Abstract
Human telomerase is a multimer containing two human telomerase RNAs (hTRs) and most likely two human telomerase reverse transcriptases (hTERTs). Telomerase synthesizes multiple telomeric repeats using a unique repeat addition form of processivity. We investigated hTR and hTERT sequences that were essential for DNA synthesis and processivity using a direct primer extension telomerase assay. We found that hTERT consists of two physically separable functional domains, a polymerase domain containing RNA interaction domain 2 (RID2), reverse transcriptase (RT), and C-terminal sequences, and a major accessory domain, RNA interaction domain 1 (RID1). RID2 mutants defective in high-affinity hTR interactions and an RT catalytic mutant exhibited comparable DNA synthesis defects. The RID2-interacting hTR P6.1 helix was also essential for DNA synthesis. RID1 interacted with the hTR pseudoknot-template domain and hTERT's RT motifs and putative thumb and was essential for processivity, but not DNA synthesis. The hTR pseudoknot was essential for processivity, but not DNA synthesis, and processivity was reduced or abolished in dimerization-defective pseudoknot mutants. trans-acting hTERTs and hTRs complemented the processivity defects of RID1 and pseudoknot mutants, respectively. These data provide novel insight into the catalytic organization of the human telomerase complex and suggest that repeat addition processivity is one of the major catalytic properties conferred by telomerase multimerization.
Telomerase is a DNA polymerase that catalyzes the de novo addition of telomeric DNA repeats to the 3′ ends of linear chromosomes, and it is essential for the long-term proliferation of most eukaryotic cells. Telomerase is minimally composed of two subunits, the telomerase reverse transcriptase (TERT) and the telomerase RNA (TR), which contains the template used to direct DNA synthesis (reviewed in reference 27). Processive telomerases add multiple DNA repeats to a single substrate by reiteratively copying the RNA template, a property referred to as reiterative, repeat addition, or type II processivity. This unusual form of processivity is unique to telomerase and is distinct from the nucleotide addition (type I) form of processivity shared by all polymerases. Repeat addition processivity entails the following: (i) initial alignment of the 3′ ends of the DNA substrate and RNA template, (ii) copying of the RNA template to its 5′ boundary, and (iii) translocation of the RNA template and/or DNA substrate within the active site so that the new DNA 3′ end realigns with the 3′ boundary of the RNA template. Repetitions of this cycle generate the periodic pattern of short DNA repeats that is characteristic of processive telomerase activity in vitro.
Many nucleic acid polymerases are functionally divided into two major domains, a core polymerase domain whose minimal function is template-directed (type I) processive DNA synthesis and a major accessory domain that confers the unique activities of individual polymerases (reviewed in reference 48). Since repeat addition (type II) processivity is unique to telomerase, telomerase-specific elements in the TERT and TR may confer this specialized catalytic property. However, human telomerase elements that mediate the enzyme's polymerase function (DNA synthesis) and the telomerase-specific property of repeat addition processivity could not be identified until recently, when a direct primer extension assay was adapted for use with recombinant vertebrate telomerases reconstituted in rabbit reticulocyte lysates (RRL) (18, 31). In this study, we examined the roles of different human TERT (hTERT) and human TR (hTR) sequences in DNA synthesis and repeat addition processivity. We use the term processivity to refer to repeat addition (type II or reiterative) processivity unless otherwise indicated.
The TRs from different organisms display limited sequence homology, but their secondary structures are conserved within families. Common features include the template and a pseudoknot structure (17, 47, 51). Vertebrate TRs contain two regions that are important for telomerase activity, the pseudoknot-template domain and the CR4-CR5 domain containing conserved region 4 (CR4) and conserved region 5 (CR5) (see Fig. 4A) (reviewed in reference 27). Mouse TR (mTR) and hTR pseudoknot-template domain sequences regulate repeat addition processivity and interact with TERT, though the specific site of interaction in TERT is unknown (6, 10, 18). Sequences in the hTR pseudoknot P3 helix mediate TR dimerization (40). The P1b helix of the hTR pseudoknot-template domain also contributes to 5′ template boundary definition (19). The mTR and hTR CR4-CR5 domains interact with TERT (6, 20, 43); specifically, the CR4-CR5 domain of hTR interacts with the RID2 region of hTERT (see below) (35). The hTR and mTR CR4-CR5 domains contain the P6.1 helix, which is important for human and mouse telomerase activity and TERT interactions (20, 43), though the specific site of P6.1 interaction in hTERT and mouse TERT has not been identified.
FIG. 4.
