Abstract
Surgery, radiation and chemotherapy are currently the most commonly used cancer therapies. Hyperthermia has been shown to work effectively with radiation and chemotherapy cancer treatments. The major obstacle faced by previous hyperthermia techniques has been the inability to deliver heat to the tumor in a precise manner. The ability to deliver cytotoxic hyperthermia to tumors (from within individual cells) via iron oxide magnetic nanoparticles (mNP) is a promising new technology that has the ability to greatly improve the therapeutic ratio of hyperthermia as an individual modality and as an adjuvant therapy in combination with other modalities. Although the parameters have yet to be conclusively defined, preliminary data suggests mNP hyperthermia can achieve greater cytotoxicity (in vitro) than conventional water bath hyperthermia methods. At this time, our theory is that intracellular nanoparticle heating is more effective in achieving the combined effect than extracellular heating techniques.1 However, understanding the importance of mNP association and uptake is critical in understanding the potential novelty of the heating modality. Our preliminary data suggests that the mNP heating technique, which did not provide time for particle uptake by the cells, resulted in similar efficacy to microwave hyperthermia. mNP hyperthermia/cisplatinum results have shown a tumor growth delay greater than either modality alone at comparable doses
Methods
One hour before nanoparticle hyperthermia, CDDP chemotherapy (5mg/kg of body mass) was delivered intraperitoneally (IP). Iron oxide nanoparticles, 7.5mg of iron per gram of tumor, were injected into MTGB flank tumors in female C3H mice immediately before activation. A 170 KHz, 400-450 Oe alternating magnetic field (AMF) was used to induce particle heating. A comparison of nanoparticle induced hyperthermia to non-nanoparticle induced hyperthermia was also made using a 915 MHz microwave generator. Treatment duration was determined by the use of the cumulative equivalent minutes (CEM) algorithm. A CEM 60 was selected as the thermal dose for all experimental groups.
Results
1) Preliminary mNP hyperthermia/cisplatinum results have shown a tumor growth delay greater than either modality alone at comparable doses.
2) mNP hyperthermia delivered 10 minutes post mNP injection and microwave hyperthermia, with the same thermal dose, demonstrate similar treatment efficacy.
Keywords: Iron oxide, nanoparticle, hyperthermia, microwave, AMF, chemotherapy, cisplatinum, MTGB
1. Introduction
1.1 Background
Within the past 30 years, the use of hyperthermia as a cancer therapy has achieved limited success, using a number of thermal delivery techniques. While some increased thermal sensitivity may exist in neoplastic tissues, this difference is not significant or consistent enough among tumors to allow for imprecise delivery of heat, and has been the fundamental difficulty faced by previous hyperthermia-based treatments.2,3 mNP hyperthermia has the potential to deliver significantly more focused heat to the cancerous tissue, either through direct injection of untargeted mNP or through anti-body directed systemic delivery. Previous efforts to improve treatment efficacy of hyperthermia-based treatments (produced by means such as microwaves, whole body hyperthermia and ultrasound) have included the use of hyperthermia as part of an adjuvant therapy, specifically in conjunction with chemotherapy and/or radiation4,5,6
The goals of this study are: 1) to determine if there is an improvement in therapeutic effect when a mild mNP-based thermal dose is delivered in conjunction with systemic chemotherapy. 2) to determine if there is a difference in thermal effect when hyperthermia is induced with mNP or microwaves. Both the thermal (CEM 60) and chemotherapy doses delivered in these experiments are significantly lower than those which would be used in optimized therapeutic conditions, in order to examine the potential potentiation of the adjuvant therapy. It should be noted that mNP activation was initiated prior to significant cellular uptake (under 5% mNP located intracellular), which improves therapeutic effect in vitro.7
1.2 Thermal dose
We use the cumulative equivalent minute relationship developed by Sapareto and Dewey to determine the delivered thermal dose. The relationship described is shown below. 8
“R” is equivalent to 0.25 when temperatures are under 43°C and 0.5 when temperatures are greater than 43°C. 9 This method allows for greater therapeutic repeatability as it is based on biologic thermal effects. However, we acknowledge that the unique properties of mNP hyperthermia may result in tissue effects different than those predicted by global temperatures measured in the tissue alone. We predict that these differences will be most significant with altered mNP distribution through tumor tissue, environmental alterations and increases in mNP uptake within the cells, with or without antibody targeting.
