Abstract
Activation of either the phosphatidylinositol 3-kinase (PI 3-kinase)/Akt or the p38 mitogen-activated protein kinase (MAPK) signaling pathways accelerates myogenesis but only when the reciprocal pathway is functional. We therefore examined the hypothesis that cross-activation between these signaling cascades occurs to orchestrate myogenesis. We reveal a novel and reciprocal cross-talk and activation between the PI 3-kinase/Akt and p38 MAPK pathways that is essential for efficient myoblast differentiation. During myoblast differentiation, Akt kinase activity correlated with S473 but not T308 phosphorylation and occurred 24 h after p38 activation. Inhibition or activation of p38 with SB203580, dominant-negative p38, or MKK6EE regulated Akt kinase activity. Analysis of Akt isoforms revealed a specific increase in Akt2 protein levels that coincided with AktS473 phosphorylation during myogenesis and an enrichment of S473-phosphorylated Akt2. Akt2 promoter activity and protein levels were regulated by p38 activation, thus providing a mechanism for communication. Subsequent Akt activation by S473 phosphorylation was PI 3-kinase dependent and specific for Akt2 rather than Akt1. Complementary to p38-mediated transactivation of Akt, activation or inhibition of PI 3-kinase regulated p38 activity upstream of MKK6, demonstrating reciprocal communication and positive feedback characteristic of myogenic regulation. Our findings have identified novel communication between p38 MAPK and PI 3-kinase/Akt via Akt2.
A hallmark of cellular differentiation in many lineages is the mutual exclusivity of proliferation and differentiation. Skeletal myogenesis is the precisely orchestrated process by which committed but proliferating myoblasts irreversibly exit from the cell cycle, acquire an apoptosis-resistant phenotype, and finally form multinucleated myotubes (44). Myogenesis therefore provides an excellent model for understanding the fundamental mechanisms that regulate cell fate specification and the apparent antagonism between cell multiplication and differentiation. Two groups of transcription factors, the myogenic determination factors (such as MyoD and myogenin) and the myocyte enhancer factor 2 (MEF2) proteins, are central to the coordination of myogenesis; these interact to modify chromatin structure and initiate muscle-specific gene expression (64).
The p38 mitogen-activated protein kinase (MAPK) family was identified as part of the mechanism by which bacterial endotoxin induces cytokine expression (25, 38); they were therefore defined as stress-activated protein kinases. The results of subsequent studies of other cell systems suggest a significant role for p38 in differentiation (reviewed in reference 42); thus, its function is not confined to stress response. p38 has also been implicated in the regulation of cell cycle exit (as evidenced by direct phosphorylation of cyclin D1) (13) and of the retinoblastoma protein independent of cdk activity (58). p38 MAPKs exist as four isoforms: p38α, p38β, p38γ, and p38δ. They are mainly activated via phosphorylation by the immediate upstream MAPK kinases MKK3 and MKK6, although mechanisms of activation of the p38 pathway have not been identified in myogenesis. p38 kinases phosphorylate and activate both cytoplasmic and nuclear proteins, including kinases such as MAPK-activated protein kinase 2 (MK2) and transcription factors. In particular, p38 transactivates MEF2A and MEF2C via direct phosphorylation (66) and transactivates MyoD (60), thus targeting the key myogenic transcription factors. A role for p38 in myogenesis was first suggested by Lechner et al. (37), who reported preferential abundant levels of p38γ in skeletal muscle and demonstrated that forced expression of p38γ could accelerate myoblast differentiation. Cuenda and Cohen (19) reported rapid activation of the p38 target MK2 during myoblast differentiation that was prevented by the presence of the p38α and p38β isoform inhibitor SB203580. The absolute importance of the p38α and p38β isoforms in the initiation of myogenesis was confirmed by direct kinase assays and transactivation studies (65).
Phosphatidylinositol 3-kinases (PI 3-kinases) are key mediators for tyrosine kinase receptor signal transduction. Inhibition or activation of PI 3-kinase demonstrated that while it is not needed for the early stages of myogenesis (myoblast elongation and alignment), PI 3-kinase is necessary and rate limiting for the later stages of fusion and terminal differentiation (26, 28). Furthermore, PI 3-kinase is essential for signaling the potent myogenic actions of the insulin-like growth factors (IGFs) (18, 29) and acts both upstream and downstream of myogenin (56). The serine-threonine protein kinase Akt (also termed protein kinase B) is a downstream target of PI 3-kinase whose activity increases during myogenesis (22). The functional necessity for Akt in myogenesis was demonstrated using a dominant-negative Akt that prevented myotube formation (27). Activation of Akt is essential for cell survival in myogenesis and (together with that of p21) forms part of a key pathway in IGF-mediated myoblast survival (22, 35, 36). Since the suppression of myogenesis induced by inhibition of PI 3-kinase is overcome by constitutively active Akt (27, 56), however, the actions of Akt in myogenesis cannot be restricted only to the regulation of apoptosis.
For full activity, Akt must be phosphorylated on T308 and S473, which are located in the central catalytic and C-terminal regulatory domains, respectively (3). Translocation of Akt to the plasma membrane occurs via interaction between an N-terminal pleckstrin homology domain and the phosphatidylinositide products of PI 3-kinase (21), which is a primary determinant of S473 and T308 phosphorylation (49). Phosphorylation of both S473 and T308 is dependent on PI 3-kinase activity though separate kinases that regulate each site. 3-Phosphoinositide-dependent kinase 1 (PDK1) has been identified as the kinase for T308 (2, 51), but the identity of the kinase for S473 (termed PDK2) remains elusive. Unequivocal evidence for independent regulation of the two kinases was provided by PDK1-null cells, in which IGF-I can robustly induce S473 phosphorylation (59). Possible candidates for PDK2 include integrin-linked kinase (ILK) (45), MK2 (47), and NIMA-related kinase 6 (10). After activation, Akt dissociates from the plasma membrane and translocates rapidly to the nucleus (8, 40). Consensus Akt downstream phosphorylation motifs have been identified previously (3, 62), and numerous targets are activated in both cytoplasm and nucleus.
The importance of both PI 3-kinase and p38 MAPK signaling in myogenesis is reinforced in rhabdomyosarcoma, an often aggressive muscle-derived soft tissue tumor of childhood. Lesions in both PI 3-kinase (61) and p38 (46) pathways have been observed in rhabdomyosarcoma cell lines, which demonstrate an inability to exit from the cell cycle and differentiate into myotubes.
Intriguingly, forced activation of p38 and PI 3-kinase/Akt can only induce skeletal myoblast differentiation when the reciprocal pathway is functional (39, 60). We therefore hypothesized that in skeletal myoblast differentiation, either (i) necessary cross-activation of the p38 and PI 3-kinase/Akt pathway occurs or (ii) each pathway activates unique myogenic targets. The aim of these studies was to examine the first theory. We demonstrate novel reciprocal cross-talk between both pathways involving initial activation of p38 MAPK and subsequent isoform-specific transcriptional regulation and differential phosphorylation of Akt.
MATERIALS AND METHODS
Reagents, plasmids, and antibodies.
Reagents were purchased as follows: kinase inhibitors SB203580 (SB), PD98059 (PD), and LY294002 (LY) were purchased from Promega (Southampton, Hampshire, United Kingdom); myelin basic protein (MBP) and dimethyl sulfoxide (DMSO) were purchased from Sigma (Poole, Dorset, United Kingdom); cell culture reagents were purchased from Invitrogen (Paisley, Scotland); and [γ-32P]ATP was purchased from Amersham Pharmacia Biotech (Little Chalfont, Buckinghamshire, United Kingdom).
Constructs and expression vectors consisting of dominant-negative p38 (p38dn) (R. Davis, University of Massachusetts Medical Center, Worcester, Mass.); myc-tagged Akt1 expression vector and dominant-negative AktT308A/S473A and single-amino-acid mutants AktT308A and AktS473A (P. Hawkins, Babraham Institute); constitutively active forms of MKK6 and PI 3-kinase (MKK6EE and PI 3-Kca, respectively) (Z. Wu, Hong Kong University of Science and Technology, Hong Kong, People's Republic of China); green fluorescent protein (GFP) expression vector (pEGFP) (Clontech Laboratories, Oxford, United Kingdom); pCDNA vector (Invitrogen); hemagglutinin (HA)-tagged Akt2 expression vector (HA-Akt2) (J. R. Testa, Fox Chase Cancer Center, Philadelphia, Pa.); MyoD-E box reporter construct (4RE-Luc) (P. L. Puri, The Salk Institute, La Jolla, Calif.); and Akt2 promoter reporter construct (pGL3Akt2/3.1-Luc) (J. Chen, University of South Florida, St. Petersburg, Fla.) were either generous gifts or purchased as indicated.
The antibodies anti-myosin heavy chain (anti-MHC) (MF20; University of Iowa, Iowa City, Iowa); anti-MEF2 (Santa Cruz, Wembley, Middlesex, United Kingdom); anti-caveolin and anti-caveolin-3 (BD Bioscience/Clontech, Cowley, Oxfordshire, United Kingdom); anti-Myc, anti-p38, anti-Akt, and phospho-Akt (both T308 and Ser 473), immobilized anti-Akt S473 (clone 1G1), and phospho-Hsp27 and phospho-ATF-2 (Thr69/71) (Cell Signaling Technologies, Hitchin, Hertfordshire, United Kingdom); phospho-p38 (Thr180/Tyr182; Promega); anti-Akt1, and anti-Akt2 (Upstate Biotechnology Inc., Milton Keynes, Buckinghamshire, United Kingdom); anti-GFP (Molecular Probes, Cambridge, United Kingdom); anti-Flag (Eastman Kodak Company, New Haven, Conn.); anti-HA (Babraham Technix Monoclonal Antibody Service, Cambridge, United Kingdom); horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) and goat anti-mouse IgG (Jackson ImmunoResearch, Luton, Bedfordshire, United Kingdom); and donkey anti-sheep IgG (Santa Cruz) were obtained from sources as indicated.
Cell culture.
C2 myoblasts (derived from mouse muscle satellite cells) were seeded (2 × 105 cells in 100-mm-diameter dishes) onto 2% gelatin-coated plates and initially grown in growth medium (GM) (Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and 10% newborn calf serum). At 80% confluence, differentiation was induced by replacing GM with differentiation medium (DM) (Dulbecco's modified Eagle's medium with 2% horse serum). When indicated, kinase inhibitors or vehicle (DMSO) was added to the medium immediately after cells were moved into DM; the medium was changed every 24 h.
Transfection.
For transient transfections, 105 C2 myoblasts were seeded in 2% gelatin-coated dishes (60-mm diameter) 24 h before transfection. Cells were transfected (using Effectene transfection reagent as recommended by the manufacturer) (QIAGEN, Crawley, West Sussex, United Kingdom) with the indicated construct or pCDNA. Transfected cells were grown in GM for 24 h and then transferred to DM for 48 to 72 h. Cells were cotransfected with a GFP expression plasmid (pEGFP) to determine transfection efficiency levels between samples. Transfection efficiencies of 40 to 60% (as assessed by fluorescence microscopy) were obtained routinely.
Whole-cell extract preparation.