RID1 sequences that mediate repeat addition processivity are required for in vitro association with the hTR template-pseudoknot domain. (A) Schematic of the predicted hTR secondary structure (17, 20, 43). Numbers indicate nucleotide positions. The P1, P3, and P6.1 helices are indicated. (B) 32P-labeled wild-type (WT) hTR or hTR nucleotides 1 to 209 (top gel) were coimmunoprecipitated with Flag-tagged, 35S-labeled proteins (indicated by asterisks) corresponding to wild-type (WT) hTERT, RID2 (amino acids 300 to 617), or N- and C-terminally truncated RID1 variants (bottom gel). hTR association with RID1 and RID2 was expressed relative to hTR association with WT hTERT. αFlag, anti-Flag antibody. (C) The specificity of hTR1-209-RID1 interactions was determined by examining the interaction of a nonspecific Flag-tagged protein (NKKβ) or a nonspecific Drosophila RNA (DART-1) with hTR1-209 or Flag-tagged RID1, respectively. One microliter of a 1:500 dilution of an input RRL reaction mixture containing the appropriate 32P-labeled RNA was loadedin input lanes. PI, preimmune serum. (D) Results of competition experiments. 35S-labeled Flag-tagged RID1 and RID2 proteins were synthesized in RRL in the presence of equal amounts of 32P-labeled hTR1-209 and increasing amounts of unlabeled wild-type (WT) competitor hTR (1.48 × 10−21, 1.48 × 10−20, and 1.48 × 10−19 M). Ribonucleoprotein complexes were immunoprecipitated and visualized using the same method as other RNA binding experiments. One microliter of a 1:1,000 dilution of an input RRL reaction mixture containing 32P-labeled hTR1-209 was loaded in the input lane. αFlag, anti-Flag antibody; PI, preimmune serum; N.C., no competition (no competitor hTR). After normalization to the amount of protein in each lane, hTR1-209 association with RID1 or RID2 in the presence of competitor hTR was expressed relative to the no-competition (N.C.) control for each protein (percent association). Average interaction and standard deviation (Std. Dev.) values for different competitor hTR concentrations relative to N.C. controls are indicated at the bottom of each lane. At least three independent experiments for both RID1 and RID2 were performed.
The TERT proteins are also phylogenetically conserved and consist of central reverse transcriptase (RT) motifs flanked by telomerase-specific N- and C-terminal sequences (Fig. 1A) (reviewed in reference 32). The Saccharomyces cerevisiae and hTERT C termini functionally resemble the thumb of the human immunodeficiency virus type 1 (HIV-1) RT because of their contributions to type I processivity, and the hTERT C terminus is also implicated in type II processivity (29, 31, 45).
FIG. 1.
Distinct hTERT sequences are essential for DNA synthesis and repeat addition processivity. hTERT variants were synthesized inRRL in the presence of wild-type (WT) hTR, and the activity of recombinant telomerases was analyzed by TRAP (C) or the direct primer extension assay (B, D, and E). Repeat addition processivity and first telomere repeat DNA synthesis (DNA synthesis) values were expressed as percentages of the corresponding values for wild-type enzyme and are indicated below the gels in panels B and D. (A) A schematic depicting conserved hTERT regions is shown at the top. Numbers indicate the amino acid boundaries defined in this study and others (see text). The other schematics depict functionally important TERT N-terminal regions identified in previous studies (references shown in parentheses to the right of the schematic). Motifs or regions identified in S. cerevisiae TERT were mapped to hTERT N-terminal sequences using the alignment reported by Xia et al. in 2000 (56). (B) Analysis of the elongation products of RID2 and RT mutants. The two gels in this panel depict independent experiments. Asterisks indicate RID2 mutants defective in high-affinity hTR interactions (44). (C) hTERT variants were synthesized in the presence (+) or absence (−) of a RID1 mutant (Δ150-159) to test for their ability to complement the activity defects of the RID1 mutant. The positions of nonspecific PCR primer dimers (arrows) and PCR internal control (IC) are indicated to the left of the top gel. Lane WT contains wild-type hTERT. Expression of 35S-labeled hTERT proteins (indicated by asterisks) was detected by SDS-polyacrylamide gel electrophoresis (bottom panel). (D) hTERT variants were analyzed for the ability to complement the repeat addition processivity defects of RID1 mutants Δ150-159 and Δ1-180. Repeat addition processivity (R.A.P.) was calculated by comparing the normalized intensity of the major product in the first and second repeats (indicated by asterisks and corresponding to the first G in the telomeric repeat TTAGGG). The processivity of each reconstituted enzyme, shown at the bottom of each lane, was expressed as a percentage of the processivity of wild-type (WT) telomerase. n/a, not applicable. (E) Longer exposure permitted the identification and quantification of second repeat products generated by RID1 mutants.
