Abstract
Background
Although the pathophysiology and treatment of adult heart failure (HF) are well studied, HF in children remains poorly understood. In adults, adrenergic receptor (AR)-mediated adaptation plays a central role in cardiac abnormalities in HF, and these patients respond well to β-blocker (BB) therapy. However, in children with HF, there is a growing body of literature suggesting a lack of efficacy of adult HF therapies. Due to these unanticipated differences in response to therapy and the paucity of data regarding the molecular adaptation of the paediatric heart, we investigated the molecular characteristics of HF in children.
Methods and results
Explanted hearts from adults and children with idiopathic dilated cardiomyopathy and non-failing controls were used in the study. Our results show that the molecular characteristics of paediatric HF are strikingly different from their adult counterparts. These differences include: (i) down-regulation of β1- and β2-AR in children, whereas β2-AR expression is maintained in adults; (ii) up-regulation of connexin43 in children, whereas down-regulation is observed in adults; (iii) no differences in phosphatase expression, whereas up-regulation is observed in adults; (iv) no decrease in the phosphorylation of phospholamban at the Ser16 or Thr17 sites in children, which are known characteristics of adult HF.
Conclusion
There is a different adaptation of β-AR and adrenergic signalling pathways in children with HF compared with adults. Our results begin to address the disparities in cardiovascular research specific to children and suggest that age-related differences in adaptation could influence the response to therapy. These findings could lead to a paradigm shift in the contemporary management of children with HF.
Keywords: Paediatric dilated cardiomyopathy, β-Adrenergic receptor, CaMK, Phosphatase, Fetal gene programme
See page 10 for the editorial comment on this article (doi:10.1093/eurheartj/ehs402)
Introduction
Heart failure (HF) is a common disorder in adults, affecting an estimated 5 million Americans and 14 million Europeans with an annual incidence of ∼15 per 1000 adults over the age of 55.1,2 Heart failure is much less common in children, with an annual incidence of ∼1.1 per 100 000 children under the age of 18 years.1 Although the majority of HF in adults is due to ischaemic heart disease, the aetiology of HF in children is very heterogeneous and includes congenital heart disease as well as various forms of cardiomyopathy. However, dilated cardiomyopathy (DCM) is a common indication for heart transplant in both children and adults and is the most common diagnosis leading to heart transplant in children over the age of 1.3 Regardless of the aetiology, paediatric HF is a challenging clinical problem with a devastating prognosis of <50% transplant-free survival at 5 years.1
Because paediatric HF is an uncommon problem, performing controlled clinical trials is very difficult. Therefore, historically the medical treatment for HF in children has been extrapolated from adult trials and lacks robust experimental evidence. Unfortunately, despite advances in medical treatment, there has not been an improvement in survival over time for children with HF. The lack of efficacy was initially demonstrated by Towbin et al.4 in a retrospective analysis of outcomes for children with HF between the early 1990s and 2000s. These findings were reproduced more recently in a retrospective review by Kantor et al.5 that showed no survival benefit with the advancement of medical therapy from the digoxin plus diuretic era in the late 1970s to the β-blocker (BB) era in the early 2000s. These data are completely contrary to the adult experience demonstrating the remarkable clinical efficacy of BB therapy and improvement in morbidity and mortality between the 1970s and 2000s.6 This differential response to treatment is further highlighted by the results of the carvedilol trial for paediatric HF published in 2007.7 Despite the marked improvement in mortality and morbidity associated with BB therapy in multiple adult trials, there was no improvement in clinical outcomes between children with symptomatic HF treated with placebo vs. the non-selective BB, carvedilol. It is important to note that the results of the paediatric carvedilol trial have been challenged due to concerns about the relatively small sample size, the heterogeneous nature of the children enrolled, and the high rate of spontaneous improvement. However, taken together, these studies suggest that the response to existing medical therapy is different between adults and children with HF.
The use of BBs for the treatment of HF in adults is supported by studies done by Bristow et al.8 demonstrating the down-regulation and desensitization of β-adrenergic receptors (β-ARs) in response to chronic catecholamine stimulation in failing (F) human heart tissue and experimental HF models.9 The adaptation of the β-adrenergic system in response to HF in children has not been previously investigated and is therefore incompletely understood. The purpose of this study is to define the response of the cardiac β-adrenergic system and to determine broader myocellular responses to HF in children with idiopathic dilated cardiomyopathy (IDC). The central hypothesis is that important age-related differences in the adaptation of β-AR and downstream signalling pathways may influence the treatment response in children with HF.
In the classical description of the β-AR pathway, β-AR activation induces the formation of cyclic adenosine monophosphate (cAMP) and the subsequent activation of protein kinase A (PKA), which in turn phosphorylates several downstream targets: L-type Ca2+ channel (LTCC), increasing Ca2+ influx; phospholamban (PLB), removing its inhibitory influence on sarcoplasmic reticulum ATPase (SERCA) and increasing sarcoplasmic reticulum Ca2+ (SR Ca2+) reuptake as well as subsequent Ca2+ release; and troponin I and myosin-binding protein C, increasing contractility.10 Recently, the works of several groups has shown that Ca2+-calmodulin kinase II (CaMKII) is activated in response to β1-AR stimulation. Ca2+-calmodulin kinase II activity is up-regulated in adult human HF,11,12 which can result in the phosphorylation of the ryanodine receptor,13 phosphorylation of LTCC,14 phosphorylation of PLB,15 and activation of various transcription factors.16 Although CaMKII and PKA phosphorylate PLB, it is dephosphorylated in adult HF, presumably through elevated phosphatase activity,17,18 resulting in decreased SERCA activity and SR Ca2+ uptake.
