Abstract
Cells of budding yeast give rise to mother and daughter cells, which differ in that only mother cells express the HO endonuclease gene and are thereby able to switch mating types. In this study, we identified the MKT1 gene as a positive regulator of HO expression. The MKT1 gene encodes a protein with two domains, XPG-N and XPG-I, which are conserved among a family of nucleases, including human XPG endonuclease. Loss of MKT1 had little effect on HO mRNA levels but resulted in decreased protein levels. This decrease was dependent on the 3′ untranslated region of the HO transcript. We screened for proteins that associate with Mkt1 and isolated Pbp1, a protein that is known to associate with Pab1, a poly(A)-binding protein. Loss of PBP1 resembles an mkt1Δ deletion, causing decreased expression of HO at the posttranscriptional level. Mkt1 and Pbp1 cosedimented with polysomes in sucrose gradients, with Mkt1 distribution in the polysomes dependent on Pbp1, but not vice versa. These observations suggest that a complex of Mkt1 and Pbp1 regulates the translation of HO mRNA.
Gene expression can be regulated at multiple steps, including transcription, splicing, mRNA transport, mRNA stability, and translation. Regulation of mRNA translation depends on a temporally and spatially orchestrated sequence of protein-protein, protein-RNA, and RNA-RNA interactions. Posttranscriptional regulation plays an important role during the development of a wide variety of organisms (7). For example, the asymmetric patterns of gene expression in Xenopus and Drosophila embryos are determined by the differential localization, stabilization, and translation of maternally synthesized mRNAs. Translational regulation also plays a key role in germ line sex determination in Caenorhabditis elegans (6, 14, 39). In most of the cases studied in detail, posttranscriptional regulation is mediated by sites in the 3′ untranslated region (UTR) of the target mRNA.
The HO endonuclease initiates mating-type switching in Saccharomyces cerevisiae by creating a double-stranded break at the MAT locus (9). The HO gene is only transcribed in mother cells, i.e., cells that have previously budded and given birth to a daughter cell. In mother cells, HO is transcribed transiently during the cell cycle, shortly before budding and DNA replication (24). Activation of HO transcription depends on at least 10 genes, named SWI1 through SWI10. SWI4 and SWI6 encode subunits of a cell cycle-regulated transcription factor, SCB-binding factor, that activates a number of genes at the G1/S boundary (17). SWI1, SWI2, SWI3, and SWI10 encode components of a large multisubunit complex that is needed for the expression of many yeast genes (26).
Asymmetric transcription of the HO gene results from the preferential accumulation of a negative regulatory protein, Ash1, in daughter nuclei (3, 32). ASH1 mRNA is targeted to the distal tip of daughter buds in postanaphase cells (18, 35). Localization of ASH1 mRNA depends on an intact actin cytoskeleton and five SHE genes (15, 18, 35). SHE1 encodes a type V myosin motor, Myo4, that colocalizes with ASH1 mRNA at the tip of daughter cells (2, 8, 23, 36). Thus, ASH1 mRNA is localized to the bud tip by an actomyosin-based transport mechanism. The RNA-binding proteins Loc1 and Khd1 are also involved in ASH1 mRNA localization and translation (12, 19). These factors interact with and are required for efficient localization of the ASH1 mRNA. Overexpression of Khd1 decreases the level of Ash1 protein, suggesting that Khd1 could be involved in the translational control of the ASH1 mRNA during its transport to the bud tip (12).
HO expression is also regulated posttranscriptionally by the yeast Puf (for Pumilio and FBF) homolog Mpt5/Puf5 (34). Mpt5 binds to the 3′ UTR of HO mRNA and represses HO expression. Repression requires the 3′ UTR of HO, which contains a tetranucleotide, UUGU, that is also found in the binding sites of Pumilio and FBF. Mpt5 may affect mRNA stability or turnover via binding to the HO 3′ UTR. Null mutation of MPT5 confers mating-type switching on daughter cells, suggesting that Mpt5 provides a second mechanism for preventing the synthesis of HO protein in daughter cells.
Here we describe the identification of another gene, MKT1, that is required for HO expression at a posttranscriptional step. MKT1 encodes a protein containing two domains, XPG-N and XPG-I, that are conserved in human XPG endonuclease. We observed that disruption of MKT1 caused a decrease in HO protein and that this inhibition required the 3′ UTR of HO. We screened for proteins that interact with Mkt1 and isolated the PBP1 gene, which was previously shown to interact with the poly(A)-binding protein Pab1 (21). Loss of PBP1 causes a phenotype similar to that caused by mkt1Δ with regard to HO expression. Mkt1 and Pbp1 cosedimented with polysomes in sucrose gradients, and the association of Mkt1 in the polysome fraction was Pbp1 dependent, but not vice versa. These observations suggest that a complex of Mkt1 and Pbp1 regulates the translation of HO mRNA.
MATERIALS AND METHODS
Strains and general methods.
Escherichia coli DH5α was used for DNA manipulations. The yeast strains used in this study are described in Table 1. Standard procedures were followed for yeast manipulations (16). The media used in this study included yeast extract-peptone-dextrose (YPD) medium, synthetic complete medium (SC), synthetic minimal medium (SD), and sporulation medium (16). SC media lacking amino acids or other nutrients (e.g., SC−Ade lacks adenine) were used to select transformants and to score ADE2 reporter activity. SG was identical to SC except that it contained 2% galactose and raffinose instead of 2% glucose. Recombinant DNA procedures were carried out as described previously (29).
TABLE 1.
Strains used in this study
| Strain | Genotypea | Reference or source |
|---|---|---|
| W303 | aho ade2 trp1 can1 leu2 his3 ura3 GAL psi+ | 32 |
| K1107 | aHOp-LacZ-HO 3′UTR | 25 |
| K5552 | α ASH1-myc | 15 |
| 10B | α HOp-ADE2-HO 3′UTR | 34 |
| YKEN104 | α HOp-ADE2-HO 3′UTR myo4Δ::CgHIS3 | This study |
| YKEN301 | α HOp-ADE2-HO 3′UTR kanMX6::GAL1p-KHD1 | 12 |
| YKEN303 | α HOp-ADE2-HO 3′UTR myo4Δ kanMX6::GAL1p-KHD1 | 12 |
| YKEN304 | α HOp-ADE2-HO 3′UTR ash1Δ | 12 |
| YKEN306 | α HOp-ADE2-HO 3′UTR myo4Δ ash1Δ | 12 |
| YKEN451 | α ASH1-myc myo4Δ::CgHIS3 | This study |
| YKEN9152 | α HOp-ADE2-HO 3′UTR mkt1-19 kanMX6::GAL1p-KHD1 | This study |
| YKEN9152a | α HOp-ADE2-HO 3′UTR mkt1-19 ash1Δ | This study |
| TTC4 | aHOp-LacZ-HO 3′UTR myo4Δ::CgLEU2 | This study |
| TTC46 | a/α HO/HO | 34 |
| TTC47 | α HOp-ADE2-ADH 3′UTR | 34 |
| TTC237 | α ASH1-myc mkt1Δ::CgHIS3 | This study |
| TTC478 | α HOp-ADE2-HO 3′UTR mkt1Δ::CgHIS3 | This study |
| TTC798 | α HOp-ADE2-HO 3′UTR pbp1Δ::CgHIS3 | This study |
| TTC848 | α HOp-ADE2-HO 3′UTR MKT1-HA::kanMX6 | This study |
| TTC929 | a/α HO/HO mkt1Δ::CgHIS3/mkt1Δ::CgHIS3 | This study |
| TTC993 | a/α HO/HO pbp1Δ::CgHIS3/pbp1Δ::CgHIS3 | This study |
| TTC996 | α HOp-ADE2-HO 3′UTR PBP1-HA::kanMX6 | This study |
| TTC999 | aHOp-ADE2-HO 3′UTR PBP1-HA::kanMX6 | This study |
| TTC1049 | aHOp-ADE2-HO 3′UTR PBP1-HA::kanMX6 MKT1-myc::kanMX6 | This study |
| TTC1085 | aHOp-LacZ-HO 3′UTR mkt1Δ::CgHIS3 | This study |
| TTC1089 | aHOp-LacZ-HO 3′UTR pbp1Δ::CgHIS3 | This study |
| TTC1163 | aHOp-LacZ-HO 3′UTR mkt1Δ::CgHIS3 pbp1Δ::CgHIS3 | This study |
| TTC1108 | α HOp-ADE2-ADH 3′UTR mkt1Δ::CgHIS3 | This study |
| TTC1233 | α HOp-ADE2-HO 3′UTR PBP1-HA::kanMX6 mkt1Δ::CgHIS3 | This study |
| TTC1234 | α HOp-ADE2-HO 3′UTR MKT1-HA::kanMX6 pbp1Δ::CgHIS3 | This study |
| TTC1257 | α HOp-ADE2-HO 3′UTR mkt1Δ::CgTRP1 pbp1Δ::CgHIS3 | This study |
| TTC1302 | aHOp-ADE2-HO 3′UTR PBP1-HA::kanMX6 MKT1ΔC-myc::kanMX6 | This study |
| TTC1313 | aho mkt1Δ::CgLEU2 | This study |
| TTC1316 | aho pbp1Δ::CgLEU2 | This study |
| TTC1319 | aho myo4Δ::CgLEU2 | This study |
| TTC1333 | α HOp-ADE2-ADH 3′UTR mkt1Δ::CgTRP1 pbp1Δ::CgHIS3 | This study |
| TTC1334 | α HOp-ADE2-ADH 3′UTR pbp1Δ::CgHIS3 | This study |
| PJ69-4A | atrp1-901 leu2-3,112 ura3-52 his3-200 gal4Δ gal80Δ GAL2-ADE2 LYS2::GAL1-HIS3 met2::GAL7-lacZ | 13 |
All strains except PJ69-4A were isogenic derivatives of W303.
