Abstract
The etiology of most human diseases involves complicated interactions of multiple environmental factors with individual genetic background which is initially generated early in human life, for example, during the processes of embryogenesis and fetal development in utero. Early embryogenesis includes a series of programming processes involving extremely accurate time-controlled gene activation/silencing expressions, and epigenetic control is believed to play a key role in regulating early embryonic development. Certain dietary components with properties in influencing epigenetic processes are believed to have preventive effects on many human diseases such as cancer. Evidence shows that in utero exposure to certain epigenetic diets may lead to reprogramming of primary epigenetic profiles such as DNA methylation and histone modifications on the key coding genes of the fetal genome, leading to different susceptibility to diseases later in life. In this review, we assess the current advances in dietary epigenetic intervention on transgenerational human disease control. Enhanced understanding of the important role of early life epigenetics control may lead to cost-effective translational chemopreventive potential by appropriate administration of prenatal and/or postnatal dietary supplements leading to early disease prevention.
KEY WORDS: diet, embryogenesis, epigenetic, human diseases, prevention, transgenerational
INTRODUCTION
The mammalian embryogenesis process involves complicated regulations that are mainly controlled by genetic and epigenetic mechanisms. Early embryonic developmental processes are controlled by highly conserved and accurate developmental genetic/epigenetic programs leading to precisely time-controlled tissue-specific gene expression, global gene silencing, and subsequent diverse phenotypes between cells, organs, as well as individuals. Recent studies have shown that epigenetic regulations such as DNA methylation and histone modification play a crucial role on genomic reprogramming during early development including gametogenesis, embryogenesis, and fetal development (1–4). Aberrant chromatin modification can result in dysregulation of normal developmental processes such as X chromosome inactivation and genomic imprinting leading to various congenital disorders as well as other human diseases in later life (5–7). It is well known that epigenetic processes are frequently influenced by environmental factors. Nevertheless, the vulnerability to environmental exposure during embryogenesis provides an excellent opportunity to re-program epigenetic profiles leading to a beneficial outcome such as disease prevention in the offspring.
An emerging theme from recent studies focused on certain groups of botanic components with bioactive properties in influencing epigenetic processes has attracted considerable attentions. These bioactive components can be consumed as part of the human diet which collectively are referred to as the “epigenetic diet” (8,9). Studies have shown that these particular diets are believed to have effects on prevention of various human diseases such as cancer and diabetes if proper dietary guidelines are followed. Many human foods contain epigenetic dietary components, such as genistein, a natural isoflavone in soybean products, sulforaphane (SFN), an isothiocyanate from cruciferous vegetables such as broccoli sprouts or cabbage, and (−)-epigallocatechin-3-gallate (EGCG), the major polyphenol in green tea, which have been found to be associated with a lower risk of developing many common cancers (10–12). These dietary epigenetic compounds are considered as potent dietary epigenetic modulators that affect key tumor-related gene expression through epigenetic regulation (13–16). In addition, some human epidemiological and animal studies have shown that early consumption of certain epigenetic diets may lead to phenotype alterations of the offspring through epigenetic modulations and therefore reduce the risk of developing certain diseases later in life (17–19). These results indicate that early-life consumption of certain diets may exhibit transgenerational effects leading to different individual health/disease outcomes later in life. Although studies on epigenetic mechanisms in dietary-associated transgenerational human disease prevention are just emerging, understanding the process of epigenetic reprogramming during embryogenesis and how maternal epigenetic dietary regimens affect this process will lead to beneficial health outcomes in the next generation.
EPIGENETIC REGULATIONS DURING EMBRYOGENESIS
DNA Methylation During Embryonic Development
DNA methylation is the best characterized epigenetic modification of chromatin. It is a stable and heritable component of epigenetic regulation that represents an important memory mechanism during embryogenesis. In mammals, DNA methylation primarily occurs on cytosine residues of CpG dinucleotides (20). A high density of CpGs frequently clustered into a certain region of genomic DNA is referred to CpG islands, and DNA methylation of these islands correlates with transcriptional repression (20). DNA methylation plays important roles during embryogenesis including genomic imprinting, regulating chromatin structure, maintaining gene expression, or silencing and X chromosome inactivation (1,2,20).
During early embryogenesis, a wave of genome-wide DNA methylation reprogramming is established. Firstly, both maternal and paternal chromosomes undergo progressive demethylation by a passive mechanism, which erases most of the epigenetic marks in the zygote (21). After implantation, global embryonic de novo methylation patterns are reestablished, which is then maintained throughout life in the somatic cells (21,22). This dynamic methylation reprogramming including global demethylation and remethylation processes is essential for fetal development during early embryogenesis. The genome-wide demethylation processes might lead to chromatin decondensation contributing to the transcriptional activation in the zygotic genes that are essential for early development. Subsequent de novo methylation processes might facilitate development of gene-specific methylation patterns, which determine tissue-specific transcription through a global silencing state. Although most genomic DNA undergoes genome-wide demethylation and de novo methylation processes during early embryogenesis, the methylation marks on imprinted genes escape from this prevailing reprogramming and thus are preserved as parental imprints leading to the differential expression of several dozen imprinted genes in the paternal and maternal alleles during development (20,23). Therefore, incorrect development of DNA methylation patterns during this critical period may lead to embryonic lethality, developmental malformations, and increased risk for certain diseases (4,24).
Maintaining DNA methylation patterns is dynamically mediated by at least three independent DNA methyltransferases (DNMTs), DNMT1, DNMT3a, and DNMT3b, which are required for cellular differentiation during early embryonic development. DNMT1 maintains genomic methylation patterns in a DNA replication-dependent manner, while DNMT3a and DNMT3b act primarily as de novo methyltransferases after DNA replication by adding a methyl moiety to the cytosine of CpG dinucleotides that are not previously methylated (25–29). Recent studies have found a new DNMT family member, DNMT3-like (DNMT3L), which encodes a protein that shares homology with DNMT3a and DNMT3b but lacks the highly conserved methyltransferase motifs and has no enzymatic activity (30). DNMT3L is believed to cooperate with DNMT3a and DNMT3b to regulate the gamete-specific methylation and genomic imprint (31).
Since DNA methylation plays important roles during early embryogenesis and development, appropriate exposure to epigenetic modulators from the diet that target DNA methylation reprogramming processes or DNMTs may lead to beneficial intervention of early epigenetic reprogramming and disease prevention in later life (Fig. 1).
Fig. 1.
