Abstract
Aims
To describe trends in incidence and case fatality among younger (18–54 years) and older (55–84 years) Swedish patients with heart failure (HF).
Methods and results
Through linking the Swedish national hospital discharge and the cause-specific death registries, we identified patients aged 18–84 years that were discharged 1987–2006 with a diagnosis of HF. Age-specific mean incidence rates per 100 000 person-years were calculated in four 5-year periods. Kaplan–Meier survival curves were plotted up to 3 years. From 1987 to 2006, there were 443 995 HF hospitalizations among adults 18–84 years. Of these, 4660 (1.0%) and 13 507 (3.0%) occurred in people aged 18–44 and 45–54 years (31.6% women), respectively. From the first to the last 5-year period, HF incidence increased by 50 and 43%, among people aged 18–34 and 35–44 years, respectively. Among people ≥45 years, incidence peaked in the mid-1990s and then decreased. Heart failure in the presence of cardiomyopathy increased more than two-fold among all age groups. Case fatality decreased for all age groups until 2001, after which no further significant decrease <55 years was observed.
Conclusion
Increasing HF hospitalization in young adults in Sweden opposes the general trend seen in older patients, a finding which may reflect true epidemiological changes. Cardiomyopathy accounted for a substantial part of this increase. High case fatality and lack of further case fatality reduction after 2001 are causes for concern.
Keywords: Heart failure, Incidence, Prognosis, Comorbidity, Young adults
See page 7 for the editorial comment on this article (doi:10.1093/eurheartj/eht360)
Introduction
Heart failure (HF) among the young is rare, but may exert a greater impact on active and income-generating individuals.1 Most HF-studies are based on older adults, and accordingly, little is known about the aetiology, incidence, and trends in HF among younger patients.2,3
While ischaemic heart disease (IHD) and hypertension are predominant causes of HF among older patients, the aetiological make-up of HF in young adults is more heterogeneous. Common causes of HF among the young include adult congenital heart disease (ACHD), different types of cardiomyopathies, myocarditis, or alcohol- or drug-related myocardial lesions.4,5
Despite improved outcomes in the management of coronary heart disease and HF,6 HF still carries a 50% 5-year mortality, parallel to that of many cancers.7,8 Studies indicate that HF incidence and mortality in Sweden, Scotland, and Australia have been decreasing since the early 1990s.6,9–12 Whether this change occurs in younger age groups has to our knowledge not been examined.
We used data from the Swedish national hospital discharge and cause-specific death registries to investigate age-specific trends in HF incidence, comorbidity and 1-year case fatality trends in ∼440 000 hospital discharges over a period of 20 years.
Methods
The registries
Sweden has universal healthcare providing low-cost hospital care to all Swedish permanent residents, with all hospitals reporting discharge diagnoses to a nationwide hospital discharge registry. The data are person based and include primary and secondary discharge diagnoses of any given hospitalized patient.13 Data from the national hospital discharge registry and cause-specific death registry were linked through a unique national personal identity number.
Procedures
Because a large percentage of younger patients with HF had other primary aetiological diagnoses, we included all patients aged 18–84 years from 1987 to 2006 who had a first-ever HF diagnosis code in any position. First admission was defined as any hospital admission with no previous admission for HF in the past 7 years. This was done to ensure the probability of being admitted for the first time was similar for any given time period. From 1987 to 1996, the International Classification of Diseases, Ninth Revision (ICD-9) was in use, and thereafter ICD-10. We defined HF by 427.10, 427.00 (ICD-8), 428A, 428B, and 428X (ICD-9) and I50 (ICD-10). The following diagnoses for concomitant or pre-existing comorbidity were included up to the point of the HF yielding hospitalization: cardiomyopathy 425 (ICD-9), I42, I43 (ICD-10); IHD 410–414 (ICD-9), I20–I25 (ICD-10); valvulopathies 391, 394–398, 421, 424 (ICD-9), I05-I09, I33-I39 (ICD-10); and congenital heart disease 745–747 (ICD-9) and Q20–Q28, Q87, Q89 (ICD-10). Because of overlapping HF aetiologies, we assigned mutually exclusive causes of HF in the following hierarchical order: (i) congenital heart disease, (ii) valvulopathy, (iii) IHD and/or diabetes and/or hypertension, (iv) cardiomyopathy, and (v) other causes.