Plasmids.
The plasmids used in this study are described in Table 2. Plasmids YCpSHE2, YCpSHE3, and YCpBNI1 were kindly provided by R. P. Jansen. pGBD-MKT1 expresses Mkt1 fused to the Gal4 DNA-binding domain. The MKT1 open reading frame (ORF) was amplified by PCR with the 5′ primer 5′-CTCAGATCTATGGCAATCAAGTCATTGGAATCG-3′, incorporating a BglII site, and the 3′ primer 5′-CTCCTCGAGTCAGCTAGATAGAGCTGTTGTAGT-3′, incorporating an XhoI site. A 2.5-kb BglII-XhoI fragment generated by PCR was inserted into the BamHI-SalI gap of the pGBD-C1 vector (13) to generate pGBD-MKT1. pGBD-MKT1ΔC1 (amino acids [aa] 1 to 760) and pGBD-MKT1ΔC2 (aa 1 to 570) express Mkt1ΔC (aa 1 to 760) and Mkt1ΔC (aa 1 to 570), respectively, fused to the Gal4 DNA-binding domain. The MKT1 ORF was amplified by PCR as described above, and the resultant fragment was digested with EcoT22I or SalI to truncate the region encoding Mkt1-C (aa 761 to 830) or Mkt1-C (aa 571 to 830). The resultant BglII-EcoT22I and BglII-SalI fragments were inserted into the BamHI-PstI and the BamHI-SalI gaps of the pGBD-C1 vector to create pGBD-MKT1ΔC1 and pGBD-MKT1ΔC2, respectively. pGAD-PBP1 expresses Pbp1 (aa 475 to 722) fused to the Gal4 transcriptional activation domain. pGAD-PBP1 was cloned from Y2HL yeast genomic two-hybrid libraries (13). pK508 is YCp50 carrying a 14.5-kb fragment of chromosome XIV that contains the MKT1 gene. pK508 was cloned from a YCp50-based genomic library (27). YCplac33MKT1 is YCplac33 carrying MKT1. The 3.5-kb fragment encoding the MKT1 5′ UTR (0.7 kb), the MKT1 ORF, and the MKT1 3′ UTR (0.3 kb) was amplified by PCR with the 5′ primer 5′-CTCCCCGGGCAATGAATTACGCGAGTCGC-3′, incorporating an SmaI site, and the 3′ primer 5′-CTCCTCGAGTTCCTTTTCTTCTCTGCCGG-3′, incorporating an XhoI site. The resultant SmaI-XhoI fragment was inserted into the SmaI-SalI gap of the YCplac33 vector to generate YCplac33MKT1. YCplac33MKT1(D32A) expresses a mutated allele of MKT1 containing one amino acid change, from Asp-32 to Ala. The 5′ UTR and mutated NH2-terminal portion of MKT1 were amplified by PCR with the 5′ primer 5′-CTCCCCGGGCAATGAATTACGCGAGTCGC-3′, incorporating an SmaI site, and the 3′ primer 5′-GGAAACATAATGGTTGACGGCTATATCCAGGGTACAATT-3′. The COOH-terminal portion and 3′ UTR of MKT1 were amplified by PCR with the 5′ primer 5′-CTCCTCGAGTTCCTTTTCTTCTCTGCCGG-3′, incorporating an XhoI site, and the 3′ primer 5′-AATTGTACCCTGGATATAGCCGTCAACCATTATGTTTCC-3′. The resulting two fragments were further amplified by PCR with the 5′ primer 5′-CTCCCCGGGCAATGAATTACGCGAGTCGC-3′, incorporating an SmaI site, and the 3′ primer 5′-CTCCTCGAGTTCCTTTTCTTCTCTGCCGG-3′, incorporating an XhoI site. The resulting fragment was inserted into the SmaI-SalI gap of YCplac33 vector to generate YCplac33MKT1(D32A).
TABLE 2.
Plasmids used in this study
| Plasmid | Relevant markers | Reference or source |
|---|---|---|
| pFA6a-kanMX6-GAL1p | kanMX6-GAL1p sequence | 20 |
| pFA6a-3HA-kanMX6 | 3HA-ADH1 3′UTR-kanMX6 sequence | 20 |
| pFA6a-13MYC-kanMX6 | 13MYC-ADH1 3′UTR-kanMX6 sequence | 20 |
| pCgHIS3 | C. glabrata HIS3 in pUC19 | 28 |
| pCgLEU2 | C. glabrata LEU2 in pUC19 | 28 |
| pCgTRP1 | C. glabrata TRP1 in pUC19 | 28 |
| YCplac33 | URA3 CEN4 | 5 |
| pK508 | PMS1 YNL083w END3 MKT1 YNL086w and YNL087w sequence in YCp50 | This study |
| YCplac33MKT1 | MKT1 sequence in YCplac33 | This study |
| YCplac33MKT1-D32A | MKT1-D32A sequence in YCplac33 | This study |
| YCpMYO4 | MYO4 sequence in YCp50 (URA3 CEN4) | This study |
| YCpSHE2 | SHE2 sequence in YCplac111 (LEU2 CEN4) | 15 |
| YCpSHE3 | SHE3 sequence in YCplac111 (LEU2 CEN4) | 15 |
| YCpSHE4 | SHE4 sequence in YCp50 (URA3 CEN4) | This study |
| YCpBNI1 | BNI1 sequence in YCplac111 (LEU2 CEN4) | 15 |
| pGAD-C1 | LEU2/2-μm GAL4-AD sequence behind ADH1 promoter | 13 |
| pGAD-PBP1 | GAL4-AD-PBP1 (amino acids 475-722) sequence in pGAD-C1 | This study |
| pGBDU-C1 | URA3/2μm GAL4-BD sequence behind ADH1 promoter | 13 |
| pGBDU-MKT1 | BD-MKT1 sequence in pGBDU-C1 | This study |
| pGBDU-MKT1ΔC1 | BD-MKT1ΔC (amino acids 1-760) sequence in pGBDU-C1 | This study |
| pGBDU-MKT1ΔC2 | BD-MKT1ΔC (amino acids 1-570) sequence in pGBDU-C1 | This study |
Isolation of mkt1 mutants.
Yeast strain YKEN301 carrying the HOp-ADE2 reporter and GAL1p-KHD1 genes was treated with ethyl methanesulfonate to 30% survival. After ethyl methanesulfonate treatment, the cells were grown for two generations in YPD medium to allow for phenotypic lag. Cells were plated onto SC−Ade containing 10 mg of adenine per liter. After 3 days, about 3,000 red or pink colonies were selected and streaked onto both SG−Ade and SC−Ade plates. Out of about 3,000 colonies, 12 mutants grew well on SG−Ade but not on SC−Ade. These 12 mutants were transformed with YCpMYO4, YCpSHE2, YCpSHE3, YCpSHE4, and YCpBNI1. The adenine auxotrophy of nine of the mutants was complemented by YCpMYO4, and that of two was complemented by YCpSHE3. The adenine auxotrophy of the remaining mutant, YKEN9152, was not complemented by YCpMYO4, YCpSHE2, YCpSHE3, YCpSHE4, or YCpBNI1. The adenine auxotrophy of YKEN9152 segregated 2:2 in a cross to a strain having the parental genotype.
Cloning of MKT1.
Strain YKEN9152 was transformed with a YCp50 yeast genomic library (27) and then replica plated to SC−UraAde plates. After 2 days of incubation, one transformant grew on SC−UraAde medium. The plasmid that complemented the adenine auxotrophy of YKEN9152, pK508, was recovered from S. cerevisiae by transformation into E. coli. The mutation site of mkt1-19 was determined as follows. Chromosomal DNA from strain YKEN9152 was isolated as described previously (16). A 3.5-kb fragment encoding the MKT1 5′ UTR (0.7 kb), the MKT1 ORF, and the MKT1 3′ UTR (0.3 kb) of YKEN9152 was amplified by PCR with the 5′ primer 5′-CTCCCCGGGCAATGAATTACGCGAGTCGC-3′, incorporating an SmaI site, and the 3′ primer 5′-CTCCTCGAGTTCCTTTTCTTCTCTGCCGG-3′, incorporating an XhoI site. Sequence analysis revealed that YKEN9152 contains a C-to-T transversion at position +385, producing a termination codon in place of the codon for Gln-129.
Gene deletions.
Deletions of MYO4, KHD1, MKT1, PBP1, and ASH1 were constructed by the PCR-based gene deletion method (1, 28, 30). Primer sets were designed such that 46 bases at the 5′ end of the primers were complementary to those at the corresponding region of the target gene and 20 bases at their 3′ end were complementary to the pUC19 sequence outside the polylinker region in plasmid pCgHIS3 containing the Candida glabrata HIS3 gene, plasmid pCgTRP1 containing the C. glabrata TRP1 gene, or plasmid pCgLEU2 containing the C. glabrata LEU2 gene as a selectable marker. Primer sets for PCR were designed to delete the ORF completely. The PCR products were used to transform the wild-type strain by selection for His+, Trp+, or Leu+. The disruption was verified by colony-PCR amplification (10) to confirm that replacement had occurred at the expected locus.
Construction of MKT1-myc, MKT1ΔC-myc, MKT1-HA, and PBP1-HA strains.