1.3 Chemotherapy and hyperthermia
Many chemotherapeutics have shown to have positive interaction with therapeutic hyperthermia. Proposed mechanisms of interaction have included, but are not limited to, increased rates of alkylation, inhibition of repair of single strand DNA breaks, and increases in drug uptake and varies with chemotherapy type.10 CDDP and related agents are chemotherapies which have been well established as strong candidates for clinical hyperthermia-adjuvant therapies.11,12 While we believe CEM is a good predictor of thermal dose and tumor response, it is important to understand that additional consideration must be taken into account when hyperthermia is used in conjunction with chemotherapy. Not only is the interaction of hyperthermia and CDDP influenced by thermal dose, it is affected by blood flow. If temperatures or CEM are too high, reduction of blood flow and drug deposition can occur. Mild hyperthermia has been shown to increase blood flow and deposition, both during and after hyperthermia.13,14
2. Methods
2.1 Magnetic Nanoparticles
The mNPs used in these experiments are composed of Fe3O4 cores with a biocompatible hydroxyethyl starch coating. These mNPs have an average hydrodynamic diameter ranging between 100 and 120 nm and are manufactured by MicroMod GmBH, Rostock, Germany and were prepared in suspension. mNPs produce heat via hysteresis when exposed to an alternating magnetic field.15
2.2 Tumor inoculation and measurement
MTGB cells, a mouse mammary adenocarcinoma, first isolated by Clifton et al.,16 were injected intradermally (100μl) in the right rear flank of C3H mice (Charles River, Wilmington, MA). Cells were suspended at a concentration of ten million cells per ml in 1× Alpha MEM. Tumors were treated when they reached a volume of 150 mm3 +/− 40 mm3. After treatment, tumors were measured every other day until their volume reached three times their initial treatment volume, which was the study endpoint. Volume was calculated using the measured perpendicular diameters of the ellipsoidal tumor, found with digital calipers. The equation of volume used is listed below.
2.3 Chemotherapy
CDDP (Teva Parenteral Medicines, Inc., Haarlem, Netherlands) was administered IP, one hour prior to hyperthermia, at 5 mg/kg.
2.4 mNP injection
7.5 mg of Fe (total mNP concentration of 42 mg/mL, containing 28 mg of Fe/mL) was injected per cm3 of tumor, in four equal quadrants. mNP injections took place 10 minutes prior to AMF exposure.
2.5 Administration of AMF, temperature recording and thermal dose
The AMF field was generated by a water cooled, whole body circular coil (Fluxtrol Inc., Auburn Hills, MI) powered by a Huttinger TIG 10/300 generator operating at 170 KHz and 450 Oe and a constant temperature of 30° C (chiller/Tek-Temo Instruments Inc.). Mouse rectal and tumor temperatures were recorded throughout the treatment using FISO fiber optic probes (FISO Inc., Quebec, Canada). At the initiation of treatment, the core body temperatures of the mice were 36°C ± 1°C. CEM was calculated real-time. Core body temperatures were held below 41.5°C. Mice were anesthetized using 1-3% isoflurane gas and 95% O2.
