Abstract
Hyperthermia has been shown to be an effective radiosensitizer. Its utility as a clinical modality has been limited by a minimally selective tumor sensitivity and the inability to be delivered in a tumor-specific manner. Recent in vivo studies (rodent and human) have shown that cancer cell-specific cytotoxicity can be effectively and safely delivered via iron oxide magnetic nanoparticles (mNP) and an appropriately matched noninvasive alternating magnetic field (AMF). To explore the tumor radiosensitization potential of mNP hyperthermia we used a syngeneic mouse breast cancer model, dextran-coated 110 nm hydrodynamic diameter mNP and a 169 kHz / 450 Oe (35.8 kA/m) AMF. Intradermally implanted (flank) tumors (150 ± 40 mm3) were treated by injection of 0.04 ml mNP (7.5 mg Fe) / cm3 into the tumor and an AMF (35.8 kA/m and 169 kHz) exposure necessary to achieve a CEM (cumulative equivalent minute) thermal dose of 60 (CEM 60). Tumors were treated with mNP hyperthermia (CEM 60), radiation alone (15 Gy, single dose) and in combination. Compared to the radiation and heat alone treatments, the combined treatment resulted in a greater than two-fold increase in tumor regrowth tripling time (tumor treatment efficacy). None of the treatments resulted in significant normal tissue toxicity or morbidity. Studies were also conducted to compare the radiosensitization effect of mNP hyperthermia with that of microwave-induced hyperthermia. The effects of incubation of nanoparticles within tumors (to allow nanoparticles to be endocytosed) before application of AMF and radiation were determined. This preliminary information suggests cancer cell specific hyperthermia (i.e. antibody-directed or anatomically-directed mNP) is capable of providing significantly greater radiosensitization / therapeutic ratio enhancement than other forms of hyperthermia delivery.
Keywords: Hyperthermia, radiation, nanoparticle, microwave, AMF, radiosensitization, intracellular hyperthermia
1. INTRODUCTION
For thousands of years, hyperthermia has been used as anti-neoplastic therapy, beginning with hot metal pokers inserted directly into tumors [1]. Over the last several decades, numerous methods for more efficiently and precisely delivering heat directly to tumors, including focused ultrasound, microwave and radio frequency ablation. One of the challenges of using hyperthermia for cancer treatment has been the relative lack of differential cytotoxicity between tumor and normal tissues when exposed to hyperthermia (temperatures greater than 41°C) [2]. Other anti-neoplastic treatments such as chemotherapy and radiation rely on different biological responses of normal and tumor tissues to effectively treat tumors. Because technologies developed to deliver clinical hyperthermia in the mid-to-late 1900s have largely been unsuccessful at delivering heat specifically to tumor cells, these therapies are not widely used for tumor therapy.
Magnetic nanoparticles (mNP) activated by an alternating magnetic field (AMF) have recently been developed to specifically deliver heat to tumor while sparing normal tissues [3]. Briefly, when magnetic nanoparticles are placed in alternating magnetic fields, they heat due to Neel relaxation and hysteretic heating [4]. Magnetic nanoparticle hyperthermia (mNPHT) is currently being investigated in clinical trials as a method of causing anti-tumor cytotoxicity in tumors [5] [6].
One of the most promising avenues for mNPHT is in combination with the traditional anti-neoplastic therapies of chemotherapy, ionization radiation and surgery [7]. Specifically, it has been shown that hyperthermia is one of the most potent radiosensitizers known [8]. By combining tumor-cell-specific mNPHT with ionizing radiation, it may be possible to dramatically improve the efficacy of lose-dose radiation therapy while simultaneously sparing normal tissues.
2. METHODOLOGY
2.1 Materials
2.1.1 Tumor Cells
The tumor cells used in this study were the murine breast adenocarcinoma MTG-B cell line characterized by Clifton and Yatvin [9]. Cells were maintained in surface-treated Falcon cell culture flasks (BD Biosciences, Bedford, MA) at 20–80% confluence at 37°C and 5% CO2 in a Queue Systems incubator (Kendro Laboratory Products, Asheville, NC). The cell culture medium used was Alpha Minimum Essential Medium (Alpha MEM, HyClone Laboratories, Inc.) with 10% fetal bovine serum (HyClone Laboratories, Inc.), 1% L-glutamine (Mediatech, Inc.) and 1% penicillin-streptomycin (HyClone Laboratories, Inc.).
