Abstract
Essential developments in the reliable and effective use of heat in medicine include: 1) the ability to model energy deposition and the resulting thermal distribution and tissue damage (Arrhenius models) over time in 3D, 2) the development of non-invasive thermometry and imaging for tissue damage monitoring, and 3) the development of clinically relevant algorithms for accurate prediction of the biological effect resulting from a delivered thermal dose in mammalian cells, tissues, and organs. The accuracy and usefulness of this information varies with the type of thermal treatment, sensitivity and accuracy of tissue assessment, and volume, shape, and heterogeneity of the tumor target and normal tissue. That said, without the development of an algorithm that has allowed the comparison and prediction of the effects of hyperthermia in a wide variety of tumor and normal tissues and settings (cumulative equivalent minutes/ CEM), hyperthermia would never have achieved clinical relevance. A new hyperthermia technology, magnetic nanoparticle-based hyperthermia (mNPH), has distinct advantages over the previous techniques: the ability to target the heat to individual cancer cells (with a nontoxic nanoparticle), and to excite the nanoparticles noninvasively with a non-injurious magnetic field, thus sparing associated normal cells and greatly improving the therapeutic ratio. As such, this modality has great potential as a primary and adjuvant cancer therapy. Although the targeted and safe nature of the noninvasive external activation (hysteretic heating) are a tremendous asset, the large number of therapy based variables and the lack of an accurate and useful method for predicting, assessing and quantifying mNP dose and treatment effect is a major obstacle to moving the technology into routine clinical practice. Among other parameters, mNPH will require the accurate determination of specific nanoparticle heating capability, the total nanoparticle content and biodistribution in the target cells/tissue, and an effective and matching alternating magnetic field (AMF) for optimal and safe excitation of the nanoparticles. Our initial studies have shown that appropriately delivered and targeted nanoparticles are capable of achieving effective tumor cytotoxicity at measured thermal doses significantly less than the understood thermal dose values necessary to achieve equivalent treatment effects using conventional heat delivery techniques. Therefore conventional CEM based thermal dose - tissues effect relationships will not hold for mNPH. The goal of this effort is to provide a platform for determining the biological and physical parameters that will be necessary for accurately planning and performing safe and effective mNPH, creating a new, viable primary or adjuvant cancer therapy.
Keywords: Iron oxide, nanoparticle, hyperthermia, dosimetry, treatment plan, CEM, thermal therapy, thermal dose, tissue assessment
1. INTRODUCTION
1.1 Background
The biological effects of traditional medical-based hyperthermia therapies, such as microwave, RF- and ultrasound, have been described, quantified and compared in terms of Cumulative Equivalent Minute (CEM) doses derived from Arrhenius models. A new method of hyperthermia delivery, achieved through the excitation of magnetic nanoparticles (mNP) by alternating magnetic fields (AMF), requires a different method of delivery, dose calculation, and dose and effect quantification. The primarily difference in convention medical hyperthermia and mNP hyperthermia is the delivery of energy (heat) in a very localized manner. Although mNP can be located between of on the exterior plasma membrane of cancer cells, most mNP hyperthermia will be associated with intracellular nanoparticles. One of the greatest challenges for assessing and standardizing mNP hyperthermia is understanding and quantifying an intracellular effect (heat or another mNP cytotoxic event) that can currently be measured. Without such measurement, treatment dosimetry and safety must determined by understanding the relevance and associations of the various tissue, biology, nanoparticle and magnetic field parameters.
The purpose of this effort is to set the stage for using the basic concepts relating the thermal dose required for conventionally delivered hyperthermia cytotoxicity and tissue damage to mNP-induced hyperthermia. Conventional CEM concepts can hopefully be used as a framework to develop regulatory guidelines for mNP hyperthermia. Factors influencing the mNP relation with time-temperature-damage relationships must be studied and used in a predictive manner that results in accurate and reproducible effects in a variety of tumor and normal tissue geometries and biology situations.