MKT1-myc, MKT1ΔC-myc, MKT1-HA, and PBP1-HA strains were prepared by the method of Longtine et al. (20), with pFA6a-13Myc-kanMX6 and pFA6a-3HA-kanMX6.
Construction of GAL1p-KHD1 strains.
The GAL1p-KHD1 strain was prepared by the method of Longtine et al. (20), with pFA6a-kanMX6-GAL1p-3HA.
Mating-type switching assays.
Pedigree analysis was performed as described previously (33), with the homothallic strains TTC85 (HO/HO), TTC929 (HO/HO mkt1Δ/mkt1Δ), and TTC993 (HO/HO pbp1Δ/pbp1Δ).
β-Galactosidase assays.
β-Galactosidase assays were performed as described previously (16).
Real-time quantitative RT-PCR.
Primers and probes of the HOp-ADE2, HOp-lacZ, and ACT1 mRNAs for TaqMan assays were designed by use of the Primer Express software program (ABI, Foster City, Calif.) and were purchased from ABI. TaqMan probes were labeled at the 5′ end with 6-carboxyfluorescein and at the 3′ end with the fluorescence quencher 6-carboxytetramethyl rhodamine. The level of ACT1 mRNA was used as a control for the amount of cDNA. For real-time reverse transcriptase PCR (RT-PCR), 1 μl of 0.3-μg/μl DNase-treated total RNA was added to 50 μl of PCR buffer, which contained the forward and reverse primers at 900 nM each and the fluorescence-labeled probe at 100 nM in TaqMan Universal master mixture reagent (ABI). Samples were incubated at 50°C for 5 min and at 95°C for 10 min, after which target amplification was carried out through 45 two-stage cycles with at 95°C for 15 s and at 60°C for 1 min in an ABI Prism 7700 Sequence Detection System (ABI). For each data point, duplicate samples were analyzed in parallel, and all assays were repeated with highly reproducible results. All of the results shown in this report represent the averages of these four measurements.
Localization of ASH1 mRNA and Ash1 protein.
In situ RNA hybridization with digoxigenin-labeled ASH1 antisense probe was performed as described previously (35). Indirect immunofluorescence microscopy of an ASH1-myc strain with anti-Myc antibody 9E10 (Santa Cruz) was done as previously described (32).
Yeast two-hybrid assays.
Two-hybrid screening with pGBDU-MKT1 and Y2HL yeast genomic two-hybrid libraries was done as previously described (13).
Immunoprecipitation.
Strains were grown in YPD medium at 30°C, and the cells were harvested by centrifugation. All cells were washed twice in XT buffer (50 mM HEPES-KOH [pH 7.3], 20 mM potassium acetate, 2 mM EDTA, 0.1% Triton X-100, 5% glycerol). Cells were resuspended in the above-described buffer containing protease inhibitors. Glass beads were added, and the samples were vortexed 12 times for 45 s each time. The supernatants were removed and centrifuged for 10 min at 4,000 × g. To immunoprecipitate Mkt1-Myc or MKT1ΔC-Myc protein, extracts were incubated with anti-Myc antibody 9E10 coupled to protein G-agarose for 1 h at 4°C. Beads were washed three times in XT buffer and eluted in elution buffer (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 10 mM EDTA, 1% sodium dodecyl sulfate [SDS]) for 10 min at 65°C. Western blot assays were performed with anti-Myc antibody 9E10 or anti-hemagglutinin (HA) antibody HA11 (Babco).
Polysome analysis.
Yeast strains were grown in 500 ml of YPD medium at 30°C to an optical density at 600 nm (OD600) of 0.8. Cells were pelleted by centrifugation, resuspended in 400 μl of lysis buffer (20 mM HEPES [pH 7.4], 2 mM magnesium acetate, 100 mM potassium acetate, 100 μg of cycloheximide per ml, 0.5 mM dithiothreitol) on ice, and transferred to 1.5-ml microcentrifuge tubes. After pelleting for 5 min at 4,000 × g, cells were resuspended in an equal volume of lysis buffer containing protease inhibitors and lysed in the presence of 1 volume of glass beads by vortexing six times for 20 s each time at 40-s intervals. Lysates were clarified briefly at 8,000 × g for 5 min, followed by a 20-min centrifugation at 8,000 × g to give the final lysate. Fifty A260 units of lysate were fractionated on 10 to 50% sucrose gradients as described previously (11). Gradients were centrifuged in an SW28 rotor (Beckman) at 27,000 rpm for 180 min at 4°C and analyzed by continuous monitoring of A260. For protein analysis, each fraction from the gradient was precipitated with 10% trichloroacetic acid, washed with cold 70% ethanol, and resuspended in SDS-polyacrylamide gel electrophoresis (PAGE) loading buffer. For RNA analysis, total RNA was isolated from each fraction with an RNeasy kit (QIAGEN) and treated with RQ1 DNase (Promega). RT-PCR was performed with 1 μl of RNA as the template and a QuickAccess RT-PCR kit (Promega) under the conditions suggested by the manufacture. The following primers were used for amplification: HO-for, 5′-GGGAACCCGTATATTTCAGC-3′; HO-rev, 5′-CTTTTATTACATACAACTTTTTAAACTAATATACAC-3′; ACT1-for, 5′-GGAATCTGCCGGTATTGACC-3′; ACT1-rev, 5′-ACATACGCGCACAAAAGCAG-3′.
Western blotting.
Cell extracts were fractionated by SDS-PAGE on 8% acrylamide gels, followed by electroblotting onto Hybond N+ membranes (Amersham). Blots were blocked by incubation for 15 min at room temperature in TBS-M (Tris-buffered saline [TBS] with 4% nonfat dry milk). Blots were then incubated with antibody in TBS-M overnight at 4°C. After three washes with TBS, blots were incubated for 2 h with a peroxidase-conjugated secondary antibody (Calbiochem) diluted 1:3,000 with TBS-M. After three final washes with TBS, blots were detected with an enhanced chemiluminescence detection kit (Amersham). The antibodies used were anti-HA antibody HA11 diluted 1:2,000, anti-Myc antibody 9E10 diluted 1:500, anti-tubulin antibody TAT-1 diluted 1:1,000, and anti-β-galactosidase polyclonal antibody (Sigma) diluted 1:1,000.
RESULTS
Isolation of mutants affecting HO expression.
To monitor HO expression, we used an HOp-ADE2 reporter system in which the ho ORF is replaced with the ADE2 ORF at the ho locus (15, 34). Expression of the reporter can thus be assayed in an ade2Δ background by growth on medium lacking adenine (SC−Ade). Since asymmetric transcription of the HO gene results from the preferential accumulation of ASH1 mRNA encoding a negative regulatory protein in daughter cells (18, 35), delocalization of ASH1 mRNA in she mutants causes a reduction in HO expression (15). Consistent with this, myo4/she1 mutants containing the HOp-ADE2 reporter failed to grow on SC−Ade plates and disruption of the ASH1 gene suppressed the growth defect associated with the myo4Δ mutation (Fig. 1A). We have previously shown that overexpression of KHD1, which encodes a protein carrying three KH RNA-binding motifs, inhibits translation of ASH1 mRNA (12). Thus, overexpression of KHD1 from the GAL1 promoter suppressed the growth defect of myo4Δ mutants on plates containing galactose and lacking adenine (SG−Ade) (Fig. 1A). With this system, it was possible to isolate she mutations or mutations affecting HO expression itself by screening for mutants that exhibited reduced growth on SC−Ade and that were suppressed by overexpression of KHD1. Mutants suppressed by KHD1 overexpression would also be expected to be suppressed by the loss of Ash1.
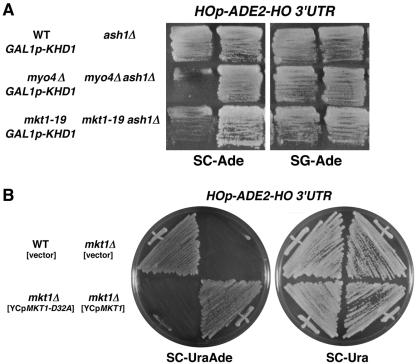
FIG. 1.
Isolation of the mkt1 mutant. (A) Effect of the mkt1-19 mutation on expression of the HOp-ADE2 reporter. Each yeast strain was streaked onto SC−Ade or SG−Ade plates and incubated for 3 days at 30°C. Yeast strains: YKEN301 (wild-type [WT] GAL1p-KHD1 HOp-ADE2-HO 3′UTR), YKEN303 (myo4Δ GAL1p-KHD1 HOp-ADE2-HO 3′UTR), YKEN9152 (mkt1-19 GAL1p-KHD1 HOp-ADE2-HO 3′UTR), YKEN304 (ash1Δ HOp-ADE2-HO 3′UTR), YKEN306 (myo4Δ ash1Δ HOp-ADE2-HO 3′UTR), and YKEN9152a (mkt1-19 ash1Δ HOp-ADE2-HO 3′UTR). (B) Effect of the mkt1Δ and mkt1-D32A mutations on expression of the HOp-ADE2 reporter. Each yeast strain was streaked onto SC−UraAde or SC−Ura plates and incubated for 3 days at 30°C. Yeast strains: 10B (wild-type HOp-ADE2-HO 3′UTR) (YCplac33), TTC478 (mkt1Δ HOp-ADE2-HO 3′UTR) (YCplac33), TTC478 (mkt1Δ HOp-ADE2-HO 3′UTR) (YCpMKT1-D32A), and TTC478 (mkt1Δ HOp-ADE2-HO 3′UTR) (YCpMKT1).