Validity of the registers
From 1987 to 1996, a primary discharge diagnosis was lacking in <1% of all admissions. The diagnosis of HF in the Swedish discharge register has been validated showing 96% accuracy for primary diagnoses in internal medicine or cardiac wards, and 86 and 91% for secondary diagnoses in internal medicine and cardiac wards, respectively.14
Statistical methods
Annual HF incidence rates per 100 000 person-years and 95% confidence intervals (CIs) were calculated using the method of direct standardization, using the median year 1996 as a standard. Descriptive statistics were applied to summarize the comorbidity prevalence within the identified HF population. In addition, we used joinpoint regression for the estimation of the annual percentage change and to find the specific years when significant changes in the trends occurred (Joinpoint Regression Program, version 3.3.1. April 2008; Statistical Research and Applications Branch, National Cancer Institute). We fitted the data in a log-linear model and set the number of possible joinpoints between 0 and 3. For each estimate of mean annual percentage change, 95% CIs were calculated. Further, 1-year, age- and sex-specific case fatality rates were calculated up to 1 year after admission in patients aged 18–34, 35–44, 45–54, and 55–84 years over the periods 1987–91, 1992–96, 1997–2001, and 2002–06. To estimate changes in 1-year case fatality, hazard ratios for each time period of hospitalization were calculated by means of Cox regression with the period of 1987–91 as reference, and adjusted for age, sex, diabetes, IHD, cardiomyopathy, and ACHD and/or valve disease. Kaplan–Meier survival curves were applied to illustrate 3-year case fatality.
Results
Between 1987 and 2006, 443 995 hospitalizations for HF were recorded among patients aged 18–84 years. Of these HFs, 4660 (1.0%) and 13 507 (3.0%) were in patients aged 18–44 and 45–54 years, respectively. About one-third of the patients were women (Table 1).
Table 1.
Characteristics of patients by age group (18–44 and 45–55 years) and period at first admission (1987–2006)
| 1987–91 | 1992–96 | 1997–2001 | 2002–06 | Total | |
|---|---|---|---|---|---|
| 18–44 | |||||
| Number, n (%) | 972 (100%) | 1187 (100%) | 1133 (100%) | 1368 (100%) | 4660 (100%) |
| Mean age, years (SD) | 37.0 (6.9) | 37.4 (6.4) | 37.1 (6.4) | 37.0 (6.6) | 37.1 (6.6) |
| Women, n (%) | 356 (36.6) | 428 (36.1) | 421 (37.2) | 461 (33.7) | 1666 (35.8) |
| Diabetes, n (%) | 165 (17.0) | 178 (15.0) | 139 (12.3) | 154 (11.3) | 636 (13.6) |
| Hypertension, n (%) | 109 (11.2) | 147 (12.4) | 153 (13.5) | 232 (17.0) | 641 (13.8) |
| Prior AMI, n (%) | 144 (14.8) | 226 (19.0) | 150 (13.2) | 157 (11.5) | 677 (14.5) |
| Any IHD, n (%) | 203 (20.9) | 305 (25.7) | 224 (19.8) | 232 (17.0) | 964 (20.7) |
| Valvular disease, n (%) | 126 (13.0) | 149 (12.6) | 134 (11.8) | 157 (11.5) | 566 (12.1) |
| Congenital heart disease, n (%) | 57 (5.9) | 56 (4.7) | 73 (6.4) | 90 (6.6) | 276 (5.9) |
| Cardiomyopathy, n (%) | 141 (14.5) | 198 (16.7) | 243 (21.4) | 335 (24.5) | 917 (19.7) |
| Prior stroke, n (%) | 40 (4.1) | 50 (4.2) | 36 (3.2) | 42 (3.1) | 168 (3.6) |
| Atrial fibrillation, n (%) | 80 (8.2) | 117 (9.9) | 133 (11.7) | 205 (15.0) | 535 (11.5) |
| Malignancy, n (%) | 102 (10.5) | 94 (7.9) | 71 (6.3) | 81 (5.9) | 348 (7.5) |
| 45–54 | |||||
| Number, n (%) | 2456 (100%) | 3830 (100%) | 3806 (100%) | 3415 (100%) | 13 507 (100%) |
| Mean age, years (SD) | 50.4 (2.8) | 50.4 (2.7) | 50.8 (2.7) | 50.5 (2.8) | 50.5 (2.7) |
| Women, n (%) | 758 (30.9) | 1220 (31.9) | 1100 (28.9) | 989 (29.0) | 4067 (30.1) |
| Diabetes, n (%) | 480 (19.5) | 750 (19.6) | 748 (19.7) | 689 (20.2) | 2667 (19.7) |
| Hypertension, n (%) | 371 (15.1) | 760 (19.8) | 830 (21.8) | 887 (26.0) | 2848 (21.1) |
| Prior AMI, n (%) | 690 (28.1) | 1093 (28.5) | 998 (26.2) | 819 (24.0) | 3600 (26.7) |
| Any IHD, n (%) | 932 (37.9) | 1601 (41.8) | 1515 (39.8) | 1243 (36.4) | 5291 (39.2) |
| Valvular disease, n (%) | 227 (9.2) | 351 (9.2) | 311 (8.2) | 323 (9.5) | 1212 (9.0) |
| Congenital heart disease, n (%) | 35 (1.4) | 46 (1.2) | 46 (1.2) | 40 (1.2) | 167 (1.2) |
| Cardiomyopathy, n (%) | 219 (8.9) | 445 (11.6) | 513 (13.5) | 523 (15.3) | 1700 (12.6) |
| Prior stroke, n (%) | 138 (5.6) | 209 (5.5) | 202 (5.3) | 172 (5.0) | 721 (5.3) |
| Atrial fibrillation, n (%) | 345 (14.0) | 588 (15.4) | 656 (17.2) | 690 (20.2) | 2279 (16.9) |
| Malignancy, n (%) | 247 (10.1) | 381 (9.9) | 372 (9.8) | 270 (7.9) | 1270 (9.4) |
The overall burden of comorbidity in this young HF population was substantial. Of patients aged <45 years, 21% had IHD with 15% having acute myocardial infarction (MI). Fourteen per cent had diabetes, 4% a prior stroke, and 8% any cancer. For patients aged 45–54 years, IHD, MI and diabetes, stroke, and cancer was found in 39, 27, and 20, 5 and 9%, respectively. Concomitant cardiomyopathy was registered in 20% of those aged <45 years and in 13% of those aged 45–54 years. Cardiomyopathy increased from 15 to 25% and from 9 to 15% over the four periods in the <45 and 45–54 age groups, respectively. Concomitant valve disease and ACHD among patients younger than 45 years remained stable throughout the study (Table 1). About 5% had no other diagnosis than HF (data not shown).
