic and/or serotonergic systems, which led to higher levels of craving and increased rates of opioid misuse.
There are limitations to the current study that must be considered when interpreting our findings. First, the cross-sectional nature of our study design precludes any firm conclusions regarding the directionality of associations between study variables. Although results of mediation analyses imply potential directional influences among variables, it cannot be determined whether NA is a precursor of craving, and whether the experience of craving is a precursor of prescription opioid misuse. Moreover, the cross-sectional nature of associations between the IV and mediator variables might have biased estimates of direct and indirect effects. Even if an alternate mediation model was ruled out based on our data, our findings should be viewed as preliminary, and longitudinal studies will be needed to replicate our findings. Studies using structural equation modeling (SEM) might allow to further elucidate pathways through which negative affect, pain, craving and other variables may lead to prescription opioid misuse in patients with pain. Second, our analyses were performed using a convenience sample, which limited our explanatory reach in accounting for some of the findings that were reported in the present study. Third, patients included in our study were recruited from a tertiary pain center and were taking relatively high doses of opioids, which places limits on the generalizability of our findings. Finally, prescription opioid misuse was assessed solely on the basis of patients’ self-reports using the COMM questionnaire. Future studies should use, if possible, multiple measurement methods (e.g., patients’ self-reports, urine toxicology screens, physician ratings) in order to assess opioid misuse. It has been argued, however, that self-report measures, when positive, represent the most reliable and direct method for assessing prescription opioid misuse.13,45
Despite these limitations, our study provided valuable new insights into the mechanisms that underlie the association between negative affect (NA) and prescription opioid misuse in patients with chronic pain. To our knowledge, our study was the first to systematically investigate the mechanisms by which NA may lead to prescription opioid misuse in patients with pain. The key finding of our study is that craving, but not pain intensity, mediated the association between NA and prescription opioid misuse. This finding could have important clinical implications for patients who are being prescribed opioid medication. From a pain management standpoint, our findings suggest that opioid craving should be routinely assessed and monitored over the course of opioid therapy, particularly among patients with high levels of NA. Our findings also suggest that treatment interventions aimed at reducing craving might lead to lower rates of prescription opioid misuse in patients with high levels of NA. Interestingly, self-reports of opioid craving in patients with chronic pain have been found to decrease following brief behavioral interventions designed to improve prescription opioid compliance,45,89 suggesting that craving is a potentially modifiable factor among patients with pain. While reducing symptoms of NA might represent one potential avenue for reducing opioid craving, interventions specifically aimed at targeting craving could also be used. For example, in the substance use literature, a number of treatment approaches for reducing craving have been described, including drug cue exposure interventions,54,74 cognitive-behavioral interventions helping patients cope with craving,10,81 and pharmacologic adjuvant therapies.58,59 Longitudinal treatment studies will be needed to determine the most efficient ways to reduce craving over the course of long-term opioid therapy. Studies are also needed to further explore the psychological and neurobiologic factors that may contribute to the experience of opioid craving in patients with pain. Advances in this domain might not only shed light on the psychological and neurobiological determinants of opioid craving and prescription opioid misuse, but might also ultimately lead to the development of new treatment interventions aimed at reducing rates of prescription opioid misuse among patients with pain conditions.
Supplementary Material
Perspective.
Our study provides new insights into the factors that underlie the association between negative affect and prescription opioid misuse in patients with chronic pain. Our findings could have important clinical implications, particularly for patients being prescribed opioid medication, and for reducing rates of opioid misuse in patients with pain.
Acknowledgements
The authors would like to thank Dr. Bernard Rosner and Dr. Wei Wang from Harvard Catalyst for their input on statistical analyses.
This study was supported in part by an investigator-initiated grant from Endo Pharmaceuticals, Chadds Ford, PA, and Grants (R21 DA024298, Jamison, PI; K23 DA020682, Wasan, PI) from the National Institute on Drug Abuse (NIDA) of the National Institutes of Health, Bethesda, MD, and the Arthritis Foundation (Investigator Award; Wasan, PI).
Appendix
Appendix 1.