Starting with an ade2Δ strain carrying the HOp-ADE2 reporter and the GAL1p-KHD1 gene, mutants with reduced HO expression were selected as red or pink colonies on SC−Ade plates containing a limited amount of adenine. These mutants were then streaked onto SG−Ade plates to isolate mutants in which the adenine auxotrophy was suppressed by KHD1 overexpression. Out of about 3,000 red or pink colonies on SC−Ade containing a limited amount of adenine, 12 mutants grew well on SG−Ade. We tested complementation with a series of low-copy-number plasmids carrying each of the SHE genes and found out that 9 of the 12 candidates could be complemented by the MYO4 gene and 2 could be complemented by the SHE3 gene. The remaining mutant, YKEN9152, contained a mutation at the MKT1 locus (see below). Genetic analysis indicated that the mkt1-19 mutation in YKEN9152 segregated 2:2 for the adenine auxotrophy when crossed to a strain with the parental genotype (data not shown). As expected, the reduced growth of the mkt1-19 mutant on an SC−Ade plate was suppressed by disruption of the ASH1 gene (Fig. 1A). We thus chose to characterize the mkt1 mutation further.
Cloning of the MKT1 gene.
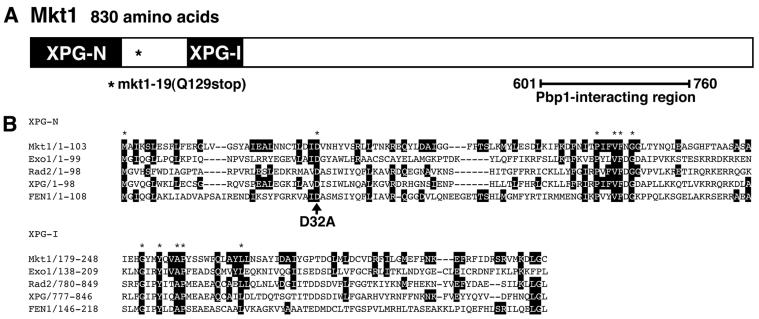
We isolated the MKT1 gene from a genomic library by virtue of its ability to complement the adenine auxotrophy of our mutant strain YKEN9152. The adenine auxotrophy of YKEN9152 was rescued by the plasmid pK508, which carries a fragment from the left arm of chromosome XIV, containing PMS1, YNL083w, END3, MKT1, YNL086w, and YNL087w. Deletion analysis of this plasmid revealed that MKT1 was responsible for complementation. The MKT1 gene encodes a protein of 830 aa (Fig. 2A) and was previously identified as a gene necessary for propagation of the M2 satellite double-stranded RNA of the L-A virus (37, 38).
FIG. 2.
Isolation of the MKT1 gene. (A) Schematic representation of the conserved XPG NH2-terminal (N) and internal (I) regions and the Pbp1-interacting region (see Fig. 5) in the Mkt1 protein. Conversion of the 369th codon from CAG to TAG (nonsense mutation) in the mkt1-19 mutant resulted in deletion of the COOH-terminal portion of the Mkt1 protein. (B) Comparison of amino acid sequences among Mkt1 and other eukaryotic XPG-like nucleases, Exo1 (accession number AAB47428), Rad2 (accession number A29839), XPG (accession number P28715), and FEN1 (accession number P39748). Amino acids identical to those of Mkt1 are indicated by black boxes. Asterisks indicate amino acids identical among all of these proteins.
To confirm that the YKEN9152 mutant bears a mutation in the MKT1 gene, we amplified the genomic region containing MKT1 by PCR. We found that the MKT1 gene in YKEN9152 has a C-to-T transversion at position +385, creating a termination codon in place of Gln-129 (Fig. 2A). To examine the behavior of the mkt1 null mutation, we disrupted the MKT1 gene in a strain harboring the HOp-ADE2 reporter gene (see Materials and Methods). The mkt1Δ mutant grew at the wild-type rate and exhibited a normal cell morphology on YPD medium or SC+Ade plates at temperatures between 23 and 37°C (Fig. 1B; data not shown). In contrast, this mutant did not grow on SC−Ade plates, similar to the originally isolated mkt1-19 mutant YKEN9152 (Fig. 1B).
We reexamined the amino acid sequence of Mkt1 and found that it has two domains in its NH2-terminal region, XPG-N and XPG-I, which are conserved among a family of nucleases that includes human XPG (xeroderma pigmentosum complementation group G) endonuclease (4, 22). The XPG protein is a DNA endonuclease with remarkable structure-specific properties, cleaving near the junctions between duplex and single-stranded DNAs with a defined polarity. Other members of this family include a group of small replication and repair nucleases such as mammalian FEN-1 and S. cerevisiae Rad2 (4, 22) (Fig. 2B). To test the functional significance of these conserved domains, site-directed mutagenesis was used to convert the highly conserved Asp-32 residue in the XPG-N domain to Ala (Fig. 2B). A previous study indicated that this conserved Asp residue is required for FEN-1 nuclease activity (31). An ade2Δ mkt1Δ strain harboring the HOp-ADE2 reporter was transformed with YCpMKT1 or YCpMKT1(D32A). As shown in Fig. 1B, the D32A mutation abolished the ability of Mkt1 to induce HO expression, indicating that the conserved residue is important for Mkt1 function. This raises the possibility that Mkt1 regulates HO expression via a nuclease activity.
Mkt1 does not affect the asymmetric localization of ASH1 mRNA or Ash1 protein.
Reduced growth of the mkt1Δ mutant harboring the HOp-ADE2 reporter on SC−Ade plates was dependent on the ASH1 gene (Fig. 1A). We therefore examined whether MKT1 might be involved in the localization of ASH1 mRNA and/or Ash1 protein. As a control, we confirmed that ASH1 mRNA and Ash1 protein were completely delocalized in myo4Δ mutants, as described previously (Tables 3 and 4) (3, 18, 35). In contrast, the mkt1Δ mutation hardly affected the proper localization of ASH1 mRNA and Ash1 protein, unlike the myo4Δ mutation (Tables 3 and 4). These results suggest that the reduction in HO expression caused by the mkt1Δ mutation is not the result of delocalization of ASH1 mRNA or Ash1 protein. It is likely that Mkt1 does not affect HO expression by acting on ASH1, even though reduced HO expression in mkt1Δ mutants was suppressed by the ash1Δ mutation.
TABLE 3.
Effect of mkt1Δ mutation on localization of ASH1 mRNA
| Genotypeb | % of 100 cellsa
|
||
|---|---|---|---|
| Anchored | Delocalized in bud | Delocalized in mother and bud | |
| Wild type | 78 | 19 | 3 |
| myo4Δ | 0 | 1 | 99 |
| mkt1Δ | 65 | 33 | 2 |
The percentage of cells that showed each pattern of ASH1 mRNA localization was determined by RNA in situ hybridization: anchored = tightly localized ASH1 mRNA at the distal tip; delocalized in the bud = delocalized ASH1 mRNA confined to the bud; delocalized in mother and bud = ASH1 mRNA in both mother cell and bud.
Yeast strains: 10B (wild type), YKEN104 (myo4Δ), and TTC478 (mkt1Δ).
TABLE 4.
Effect of mkt1Δ mutation on localization of Ash1 protein
| Genotypeb | % of 100 cellsa
|
||
|---|---|---|---|
| Daughter only | Predominantly daughter | Mother and daughter | |
| Wild type | 92 | 6 | 2 |
| myo4Δ | 2 | 4 | 94 |
| mkt1Δ | 72 | 14 | 14 |
The percentage of cells that showed each pattern of Ash1 protein localization was determined by staining with anti-Myc antibody in cells expressing Ash1-Myc: daughter only = visible Ash1 only in the daughter; predominantly daughter = Ash1 staining predominantly in the daughter nucleus with an intermediate amount of staining in the mother nucleus; mother and daughter = equivalent levels of Ash1 in both mother and daughter nuclei.
Yeast strains: K5552 (wild-type ASH1-myc), YKEN451 (myo4Δ ASH1-myc), and TTC237 (mkt1Δ ASH1-myc).
Mkt1 regulates HO expression at the posttranscriptional level via the HO 3′ UTR.
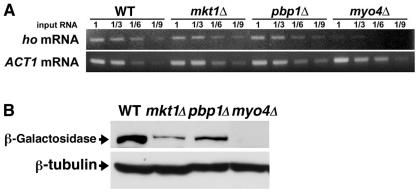
To determine at which stage Mkt1 regulates HO expression, we tested the effect of the mkt1Δ mutation on ho mRNA levels. In contrast to the myo4Δ mutation, which abolished transcription of the ho gene, the mkt1Δ mutation had no obvious effect on the concentration of ho mRNA (Fig. 3A). This suggests that Mkt1 may regulate HO expression posttranscriptionally.
FIG. 3.
Effect of the mkt1Δ and pbp1Δ mutations on levels of ho mRNA and β-galactosidase protein from the HOp-lacZ reporter. (A) Effects of the mkt1Δ and pbp1Δ mutations on ho mRNA levels. Each yeast strain was grown in YPD medium at 30°C and harvested. Total RNA samples were prepared from each strain and assayed by RT-PCR for ho mRNA levels (top) and ACT1 mRNA levels (bottom) as a quantity control. The values above the lanes are template RNA dilution factors. Yeast strains: W303 (wild-type [WT] ho), TTC1313 (mkt1Δ ho), TTC1316 (pbp1Δ ho), and TTC1319 (myo4Δ ho). (B) Effects of the mkt1Δ and pbp1Δ mutations on β-galactosidase protein levels from the HOp-lacZ reporter. Each yeast strain was grown in YPD medium at 30°C and harvested. Western blot analysis was performed to quantitate the level of β-galactosidase protein (top) and tubulin protein (bottom) as a quantity control. Yeast strains: K1107 (wild-type HOp-lacZ), TTC1085 (mkt1Δ HOp-lacZ) TTC1089 (pbp1Δ HOp-lacZ), and TTC4 (myo4Δ HOp-lacZ).