2.1.2 Nanoparticles
The nanoparticles used in these experiments with BNF magnetic nanoparticles (product number 10-00-801, Micromod Partikeltechnologie GmBH, Rostock, Germany). These mNP are in a core-shell composition with hematite crystals in the core with average diameter of approximately 20 nm (total core diameter approximately 40 nm), coated with hydroxyethyl starch to a final average hydrodynamic diameter of 117 nm. The mNP particle concentration was 44 mg/ml and the iron concentration 28 mg/ml.
2.1.3 Mouse Model
The Dartmouth Institutional Animal Care and Use Committee approved these experiments. Six-eight week old female C3H/He mice were shaved on the right flank. Mice were anesthetized with 1% isoflurane in 99% oxygen. MTG-B cells were removed from the cell culture flasks using trypsin (0.25% trypsin in EDTA, HyClone Laboratory, Inc.), counted using trypan blue (Hyclone Laboratories, Inc.) and a hemacytometer (Fisher Scientific, Inc.) and resuspended in Alpha MEM at a concentration of 107 cells/ml. Using a 25-gauge needle, 0.1 ml of this cell solution was injected into the right flank of the mouse. After two-three weeks, tumors grew to a volume of 150 ± 40 mm3. Tumor volume was evaluated using the equation for the volume of an ellipsoid:
| (1) |
2.1.4 AMF Equipment
A 3.5 cm inner diameter cylindrical solenoid (Fluxtrol Inc., Auburn Hills, MI) was used to apply whole-body AMF at 169 kHz to the mice. The AMF coil was powered by a TIG 10/300 generator (Huttinger Elektronik GmbH, Freiburg, Germany). The AMF coil and generator were cooled by a chiller (Tek-Temp Instruments, Croydon PA) operating at 30°C and four gallons per minute.
2.1.5 Microwave Applicator
Microwaves were applied to the tumor at a frequency of 915 MHz using a water-cooled cylindrical applicator. The design of the applicator and generator is described by Trembly, et al. [10].
2.2 Methods
2.2.1 Tumor Therapy
All mice were anesthetized with 1% isoflurane in 99% O2. Nanoparticles were injected directly into tumors with a 30-gauge needle to a target iron concentration of 7.5 mg Fe / cm3 tumor. When mice were exposed to AMF or microwave, fiber optic temperature probes (FISO Inc., Quebec, Canada) with an outer diameter of 0.5 mm were placed in the manner shown in Figure 1: one probe was placed at the medial margin of the tumor, another placed directly into the center of the tumor and a third probed placed at the lateral margin of the tumor. A fourth temperature probe was placed in the rectum of the mouse. Treatments were started when mouse rectal temperatures were at 36.5°C ± 1.5°C. Temperatures were recorded during treatment and heat dose simultaneously quantified using the CEM equation:
| (2) |
Where Δt is the time interval, T is the temperature and R = 0.25 when T is below 43°C and R = 0.45 when T is above 43°C [11]. For mice receiving CEM 60 hyperthermia, the AMF or microwave was applied until the tumor center prove registered CEM 60. AMF was started at 169 kHz and 450 Oe and was increased to 500 Oe if the tumor temperature did not reach 43°C within 10 minutes. AMF was decreased to 400 Oe if the mouse rectal temperature reached 40.5°C.
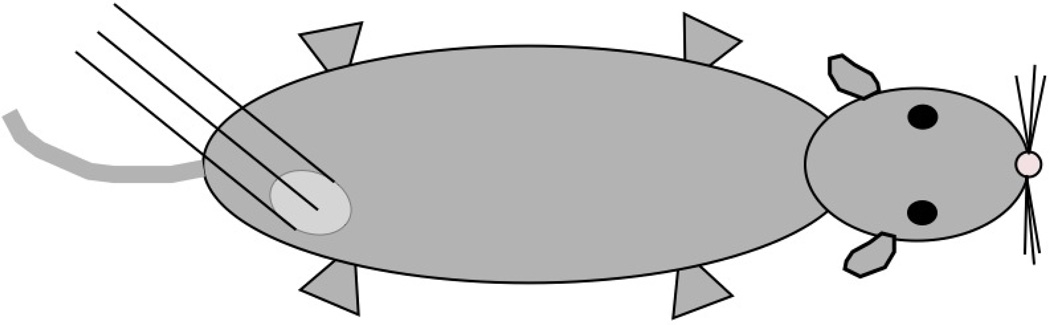
Figure 1.