The hTERT N terminus can be subdivided into two regions, RNA interaction domains 1 and 2 (RID1 and RID2, respectively), which are separated by a nonconserved linker that is not required for telomerase activity (Fig. 1A) (3, 44, 56). RID1 encompasses the previously identified GQ and T2 motifs, and is also referred to as region I (for a review of alternative nomenclature, see reference 32) (Fig. 1A). RID2 includes the CP, QFP, and T motifs, alternatively referred to as regions II, III, and IV, respectively (for a review, see reference 32) (Fig. 1A). Both RID1 and RID2 are required for wild-type levels of TR interaction and telomerase activity in yeast, Tetrahymena, and humans (for a review, see reference 32). The RT-adjacent RID2 is a site of high-affinity TR binding (35). The catalytic function of the more N-terminal RID1 is poorly understood. The RID1 of S. cerevisiae TERT (Est2p) is important for telomerase activity and interacts nonsequence specifically with both DNA and RNA (56). Deletion of the hTERT RID1 greatly reduces but does not abolish telomerase activity and affects the ability of human telomerase to extend nontelomeric primers (9). These observations suggest that although RID1 is not essential for catalysis, it may be an important site of DNA interaction in both yeast and human TERTs (9, 56). RID1 also contains residues that are not required for wild-type levels of in vitro telomerase activity but that are important for telomere length maintenance in S. cerevisiae and human cells (3, 22, 56). N-terminal hTERT sequences that are important for in vivo function are referred to as the N-DAT domain (Fig. 1A) (3). Though mutation of N-DAT sequences does not appear to affect catalytic function when telomerase activity is measured using the telomeric repeat amplification protocol (TRAP) (3, 44), recent work indicates that some N-DAT mutants are less active than wild-type hTERT when activity is measured using a direct primer extension (conventional) assay or nontelomeric DNA primers (37). Similarly, the direct primer extension assay reveals catalytic defects in hTERT C-terminal mutants that are undetected by the PCR-based TRAP assay (31).
The human telomerase enzyme appears to be an obligate dimer. Stringently purified human telomerase complexes contain two hTRs and are predicted to contain two hTERTs on the basis of size measurements (55). S. cerevisiae telomerase also contains at least two functionally interacting TRs, but Tetrahymena telomerase is active as a monomer (15, 46). hTR monomers are interdependent, and reconstitution of hTR-hTR interactions restores telomerase activity to dimerization-defective hTR mutants (40, 55). Similarly, distinct, overlapping hTERT mutants can functionally complement each other's activity defects, indicating that hTERT monomers can function in trans (9, 44). RT and RNA template dimerization are essential for replication of the double-stranded RNA genomes of retroviruses such as HIV-1 (references 8 and 24 and references therein). However, the catalytic functions of telomerase multimerization have not been defined.
Previous work indicates that the TERT C-terminal and RT sequences functionally resemble the thumb and RT motifs of the HIV-1 RT and other nucleic acid polymerases (32). However, telomerase contains a TERT-specific N terminus and employs a stably integrated, structurally complex RNA as a template. Many telomerases also exhibit a unique, repeat addition form of processivity. In this study, we used a recently adapted direct primer extension assay and recombinant human telomerases reconstituted in RRL to characterize the telomerase-specific elements in hTERT and hTR that are essential for polymerase function (DNA synthesis) and the telomerase-specific property of repeat addition processivity. We also investigated the roles of hTERT-hTERT, hTR-hTR, and hTERT-hTR interactions in mediating these catalytic functions. Our results indicated that the functional domain organization of the entire hTERT protein resembles that of other nucleic acid polymerases and that TR and a major nonpolymerase domain of TERT (RID1) confer repeat addition processivity to human telomerase. Furthermore, TR and TERT multimerization played important roles in repeat addition processivity. These data provided novel insight into the catalytic organization of the human telomerase complex. We propose that the mechanism mediating repeat addition processivity may represent a variation on a catalytic mechanism shared by other RTs.
MATERIALS AND METHODS
Constructs.
pET28-hTERT wild-type and D868N (5), 595-1132, Δ1-180, 595-946, and 1-946 (6), Δ150-159, Δ350-359, Δ390-399, Δ481-490, Δ508-517, W547A,and W547F (44), Δ936-945, Δ963-972, Δ993-1002, Δ1020-1029, and Δ1077-1086 (31) constructs were previously described. pET28-hTERT constructs 1-595 and 946-1132 were cloned by PCR using pET28-hTERT as a DNA template. The pCR3.1-Flag-hTERT wild-type construct was previously described (10). pCR3.1-Flag-hTERT 1-300, 1-250, 1-200, 1-150, 25-300, and 300-617 constructs were cloned by PCR using pCR3.1-Flag-hTERT as a template. The luciferase-expressing construct is provided with the RRL kit (Promega). phTR wild-type, hTR170, hTR180, and hTR190 constructs were previously described (4). During the final revision of this manuscript, we discovered that phTR180 and phTR190 also contain a deletion of nucleotides 200 and 201. These deletions were inadvertently introduced into the constructs when they were made in 1995, as the oligonucleotides used for PCR mutagenesis were designed on the basis of an early incomplete version of the hTR gene sequence. The phTR 1-209, 207-451, Δ1-64, Δ65-144, and Δ145-208 constructs were cloned by PCR with a T7 promoter from the pGRN33 construct into the puc119 vector using a previously described strategy (4). phTR GC107/108AG, P6.1.302UCUC, P6.256GCGCC, P6.296GGCGC, 306AAAAA, 315UUCAUU, 316AGU, P6.1.311GAGA, P6.1.302UCUCcomp, 36-45, and 122-129 constructs were cloned by site-directed mutagenesis, using phTR + 1 (wild type) as a template. The identity of all hTERT and hTR constructs was confirmed by restriction digestion and/or sequencing.