2.6 Administration of microwave, temperature recording and thermal dose
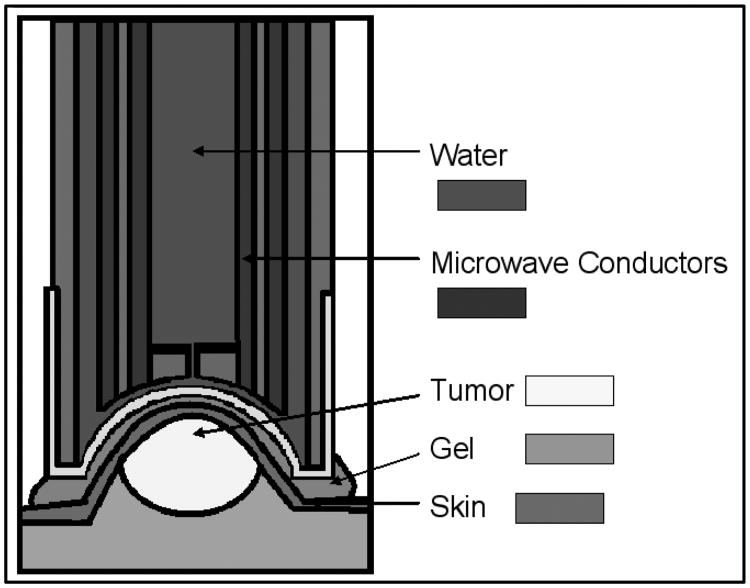
The microwave applicator utilized for tumor treatment was modified from its original purpose as a microwave thermal keratoplasty device.17 It consists of an open-ended pair of coaxial conductors, driven by a 915 MHz microwave generator. The applicator surface, made of vinyl, is cooled by circulating water as illustrated in the image below (figure 4).
Figure 4.
Microwave applicator: cross section, adapted from Trembly et al.18
The device is technically a near field applicator, not an antenna, because the spacing between the microwave conductors is much shorter than the wavelength at 915 MHz. The penetration depth of the microwave energy is therefore not dependent on the frequency used, instead it is dependent upon the spacing between the conductors.19,20 This applicator was designed to apply sub-millimeter energy penetration over short durations (2 seconds) to prevent thermal conduction.21 The device was used in these experiments to provide uniform heating by taking advantage of thermal conduction through the entire tumor, by utilizing long treatment durations (20-30 minutes). Through the use of tissue equivalent phantoms, ex vivo samples and characterization of in vivo specimens, it was determined that the global thermal profile of the tumors treated with microwave hyperthermia was comparable to that generated with mNP (heating immediately after injection).
An injection of PBS corresponding to the volume of mNP was used as a control. As with mNP hyperthermia, temperature measurements were recorded in three tumor positions, and the rectum (core temperature). The central CEM of the tumor acted as the treatment duration determinate. Core temperatures were maintained within 38°C ± 2°C.
All animal experiments were approved by the Dartmouth College Institutional Animal Care and Use Committee (IACUC), in accordance with all federal, institutional and AAALAC (Association for Assessment and Accreditation of Laboratory Animal Care) guidelines.
3. Results
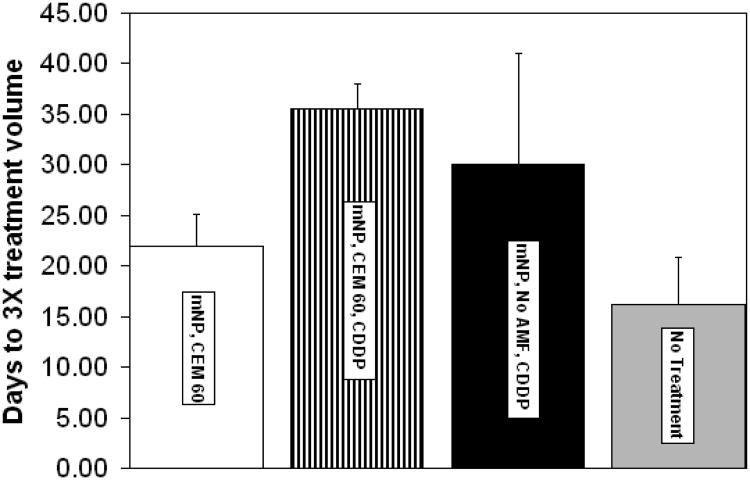
These data suggest that the combination of mNP hyperthermia and CDDP result in tumor treatment efficacy that is 18% greater than CDDP alone and 61% greater than mNP hyperthermia alone.