At the cellular level, myocardial failure is characterized by changes in the gene expression of many components of the heart, described as a recapitulation of a ‘fetal’ gene programme (FGP), because many embryonically expressed genes that are down-regulated post-natally are reactivated, while several ‘adult’ genes are repressed.19 Of the changes that are observed in failing adult hearts, increases in β-myosin heavy chain (MyHC), skeletal α-actin, B-type natriuretic peptide (BNP), and atrial natriuretic peptide (ANP), with coordinate decreases in αMyHC and SERCA2, are perhaps the most widely recognized.
The goal of this study was to analyse cardiac myocellular changes that occur in children with HF. Our results suggest that there are differences in the molecular regulation of HF in children when compared with adults.
Methods
A detailed description of the methods can be found in Supplementary material online.
Tissue procurement
Human subjects were males and females of all ages, races, and ethnic background who donated their heart to the institutional review board-approved adult and paediatric transplant tissue banks at the University of Colorado. Non-failing (NF) control hearts were obtained from donors whose heart could not be placed for technical reasons. A detailed description of all paediatric patients and a summary of all adult patients can be found in Supplementary material online, Tables S1 and S2.
β-Adrenergic receptor density
β-Adrenergic receptor density was determined using crude membrane preparations and [125I]-iodocyanopindolol binding by the Engel method20: 9 NF paediatric, 13 IDC paediatric, 9 NF adult, and 9 IDC adult.
Real-time polymerase chain reaction
Real-time polymerase chain reaction was performed as described previously21: 9 NF paediatric, 31 IDC paediatric, 26 NF adult, and 22 IDC adult (β-AR and FGP) and 8 NF paediatric, 9 IDC paediatric, 10 NF adult, and 10 IDC adult (Phosphatases).
Western blots
Western blots were performed as described previously22: 12 NF paediatric, 23 IDC paediatric, 4 NF adult, and 4 IDC adult (PLB); 5 NF adult and 5 IDC adult (αMyHC); and 7 NF paediatric, 8 IDC paediatric, 8 NF adult and 8 IDC adult (p-CaMKII and CaMKIIδ).
Cyclic adenosine monophosphate assay
Enzyme-linked immunosorbent assay was performed by the core facility at Children's Hospital Colorado, Aurora, CO, USA. The total number of patients in each category is as follows: 10 NF paediatric, 19 IDC paediatric, 6 NF adult, and 16 IDC adult.
Statistical analysis
Statistical analyses were performed using Statview software (SAS Institute, Cary, NC, USA). Statistical significance was set a priori at P< 0.05 and all data are presented as mean ± SD in the text and tables unless otherwise indicated and mean ± SEM in the figures. For analyses using log-transformed data, the means and error measures (SD or SEM) are given for the raw values for visual clarity.
Results
β2-Adrenergic receptor is down-regulated in paediatric idiopathic dilated cardiomyopathy
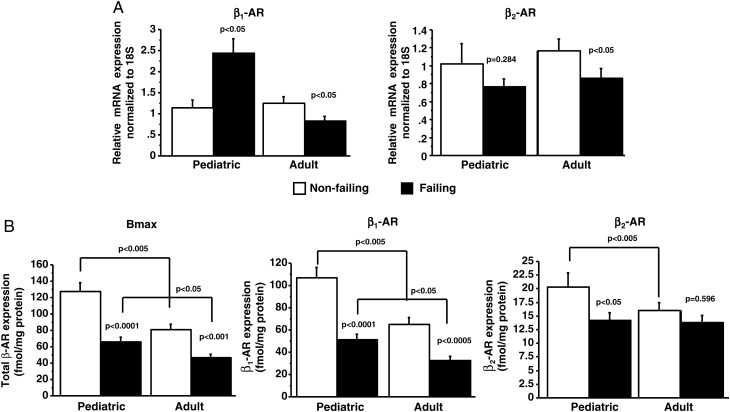
In preparations from the left ventricle, β-AR mRNA levels were determined. As shown in Figure 1A, whereas β1-AR and β2-AR mRNA expression are down-regulated in adult HF [β1-AR: NF 1.2 ± 0.8/failing (F) 0.8 ± 0.5; β2-AR: NF 1.2 ± 0.6/F 0.9 ± 0.5], β1-AR mRNA expression is up-regulated and β2-AR mRNA expression does not change in paediatric HF (β1-AR: NF 1.1 ± 0.6/F 2.4 ± 1.9; β2-AR: NF 1.0 ± 0.7/F 0.8 ± 0.5).
Figure 1.
β1- and β2-adrenergic receptor levels are down-regulated in paediatric heart failure patients. (A) Antithetical regulation of β1- and β2-adrenergic receptor mRNA levels in paediatric and adult heart failure patients. P-values correspond to failing-to-non-failing comparisons unless otherwise noted in the figure. (B) β-Adrenergic receptor levels were determined by [125I]-iodocyanopindolol binding. AR, adrenergic receptor; F, failing; NF, non-failing; Bmax, total β-adrenergic receptor.