In vitro reconstitution of telomerase, TRAP, and direct primer extension assays.
hTERT proteins were expressed in RRL in the presence of in vitro synthesized and purified hTRs (44). When hybrid telomerases were reconstituted using mixtures of hTR or hTERT variants, equal amounts (micrograms) of hTR- or hTERT-encoding plasmid DNA were added to the RRL reaction mixtures. TRAP and direct primer extension assays and quantification of repeat addition processivity were performed as described previously (31), except that conventional assays were performed with 1 μM biotinylated (TTAGGG)3 primer. First repeat DNA synthesis was quantified by expressing the total signal of the first six telomerase products (+1 to +6) generated by any mutant as a percentage of the total signal of the first six telomerase products generated by wild-type telomerase tested in the same experiment. Repeat addition processivity was quantified by a previously described method (26). Repeat addition processivity values for mutant telomerases were usually expressed as a percentage of the repeat addition processivity value for wild-type telomerase tested in the same experiment (see Fig. 1, 5, and 6). The products of wild-type and variant telomerases quantified by this method were loaded on the same gel.
FIG. 5.
Distinct hTR regions are essential for DNA synthesis and repeat addition processivity. (A and B) Schematics of the predicted secondary structures of the hTR CR4-CR5 domain (A) and pseudoknot P3 base pairing region (B) (17, 20, 43). (A) The starting positions of mutations analyzed in this study are indicated. (B) The starting positions of the hTR170, hTR180, and hTR190 10-nucleotide substitutions are numbered, and the location of the GC107-108AG (DKC) mutation is underlined. P3 residues implicated in hTR dimerization are indicated by uppercase letters (40). CR2 and CR3 are boxed. (C and D) The elongation products of telomerases reconstituted with wild-type (WT) hTERT and the indicated CR4-CR5 (C) or pseudoknot (D) substitution mutants were examined using the direct primer extension assay. Repeat addition processivity (R.A.P.) and first telomere repeat DNA synthesis (DNA synthesis) values were expressed as percentages of the corresponding values for wild-type enzyme and are indicated below the gels. n/a, not applicable. (E) Interaction of wild-type (WT) hTR and P6.1 helix mutants with wild-type hTERT and RID2 (amino acids 300 to 617). The WT hTERT proteinsignal in lane 4 appears stronger than the WT hTERT protein signals in lanes 7 and 10 due to comigration of the efficiently coprecipitated WT hTR with hTERT in SDS-12% polyacrylamide gels. One microliter of a 1:500 dilution of an input RRL reaction mixture containing the appropriate 32P-labeled hTR variant was loaded in input lanes. PI, preimmune serum. (F) Quantification of in vitro hTR dimerization. At least three independent experiments were performed for each hTR. Mean dimerization values that differed significantly from the value for wild-type (WT) hTR in a Student's t test are indicated by one asterisk (P < 0.05) or two asterisks (P < 0.01). (G) Representative hTR dimerization results. hTRs were incubated on ice (−) or at 37°C (+) for 2 h prior to electrophoresis on 5% nondenaturing polyacrylamide gels. The positions of monomers (one asterisk) and dimers (two asterisks) are indicated to the right of the gel.
FIG. 6.
Reconstitution of P3 base pairing potential in trans restores repeat addition processivity and hTR dimerization to a P3 mutant. (A) Recombinant telomerases were reconstituted in the presence or absence of hTR170, and elongation products were examined using the direct primer extension assay. Repeat addition processivity (R.A.P.) and first telomere repeat DNA synthesis (DNA synth.) values were expressed as percentages of the corresponding values for wild-type (WT) enzyme and are indicated below the gel. n/a, not applicable. (B and C) In vitro dimerization of hTR mixtures. (B) Percent dimer yield for mixtures of hTR mutants was calculated by measuring the combined homo- and heterodimer signal, which was expressed as a percentage of the total signal for monomers and dimers. Mean dimerization values that were significantly different (P < 0.01) from the wild-type (WT) hTR value are indicated by two asterisks. (C) Representative dimerization results. The positions of monomers (one asterisk) and homo- and heterodimers (which comigrated extensively) (collectively identified by two asterisks) are indicated to the right of the gel. DNA Marker V (Roche) was used as a size indicator.
Pulse-chase time course experiments.
Pulse-chase time course reaction conditions were adapted from reference 13. Direct primer extension assays were performed as described above. Biotinylated (TTAGGG)3 pulse primer and nonbiotinylated (TTAGGG)3 chase primer were included at a final concentration of 1 and 150 μM, respectively. Reactions were stopped with a buffer containing RNase A and EDTA, as previously described (31). Quantification of first repeat DNA synthesis and processivity were performed as described above.
In vitro RNA binding assay.
RNA binding and quantification were performed as described previously (44), except that 3,000 Ci/mmol of UTP was used for hTR synthesis, and 1 μg of competitor bovine serum albumin and tRNA per ml and 0.45 μg/ml of Flag M2 antibody (Sigma) per ml were used in immunoprecipitations. RNA and protein signals were distinguished by exposing sodium dodecyl sulfate (SDS)-polyacrylamide gels on two sheets of film simultaneously, as previously described (14). For competition experiments, protein A Sepharose beads (Sigma) were blocked extensively with bovine serum albumin and tRNA prior to addition of RRL-reconstituted telomerase complexes, but these competitors were not included during immunoprecipitation.