Subset of COMM items (COMM-s) assessing prescription opioid misuse
|
Item 3) How often have you had to go to someone other than your prescribing physician to get sufficient pain relief from your medications? (ie, another doctor, the Emergency Room) |
|
Item 4) How often have you taken your medications differently from how they are prescribed? |
|
Item 9) How often have you needed to take pain medications belonging to someone else? |
| Item 10) How often have you been worried about how you’re handling your medications? |
| Item 14) How often have you had to take more of your medication than prescribed? |
| Item 15) How often have you borrowed pain medication from someone else? |
|
Item 16) How often have you used your pain medicine for symptoms other than for pain (eg, to help you sleep, improve your mood, or relieve stress)? |
Note. Items were rated from 0 (never) to 4 (very often).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no financial interests in the results of this research and no conflicts of interest.
References
- 1.Abel KM, O'Keane V, Murray RM, Cleare AJ. Serotonergic function and negative and depressive symptomatology in schizophrenia and major depression. Psychoneuroendocrinology. 1997;22:539–548. doi: 10.1016/s0306-4530(97)00050-4. [DOI] [PubMed] [Google Scholar]
- 2.Adams LL, Gatchel RJ, Robinson RC, Polatin P, Gajraj N, Deschner M, Noe C. Development of a self-report screening instrument for assessing potential opioid medication misuse in chronic pain patients. J Pain Symptom Manage. 2004;27:440–459. doi: 10.1016/j.jpainsymman.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Affleck G, Tennen H, Urrows S, Higgins P. Person and contextual features of daily stress reactivity: individual differences in relations of undesirable daily events with mood disturbance and chronic pain intensity. J Pers Soc Psychol. 1994;66:329–340. doi: 10.1037//0022-3514.66.2.329. [DOI] [PubMed] [Google Scholar]
- 4.Medicine ASoA, editor. ASAM. Maryland: Chevy Chase; 2001. American Academy of Pain Medicine, The American Society of Addiction Medicine: Definitions related to the use of opioids for the treatment of pain: a consensus document from the American Academy of Pain Medicine, the American Pain Society, and the American Society of Addiction Medicine. [Google Scholar]
- 5.American Psychological Association. Diagnostic and Statistical Manual of Mental Disorders. 4th edition. Washington DC: APA; 2000. [Google Scholar]
- 6.Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- 7.Ballantyne JC. Opioid controls: regulate to educate. Pain Med. 2010;11:480–481. doi: 10.1111/j.1526-4637.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 8.Ballantyne JC, LaForge KS. Opioid dependence and addiction during opioid treatment of chronic pain. Pain. 2007;129:235–255. doi: 10.1016/j.pain.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 9.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 10.Beck AT, Newman CF, Liese BS. Cognitive Therapy of Substance Abuse. New York: Guilford Press; 1993. [PubMed] [Google Scholar]
- 11.Becker WC, Sullivan LE, Tetrault JM, Desai RA, Fiellin DA. Non-medical use, abuse and dependence on prescription opioids among U.S. adults: psychiatric, medical and substance use correlates. Drug Alcohol Depend. 2008;94:38–47. doi: 10.1016/j.drugalcdep.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 12.Burns JW, Quartana P, Gilliam W, Gray E, Matsuura J, Nappi C, Wolfe B, Lofland K. Effects of anger suppression on pain severity and pain behaviors among chronic pain patients: evaluation of an ironic process model. Health Psychol. 2008;27:645–652. doi: 10.1037/a0013044. [DOI] [PubMed] [Google Scholar]
- 13.Butler SF, Budman SH, Fanciullo GJ, Jamison RN. Cross validation of the current opioid misuse measure to monitor chronic pain patients on opioid therapy. Clin J Pain. 2010;26:770–776. doi: 10.1097/AJP.0b013e3181f195ba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112:65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 15.Butler SF, Budman SH, Fernandez KC, Houle B, Benoit C, Katz N, Jamison RN. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Butler SF, Fernandez K, Benoit C, Budman SH, Jamison RN. Validation of the revised Screener and Opioid Assessment for Patients with Pain (SOAPP-R) J Pain. 2008;9:360–372. doi: 10.1016/j.jpain.2007.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychol. 2000;19:124–133. [PubMed] [Google Scholar]