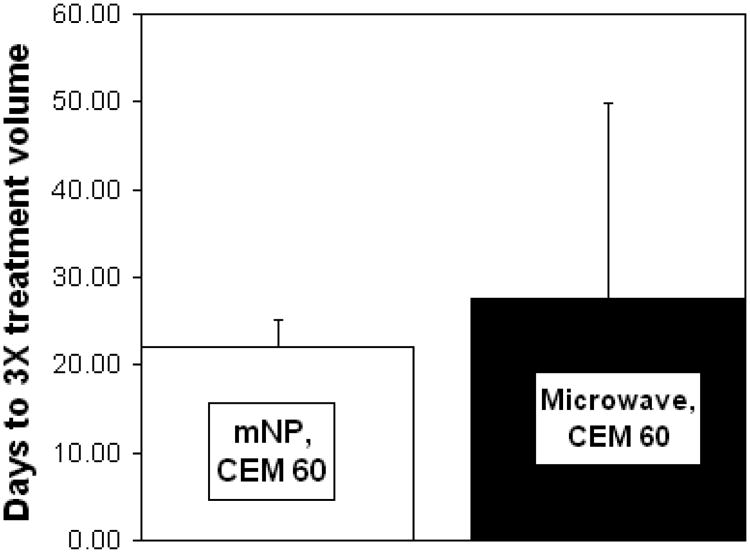
Preliminary data examining the regrowth delay of mNP hyperthermia immediately after mNP injection and microwave hyperthermia suggests that the thermal effects generated by both methods are similar.
4. Conclusions
Our data suggests the combination of mNP hyperthermia and standard CDDP chemotherapy is as safe and a more effective tumor treatment (tumor regrowth delay) than mNP hyperthermia, microwave hyperthermia or CDDP alone at similar doses. It is important to note that the mNP therapy used in these experiments was based on the extracellular location of the mNP, which according to “in press” data from our group is less efficient and less effective than intracellular mNP hyperthermia (at the same mNP/AMF levels). As such, cellular uptake of mNPs becomes a very important aspect and variable of this treatment. Additional data from our group suggests an increase in mNP may be acquired through decreases in intratumoral pressures, optimization of incubation times (both mNP and CDDP), antibody targeting and/or radiation.
Our data further suggests that interstitial/extracellular mNP hyperthermia and externally delivered 915 MHz microwave hyperthermia, delivered to the same thermal dose (0.3 mm fiber-optic intratumoral temperature assessment) result in a very similar tumor treatment effect. Although the effects of extracellular mNP hyperthermia cancer treatment compare favorably with, or are likely superior to, microwave heating, via improved targeting, the most significant advances in therapeutic ratio (outcome and safety) will likely come from the combination of intracellular heat and systemically delivered chemotherapy.
Figure 1. Cumulative equivalent minute relationship.
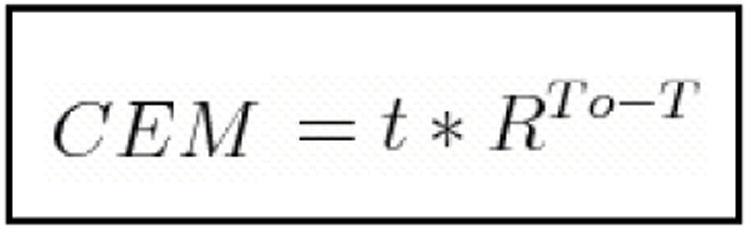
Figure 2.
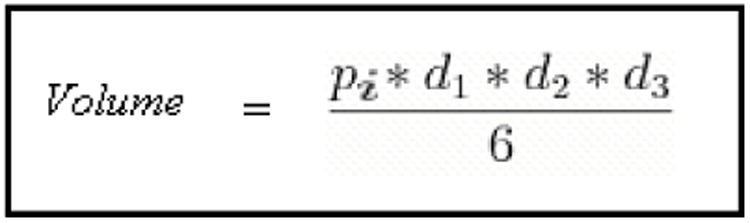
Volume of ellipsoidal tumor, “d”=diameter.