In vitro hTR dimerization assay.
hTR dimerization experiments were performed as described previously (40).
hTERT protein interaction assay.
Flag-tagged, unlabeled hTERT1-200 was prebound to Flag M2 antibody-coupled protein G Sepharose beads (Sigma), followed by extensive washing to remove unbound proteins. 35S-labeled hTERT proteins were added to the immunoprecipitation mixture, with or without 300 ng of hTR, and immunoprecipitations were performed for 4 h or overnight. Immunoprecipitation buffers, competitors, and wash conditions have previously been described (44). All proteins were synthesized in RRL.
RESULTS
The hTERT RID2, RT, and C-terminal sequences function in cis as a single domain.
The function of many multidomain proteins, such as lac permease or the HIV-1 RT, is reconstituted when different domains of the proteins are expressed from discontinuous mRNAs (11, 30). Functional complementation experiments have been used to define the boundaries of lac permease domains (57). We examined the domain organization of hTERT using a functional complementation approach and a series of overlapping hTERT proteins containing small internal deletions and substitutions. This method minimizes potential artifacts caused by misfolding of truncated proteins or deletion of whole domains. Furthermore, the use of overlapping proteins permits analysis of trans-acting sequences in the hTERT multimer.
To distinguish the hTERT sequences that function cooperatively in the same domain (in cis) from those which can function in trans, we cosynthesized pairs of inactive hTERT variants in RRL in the presence of wild-type hTR. Hybrid enzymes were tested for the ability to reconstitute telomerase activity using the TRAP assay (Table 1). We found that inactive RID2, RT, and C-terminal variants could not complement other hTERT variants with distinct mutations in RID2, RT, or C-terminal sequences, whereas all inactive RID2, RT, and C-terminal mutants could complement the activity defects of a RID1 mutant (Table 1) (31, 44).
TABLE 1.
Functional complementation of activity defects of hTERT mutants in cosynthesis experiments
| Mutated hTERT region | Coexpressed hTERT mutanta | Region | Complementationf |
|---|---|---|---|
| RID1 (Δ150-159) | Δ350-359,b Δ390-399,b Δ481-490,b Δ508-517,b W547Ab | RID2 | + |
| D868Nc | RT | + | |
| Δ936-945,bΔ963-972,b Δ993-1002,b Δ1020-1029,bΔ1077-1086b | Ce | + | |
| RID2 (W547A) | Δ150-159b | RID1 | + |
| D868Nc | RT | − | |
| Δ936-945,bΔ963-972,b Δ993-1002,b Δ1020-1029,bΔ1077-1086b | C | − | |
| RT (D868N) | Δ150-159c | RID1 | + |
| Δ350-359,c Δ390-399,c Δ481-490,c Δ508-517,c W547Ac | RID2 | − | |
| Δ936-945,dΔ963-972,d Δ993-1002,d Δ1020-1029,dΔ1077-1086d | C | − |
hTERT mutants were cosynthesized with RID1, RID2, or RT variants, as indicated in the leftmost column. Repeat addition processivity-defective C-terminal mutants identified in a previous study (31) are underlined.
Cosynthesis experiments performed for this study.
Previously reported cosynthesis experiments (44).
Previously reported cosynthesis experiments (31).
C, C terminus.
The ability of two distinct hTERT mutants to complement each others' activity defects when cosynthesized in RRL in the presence of wild-type hTR was evaluated by TRAP assay. Symbols: −, absence of complementation; +, reconstitution of telomerase activity by complementation.
In a multimeric protein, the ability of two distinct, overlapping mutants to complement each other's activity defects suggests that the complementing sequences in these proteins can function in trans. Conversely, the inability of two distinct mutants to reconstitute catalytic function in a multimeric complex can be interpreted in several ways. First, one or both mutations may disrupt the ability of monomers to physically interact. We previously reported that hTERT RID2 and C-terminal mutants cannot function in trans with an RT catalytic mutant, though RID2 and C-terminal variants coprecipitate with the RT mutant as efficiently as wild-type hTERT (Table 1) (31, 44), suggesting that the inability of these variants to complement each other's activity defects is not attributable to loss of hTERT-hTERT interactions. Second, two distinct mutants may be unable to reconstitute catalytic function if more than one functional copy of either mutated region is required for activity. However, the ability of a RID1 mutant to complement the activity defects of RID2, RT, or C-terminal variants suggests that multiple functional copies of RID2, the RT motifs, or the C terminus are not required for telomerase activity (Table 1). A third explanation for the inability of distinct mutants to complement each other's activity defects is that the sequences altered in these mutants normally function in cis; that is, they are part of the same functional domain (57). Since the inactive RID2, RT, and C-terminal hTERT variants we examined did not demonstrate defects in hTERT-hTERT protein interactions (31, 44) and since mutations in these regions could be complemented by a RID1 variant (Table 1), we concluded that RID2, the RT motifs, and C terminus of hTERT likely function in cis as part of a single functional domain. The hTERT RID2, RT, and C-terminal sequences are predicted by the PSIPRED secondary structure prediction program (http://bioinf.cs.ucl.ac.uk) (42) to form a continuous and closely spaced series of alpha-helices and beta sheets, whereas RID1 is separated from other catalytically important regions of hTERT by a nonconserved linker of low sequence complexity (data not shown) (3, 56). Collectively, these observations suggested that the RID2, RT, and C-terminal regions of hTERT act cooperatively within the same hTERT molecule.