- 18.Chabal C, Erjavec MK, Jacobson L, Mariano A, Chaney E. Prescription opiate abuse in chronic pain patients: clinical criteria, incidence, and predictors. Clin J Pain. 1997;13:150–155. doi: 10.1097/00002508-199706000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Childress AR, Ehrman R, McLellan AT, MacRae J, Natale M, O'Brien CP. Can induced moods trigger drug-related responses in opiate abuse patients? J Subst Abuse Treat. 1994;11:17–23. doi: 10.1016/0740-5472(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 20.Chou R, Fanciullo GJ, Fine PG, Miaskowski C, Passik SD, Portenoy RK. Opioids for chronic noncancer pain: prediction and identification of aberrant drug-related behaviors: a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009;10:131–146. doi: 10.1016/j.jpain.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Ciccocioppo R. The role of serotonin in craving: from basic research to human studies. Alcohol Alcohol. 1999;34:244–253. doi: 10.1093/alcalc/34.2.244. [DOI] [PubMed] [Google Scholar]
- 22.Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16:355–363. doi: 10.1016/s0885-3924(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 23.de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drummond DC. Theories of drug craving, ancient and modern. Addiction. 2001;96:33–46. doi: 10.1046/j.1360-0443.2001.961333.x. [DOI] [PubMed] [Google Scholar]
- 25.Dunbar-Jacob J, Mortimer-Stephens MK. Treatment adherence in chronic disease. J Clin Epidemiol. 2001;54(Suppl 1):S57–S60. doi: 10.1016/s0895-4356(01)00457-7. [DOI] [PubMed] [Google Scholar]
- 26.Edlund MJ. Chronic opioid therapy for chronic noncancer pain in the United States: Long Day's Journey into Night? Gen Hosp Psychiatry. 2011;33:416–418. doi: 10.1016/j.genhosppsych.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 27.Edlund MJ, Sullivan M, Steffick D, Harris KM, Wells KB. Do users of regularly prescribed opioids have higher rates of substance use problems than nonusers? Pain Med. 2007;8:647–656. doi: 10.1111/j.1526-4637.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 28.Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin JL, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry. 2009;66:88–94. doi: 10.1001/archgenpsychiatry.2008.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Erhardt S, Olsson SK, Engberg G. Pharmacological manipulation of kynurenic acid: potential in the treatment of psychiatric disorders. CNS Drugs. 2009;23:91–101. doi: 10.2165/00023210-200923020-00001. [DOI] [PubMed] [Google Scholar]
- 30.Ersche KD, Turton AJ, Pradhan S, Bullmore ET, Robbins TW. Drug addiction endophenotypes: impulsive versus sensation-seeking personality traits. Biol Psychiatry. 2010;68:770–773. doi: 10.1016/j.biopsych.2010.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frokjaer VG, Mortensen EL, Nielsen FA, Haugbol S, Pinborg LH, Adams KH, Svarer C, Hasselbalch SG, Holm S, Paulson OB, Knudsen GM. Frontolimbic serotonin 2A receptor binding in healthy subjects is associated with personality risk factors for affective disorder. Biol Psychiatry. 2008;63:569–576. doi: 10.1016/j.biopsych.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 32.Frokjaer VG, Vinberg M, Erritzoe D, Baare W, Holst KK, Mortensen EL, Arfan H, Madsen J, Jernigan TL, Kessing LV, Knudsen GM. Familial risk for mood disorder and the personality risk factor, neuroticism, interact in their association with frontolimbic serotonin 2A receptor binding. Neuropsychopharmacology. 2010;35:1129–1137. doi: 10.1038/npp.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gao K, Kemp DE, Conroy C, Ganocy SJ, Findling RL, Calabrese JR. Comorbid anxiety and substance use disorders associated with a lower use of mood stabilisers in patients with rapid cycling bipolar disorder: a descriptive analysis of the cross-sectional data of 566 patients. Int J Clin Pract. 2010;64:336–344. doi: 10.1111/j.1742-1241.2009.02284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gatchel RJ, Peng YB, Peters ML, Fuchs PN, Turk DC. The biopsychosocial approach to chronic pain: scientific advances and future directions. Psychol Bull. 2007;133:581–624. doi: 10.1037/0033-2909.133.4.581. [DOI] [PubMed] [Google Scholar]