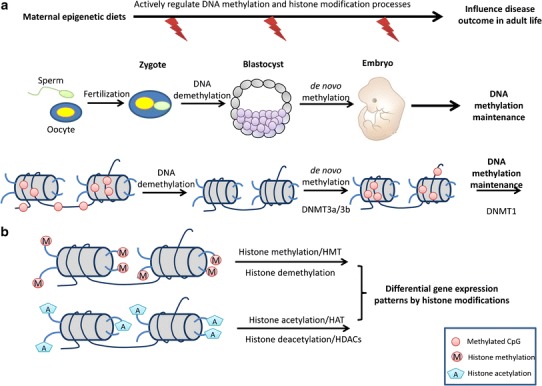
Maternal epigenetic diets regulate DNA methylation and histone modifications during embryogenesis. a DNA methylation reprogramming during early embryonic development. After fertilization, genomic DNA undergoes a passive demethylation process and parental DNA methylation markers are erased except imprinting genes. The methylation level of a blastocyst reaches the lowest point. After implantation, a genome-wide remethylation phase occurs through an active de novo methylation regulated by DNMT3a/3b. Cellular and organ-specific methylation patterns are maintained by DNMT1 throughout life in the somatic cells. b Histone modification during embryogenesis. Transcriptional regulators of cell differentiation lineages are mainly regulated by histone methylation and acetylation. Histone methylation is mediated by HMT, and either gene activation or repression by histone methylation is dependent upon the particular lysine residue that is modified. Histone acetylation is mediated by HAT and deacetylation is catalyzed by the HDAC family. Histone acetylation causes an open chromatin structure leading to active transcription, whereas histone deacetylation is always associated with transcriptional repression. DNMT DNA methyltransferases, HAT histone acetyltransferases, HDAC histone deacetylase, HMT histone methyltransferase
Histone Modifications During Embryonic Development
In addition to DNA methylation, changes in gene expression governed by the plasticity of chromatin add another layer of epigenetic control in embryogenesis (Fig. 1). The dynamic structure of chromatin is maintained by modification of core histones at their amino-terminal tails through adding molecular groups such as acetylation, phosphorylation, methylation, and ubiquitylation (32). Prior to fetal development, the zygotic genome is reprogrammed by changes in the epigenetic landscape mediated by key genes and histone marks that dictate correct lineage specification and terminal differentiation (33). Methylation of histone H3 lysine and arginine residues in conjunction with protein complexes such as trithorax (trxG) and polycomb (PcG) group influences the epigenetic landscape required for imprinting of genes and programming of cells (34–39). Trimethylation of histone H3 lysine 27 (H3K27me3) with PcG complex and trimethylation of histone H3 lysine 4 (H3K4me3) with trxG establish inactive and active chromatin states, respectively. Histone H3 lysine 9 acetylation and trimethylation (H3K9me3) constitute active and repressive marks, respectively (40,41). Transcriptional regulators of cell differentiation lineages are marked by H3K4me3 and are repressed in the presence of H3K27me3 in the embryonic stem cells (ESCs) (39,42). The progressive loss of H3K27me3 can activate these regulators that are marked by H3K4me3. Therefore, such bivalent marks regulate the differential potential of ESCs. Genes of pluripotent cells derived from the blastocyst require the activation of pluripotent factors: octamer-binding transcription factor 4 (Oct4), sex-determining region Y-box 2 (Sox2), and NanoG (43). However, monomethylation states of histone H3 lysine residues (H3K4me1, H3K9me1, and H3K27me1) direct changes from multipotent to differentiated unipotent cells; an example of this is the differentiation into erythrocyte precursors from human primary hematopoietic stem cells/progenitor cells (44). The epigenetic landscape changes as developmental and differentiation potentials decrease from totipotent to unipotent cell populations. Therefore, specific histone marks may correlate to defined embryonic stages governed by the expression or repression of pluripotent factors or lineage regulators.
IN UTERO EPIGENETIC DIET EXPOSURE
Aberrant patterns and dysregulation of DNA methylation and histone modifications may cause stable, heritable transcriptional abnormality of the associated gene during embryogenesis. Epigenetic variability appears to be susceptible to modulation by nutritional changes such as diets (45). Epigenetic diets refer to a broad range of diets that have been identified to mediate epigenetic processes (8,9). The epigenetic diets such as green tea, broccoli sprouts, and soybean and the bioactive compounds extracted from these diets have received extensive attention due to their profound actions on the prevention of various human diseases such as cancer, diabetes, and cardiovascular diseases by altering aberrant epigenetic profiles in cells (13–16,46). Recent studies have shown that epigenetic diets may also have an impact on developmental processes leading to disease prevention in later life (17–19) (Table I). Although the precise epigenetic mechanism-regulated developmental pathways and target genes are not fully understood, better understanding the effects of epigenetic diets on embryogenesis shows promising prospects in developing a novel maternal dietary regimen to prenatally prevent human diseases.
Table I.
Transgenerational Effects of Epigenetic Dietary Compounds
| Dietary components | Food resource | Epigenetic effects | Transgenerational effects | References |
|---|---|---|---|---|
| Folate | Leafy vegetables; egg yolk; liver products | Methyl donor diet (providing methyl group for SAM synthesis) | Folate insufficiency increased the risk for neural tube defects in the fetus and genome-wide DNA hypomethylation and genomic instability in agouti mice | (47–49,51,52) |
| Genistein | Soybean products | DNMT inhibitor; HDACs inhibitor | Maternal genistein may cause diverse effects of breast cancer development in later life; maternal genistein leads to genome-wide DNA hypermethylation in agouti mice | (61,66–68) |
| Sulforaphane | Cruciferous vegetables such as broccoli sprouts and cabbage | Potent HDAC inhibitor; DNMT inhibitor | Early-life consumption of cruciferous vegetables exhibits more effective prevention effects against cancers | (18,73) |
| EGCG | Green tea | Potent DNMT inhibitor; HDAC inhibitor | Maternal ingestion of green tea may provide transplacental protection against carcinogenesis and chronic diseases | (82) |
| Butyrate | Cheese | HDAC inhibitor | In utero exposure to butyrate delays the developmental switch from γ- to β-globin gene expression in sheep fetuses | (87) |
| Indole-3-carbinol | Cruciferous vegetables | Regulate microRNA expression | Maternal ingestion of indole-3-carbinol provides transplacental protection against carcinogen-induced lung cancer | (88) |
DNMT DNA methyltransferases, HDAC histone deacetylase, SAM S-adenosylmethionine
Folate
Folate, a water-soluble B vitamin, which must be obtained from dietary sources or supplements, is of fundamental importance for normal DNA synthesis and repair (47). Folate is essential for the transfer of one-carbon units and believed to act as a methyl donor diet for synthesis of S-adenosylmethionine (SAM), the universal methyl donor of biological methylation (48). Folate deficiency leads to a decrease in SAM and is associated to genome-wide hypomethylation both in humans and in animal models (45,48–51).
Evidence has indicated that folate deficiency is associated with developing several human tumors (50), which may be due to the abnormal process of DNA synthesis and methylation caused by low folate status. More importantly, it is well recognized that folate insufficiency during pregnancy increases the risk for development of NTDs in the fetus and may also influence risk for the other human diseases in later life owing to the critical role of folate in DNA methylation and synthesis of purines and pyrimidines (51). The agouti mouse model system has been successfully used to detect the methylation status in mammals when administrating folate-deficient dietary supplementation (52,53). The coat color of mice carrying the agouti viable yellow gene varies from yellow to mottle to pseudoagouti, which is dependent on the methylation status of the transgene. Studies have shown that agouti dams fed with methyl donor supplementation (folate, methionine, choline, and vitamin B12) during pregnancy prevent the ectopic expression of agouti protein due to the methylation of intra-cisternal A particle retrotransposon inserted upstream of the agouti gene. The expression of agouti results in yellow coat color, obesity, diabetes, and tumor susceptibility in the offspring and absence of the protein shifts towards the production of the pseudoagouti phenotype (54,55). Therefore, maternal diet enriched with methyl donors possibly through folate supplementation can have a significant effect on DNA methylation.