C2 cells were washed three times with phosphate-buffered saline (PBS) and harvested in 500 μl of lysis buffer (20 mM Tris-HCl, pH 7.5; 137 mM NaCl; 1 mM EGTA, pH 8; 1% Triton X-100; 10% glycerol; 1.5 mM MgCl2) containing protease and phosphatase inhibitors (10 mM NaF; 1 mM phenylmethylsulfonyl fluoride; 1 mM sodium metavanadate; 5 μg of aprotinin/ml; 10 μg of leupeptin/ml). Cellular debris were removed by centrifugation at 13,000 rpm (Heraeus Biofuge 13) for 2 min. Cell extracts were snap frozen in liquid nitrogen and stored at −80°C until use. Total protein in the extracts was determined by the Bradford assay (Bio-Rad, Hemel Hempstead, Hertfordshire, United Kingdom).
Cell morphology.
C2 cells were analyzed morphologically by Gill's hematoxylin and eosin (H and E) staining as described previously (33). Myoblasts were washed three times with PBS and fixed with 4% paraformaldehyde for 10 to 15 min at room temperature. Stained cells were photographed using a Leica MZ6 microscope. Unstained cells were photographed using bright-field microscopy.
Immunofluorescence.
Myoblasts were seeded on gelatin-coated single slide chambers (Nunc-Life Technologies, Wilford, Nottinghamshire, United Kingdom) 48 h before induction of differentiation in the presence or absence of kinase inhibitors or 24 h before transfection. At the indicated time points, slides were washed with PBS, fixed in 4% paraformaldehyde for 10 min, and permeabilized with 0.2% Triton X-100-PBS (PBS-TX) for 5 min. After being blocked with 3% bovine serum albumin in PBS-TX, cells were incubated for 3 h at room temperature with anti-MHC antibody (MF20; University of Iowa) diluted in 3% bovine serum albumin-PBS-TX. After the cells were washed in PBS-TX, immunostaining was detected using a Texas red-conjugated goat anti-mouse antibody (Jackson ImmunoResearch). Nuclei were stained with DAPI (4′,6′-diamidino-2-phenylindole) (Vector Laboratories, Orton Southgate, Peterborough, United Kingdom). Cells were visualized using an Olympus BX41 fluorescence microscope.
mRNA detection by reverse transcription-PTR (RT-PCR).
Studies of Akt2 gene expression were done by semiquantitative RT-PCR. Total RNA from C2 cells was obtained by using an RNeasy Mini protocol according to the instructions of the manufacturer (QIAGEN) and digested with DNase I (Promega). cDNA was synthesized using Superscript II (Invitrogen) according to the manufacturer's instructions. The primer sequences were as follows: for Akt2, 5′-GCGGGCTATCCAGATGGTCGC-3′ and 5′-GCCCGTGCCTTGTTGACAGCT-3′, which amplify a 136-bp unique region; for β-actin, 5′-CAGGTCATCACTATTGGCAACGAG-3′ and 5′-ACGGATGTCAACGTCACACTTCAT-3′, which amplify a 134-bp fragment.
Western blot analysis.
Protein (50 μg) from cell lysates was resolved by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to an Immobilon-P membrane (Millipore, Watford, Hertfordshire, United Kingdom) by electroblotting. The membranes were blocked with 0.2% I-block (Applied Biosystems, Warrington, Cheshire, United Kingdom)-0.1% Tween 20-Tris-buffered saline overnight at 4°C and probed with corresponding primary and secondary antibodies. Blots were washed with Tris-buffered saline-0.1% Tween 20, and antigen-antibody complexes were visualized using ECL reagents (Amersham Pharmacia Biotech) according to the manufacturer's instructions. Where reprobing of the blots was performed, membranes were stripped with 5% acetic acid (pH 2.8) for 7 min at room temperature before being washed and blocked as described above.
Immune complex kinase assays.
Protein (200 μg) from whole-cell extracts was incubated for 12 h at 4°C with continuous rotation with 5 μl of antibodies against p38, Akt, or phospho-Akt (S473). Extracts were then incubated with 50 μl of protein A-Sepharose bead suspension (Amersham Pharmacia Biotech) for another 2 h. The immune complexes were washed three times with lysis buffer and once with kinase reaction buffer (30 mM Tris, pH 8; 20 mM MgCl2; 2 mM MnCl2; 25 mM β-glycerol phosphate; 0.1 mM sodium vanadate). Beads were resuspended with 30 μl of kinase reaction buffer (7 μg of MBP, 3 μCi of [γ-32P]ATP, 10 μM ATP) and incubated at 30°C for 30 min in a shaking incubator. When indicated, kinase inhibitors were added to the kinase reaction mixtures. Reactions were stopped by addition of 10 μl of 4× Laemmli SDS sample buffer. Samples were subjected to SDS-12.5% PAGE, and products were visualized by autoradiography. Results were quantified using a phosphorimager.
Immunoprecipitation.
After 72 h in DM, C2 cells were harvested in lysis buffer and 200 μg of extracts were incubated for 12 h at 4°C on a rocker with 10 μl of immobilized anti-AktSer473 antibodies. Immunoprecipitates were washed three times with lysis buffer, resuspended in 30 μl of 4× Laemmli SDS sample buffer, heat denatured, and analyzed by SDS-PAGE. The presence of Akt isoforms in the complexes was determined by immunoblotting with isoform-specific Akt antibodies.
Luciferase reporter assays.
C2 cells transfected with either 4RE-Luc or pGL3Akt2/3.1-Luc were grown in GM for 24 h after transfection and then transferred to DM for 36 h as described previously (60). These luciferase reporters were cotransfected with MKK6EE or control empty vector (pCDNA). pEGFP was cotransfected to assess transfection efficiency between samples. Cells were harvested (using a luciferase assay system according to instructions of the manufacturer) (Promega) to measure luciferase activity. Expression of GFP was quantified by immunoblotting and phosphorimaging; luciferase activity was normalized for protein concentration and GFP expression. When used, SB203580 was added to the medium immediately after transfection and the medium was changed every 24 h with fresh drug or vehicle.
Statistical analysis.
Statistical differences between specific treatments were analyzed using Student's t test. The corresponding values represent means ± standard errors. All experiments were performed at least three times.
RESULTS
The PI 3-kinase/Akt and p38 MAPK pathways are essential for skeletal muscle differentiation.
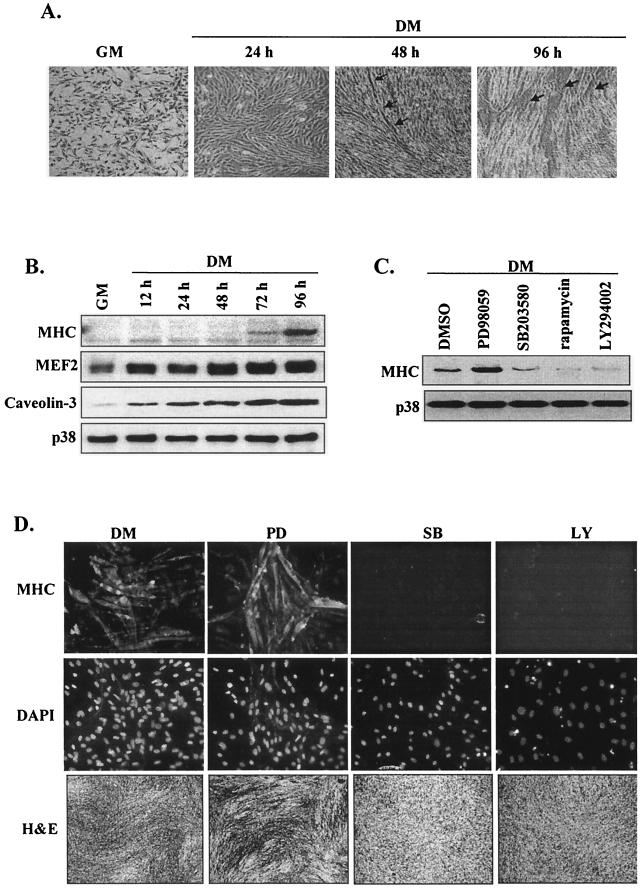
Mouse C2 myoblasts were induced to differentiate by transfer at 80% confluence into low-serum medium. Differentiation was observed morphologically by the alignment and elongation of cells by 24 h followed by fusion of myoblasts into multinucleated myotubes by 48 h (Fig. 1A and D [DM panels]) and by significant increases in the established myogenic differentiation markers MHC, MEF2, and caveolin-3 (Fig. 1B).
FIG. 1.
PI 3-kinase and p38 MAPK signaling pathways are essential for myogenesis. (A) Phase-contrast photomicrographs (×20 magnification) of C2 myoblasts in proliferative (GM) and differentiating (DM [24 to 96 h]) phases; arrows highlight myotube formations. (B) Western blot detection of MHC, MEF2, and caveolin-3 in differentiating C2 cells; total p38 protein levels are shown as a loading control. (C) Analysis (by Western blotting) of MHC expression after 72 h in DM in the presence or absence of the following kinase inhibitors: PD98059 (20 μM), SB203580 (5 μM), rapamycin (2 ng/ml), and LY294002 (10 μM). As a control, cells were also treated with vehicle alone (DMSO); total p38 protein levels are shown as a loading control. (D) Myoblast differentiation (in the presence or absence of the indicated inhibitors) was assessed by immunofluorescence (×20 magnification) with anti-MHC antibody (upper panels); cell nuclei were visualized by DAPI staining (middle panels), and cell morphology was examined by H and E staining (lower panels) (×5 magnification).
To confirm the role of the ERK, PI 3-kinase/Akt, and p38 MAPK pathways in myogenic differentiation, we initially examined the effects of specific pharmacological inhibitors. PD98059 augmented (but both LY294002 and SB203580 inhibited) myotube formation (Fig. 1D) and MHC expression (Fig. 1C), corroborating previous conclusions that ERK1/2 inhibits myoblast differentiation whereas PI 3-kinase and p38 MAPK accelerate the myogenic program. As previously reported (19), treatment of C2 myoblasts with the inhibitor rapamycin (p70S6K and FRAP/mTOR inhibitor) also significantly reduced MHC expression (Fig. 1C). These results therefore establish temporal aspects of myogenic regulation in our system and confirm the importance of the PI 3-kinase and p38 MAPK pathways in myogenesis.
Akt is differentially phosphorylated during myogenic differentiation.
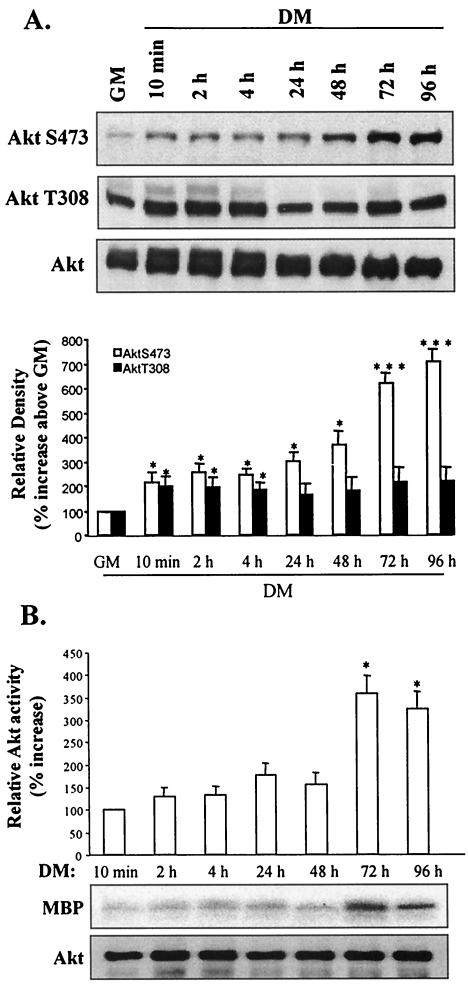
Since Akt is a key downstream target of PI 3-kinase in myogenesis, we examined the mechanism of Akt activation during myoblast differentiation. A modest increase in phosphorylation of AktS473 (which increased substantially by late myogenic differentiation) was initially observed after 48 h in DM (Fig. 2A). In contrast and even though a similar severalfold increase in phosphorylation of AktT308 occurred after 10 min in DM, the level of phosphorylation was not subsequently increased; these data do not necessarily imply that AktT308 is not phosphorylated during myogenesis but rather imply that phosphorylation was not substantially changed.