2. PREDICTION AND QUANTIFICATION OF HYPERTHERMIA CYTTOXICITY: THEORY AND EVIDENCE
2.1 Kinetics of cell killing by moderate hyperthermia: The Arrhenius Relationship
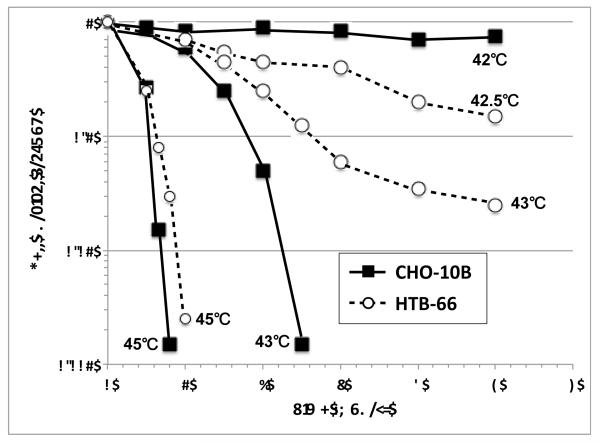
Numerous in vitro studies show that the rate of cell killing during exposure to moderate heat doses is exponential and dependent on the temperature and length of exposure. Figure 1 shows a family of survival curves for Chinese Hamster Ovary (CHO) and Human melanoma cells (HTB-66) covering the range from 42-45°C with heating times up to five hours [1]. At temperatures greater than 42°C these survival curves typically have a shoulder. The width of the shoulder region varies with cell line and is also dependent upon individual cell thermal tolerance. The shoulder region shows that there is a similar but not identical threshold for thermal damage to various cancer and normal cells. Since most cytotoxicity, at the moderate thermal doses, is thought to be based on protein denaturation, it remains somewhat unclear why these differences exist. It is assumed that the basic pathophysiology of hyperthermia cytotoxicity, at modest doses, is similar. This situation is likely different, however, for mNP where global tumor temperatures will be much lower, but targeted, highly-localized intracellular temperatures much higher. Once conventional hyperthermia starts to occur, the rate of cell killing, which is exponential with time of heating, is dependent on temperature. For the example with CHO cells there is very little cytotoxicity for up to five hours of heating at 42°C. At 42.5°C, however three logs of cell kill are achieved after five hours of heating. For comparison purposes, a similar family of survival curves is plotted for a human tumor cell line.
Figure 1.
Survival fraction curves CHO-10B and HTB-66 cells at different temperatures, adapted from Roizin-Towle and Pirro [1]
CHO and HTB-66 cell curves in Figure 1 show a reduction in slope after four hours of heating at 42.5°C and three hours of heating at either 42.5 and 43°C, respectively. This reduction in slope is due to acquired resistance to heating, or thermotolerance. Although thermotolerance may not be an important component of mNP hyperthermia, its basic mechanisms must be considered.
A number of authors have used an Arrhenius analysis to determine the heat of activation of cells. This analysis is done by plotting the rate of cell killing (1/Do; where Do is defined at the number of minutes to reduce survival by 63% on the exponential portion of the survival curve) vs. 1/temperature (°K). Using equation 1, the heat of inactivation can be calculated;
Where E = heat of activation in kcal/mole, A is a constant that is assumed to be unchanged over the temperature range studied, R = molar gas constant (1.987 × 10-3 Kcal/mole-°K) and T is the absolute temperature in °K.
The slope of the Arrhenius plot is typically biphasic. The curves have a threshold and the slope tends to be steeper below the threshold than above it. The activation energy for the temperature range above the threshold is typically around 120-150 kcal/mole, which is consistent with the heat of inactivation of proteins and enzymes. The change in slope of the Arrhenius plot below the threshold is generally thought to be related to the development of thermotolerance (acquired thermal resistance) during heating. When heating is delivered at temperatures above the threshold, thermotolerance does not occur during the heating period. It should be noted that thermotolerance does develop after heating at temperatures above or below the threshold. Above the threshold of the Arrhenius plot, the rate of cell killing essentially doubles for every degree increase in temperature. This means for a given isoeffect, such as a defined level of survival, the time at temperature needed to achieve that isoeffect is halved for each degree increase in temperature. At temperatures below the threshold the rate of cell killing decreases by a factor of 4-6 for every degree decrease in temperature.