Although extensive evidence has indicated the vital roles of folate in regulation of epigenetic profiles during development in utero, studies on how folate supply during development affects chronic human diseases in later life are still under investigation. Thompson et al. have reported a case–control study indicating that folate supplementation in pregnancy reduces the risk of common acute lymphoblastic leukemia in the child (56). However, an animal study in sheep has shown periconceptional restriction of maternal folate intake leads to widespread changes in the fetal epigenome and the offspring are more resistant to insulin and have higher blood pressure by adult age (57). These results indicate that early-life exposure to folate may be beneficial to changing the susceptibility to certain human chronic diseases in adulthood.
Soybean Genistein
Soy products have been of particular interest because of their bioactive roles on inhibiting various human cancers and other chronic noncommunicable diseases. Genistein is a botanical isoflavone enriched in soybean products, such as soymilk and tofu (58). Genistein is believed to act as a phytoestrogen to compete with estradiol and interact with the ER-associated signal pathway (59). Epidemiological studies also indicate that the incidences of breast and prostate cancer in Asian countries, where the genistein-rich soy products are their traditional daily diet, are much lower than in the USA suggesting that genistein is a potent dietary chemopreventive compound against human breast and prostate cancers (59,60). Besides, its notable effects on suppressing carcinogenesis, early exposure to genistein in utero has recently been found to alter multiple phenotypes of the offspring of the agouti mice such as reduction of prevalence of obesity and brown coat due to hypermethylation of the agouti gene (61). This result suggests that soybean genistein can act as an epigenetic modulator to interfere with early epigenetic reprogramming leading to altered phenotypes such as different susceptibility to diseases in the offspring. Our work on genistein also confirmed that genistein can influence key gene expression and signal pathways through dynamic regulation of epigenetic pathways via both DNA methylation and histone modification pathways (13,14,62).
Although strong evidence has shown a clear correlation of postnatal consumption of soybean genistein with a low incidence of human cancer, an appropriate exposure window has been considered as a crucial factor for genistein to implement its effects on inhibiting tumorigenesis. For example, most existing literature suggests that consumption of soy isoflavone genistein during childhood and adolescence in women reduces later mammary cancer risk, whereas adult exposure to soy genistein is not protective but considered dangerous due to a weak estradiol character of genistein (63–65). There has also been controversy with respect to whether maternal (in utero) exposure to soybean genistein can actually affect breast cancer incidence of the offspring in adult life. The findings obtained in rats exposed to purified genistein during the fetal–perinatal period vary from study to study (66–68), indicating appropriate exposure window during pregnancy could be a key factor for transgenerational effects of genistein on mammary cancer prevention (Fig. 2). However, little evidence is available for a correlation of a maternal genistein diet to incidence of chronic noncommunicable diseases such as cardiovascular disease and diabetes. The result showing a change of obesity status in the offspring of the agouti mice fed with a maternal genistein diet (61) suggests that early exposure to genistein may influence adipogenesis process in later life; however, this speculation requires further investigation.
Fig. 2.
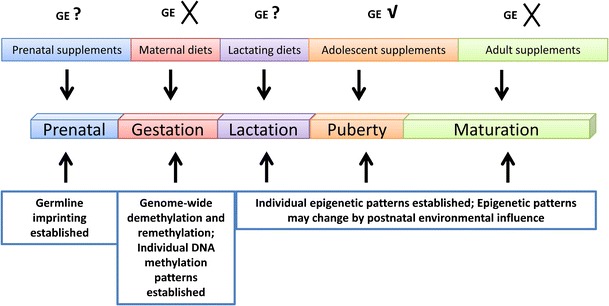
The timing of epigenetic diet exposure throughout life. The top row indicates the importance of different epigenetic dietary exposure that may affect the epigenome and have later-life consequences. For example, early consumption of genistein during childhood and adolescence in women reduces later mammary cancer risk, whereas maternal/adult exposure to GE may be considered to be a risk factor to induce breast cancer. The middle row represents different lifetime periods including prenatal, gestation, lactation, puberty, and maturation periods. The bottom row indicates important epigenetic events in different stages of life. GE genistein; check mark indicates potential protective effects of GE consumption on prevention of breast cancer; cross indicates the potential risk factors of GE consumption to the development of breast cancer; question mark indicates an unknown effect of GE consumption on breast cancer development
Cruciferous Vegetable Sulforaphane
SFN (CH3−SO−(CH2)4−N=C=S), an isothiocyanate from cruciferous vegetables such as broccoli sprouts, cabbage, and kale, is a common dietary natural plant product that can reduce the risk of developing many human cancers (11). SFN mediates chemoprevention through several mechanisms including cell cycle arrest, induction of apoptosis, and phase 2 detoxifying enzymes (69,70). Recent studies on investigation of epigenetic regulation by SFN in chemoprevention have drawn extensive attention due to its inhibitory property on histone deacetylase (HDAC) enzymatic activity (8,9,71) which leads to an increase in the global and local histone acetylation of a number of genes (15,72). In addition, both animal work and human studies indicate that SFN is a potent HDAC inhibitor and is readily bioavailable to prevent various human diseases.
Environmental factors play a major role in the interaction between genomic background and individual phenotypes through epigenetic regulations. Epidemiological studies have also suggested that early-life consumption of SFN through dietary cruciferous vegetables is more effective than later-life consumption (18,73). Asian populations who consume more cruciferous vegetables at earlier ages than Caucasians have less incidence of certain diseases such as breast cancer and prostate cancer, suggesting that maternal consumption of cruciferous vegetables during pregnancy may reduce the subsequent risk of cancer in their children (74). A recent study shows that in utero exposure to SFN prevents skin blistering associated with keratin 14 mutations in epidermolysis bullosa simplex, a rare inherited condition in which the epidermis loses its integrity after mechanical trauma (75). Our pilot study shows that maternal SFN diet can prevent the onset of breast tumorigenesis in a spontaneous breast cancer mouse model. Although the chemopreventive mechanisms for early exposure to broccoli sprout sulforaphane during pregnancy are currently not clear, the key characteristic of HDAC inhibitor of SFN may represent an attractive target for epigenetic reprogramming during embryogenesis. Further studies aimed towards elucidating the detailed mechanisms for how SFN interferes with early developmental processes are urgently needed.
Green Tea Polyphenols
Tea is consumed worldwide and has shown many beneficial effects on human health. EGCG, the most abundant polyphenol, is considered the most effective content for prevention of various human diseases in green tea (12). Green tea EGCG has been extensively studied as a bioactive dietary component against various types of human diseases such as cancer, cardiovascular diseases, hypertension, and hyperlipidemia through multiple mechanisms such as anti-oxidation and induction of apoptosis (76–78). Recently, studies indicate that EGCG can modulate gene expression by influencing epigenetic processes such as DNA methylation and/or histone modification (79–81). EGCG can alter DNA methylation patterns by directly and indirectly inhibiting the enzymatic activities of DNMTs. Furthermore, it is believed that EGCG-induced remodeling of chromatin structure is a key epigenetic mechanism for regulating tumor-related gene transcription (16,79).