- 35.Gil KM, Carson JW, Porter LS, Scipio C, Bediako SM, Orringer E. Daily mood and stress predict pain, health care use, and work activity in African American adults with sickle-cell disease. Health Psychol. 2004;23:267–274. doi: 10.1037/0278-6133.23.3.267. [DOI] [PubMed] [Google Scholar]
- 36.Grattan A, Sullivan MD, Saunders KW, Campbell CI, Von Korff MR. Depression and prescription opioid misuse among chronic opioid therapy recipients with no history of substance abuse. Ann Fam Med. 2012;10:304–311. doi: 10.1370/afm.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayes A. Beyond Baron and Kenny: Statistical mediation analysis in the new millennium. Communication Monographs. 2009;76:408–420. [Google Scholar]
- 38.Heckman BD, Catz SL, Heckman TG, Miller JG, Kalichman SC. Adherence to antiretroviral therapy in rural persons living with HIV disease in the United States. AIDS Care. 2004;16:219–230. doi: 10.1080/09540120410001641066. [DOI] [PubMed] [Google Scholar]
- 39.Hyman SM, Fox H, Hong KI, Doebrick C, Sinha R. Stress and drug-cue-induced craving in opioid-dependent individuals in naltrexone treatment. Exp Clin Psychopharmacol. 2007;15:134–143. doi: 10.1037/1064-1297.15.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ives TJ, Chelminski PR, Hammett-Stabler CA, Malone RM, Perhac JS, Potisek NM, Shilliday BB, DeWalt DA, Pignone MP. Predictors of opioid misuse in patients with chronic pain: a prospective cohort study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobsen JP, Medvedev IO, Caron MG. The 5-HT deficiency theory of depression: perspectives from a naturalistic 5-HT deficiency model, the tryptophan hydroxylase 2Arg439His knockin mouse. Philos Trans R Soc Lond B Biol Sci. 2012;367:2444–2459. doi: 10.1098/rstb.2012.0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jamison RN, Edwards RR, Liu X, Ross EL, Michna E, Warnick M, Wasan AD. Relationship of negative affect and outcome of an opioid therapy trial among low back pain patients. Pain Pract. 2013;13:173–181. doi: 10.1111/j.1533-2500.2012.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jamison RN, Link CL, Marceau LD. Do pain patients at high risk for substance misuse experience more pain? A longitudinal outcomes study. Pain Med. 2009;10:1084–1094. doi: 10.1111/j.1526-4637.2009.00679.x. [DOI] [PubMed] [Google Scholar]
- 44.Jamison RN, Raymond SA, Levine JG, Slawsby EA, Nedeljkovic SS, Katz NP. Electronic diaries for monitoring chronic pain: 1-year validation study. Pain. 2001;91:277–285. doi: 10.1016/S0304-3959(00)00450-4. [DOI] [PubMed] [Google Scholar]
- 45.Jamison RN, Ross EL, Michna E, Chen LQ, Holcomb C, Wasan AD. Substance misuse treatment for high-risk chronic pain patients on opioid therapy: a randomized trial. Pain. 2010;150:390–400. doi: 10.1016/j.pain.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jamison RN, Serraillier J, Michna E. Assessment and treatment of abuse risk in opioid prescribing for chronic pain. Pain Res Treat. 2011;2011:941808. doi: 10.1155/2011/941808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen MP, Turner JA, Romano JM, Karoly P. Coping with chronic pain: a critical review of the literature. Pain. 1991;47:249–283. doi: 10.1016/0304-3959(91)90216-K. [DOI] [PubMed] [Google Scholar]
- 48.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol. 2007;58:593–614. doi: 10.1146/annurev.psych.58.110405.085542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.MacKinnon DP, Lockwood CM, Hoffman JM, West SG, Sheets V. A comparison of methods to test mediation and other intervening variable effects. Psychol Methods. 2002;7:83–104. doi: 10.1037/1082-989x.7.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Manchikanti L, Giordano J, Boswell MV, Fellows B, Manchukonda R, Pampati V. Psychological factors as predictors of opioid abuse and illicit drug use in chronic pain patients. J Opioid Manag. 2007;3:89–100. doi: 10.5055/jom.2007.0045. [DOI] [PubMed] [Google Scholar]