FIG. 2.
Differential phosphorylation of Akt at residues T308 and S473 during myoblast differentiation. (A) Western blot detection of phospho-AktS473, phospho-AktT308, and total Akt levels from 10 min to 96 h following transfer of myoblasts from GM to DM (upper three panels). The results of quantification of the immunoblots by scanning densitometry are shown (lower panel); data are expressed as percentages of Akt phosphorylation above basal levels in GM. (B) Immune complex kinase assay (using MBP as a substrate) of the samples described above; the results of quantification (by scanning densitometry) of the in vitro kinase data are expressed as percentages of Akt kinase activity above basal levels in DM (10 min). Assay Akt levels were determined by Western blotting with anti-Akt antibody. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Since each phosphorylation site contributes to Akt activity, we determined Akt kinase activity during myogenesis (Fig. 2B). Akt kinase activity remained low during the initial stages of myogenesis but increased almost fourfold by 72 h, correlating with the substantial increase in Akt phosphorylation at S473. These observations suggest that Akt phosphorylation activities (or inhibition of dephosphorylation) at residues T308 and S473 are differentially regulated throughout myogenesis.
Inhibition of PI 3-kinase activity differentially inhibits Akt phosphorylation.
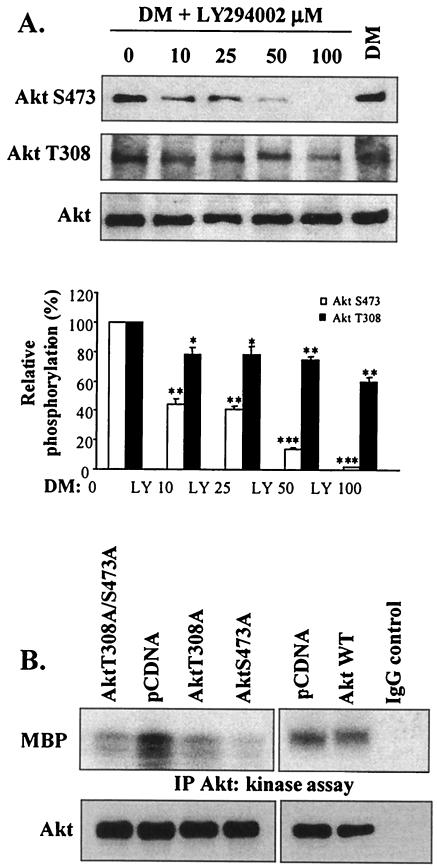
We addressed the role of PI 3-kinase in the differential phosphorylation of Akt by treating myoblasts with increasing concentrations of LY294002 (Fig. 3A). LY294002 at a concentration as low as 10 μM reduced AktS473 phosphorylation to 65% of control (DM) levels, and 50 μM LY294002 caused a substantial inhibition (about 85%) of AktS473 phosphorylation. In marked contrast, 10 μM LY294002 caused a modest though significant decrease in AktT308 phosphorylation (22%) and 100 μM LY294002 reduced AktT308 phosphorylation by 40%. Therefore, phosphorylation activities of AktT308 and AktS473 are dependent on PI 3-kinase-dependent mechanisms but exhibit different sensitivities. It is noteworthy that the lowest concentration (10 μM) of LY294002 examined was sufficient to substantially delay myogenesis (Fig. 1), implying either that full Akt activity is essential or that other myogenic PI 3-kinase-dependent mechanisms are inhibited at this concentration.
FIG. 3.
Phosphorylation of both sites in Akt (which are differentially regulated by PI 3-kinase) is essential in myogenesis. (A) Western blot analysis of Akt phosphorylation and total Akt levels in the presence of increasing concentrations of LY294002 after 72 h in DM. As a control, cells were treated with vehicle alone (DMSO; first lane). Quantification (by scanning densitometry) of the immunoblots was performed; data are expressed as percentages of Akt phosphorylation above control sample results. *, P < 0.05; **, P < 0.01; ***, P < 0.001 (compared with DMSO results). (B) Akt kinase activity (after 72 h in DM) in lysates of C2 cells transiently transfected with AktT308A or AktS473A; as controls, C2 cells were also transfected with either empty vector (pCDNA) or the dominant-negative Akt double mutant AktT308A/S473A or wild-type Akt1 (Akt1 WT). As a control, kinase activity was examined using non-Akt binding IgG substituted for the anti-Akt antibody; assay Akt levels were determined by Western blotting with anti-Akt antibody (lower panels). IP, immunoprecipitation.
To further examine the requirement of Akt phosphorylation at residues T308 and S473 for Akt activity in myogenesis, kinase activity assays were performed using lysates from myoblasts transiently transfected with the single-amino-acid mutants (AktT308A and AktS473A). Myoblasts were also transfected with the Akt double mutant AktT308A/S473A (which functions as a dominant-negative Akt) (4), control empty vector (pCDNA), and wild-type Akt1. Mutation of either T308 or S473 to alanine significantly reduced Akt activity to levels similar to those seen with the double mutant and fivefold lower than those seen with endogenous Akt or wild-type Akt1 (Fig. 3B), suggesting that Akt functions as an active kinase in myogenesis only when it is phosphorylated by both PDK1 and PDK2 and confirming the observations of Alessi et al. (4).
Activation of p38 MAPK precedes Akt phosphorylation during myoblast differentiation.
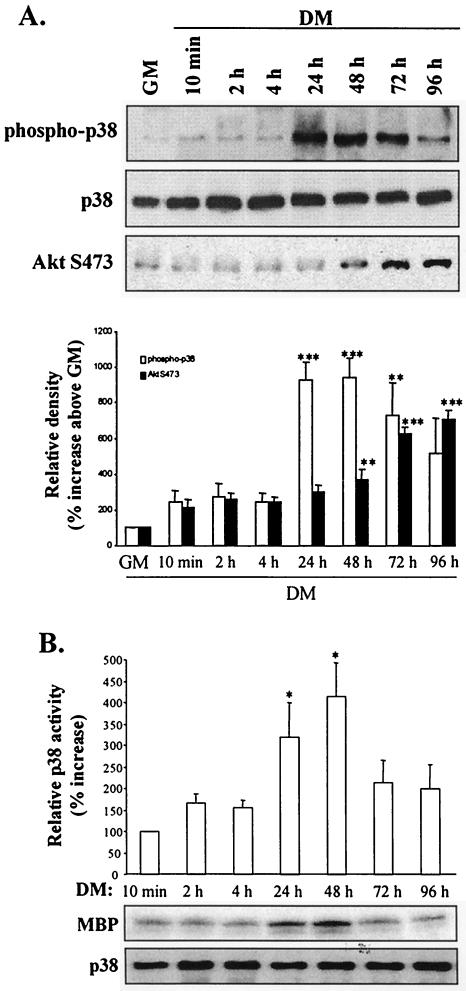
p38 phosphorylation increased modestly upon induction of differentiation (10 min) (Fig. 4A). However, a 10-fold increase in phospho-p38 levels was observed by 24 h; the increase was sustained until 72 h when it began to decline. The in vitro kinase activity of p38 also increased at 24 h, reaching a peak at 48 h (Fig. 4B) and decreasing thereafter. Comparison of p38 and AktS473 phosphorylation profiles during myogenesis revealed that activation of p38 preceded AktS473 phosphorylation by approximately 24 h, suggesting that p38 might have a role in the activation of Akt activity.
FIG. 4.
p38 MAPK activation precedes Akt activation during myogenic differentiation. (A) Western blot detection of p38, AktS473 phosphorylation, and total p38 levels from 10 min to 96 h following transfer of myoblasts to DM (upper three panels). The results of quantification (by scanning densitometry) of the immunoblots presented in the top three panels are shown in the lower panel; data are expressed as percentages of p38 phosphorylation above basal levels (GM). (B) p38 kinase activity determined by immune complex kinase assays using MBP as a substrate. The results of quantification (by scanning densitometry) of in vitro kinase data are shown. Data are expressed as percentages of p38 kinase activity above DM results (after 10 min of incubation); assay p38 levels were determined by Western blotting with anti-p38 antibody. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
The p38 MAPK pathway regulates Akt phosphorylation in myogenesis.
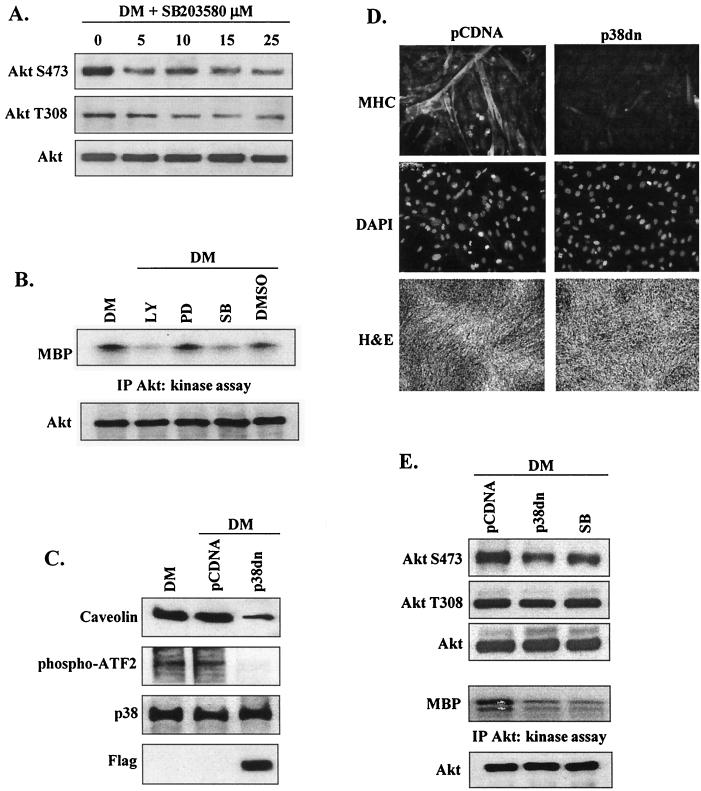
To determine the role of p38 MAPK in Akt activation during myogenesis, we initially examined the effect of the presence of the p38-specific (α/β isoform) inhibitor SB203580 on Akt phosphorylation and kinase activity. SB203580 reduced AktS473 phosphorylation (Fig. 5A) at low concentrations (5 μM); an inhibitor concentration of at least 15 μM was required for a similar reduction in AktT308 phosphorylation, although at higher concentrations SB203580 may directly interact with Akt (32). These data suggest that inhibition of p38 activity has a role in the regulation of Akt.