β-Adrenergic receptor density measurements were also performed in these tissues and showed a similar decrease in the total β-AR number (Bmax) due to HF in adult (NF 80.8 ± 20.9/F 46.3 ± 13.7) and paediatric (NF 127.4 ± 33.4/F 65.5 ± 22.4) patients (Figure 1B). However, the total β-AR receptor density was ∼1.5-fold higher in NF paediatric subjects when compared with adults. As shown in Figure 1B, β1-AR density is decreased in children (NF 107.1 ± 27.9/F 51.3 ± 18.7) and adults (NF 64.9 ± 19.5/F 32.5 ± 11.3) with HF. However, β2-AR density is decreased only in paediatric HF patients (NF 20.3 ± 7.8/F 14.2 ± 4.9; adult: NF 16.0 ± 4.3/F 13.7 ± 3.9) and represents a novel characteristic of paediatric HF. Not only is the absolute amount of each receptor subtype important, but the stoichiometry between the two subtypes (represented as the β1:β2 ratio) also contributes to the phenotype due to redundancies of the intracellular pathways. The β1:β2 ratio significantly changes with HF in both adult patients (NF 80 ± 5:20 ± 5%/F 69 ± 7:31 ± 7%, P< 0.005) and paediatric patients (NF 84 ± 4:16 ± 4%/F 78 ± 5:22 ± 5%, P< 0.01). Under NF conditions, the β1:β2 ratio is similar between children and adults. However, the magnitude of the change in response to HF is almost 2-fold greater in adults compared with children, creating a significant difference in the β1:β2 ratio between the failing adult and failing paediatric hearts (P< 0.005).
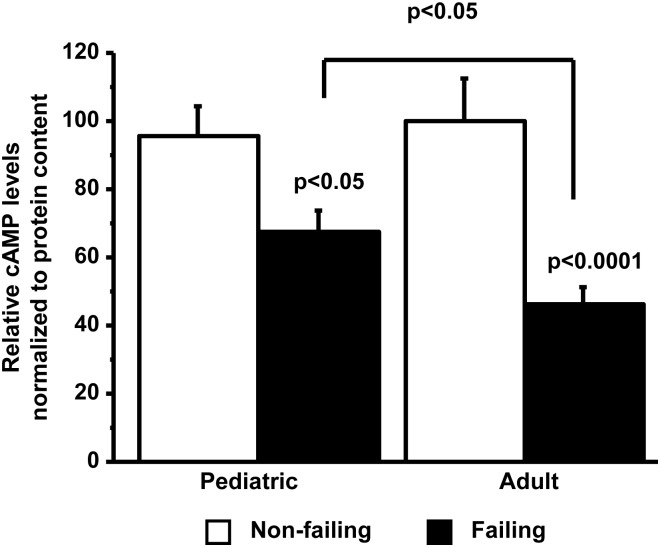
Decrease in cyclic adenosine monophosphate levels is blunted in paediatric heart failure
In the classical description of the β1-AR signalling pathway, β1-AR stimulation activates PKA via an increase in the adenylyl cyclase generation of cAMP. Cyclic adenosine monophosphate levels are decreased in HF most likely due to the desensitization of the β-AR system.23 We investigated whether cAMP levels are also decreased in paediatric IDC. As shown in Figure 2, although cAMP levels are decreased in paediatric IDC (NF 95.8 ± 27.3/F 67.2 ± 27.7), they were significantly higher than the levels noted in adult IDC (NF 100.0 ± 30.1/F 46.8 ± 20.6).
Figure 2.
Down-regulation of cyclic adenosine monophosphate levels is greater in adult than in paediatric HF patients. Cyclic adenosine monophosphate levels were measured by enzyme-linked immunosorbent assay. cAMP, cyclic adenosine monophosphate, F, failing; NF, non-failing.
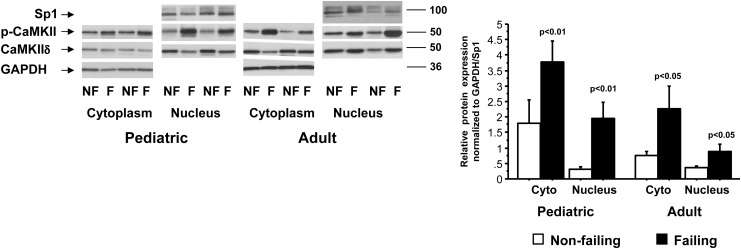
Ca2+-calmodulin kinase phosphorylation levels are increased in paediatric and adult heart failure
We tested phosphorylation levels of CaMKIIδ in the nuclear and cytoplasmic fractions of adult and paediatric IDC patients and NF subjects. As shown in Figure 3, the phosphorylation of CaMKIIδ is increased in the nuclear and cytoplasmic fractions of adult (cyto: NF 0.7 ± 0.3/F 2.3 ± 2.0; nuclear: NF 0.4 ± 0.2/F 0.8 ± 0.4) and paediatric IDC patients (cyto: NF 1.8 ± 2.2/F 3.8 ± 1.9; nuclear: NF 0.3 ± 0.2/F 1.9 ± 1.5). There was no difference in total CaMKIIδ based on age or the presence of HF.
Figure 3.
Ca2+-calmodulin kinase II phosphorylation is increased in paediatric and adult nuclear fractions but only in the adult cytoplasmic fraction. F, failing; NF, non-failing; cyto, cytoplasm.