Figure 1 and Supplementary material online, Table S1 show the mutually exclusive categories of comorbidities by age over the entire study period. The proportion of IHD-related HF hospitalization increased substantially with age, whereas cardiomyopathies decreased. The proportion of patients diagnosed with ACHD was 12.0% among the youngest age group and 0.3% among the oldest. The proportion with valve disease and ACHD did not change materially over time in any age group. The prevalence of the combined common aetiologies of IHD, diabetes, and hypertension below the age of 55 years was stable throughout the study period in patients <55, but not in older patients (increase 54 to 66%), chiefly as a result of a decrease in other causes. Cardiomyopathy increased across all age groups. A closer look at the large and heterogeneous group of patients labelled ‘other’ revealed no qualitative differences between the first and the last periods, the most common diagnoses being malignancy, perimyocarditis, and infectious diseases.
Figure 1.
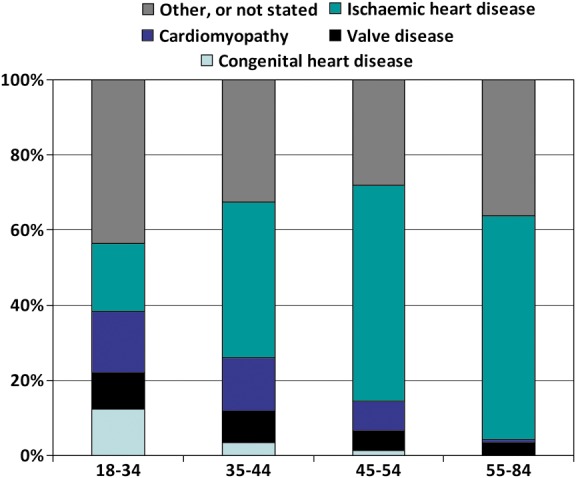
Comorbid prevalence (%) of mutually exclusive diagnoses among heart failure patients of different ages.
Table 2 illustrates the divergent trends in HF hospitalization between younger and older patients. Whereas hospitalization in the 55–84 year age group peaked in 1992–96 at 854 per 100 000 decreasing to 603 per 100 000 in 2002–06, HF hospitalization among persons <45 years increased throughout the entire observation period. Trends among persons aged 45–54 years were similar to those aged 55–84 years with a less marked decrease after 1996. A joinpoint analysis confirmed the continuous increase throughout the period for the 18–34 year age group, and from 1999 to 2006 for the age group 35–44 years. Further in adults ≥45 years a rising then falling pattern was seen 1994 signifying the marked breaking point for a decline in HF rates (Supplementary material online, Figure and Table S2).
Table 2.