2.2 Thermal Isoeffective Dose
The recognition that the rate of cell killing is related to time and temperature has led to several different methods for normalizing time-at-temperature data to a common unit that would allow for comparison of different heating regimes. For clinical applications of conventional hyperthermia, this is particularly important, since temperatures during heating are typically non-uniform and temporally unstable. Thus, a simple thermal prescription defining a desired temperature for a defined period of time is difficult if not impossible to achieve. In a classic paper, Sapareto and Dewey proposed a simple method for converting one time-temperature combination to another. This method is termed “thermal isoeffective dose”. Typically, the time-temperature data are converted to an equivalent number of minutes at 43°C. There was no particular reason for choosing 43°C as the index temperature, aside from the fact that it is near the threshold level for CHO and several other cell lines. The equation for doing this conversion is:
where CEM 43°C = cumulative number of equivalent minutes at 43°C, t = time interval (min), T = average temperature during time interval t. R is the number of minutes needed to compensate for a one degree temperature change either above or below the breakpoint. When there is temporal variation in the temperature of a specific tissue, the time at each temperature must be determined and the CEM 43°C summed over contiguous intervals where temperature is relatively constant. The resultant CEM 43°C value represents the entire history of the exposure.
2.3 Relevance of the R-value for establishing conventional and mNP thermal sensitivity of tissues
There is uncertainty about the slope of the Arrhenius plot below the threshold. The method of Sapareto and Dewey assumes that it is 0.25, but there are others who reported that it could be as low as 0.125 indicating that the time to achieve an isoeffect at a defined temperature is increased by a factor of 8, as opposed to 4, for every degree drop below the threshold. Most rodent data, however, suggest that the R-value below the threshold is between 0.25 and 0.17. For regulatory purposes, precise knowledge of the slope of the Arrhenius plot below the threshold would be advantageous, since it would allow for more detailed description of the thermal limits to achieve a specific tissue endpoint or to avoid tissue damage. The differences in the slopes of the Arrhenius plots affect the resultant isoeffect curves significantly, particularly at the extremes of the temperature range.
There is a temperature boundary for most tissues, below which no clinically detectable injury occurs, even for infinitely long heating periods. Likewise there is likely a focal organelle temperature boundary at which every very short exposures result in cytotoxicity. It is this type of organelle specific hyperthermia parameter that may provide the most innovative and therapeutically relevant component the mNP hyperthermia application.
2.4 Why the Arrhenius relationship for conventionally delivered hyperthermia (global hyperthermia) will not work for mNP hyperthermia
Due to higher (intracellular) and more focused temperatures, it appears very unlikely mNP hyperthermia will induced thermotolerance or be able to employ the type of therapeutic dosimetry predictions being used for conventional hyperthermia (CEM). The inability to accurately measure thermal dose in the targeted cells is extremely limiting. Without knowledge of a hyperthermia based Arrhenius relationship, isoeffective thermal dose or “R” values the quantification and accurate assessment of mNP hyperthermia will have to depend on material, tumor /normal tissue geometries / sensitivities, and pre-excitation parameters. Although many of these parameters have yet to be determined or used in the appropriate context, they will be need to understood and used in concert for mNP hyperthermia to become clinically relevant in the heterogeneous cancer setting.
3. CRICTICAL COMPOMENTS FOR SUCCESFUL IONP TREATMENT PLANNING AND USE
3.1: Global vs. intracellular mNP heating heat effect
We and others have shown that successful mNP hyperthermia tumor treatment can be achieved via the creation of an overall (global) cytotoxic thermal dose or an intracellular thermal dose. The global thermal dose can be measured and quantified in the same manner as conventionally delivered thermal hyperthermia, whereas mNP based intracellular hyperthermia can not measured or assessed in that manner. How then can mNP hyperthermia be prescribed and used in a reproducible and successful manner in vivo? Currently such a mechanism is unclear.