FIG. 5.
p38 regulates Akt activation in differentiating myoblasts. (A) Western blot detection of Akt phosphorylation and total Akt levels in the presence of increasing concentrations of the p38 inhibitor SB203580 after 72 h in DM. (B) Akt kinase activity in myoblasts after 72 h in DM in the presence or absence of LY294002 (10 μM), PD98059 (20 μM), SB203580 (5 μM), or vehicle alone (DMSO). The level of Akt was determined by Western blotting with anti-Akt antibody. IP, immunoprecipitation. (C) Western blot analysis performed to demonstrate inhibition (induced by the presence of p38dn) of the expression and phosphorylation of the p38 targets caveolin and ATF-2. C2 myoblasts were transfected with either p38dn or control vector (pCDNA) and harvested after 72 h in DM. Total p38 protein levels are shown as a loading control. Expression of p38dn was detected by the presence of its Flag tag. (D) Myoblast differentiation in cells transfected with p38dn was assessed by immunofluorescence (×20 magnification) with anti-MHC antibody (upper panels), cell nuclei were visualized by DAPI staining (middle panels), and cell morphology was examined by H and E staining (×5 magnification) (lower panels). (E) Western blot detection of Akt phosphorylation and total Akt levels in myoblasts transfected with either p38dn or pCDNA or treated with SB203580 (5 μM) after 72 h in DM (upper three panels). Akt kinase activity in the same experiment was examined; the level of Akt was determined by Western blotting with anti-Akt antibody (lower two panels).
The role of p38 MAPK in Akt kinase activity was also examined (Fig. 5B). Akt activation was not significantly affected by the addition of vehicle (DMSO) or PD98059, implying that (at least at this stage of myogenesis) p44/42 MAPK kinase does not participate in the Akt signaling pathway. In consistency with its role in Akt activation, inhibition of PI 3-kinase with LY294002 severely reduced Akt kinase activity. Inhibition of p38 activity with SB203580 also strongly attenuated Akt kinase activation, suggesting that p38 MAPK positively regulates Akt signaling during myogenic differentiation.
As SB203580 may exert nonspecific pharmacological effects, myoblasts were transiently transfected with a dominant-negative form of p38α (p38dn). Expression of p38dn was demonstrated by presence of its Flag tag (Fig. 5C). p38dn also induced a marked decrease in phosphorylation of its established target (ATF2) and in the protein levels of caveolin compared with the results seen with control cells (pCDNA) and significantly inhibited myotube formation and MHC expression (Fig. 5D). Confirming the effects of SB203580, expression of p38dn reduced AktS473, but not AktT308, phosphorylation in differentiating myoblasts and, importantly, p38dn also inhibited Akt kinase activity (Fig. 5E).
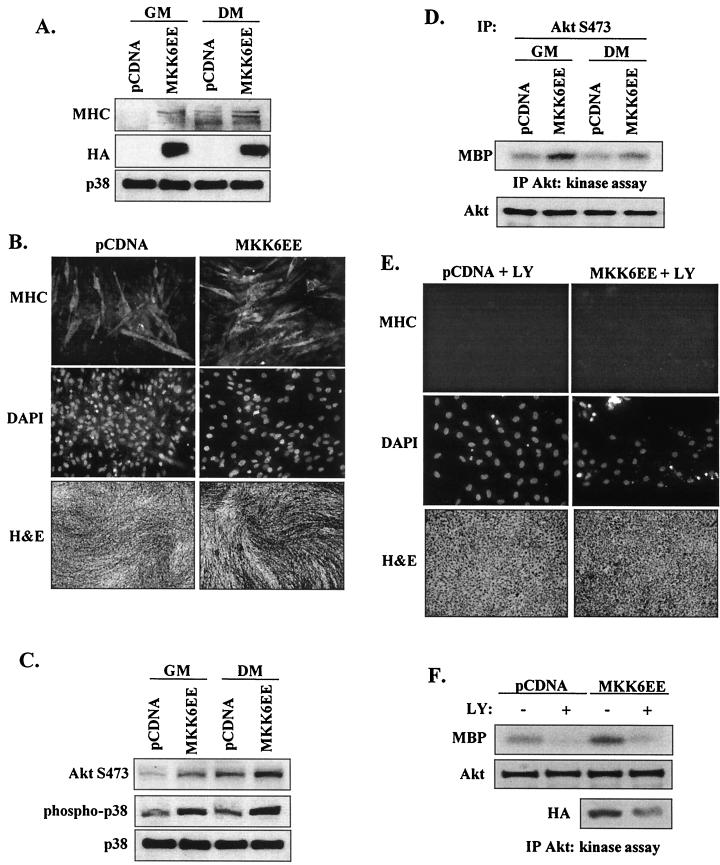
To complement these inhibition studies, myoblasts were also transfected with the constitutively active upstream p38 activator MKK6EE. The presence of active MKK6 was demonstrated in transfected cells by expression of its HA tag and increased phospho-p38 levels (Fig. 6A). As previously reported (60), MKK6EE induced differentiation even in GM (as assessed by the increase in MHC protein levels and the increased density of myotubes) (Fig. 6B). MKK6EE also stimulated AktS473 phosphorylation in both proliferative and differentiating myoblasts (Fig. 6C). Nevertheless, MKK6EE did not affect AktT308 phosphorylation under any conditions tested (data not shown). Significantly, MKK6EE stimulated the kinase activity of Akt phosphorylated at S473 (Fig. 6D). Taken together, these results indicate that p38 has a role in Akt activation during myogenic differentiation.
FIG. 6.
Activation of Akt by p38 MAPK requires PI 3-kinase activity. (A) Constitutively active MKK6EE stimulates myoblast differentiation of C2 cells. Proliferative (GM) and differentiating (DM) myoblasts were transfected with MKK6EE or control vector (pCDNA); after 48 h, expression of MHC was determined by Western blotting. The results of expression of HA-tagged MKK6EE in the cell lysates and total p38 levels are shown. (B) Myoblast differentiation in cells transfected with MKK6EE was assessed after 48 h in DM by immunofluorescence (×20 magnification) with anti-MHC antibody (upper panels), cell nuclei were visualized by DAPI staining (middle panels), and cell morphology was examined by H and E staining (×5 magnification) (lower panels). (C) Phosphorylation of AktS473 and p38 in proliferative (GM) and differentiating (DM) myoblasts transfected with MKK6EE or control vector (pCDNA) was determined by Western blotting after 48 h. Total p38 levels were measured to confirm even loading. (D) Akt kinase activity in proliferative (GM) and differentiating (DM) myoblasts transfected with MKK6EE or pCDNA after 48 h. C2 cells were harvested, and lysates were immunoprecipitated (IP) with an AktS473-phosphospecific antibody; total Akt levels were determined by Western blotting with anti-Akt antibody (lower panel). (E) Myoblast differentiation and morphology in cells transfected with MKK6EE or pCDNA in the presence or absence of LY294002 (10 μM), as assessed by immunofluorescence with anti-MHC antibody and by H and E staining. (F) Akt kinase activity in cells lysates from myoblasts transfected with MKK6EE or pCDNA in the presence (+) or absence (−) of LY and incubated in DM for 48 h. Total Akt and HA-tagged MKK6EE levels were determined by Western blotting (lower two panels).
To address whether forced activation of the p38 pathway could overcome the inhibition of myogenesis when PI 3-kinase was inhibited by LY294002 (Fig. 1), myoblasts were transfected with MKK6EE or empty pCDNA vector and simultaneously treated with LY. MKK6EE expression could not overcome the profound inhibition of myogenesis (as assessed by MHC expression and myotube formation) induced by blocking PI 3-kinase activity (Fig. 6E). In vector-only transfected cells, the presence of LY dramatically reduced Akt kinase activity and (as expected) transfection with MKK6EE increased Akt kinase activity. Unfortunately, transfection efficiency was always decreased in myoblasts treated with LY; even when this was taken into account, the reduction in Akt kinase activity was not overcome by MKK6EE overexpression, suggesting that activation of Akt activity and acceleration of myogenesis by p38 requires PI 3-kinase signaling.
Akt2 is upregulated during myogenic differentiation.
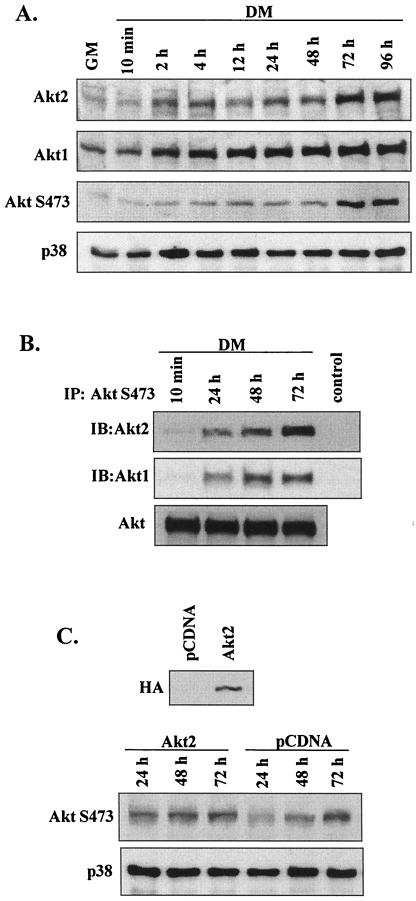
To date, three isoforms of Akt, Akt1/PKBα, Akt2/PKBβ, and Akt3/PKBγ, have been identified (1). Since Akt1 and Akt2 are the major isoforms in muscle (43), we compared their protein profiles at different stages of myogenesis (Fig. 7A). Both Akt1 and Akt2 were upregulated modestly by 12 h after induction of differentiation. Akt2 abundance subsequently increased markedly at 72 h (coinciding with increased AktS473 phosphorylation), whereas changes in Akt1 levels did not correlate with AktS473 phosphorylation. These results support previous suggestions that Akt2 is the key Akt isoform upregulated in myogenesis.
FIG. 7.
Akt2 upregulation in myogenesis. (A) Western blot detection of Akt1, Akt2, phospho-AktS473, and total p38 from 10 min to 96 h after transfer of myoblasts from GM to DM. (B) Western blot analysis of Akt isoforms (Akt1 and Akt2) in myoblasts following immunoprecipitation (IP) with immobilized AktS473-phosphospecific antibodies; total Akt was determined by Western blotting. In the control lanes, immunoprecipitation was performed using non-Akt-binding IgG. IB, immunoblot. (C) Western blot detection of phospho-AktS473 and total p38 in C2 cells transfected with Akt2 or pCDNA and transferred to DM. Expression of HA-tagged Akt2 in the cell lysates is shown in the upper panel.
The concomitant increase in Akt2 protein levels and total AktS473 phosphorylation during myogenesis suggest that the proportion of Akt2 phosphorylated on S473 should increase. We tested this by immunoprecipitating either total Akt or S473-phosphorylated Akt and subsequently detecting Akt1 or Akt2 levels by Western blotting during myogenesis (Fig. 7B). After 24 h in DM, modest increases in both S473-phosphorylated Akt1 and Akt2 were observed. After 72 h in DM (when total Akt kinase activity was maximal), however, the amount of S473-phosphorylated Akt2 considerably exceeded that seen with Akt1. To further examine the relationship between the increase in Akt2 protein levels and the regulation of its phosphorylation, myoblasts were transfected with Akt2 (as shown by the results obtained with an HA tag) (Fig. 7C). In the Akt2-transfected cells, AktS473 phosphorylation was accelerated (exceeding that seen with cells transfected with vector alone at 24 h). Thus, once Akt2 protein is expressed the mechanisms for its phosphorylation exist by 24 h in DM.