However, there has been some controversy with respect to consumption of tea products during pregnancy due to the adverse effects of caffeine from the tea for fetal development. Recent studies, however, have proven that consumption of green tea in a small amount (<2 cups per day) is safe during pregnancy (82), and maternal ingestion of green tea during pregnancy and nursing may provide transplacental protection against carcinogenesis (82). Studies from Yang et al. have indicated that maternal green tea EGCG prevents hyperglycemia-induced embryonic vasculopathy and malformation via inhibition of oxidative stress signaling (83). A similar work by Long et al. indicated that consumption of green tea prevents ethanol-induced embryonic growth retardation (84). These studies provide insights that a diet rich in chemopreventive compounds consumed early in life may modulate the genetic/epigenetic profiles to confer beneficial effects against the risk of developing several types of diseases later in life. However, the epigenetic mechanisms for these changes and the role of green tea in these processes are not known.
Other Epigenetic Diets
Most in vitro and in vivo studies analyze the effect of single epinutrients on disease outcome. Maternal diet varies, and the altered state would depend on nutrient–nutrient interactions, nutrient–gene interactions, placental transport, and concentration. Therefore, the influence of epimolecules in embryonic development contributing to altered physiological and diseased states is complex. The impact of the epigenetic maternal diet on embryogenesis occurs at the pre-pregnancy and gestational stage, both of which influence in utero environment and availability of nutrients through the placenta. A study of female rats fed with a high-fat diet showed that changes in the uterine environment affected developmental outcomes, and in the study, impaired bone developmental was observed due to hypermethylation of homeobox protein Hox-A10 (HOX-A10) promoter and down-regulation of the gene (85). Diets that contain molecules that target histone deacetylases such as butyrate (cheese), diallyl sulfide (garlic), indole-3-carbinol (cruciferous vegetables), and histone acetylases such as curcumin (turmeric, an Asian spice) can potentially influence histone markers, thus affecting embryological development and further different risk against diseases (86–89). Therefore, the time of consumption of foods rich in the aforementioned biomolecules and the placental availability of these compounds determine the phenotypic outcome during embryogenesis.
TRANSGENERATIONAL PREVENTION OF HUMAN DISEASES
Early evidence has revealed that developmental plasticity is affected, at least in part, by epigenetic changes that are established in early life and modulate gene expression during embryogenesis. It is thought that maternal dietary epigenetic modulations acting as fetal environmental factors that can be transmitted maternally to the embryo, fetus, and breast-feeding neonates can influence subsequent susceptibility to certain congenital birth defects as well as chronic disorders in later life (Table II).
Table II.
Transgenerational Prevention of Human Diseases by Epigenetic Maternal Diet
| Diseases | Diet | Findings | References |
|---|---|---|---|
| Congenital diseases | Folate; SFN | Maternal folate prevents neural tube defects, congenital heart defects, and urinary tract anomalies; in utero exposure to SFN prevents a rare inherited condition, epidermolysis bullosa simplex | (51,75,90,91) |
| Cancer | Folate; GE; SFN; EGCG; I3C | Maternal folate reduces the risk of acute lymphoblastic leukemia in the child; maternal GE may promote carcinogen-induced breast cancer development in rats; early-life consumption of cruciferous vegetables exhibits more effective prevention effects against cancers; maternal ingestion of green tea may provide transplacental protection against carcinogenesis; maternal ingestion of I3C provides transplacental protection against carcinogen-induced lung cancer | (18,56,68,82,88) |
| Diabetes | Folate; genistein; EGCG | Restriction of maternal folate may increase risk of diabetes by adult age; studies in agouti mice suggest that early exposure to genistein may influence the adipogenesis processes and obesity-related diseases such as type 2 diabetes; maternal green tea extract attenuates a high-fat diet-induced insulin resistance in adult male offspring | (57,61,100) |
| Cardiovascular diseases | Folate | Restriction of maternal folate may increase risk of high blood pressure in adulthood | (57) |
GE genistein, SFN S-adenosylmethionine, EGCG (−)-epigallocatechin-3-gallate, I3C indole-3-carbinol
Congenital Birth Defects
Although the potential mechanisms that cause congenital disorders can be complex, the intrauterine environment such as maternal dietary intake is considered as one of the most important factors that can influence embryogenesis and fetal development leading to either beneficial prevention or adverse effects contributing to certain birth defects. Folate is by far the most effective prenatal supplement to prevent neural tube defects such as spinal bifida and anencephaly in fetus (51). It also has been shown that folate can reduce the risk of other congenital disorders such as congenital heart defects and urinary tract anomalies (90,91). Despite its major roles on DNA and RNA synthesis for cellular proliferation, folate is essential to carry one-carbon units and plays an important role on DNA methylation. The direct evidence for maternal folate influencing methylation status in the offspring comes from the agouti mouse model as we discussed previously (52), which suggests that folate-induced epigenetic reprogramming during early embryonic development may contribute to its preventive properties of certain congenital disorders. Early exposure to another epigenetic diet, soybean genistein, results in alterations of multiple phenotypes of the agouti offspring such as reduction of prevalence of obesity and brown coat due to hypermethylation of agouti gene (61). However, direct pieces of evidence including animal experimentation and epidemiological data connected to other important epigenetic dietary components, such as soybean genistein, broccoli sprout sulforaphane, and green tea polyphenol EGCG, to prevention of congenital defects are largely lacking.
Noncommunicable Diseases
Turbulent epigenetic rewriting occurs during early embryogenesis, whereas global demethylation and subsequent de novo remethylation processes as well as concomitant histone modification changes establish a new epigenetic pattern in the offspring, most of which will be maintained throughout life in the somatic cells. Therefore, the early impact on epigenetic profile induced by maternal epigenetic diets could be stably inherited and then influence genetic susceptibility to certain human disease in the adult life. Noncommunicable diseases (NCDs) refer to noninfectious and non-transmissible medical condition, and most of NCDs are chronic human diseases such as cancer, diabetes, and cardiovascular diseases with defined genetic predisposition. Although direct evidence on how epigenetic diets affect chronic human diseases in later life is still insufficient, many epidemiological studies provide first-hand evidence indicating a tight correlation of maternal epigenetic diet consumption with disease prevention in the progeny. For example, maternal consumption of folate supplementation, a methyl-donor diet, has been shown to reduce the risk of common acute lymphoblastic leukemia in later life (56); whereas long-term consumption of cruciferous vegetables enriched SFN is correlated with low incidence of breast cancer in their children (73). In addition, study shows that deficiency of maternal folate leads to genome-wide changes in the fetal epigenome and the offspring are more prone to develop diabetes and high blood pressure by adult age (57), indicating a potential role of maternal folate on prevention of diabetes and cardiovascular diseases in adult life.