3.2 Particle heating capability
Properties assumed to be most important for the generation of mNP hyperthermia includes saturation magnetization of the particles and amplitude and frequency of external magnetic field. These factors originate from theoretical models that assume non-interacting nanoparticles. Experimental evidence demonstrates that for interacting magnetite nanoparticles, determined by their spacing and anisotropy, the resulting collective behavior in the kilohertz frequency regime generates significant heat. Key components in the generation of a high specific absorption rate (SAR) include the balance between magnetic dipole interactions, Brownian motion and polymer-polymer steric interactions. Appropriately-treated, a loose aggregation of particles may act like a large particle without the biological issues presented by rigid large particles. This collective behavior enhances the efficacy of the interacting nanoparticles,
3.3 Internal vs. external cellular location of the mNP and intracellular location of the nanoparticles
Developing mNP and associated delivery techniques that allow for preferential tumor and tumor cell uptake is a great challenge. It is well know that many cancer cells selectively uptake and aggregate mNPs within vesicles. This mNP uptake and aggregation capability, fortunately, appears better developed in many cancer cells than in many normal cells, excluding hepatic Kupffer cells and some types of reticuloendothelial (RE) cells, which have enhanced phagocytic capabilities.
Our own studies have shown that that within the first 4 hrs following intra-tumoral injection of starch-coated 110nm mNP into tumors, approximately 95% of the mNP have undergone endocytosis, are located within the cell. This type of information for antibody directed and systemically administered mNP will be critical to the treatment success of these applications, but has yet to be determined.
Although most mNP therapy efforts have been directed at the intracellular location of mNP, there may well be applications for mNP locations in the tumor interstitium or on the external plasma membrane of the tumor cells. As mentioned above these locations may be more applicable for inducing global tumor heating or altering tumor cell membrane permeability and improving the uptake of therapies such as chemotherapy, respectively.
Finally there is much discussion, but little documented evidence regarding the optimal intracellular location for aggregates of mNPs. One might intuitively believe that mNP aggregate locations near critical structures such as the external plasma membrane, H2O2 containing lysosomes or the nuclear envelope would result in enhanced cell killing at potentially lower doses, however this has yet to be conclusively investigated.
3.4 AMF mNP excitation parameters, including frequency, field strength, penetration depth, tumor and normal tissue geometry
It is well understood that the excitation of specific mNPs varies with AMF frequency and field strength. It is also well understood that the eddy currents associated with the delivery of significant AMF to tissues can result in non-targeted and dose limited tissue heating. Therefore, for mNP-AMF therapy to be effective, especially in tumor at depth, which require significant AMF dose, it is and will be critical to match the AMF parameters with mNP SAR capability and the tissue geometry. The development of relevant and appropriate algorithm will be essential for delivery of an effective and safe AMF dose.
3.5 mNP hyperthermia as a primary or adjuvant therapy
Although there are clear limitations in mNP hyperthermia cancer therapy, there is strong indication that appropriately excited mNPs alone can result in effective targeted cancer cell cytotoxicity. Issues associated with getting enough mNPs to the tumor and exciting the nanoparticles to an effective level in non-superficial tumors provide the greatest challenge. In many situations these factors may prove dose limitation for mNP therapy alone. One method of increasing the efficacy of yet non-optimized treatment methods is to combine them with a proven therapy. Many previous studies have established that there is great potential in combining hyperthermia with radiation and/or chemotherapy as part of an adjuvant therapy for cancer. [2,3] Despite the promise of multi-modality therapies, the use of traditional forms of hyperthermia in combination with radiation and chemotherapy has had modest clinical success due, at least in part, to the inability to deliver adequate thermal doses to the tumor while sparing the normal tissue. mNP hyperthermia has the potential to improve the therapeutic ratio beyond what has been achieved by providing a more focused delivery of heat (intracellular and or intra-tumoral), in addition to acting as a carrier of chemotherapeutic agents, which can be induced to release their payload with thermal activation. [4]
The development of mNP-based adjuvant therapies have further parameters, beyond those already associated with traditional forms of hyperthermia/chemotherapy adjuvant therapies. Studies suggest that the biodistribution of systemically delivered drugs are influenced by the blood flow, which can be altered by elevated temperatures. [5] One potential application of mNP technology is the use of tumor targeted mNP hyperthermia to improve the uptake and efficacy of systemic chemotherapy. Further opportunities exist with chemotherapy baring mNPs. However, optimizing their application will require significant investigation of the biodistribution, toxicity and kinetics for this novel and dual form of modality delivery.