This figure shows the placement of the three fiber optic temperature probes (black lines) relative to the tumor (light gray oval).
Treatment groups are described in Table 2. Mice receiving ionizing radiation received 15 Gy of 6 MeV electrons from a Clincac 2100C linear accelerator (Varian Medical Systems, Palo Alto, CA) within 30 minutes of AMF or microwave exposure in a single fraction. After tumor therapy, tumor volume was measured every two days until tumors grew to triple their treatment volumes.
Table 2.
Description of and results from the mice used in this study.
| Group | Therapy | Mice | Mean Tumor Tripling Time (Days) |
|---|---|---|---|
| 1 | mNP then AMF to CEM 60 (within 30 minutes) | 5 | 22 |
| 2 | mNP then AMF to CEM 60 (within 30 minutes) then 15 Gy radiation | 6 | 33.7 |
| 3 | mNP then 15 Gy radiation (within 30 minutes) then AMF to CEM 60 | 5 | 31.8 |
| 4 | mNP incubated for 24 hours then AMF to CEM 60 then 15 Gy radiation | 5 | 42.6 |
| 5 | mNP incubated for 24 hours then AMF to CEM 60 | 3 | 18.7 |
| 6 | PBS then AMF for 30 minutes (within 30 minutes) then 15 Gy radiation | 3 | 22 |
| 7 | PBS then 15 Gy then AMF for 30 minutes | 4 | 20 |