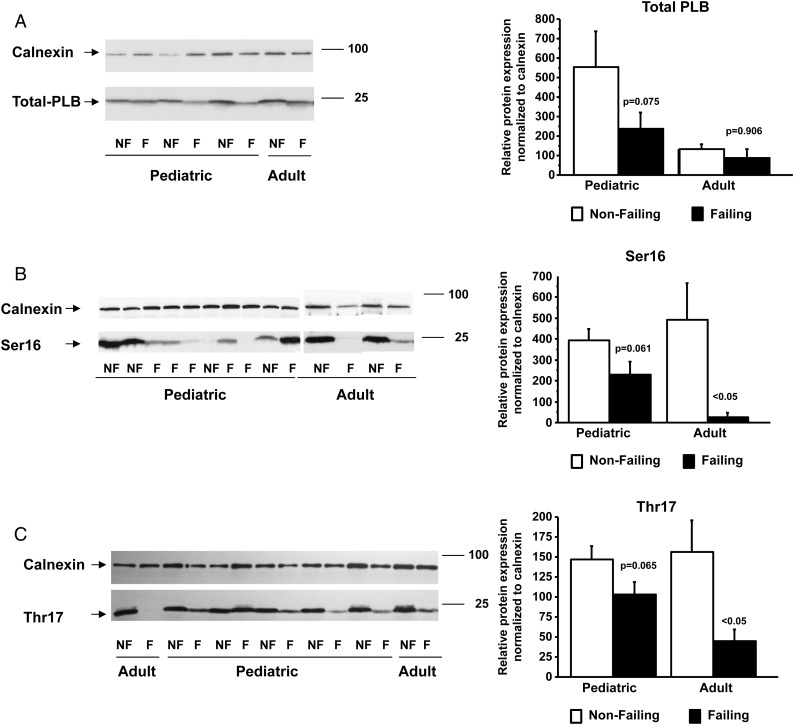
Phospholamban phosphorylation is unchanged in paediatric heart failure
Phospholamban is phosphorylated by PKA on Ser16 and CaMKII on Thr17. We measured total PLB levels and Ser16 and Thr17 phosphorylation of PLB in paediatric and adult IDC samples. As shown in Figure 4, total PLB levels were unchanged in adult (NF 132.7 ± 50.2/F 87.3 ± 89.1) and paediatric (NF 552.5 ± 762.2/F 237.6 ± 398.6) HF patients. In addition, Ser16 and Thr17 phosphorylation did not change in paediatric IDC samples (Ser16: NF 392.3 ± 230.5/F 231.3 ± 270.0; Thr17: NF 146.8 ± 69.1/F 103.3 ± 73.5) but is decreased in adult HF (Ser16: NF 492.6 ± 346.9/F 26.9 ± 40.5; Thr17: NF 156.0 ± 79.7/F 45.0 ± 29.2).
Figure 4.
Phosphorylation of phospholamban levels are down-regulated in adult but not in paediatric HF patients. Total phospholamban (A), Ser16 (B), and Thr17 (C) phosphorylation levels were measured by western blot and normalized to calnexin. The measurement of total phospholamban was done on Ser16 and Thr17 blots. PLB, phospholamban; F, failing; NF, non-failing.
Expression of PP1β and PP2A is increased in adult but not in paediatric heart failure
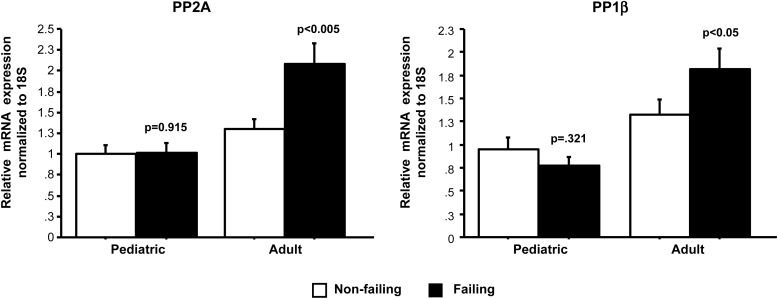
The decreased phosphorylation of PLB in the setting of increased CaMK phosphorylation is likely due to an increase in phosphatase activity.17 Since PLB phosphorylation was only decreased in adults but not children with HF, we examined the levels of PP2A and PP1β phosphatases involved in PLB phosphorylation. As shown in Figure 5, PP2A and PP1β mRNA levels were increased in adult (PP2A: NF 1.3 ± 0.3/F 2.1 ± 0.7; PP1β: NF 1.2 ± 0.5/F 1.8 ± 0.7) but not in a paediatric (PP2A: NF 1.0 ± 0.3/F 1.0 ± 0.4; PP1β: NF 1.0 ± 0.4/F 0.8 ± 0.3) HF patients.
Figure 5.
PP2A and PP1β expression analysis. The mRNA expression of PP2A and PP1β is increased in adult but not in paediatric HF. P-values correspond to failing-to-non-failing comparisons. F, failing; NF, non-failing.
B-type natriuretic peptide and Connexin43 are antithetically regulated in paediatric and adult HF
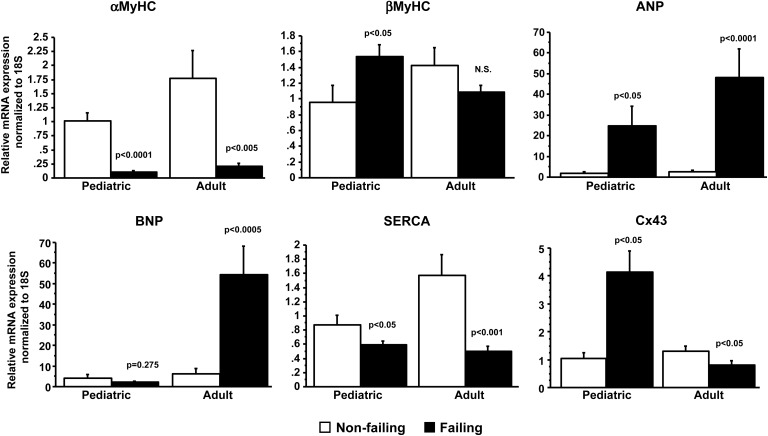
To test if paediatric HF induced the expression of the pathological gene programme, mRNA levels of αMyHC, βMyHC, ANP, BNP, SERCA, and connexin43 (Cx43) were determined (Figure 6). As shown in Figure 6, the expression of several markers is similarly regulated in paediatric (αMyHC: NF 1.0 ± 0.4/F 0.1 ± 0.1; βMyHC: NF 1.0 ± 0.7/F 1.5 ± 0.8; ANP: NF 1.8 ± 2.5/F 24.9 ± 51.3; SERCA: NF 0.9 ± 0.4/F 0.6 ± 0.3) and adult (αMyHC: NF 1.8 ± 2.5/F 0.2 ± 0.2; βMyHC: NF 1.4 ± 1.1/F 1.1 ± 0.4; ANP: NF 2.5 ± 3.5/F 48.2 ± 65.1; SERCA: NF 1.6 ± 1.5/F 0.5 ± 0.3) HF. However, BNP and Cx43 are antithetically regulated in these samples (paediatric: BNP: NF 4.0 ± 5.5/F 2.1 ± 3.3; Cx43: NF 1.0 ± 0.7/F 4.1 ± 4.2; adult: BNP: NF 6.1 ± 13.4/F 54.4 ± 64.9; Cx43: NF 1.3 ± 0.9/F 0.8 ± 0.8).