Myogenic induction of Akt2 requires activation of p38.
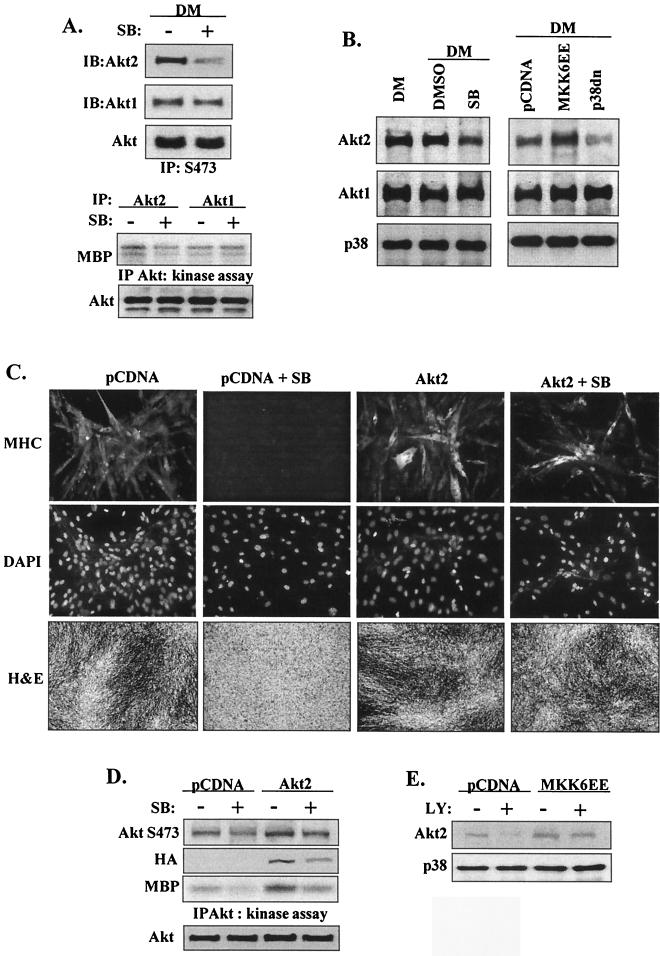
We next examined the relationship between p38 and the activation of Akt1 and Akt2 isoforms. In the presence of SB203580, Akt2 levels decreased in Akt phospho-S473 immunoprecipitates whereas there was no change in Akt1 levels (Fig. 8A). Further, Akt2 kinase activity exceeded Akt1 kinase activity after 72 h in DM and Akt2 kinase activity but not Akt1 kinase activity was inhibited by the presence of SB. The role of p38 was examined further by transfecting myoblasts with either p38dn or MKK6EE (Fig. 8B). In confirmation of the importance of p38 in the regulation of Akt2 protein levels, the presence of MKK6EE increased whereas that of p38dn decreased Akt2 protein levels but not Akt1 protein levels. These data suggest that in differentiating myoblasts, expression of Akt2, but not that of Akt1, requires activation of the p38 MAPK pathway.
FIG. 8.
Myogenic induction of Akt2 requires p38 activation. (A) Western blot analysis of Akt isoforms (Akt1 and Akt2) in myoblasts after 72 h in DM in the presence (+) or absence (−) of SB203580 (5 μM) following immunoprecipitation (IP) with immobilized AktS473-phosphospecific antibodies. Kinase activity of Akt1 and Akt2 in myoblasts after 72 h in DM in the presence or absence of SB203580 is shown in the lower panels. Total Akt levels were determined by Western blotting. IB, immunoblotting. (B) Akt2 expression is more sensitive to p38 inhibition than is Akt1. The results of Western blot detection of Akt1, Akt2, and total p38 in lysates (after 72 h in DM) from myoblasts treated with SB203580 or DMSO or transfected with either p38dn or MKK6EE are shown. (C) Differentiation (as assessed by immunofluorescence with anti-MHC antibody) (×20magnification) of myoblasts transfected with Akt2 in the presence or absence of SB203580 after 72 h in DM (upper panels). Cell nuclei were visualized by DAPI staining (middle panels), and cell morphology was examined by H and E staining (×5 magnification) (lower panels). (D) Akt S473 phosphorylation and Akt kinase activity (after 72 h in DM) in myoblasts transfected with Akt2 or pCDNA in the presence or absence of SB203580; total Akt protein levels and expression of HA-tagged Akt2 in the cell lysates are shown. (E) Western blot detection of Akt2 and total p38 levels (after 48 h in DM) in myoblasts transfected with MKK6EE or pCDNA in the presence or absence of LY294002 (10 μM).
We next investigated whether forced expression of Akt could overcome the inhibition of myogenesis induced by SB203580 inhibition of p38 activity (Fig. 1). Myoblasts were transfected with Akt1, Akt2, or empty vector and treated with SB. Even though SB completely prevented myotube formation and MHC expression, transfection with Akt2 could substantially overcome this effect (Fig. 8C) (though myoblast fusion was minimal). Interestingly, forced expression of Akt1 did not rescue MHC expression but did partially reverse SB-induced cell death (data not shown) (even though Western blotting for its myc tag indicated considerable overexpression). Even without taking into account differences in transfection efficiency, overexpression of Akt2 was able to largely compensate for the decreased total AktS473 phosphorylation and kinase activity observed with SB-treated myoblasts (Fig. 8D). These data imply that Akt2 can rescue some, though not all (as myoblast fusion was not normal), of the effects of p38 inhibition on myogenesis. This conclusion is in marked contrast with the results seen with the reciprocal study (presented in Fig. 6E and F), in which activation of the p38 pathway apparently could not overcome the effects of PI 3-kinase inhibition of myoblast differentiation.
Since our data suggest that Akt2 is the key Akt isoform in myoblast differentiation and since its protein levels appear to be regulated by p38, we investigated whether overexpression of MKK6EE could stimulate increased Akt2 protein levels when PI 3-kinase activity is inhibited by LY. The samples presented initially as described for Fig. 6 were used to determine Akt2 levels (Fig. 8E). MKK6EE could indeed stimulate increased Akt2 levels in the presence of LY; when the differences in MKK6EE transfection efficiency induced by LY are taken into account, this increase exceeded the modest changes in AktS473 phosphorylation seen with the equivalent samples whose results are presented in Fig. 6F. These observations imply that MKK6EE-stimulated pathways could be largely responsible for Akt2 protein levels but that additional PI 3-kinase-dependent signaling is required for activation of Akt.
Transactivation of Akt2 is mediated by the p38 MAPK pathway.
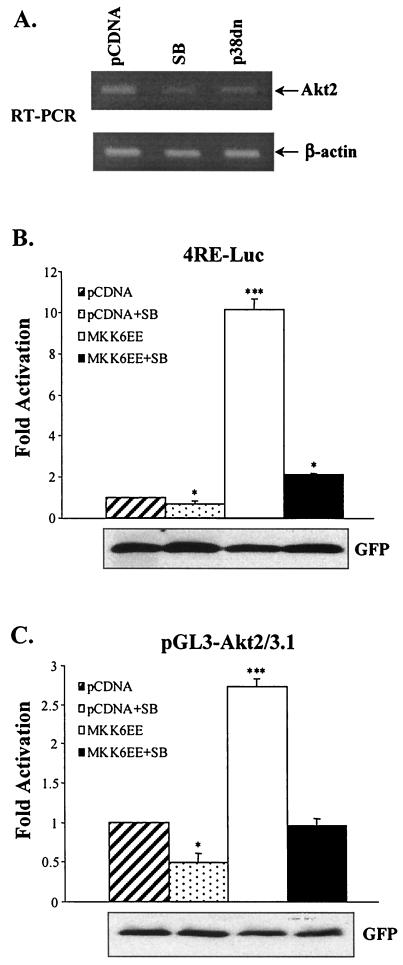
As the data presented in Fig. 8 indicated that p38 has a role in determining Akt2 protein levels, the effects of the presence of either SB or p38dn on Akt2 mRNA levels were investigated using RT-PCR (Fig. 9A). Both SB and p38dn decreased Akt2 mRNA levels. Therefore, the role of p38 MAPK in Akt2 gene transcription was examined using luciferase reporter assays with the Akt2 promoter-reporter (pGL3-AKT2/3.1). The promoter sequence of the pGL3-AKT2/3.1 reporter corresponds to bases −2898 to +220 of the Akt2 gene, which contains multiple binding sites for MyoD; MyoD transactivation of the Akt2 promoter and Akt2 expression during myogenesis was observed recently (30). Deliberate p38 MAPK activation was achieved by cotransfection of the constitutively active p38 activator (MKK6EE) in the presence or absence of SB203580. Since p38 may stimulate MyoD transcriptional activity (60), we also performed transcription assays with a luciferase reporter containing four MyoD binding sites or E boxes (4RE-Luc). In agreement with the results presented in previous reports, SB203580 inhibited the induction of 4RE-Luc activity by DM and MKK6EE enhanced MyoD-dependent activation of 4RE-Luc in an SB203580-sensitive manner (Fig. 9B). Cotransfection of pGL3-AKT2/3.1 with MKK6EE resulted in a marked increase in activation of the Akt2 reporter compared with the results seen with control cells cotransfected with reporter and control vector (Fig. 9C). Furthermore, SB203580 significantly reduced the activation of pGL3-AKT2/3.1 mediated either by DM or by MKK6EE. Thus, activation of the Akt2 promoter provides a mechanism by which p38 mediates increased Akt activity in myogenesis.
FIG. 9.
Activation of p38 stimulates Akt2 transcription. (A) Akt2 mRNA levels (as determined by RT-PCR after 72 h in DM) in myoblasts transfected with p38dn and pCDNA in the presence or absence of SB203580; the results of RT-PCR of β-actin are shown as a control for PCRs. (B) Activation of p38 enhances myogenic transcription of 4RE-Luc, the MyoD-dependent promoter. Myoblasts were cotransfected with the 4RE-Luc reporter and constitutively active MKK6EE or control vector (pCDNA); cells were grown for 24 h in GM and then shifted to DM for another 36 h in the presence or absence of SB203580 (5 μM), after which luciferase activity levels were determined. Transfection efficiency was determined by cotransfection with an expressionvector for GFP and by counting the proportion of GFP-positive cells; GFP was also detected by Western immunoblotting. Severalfold activation levels are expressed as the ratio of luciferase activity in MKK6EE-transfected cells to that in cells transfected with pCDNA; values were normalized to the relative GFP expression levels per unit of protein. (C) p38 stimulates MyoD-dependent transcription of the Akt-2 promoter. Cells were transfected with the pGL3-Akt2/3.1 luciferase reporter as described for panel B. ***, P < 0.001; * P < 0.05 (compared with an activation value of 1.0).
Activation of p38 requires the PI 3-kinase pathway: bidirectional regulation between PI 3-kinase and p38 MAPK pathways.