To test the effect of the mkt1Δ mutation on the translation of the ho gene, we used an HOp-lacZ reporter, which replaces the ho ORF with the lacZ ORF at the ho locus but retains the 5′ and 3′ UTRs of the HO mRNA (25, 34). We monitored the translation of the HOp-lacZ reporter with an antibody against β-galactosidase. First, we confirmed that the mkt1Δ mutation caused decreased expression of HOp-lacZ and observed an about threefold decrease in HO expression in mkt1Δ mutants compared with that in wild-type cells (Table 5). Western blotting analysis revealed that the mkt1Δ mutation reduced the levels of β-galactosidase protein (Fig. 3B). To eliminate the possibility that the reporter mRNA behaves differently from the authentic transcript, we examined whether the mkt1Δ mutation had an effect on the levels of HOp-lacZ and HOp-ADE2 reporter mRNAs by real-time quantitative RT-PCR. The mkt1Δ mutation had no effect on the levels of the reporter mRNAs (105 and 93% of the HOp-lacZ and HOp-ADE2 reporter mRNAs, respectively, in the mkt1Δ mutant cells compared with those in wild-type cells), while the myo4Δ mutation reduced the levels of those mRNAs (12 and 32% of the HOp-lacZ and HOp-ADE2 reporter mRNAs, respectively, in the myo4Δ mutant cells compared with those in wild-type cells). Furthermore, Northern blotting also confirmed that the level and size of the HOp-lacZ reporter mRNA were not altered by the mkt1Δ mutation (data not shown). These results suggest that Mkt1 is involved in the translational control of HO mRNA.
TABLE 5.
Effects of mkt1Δ and pbp1Δ mutations on expression of the HOp-LacZ reporter
| Genotypea | β-Galactosidase activity (nmol/min/mg)b |
|---|---|
| Wild type | 36.6 |
| myo4Δ | 4.7 |
| mkt1Δ | 13.4 |
| pbp1Δ | 12.1 |
| mkt1Δ pbp1Δ | 14.3 |
Yeast strains: K1107 (wild-type HOp-LacZ), TTC4 (myo4Δ HOp-LacZ), TTC1085 (mkt1Δ HOp-LacZ), TTC1089 (pbp1Δ HOp-LacZ), and TTC1163 (mkt1Δ pbp1Δ HOp-LacZ).
Yeast strains were grown in YPD medium and assayed for β-galactosidase activity. Values shown are averages of three experiments.
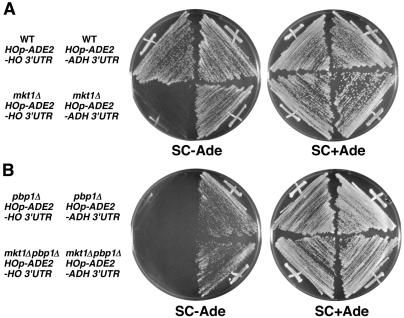
To further localize the level of Mkt1 regulation, we examined the involvement of the HO 3′ UTR in Mkt1-mediated regulation of HO expression. We used two reporters that differ only in their 3′ UTRs, HOp-ADE2-HO 3′UTR and HOp-ADE2-ADH1 3′UTR. In the latter, the HO 3′ UTR has been replaced with the ADH1 3′ UTR (34). When the HO-ADE2-HO 3′UTR and HO-ADE2-ADH 3′UTR reporters were expressed in ade2Δ strains, their mRNA levels were similar (data not shown). Consistent with this, ade2Δ strains harboring either HOp-ADE2-HO 3′UTR or HOp-ADE2-ADH1 3′UTR grew normally on SC−Ade plates, similar to the ADE2 strain (Fig. 4A). However, the mkt1Δ mutant harboring HOp-ADE2-HO 3′UTR grew poorly on SC−Ade plates whereas the mkt1Δ mutant harboring HOp-ADE2-ADH1 3′UTR grew normally, similar to the wild-type strain harboring HOp-ADE2-ADH1 3′UTR (Fig. 4A). These results suggest that Mkt1 is involved in the regulation of HO expression through the HO 3′ UTR.
FIG. 4.
The phenotype of mkt1Δ and pbp1Δ mutants is dependent on the HO 3′ UTR. Each yeast strain was streaked onto SC−Ade or SC+Ade plates and incubated for 3 days at 30°C. (A) Yeast strains: 10B (wild-type [WT] HOp-ADE2-HO 3′UTR), TTC47 (wild-type HOp-ADE2-ADH1 3′UTR), TTC478 (mkt1Δ HOp-ADE2-HO3′UTR), and TT1108 (mkt1Δ HOp-ADE2-ADH1 3′UTR). (B) Yeast strains: TTC798 (pbp1Δ HOp-ADE2-HO 3′UTR), TTC1334 (pbp1Δ HOp-ADE2-ADH1 3′UTR), TTC1257 (mkt1Δ pbp1Δ HOp-ADE2-HO 3′UTR), and TTC1333 (mkt1Δ pbp1Δ HOp-ADE2-ADH1 3′UTR).
Mkt1 interacts with the poly(A)-binding protein-binding protein Pbp1.
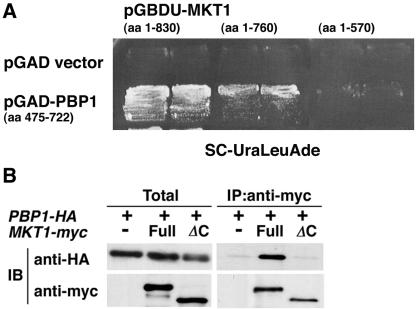
To examine Mkt1-mediated regulation of HO expression further, we screened for an Mkt1-binding protein(s) by using the yeast two-hybrid screen with Mkt1 as bait. We screened 7 × 105 clones from a yeast genomic library and obtained 39 positive clones. All 39 clones encoded different COOH-terminal portions of the same protein, Pbp1 (aa 198 to 722, 357 to 722, and 475 to 722) (Fig. 5A; data not shown). Thus, the COOH-terminal half of the Pbp1 protein is able to interact with Mkt1. Pbp1 was previously identified as a protein that binds to Pab1, a poly(A)-binding protein (21). Two-hybrid analysis revealed that the COOH-terminal region of Mkt1 is required for its interaction with Pbp1 (Fig. 5A).
FIG. 5.
Interaction of Mkt1 with Pbp1. (A) Two-hybrid analysis. Yeast strain PJ69-4A harboring GAL2p-ADE2 and GAL1p-HIS3 reporters was transformed with the indicated plasmids, and the transformants were streaked onto SC−UraLeuAde plates and incubated for 3 days at 30°C. The same results were obtained with the HIS3 reporter assay (data not shown). Plasmids: pGAD-C1 (vector), pGAD-PBP1 (aa 475 to 722), pGBDU-MKT1-Full (aa 1 to 830), pGBDU-MKT1ΔC1(aa 1 to 760), and pGBDU-MKT1ΔC2 (aa 1 to 570). (B) Coimmunoprecipitation (IP) analysis. Extracts prepared from strains TTC999 (PBP1-HA), TTC1049 (PBP1-HA MKT1-myc) and TTC1302 (PBP1-HA MKT1ΔC-myc) were incubated with anti-Myc antibody coupled to protein G-Sepharose. Each immunopellet was separated by SDS-10% PAGE, blotted, and probed with anti-HA or anti-Myc antibody for the presence of Pbp1-HA or Mkt1-Myc protein. Total extracts were also immunoblotted (IB) with anti-HA or anti-Myc antibody. Mkt1ΔC-Myc lacks the COOH-terminal 230 aa.
To confirm that Mkt1 binds Pbp1 in vivo, we performed immunoprecipitation analyses. We constructed strains harboring a Myc-tagged version of Mkt1, Mkt1-Myc, and an HA-tagged version of Pbp1, Pbp1-HA, as described in Materials and Methods. When Mkt1-Myc was immunoprecipitated from cell extracts with an anti-Myc antibody, Pbp1-HA was coimmunoprecipitated, as detected by anti-HA antibody in Western blot assays (Fig. 5B). When Mkt1ΔC-Myc lacking the COOH-terminal 230 aa was coexpressed with Pbp1-HA, coimmunoprecipitation analysis revealed that Mkt1ΔC-Myc failed to interact with Pbp1-HA (Fig. 5B). These results indicate that Mkt1 interacts with Pbp1 in vivo and that the COOH-terminal region of Mkt1 is required for its interaction with Pbp1.
Effect of the pbp1Δ mutation on HO expression.