Figure 6.
Pathological gene expression analysis. Several components of a pathological gene programme change in accordance with the adult literature and with the adult profile demonstrated by the current experiments with the exception of B-type natriuretic peptide which is unchanged in paediatric HF, and cx43, which is antithetically regulated when compared with adult HF patients. P-values correspond to failing-to-non-failing comparisons. F, failing; NF, non-failing.
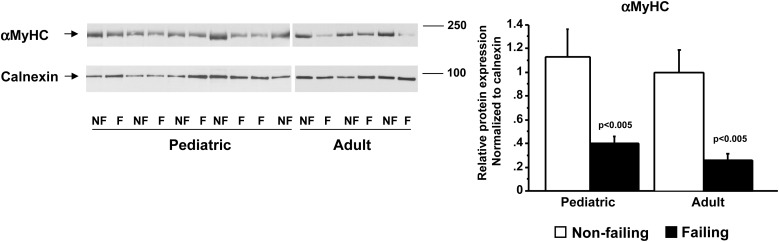
The down-regulation of αMyHC is thought to have important consequences for contractility, and small decreases in αMyHC protein can have a major effect on power output.24 Previous studies have estimated that 2–7% of the total MyHC in adult NF hearts was composed of αMyHC, and its levels were very low to undetected in failing hearts.25 Our results show a 2-fold reduction in αMyHC protein levels in the failing paediatric (NF 1.1 ± 1.0/F 0.4 ± 0.4) and adult (NF 1.0 ± 0.4/F 0.3 ± 0.1) hearts (Figure 7).
Figure 7.
αMyHC protein levels are increased in paediatric HF patients. αMyHC protein levels were measured by western blot using a specific antibody and normalized to calnexin. F, failing; NF, non-failing.
Discussion
Molecular changes in response to HF in adults are well characterized. Changes in β-AR levels were first described over 20 years ago, and although there are still many unknowns, the now standard use of BB treatment in adults is at least in part supported by investigations into how the β-AR system is regulated in HF. However, the results of the only randomized multicentre BB clinical trial in symptomatic paediatric HF failed to demonstrate any benefit from non-specific BB treatment in this population. Although this trial was challenged by the heterogeneity of the patients enrolled and the high rate of spontaneous improvement in enrolled subjects, these results were unexpected and led us to determine the influence of HF on the regulation of the β-AR system in the paediatric population. In an effort to minimize the confounding difficulties related to the heterogeneity of paediatric HF on these investigations, we chose to investigate children and adults with end-stage IDC only. In this population, we demonstrate that the influence of HF on β-ARs and their intracellular signalling molecules is unique between children and adults. Specifically, we showed that β2-AR expression, phosphorylation of PLB, and expression of phosphatases and components of the pathological gene programme are uniquely regulated in paediatric HF patients. These results suggest a distinct molecular profile in children with HF. Given that the current treatments for HF in adults are supported by several randomized clinical trials associated with reported changes in molecular responses to BB therapy,26 the observed differences in the molecular profile of children with HF suggests that paediatric-specific therapies may be needed to treat this population.
While our data recapitulate two established characteristics of cardiac β-AR expression: (i) an age-related decline in total β-AR expression26 and (ii) down-regulation of β1-AR expression with no changes in β2-AR in response to HF in adults,27 our results demonstrate that the down-regulation of both receptors is a unique characteristic of the paediatric HF population. These age-related differences in β-AR adaptation occur in the setting of similar plasma norepinephrine levels between adults and children,28 suggesting age-specific contributions of myocellular mechanisms. Although speculative in nature, it is possible that β1-AR stimulation in HF is pathological in both populations, but that some preservation of β2-AR function is beneficial.29,30 Inhibition of the already down-regulated β2-ARs may override the benefits of β1-AR inhibition in children, limiting the efficacy of non-specific blockade (eg. carvedilol) in paediatric HF. Interestingly, although the β1-AR is desensitized and down-regulated, mRNA levels are increased in paediatric HF. These results suggest different post-transcriptional regulation in paediatric and adult HF. It is well known that several RNA-binding proteins or miRNAs can bind and regulate the 3′UTR of the human β1- or β2-AR resulting in decreased protein levels.31,32 It is also possible that autoantibodies target both β1- and β2-ARs in the paediatric HF population.