RID2, RT, and C-terminal sequences constitute the core polymerase domain of hTERT.
Though it has previously been established using the TRAP assay that RID2 and the RT motifs are important for telomerase activity (for a review, see reference 32), the PCR-based TRAP technique does not always distinguish between catalytic defects caused by poor efficiency of nucleotide addition (DNA synthesis) and those that are attributable to defects in the telomerase-specific property of repeat addition processivity. The shortest PCR products detected using the TRAP assay are 40 to 50 nucleotides long (including the 18-nucleotide substrate primer) and contain at least three telomere DNA repeats (23, 33, 34, 49). Therefore, when the TRAP technique is used, nonprocessive enzymes that synthesize less than three telomere repeats cannot be distinguished from enzymes with severe defects in DNA synthesis.
To characterize the specific catalytic functions mediated by the hTERT RID2 and RT regions, we therefore examined the elongation products of reconstituted telomerases using a direct primer extension assay. The catalytic defects associated with hTERT C-terminal mutations were recently described (31). We previously demonstrated that most sequences in the hTERT RID2 are required for high-affinity interaction with hTR (44). We found that all RID2 mutations that disrupt hTR binding abolished DNA synthesis (<1% wild-type first repeat DNA synthesis) (Fig. 1B, lanes 5 and 8 to 12), whereas a RID2 mutation that does not impair hTR interactions did not affect the ability of recombinant telomerase to synthesize DNA (Fig. 1B, lane 13). Nonspecific signals were occasionally detected, for example in reaction mixtures with hTR alone, hTERT alone, or an hTR without a template (Fig. 1B, lanes 2 and 3; also see Fig. 6A, lane 11). As expected, substitution of a highly conserved aspartate that is essential for the catalytic activity of all RTs abolished DNA synthesis (<1% wild-type first repeat DNA synthesis) (Fig. 1B, lane 6) (32). The DNA synthesis defects of RID2 variants and this RT catalytic mutant were comparable (Fig. 1B, compare lane 6 to lanes 5 and 8 to 12), supporting the conclusion that hTR-interacting RID2 sequences are essential for DNA synthesis. The core polymerase domain of nucleic acid polymerases is composed of the catalytically essential palm and fingers subdomains, and a thumb subdomain that regulates nucleotide addition (type I) processivity (48). The function of the core polymerase domain is confined to template-directed type I processive DNA synthesis (48). Since the hTERT C terminus is also implicated in nucleotide addition (type I) processivity and is not physically separable from RID2 and the RT motifs (Table 1) (31) and as RID2 and the RT motifs are physically inseparable and catalytically essential (Table 1; Fig. 1B), we propose that RID2, RT, and C-terminal sequences constitute the core polymerase domain of hTERT and that these sequences function catalytically in cis.
The hTERT RID1 domain functions in trans.
Next, we examined the hTERT sequences that can function in trans in hTERT multimers. Cosynthesis of an inactive RID1 mutant (Δ150-159) with numerous, overlapping inactive RID2, RT, and C-terminal mutants reconstituted telomerase activity, indicating that RID1 can function in trans (Table 1; Fig. 1C). The hTERT N terminus alone was both necessary and sufficient for complementation of the RID1 mutant's activity defect (Fig. 1C, lanes 3 and 7), and reconstitution of activity was not dependent on RID2 sequences (Fig. 1C, lane 5). Deletion of the first 180 hTERT residues (RID1) prevented complementation, and amino acids 1 to 200 were sufficient to restore telomerase activity (Fig. 1C, lanes 9 and 21). Deletion of RID1 residues 1 to 24 greatly reduced complementation (Fig. 1C, lane 23). These data demonstrated that the RID1 region is responsible for functional complementation of the Δ150-159 mutant's activity defects and that RID1 sequences can function in trans.
RID1 is an essential determinant of repeat addition processivity.
In contrast to RID2, RT, and C-terminal sequences, RID1 is physically separable from the remainder of hTERT and can fulfill its catalytic role in trans (Table 1; Fig. 1C). Similarly, isolated accessory and polymerase domains can assemble in vitro to reconstitute the catalytic function of HIV-1 RT (30). Since major accessory domains confer unique catalytic properties to nucleic acid polymerases (48), we investigated whether RID1 was important for telomerase-specific catalytic functions.