Incidence of first hospital admissions for heart failure per 100 000 person-years in Sweden 1987–2006 by age, sex, and period
| Age group | 18–34 | Per 100 000 | Hazards ratio (95% CI) | 35–44 | Per 100 000 | Hazards ratio (95% CI) | 45–54 | Per 100 000 | Hazards ratio (95% CI) | 55–84 | Per 100 000 | Hazards ratio (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | n | n | n | n | ||||||||
| 1987–91 | 266 | 2.5 | 1.00 | 706 | 10.2 | 1.00 | 2456 | 46.2 | 1.00 | 105 741 | 719 | 1.00 |
| 1992–96 | 312 | 2.8 | 1.15 (1.00–1.33) | 875 | 13.6 | 1.33 (1.22–1.45) | 3830 | 59.6 | 1.29 (1.22–1.36) | 125 138 | 854 | 1.19 (1.15–1.23) |
| 1997–2001 | 319 | 2.9 | 1.19 (1.03–1.37) | 814 | 12.7 | 1.25 (1.14–1.36) | 3806 | 55.6 | 1.20 (1.14–1.27) | 102 067 | 688 | 0.96 (0.92–0.99) |
| 2002–06 | 387 | 3.7 | 1.50 (1.31–1.71) | 981 | 14.6 | 1.43 (1.31–1.55) | 3415 | 53.6 | 1.16 (1.10–1.22) | 92 882 | 603 | 0.84 (0.81–0.87) |
| Men | ||||||||||||
| 1987–91 | 150 | 2.7 | 1.00 | 466 | 13.0 | 1.00 | 1698 | 63.1 | 1.00 | 57 399 | 947 | 1.00 |
| 1992–96 | 183 | 3.2 | 1.18 (0.94–1.47) | 576 | 17.3 | 1.33 (1.19–1.49) | 2610 | 79.9 | 1.27 (1.20–1.34) | 66 766 | 1098 | 1.16 (1.12–1.20) |
| 1997–2001 | 178 | 3.2 | 1.16 (0.93–1.46) | 534 | 16.1 | 1.24 (1.10–1.39) | 2706 | 77.8 | 1.23 (1.17;1.30) | 55 847 | 896 | 0.95 (0.91–0.98) |
| 2002–06 | 233 | 4.4 | 1.61 (1.30–1.99) | 674 | 19.2 | 1.48 (1.33–1.65) | 2426 | 74.4 | 1.18 (1.11–1.25) | 51 244 | 777 | 0.82 (0.79–0.85) |
| Women | ||||||||||||
| 1987–91 | 116 | 2.2 | 1.00 | 240 | 7.4 | 1.00 | 758 | 29 | 1.00 | 48 342 | 522 | 1.00 |
| 1992–96 | 129 | 2.4 | 1.09 (0.88–1.36) | 299 | 9.8 | 1.33 (1.11–1.59) | 1220 | 38.9 | 1.34 (1.22–1.48) | 58 372 | 631 | 1.22 (1.17–1.27) |
| 1997–01 | 141 | 2.6 | 1.19 (0.96–1.46) | 280 | 9.2 | 1.25 (1.04–1.50) | 1100 | 32.6 | 1.12 (1.02–1.24) | 46 220 | 499 | 0.96 (0.92–1.00) |
| 2002–06 | 154 | 3.1 | 1.38 (1.13–1.70) | 307 | 9.5 | 1.30 (1.08–1.55) | 989 | 31.4 | 1.08 (0.98–1.20) | 41 638 | 436 | 0.84 (0.80–0.87) |
Table 3 shows trends in the incidence of HF with concomitant cardiomyopathy, IHD, and all other causes. From 1987–1991 to 2002–2006, the mean incidence of HF with cardiomyopathy more than doubled in all age groups (HRs 2.0–3.0). In patients ≥55 years, this was not at the expense of other diagnoses as the incidence of HF from other causes not related to IHD or cardiomyopathy also increased.
Table 3.
Incidence of heart failure and concomitant cardiomyopathy, ischaemic heart disease and all other causes per 100 000 person-years by age, sex, and period (1987–2006)
| Age | 18–34 |
35–44 |
45–54 |
55–84 |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Period | n | Inc* | HR | 95% CI | n | Inc* | HR | 95% CI | n | Inc* | HR | 95% CI | n | Inc* | HR | 95% CI |
| Cardiomyopathy | ||||||||||||||||
| 1987–91 | 37 | 0.3 | 1.00 | — | 104 | 1.5 | 1.00 | — | 219 | 4.3 | 1.00 | — | 925 | 8.3 | 1.00 | — |
| 1992–96 | 58 | 0.5 | 1.51 | (1.00–2.28) | 140 | 2.2 | 1.46 | (1.15–1.84) | 445 | 7.2 | 1.69 | (1.44–1.99) | 1708 | 15.4 | 1.85 | (1.70–2.02) |
| 1997–01 | 69 | 0.6 | 1.82 | (1.21–2.71) | 174 | 2.8 | 1.80 | (1.45–2.24) | 513 | 8.0 | 1.88 | (1.60–2.20) | 1974 | 17.1 | 2.06 | (1.89–2.25) |
| 2002–06 | 108 | 1.0 | 2.98 | (2.05–4.33) | 227 | 3.4 | 2.23 | (1.81–2.74) | 523 | 8.7 | 2.04 | (1.74–2.39) | 2305 | 18.7 | 2.25 | (2.07–2.45) |
| Ischaemic heart disease, IHD | ||||||||||||||||
| 1987–91 | 16 | 0.2 | 1.00 | 177 | 2.3 | 1.00 | 901 | 16.6 | 1.00 | 42 928 | 300 | 1.00 | ||||
| 1992–96 | 34 | 0.3 | 1.98 | (1.20–3.27) | 262 | 3.6 | 1.58 | (1.27–1.96) | 1540 | 23.4 | 1.41 | (1.30–1.53) | 54 446 | 380 | 1.27 | (1.20–1.34) |
| 1997–01 | 21 | 0.2 | 1.20 | (0.72–2.00) | 187 | 2.6 | 1.14 | (0.91–1.43) | 1443 | 20.4 | 1.23 | (1.13–1.34) | 44 817 | 303 | 1.01 | (0.96–1.07) |
| 2002–06 | 25 | 0.2 | 1.45 | (0.89–2.36) | 188 | 2.5 | 1.09 | (0.87–1.37) | 1177 | 17.9 | 1.08 | (0.98–1.18) | 41 675 | 266 | 0.89 | (0.84–0.94) |
| All other diagnoses | ||||||||||||||||
| 1987–91 | 213 | 2.0 | 1.00 | 425 | 6.4 | 1.00 | 1336 | 25.3 | 1.00 | 61 888 | 406 | 1.00 | ||||
| 1992–96 | 220 | 2.0 | 1.02 | (0.86–1.19) | 473 | 7.5 | 1.17 | (1.05–1.31) | 1845 | 28.8 | 1.14 | (1.07–1. 22) | 68 984 | 452 | 1.11 | (1.08–1.15) |
| 1997–01 | 229 | 2.2 | 1.08 | (0.92–1.27) | 453 | 7.3 | 1.14 | (1.02–1.27) | 1850 | 27.1 | 1.07 | (1.01–1.15) | 55 276 | 361 | 0.89 | (0.86–0.91) |
| 2002–06 | 254 | 2.5 | 1.25 | (1.07–1.45) | 566 | 8.6 | 1.35 | (1.22–1.49) | 1715 | 26.7 | 1.06 | (0.99–1.13) | 48 902 | 311 | 0.76 | (0.74–0.79) |
HR, hazard ratio for HF hospitalization. Inc*, incidence per 100 000 person-years.