- 53.Marceau LD, Link C, Jamison RN, Carolan S. Electronic diaries as a tool to improve pain management: is there any evidence? Pain Med. 2007;8(Suppl 3):S101–S109. doi: 10.1111/j.1526-4637.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 54.Marlatt GA. Cue exposure and relapse prevention in the treatment of addictive behaviors. Addict Behav. 1990;15:395–399. doi: 10.1016/0306-4603(90)90048-3. [DOI] [PubMed] [Google Scholar]
- 55.Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Palombi D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. J Pain Symptom Manage. 2004;28:250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 56.Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- 57.O'Brien C. Addiction and dependence in DSM-V. Addiction. 2011;106:866–867. doi: 10.1111/j.1360-0443.2010.03144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.O'Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. Am J Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- 59.O'Brien CP. Review. Evidence-based treatments of addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3277–3286. doi: 10.1098/rstb.2008.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Passik SDKKL, Webster L. Pseudoaddiction revisited: a commentary on clinical and historical considerations. Pain Management. 2011;1:239–248. doi: 10.2217/pmt.11.12. [DOI] [PubMed] [Google Scholar]
- 61.Passik SD, Kirsh KL. Addictions in pain clinics and pain treatment. Ann N Y Acad Sci. 2011;1216:138–143. doi: 10.1111/j.1749-6632.2010.05897.x. [DOI] [PubMed] [Google Scholar]
- 62.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36:717–731. doi: 10.3758/bf03206553. [DOI] [PubMed] [Google Scholar]
- 63.Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40:879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- 64.Reme SE, Tangen T, Moe T, Eriksen HR. Prevalence of psychiatric disorders in sick listed chronic low back pain patients. Eur J Pain. 2011;15:1075–1080. doi: 10.1016/j.ejpain.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 65.Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- 66.Schieffer BM, Pham Q, Labus J, Baria A, Van Vort W, Davis P, Davis F, Naliboff BD. Pain medication beliefs and medication misuse in chronic pain. J Pain. 2005;6:620–629. doi: 10.1016/j.jpain.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 67.SD Passik KK. Pain in the substance abuse population. In: Smith H, editor. Current therapy in pain. Philadelphia: Saunders Elsevier Press; 2009. pp. 392–395. [Google Scholar]
- 68.Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15:ES67–ES92. [PubMed] [Google Scholar]
- 69.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 70.Shrout PE, Bolger N. Mediation in experimental and nonexperimental studies: new procedures and recommendations. Psychol Methods. 2002;7:422–445. [PubMed] [Google Scholar]
- 71.Sinha R. Modeling stress and drug craving in the laboratory: implications for addiction treatment development. Addict Biol. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sinha R, Catapano D, O'Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) 1999;142:343–351. doi: 10.1007/s002130050898. [DOI] [PubMed] [Google Scholar]
- 73.Sinha R, Garcia M, Paliwal P, Kreek MJ, Rounsaville BJ. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch Gen Psychiatry. 2006;63:324–331. doi: 10.1001/archpsyc.63.3.324. [DOI] [PubMed] [Google Scholar]
- 74.Skinner MD, Aubin HJ. Craving's place in addiction theory: contributions of the major models. Neurosci Biobehav Rev. 2010;34:606–623. doi: 10.1016/j.neubiorev.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 75.Smalls BL, Walker RJ, Hernandez-Tejada MA, Campbell JA, Davis KS, Egede LE. Associations between coping, diabetes knowledge, medication adherence and self-care behaviors in adults with type 2 diabetes. Gen Hosp Psychiatry. 2012;34:385–389. doi: 10.1016/j.genhosppsych.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan MD, Ballantyne JC. What are we treating with long-term opioid therapy? Arch Intern Med. 2012;172:433–434. doi: 10.1001/archinternmed.2011.2156. [DOI] [PubMed] [Google Scholar]