In HF, desensitization and down-regulation of β1-AR results in decreased levels of the second messenger cAMP.23 Although we observed a decrease in cAMP levels in paediatric HF patients, this decrease was less dramatic than in adult patients. The blunted down-regulation of cAMP levels in the paediatric population can be due to decreased β2-AR levels and subsequent Gi-mediated inhibition of β1-AR signalling.33 Alternatively, it is possible that phosphodiesterase or adenylyl cyclase activities are different in paediatric and adult HF. One of the effects of decreased cAMP levels is the dephosphorylation of PLB. This results in the repression of SERCA2 activity and a decrease in SR Ca2+ uptake. In the paediatric population, consistent with the attenuated decrease in cAMP, PLB phosphorylation is unchanged, which could result in improved SR Ca2+ handling in paediatric HF patients. At present, mechanisms for preserved PLB phosphorylation in a setting of decreased (although blunted) levels of cAMP are unknown.
The other pathway regulated in response to the chronic stimulation of the β-AR receptor is the CaMKII pathway. As described in adults with HF, in paediatric HF, CaMKII phosphorylation is increased in the nuclear and cytoplasmic fractions. Ca2+-calmodulin kinase II influences pathological changes in gene expression, increases apoptosis and myocyte hypertrophy, and increases the phosphorylation of PLB.34 An increase in CaMKII activity is detrimental to the heart, and transgenic animals expressing a CaMKII peptide inhibitor or CaMKIIδ knockout animals are protected against cardiomyopathy.35,36 Although CaMKII activity is increased in adult HF, PLB phosphorylation is decreased, most likely due to an increase in phosphatase activity.37 As we did not observe a decrease in the phosphorylation of PLB in paediatric HF, we tested the expression levels of PP1β and PP2A. Previous studies have shown that PP1 (primarily PP1β) and PP2A are the main phosphatases responsible for the dephosphorylation of PLB.17,18 Our results show an increase in PP2A and PP1β expression in adult but not paediatric HF patients. These results suggest that a differential increase in phosphatase levels may be responsible for the lack of PLB dephosphorylation in paediatric HF. It is possible that the deregulation of SERCA2 activity and SR Ca2+ uptake is not a characteristic of paediatric HF.
Regulation of the FGP is a hallmark of cardiac pathology, and the directionality of expression is very similar in adult and paediatric patients overall. However, we observed an antithetical regulation of BNP and Cx43 in paediatric patients. Connexin43 expression decreases in the adult heart in response to HF and myocardial infarction.38 Being the primary ventricular gap junction protein, this decrease produces heterogeneities in cell-to-cell communication, pre-disposing the heart to ventricular arrhythmias.38 Even in the setting of severely impaired ventricular function, paediatric patients with HF rarely develop ventricular arrhythmias.39 The difference in Cx43 regulation could contribute to the decreased arrhythmias observed in this population. Circulating BNP is increased in adult and paediatric HF,40,41 and localized differences in BNP regulation have not been described previously. Initiation of medical therapy in paediatric IDC patients generally results in a transition to a compensated state and circulating BNP decreases accordingly. This trend in BNP was observed in the paediatric carvedilol trial with a significant decrease in BNP from screening to 6 months of maintenance treatment even in the placebo group.7 A post hoc analysis of the paediatric carvedilol trial demonstrated that BNP was not different among HF classes and the median BNP was quite low at enrolment compared with adults with HF.42 The lower circulating BNP levels in children may correlate with the decreased BNP mRNA levels observed.
There are several limitations of the current study. First, due to the characteristics of tissue bank-based studies, these investigations are cross-sectional. Therefore, the results can only ascertain associations not mechanisms. However, this is a necessary first step due to the paucity of knowledge in the area of the adaptation of the β-adrenergic system in paediatric HF. It is important to recognize that longitudinal and interventional studies are difficult given the invasive nature of the experiments that would be necessary (serial endomyocardial biopsies) in this vulnerable population. In addition, because it is impossible to obtain heart tissue from ‘normal’ children, the NF control hearts used in this study are from brain dead donors that could be influenced by underlying physiological and metabolic alterations related to their cause of death. Second, age-related development may influence our results. We minimized the influence of this potential confounder by only using heart tissue from pre-pubertal children (≤12 years of age). Third, although a comprehensive evaluation of children and adults is done at our institution to identify the cause of DCM, none of the patients included in this study had an identifiable genetic abnormality. Therefore, they could have undiagnosed sarcomeric or other genetic mutations as a cause of their cardiomyopathy and are not truly ‘idiopathic’ in nature. Finally, although only patients diagnosed with IDC in both age groups were studied, there are clearly differences in HF aetiologies between children and adults. One could argue that the adult population should not be used as a comparator due to these aetiological differences. This argument strengthens the importance of our molecular findings given that currently both populations are treated in an identical fashion based solely on existing adult data.
In conclusion, the results of this study demonstrate the influence of HF on some aspects of the β-adrenergic system in children. We have demonstrated significant differences in the molecular remodelling that occurs due to HF when compared with adult hearts. Our findings support the concept that adult treatment paradigms may not be perfectly extrapolated to children with HF and highlight the need for further investigation into age-specific HF therapies in children.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
This work was supported by the National Institutes of Health grants: K01HL088708 to C.C.S.; K08 HL080212 to B.L.S.; R21 HL097123 to S.D.M., B.L.S., C.C.S.; and R01 HL107715 to B.L.S.; and also supported by National Institute of Health/National Center for Research Resources, Colorado Clinical and Translational Sciences Institute (UL1 RR025780).
Conflict of interest: C.C.S.: Equity in miRagen, Inc.; B.L.S.: Research support from Forest Laboratories, Inc.