As with chemotherapy, radiotherapy has been shown to enhance the efficacy of traditional forms of hyperthermia. [6] This mechanism of enhancement is believed to be associated with the ability of modest heat (40-42°C) to limit the cancer cells ability to repair radiation damage. It has also been demonstrated that hyperthermia is able to improve tumor blood flow/oxygenation thereby increasing radiation induced free radical cytotoxicity.[7] mNP hyperthermia offers the potential to enhance such radiation repair phenomena at the individual cancer cell level (while sparing associated normal tissues).
Early studies of mNPH as an adjuvant therapy with traditional cancer treatment methods supports that combination treatments results in greater thermal efficacy than any of these treatments applied alone. In a study investigating mNPH in conjunction with CDDP, a thermal dose of CEM 60 resulted in an average tumor volume tripling time 1.4 times longer than unaltered tumor growth (Figure 3). The combined treatment of CEM 60 and CDDP resulted in an average tumor volume tripling time 2.2 longer than unaltered tumor growth.
Figure 3.
Comparison of CDDP with and without mNP hyperthermia at CEM 60. Preliminary data supports increased efficacy with adjuvant therapy. n=4 per group.
Studies have also suggested that mNP hyperthermia results in greater efficacy than other forms of hyperthermia when used in conjunction with radiation. In a study examining the effect of mNP hyperthermia/radiation, in comparison to traditional (microwave) hyperthermia /radiation, it was determined that mNPH, with mNP incubated in the tumor for 24hrs, combined with15 Gy, resulted in 1.4 times the growth delay than microwave hyperthermia with 15 Gy. This study also showed that particle incubation, which is associated with uptake within the cell, has a significant effect on efficacy (Figure 4).
Figure 4.
Tumors incubated with mNP for 24 hours prior to AMF and 15 Gy radiation exposure demonstrate greater anti-tumor efficacy than tumors exposed to radiation and microwave hyperthermia. These data suggest that intracellular mNPHT is a more potent radiosensitizer than traditional hyperthermia.
4. CONCLUSIONS
We developed the following flow chart to summarized the various aspect mNP hyperthermia that are necessary for optimizing a reproducible, safe and effective treatment plan the in vivo/clinical setting. We propose the generation of a mNP Hyperthermia Treatment Unit (NHTU) which, similar to the cumulative equivalent minutes (CEM) algorithm used in conventional hyperthermia, will allow researchers and clinician quantify and compare the effects, outcomes and toxicities associated with various types and deliveries of mNPs and AMF.
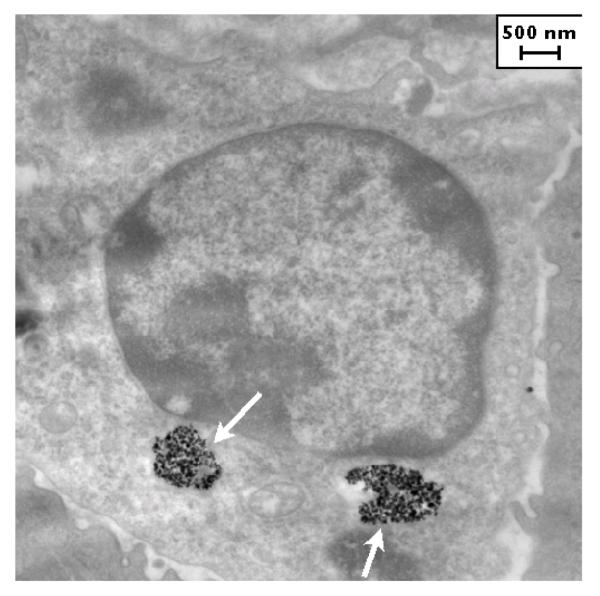
Figure 2.