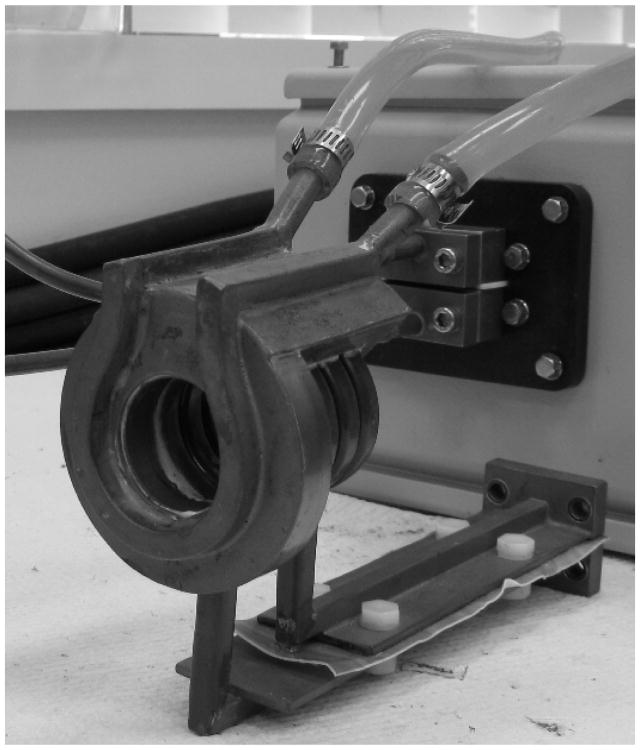
Figure 3. Water cooled, whole body circular coil.
Figure 5. Microwave applicator, end view without vinyl covering.
Figure 6.
Comparison of CDDP with and without mNP hyperthermia at CEM 60. Data support increased efficacy of CDDP/mNP hyperthermia at 18% for CDDP alone and 61% for mNP hyperthermia alone. These data were determined using 3× tumor growth delay assessments. n=4 per group. Error bars represent standard deviation.
Figure 7.
A comparison of nanoparticle hyperthermia and microwave hyperthermia at CEM 60. These data suggest similar thermal effects between hyperthermia created with non-incubation mNP and microwaves. n=4 per group. Error bars represent standard deviation.
References
- 1.Giustini AJ, Gottesman RE, Rauwerdink KM, Rauwerdink AM, Petryk AA, Weaver JB, Hoopes PJ. Kinetics and pathogenesis of intracellular iron-oxide nanoparticle hyperthermia. Presented at BiOS SPIE Photonics West, San Francisco, CA: Proc SPIE; 2011. pp. 7901–43. [Google Scholar]
- 2.Maehara Y, Kusumoto T, Kusumoto H, Anai H, Akazawa K, Sugimachi K. Excised Human Neoplastic Tissues Are More Sensitive to Heat than the Adjacent Normal Tissues. Eur Surg Res. 1988;20(4):254–259. doi: 10.1159/000128770. [DOI] [PubMed] [Google Scholar]
- 3.Dewhirst MW, Sim DA. The Utility of Thermal Dose as a Predictor of Tumor and Normal Tissue Responses to Combined Radiation and Hyperthermia. Cancer Research. 1984;44:4772s–4780s. [PubMed] [Google Scholar]
- 4.Mori M, Maehara Y, Inoue T, Shimono R, Kuwano H, Sugimachi K. Sensitivity to Heat and Radiation of Human Rectal Malignant Tissues In Vitro. Dis Colon Rectum. 1990;33(7):590–593. doi: 10.1007/BF02052213. [DOI] [PubMed] [Google Scholar]
- 5.Wust P, Hildebrandt B, Sreenivasa G, Rau B, Gellermann J, Riess H, Felix R, Schlag PM. Hyperthermia in combined treatment of cancer. The Lancet Oncology. 2002;3(8):487–497. doi: 10.1016/s1470-2045(02)00818-5. [DOI] [PubMed] [Google Scholar]
- 6.Issels RD, Lindner LH, Verweij J, Wust P, Reichardt P, Schem BC, Abdel-Rahman S, Daugaard S, Salat C, Wendtner CM, Vujaskovic Z, Wessalowski R, Jauch KW, Dürr HR, Ploner F, Baur-Melnyk A, Mansmann U, Hiddemann W, Blay JY, Hohenberger P. Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. The Lancet Oncology. 2010;11(6):561–570. doi: 10.1016/S1470-2045(10)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giustini Kinetics and pathogenesis of intracellular. :2011. doi: 10.1117/12.876519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sapareto SA, Dewey WC. Thermal Dose Determination in Cancer Therapy. International Journal of Radiation Oncology, Biology, Physics. 