Animal studies have demonstrated that when pattern changes in DNA methylation or histone modification on obesity- or adipogenesis-related genes such as leptin, leptin receptor, glucocorticoid, and peroxisome proliferator-activated receptor γ, a proclivity towards an obese phenotype is observed in adult life (92–96). Diet enrichment of methyl donors (folic acid intake, selenium choline, vitamin B12, diallyl sulfide) has been shown to prevent transgenerational obesity (97). Dietary inhibitors of histone acetylases (HATs) and HDACs such as curcumin and diallyl sulfide may influence chromatin states as well affect genes of the adipogenic pathways (98,99). In addition, a change of obesity status in the offspring of the agouti mice fed with another epigenetic diet, soybean genistein, suggests that early exposure to genistein may influence the adipogenesis processes in later life, thus influencing the susceptibility to certain human diseases that are closely related to obesity, such as type 2 diabetes (61). Evidence also indicates that maternal green tea attenuates a high-fat diet-induced insulin resistance in adult male offspring suggesting a transplacental prevention effect of green tea EGCG against diabetes (100). Further investigation focused on determining actual transgenerational epigenetic alterations and precise epigenetic loci changes that are connected to disease incidence is highly needed.
WINDOW OF EPIGENETIC DIET EXPOSURE
The importance of the exposure period to soybean genistein during pregnancy and lifetime on prevention of mammary cancer is well illustrated by experimental animal and human epidemiological studies (63–65). In postnatal life, early consumption of genistein during childhood and adolescence in women reduces later mammary cancer risk, whereas adult exposure to soy genistein is not considered as a protective factor (63–65). Although the findings obtained in rats and mice exposed to maternal genistein on later breast cancer prevention vary from study to study, our recent pilot study indicates a clear adverse effect of maternal genistein exposure during pregnancy on breast cancer development in a spontaneous breast cancer mouse model, indicating that an appropriate exposure window could be a key factor for prevention of human diseases (Fig. 2). Although the precise mechanisms that cause these differences are still not clear, two important factors including interfering with estrogen signaling and epigenetic pathways may contribute to diverse effects of genistein on prevention of mammary cancer. Further investigation focused on determining accurate exposure time that maximizes beneficial effects of epigenetic diets will provide key evidence for guiding optimal maternal dietary regimens in humans.
CONCLUSIONS
The available data provide a molecular basis for epidemiological and experimental evidence that shows that the early period of life is critical in determining susceptibility to many human diseases. Early embryogenesis involving accurately designed sequential epigenetic reprogramming provides an excellent opportunity for later intervention on several human diseases when there is appropriate exposure to epigenetic diets such as folate, soybean genistein, broccoli sprout sulforaphane, green tea polyphenols, etc. (Fig. 1). Although our knowledge of the role of epigenetic mechanisms in early dietary prevention on human diseases is relatively limited at the present, further studies will likely provide more precise interpretation. Moreover, better understanding the transgenerational effects of bioactive dietary compounds as well as the precise mechanisms during this process will facilitate the discovery of the novel approaches linking early dietary or pharmaceutical interventions to human disease prevention.
ACKNOWLEDGMENTS
This work was supported by grants from the American Institute for Cancer Research.
Conflict of Interest
The authors declare that they have no competing interests.
Abbreviations
- DNMT
DNA methyltransferase
- HDACs
Histone deacetylases
- HATs
Histone acetyltransferases
- SFN
Sulforaphane
- EGCG
(−)-Epigallocatechin-3-gallate
- SAM
S-adenosylmethionine
- NTDs
Neural tube defects
- ER
Estrogen receptor
- NCDs
Noncommunicable diseases
REFERENCES
- 1.Jablonka A, Raz G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Quart Rev Biol. 2009;84(2):131–176. doi: 10.1086/598822. [DOI] [PubMed] [Google Scholar]
- 2.Cantone I, Fisher AG. Epigenetic programming and reprogramming during development. Nat Struct Mol Biol. 2013;20(3):282–289. doi: 10.1038/nsmb.2489. [DOI] [PubMed] [Google Scholar]
- 3.Ho L, Crabtree GR. Chromatin remodeling during development. Nature. 2010;463(7280):474–484. doi: 10.1038/nature08911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gopalakrishnan S, Van Emburgh BO, Robertson KD. DNA methylation in development and human disease. Mutat Res. 2008;647(1–2):30–38. doi: 10.1016/j.mrfmmm.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avner P, Heard E. X-chromosome inactivation: counting, choice and initiation. Nat Rev Genet. 2001;2(1):59–67. doi: 10.1038/35047580. [DOI] [PubMed] [Google Scholar]
- 6.Lee JT, Bartolomei MS. X-inactivation, imprinting, and long noncoding RNAs in health and disease. Cell. 2013;152(6):1308–1323. doi: 10.1016/j.cell.2013.02.016. [DOI] [PubMed] [Google Scholar]
- 7.Tomizawa S, Sasaki H. Genomic imprinting and its relevance to congenital disease, infertility, molar pregnancy and induced pluripotent stem cell. J Hum Genet. 2012;57(2):84–91. doi: 10.1038/jhg.2011.151. [DOI] [PubMed] [Google Scholar]
- 8.Meeran SM, Ahmed A, Tollefsbol TO. Epigenetic targets of bioactive dietary components for cancer prevention and therapy. Clin Epigenetics. 2010;1(3–4):101–116. doi: 10.1007/s13148-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hardy TM, Tollefsbol TO. Epigenetic diet: impact on the epigenome and cancer. Epigenomics. 2011;3(4):503–518. doi: 10.2217/epi.11.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21(2):113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 11.Cheung KL, Kong AN. Molecular targets of dietary phenethyl isothiocyanate and sulforaphane for cancer chemoprevention. AAPS J. 2010;12(1):87–97. doi: 10.1208/s12248-009-9162-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82(12):1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Liu L, Andrews L, Tollefsbol T. Genistein depletes telomerase activity through cross-talk between genetic and epigenetic mechanisms. Int J Cancer. 2009;125(2):286–296. doi: 10.1002/ijc.24398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berghe WV. Epigenetic impact of dietary polyphenols in cancer chemoprevention: lifelong remodeling of our epigenomes. Pharmacol Res. 2012;65:565–576. doi: 10.1016/j.phrs.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Canani RB, Di Costanzo M, Leone L, Bedogni G, Brambilla P, Cianfarani S, et al. Epigenetic mechanisms elicited by nutrition in early life. Nutr Res Rev. 2011;24:198–205. doi: 10.1017/S0954422411000102. [DOI] [PubMed] [Google Scholar]