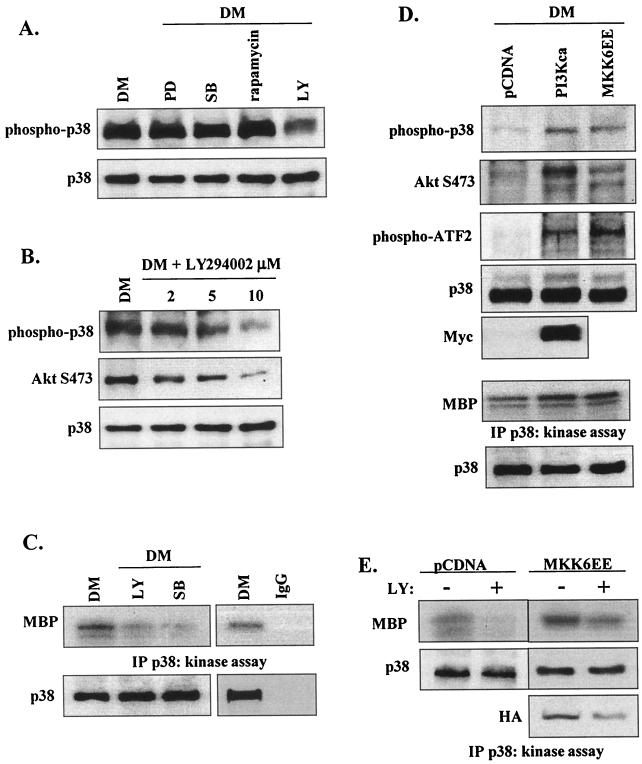
The signaling pathways upstream of p38 MAPK are not defined in myogenesis. We examined the effect of kinase inhibitors on p38 phosphorylation; phosphorylation of p38 was not affected by PD98059, SB203580, or rapamycin, whereas LY294002 markedly reduced p38 phosphorylation in a dose-dependent manner (Fig. 10A and B). Moreover, in vitro kinase assays revealed that LY294002 could substantially inhibit p38 kinase activity (Fig. 10C).
FIG. 10.
PI 3-kinase is required for p38 activation in differentiating myoblasts. (A) Western blot detection of phospho-p38 and total p38 in the presence of PD98059 (20 μM), SB203580 (5 μM), rapamycin (2 ng/ml), and LY294002 (10 μM) after 48 h in DM. (B) Western blot detection of phospho-p38, phospho-AktS473, and total p38 in the presence of increasing concentrations of LY294002 in myoblasts after 48 h in DM. (C) p38 kinase activity in myoblasts after 48 h in DM in the presence or absence of LY294002 (10 μM) and SB203580 (5 μM); total p38 levels were determined by Western blotting (lower panel). Nonspecific phosphorylation was assessed using a non-p38-binding IgG in place of the anti-p38 antiserum. IP, immunoprecipitation. (D) Western blot detection of phospho-p38, phospho-AktSer473, and phospho-ATF-2 after 48 h in GM in myoblasts transfected with constitutively active PI 3-kinase (PI 3Kca), MKK6EE, or pCDNA (upper three panels). Total p38 protein levels are shown as a loading control; the presence of PI 3Kca was detected by Western blotting of its Myc tag. p38 kinase activity and total p38 levels were determined (lower two panels). (E) p38 kinase activity in myoblasts transfected with MKK6EE in the presence (+) or absence (−) of LY294002 (10 μM) after 48 h in DM. Total p38 levels were determined by Western blotting with anti-p38 antibody; expression of HA-tagged MKK6EE was determined by Western blotting.
To verify that LY294002 effects on p38 activation are PI 3-kinase-mediated events, myoblasts were transiently transfected with a constitutively activated PI 3-kinase (PI 3Kca) (expression was verified by expression of its Myc tag) (Fig. 10D); MKK6EE-transfected cells were used as a positive control. In comparison with the results seen with control vector (pCDNA), PI 3Kca and MKK6EE markedly induced p38 phosphorylation (Fig. 10D). In addition to increasing p38 phosphorylation, PI 3Kca (as expected) enhanced AktS473 phosphorylation. Furthermore, PI 3Kca increased phosphorylation of ATF-2 (a known downstream target of p38) and p38 kinase activity (Fig. 10D), supporting the suggestion that PI 3-kinase facilitates p38 function in myoblasts and confirming bidirectional cross-talk between PI 3-kinase/Akt and p38 MAPK.
To complement the studies that demonstrated that MKK6EE activation of p38 could not overcome the inhibition of myogenesis induced by blocking PI 3-kinase activity (Fig. 6E and F), the effects of MKK6EE expression on p38 activation in the presence of LY was investigated in those samples. Even without taking into account the reduced transfection efficiency caused by the presence of LY, p38 kinase activity was restored by MKK6EE to almost pCDNA levels in LY-treated cells, indicating that the level of PI 3-kinase regulation of the p38 pathway is controlled upstream of MKK6. Overall, these findings and those presented in Fig. 6 suggest that PI 3-kinase acts at multiple levels in myogenesis, regulating p38 pathway activation mechanisms upstream as well as downstream of p38 in terms of Akt2 phosphorylation.
DISCUSSION
These studies further characterized the important role of the PI 3-kinase and p38 MAPK pathways in myogenesis, revealing a reciprocal cross-talk and activation that is essential for efficient myoblast differentiation. We demonstrate that Akt is differentially phosphorylated during myogenesis and that its kinase activity only increases when both sites are phosphorylated. Whereas phosphorylation of AktT308 is relatively constant during myogenesis, AktS473 phosphorylation changes dramatically and has a key role in determining Akt kinase activity. Our findings also highlight a key role for Akt2 and not Akt1 in myoblast differentiation. Overall Akt2 activity was sensitive to both p38 and PI 3-kinases but via different mechanisms. Whereas p38 regulates transcription from the Akt2 gene, PI 3-kinase appears essential for subsequent Akt2 phosphorylation. Complementary to p38-mediated transactivation of Akt2, PI 3-kinase also regulated p38 activity upstream of MKK6, demonstrating bidirectional communication and positive feedback characteristic of myogenic regulation. Further, Kaneko et al. (30) have demonstrated that Akt2 (once activated) can feed forward and activate its own promoter, thus providing a mechanism for sustained Akt activation.
The idea of communication between PI 3-kinase and p38 MAPK in myogenesis has been excluded in previous studies (39, 52, 60), which concluded that the pathways were independent and parallel and exhibited no cross-activation or cross-inhibition. In support of this, cooperative but independent signaling by PI 3-kinase and p38 has been observed in other systems (see, e.g., reference 41). Our observations suggest that activation is strictly temporally regulated, with p38 activation preceding increased Akt kinase activity by 24 h; it is therefore possible that cross-activation only occurs at specific time points. Given this time delay in p38 and Akt activation, it is difficult to reconcile this idea with a hypothesis according to which p38 directly activates Akt. However, our observation that a key role of p38 is that of stimulating Akt transcription is compatible with these temporal observations.
The embryonic death of p38α-null mice demonstrates the importance of p38 in development and that other p38 isoforms cannot compensate (5, 53). Targets for p38 include the key myogenic transcription factors MyoD (46, 60) and MEF2 (66) and the structural protein caveolin-3 (which is essential for myoblast fusion) (24). The p38 kinase target MK2 and p38 itself mutually enhance each other's functionality: MK2 activity is very much reduced in p38α-null mice (5), and MK2 stabilizes p38 and can dictate its cellular localization (31). However, the precise role of each of the p38 isoforms is not well defined. The inhibition of myoblast differentiation by SB203580 indicates that the p38α and p38β isoforms are important, and microarray analysis has defined MyoD- and p38α/p38β-activated genes in myogenesis (11); use of SB203580-resistant forms of p38α and p38β demonstrated that both are required for maximal induction of myogenic target genes (39). Further, the p38γ isoform may also induce myoblast differentiation (37). Each isoform may therefore exhibit specific temporal or spatial expression or may activate nonoverlapping downstream targets. For example, p38α and p38β are more effective than the p38γ and p38δ isoforms in MEF2A and C phosphorylation (63) but the p38γ and p38δ isoforms have been reported to be more efficient at inducing myogenic differentation of myoblasts cotransfected with MyoD (65). It has also been suggested that p38α and p38β have opposing roles with respect to effects on apoptosis (reviewed in reference 42). Even though the dominant-negative p38 construct used in the study presented here was a p38α sequence, it may well have sequestered key p38 partner molecules (e.g., scaffold proteins) and inhibited activity of other isoforms. In theory, high-level expression of MKK6EE would activate all isoforms (6). Therefore, our studies confirm the importance of p38α and p38β but have not excluded functions for p38γ and p38δ in myogenesis.
In terms of Akt activation, the identity of the kinase responsible for AktT308 phosphorylation (PDK1) is unequivocally established whereas that for AktS473, the putative PDK2, has not been determined. It is not known whether a novel kinase remains to be cloned or whether existing kinases have the role of AktS473 phosphorylation. Alessi et al. (4) first demonstrated that MK2 was capable of phosphorylating AktS473 in cell extracts. Rane et al. (47) confirmed p38 MAPK-mediated phosphorylation of AktS473 in stimulated neutrophils and coimmunoprecipitation of Akt with p38, MK2, and Hsp27. Even though our study strongly supports the idea of a p38-mediated induction of Akt2 activity in myogenesis via transcriptional mechanisms, we do not have evidence that the p38 or its targets are acting as a PDK2. Our data do suggest that once Akt2 is synthesized, appropriate PDK2-like mechanisms exist in differentiating myoblasts that are highly PI 3-kinase dependent and appear to be more active towards Akt2 than Akt1. As far as we are aware, the specificity of PDK1 or putative PDK2 enzymes for different Akt isoforms has not been investigated. Related observations of ovarian cancer cells (in which lysophosphatidic acid and sphingosine-1-phosphate induce coordinated AktS473 and AktT308 phosphorylation) support the idea of p38-mediated activation of Akt (9); regulation of AktS473 phosphorylation was p38 dependent but (in marked contrast to the results seen with myoblasts) also required MEK activation, which inhibits myogenesis. Inhibition (using SB203580 blockage of p38 activation) of cardiac myocyte differentiation did not reduce PI 3-kinase activity (17); Akt activity was not determined, but these observations are not incompatible with p38 regulation of the PI 3-kinase pathway downstream of PI 3-kinase itself.
Additional candidates besides p38 MAPK exist for the role of PDK2. For example, stress activation of Akt is independent of p38 (50). Insulin also induces Akt phosphorylation that is not dependent on p38 activity, and MK2 activity is minimally stimulated by insulin (4, 9). ILK has been proposed as a possible PDK2 in prostate cancer cells (45) and in immortalized macrophages (55), and insulin indeed stimulates ILK-mediated phosphorylation of AktS473 (20). Transfection of myoblasts with dominant-negative ILK partially inhibited AktS473 phosphorylation (I. Gonzalez and J. M. Pell, unpublished observations), indicating that this might be a mechanism for Akt phosphorylation during myogenesis. One proposal is therefore that several kinases exist that can assume a PDK2-like function and whose specificity is stimulus or cell dependent or is dictated by a distinct affinity for a particular Akt isoform. Alternatively, it is possible that a single PDK2-type enzyme exists but that several phosphatases differentially regulate the AktS473 site.