1984;10(6):787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 9.Johannsen M, Gneveckow U, Thiesen B, Taymoorian K, Cho CH, Waldöfner N, Scholz R, Jordan A, Loening SA, Wust P. Thermotherapy of prostate cancer using magnetic nanoparticles: feasibility, imaging, and three-dimensional temperature distribution. Eur Urol. 2007;52(6):1661–1662. doi: 10.1016/j.eururo.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Hall EJ. Radiobiology for the Radiologist. 5th. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 293–329. [Google Scholar]
- 11.Hall Radiobiology for the Radiologist. :293–329. [Google Scholar]
- 12.Herman TS, Teicher BA, Collins LS. Effect of Hypoxia and Acidosis on the Cytotoxicity of Four Platinum Complexes at Normal and Hyperthermic Temperatures. Cancer Research. 1988;48:2342–2347. [PubMed] [Google Scholar]
- 13.Hall Radiobiology for the Radiologist. :293–329. [Google Scholar]
- 14.Ausmus PL, Wilke AV, Frazier DL. Effects of Hyperthermia on Blood Flow and cw-Diamminedichloroplatinum(II) Pharmacokinetics in Murine Mammary Adenocarcinomas. Cancer Research. 1992;52:4965–4968. [PubMed] [Google Scholar]
- 15.Giustini AJ, Petryk AP, Cassim SM, Tate JA, Baker I. Magnetic Nanoparticle Hyperthermia in Cancer Treatment. Nano LIFE. 2010;1&2:17–32. doi: 10.1142/S1793984410000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clifton KH, Drapers NR. Survival-curves of Solid Transplantable Tumor Cells Irradiated in vivo: A Method of Determination and Statistical Evaluation; Comparison of Cell-survival and 32P-uptake into DNA. International Journal of Radiation Biology. 1963;7:515–535. doi: 10.1080/09553006314551561. [DOI] [PubMed] [Google Scholar]
- 17.Trembly BS, Hashizume N, Moodie KL, Cohen KL, Tripoli NK, Hoopes PJ. Microwave Thermal Keratoplasty for Myopia: Keratoscopic Evaluation in Porcine Eyes. J Refrac Surg. 2001;17:682–688. doi: 10.3928/1081-597X-20011101-08. [DOI] [PubMed] [Google Scholar]
- 18.Trembly Microwave Thermal Keratoplasty. :682–688. doi: 10.3928/1081-597X-20011101-08. [DOI] [PubMed] [Google Scholar]
- 19.Trembly Microwave Thermal Keratoplasty. :682–688. doi: 10.3928/1081-597X-20011101-08. [DOI] [PubMed] [Google Scholar]
- 20.Swicord ML, Davis CC. Energy absorption from small radiating coaxial probes in lossy media. IEEE Tran on Microwave Theory and Technique. 1981;29:1202–1209. [Google Scholar]
- 21.Trembly Microwave Thermal Keratoplasty. :682–688. doi: 10.3928/1081-597X-20011101-08. [DOI] [PubMed] [Google Scholar]