- 16.Ordovás JM, Smith CE. Epigenetics and cardiovascular disease. Nat Rev Cardiol. 2010;7(9):510–519. doi: 10.1038/nrcardio.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanhees K, Coort S, Ruijters EJ, Godschalk RW, van Schooten FJ, van Waalwijk B, et al. Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011;25(2):797–807. doi: 10.1096/fj.10-172155. [DOI] [PubMed] [Google Scholar]
- 18.Nelson NJ. Migrant studies aid the search for factors linked to breast cancer risk. J Natl Cancer Inst. 2006;98(7):436–438. doi: 10.1093/jnci/djj147. [DOI] [PubMed] [Google Scholar]
- 19.Mosley BS, Cleves MA, Siega-Riz AM, Shaw GM, Canfield MA, Waller DK, et al. Neural tube defects and maternal folate intake among pregnancies conceived after folic acid fortification in the United States. Am J Epidemiol. 2009;169(1):9–17. doi: 10.1093/aje/kwn331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strogantsev R, Ferguson-Smith AC. Proteins involved in establishment and maintenance of imprinted methylation marks. Brief Funct Genomics. 2012;11(3):227–239. doi: 10.1093/bfgp/els018. [DOI] [PubMed] [Google Scholar]
- 21.Robertson KD. DNA methylation and chromatin—unraveling the tangled web. Oncogene. 2002;21(35):5361–5379. doi: 10.1038/sj.onc.1205609. [DOI] [PubMed] [Google Scholar]
- 22.Deaton A, Bird A. CpG islands and the regulation of transcription. Genes and Dev. 2011;25:1010–1022. doi: 10.1101/gad.2037511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan HD, Santos F, Green K, Dean W, Reik W. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14(1):R47–R58. doi: 10.1093/hmg/ddi114. [DOI] [PubMed] [Google Scholar]
- 24.Haaf T. Methylation dynamics in the early mammalian embryo: implications of genome reprogramming defects for development. Curr Top Microbiol Immunol. 2006;310:13–22. doi: 10.1007/3-540-31181-5_2. [DOI] [PubMed] [Google Scholar]
- 25.Goll MG, Bestor TH. Eukaryotic cytosine methyltransferases. Annu Rev Biochem. 2005;74:481–514. doi: 10.1146/annurev.biochem.74.010904.153721. [DOI] [PubMed] [Google Scholar]
- 26.Chen T, Tsujimoto N, Li E. The PWWP domain of Dnmt3a and Dnmt3b is required for directing DNA methylation to the major satellite repeats at pericentric heterochromatin. Mol Cell Biol. 2004;24(20):9048–9058. doi: 10.1128/MCB.24.20.9048-9058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okano M, Bell D, Haber D, Li E. DNA methyltransferases Dnmt3a and Dnmt3b are essential for de novo methylation and mammalian development. Cell. 1999;99(3):247–257. doi: 10.1016/S0092-8674(00)81656-6. [DOI] [PubMed] [Google Scholar]
- 28.Okano M, Takebayashi S, Okumura K, Li E. Assignment of cytosine-5 DNA methyltransferases Dnmt3a and Dnmt3b to mouse chromosome bands 12A2-A3 and 2H1 by in situ hybridization. Cytogenet Cell Genet. 1999;86(3–4):333–334. doi: 10.1159/000015331. [DOI] [PubMed] [Google Scholar]
- 29.Okano M, Xie S, Li E. Cloning and characterization of a family of novel mammalian DNA (cytosine-5) methyltransferases. Nat Genet. 1998;19(3):219–220. doi: 10.1038/890. [DOI] [PubMed] [Google Scholar]
- 30.Bourc’his D, Xu GL, Lin CS, Bollman B, Bestor TH. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294(5551):2536–2539. doi: 10.1126/science.1065848. [DOI] [PubMed] [Google Scholar]
- 31.Hata K, Okano M, Lei H, Li E. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development. 2002;129(8):1983–1993. doi: 10.1242/dev.129.8.1983. [DOI] [PubMed] [Google Scholar]
- 32.Berger SL. Histone modifications in transcriptional regulation. Curr Opin Genet Dev. 2002;12(2):142–148. doi: 10.1016/S0959-437X(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 33.Rugg-Gunn PJ, Cox BJ, Ralston A, Rossant J. Distinct histone modifications in stem cell lines and tissue lineages from the early mouse embryo. Proc Natl Acad Sci U S A. 2010;107(24):10783–10790. doi: 10.1073/pnas.0914507107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres-Padilla ME, Parfitt DE, Kouzarides T, Zernicka-Goetz M. Histone arginine methylation regulates pluripotency in the early mouse embryo. Nature. 2007;445(7124):214–218. doi: 10.1038/nature05458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bedford MT. Arginine methylation at a glance. J Cell Sci. 2007;120(Pt 24):4243–4246. doi: 10.1242/jcs.019885. [DOI] [PubMed] [Google Scholar]
- 36.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in polycomb-group silencing. Science. 2002;298(5595):1039–1043. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 37.Byrd KN, Shearn A. ASH1, a Drosophila trithorax group protein, is required for methylation of lysine 4 residues on histone H3. Proc Natl Acad Sci U S A. 2003;100(20):11535–11540. doi: 10.1073/pnas.1933593100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schnerch A, Lee JB, Graham M, Guezguez B, Bhatia M. Human embryonic stem cell-derived hematopoietic cells maintain core epigenetic machinery of the polycomb group/trithorax group complexes distinctly from functional adult hematopoietic stem cells. Stem Cells Dev. 2013;22(1):73–89. doi: 10.1089/scd.2012.0204. [DOI] [PubMed] [Google Scholar]
- 39.Hochedlinger K, Plath K. Epigenetic reprogramming and induced pluripotency. Development. 2009;136(4):509–523. doi: 10.1242/dev.020867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindeman LC, Winata CL, Aanes H, Mathavan S, Alestrom P, Collas P. Chromatin states of developmentally-regulated genes revealed by DNA and histone methylation patterns in zebrafish embryos. Int J Dev Biol. 2010;54(5):803–813. doi: 10.1387/ijdb.103081ll. [DOI] [PubMed] [Google Scholar]