To examine whether Pbp1 also regulates HO expression in a manner similar to that of Mkt1, we disrupted the PBP1 gene in a strain harboring the HOp-ADE2-HO 3′UTR or HOp-ADE2-ADH1 3′UTR reporter (see Materials and Methods). The pbp1Δ mutant harboring HOp-ADE2-HO 3′UTR did not grow on SC−Ade plates, similar to the mkt1Δ mutant (Fig. 4B). In contrast, the pbp1Δ mutant harboring HOp-ADE2-ADH1 3′UTR grew normally, similar to the wild-type strain, suggesting that Pbp1 regulates HO expression via the HO 3′ UTR. The pbp1Δ mutation decreased expression of the HOp-lacZ reporter (Table 5). Furthermore, an mkt1Δ pbp1Δ double mutant showed β-galactosidase activity similar to that of each single mutant, indicating that the effects of the mkt1Δ and pbp1Δ mutations on HO expression are nonadditive. Together with the observation that Mkt1 directly associates with Pbp1, our results suggest that Mkt1 and Pbp1 regulate HO expression at the same step. Consistent with this, the pbp1Δ mutation had no effect on ho mRNA levels (Fig. 3A) but caused a reduction in the levels of β-galactosidase protein in a strain carrying the HOp-lacZ reporter (Fig. 3B). Furthermore, the real-time quantitative RT-PCR analysis revealed that HOp-lacZ reporter mRNA levels were not affected by the pbp1Δ mutation (109% in pbp1Δ mutant cells compared with those in wild-type cells). Thus, the effect of Pbp1 on HO expression is similar to that of Mkt1. These results suggest that Mkt1 and Pbp1 are involved in the translational control of HO mRNA.
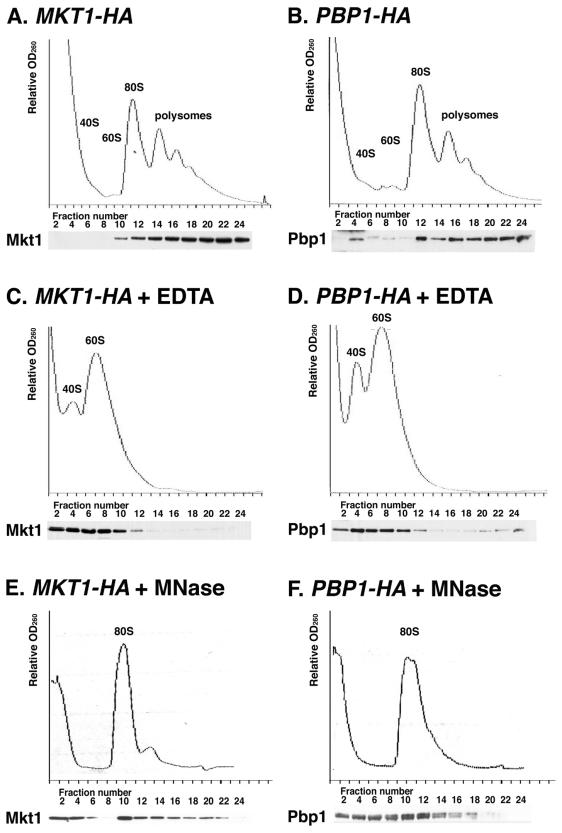
Mkt1 and Pbp1 cosediment with polysomes.
It has been reported that Pbp1 cofractionates with polysomes (21). We therefore examined the subcellular localization of Mkt1. The subcellular localizations of Mkt1-HA and Pbp1-HA were assayed by fractionating cytoplasmic extracts on sucrose gradients and analyzing the fractions by Western blotting. As observed previously (21), Pbp1-HA cosedimented with polysomes. We observed that Mkt1-HA and Pbp1-HA were distributed similarly in the gradient (Fig. 6A and B). Since the formation and maintenance of 80S ribosomes require the presence of Mg2+ ions, polysomes can be disrupted by adding EDTA to the extracts. EDTA treatment caused a loss of polysomes and a large increase in the number of free 40S and 60S subunits (Fig. 6C and D). Concomitantly, there was a shift in the sedimentation pattern of Mkt1-HA and Pbp1-HA from polysomes to the lighter fractions of the gradient. Furthermore, when extracts were treated with micrococcal nuclease, which collapses polysomes into a single monosome peak (80S), the distribution of Mkt1-HA and Pbp1-HA was also disrupted (Fig. 6E and F). These results confirm that Mkt1 and Pbp1 cosediment with polysomes. Mkt1 and Pbp1 were still distributed in the ribosomal subunits or the monosome fraction after treatment with EDTA or micrococcal nuclease (Fig. 6C, D, E, and F), suggesting that the Mkt1-Pbp1 complex may associate with ribosome subunits.
FIG. 6.
Mkt1 and Pbp1 cofractionate with polysomes on sucrose gradients. Extracts prepared from strains expressing Mkt1-HA (A, C, and E) or Pbp1-HA (B, D, and F) were fractionated on 10 to 50% sucrose gradients. Extracts were pretreated with 30 mM EDTA (C and D) or 1 U of micrococcal nuclease (MNase) per μl (E and F) for 20 min before being loaded onto the gradients. The upper part of each panel shows the OD260 profile of the gradient. Peaks representing small (40S) and large (60S) ribosomal subunits, a single ribosome (80S), and polysomes (>80S) are indicated. The lower part of each panel shows an anti-HA Western blot of gradient fractions concentrated and subjected to SDS-8% PAGE. Yeast strains: TTC848 (MKT1-HA) and TTC996 (PBP1-HA).
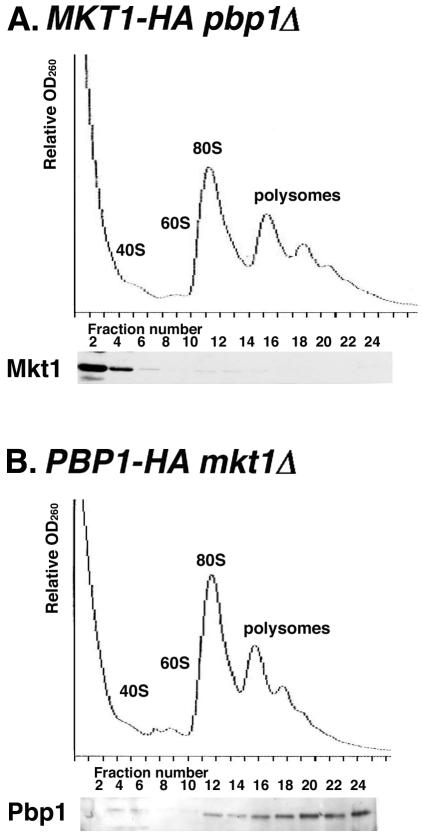
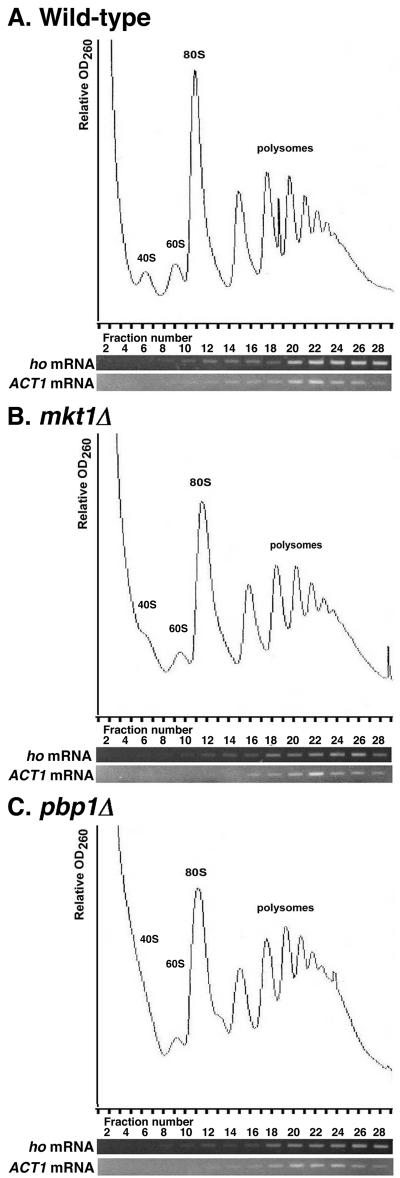
Since Mkt1 associates with Pbp1, we examined whether the association of Mkt1 and/or Pbp1 with polysomes depends on the presence of the other protein. Fractionation of extracts prepared from a pbp1Δ mutant expressing Mkt1-HA revealed that Mkt1 was not associated with polysomes (Fig. 7A). This indicates that the association of Mkt1 with polysomes is indirect and Pbp1 dependent. In contrast, Pbp1 was associated with polysomes even in the absence of Mkt1 (Fig. 7B). These results suggest that Pbp1 mediates the association of Mkt1 with polysomes. Finally, we examined the effects of the mkt1Δ and pbp1Δ mutations on the distribution of HO mRNA in the polysome fraction. HO mRNA was quantitatively incorporated into the polysome fractions (Fig. 8A). Neither the mkt1Δ nor the pbp1Δ mutation had any effect on HO mRNA polysome size distribution (Fig. 8B and C). These results suggest that Mkt1 may regulate the elongation or termination of translation, but not the initiation, of HO mRNA.
FIG. 7.
Pbp1-dependent cofractionation of Mkt1 with polysomes. Extracts prepared from strains expressing Mkt1-HA (A) or Pbp1-HA (B) were fractionated on 10 to 50% sucrose gradients. The upper part of each panel shows the OD260 profile of the gradient. Peaks representing small (40S) and large (60S) ribosomal subunits, a single ribosome (80S), and polysomes (>80S) are indicated. The lower part of each panel shows an anti-HA Western blot of gradient fractions concentrated and subjected to SDS-8% PAGE. Yeast strains: TTC1234 (MKT1-HA pbp1Δ) and TTC1233 (PBP1-HA mkt1Δ).
FIG.8.