| 8 | PBS then CEM 60 with microwave (within 30 minutes) | 4 | 25 |
| 9 | PBS then CEM 60 with microwave (within 30 minutes) then 15 Gy | 5 | 31.4 |
| 10 | No Treatment | 7 | 13 |
3. RESULTS
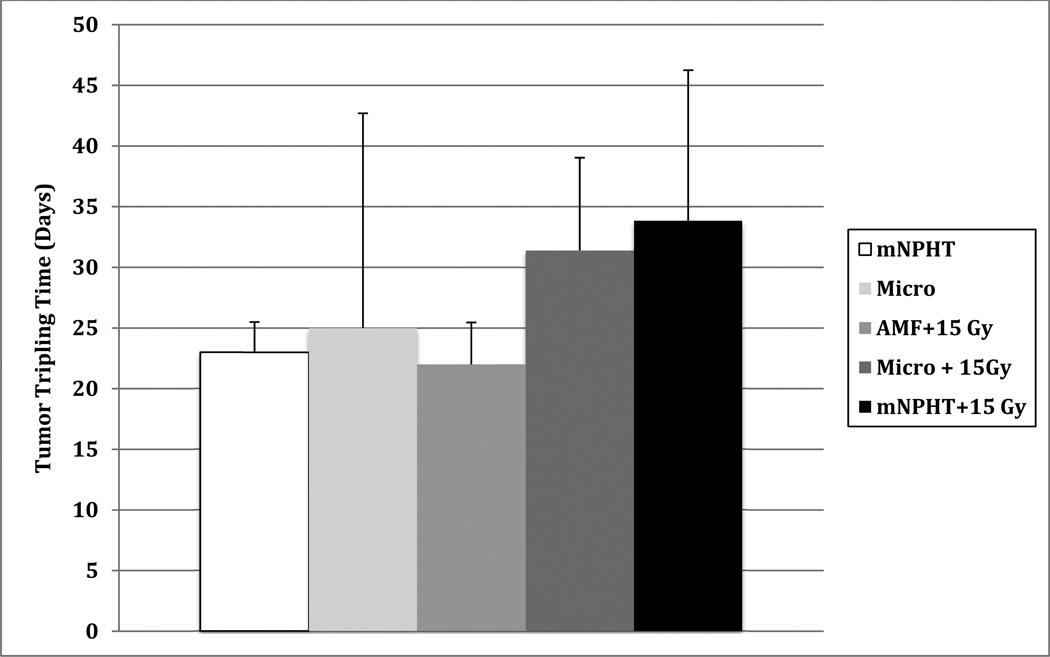
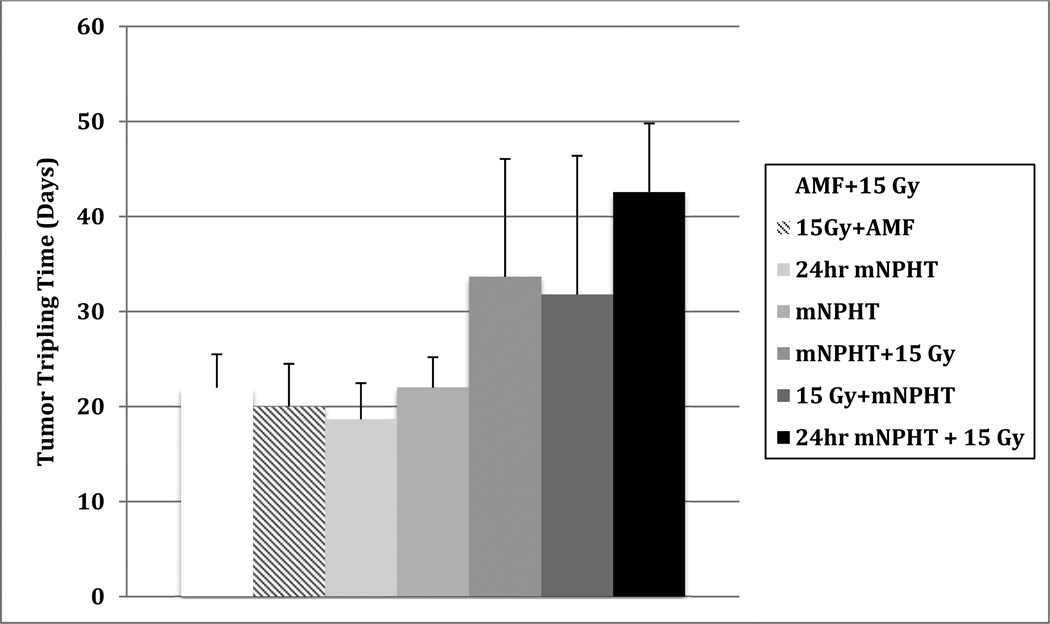
Mean tumor volume tripling times are shown in Table 2 and in Figures 2–4. Average tumor tripling time when no treatment was given was 13 ± 1.7 days. Mice tumors receiving one therapy (radiation or hyperthermia) had mean tumor volume tripling times of 18.7–25 days. When immediate hyperthermia and 15 Gy of radiation were combined, mean tumor volume tripling times ranged from 31.4 to 33.9 d days. When nanoparticles were allowed to incubate in tumors for 24 hours before application of AMF and radiation, mean tumor tripling time increased significantly to 42.6 days.
Figure 2.
This figure shows mean tumor tripling times (with standard deviations) of five tumor treatment groups. MNPHT combined with radiation demonstrates improved tumor response when compared to either hyperthermia or radiation alone.
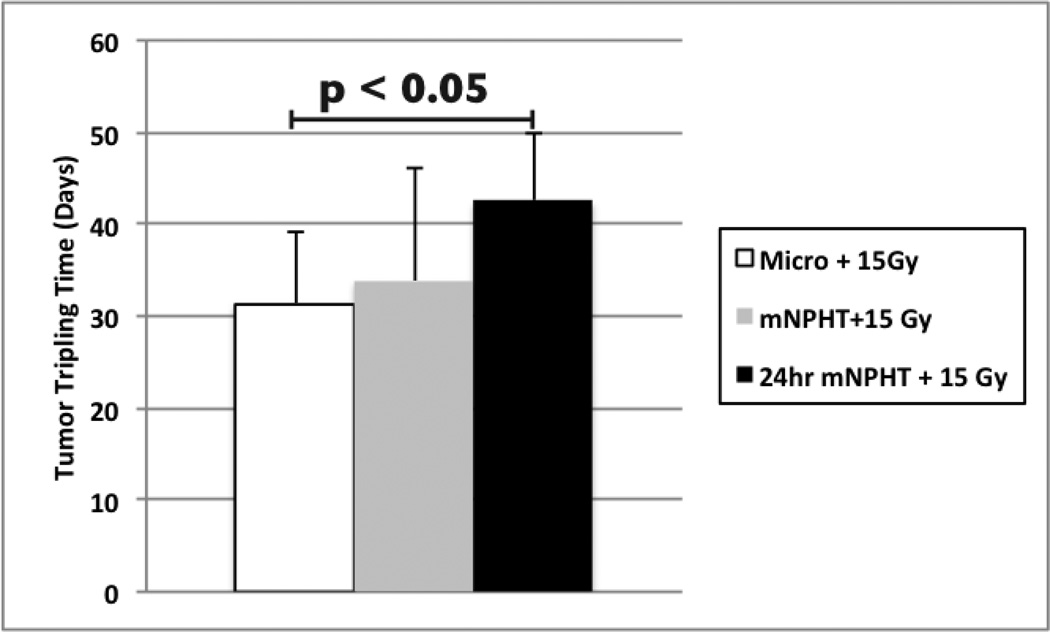
Figure 4.