Case fatality
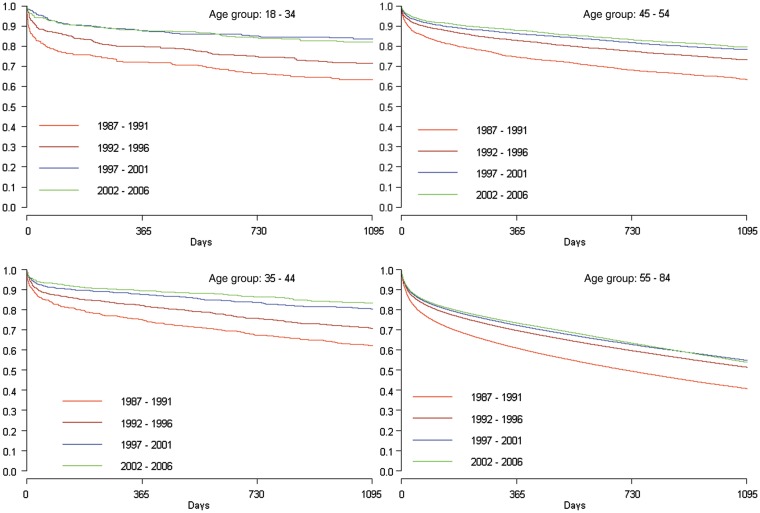
One-year case fatality at the beginning of the study period was high in all age groups, with about one in four dead at ages 18–54 and 39% ≥55 years (Table 4). From 1987–1991 to 2002–06 a marked adjusted 1-year relative case fatality reduction of 60–62% at 18–44 years was observed, and correspondingly 56 and 38% at 45–54 and 55–84 years, respectively. Figure 2 demonstrates the survival benefit was sustained up to 3 years, but that no further significant improvement in case fatality occurred in the <55 year age group after 2001.
Table 4.
One-year case fatality (%) in first-time hospitalization for HF in Sweden 1987 to 2006 by age and period
| Period |
n |
One-year case fatality (%) |
HR* (95% CI) |
n |
One-year case fatality (%) |
HR* (95% CI) |
n |
One-year case fatality (%) |
HR* (95% CI) |
n |
One-year case fatality (%) |
HR* (95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 18–34 |
35–44 |
45–54 |
55–84 |
||||||||
| 1987–91 | 266 | 28.3 | 1 | 706 | 25.1 | 1 | 2456 | 25.4 | 1 | 105 741 | 39.1 | 1 |
| 1992–96 | 312 | 19.9 | 0.65 (0.46–0.91) | 875 | 18.1 | 0.69 (0.56–0.86) | 3830 | 17.2 | 0.64 (0.58–0.72) | 125 138 | 30.4 | 0.73 (0.72–0.74) |
| 1997–2001 | 319 | 12.3 | 0.39 (0.26–0.57) | 814 | 12.6 | 0.48 (0.37;0.61) | 3806 | 13.7 | 0.50 (0.44–0.56) | 102 067 | 27.8 | 0.66 (0.65–0.67) |
| 2002–06 | 387 | 12.2 | 0.38 (0.27–0.55) | 981 | 10.6 | 0.40 (0.32–0.51) | 3415 | 12.2 | 0.44 (0.39–0.50) | 92 882 | 26.6 | 0.62 (0.61–0.63) |
HR*, hazard ratio adjusted for age, sex, diabetes, ischaemic heart disease, cardiomyopathy, and adult congenital heart disease and/or valve disease.
Figure 2.
Kaplan–Meier survival curves. One-year case fatality after first heart failure hospitalization.