Of the three Akt isoforms (Akt1, Akt2, and Akt3), Akt2 is highly expressed in insulin-responsive tissues such as skeletal muscle, heart, liver, and adipose tissue (7). Akt2 protein and mRNA levels specifically increase during myoblast and adipocyte differentiation (7, 12, 23), suggesting that Akt2 rather than Akt1 is important during myoblast differentiation in physiological conditions. Studies in vivo and in vitro have demonstrated that Akt1 and Akt2 have different functions and do not compensate for each other. Akt1-null mice exhibit modest growth retardation and increased susceptibility to apoptotic stimuli (14, 15), whereas Akt2-null mice grow normally but develop type II diabetes (16). Akt1 and Akt2 double-null mice die at birth and exhibit severe muscle hypoplasia (43). In consistency with these observations, microinjection of myoblasts with antibodies specific to either Akt2 or Akt1 demonstrated that Akt1 is important for cell cycle progression whereas Akt2 stimulated myoblast differentiation (57). Akt2, but not Akt1, localizes to the nuclei of myoblasts (40, 57), and IGFs can stimulate nuclear localization of wild-type Akt (8). This finding casts doubt on the validity of studies conducted using constitutively active myristoylated Akt (which may remain membrane bound) (8). p21 phosphorylation status (48) and localization (67) may be determined by Akt localization, with cytoplasmic p21 stimulating cell survival and nuclear p21 inducing cell cycle withdrawal. Finally, the Akt2 promoter (but not the Akt1 promoter) contains multiple E boxes that are transactivated by MyoD, leading to downstream activation of MEF2 proteins and the formation of highly transcriptionally active MyoD/MEF2 complexes (30). These recent findings suggest a role for Akt2 rather than Akt1 in myogenesis. Our data support and extend this hypothesis, suggesting differential roles for Akt isoforms and demonstrating a novel functional link between the PI 3-kinase and p38 pathways, Akt2 transcription, and Akt activity in myoblast differentiation.
Our findings suggest not only p38 transactivation of Akt2 but also reciprocal activation of p38 MAPK pathways upstream of MKK6 by PI 3-kinase. Thus, constitutively active PI 3-kinase stimulated p38 phosphorylation and kinase activity. Communication between PI 3-kinase and p38 in myogenesis is controversial; increases in p38 activation independent of PI 3-kinase activation have been reported for skeletal myoblasts (52, 60), but PI 3-kinase-regulated p38 activity has been observed in cardiac myoblasts (17). It has been suggested that PI 3-kinase can activate p38 via Rac/Cdc42 (54); further, PI 3-kinase is activated via E-cadherin-mediated cell-cell contact in enterocytes, activating p38 and stimulating differentiation (34).
In conclusion, we have revealed novel links between the p38 MAPK and the PI 3-kinase/Akt pathways that occur at several levels. PI 3-kinase can regulate activity of the p38 MAPK pathway (which then induces transactivation of the Akt2 promoter). Subsequent Akt2 kinase activity is mainly determined by phosphorylation of S473 and is PI 3-kinase dependent. Intriguingly, both Akt transcription and activation mechanisms appear to be specific for Akt2 but not for Akt1. We thus demonstrate reciprocal feedforward activation mechanisms that are characteristic of myogenic regulation.
Acknowledgments
This work was funded by a Competitive Strategic Grant to The Babraham Institute from the Biotechnology and Biological Sciences Research Council.
We thank Simon Cook and Len Stephens (The Babraham Institute) for critical evaluation of the manuscript.
REFERENCES
- 1.Alessi, D. R., and P. Cohen. 1998. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 8:55-62. [DOI] [PubMed] [Google Scholar]
- 2.Alessi, D. R., M. Deak, A. Casamayor, F. B. Caudwell, N. Morrice, D. G. Norman, P. Gaffney, C. B. Reese, C. N. MacDougall, D. Harbison, A. Ashworth, and B. Bownes. 1997. 3-Phosphoinositide-dependent kinase 1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 7:776-789. [DOI] [PubMed] [Google Scholar]
- 3.Alessi, D. R., F. B. Caudwell, M. Andjelkovic, B. A. Hemmings, and P. Cohen. 1996. Molecular basis for substrate specificity of protein kinase B: comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett. 399:333-338. [DOI] [PubMed] [Google Scholar]
- 4.Alessi, D. R., M. Andjelkovic, B. Caudwell, P. Cron, N. Morrice, P. Cohen, and B. A. Hemmings. 1996. Mechanism of activation of protein kinase B by insulin and IGF-I. EMBO J. 15:6541-6551. [PMC free article] [PubMed] [Google Scholar]
- 5.Allen, M., L. Svensson, M. Roach, J. Hambar, J. McNeish, and G. A. Gabel. 2000. Deficiency of the stress kinase p38α results in embryonic lethality: characterization of the kinase dependence of stress responses of enzyme-deficient embryonic stem cells. J. Exp. Med. 191:859-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso, G., C. Ambrosino, M. Jones, and A. R. Nebrada. 2000. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J. Biol. Chem. 275:40641-40648. [DOI] [PubMed] [Google Scholar]
- 7.Altomare, D. A., G. E. Lyons, Y. Mitsuuchi, J. Q. Cheng, and J. R. Testa. 1998. Akt2 mRNA is highly expressed in embryonic brown fat and the Akt2 kinase is activated by insulin. Oncogene 16:2407-2411. [DOI] [PubMed] [Google Scholar]
- 8.Andjelkovic, M., D. R. Alessi, R. Meier, A. Fernandez, N. J. C. Lamb, M. Frech, P. Cron, P. Cohen, J. M. Lucocq, and B. A. Hemmings. 1997. Role of translocation in the activation and function of protein kinase B. J. Biol. Chem. 272:31515-31524. [DOI] [PubMed] [Google Scholar]
- 9.Baudhuin, L. M., K. L. Kristina, J. Lu, and Y. Xu. 2002. Akt activation induced by lysophosphatidic acid and sphingosine-1-phosphate requires both mitogen-activated protein kinase kinase and p38 mitogen-activated protein kinase and is cell-line specific. Mol. Pharmacol. 62:660-672. [DOI] [PubMed] [Google Scholar]
- 10.Belham, C., M. J. Comb, and J. Avruch. 2001. Identification of the NIMA family kinases NEK6/7 as regulators of the p70 ribosomal S6 kinase. Curr. Biol. 11:1155-1167. [DOI] [PubMed] [Google Scholar]
- 11.Bergstrom, D. A., B. H. Penn, A. Strand, R. L. S. Perry, M. A. Rudnicki, and S. J. Tapscott. 2002. Promoter-specific regulation of MyoD binding and signal transduction cooperate to pattern gene expression. Mol. Cell 9:587-600. [DOI] [PubMed] [Google Scholar]
- 12.Calera, M., and P. F. Pilch. 1998. Induction of Akt2 correlates with differentiation in Sol8 muscle cells. Biochem. Biophys. Res. Commun. 251:835-841. [DOI] [PubMed] [Google Scholar]
- 13.Casanovas, O., F. Miro, J. M. Estanyol, E. Itarte, N. Agell, and O. Bachs. 2000. Osmotic stress regulated the stability of cyclin D1 in a p38SAPK2-dependent manner. J. Biol. Chem. 275:35091-35097. [DOI] [PubMed] [Google Scholar]
- 14.Chen, W. S., P.-Y. Xu, K. Gottlob, M.-L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadwaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho, H., J. L. Thorvaldson, Q. Chu, F. Feng, and M. J. Birnbaum. 2001. Akt1/PKBα is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 276:38349-38352. [DOI] [PubMed] [Google Scholar]
- 16.Cho, H., J. Mu, J. K. Kim, J. L. Thorvaldsen, Q. Chu, E. B. Crenshaw III, K. H. Kaestner, M. S. Bartolmei, G. I. Shulman, and M. J. Birnbaum. 2001. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKBβ). Science 292:1728-1731. [DOI] [PubMed] [Google Scholar]
- 17.Chun, Y. K., J. Kim, S. Kwon, S. H. Choi, F. Hong, K. Moon, J. M. Kim, S. L. Choi, B. S. Kim, J. Ha, and S. S. Kim. 2000. Phosphatidylinositol 3-kinase stimulates muscle differentiation by activating p38 mitogen-activated protein kinase. Biochem. Biophys. Res. Commun. 276:502-507. [DOI] [PubMed] [Google Scholar]
- 18.Coolican, S. A., D. S. Samuel, D. Z. Ewton, F. J. McWade, and J. R. Florini. 1997. The mitogenic and myogenic actions of the insulin-like growth factors utilize distinct signalling pathways. J. Biol. Chem. 272:6653-6662. [DOI] [PubMed] [Google Scholar]
- 19.Cuenda, C., and P. Cohen. 1999. Stress-activated protein kinase-2/p38 and a rapamycin-sensitive pathway are required for C2 myogenesis. J. Biol. Chem. 274:4341-4346. [DOI] [PubMed] [Google Scholar]
- 20.Delcommenne, M., C. Tan, V. Gray, L. Rue, J. Woogett, and S. Dedhar. 1998. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/Akt by the integrin-linked kinase. Proc. Natl. Acad. Sci. USA 95:11211-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frech, M., M. Andjelkovic, E. Ingley, K. K. Reddy, J. R. Falck, and B. A. Hemmings. 1997. High affinity binding of inositol phosphates and phosphoinositides to the pleckstrin homology domain of RAC/protein kinase B and their influence on kinase activity. J. Biol. Chem. 272:8474-8481. [DOI] [PubMed] [Google Scholar]
- 22.Fujio, Y., K. Guo, T. Mano, Y. Mitsuuchi, J. R. Testa, and K. Walsh. 1999. Cell cycle withdrawal promotes myogenic induction of Akt, a positive modulator of myocyte survival. Mol. Cell. Biol. 19:5073-5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujio, Y., Y. Mitsuuchi, J. R. Testa, and K. Walsh. 2001. Activation of Akt2 inhibits anoikis and apoptosis induced by myogenic differentiation. Cell Death Differ. 8:1207-1212. [DOI] [PubMed] [Google Scholar]
- 24.Galbiati, F., D. Volonte, J. A. Engelman, P. E. Scherer, and M. P. Lisanti. 1999. Targeted down-regulation of caveolin-3 is sufficient to inhibit myotube formation in differentiating C2 myoblasts. Transient activation of p38 mitogen-activated protein kinase is required for induction of caveolin-3 expression and subsequent myotube formation. J. Biol. Chem. 274:30315-30321. [DOI] [PubMed] [Google Scholar]
- 25.Han, J., J.-D. Lee, L. Bibbs, and R. J. Ulevitch. 1994. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science 265:808-811. [DOI] [PubMed] [Google Scholar]
- 26.Jiang, B.-H., J. Z. Zheng, and P. K. Vogt. 1998. An essential role of phosphatidylinositol 3-kinase in myogenic differentiation. Proc. Natl. Acad. Sci. USA 95:14179-14183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang, B.-H., M. Aoki, J. Z. Zheng, J. Li, and P. K. Vogt. 1999. Myogenic signalling of phosphatidylinositol 3-kinase requires the serine-threonine kinase Akt/protein kinase B. Proc. Natl. Acad. Sci. USA 96:2077-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaliman, P., F. Viñals, X. Tester, M. Palacin, and A. Zorzano. 1996. Phosphatidylinositol 3-kinase inhibitors block differentiation of skeletal muscle cells. J. Biol. Chem. 271:19146-19151. [DOI] [PubMed] [Google Scholar]