Distribution of ho mRNA in the polysome fraction. Extracts prepared from wild-type (A), mkt1Δ (B), and pbp1Δ (C) strains were fractionated on 10 to 50% sucrose gradients. Total RNA samples were prepared from each fraction and assayed by RT-PCR for ho and ACT1 mRNA levels. The upper part of each panel shows the OD260 profile of the gradient. Peaks representing small (40S) and large (60S) ribosomal subunits, a single ribosome (80S), and polysomes (>80S) are indicated. The lower part of each panel shows the levels of ho and ACT1 mRNAs. Yeast strains: W303 (wild-type ho), TTC1313 (mkt1Δ ho), and TTC1316 (pbp1Δ ho).
MKT1 and PBP1 affect mating-type switching in mother cells.
The HO gene encodes an endonuclease that stimulates mating-type switching in budding yeast (9). The ability of cells in a cell lineage to undergo mating-type switching has a very precise pattern in which mother cells switch efficiently (typically 70% of cell divisions) but daughter cells do not (<0.1% of cell divisions) (33). The basis for this difference is the presence of HO mRNA in mother cells but not in daughter cells (24). Since Mkt1 and Pbp1 are required for efficient HO expression, we tested whether the mkt1Δ or pbp1Δ mutation affects the regulation of mating-type switching. We introduced the mkt1Δ or pbp1Δ mutation into an HO background and measured its effect on mating-type switching by pedigree analysis (Table 6). In wild-type cells, we observed that 72% of the mother cells and none of the daughter cells switched mating type. Mutations in MKT1 and PBP1 reduced the frequency of mating-type switching in mother cells to 52 and 37%, respectively, but had no effect on daughter cells. Although substantial, the effects of these mutations on mother cell switching are not as strong as those observed in swi or she mutants. These results indicate that Mkt1 and Pbp1 are required for efficient mating-type switching in mother cells.
TABLE 6.
Mating-type switching in mkt1Δ and pbp1Δ mutant cells
| Genotypeb | Mating-type switching frequency (%)a
|
|
|---|---|---|
| Mother | Daughter | |
| Wild type | 72 | 0 |
| mkt1Δ | 52c | 0 |
| pbp1Δ | 37d | 0 |
Mean percentages of three independent experiments are indicated. More than 80 cell divisions were examined in each experiment. Note the significant differences between wild-type and mutant cells.
Yeast strains: TTC46 (wild-type HO), TTC929 (mkt1Δ HO), and TTC993 (pbp1Δ HO).
P < 0.002.
P < 0.0005.
DISCUSSION
HO expression is regulated at the transcriptional level by a negative regulator, Ash1 (3, 32). Ash1 is a specific repressor of transcription that localizes asymmetrically to the daughter cell nucleus through the localization of ASH1 mRNA to the distal tip of the daughter cell (18, 35). This localization depends on the actin cytoskeleton and five She proteins, one of which is a type V myosin motor, Myo4. Delocalization of ASH1 mRNA in she mutants causes a reduction in HO expression (15). Recently, we have shown that an RNA-binding protein, Khd1 (KH domain protein 1), is also required for efficient localization of ASH1 mRNA to the distal tip of the daughter cell, by associating with the N element of ASH1 mRNA (12). Furthermore, overexpression of KHD1 decreases the concentration of Ash1 protein and restores HO expression to she mutants. Therefore, we expected that by screening for mutants that had reduced expression of the HOp-ADE2 reporter and that could be suppressed by KHD1 overexpression, we could isolates she mutations or mutations affecting HO expression itself. Indeed, we isolated myo4 and she3 mutations by this screening method. Thus, screening with KHD1 overexpression is useful for the isolation of she mutations. However, we failed to identify novel SHE genes. Since we did not isolate a she2, she4, or she5 mutation, our screening has not been saturated. Alternatively, such novel SHE genes, if present, might be essential for yeast cell growth.
In this study, we isolated mkt1 as a mutation that caused reduced expression of an HOp-ADE2 reporter and that was in turn suppressed by overexpression of the KHD1 gene. In contrast to she mutants, the mkt1Δ mutation had little effect on the localization of ASH1 mRNA or Ash1 protein. This indicates that Mkt1 affects HO expression at a step different from that of the She proteins. In addition, we isolated the PBP1 gene as an Mkt1-interacting protein. Pbp1 was previously identified as a protein that associates with the poly(A)-binding protein Pab1 (21). We have presented several pieces of evidence suggesting that Mkt1 regulates the translation of HO mRNA by forming a complex with Pbp1. First, loss of the MKT1 or PBP1 gene has little effect on HO mRNA levels but decreases HO protein levels. Second, regulation of HO expression by Mkt1 and Pbp1 is mediated by the HO 3′ UTR. Third, Mkt1 binds to Pbp1 in vivo, and they cosediment with polysomes. Association of Mkt1 in the polysome fraction is dependent on Pbp1. Finally, both Mkt1 and Pbp1 are required for effective mating-type switching in mother cells. We thus propose that the Mkt1-Pbp1 complex regulates HO translation via the 3′ UTR of HO mRNA. Reduced expression of HO in mkt1 mutants is suppressed by KHD1 overexpression or the ash1Δ mutation. This suggests that an increase in HO mRNA caused by inactivation of the Ash1 repressor can overcome inefficient translation caused by the mkt1 mutation.
The observation that Mkt1 and Pbp1 cosediment with polysomes in similar distribution patterns in sucrose gradients is consistent with the idea that these two proteins associate. Furthermore, the association of Mkt1 with polysomes is dependent on Pbp1. These results suggest that Pbp1 interacts with and recruits Mkt1 to the polysomes. Pbp1 interacts with Pab1, poly(A)-binding protein, and Pab1 also cosediments with polysomes (21). However, Pbp1 is still associated with polysomes in the absence of Pab1 (21). Furthermore, Pbp1 and Mkt1 are distributed in the monosome fractions even after micrococcal nuclease treatment. Thus, it is likely that the Mkt1-Pbp1 complex associates with ribosome subunits but not with mRNA. Consistent with this, we could not detect an interaction of Mkt1 with the 3′ UTR of HO mRNA by coimmunoprecipitation and three-hybrid analyses (T. Tadauchi, T. Inada, K. Matsumoto, and K. Irie, unpublished data). The presence of Mkt1 and Pbp1 on polysomes suggests that the Mkt1-Pbp1 complex regulates the translation of HO mRNA through its interaction with polysomes. In fact, we observed that HO mRNA is quantitatively incorporated into the polysome fractions. The observation that neither the mkt1Δ nor the pbp1Δ mutation has any effect on the distribution of HO mRNA in the polysome fractions suggests that the Mkt1-Pbp1 complex may regulate the elongation or termination of translation but not the translational initiation or nuclear export of HO mRNA.
How does Mkt1 regulate HO expression at the translational level? We reexamined the amino acid sequence of the Mkt1 protein and found that Mkt1 has two domains, XPG-N and XPG-I, in its NH2-terminal region. These domains are conserved within a family of nucleases that includes the XPG endonuclease (4, 22). The conserved residue essential for the nuclease activity in the XPG-N domain of Mkt1 is required for its ability to regulate HO expression. The XPG family members have exo- or endonuclease activities with different substrate specificities and are involved in repair, recombination, and replication (4, 22). Although we have not determined whether Mkt1 has nuclease activity, it might function in the processing of the HO mRNA. This processing, presumably involving the HO 3′ UTR, would be required for efficient translation of HO mRNA at the elongation or termination step. The amino acid residues that are required for XPG nuclease activity, Asp-77 and Glu-791, are not conserved in Mkt1 (Asn-82 and Trp-193), suggesting that Mkt1 might have different activity or substrate specificity. One possibility is that Mkt1 accesses the 3′ UTR of the HO mRNA in the polysome fraction through an interaction with Pbp1 and modification via the Mkt1 nuclease activity leads to the efficient elongation or termination of HO mRNA translation. There still remains the possibility that an unidentified factor that requires the Mkt1-Pbp1 complex for its function could be responsible for the control of translation.
Regulation of HO expression by Mkt1 and Pbp1 is mediated by the 3′ UTR of the HO mRNA. The reduction in HO expression seen in mkt1Δ or pbp1Δ mutant cells is eliminated by replacement of the HO 3′ UTR with the ADH1 3′ UTR. HO expression is also regulated posttranscriptionally by the yeast Puf homolog Mpt5/Puf5 via the HO 3′ UTR (34). Mpt5 directly binds to the 3′ UTR of HO mRNA and represses HO expression. Analysis of mpt5Δ mkt1Δ double mutants revealed that the mpt5Δ mutation suppressed the defect in HO expression caused by the mkt1Δ mutation (Tadauchi et al., unpublished). This genetic analysis perhaps suggests that the Mkt1-Pbp1 complex could positively regulate HO expression by negatively regulating Mpt5 function. However, this possibility is unlikely because the mechanism by which MPT5 overexpression affects HO mRNA is different from that by which deletion of the MKT1 or PBP1 gene affects HO mRNA. Overexpression of the MPT5 gene promotes degradation of the reporter mRNA containing the HO 3′ UTR, an observation that suggests that Mpt5 may affect mRNA stability or turnover via binding to the HO 3′ UTR (34). On the other hand, loss of the MKT1 or PBP1 gene has little effect on HO mRNA levels. Indeed, we observed that disruption or overexpression of the MKT1 gene had no effect on the ability of Mpt5 to bind to the 3′ UTR of the HO mRNA (Tadauchi et al., unpublished). Thus, it is likely that the Mkt1-Pbp1 complex and Mpt5 independently regulate HO expression posttranscriptionally via the HO mRNA 3′ UTR. The observation that mpt5Δ suppresses the mkt1Δ defect might be explained by simple additivity, i.e., that an increase in HO mRNA caused by inactivation of Mpt5 may overcome the inefficient translation caused by the mkt1Δ mutation.