Discussion
During the study period, we observed an increase in hospitalization for HF among people <45 years of age which was divergent from the continuing decrease after 1992–96 seen among persons ≥54 years of age. The incidence of concomitant cardiomyopathy more than doubled during this period in all age groups. Case fatality in young patients was reduced by more than half, but with no further decrease after 2001.
Previous data from Sweden, Netherlands, and Scotland show that HF admissions have levelled off and eventually declined.9,10,12,15 This may now also be the case in the USA.16 Because HF is predominantly a disorder of the elderly, there is a lack of data on trends in younger people.
Decreasing mortality and increasing readmission rates influence hospitalization rates, but in our data 97.8% of admissions were unique individuals. For the same reason mortality reduction cannot explain the increasing hospitalization rates. Conversely, fiscal measures in Sweden during the past three decades have reduced hospital bed supply by two-thirds since 1980 leading to higher hospitalization thresholds.17 Because the national discharge registry only reached full coverage from 1987, it was not possible to exclude all readmissions within the 7 years prior to 1987. However, the expected effect on our data set would be an overestimation, not an underestimation of the incidence in the first 7 years.
Epidemiological studies of HF in patients <40 years of age are scarce. The Framingham cohort comprised patients aged 28–62 years, but none of the analyses from that cohort identified enough cases to appropriately address trends in HF in this age group. In a substudy of the CARDIA cohort, a 20-year follow-up prospective analysis of 5115 patients 18–30 years revealed new onset HF in a mere 27 subjects.5
Hypertension, diabetes, and IHD are major targets for reduction of HF. In the CARDIA study, hypertension was a major aetiological risk factor in the development of early HF.5 In our HF population, diabetes and IHD were common and increased with age, but not over time. The rising prevalence of hypertension in HF patients may influence the incidence of HF, but as the validity of this diagnosis is poor,13 this conjecture remains speculative. The introduction of reimbursement by diagnosis-related groups systems in 1992 may explain some of the increase in HF seen in our data between the first two periods, but does not explain the continued and accelerating increase in hospitalization for patients <45 years seen in the two latter periods.
Cardiomyopathy in heart failure
The incidence of cardiomyopathy more than doubled in all age groups, and the largest share of the increase in HF incidence among patients <45 years of age could be directly ascribed to increasing concomitant cardiomyopathy. Before 1995, cardiomyopathy was defined as ‘heart muscle disease of unknown cause’, but was later redefined to encompass ‘all disease affecting the heart muscle’. This changed coding practices affecting perceived admission and incidence rates,18 but cannot account for the continuous increase in cardiomyopathy throughout the 20-year period.
Only a few cross-sectional studies have described the epidemiology of cardiomyopathy.3,19–21 In a US population-based study, the sex- and age-adjusted incidence of idiopathic dilated cardiomyopathy in patients <55 years was 17.9/100 000 person-years (n = 32).3 Swedish population-based studies, using different definitions and age bands have shown varying but lower incidence rates more similar to our study ranging from 1.0/100 000 at 18–44 to 8.7/100 000 at 45–54 years.
The underlying cause of the observed increase of HF in the young is not known. Given the seriousness of HF diagnosis in young patients, these patients are highly likely to have been admitted for investigation regardless the time period. Accordingly, our findings may suggest a change in phenotype, with an increasing proportion of HF due to cardiomyopathy, most notably in the youngest patients in whom IHD is rare.
Obesity, which has more than tripled in Sweden among the young 1985–2002,22 changes in drug abuse patterns and excessive alcohol consumption are all potentially contributing factors, but their impact could not be assessed in our population.
Decreasing case fatality among young adults
One-year case fatality was substantially reduced in all age groups. Mortality data among persons <55 years of age with HF are exiguous. Rochester population-based data show stable 1-year mortality ranging from 23 to 28% (mean age 74 years).23 In contrast, another survey from the same population (1979–2000) reported a 52% survival improvement among men in their 60 s, but only 28% among men in their 80 s, a finding congruent to the more pronounced case fatality reduction observed among younger subjects in our population.24
Our data did not show any further improvement in fatality after 2001. Neurohormonal blockade has improved survival in patients with systolic HF.25–27 The largest relative reduction in case fatality occurred from 1987–1991 to 1997–2001, i.e. prior to the publication of the landmark trials validating beta-blocking agents in HF treatment, suggesting either that beta-blocker treatment had not been fully implemented,26,27 or did not impact fatality in this population. Beta-blockers and ACE-inhibitors were, however, at the time widely employed in the treatment of hypertension and IHD and may have curtailed the emergence or at least a further increase in HF during this time period.
Limitations
The study has several advantages, such as an unselected and large population and near-complete follow-up. However, there are also some limitations. First, using discharge diagnosis codes poses a risk of underestimation.28 Secondly, hospital admission rates and not true incidence were employed. It is reasonable to believe that young patients with new onset HF undergo comprehensive assessment, including echocardiography in a cardiac ward where the validity of the HF diagnosis is higher than stated by Ingelsson et al.14 in their study of elderly men. Additionally the lack of further mortality reduction over the last period would not indicate a spurious inflation in incidence because of inclusion of milder cases. Coding practices may certainly have influenced the data, but if so most likely at all ages.