Supplementary Material
References
- 1.Wilkinson JD, Landy DC, Colan SD, Towbin JA, Sleeper LA, Orav EJ, Cox GF, Canter CE, Hsu DT, Webber SA, Lipshultz SE. The pediatric cardiomyopathy registry and heart failure: key results from the first 15 years. Heart Fail Clin. 2010;6:401–413. doi: 10.1016/j.hfc.2010.05.002. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Giuli F, Khaw KT, Cowie MR, Sutton GC, Ferrari R, Poole-Wilson PA. Incidence and outcome of persons with a clinical diagnosis of heart failure in a general practice population of 696 884 in the united kingdom. Eur J Heart Fail. 2005;7:295–302. doi: 10.1016/j.ejheart.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 3.Kirk R, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Dobbels F, Rahmel AO, Stehlik J, Hertz MI. The registry of the international society for heart and lung transplantation: thirteenth official pediatric heart transplantation report–2010. J Heart Lung Transplant. 2010;29:1119–1128. doi: 10.1016/j.healun.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 4.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 5.Kantor PF, Abraham JR, Dipchand AI, Benson LN, Redington AN. The impact of changing medical therapy on transplantation-free survival in pediatric dilated cardiomyopathy. J Am Coll Cardiol. 2010;55:1377–1384. doi: 10.1016/j.jacc.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 6.Konstam MA. Improving clinical outcomes with drug treatment in heart failure: what have trials taught? Am J Cardiol. 2003;91:9D–14D. doi: 10.1016/s0002-9149(02)03374-x. [DOI] [PubMed] [Google Scholar]
- 7.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 8.Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta-adrenergic-receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. doi: 10.1056/NEJM198207223070401. [DOI] [PubMed] [Google Scholar]
- 9.Staley NA, Noren GR, Einzig S, Rublein TG. Effect of early propranolol treatment in an animal model of congestive cardiomyopathy: I mortality and Ca2+ transport in sarcoplasmic reticulum. Cardiovasc Res. 1984;18:371–376. doi: 10.1093/cvr/18.6.371. [DOI] [PubMed] [Google Scholar]
- 10.Lohse MJ, Engelhardt S, Eschenhagen T. What is the role of beta-adrenergic signaling in heart failure? Circ Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- 11.Hoch B, Meyer R, Hetzer R, Krause EG, Karczewski P. Identification and expression of delta-isoforms of the multifunctional Ca2+/calmodulin-dependent protein kinase in failing and nonfailing human myocardium. Circ Res. 1999;84:713–721. doi: 10.1161/01.res.84.6.713. [DOI] [PubMed] [Google Scholar]
- 12.Calalb MB, McKinsey TA, Newkirk S, Huynh K, Sucharov CC, Bristow MR. Increased phosphorylation-dependent nuclear export of class II histone deacetylases in failing human heart. Clin Transl Sci. 2009;2:325–332. doi: 10.1111/j.1752-8062.2009.00141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–11152. [PubMed] [Google Scholar]
- 14.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of l-type calcium channels. Nat Cell Biol. 2000;2:173–177. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 15.Le Peuch CJ, Haiech J, Demaille JG. Concerted regulation of cardiac sarcoplasmic reticulum calcium transport by cyclic adenosine monophosphate dependent and calcium–calmodulin-dependent phosphorylations. Biochemistry. 1979;18:5150–5157. doi: 10.1021/bi00590a019. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T, Miyamoto S, Brown JH. Cardiomyocyte calcium and calcium/calmodulin-dependent protein kinase II: friends or foes? Recent Prog Horm Res. 2004;59:141–168. doi: 10.1210/rp.59.1.141. [DOI] [PubMed] [Google Scholar]
- 17.Boknik P, Fockenbrock M, Herzig S, Knapp J, Linck B, Luss H, Muller FU, Muller T, Schmitz W, Schroder F, Neumann J. Protein phosphatase activity is increased in a rat model of long-term beta-adrenergic stimulation. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:222–231. doi: 10.1007/s002100000283. [DOI] [PubMed] [Google Scholar]
- 18.Aoyama H, Ikeda Y, Miyazaki Y, Yoshimura K, Nishino S, Yamamoto T, Yano M, Inui M, Aoki H, Matsuzaki M. Isoform-specific roles of protein phosphatase 1 catalytic subunits in sarcoplasmic reticulum-mediated Ca2+ cycling. Cardiovasc Res. 2011;89:79–88. doi: 10.1093/cvr/cvq252. [DOI] [PubMed] [Google Scholar]
- 19.Lompre AM, Schwartz K, d'Albis A, Lacombe G, Van Thiem N, Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979;282:105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- 20.Engel G, Hoyer D, Berthold R, Wagner H. (+/-)[125iodo] cyanopindolol, a new ligand for beta-adrenoceptors: identification and quantitation of subclasses of beta-adrenoceptors in guinea pig. Naunyn Schmiedebergs Arch Pharmacol. 1981;317:277–285. doi: 10.1007/BF00501307. [DOI] [PubMed] [Google Scholar]
- 21.Sucharov CC, Dockstader K, McKinsey TA. Yy1 protects cardiac myocytes from pathologic hypertrophy by interacting with hdac5. Mol Biol Cell. 2008;19:4141–4153. doi: 10.1091/mbc.E07-12-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sucharov CC, Mariner P, Long C, Bristow M, Leinwand L. Yin yang 1 is increased in human heart failure and represses the activity of the human alpha-myosin heavy chain promoter. J Biol Chem. 2003;278:31233–31239. doi: 10.1074/jbc.M301917200. [DOI] [PubMed] [Google Scholar]