- 77.Sullivan MD, Edlund MJ, Fan MY, Devries A, Brennan Braden J, Martin BC. Risks for possible and probable opioid misuse among recipients of chronic opioid therapy in commercial and medicaid insurance plans: The TROUP Study. Pain. 2010;150:332–339. doi: 10.1016/j.pain.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Swift RM, Stout RL. The relationship between craving, anxiety, and other symptoms in opioid withdrawal. J Subst Abuse. 1992;4:19–26. doi: 10.1016/0899-3289(92)90024-r. [DOI] [PubMed] [Google Scholar]
- 79.Tang NK, Salkovskis PM, Hodges A, Wright KJ, Hanna M, Hester J. Effects of mood on pain responses and pain tolerance: an experimental study in chronic back pain patients. Pain. 2008;138:392–401. doi: 10.1016/j.pain.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 80.Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19–28. doi: 10.1016/0376-8716(93)90042-o. [DOI] [PubMed] [Google Scholar]
- 81.Tiffany ST, Wray JM. The clinical significance of drug craving. Ann N Y Acad Sci. 2012;1248:1–17. doi: 10.1111/j.1749-6632.2011.06298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Turk DC, Swanson KS, Gatchel RJ. Predicting opioid misuse by chronic pain patients: a systematic review and literature synthesis. Clin J Pain. 2008;24:497–508. doi: 10.1097/AJP.0b013e31816b1070. [DOI] [PubMed] [Google Scholar]
- 83.Tziortzis D, Mahoney JJ, 3rd, Kalechstein AD, Newton TF, De la Garza R., 2nd The relationship between impulsivity and craving in cocaine- and methamphetamine-dependent volunteers. Pharmacol Biochem Behav. 2011;98:196–202. doi: 10.1016/j.pbb.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 84.Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol Alcohol. 1999;34:197–222. doi: 10.1093/alcalc/34.2.197. [DOI] [PubMed] [Google Scholar]
- 85.Wasan AD, Butler SF, Budman SH, Benoit C, Fernandez K, Jamison RN. Psychiatric history and psychologic adjustment as risk factors for aberrant drug-related behavior among patients with chronic pain. Clin J Pain. 2007;23:307–315. doi: 10.1097/AJP.0b013e3180330dc5. [DOI] [PubMed] [Google Scholar]
- 86.Wasan AD, Butler SF, Budman SH, Fernandez K, Weiss RD, Greenfield SF, Jamison RN. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clin J Pain. 2009;25:193–198. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wasan AD, Davar G, Jamison R. The association between negative affect and opioid analgesia in patients with discogenic low back pain. Pain. 2005;117:450–461. doi: 10.1016/j.pain.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 88.Wasan AD, Jamison RN, Pham L, Tipirneni N, Nedeljkovic SS, Katz JN. Psychopathology predicts the outcome of medial branch blocks with corticosteroid for chronic axial low back or cervical pain: a prospective cohort study. BMC Musculoskelet Disord. 2009;10:22. doi: 10.1186/1471-2474-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wasan AD, Ross EL, Michna E, Chibnik L, Greenfield SF, Weiss RD, Jamison RN. Craving of prescription opioids in patients with chronic pain: a longitudinal outcomes trial. J Pain. 2012;13:146–154. doi: 10.1016/j.jpain.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Webster LR, Webster RM. Predicting aberrant behaviors in opioid-treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432–442. doi: 10.1111/j.1526-4637.2005.00072.x. [DOI] [PubMed] [Google Scholar]
- 91.Weiss F. Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol. 2005;5:9–19. doi: 10.1016/j.coph.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 92.Weiss RD, Griffin ML, Mazurick C, Berkman B, Gastfriend DR, Frank A, Barber JP, Blaine J, Salloum I, Moras K. The relationship between cocaine craving, psychosocial treatment, and subsequent cocaine use. Am J Psychiatry. 2003;160:1320–1325. doi: 10.1176/appi.ajp.160.7.1320. [DOI] [PubMed] [Google Scholar]
- 93.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34:1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu SM, Compton P, Bolus R, Schieffer B, Pham Q, Baria A, Van Vort W, Davis F, Shekelle P, Naliboff BD. The addiction behaviors checklist: validation of a new clinician-based measure of inappropriate opioid use in chronic pain. J Pain Symptom Manage. 2006;32:342–351. doi: 10.1016/j.jpainsymman.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 95.Zautra AJ, Johnson LM, Davis MC. Positive affect as a source of resilience for women in chronic pain. J Consult Clin Psychol. 2005;73:212–220. doi: 10.1037/0022-006X.73.2.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.