A further limitation is the lack of important biological variables, increasingly used over the period, such as the measurement of natriuretic peptides, or ejection fraction. The absence of such objective measurements obviously detracts from the comparability of HF rates over time.
HF is, however, largely a clinical diagnosis, and though we contend that the lack of biological variables is unlikely to explain the diverging changes in HF incidence over time in the different age groups, we acknowledge that their addition would have added value to the study.
Diagnosing practices for cardiomyopathy changed in the mid-1990s.18 Accordingly, the group classified as other, could have harboured undiagnosed cases of cardiomyopathy from earlier periods. Recent unpublished data from an on-going validation study by our group showed that a diagnosis of DCM could be confirmed in 92% (202 out of 219 cases, using data from medical records from five calendar years over the period 1989–2009) with a further 4% classified as suspected DCM, and, importantly, no change in diagnostic accuracy over time. A systematic and variable underreporting of hypertension, a major cause of HF, was probably present, but this is unlikely to have influenced hospitalization rates differentially in different age groups. The stagnating mortality reduction observed does not support a falsely inflated incidence rate in the young. Thus, potential drifts in diagnoses and classifications are unlikely to explain the opposing trends in HF incidence in younger and older patients.
Conclusion
Increasing HF admission in young adults opposes the general trend noted in older patients. Although the cause for this is not known, it may reflect true epidemiological changes and may serve as an ominous sign of stagnating morbidity reduction among young adults. Declining case fatality reduction with lack of further improvement after 2001 is a cause for concern.
Supplementary material
Supplementary material is available at European Heart Journal online.
Funding
The study was funded by the Swedish Research Council (VR 521-2010-2984); the Swedish Heart-Lung Foundation (2009-0390); and the Swedish Council for Working Life and Social Research (Epilife 2006-1506).
Conflict of interest: none declared.
Supplementary Material
References
- 1.McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez-Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail (Practice Guideline) 2012;14:803–869. doi: 10.1093/eurjhf/hfs105. doi:10.1093/eurjhf/hfs105. [DOI] [PubMed] [Google Scholar]
- 2.Lloyd-Jones DM, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JM, Vasan RS, Benjamin EJ, Levy D. Lifetime risk for developing congestive heart failure: the Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. doi:10.1161/01.CIR.0000039105.49749.6F. [DOI] [PubMed] [Google Scholar]
- 3.Codd MB, Sugrue DD, Gersh BJ, Melton LJ., III Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy. A population-based study in Olmsted County, Minnesota, 1975–1984. Circulation. 1989;80:564–572. doi: 10.1161/01.cir.80.3.564. doi:10.1161/01.CIR.80.3.564. [DOI] [PubMed] [Google Scholar]
- 4.Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi: 10.1056/NEJM200004133421502. doi:10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 5.Bibbins-Domingo K, Pletcher MJ, Lin F, Vittinghoff E, Gardin JM, Arynchyn A, Lewis CE, Williams OD, Hulley SB. Racial differences in incident heart failure among young adults. N Engl J Med. 2009;360:1179–1190. doi: 10.1056/NEJMoa0807265. doi:10.1056/NEJMoa0807265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shafazand M, Rosengren A, Lappas G, Swedberg K, Schaufelberger M. Decreasing trends in the incidence of heart failure after acute myocardial infarction from 1993–2004: a study of 175,216 patients with a first acute myocardial infarction in Sweden. Eur J Heart Fail. 2011;13:135–141. doi: 10.1093/eurjhf/hfq205. doi:10.1093/eurjhf/hfq205. [DOI] [PubMed] [Google Scholar]
- 7.Stewart S, Ekman I, Ekman T, Oden A, Rosengren A. Population impact of heart failure and the most common forms of cancer: a study of 1 162 309 hospital cases in Sweden (1988 to 2004) Circ Cardiovasc Qual Outcomes. 2010;3:573–580. doi: 10.1161/CIRCOUTCOMES.110.957571. doi:10.1161/CIRCOUTCOMES.110.957571. [DOI] [PubMed] [Google Scholar]