- 29.Kaliman, P., J. Canicio, P. R. Shepher, C. A. Beeton, X. Testar, M. Palacin, and A. Zorzano. 1998. Insulin-like growth factors require phosphatidylinositol 3-kinase to signal myogenesis: dominant negative p85 expression blocks differentiation of L6E9 muscle cells. Mol. Endocrinol. 12:66-77. [DOI] [PubMed] [Google Scholar]
- 30.Kaneko, S., R. I. Feldman, L. Yu, Z. Wu, T. Gritsko, S. A. Shelley, S. V. Nicosia, T. Nobori, and J. Q. Cheng. 2002. Positive feedback regulation between Akt2 and MyoD during muscle differentiation: cloning of the Akt2 promoter. J. Biol. Chem. 277:23230-23235. [DOI] [PubMed] [Google Scholar]
- 31.Kotlyarov, A., Y. Yannoni, S. Fritz, K. Laass, J.-B. Teliez, D. Pitman, and M. Gaestel. 2002. Distinct cellular function of MK2. Mol. Cell. Biol. 22:4827-4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lali, F. V., A. E. Hunt, S. J. Turner, and B. M. J. Foxwell. 2000. The pyridinyl imidazole inhibitor SB203580 blocks phosphoinositide-dependent protein kinase activity, protein kinase B phosphorylation, and retinoblastoma hyperphosphorylation in interleukin-2-stimulated T cells independently of p38 mitogen-activated protein kinase. J. Biol. Chem. 275:7395-7402. [DOI] [PubMed] [Google Scholar]
- 33.Langley, B., M. Thomas, A. Bishop, M. Sharma, S. Gilmour, and R. Kambadur. 2002. Myostatin inhibits myoblast differentiation by down-regulating MyoD expression. J. Biol. Chem. 277:49831-49840. [DOI] [PubMed] [Google Scholar]
- 34.Laprise, P., P. Chailler, M. Houde, J.-F. Beaulieu, M.-J. Boucher, and N. Rivard. 2002. Phosphatidylinositol-3-kinase controls human intestinal epithelial cell differentiation by promoting adherens junction assembly and p38 MAPK activation. J. Biol. Chem. 277:8226-8234. [DOI] [PubMed] [Google Scholar]
- 35.Lawlor, M. A., and P. Rotwein. 2000. Insulin-like growth factor-mediated muscle cell survival: central roles for Akt and cyclin-dependent kinase inhibitor p21. Mol. Cell. Biol. 20:8983-8995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lawlor, M. A., X. Feng, D. R. Everding, K. Sieger, C. E. H. Stewart, and P. Rotwein. 2000. Dual control of muscle cell survival by distinct growth factor-regulated signaling pathways. Mol. Cell. Biol. 20:3256-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lechner, C., M. A. Zahalka, J.-F. Giot, N. P. H. Moller, and A. Ulrich. 1996. ERK6, a mitogen-activated protein kinase involved in C2 myoblast differentiation. J. Biol. Chem. 93:4355-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee, J. C., J. T. Laydon, P. C. McDonnell, T. F. Gallagher, S. Kumar, D. Green, D. McNulty, M. J. Blumenthal, R. J. Hayes, S. W. Landvatter, J. E. Strickler, M. M. McLaughlin, I. Siemens, S. Fisher, G. P. Livi, J. R. White, J. L. Adams, and P. R. Young. 1994. Identification and characterization of a novel protein kinase involved in regulation of inflammatory cytokine biosynthesis. Nature 372:739-746. [DOI] [PubMed] [Google Scholar]
- 39.Li, Y., B.-H. Jiang, W. Y. Ensign, P. K. Vogt, and J. Han. 2000. Myogenic differentiation requires signalling through both phosphatidylinositol 3-kinase and p38 MAP kinase. Cell. Signal. 12:751-757. [DOI] [PubMed] [Google Scholar]
- 40.Meier, R., D. R. Alessi, P. Cron, M. Andjelkovic, and B. A. Hemmings. 1997. Mitogenic activation, phosphorylation, and nuclear translocation of protein kinase Bβ. J. Biol. Chem. 272:30491-30497. [DOI] [PubMed] [Google Scholar]
- 41.Ming, X.-F., G. Stoeklin, M. Lu, R. Looser, and C. Moroni. 2001. Parallel and independent regulation of interleukin-3 mRNA turnover by phosphatidylinositol 3-kinase and p38 mitogen-activated protein kinase. Mol. Cell. Biol. 21:5778-5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nebrada, A. R., and A. Porras. 2000. p38 MAP kinases: beyond the stress response. Trends Biochem. Sci. 25:257-260. [DOI] [PubMed] [Google Scholar]
- 43.Peng, X., P.-Z. Xu, M.-L. Chen, A. Hahn-Windgassen, J. Skeen, J. Jacobs, D. Sundararajan, W. S. Chen, S. E. Crawford, K. Coleman, and N. Hay. 2003. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 17:1352-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perry, R. L. S., and M. A. Rudnicki. 2000. Molecular mechanisms regulating myogenic determination and differentiation. Front. Biosci. 5:750-767. [DOI] [PubMed] [Google Scholar]
- 45.Persad, S., S. Attwell, V. Gray, N. Mawji, J. T. Deng, D. Leung, J. Yan, J. Sanghera, M. P. Walsh, and S. Dedhar. 2001. Regulation of protein kinase B/Akt-serine 473 phosphorylation by integrin-linked kinase: critical roles for kinase and amino acids arginine 211 and serine 343. J. Biol. Chem. 276:27462-27469. [DOI] [PubMed] [Google Scholar]
- 46.Puri, P. L., Z. Wu, P. Xhang, L. D. Wood, K. S. Bhakta, J. Han, J. R. Feramisco, M. Karin, and J. Y. J. Wang. 2000. Induction of terminal differentiation by constitutive activation of p38 MAP kinase in human rhabdomyosarcoma cells. Genes Dev. 14:574-584. [PMC free article] [PubMed] [Google Scholar]
- 47.Rane, M. J., P. Y. Coxon, D. W. Powell, R. Webster, J. B. Klein, W. Pierce, P. Ping, and K. R. McLeish. 2001. p38 kinase-dependent MAPKAPK-2 activation functions as 3-phosphoinositide-dependent kinase-2 for Akt in human neutrophils. J. Biol. Chem. 276:3517-3523. [DOI] [PubMed] [Google Scholar]
- 48.Rössig, L., A. S. Jadidi, C. Urbich, C. Badorff, A. M. Zeiher, and S. Dimmeler. 2001. Akt-dependent phosphorylation of p21CIP1 regulates PCNA binding and proliferation of endothelial cells. Mol. Cell. Biol. 21:5644-5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Scheid, M. P., P. A. Marignani, and J. R. Woodgett. 2002. Multiple phosphoinositide 3-kinase-dependent steps in activation of protein kinase B. Mol. Cell. Biol. 22:6247-6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shaw, M., P. Cohen, and D. R. Alessi. 1998. The activation of protein kinase B by H2O2 or heat shock is mediated by phosphoinositide-3-kinase and not by mitogen-activated protein kinase-activated protein kinase-2. Biochem. J. 336:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stokoe, D., L. R. Stephens, T. Copeland, P. R. Gaffney, C. B. Reese, G. F. Painter, A. B. Holmes, F. McCormick, and P. T. Hawkins. 1997. Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277:567-570. [DOI] [PubMed] [Google Scholar]
- 52.Tamir, Y., and E. Bengal. 2000. Phosphoinositide 3-kinase induces the transcriptional activity of MEF2 proteins during muscle differentiation. J. Biol. Chem. 275:34424-34432. [DOI] [PubMed] [Google Scholar]
- 53.Tamura, K., T. Sudo, U. Senttleben, A. Dadak, R. Johnson, and M. Karin. 2000. Requirement of p38α in erythropoietin expression: a role for stress kinases in erythropoiesis. Cell 102:221-231. [DOI] [PubMed] [Google Scholar]
- 54.Tang, Y., J. Yu, and J. Field. 1999. Signals from Ras, Rac, and Rho GTPases converge on the Pak protein kinase in Rat-1 fibroblasts. Mol. Cell. Biol. 19:1881-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Troussard, A. A., N. M. Mawji, C. Ong, A. Mui, R. St.-Arnaud, and S. Dedhar. 2003. Conditional knock-out of integrin-linked kinase demonstrates an essential role in protein kinase B/Akt activation. J. Biol. Chem. 278:22374-22378. [DOI] [PubMed] [Google Scholar]
- 56.Tureckova, J., E. M. Wilson, J. L. Cappalonga, and P. Rotwein. 2001. Insulin-like growth factor-mediated muscle differentiation. J. Biol. Chem. 276:39264-39270. [DOI] [PubMed] [Google Scholar]
- 57.Vandromme, M., A. Roche, R. Meier, G. Carnac, D. Besser, B. A. Hemmings, A. Fernandez, and N. J. C. Lamb. 2001. Protein kinase Bβ/Akt2 plays a specific role in muscle differentiation. J. Biol. Chem. 276:8173-8179. [DOI] [PubMed] [Google Scholar]
- 58.Wang, S., N. Nath, A. Minden, and S. Chellappan. 1999. Regulation of Rb and E2F by signal transduction cascades: divergent effects of JNK1 and p38 kinases. EMBO J. 18:1559-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams, M. R., J. S. Arthur, A. Balendran, J. van der Kaay, V. Poli, P. Cohen, and D. R. Alessi. 2000. The role of 3-phosphoinositide-dependent protein kinase 1 in activating AGC kinases defined in embryonic stem cells. Curr. Biol. 10:439-448. [DOI] [PubMed] [Google Scholar]
- 60.Wu, Z., P. J. Woodring, K. S. Bhakta, K. Tamura, F. Wen, J. R. Feramisco, M. Karin, J. Y. J. Wang, and P. L. Puri. 2000. p38 and extracellular signal-related kinases regulate the myogenic program at multiple stages. Mol. Cell. Biol. 20:3951-3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xu, Q., and Z. Wu. 2000. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signalling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J. Biol. Chem. 275:36750-36757. [DOI] [PubMed] [Google Scholar]
- 62.Yaffe, M. B., G. G. Leparc, J. Lai, T. Obata, S. Volinia, and L. C. Cantley. 2001. A motif-based profile scanning approach for genome-wide prediction of signalling pathways. Nat. Biotechnol. 19:348-353. [DOI] [PubMed] [Google Scholar]
- 63.Yang, S.-H., A. Galamis, and A. D. Sharrocks. 1999. Targeting of p38 mitogen-activated protein kinase to MEF2 transcription factors. Mol. Cell. Biol. 19:4028-4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yun, K., and B. Wold. 1996. Skeletal muscle determination and differentiation: story of a core regulatory network and its context. Curr. Opin. Cell Biol. 8:877-889. [DOI] [PubMed] [Google Scholar]
- 65.Zetser, A., E. Gredinger, and E. Bengal. 1999. p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation: participation of the MEF2C transcription factor. J. Biol. Chem. 274:5193-5200. [DOI] [PubMed] [Google Scholar]
- 66.Zhao, M., L. New, V. V. Kravchenko, Y. Kato, H. Gram, F. di Padova, E. N. Olsen, R. J. Ulevitch, and J. Han. 1999. Regulation of MEF2 transcription factor family by p38. Mol. Cell. Biol. 19:21-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhou, B. P., Y. Liao, W. Xia, B. Spohn, M.-H. Lee, and M.-C. Hung. 2001. Cytoplasmic localization of p21Cip1/WAF1 by Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat. Cell Biol. 3:245-252. [DOI] [PubMed] [Google Scholar]