Tumors incubated with mNP for 24 hours prior to AMF and 15 Gy radiation exposure demonstrate greater anti-tumor efficacy than tumors exposed to radiation and microwave hyperthermia. These data suggest that intracellular mNPHT is a more potent radiosensitizer than traditional hyperthermia.
4. CONCLUSIONS
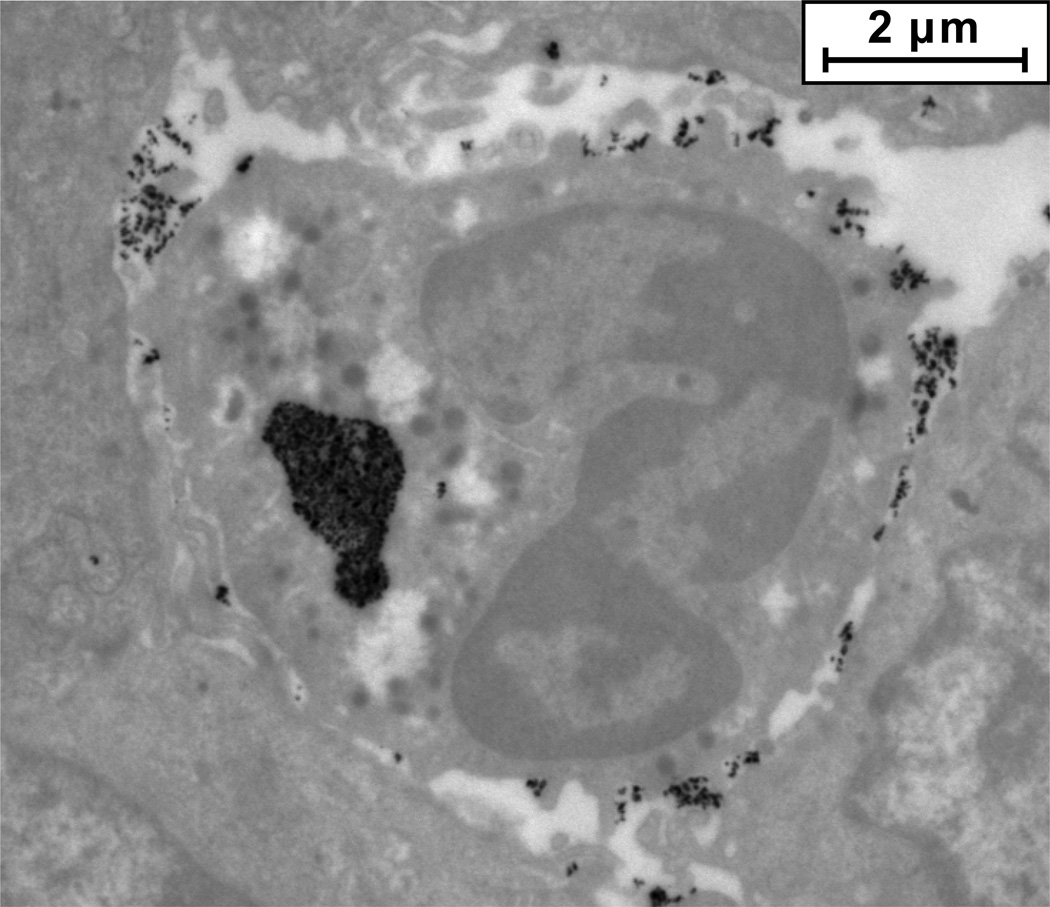
There is a statistically significant, 36% improvement in tumor tripling time (p < 0.05, two-sided t-test), 31.4 days vs. 42.6 days for microwave CEM 60 and 15 Gy radiation vs. AMF to CEM 60 and 15 Gy radiation when nanoparticles are allowed to incubate in tumors for 24 hours. This significant difference in tumor tripling time is not seen when AMF to CEM 60 and radiation are applied to the tumor directly after injection of mNP. In our previous work [12], we demonstrated that virtually all mNP present within tumors are extracellular until approximately one hour post-injection. After three hours post-injection, however, most mNP present within cells have been taken up and trafficked into vesicles within the cells (Figure 5). Our preliminary data presented in this report suggest that this difference in nanoparticle position relative to the cells demonstrates that intracellular mNP activated by AMF improve the efficacy of ionizing radiation therapy more than extracellular AMF or tumors heated by microwaves to the same CEM.