- 41.Tachibana M, Sugimoto K, Nozaki M, Ueda J, Ohta T, Ohki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16(14):1779–1791. doi: 10.1101/gad.989402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernstein BE, Mikkelsen TS, Xie X, Kamal M, Huebert DJ, Cuff J, et al. A bivalent chromatin structure marks key developmental genes in embryonic stem cells. Cell. 2006;125(2):315–326. doi: 10.1016/j.cell.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 43.Aranda P, Agirre X, Ballestar E, Andreu EJ, Román-Gómez J, Prieto I, et al. Epigenetic signatures associated with different levels of differentiation potential in human stem cells. PLoS One. 2009;4(11):e7809. doi: 10.1371/journal.pone.0007809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cui K, Zang C, Roh TY, Schones DE, Childs RW, Peng W, et al. Chromatin signatures in multipotent human hematopoietic stem cells indicate the fate of bivalent genes during differentiation. Cell Stem Cell. 2009;4(1):80–93. doi: 10.1016/j.stem.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, Tollefsbol TO. Impact on DNA methylation in cancer prevention and therapy by bioactive dietary components. Curr Med Chem. 2010;17(20):2141–2151. doi: 10.2174/092986710791299966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition. 2004;20(1):63–68. doi: 10.1016/j.nut.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 47.Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71(1–2):121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman DR, Cornatzer WE, Duerre JA. Relationship between tissue levels of S-adenosylmethionine, S-adenylhomocysteine, and transmethylation reactions. Can J Biochem. 1979;57(1):56–65. doi: 10.1139/o79-007. [DOI] [PubMed] [Google Scholar]
- 49.McKay JA, Williams EA, Mathers JC. Folate and DNA methylation during in utero development and aging. Biochem Soc Trans. 2004;32(Pt 6):1006–1007. doi: 10.1042/BST0321006. [DOI] [PubMed] [Google Scholar]
- 50.Kim YI. Methylenetetrahydrofolate reductase polymorphisms, folate, and cancer risk: a paradigm of gene-nutrient interactions in carcinogenesis. Nutr Rev. 2000;58(7):205–209. doi: 10.1111/j.1753-4887.2000.tb01863.x. [DOI] [PubMed] [Google Scholar]
- 51.Pitkin RM. Folate and neural tube defects. Am J Clin Nutr. 2007;85(1):285S–288S. doi: 10.1093/ajcn/85.1.285S. [DOI] [PubMed] [Google Scholar]
- 52.Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. FASEB J. 1998;12(11):949–957. [PubMed] [Google Scholar]
- 53.Yen TT, Gill AM, Frigeri LG, Barsh GS, Wolff GL. Obesity, diabetes, and neoplasia in yellow A(vy)/-mice: ectopic expression of the agouti gene. FASEB J. 1994;8(8):479–488. doi: 10.1096/fasebj.8.8.8181666. [DOI] [PubMed] [Google Scholar]
- 54.Kim YI. Nutritional epigenetics: impact of folate deficiency on DNA methylation and colon cancer susceptibility. J Nutr. 2005;135(11):2703–2709. doi: 10.1093/jn/135.11.2703. [DOI] [PubMed] [Google Scholar]
- 55.Balaghi M, Wagner C. DNA methylation in folate deficiency: use of CpG methylase. Biochem Biophys Res Commun. 1993;193:1184–1190. doi: 10.1006/bbrc.1993.1750. [DOI] [PubMed] [Google Scholar]
- 56.Thompson JR, Gerald PF, Willoughby ML, Armstrong BK. Maternal folate supplementation in pregnancy and protection against acute lymphoblastic leukaemia in childhood: a case–control study. Lancet. 2001;358(9297):1935–1940. doi: 10.1016/S0140-6736(01)06959-8. [DOI] [PubMed] [Google Scholar]
- 57.Sinclair KD, Allegrucci C, Singh R, Gardner DS, Sebastian S, Bispham J, et al. DNA methylation, insulin resistance, and blood pressure in offspring determined by maternal periconceptional B vitamin and methionine status. Proc Natl Acad Sci U S A. 2007;104(49):19351–19356. doi: 10.1073/pnas.0707258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Munro IC, Harwood M, Hlywka JJ, Stephen AM, Doull J, Flamm WG, et al. Soy isoflavones: a safety review. Nutr Rev. 2003;61(1):1–33. doi: 10.1301/nr.2003.janr.1-33. [DOI] [PubMed] [Google Scholar]
- 59.Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17(2):271–275. doi: 10.1093/carcin/17.2.271. [DOI] [PubMed] [Google Scholar]
- 60.Barnes S. Effect of genistein on in vitro and in vivo models of cancer. J Nutr. 1995;125(3 Suppl):777S–783S. doi: 10.1093/jn/125.3_Suppl.777S. [DOI] [PubMed] [Google Scholar]
- 61.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114(4):567–572. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li Y, Meeran SM, Patel SN, Chen H, Hardy TM, Tollefsbol TO. Epigenetic reactivation of estrogen receptor-α (ERα) by genistein enhances hormonal therapy sensitivity in ERα-negative breast cancer. Mol Cancer. 2013;12:19. doi: 10.1186/1476-4598-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu AH, Wan P, Hankin J, Tseng CC, Yu MC, Pike MC. Adolescent and adult soy intake and risk of breast cancer in Asian-Americans. Carcinogenesis. 2002;23(9):1491–1496. doi: 10.1093/carcin/23.9.1491. [DOI] [PubMed] [Google Scholar]
- 64.Warri A, Saarinen NM, Makela S, Hilakivi-Clarke L. The role of early life genistein exposures in modifying breast cancer risk. Br J Cancer. 2008;98(9):1485–1493. doi: 10.1038/sj.bjc.6604321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Assis S, Hilakivi-Clarke L. Timing of dietary estrogenic exposures and breast cancer risk. Ann N Y Acad Sci. 2006;1089:14–35. doi: 10.1196/annals.1386.039. [DOI] [PubMed] [Google Scholar]
- 66.Hilakivi-Clarke L, Cho E, Onojafe I, Raygada M, Clarke R. Maternal exposure to genistein during pregnancy increases carcinogen-induced mammary tumorigenesis in female rat offspring. Oncol Rep. 1999;6(5):1089–1095. doi: 10.3892/or.6.5.1089. [DOI] [PubMed] [Google Scholar]
- 67.Foster WG, Younglai EV, Boutross-Tadross O, Hughes CL, Wade MG. Mammary gland morphology in Sprague–Dawley rats following treatment with an organochlorine mixture in utero and neonatal genistein. Toxicol Sci. 2004;77(1):91–100. doi: 10.1093/toxsci/kfg247. [DOI] [PubMed] [Google Scholar]
- 68.Su Y, Eason RR, Geng Y, Till SR, Badger TM, Simmen RC. In utero exposure to maternal diets containing soy protein isolate, but not genistein alone, protects young adult rat offspring from NMU-induced mammary tumorigenesis. Carcinogenesis. 2007;28(5):1046–1051. doi: 10.1093/carcin/bgl240. [DOI] [PubMed] [Google Scholar]
- 69.Parnaud G, Li P, Cassar G, Rouimi P, Tulliez J, Combaret L, et al. Mechanism of sulforaphane-induced cell cycle arrest and apoptosis in human colon cancer cells. Nutr Cancer. 2004;48(2):198–206. doi: 10.1207/s15327914nc4802_10. [DOI] [PubMed] [Google Scholar]
- 70.Bertl E, Bartsch H, Gerhäuser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5(3):575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 71.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64(16):5767–5774. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 72.Meeran SM, Patel SN, Li Y, Shukla S, Tollefsbol TO. Bioactive dietary supplements reactivate ER expression in ER-negative breast cancer cells by active chromatin modifications. PLoS One. 2012;7(5):e37748. doi: 10.1371/journal.pone.0037748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ziegler RG, Hoover RN, Pike MC, Hildesheim A, Nomura AM, West DW, et al. Migration patterns and breast cancer risk in Asian-American women. J Natl Cancer Inst. 1993;85(22):1819–1827. doi: 10.1093/jnci/85.22.1819. [DOI] [PubMed] [Google Scholar]