Acknowledgments
We thank M. Ota for technical assistance and R. P. Jansen for materials.
These studies were supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, and Science of Japan (K.I.) and by special grants for CREST, Advanced Research on Cancer, from the Ministry of Education, Culture, and Science of Japan (K.M.).
Footnotes
This paper is dedicated to the memory of Ira Herskowitz (deceased 28 April 2003).
REFERENCES
- 1.Baudin, A., K. O. Ozier, A. Denouel, F. Lacroute, and C. Cullin. 1993. A simple and efficient method for direct gene deletion in Saccharomyces cerevisiae. Nucleic Acids Res. 21:3329-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertrand, E., P. Chartrand, M. Schaefer, S. M. Shenoy, R. H. Singer, and R. M. Long. 1998. Localization of ASH1 mRNA particles in living yeast. Mol. Cell 2:437-445. [DOI] [PubMed] [Google Scholar]
- 3.Bobola, N., R. P. Jansen, T. H. Shin, and K. Nasmyth. 1996. Asymmetric accumulation of Ash1p in postanaphase nuclei depends on a myosin and restricts yeast mating-type switching to mother cells. Cell 84:699-709. [DOI] [PubMed] [Google Scholar]
- 4.Constantinou, A., D. Gunz, E. Evans, P. Lalle, P. A. Bates, R. D. Wood, and S. G. Clarkson. 1999. Conserved residues of human XPG protein important for nuclease activity and function in nucleotide excision repair. J. Biol. Chem. 274:5637-5648. [DOI] [PubMed] [Google Scholar]
- 5.Gietz, R. D., and A. Sugino. 1988. New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74:527-534. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin, E. B., P. G. Okkema, T. C. Evans, and J. Kimble. 1993. Translational regulation of tra-2 by its 3′ untranslated region controls sexual identity in C. elegans. Cell 75:329-339. [DOI] [PubMed] [Google Scholar]
- 7.Gray, N. K., and M. Wickens. 1998. Control of translation initiation in animals. Annu. Rev. Cell Dev. Biol. 14:399-458. [DOI] [PubMed] [Google Scholar]
- 8.Haarer, B. K., A. Petzold, S. H. Lillie, and S. S. Brown. 1994. Identification of MYO4, a second class V myosin gene in yeast. J. Cell Sci. 107:1055-1064. [DOI] [PubMed] [Google Scholar]
- 9.Herskowitz, I. 1988. Life cycle of the budding yeast Saccharomyces cerevisiae. Microbiol. Rev. 52:536-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huxley, C., E. D. Green, and I. Dunham. 1990. Rapid assessment of S. cerevisiae mating type by PCR. Trends Genet. 6:236. [DOI] [PubMed] [Google Scholar]
- 11.Inada, T., E. Winstall, S. Z. Tarun, Jr., J. R. Yates III, D. Schieltz, and A. B. Sachs. 2002. One-step affinity purification of the yeast ribosome and its associated proteins and mRNAs. RNA 8:948-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irie, K., T. Tadauchi, P. A. Takizawa, R. D. Vale, K. Matsumoto, and I. Herskowitz. 2002. The Khd1 protein, which has three KH RNA-binding motifs, is required for proper localization of ASH1 mRNA in yeast. EMBO J. 21:1158-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.James, P., J. Hallady, and E. A. Craig. 1996. Genomic libraries and a host strain designed for highly efficient two-hybrid selection in yeast. Genetics 144:1425-1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jan, E., C. K. Motzny, L. E. Graves, and E. B. Goodwin. 1999. The STAR protein, GLD-1, is a translational regulator of sexual identity in Caenorhabditis elegans. EMBO J. 18:258-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen, R. P., C. Dowzer, C. Michaelis, M. Galova, and K. Nasmyth. 1996. Mother cell-specific HO expression in budding yeast depends on the unconventional myosin Myo4p and other cytoplasmic proteins. Cell 84:687-697. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser, C. A., A. Adams, and D. E. Gottschling. 1994. Methods in yeast genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 17.Koch, C., and K. Nasmyth. 1994. Cell cycle regulated transcription in yeast. Curr. Opin. Cell Biol. 6:451-459. [DOI] [PubMed] [Google Scholar]
- 18.Long, R. M., R. H. Singer, X. Meng, I. Gonzalez, K. Nasmyth, and R. P. Jansen. 1997. Mating type switching in yeast controlled by asymmetric localization of ASH1 mRNA. Science 277:383-387. [DOI] [PubMed] [Google Scholar]
- 19.Long, R. M., W. Gu, X. Meng, G. Gonsalvez, R. H. Singer, and P. Chartrand. 2001. An exclusively nuclear RNA-binding protein affects asymmetric localization of ASH1 mRNA and Ash1p in yeast. J. Cell Biol. 153:307-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Longtine, M. S., A. R. McKenzie, D. J. Demarini, N. G. Shah, A. Wach, A. Brachat, P. Philippsen, and J. R. Pringle. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14:953-961. [DOI] [PubMed] [Google Scholar]
- 21.Mangus, D. A., N. Amrani, and A. Jacobson. 1998. Pbp1p, a factor interacting with Saccharomyces cerevisiae poly(A)-binding protein, regulates polyadenylation. Mol. Cell. Biol. 18:7383-7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mueser, T. C., N. G. Nossal, and C. C. Hyde. 1996. Structure of bacteriophage T4 RNase H, a 5′ to 3′ RNA-DNA and DNA-DNA exonuclease with sequence similarity to the RAD2 family of eukaryotic proteins. Cell 85:1101-1112. [DOI] [PubMed] [Google Scholar]
- 23.Munchow, S., C. Sauter, and R. P. Jansen. 1999. Association of the class V myosin Myo4p with a localised messenger RNA in budding yeast depends on She proteins. J. Cell Sci. 112:1511-1518. [DOI] [PubMed] [Google Scholar]
- 24.Nasmyth, K. 1983. Molecular analysis of a cell lineage. Nature 302:670-676. [DOI] [PubMed] [Google Scholar]
- 25.Nasmyth, K. 1987. The determination of mother cell-specific mating type switching in yeast by a specific regulator of HO transcription. EMBO J. 6:243-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peterson, C. L., and I. Herskowitz. 1992. Characterization of the yeast SWI1, SWI2, and SWI3 genes, which encode a global activator of transcription. Cell 68:573-583. [DOI] [PubMed] [Google Scholar]
- 27.Rose, M. D., P. Novick, J. H. Thomas, D. Botstein, and G. R. Fink. 1987. A Saccharomyces cerevisiae genomic bank based on a centromere-containing shuttle vector. Gene 60:237-243. [DOI] [PubMed] [Google Scholar]
- 28.Sakumoto, N., Y. Mukai, K. Uchida, T. Kouchi, J. Kuwajima, Y. Nakagawa, S. Sugioka, E. Yamamoto, T. Furuyama, H. Mizubuchi, N. Ohsugi, T. Sakuno, K. Kikuchi, I. Matsuoka, N. Ogawa, Y. Kaneko, and S. Harashima. 1999. A series of protein phosphatase gene disruptants in Saccharomyces cerevisiae. Yeast 15:1669-1679. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning; a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 30.Schneider, D., C. J. Bruton, and K. F. Chater. 1996. Characterization of spaA, a Streptomyces coelicolor gene homologous to a gene involved in sensing starvation in Escherichia coli. Gene 177:243-251. [DOI] [PubMed] [Google Scholar]
- 31.Shen, B., J. P. Nolan, L. A. Sklar, and M. S. Park. 1997. Functional analysis of point mutations in human flap endonuclease-1 active site. Nucleic Acids Res. 25:3332-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sil, A., and I. Herskowitz. 1996. Identification of asymmetrically localized determinant, Ash1p, required for lineage-specific transcription of the yeast HO gene. Cell 84:711-722. [DOI] [PubMed] [Google Scholar]
- 33.Strathern, J. N., and I. Herskowitz. 1979. Asymmetry and directionality in production of new cell types during clonal growth: the switching pattern of homothallic yeast. Cell 17:371-381. [DOI] [PubMed] [Google Scholar]
- 34.Tadauchi, T., K. Matsumoto, I. Herskowitz, and K. Irie. 2001. Post-transcriptional regulation through the HO 3′-UTR by Mpt5, a yeast homolog of Pumilio and FBF. EMBO J. 20:552-561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takizawa, P. A., A. Sil, J. R. Swedlow, I. Herskowitz, and R. D. Vale. 1997. Actin-dependent localization of an RNA encoding a cell-fate determinant in yeast. Nature 389:90-93. [DOI] [PubMed] [Google Scholar]
- 36.Takizawa, P. A., and R. D. Vale. 2000. The myosin motor, Myo4p, binds Ash1 mRNA via the adapter protein, She3p. Proc. Natl. Acad. Sci. USA 97:5273-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vermut, M., W. R. Widner, J. D. Dinman, and R. B. Wickner. 1994. Sequence of MKT1, needed for propagation of M2 satellite dsRNA of the L-A virus of Saccharomyces cerevisiae. Yeast 10:1477-1479. [DOI] [PubMed] [Google Scholar]
- 38.Wickner, R. B. 1987. MKT1, a nonessential Saccharomyces cerevisiae gene with a temperature-dependent effect on replication of M2 double-stranded RNA. J. Bacteriol. 169:4941-4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, B., M. Gallegos, A. Puoti, E. Durkin, S. Fields, J. Kimble, and M. P. Wickens. 1997. A conserved RNA-binding protein that regulates sexual fates in the C. elegans hermaphrodite germ line. Nature 390:477-484. [DOI] [PubMed] [Google Scholar]