MTG-B tumor, three hours post injection of nanoparticles. Aggregates of nanoparticles are observed within the cell (white arrows).
REFERENCES
- [1].Roizin-Towle L, Pirro JP. The response of human and rodent cells to hyperthermia. International journal of radiation oncology, biology, physics. 1991;20(4):751–756. doi: 10.1016/0360-3016(91)90018-y. [DOI] [PubMed] [Google Scholar]
- [2].Oleson JR, Calderwood SK, Coughlin CT, Dewhirst MW, Gerweck LE, Gibbs FA, Kapp DS. Biological and Clinical Aspects of Hyperthermia in Cancer Therapy. Amer. J. Clin. Oncology. 1988;11:368–380. doi: 10.1097/00000421-198806000-00013. [DOI] [PubMed] [Google Scholar]
- [3].Horsman MR, Overgaard J. Hyperthermia: a Potent Enhancer of Radiotherpay. Clin. Oncol. 2007;19:418–426. doi: 10.1016/j.clon.2007.03.015. [DOI] [PubMed] [Google Scholar]
- [4].Zhang J, Misra RDK. Magnetic drug-targeting carrier encapsulated with thermosensitive smart polymer: Core shell nanoparticle carrier and drug release response. Acta Biomaterialia. 2007;3(6):838–850. doi: 10.1016/j.actbio.2007.05.011. [DOI] [PubMed] [Google Scholar]
- [5].Hall EJ. Radiobiology for the Radiologist. 5th ed. Lippincott Williams & Wilkins; Philadelphia: 2000. pp. 293–329. [Google Scholar]
- [6].Sekhar KR, Vijayakumar NS, Muthusamy V, Sasi S, Laszlo A, Sawani J, Horikoshi N, Higashikubo R, Bristow RG, Borrelli MJ, Crooks PA, Lepock JR, Roti Roti JL, Freeman ML. Novel Chemical Enhancers of Heat Shock Increase Thermal Radiosensitization through a Mitotic Catastrophe Pathway. Cancer Res. 2007;67(2):695–70. doi: 10.1158/0008-5472.CAN-06-3212. [DOI] [PubMed] [Google Scholar]
- [7].Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. Int. J. Rad. Biol. 2001;77(4):399–408. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- [8].Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int. J. Hyperthermia. 2003;19:267–294. doi: 10.1080/0265673031000119006. [DOI] [PubMed] [Google Scholar]
- [9].Dewey WC. Arrhenius relationships from the molecule and cell to the clinic. Int. J. Hyperthermia. 1994;10(4):457–483. doi: 10.3109/02656739409009351. [DOI] [PubMed] [Google Scholar]
- [10].Roizin-Towle L, Pirro PJ. The response of human and rodent cells to hyperthermia. Int. J. Rad. Oncol, Biol Phys. 1991;20:751–756. doi: 10.1016/0360-3016(91)90018-y. [DOI] [PubMed] [Google Scholar]
- [11].Dennis CL, Jackson AJ, Borchers JA, Hoopes PJ, Strawbridge R, Foreman AR, van Lierop J, Grüttner C, Ivkov R. Nearly complete regression of tumors via collective behavior of magnetic nanoparticles in hyperthermia. Nanotechnology. 2009;20(39):395103. doi: 10.1088/0957-4484/20/39/395103. [DOI] [PMC free article] [PubMed] [Google Scholar]