Figure 5.
As shown by this TEM, when allowed to incubate in tumors for more than three hours, most mNP (black pixels) within tumors are taken up by cells in the tumor.
Figure 3.
This figure shows mean tumor tripling times (with standard deviation) of three of the combined radiation and hyperthermia groups and representative controls.
Table 1.
This table defines the acronyms used in this report.
| Acronym | Definition |
|---|---|
| Alpha MEM | Alpha Minimum Essential Cell Culture Medium |
| AMF | Alternating Magnetic Field |
| CEM | Cumulative Equivalent Minutes of Hyperthermia |
| mNP | Magnetic Nanoparticle |
| mNPHT | Magnetic Nanoparticle Hyperthermia |
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the assistance of Rendall Strawbridge and Rachel Gottesman in conducting these studies. The authors also gratefully acknowledge the Dartmouth-Hitchcock Medical Center Section of Radiation Oncology for allowing the use of their Clinac 2100C linear accelerator. The NIH NCI grant 1U54CA151662-01 and the Dartmouth Center of Cancer Nanotechnology Excellence (DCCNE) supported this work. AJG also received support from the Thayer School of Engineering Innovation Fellowship.
REFERENCES
- 1.Horsman MR, Overgaard J. Hyperthermia: a Potent Enhancer of Radiotherapy. Clin. Oncol. 2007;19:418. doi: 10.1016/j.clon.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Roizin-Towle L, Pirro JP. The Response of Human and Rodent Cells to Hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1991;20:751. doi: 10.1016/0360-3016(91)90018-y. [DOI] [PubMed] [Google Scholar]
- 3.Jordan A, et al. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. Journal Of Magnetism And Magnetic Materials. 1999;201:413–419. [Google Scholar]
- 4.Sonvico F, et al. Folate-conjugated iron-oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physiochemical characterization, and in vitro experiments. Bioconj Chem. 2005;16:1181–1188. doi: 10.1021/bc050050z. [DOI] [PubMed] [Google Scholar]
- 5.Maier-Hauff K, et al. Intracranial Thermotherapy using Magnetic Nanoparticles Combined with External Beam Radiotherapy: Results of a Feasibility Study on Patients with Glioblastoma Multiforme. Journal of Neuro-Oncology. 2006;81(1):53–60. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 6.Johannsen M, et al. Morbidity and quality of life during thermotherapy using magnetic nanoparticles in locally recurrent prostate cancer: Results of a prospective phase I trial. International Journal of Hyperthermia. 2007;23(3):315–323. doi: 10.1080/02656730601175479. [DOI] [PubMed] [Google Scholar]
- 7.Giustini AJ, et al. Magnetic Nanoparticle Hyperthermia in Cancer Treatment. Nano LIFE. 2010;1(1–2):17–32. doi: 10.1142/S1793984410000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kampinga HH, Dikomey E. Hyperthermic radiosensitization: mode of action and clinical relevance. International journal of radiation biology. 2001;77(4):399–408. doi: 10.1080/09553000010024687. [DOI] [PubMed] [Google Scholar]
- 9.Clifton KH, Yatvin MB. Cell population growth and cell loss in the MTG-B mouse mammary carcinoma. Cancer Research. 1970;30(3):658–64. [PubMed] [Google Scholar]
- 10.Trembly BS, et al. Microwave Thermal Keratoplasty for Myopia: Keratoscopic Evaluation in Porcine Eyes. J. Refrac. Surg. 2001;17:682–688. doi: 10.3928/1081-597X-20011101-08. [DOI] [PubMed] [Google Scholar]
- 11.Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. International journal of radiation oncology, biology, physics. 1984;10(6):787–800. doi: 10.1016/0360-3016(84)90379-1. [DOI] [PubMed] [Google Scholar]
- 12.Giustini AJ, Ivkov R, Hoopes PJ. An in vivo transmission electron microscopy study of injected dextrancoated iron-oxide nanoparticle location in murine breast adenocarcinoma tumors versus time. Proc. SPIE Vol. 2009 Feb 23;7181 doi: 10.1117/12.809868. 71810M. [DOI] [PMC free article] [PubMed] [Google Scholar]