- 8.Piller LB, Baraniuk S, Simpson LM, Cushman WC, Massie BM, Einhorn PT, Oparil S, Ford CE, Graumlich JF, Dart RA, Parish DC, Retta TM, Cuyjet AB, Jafri SZ, Furberg CD, Saklayen MG, Thadani U, Probstfield JL, Davis BR. Long-Term follow-up of participants with heart failure in the Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Circulation. 2011;124:1811–1818. doi: 10.1161/CIRCULATIONAHA.110.012575. doi:10.1161/CIRCULATIONAHA.110.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schaufelberger M, Swedberg K, Koster M, Rosen M, Rosengren A. Decreasing one-year mortality and hospitalization rates for heart failure in Sweden; Data from the Swedish Hospital Discharge Registry 1988 to 2000. Eur Heart J. 2004;25:300–307. doi: 10.1016/j.ehj.2003.12.012. doi:10.1016/j.ehj.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 10.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan RS. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. doi:10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 11.Teng TH, Finn J, Hobbs M, Hung J. Heart failure: incidence, case fatality, and hospitalization rates in Western Australia between 1990 and 2005. Circ Heart Fail. 2010;3:236–243. doi: 10.1161/CIRCHEARTFAILURE.109.879239. doi:10.1161/CIRCHEARTFAILURE.109.879239. [DOI] [PubMed] [Google Scholar]
- 12.Stewart S, MacIntyre K, MacLeod MM, Bailey AE, Capewell S, McMurray JJ. Trends in hospitalization for heart failure in Scotland, 1990–1996. An epidemic that has reached its peak? Eur Heart J. 2001;22:209–217. doi: 10.1053/euhj.2000.2291. doi:10.1053/euhj.2000.2291. [DOI] [PubMed] [Google Scholar]
- 13.Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. doi: 10.1186/1471-2458-11-450. doi:10.1186/1471-2458-11-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ingelsson E, Arnlov J, Sundstrom J, Lind L. The validity of a diagnosis of heart failure in a hospital discharge register. Eur J Heart Fail. 2005;7:787–791. doi: 10.1016/j.ejheart.2004.12.007. doi:10.1016/j.ejheart.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. doi:10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis LH, Whellan DJ, Hammill BG, Hernandez AF, Anstrom KJ, Shea AM, Schulman KA. Incidence and prevalence of heart failure in elderly persons, 1994–2003. Arch Intern Med. 2008;168:418–424. doi: 10.1001/archinternmed.2007.80. doi:10.1001/archinternmed.2007.80. [DOI] [PubMed] [Google Scholar]
- 17.Kroneman M, Siegers JJ. The effect of hospital bed reduction on the use of beds: a comparative study of 10 European countries. Soc Sci Med. 2004;59:1731–1740. doi: 10.1016/j.socscimed.2004.01.036. doi:10.1016/j.socscimed.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 18.Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. Circulation. 1996;93:841–842. doi: 10.1161/01.cir.93.5.841. doi:10.1161/01.CIR.93.5.841. [DOI] [PubMed] [Google Scholar]
- 19.Torp A. Incidence of congestive cardiomyopathy. Postgrad Med J. 1978;54:435–439. doi: 10.1136/pgmj.54.633.435. doi:10.1136/pgmj.54.633.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coughlin SS, Comstock GW, Baughman KL. Descriptive epidemiology of idiopathic dilated cardiomyopathy in Washington County, Maryland, 1975–1991. J Clin Epidemiol. 1993;46:1003–1008. doi: 10.1016/0895-4356(93)90167-y. doi:10.1016/0895-4356(93)90167-Y. [DOI] [PubMed] [Google Scholar]
- 21.Andersson B, Caidahl K, Waagstein F. Idiopathic dilated cardiomyopathy among Swedish patients with congestive heart failure. Eur Heart J. 1995;16:53–60. doi: 10.1093/eurheartj/16.1.53. doi:10.1093/eurheartj/16.1.53. [DOI] [PubMed] [Google Scholar]
- 22.Berg C, Rosengren A, Aires N, Lappas G, Toren K, Thelle D, Lissner L. Trends in overweight and obesity from 1985 to 2002 in Goteborg, west Sweden. Int J Obes (Lond) 2005;29:916–924. doi: 10.1038/sj.ijo.0802964. doi:10.1038/sj.ijo.0802964. [DOI] [PubMed] [Google Scholar]
- 23.Senni M, Tribouilloy CM, Rodeheffer RJ, Jacobsen SJ, Evans JM, Bailey KR, Redfield MM. Congestive heart failure in the community: trends in incidence and survival in a 10-year period. Arch Intern Med. 1999;159:29–34. doi: 10.1001/archinte.159.1.29. doi:10.1001/archinte.159.1.29. [DOI] [PubMed] [Google Scholar]
- 24.Roger VL, Weston SA, Redfield MM, Hellermann-Homan JP, Killian J, Yawn BP, Jacobsen SJ. Trends in heart failure incidence and survival in a community-based population. JAMA. 2004;292:344–350. doi: 10.1001/jama.292.3.344. doi:10.1001/jama.292.3.344. [DOI] [PubMed] [Google Scholar]
- 25.Garg R, Yusuf S. Overview of randomized trials of angiotensin-converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA. 1995;273:1450–1456. doi:10.1001/jama.1995.03520420066040. [PubMed] [Google Scholar]
- 26.The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. doi:10.1016/S0140-6736(98)11181-9. [PubMed] [Google Scholar]
- 27.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. doi:10.1016/S0140-6736(99)04440-2. [PubMed] [Google Scholar]
- 28.Kumler T, Gislason GH, Kirk V, Bay M, Nielsen OW, Kober L, Torp-Pedersen C. Accuracy of a heart failure diagnosis in administrative registers. Eur J Heart Fail. 2008;10:658–660. doi: 10.1016/j.ejheart.2008.05.006. doi:10.1016/j.ejheart.2008.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.