- 23.Bristow MR, Feldman AM. Changes in the receptor-g protein-adenylyl cyclase system in heart failure from various types of heart muscle disease. Basic Res Cardiol. 1992;87(Suppl. 1):15–35. doi: 10.1007/978-3-642-72474-9_2. [DOI] [PubMed] [Google Scholar]
- 24.Herron TJ, McDonald KS. Small amounts of alpha-myosin heavy chain isoform expression significantly increase power output of rat cardiac myocyte fragments. Circ Res. 2002;90:1150–1152. doi: 10.1161/01.res.0000022879.57270.11. [DOI] [PubMed] [Google Scholar]
- 25.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res. 2000;86:386–390. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- 26.White M, Roden R, Minobe W, Khan MF, Larrabee P, Wollmering M, Port JD, Anderson F, Campbell D, Feldman AM. Age-related changes in beta-adrenergic neuroeffector systems in the human heart. Circulation. 1994;90:1225–1238. doi: 10.1161/01.cir.90.3.1225. [DOI] [PubMed] [Google Scholar]
- 27.Bristow MR, Ginsburg R, Umans V, Fowler M, Minobe W, Rasmussen R, Zera P, Menlove R, Shah P, Jamieson S. Beta 1- and beta 2-adrenergic-receptor subpopulations in nonfailing and failing human ventricular myocardium: coupling of both receptor subtypes to muscle contraction and selective beta 1-receptor down-regulation in heart failure. Circ Res. 1986;59:297–309. doi: 10.1161/01.res.59.3.297. [DOI] [PubMed] [Google Scholar]
- 28.Ross RD, Daniels SR, Schwartz DC, Hannon DW, Shukla R, Kaplan S. Plasma norepinephrine levels in infants and children with congestive heart failure. Am J Cardiol. 1987;59:911–914. doi: 10.1016/0002-9149(87)91118-0. [DOI] [PubMed] [Google Scholar]
- 29.Chesley A, Lundberg MS, Asai T, Xiao RP, Ohtani S, Lakatta EG, Crow MT. The beta(2)-adrenergic receptor delivers an antiapoptotic signal to cardiac myocytes through G(i)-dependent coupling to phosphatidylinositol 3′-kinase. Circ Res. 2000;87:1172–1179. doi: 10.1161/01.res.87.12.1172. [DOI] [PubMed] [Google Scholar]
- 30.Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : role of a pertussis toxin-sensitive g protein. Circulation. 1999;100:2210–2212. doi: 10.1161/01.cir.100.22.2210. [DOI] [PubMed] [Google Scholar]
- 31.Port JD, Bristow MR. Adrenergic receptor coupling and uncoupling in heart failure. In: Richard A, Walsh MD, editors. Molecular Mechanisms for Cardiac Hypertrophy and Failure. 2005. pp. 323–340. Oxford: Taylor & Francis. [Google Scholar]
- 32.Wang WC, Juan AH, Panebra A, Liggett SB. Microrna let-7 establishes expression of beta2-adrenergic receptors and dynamically down-regulates agonist-promoted down-regulation. Proc Natl Acad Sci USA. 2011;108:6246–6251. doi: 10.1073/pnas.1101439108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He JQ, Balijepalli RC, Haworth RA, Kamp TJ. Crosstalk of beta-adrenergic receptor subtypes through Gi blunts beta-adrenergic stimulation of l-type Ca2+ channels in canine heart failure. Circ Res. 2005;97:566–573. doi: 10.1161/01.RES.0000181160.31851.05. [DOI] [PubMed] [Google Scholar]
- 34.Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–473. doi: 10.1016/j.yjmcc.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase ii inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 36.Ling H, Zhang T, Pereira L, Means CK, Cheng H, Gu Y, Dalton ND, Peterson KL, Chen J, Bers D, Heller Brown J. Requirement for Ca2+/calmodulin-dependent kinase ii in the transition from pressure overload-induced cardiac hypertrophy to heart failure in mice. J Clin Invest. 2009;119:1230–1240. doi: 10.1172/JCI38022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neumann J, Eschenhagen T, Jones LR, Linck B, Schmitz W, Scholz H, Zimmermann N. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J Mol Cell Cardiol. 1997;29:265–272. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- 38.Saffitz JE. Douglas p. Zipes lecture. Biology and pathobiology of cardiac connexins: from cell to bedside. Heart Rhythm. 2006;3:102–107. doi: 10.1016/j.hrthm.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 39.Rhee EK, Canter CE, Basile S, Webber SA, Naftel DC. Sudden death prior to pediatric heart transplantation: would implantable defibrillators improve outcome? J Heart Lung Transplant. 2007;26:447–452. doi: 10.1016/j.healun.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 40.Ationu A, Sorensen K, Whitehead B, Singer D, Burch M, Carter ND. Ventricular expression of brain natriuretic peptide gene following orthotopic cardiac transplantation in children—a three year follow up. Cardiovasc Res. 1993;27:2135–2139. doi: 10.1093/cvr/27.12.2135. [DOI] [PubMed] [Google Scholar]
- 41.Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J Clin Invest. 1991;87:1402–1412. doi: 10.1172/JCI115146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Auerbach SR, Richmond ME, Lamour JM, Blume ED, Addonizio LJ, Shaddy RE, Mahony L, Pahl E, Hsu DT. Bnp levels predict outcome in pediatric heart failure patients: post hoc analysis of the pediatric carvedilol trial. Circ Heart Fail. 2010;3:606–611. doi: 10.1161/CIRCHEARTFAILURE.109.906875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.