- 74.Kakehi Y. Epidemiology and clinical features of prostate cancer in Japan. Nihon Rinsho. 1998;56(8):1969–1973. [PubMed] [Google Scholar]
- 75.Kerns ML, DePianto D, Dinkova-Kostova AT, Talalay P, Coulombe PA. Reprogramming of keratin biosynthesis by sulforaphane restores skin integrity in epidermolysis bullosa simplex. Proc Natl Acad Sci U S A. 2007;104(36):14460–14465. doi: 10.1073/pnas.0706486104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bushman JL. Green tea and cancer in humans: a review of the literature. Nutr Cancer. 1998;31(3):151–159. doi: 10.1080/01635589809514697. [DOI] [PubMed] [Google Scholar]
- 77.Tipoe GL, Leung TM, Hung MW, Fung ML. Green tea polyphenols as an anti-oxidant and anti-inflammatory agent for cardiovascular protection. Cardiovasc Hematol Disord Drug Targets. 2007;7(2):135–144. doi: 10.2174/187152907780830905. [DOI] [PubMed] [Google Scholar]
- 78.Raederstorff DG, Schlachter MF, Elste V, Weber P. Effect of EGCG on lipid absorption and plasma lipid levels in rats. J Nutr Biochem. 2003;14(6):326–332. doi: 10.1016/S0955-2863(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 79.Berletch JB, Liu C, Love WK, Andrews LG, Katiyar SK, Tollefsbol TO. Epigenetic and genetic mechanisms contribute to telomerase inhibition by EGCG. J Cell Biochem. 2008;103(2):509–519. doi: 10.1002/jcb.21417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fang MZ, Wang Y, Ai N, Hou Z, Sun Y, Lu H, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63(22):7563–7570. [PubMed] [Google Scholar]
- 81.Fang M, Chen D, Yang CS. Dietary polyphenols may affect DNA methylation. J Nutr. 2007;137(1 Suppl):223S–228S. doi: 10.1093/jn/137.1.223S. [DOI] [PubMed] [Google Scholar]
- 82.Castro DJ, Yu Z, Löhr CV, Pereira CB, Giovanini JN, Fischer KA, et al. Chemoprevention of dibenzo[a, l]pyrene transplacental carcinogenesis in mice born to mothers administered green tea: primary role of caffeine. Carcinogenesis. 2008;29(8):1581–1586. doi: 10.1093/carcin/bgm237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang P, Li H. Epigallocatechin-3-gallate ameliorates hyperglycemia-induced embryonic vasculopathy and malformation by inhibition of Foxo3a activation. Am J Obstet Gynecol. 2010;203(1):75.e1–6. doi: 10.1016/j.ajog.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Long L, Li Y, Wang YD, He QY, Li M, Cai XD, et al. The preventive effect of oral EGCG in a fetal alcohol spectrum disorder mouse model. Alcohol Clin Exp Res. 2010;34(11):1929–1936. doi: 10.1111/j.1530-0277.2010.01282.x. [DOI] [PubMed] [Google Scholar]
- 85.Chen JR, Zhang J, Lazarenko OP, Kang P, Blackburn ML, Ronis MJ, et al. Inhibition of fetal bone development through epigenetic down-regulation of HoxA10 in obese rats fed high-fat diet. FASEB J. 2012;26(3):1131–1141. doi: 10.1096/fj.11-197822. [DOI] [PubMed] [Google Scholar]
- 86.Choi SW, Friso S. Epigenetics: a new bridge between nutrition and health. Adv Nutr. 2010;1(1):8–16. doi: 10.3945/an.110.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perrine SP, Rudolph A, Faller DV, Roman C, Cohen RA, Chen SJ, et al. Butyrate infusions in the ovine fetus delay the biologic clock for globin gene switching. Proc Natl Acad Sci U S A. 1988;85(22):8540–8542. doi: 10.1073/pnas.85.22.8540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yu Z, Mahadevan B, Löhr CV, Fischer KA, Louderback MA, Krueger SK, et al. Indole-3-carbinol in the maternal diet provides chemoprotection for the fetus against transplacental carcinogenesis by the polycyclic aromatic hydrocarbon dibenzo[a, l]pyrene. Carcinogenesis. 2006;27(10):2116–2123. doi: 10.1093/carcin/bgl072. [DOI] [PubMed] [Google Scholar]
- 89.Xia X, Cai H, Qin S, Xu C. Histone acetylase inhibitor curcumin impairs mouse spermiogenesis—an in vitro study. PLoS One. 2012;7(11):e48673. doi: 10.1371/journal.pone.0048673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kapusta L, Haagmans ML, Steegers EA, Cuypers MH, Blom HJ, Eskes TK. Congenital heart defects and maternal derangement of homocysteine metabolism. J Pediatr. 1999;135(6):773–774. doi: 10.1016/S0022-3476(99)70102-2. [DOI] [PubMed] [Google Scholar]
- 91.Li DK, Daling JR, Mueller BA, Hickok DE, Fantel AG, Weiss NS. Periconceptional multivitamin use in relation to the risk of congenital urinary tract anomalies. Epidemiology. 1995;6(3):212–218. doi: 10.1097/00001648-199505000-00004. [DOI] [PubMed] [Google Scholar]
- 92.Frias AE, Grove KL. Obesity: a transgenerational problem linked to nutrition during pregnancy. Semin Reprod Med. 2012;30(6):472–478. doi: 10.1055/s-0032-1328875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dabelea D, Crume T. Maternal environment and the transgenerational cycle of obesity and diabetes. Diabetes. 2011;60(7):1849–1855. doi: 10.2337/db11-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boqué N, de la Iglesia R, de la Garza AL, Milagro FI, Olivares M, Bañuelos O, et al. Prevention of diet-induced obesity by apple polyphenols in Wistar rats through regulation of adipocyte gene expression and DNA methylation patterns. Mol Nutr Food Res. 2013;57(8):1473–1478. doi: 10.1002/mnfr.201200686. [DOI] [PubMed] [Google Scholar]
- 95.Milagro FI, Campión J, García-Díaz DF, Goyenechea E, Paternain L, Martínez JA. High fat diet-induced obesity modifies the methylation pattern of leptin promoter in rats. J Physiol Biochem. 2009;65(1):1–9. doi: 10.1007/BF03165964. [DOI] [PubMed] [Google Scholar]
- 96.Takada I, Mihara M, Suzawa M, Ohtake F, Kobayashi S, Igarashi M, et al. A histone lysine methyltransferase activated by non-canonical Wnt signalling suppresses PPAR-gamma transactivation. Nat Cell Biol. 2007;9(11):1273–1285. doi: 10.1038/ncb1647. [DOI] [PubMed] [Google Scholar]
- 97.Waterland RA, Travisano M, Tahiliani KG, Rached MT, Mirza S. Methyl donor supplementation prevents transgenerational amplification of obesity. Int J Obes (Lond) 2008;32(9):1373–1379. doi: 10.1038/ijo.2008.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. J Nutr. 2009;139(5):919–925. doi: 10.3945/jn.108.100966. [DOI] [PubMed] [Google Scholar]
- 99.Campión J, Milagro FI, Martínez JA. Individuality and epigenetics in obesity. Obes Rev. 2009;10(4):383–392. doi: 10.1111/j.1467-789X.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- 100.Li S, Tse IM, Li ET. Maternal green tea extract supplementation to rats fed a high-fat diet ameliorates insulin resistance in adult male offspring. J Nutr Biochem. 2012;23(12):1655–1660. doi: 10.1016/j.jnutbio.2011.11.008. [DOI] [PubMed] [Google Scholar]