We examined the elongation products of RID1 mutants Δ150-159 and Δ1-180 using the direct primer extension assay and compared them to the products generated by wild-type hTERT and RID2 and RT variants (Fig. 1B and D to E). RID1 mutants synthesized DNA less efficiently than wild-type enzyme; however, unlike RID2 and the RT motifs, RID1 was not essential for DNA synthesis (compare Fig. 1B, lanes 5 and 8 to 12, <1% wild-type DNA synthesis, with Fig. 1D, lanes 2 and 10, 6 to 16% wild-type DNA synthesis). Similarly, the major accessory domains of HIV-1 RT (RNase H) and other nucleic acid polymerases stimulate but are not required for DNA synthesis (30). The pausing pattern of the first repeat elongation products generated by RID1 mutants resembled the wild-type profile (Fig. 1D, lanes 1, 2, and 10). However, both variants synthesized predominantly only one telomere repeat. The second repeat addition products generated by these mutants could be reliably detected and quantified after longer exposure (Fig. 1E).
Repeat addition processivity values were determined by calculating the ratio of the intensity of the major second repeat telomerase product to the intensity of the corresponding first repeat telomerase product (26). This ratio was then compared to the ratio for wild-type enzyme. Comparison of these ratios permits quantification of processivity independent of differences in DNA synthesis efficiency, and this quantification method detected 40-fold differences in processivity even in enzymes with similar, low levels of DNA synthesis (Fig. 1D, lanes 10 and 13). Processivity quantification confirmed that both RID1 mutants were nonprocessive (<1% of wild-type processivity). The Δ1-180 and Δ150-159 mutants exhibited similar catalytic defects, implying that amino acids 150 to 159 play a critical role in RID1 function. RID1 mutations cause a small reduction in the ability of hTERT to interact with wild-type hTR (10, 44); however, RID1 mutations do not inhibit hTERT-hTR interactions as extensively as RID2 mutations (6, 10, 44). Furthermore, complexes containing RID1 mutants were sufficiently stable to permit complementation of activity defects by an isolated trans-acting RID1 domain or a RID2 mutant that inhibits ribonucleoprotein (RNP) assembly (Fig. 1C, lanes 19 and 11, and D), suggesting that major defects in RNP assembly do not account for the catalytic properties of RID1 mutants. We concluded that the hTERT RID1 is essential for repeat addition processivity and that it is important but not essential for DNA synthesis.
Repeat addition processivity can be conferred by a trans-acting RID1.
The results of complementation experiments performed using the TRAP assay and overlapping hTERT mutants indicated that RID1 can function in trans (Table 1; Fig. 1C). We coexpressed a panel of hTERT mutants with the Δ150-159 protein to determine whether trans-acting hTERTs could confer repeat addition processivity to this RID1 variant. Addition of amino acids 1 to 300 in trans stimulated DNA synthesis twofold but enhanced processivity more than 60-fold (Fig. 1D, compare lanes 2 and 4). Deletion of the first 180 residues of hTERT prevented complementation (Fig. 1D, lane 8). A RID2 point mutant and RT and C-terminal mutants also restored processivity to Δ150-159, though not as efficiently as residues 1 to 300 (Fig. 1D, lane 6; also data not shown). The ability of these overlapping hTERT proteins to complement the Δ150-159 processivity defect indicated that RID1 can mediate repeat addition processivity in trans.
To map the sequences required for complementation of the processivity defects of RID1 mutants, we cosynthesized the Δ1-180 mutant with fragments containing residues from the RID1 and linker regions. Fragment 1-250 (fragment consisting of amino acids 1 to 250) restored processivity to nearly wild-type levels, but amino acids 1 to 150 and 25 to 300 were not sufficient for complementation (Fig. 1D, lanes 12, 14, and 15). Residues 1 to 200 also restored processivity to hTERT Δ1-180, albeit less efficiently than fragment 1-250 (Fig. 1D, compare lanes 12 and 13). These results suggested that RID1 functions as a single domain spanning amino acids 1 to 250. Fragment 1-250, but not 1-200, also stimulated the efficiency of DNA synthesis approximately twofold (Fig. 1D, compare lane 10 to lanes 12 and 13). The complementation defect of fragment 1-200 was not detected when telomerase activity was measured by TRAP (Fig. 1C, lane 21). Previous mutagenesis studies using the TRAP assay reported that hTERT residues 200 to 250 are not implicated in telomerase activity, though mutations in this region cause small reductions in hTR interaction (3, 44). In contrast, the direct primer extension assay suggests a role for these residues in both repeat addition processivity and efficient DNA synthesis.
To determine whether sequences between residues 200 to 250 and also the N-DAT region were important for processivity, we examined the catalytic activity of two hTERT variants containing small internal deletions of amino acids 230 to 239 and 110 to 119, respectively (data not shown) (44). Both mutants exhibited wild-type levels of first repeat DNA synthesis and processivity when activity was detected using the direct primer extension assay and a telomeric primer (data not shown). These data indicate that this region of N-DAT and some residues between amino acids 200 and 250 are not required for processive elongation of telomeric primers. Secondary structure predictions for hTERT indicate that amino acids 206 to 250 are unlikely to form secondary structures (http://bioinf.cs.ucl.ac.uk). Furthermore, these sequences are not conserved, even among vertebrate TERTs (44, 56). Therefore, secondary structure predictions and the activity profiles of the Δ230-239 mutant and 1-200 and 1-250 RID1 fragments suggest that the functional C-terminal boundary of RID1 falls after hTERT residue 200 and likely does not include amino acids 230 to 250 (Fig. 1A).
The repeat addition processivity defects of RID1 mutants are not attributable to reduced elongation kinetics.
To determine whether the apparent processivity defects of the Δ1-180 and Δ150-159 RID1 mutants could be explained by reduced elongation kinetics, we examined the product profiles of these mutants in pulse-chase time course experiments (Fig. 2). Fewer wild-type telomerase elongation products were generated during a 5-min pulse-labeling than during a longer pulse-labeling (Fig. 2A, lanes 1 and 2). Since RID1 mutants were less active than wild-type hTERT (Fig. 1D), we performed RID1 pulse-chase experiments using three times more RRL-reconstituted telomerase and primer extension reagents. Under these conditions, the elongation products of RID1 mutants were readily detected after a 5-min pulse-labeling (Fig. 2B and C, lanes 1). Like wild-type enzyme, the intensity of first repeat DNA synthesis products generated by RID1 mutants did not increase after the addition of chase primer (Fig. 2A to C, lanes 1 and 5 to 8). Furthermore, the pattern of elongation products was the same after 0, 15, 30, 60, and 120 min of incubation with chase primer (Fig. 2A to C, lanes 1 and 5 to 8), indicating that RID1 mutations do not affect the kinetics of primer elongation. As previously observed for Tetrahymena telomerase (13), the high concentration of chase primer (150 μM) used in these assays did not prevent processive elongation of biotinylated pulse primers prebound to wild-type telomerase at a final concentration of 1 μM (data not shown). Therefore, the inability of RID1 mutants to synthesize more than one telomere repeat under pulse-chase conditions is unlikely to be caused by nonspecific telomerase inhibition at high concentrations of chase primer. Pulse-chase experiments also confirmed that the major property conferred by trans-acting RID1 sequences is repeat addition processivity (Fig. 2D and E). Reconstitution of RID1 mutant telomerases in the presence of RID1 residues 1 to 250 strongly stimulated processivity and did not alter primer elongation kinetics (Fig. 2, compare panel B to D and panel C to E). Therefore, we concluded that deletion of RID1 residues 150 to 159 or 1 to 180 specifically impairs repeat addition processivity and that trans-acting RID1 sequences confer this property to RID1 mutants.
FIG. 2.
RID1 mutants display specific defects in repeat addition processivity. Pulse-chase time course experiments were performed to determine whether the product profiles of RID1 mutants were attributable to defects in repeat addition processivity or elongation kinetics. Wild-type (WT) hTERT (A) and RID1 variants (B to E) were synthesized in RRL in the presence of wild-type hTR and in the absence (B and C) or presence (D and E) of DNA encoding the minimal RID1 region required for complementation of processivity defects (amino acids 1 to 250). Since RID1 mutants were less active than wild-type hTERT (Fig. 1D), we performed RID1 pulse-chase experiments using three times more RRL-reconstituted telomerase and primer extension reagents (B to E). Experiments were performed with wild-type telomerase using standard assay conditions (A). Reconstituted telomerases were incubated with biotinylated (TTAGGG)3 pulse primer for 5 min (5′) at 30°C in the presence of dATP, dTTP, and [α-32P]dGTP (direct primer extension assay). A 150-fold excess of nonbiotinylated (TTAGGG)3 chase primer was added, and the reactions continued for 15, 30, 60, or 120 min. Elongated biotinylated pulse primers were separated from the products of chase primer elongation using magnetic streptavidin beads. Two controls were used. In control 1 (lane 3), pulse and chase primers were added simultaneously to demonstrate the efficiency of chase conditions. In control 2 (lane 4), reactions were performed with chase primer alone to demonstrate that nonbiotinylated chase primer elongation products are not purified by streptavidin beads. A 3′ radiolabeled biotinylated TTAGGGT primer was added to stopped reaction mixtures prior to purification on streptavidin beads as a purification and loading control (LC). First repeat DNA synthesis and repeat addition processivity (R.A.P.) values for individual samples (expressed in arbitrary units) are indicated below the gels. All experiments were performed twice.
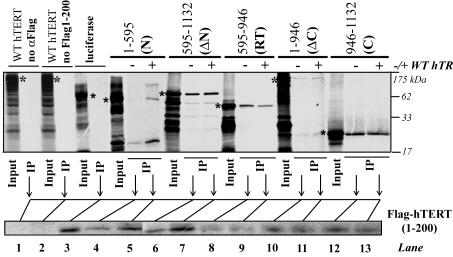
RID1 interacts with the hTERT RT motifs and putative thumb.
Since RID1 was physically separable from the hTERT polymerase domain and stimulated DNA synthesis and repeat addition processivity in trans (Fig. 1D), we investigated whether RID1 associated with hTERT in vitro and whether these interactions were dependent on hTR (Fig. 3). RID1 associated most efficiently with fragments containing both the RT motifs and C terminus, and coprecipitation of these regions was not affected by hTR (Fig. 3, lanes 8 to 13). Interactions were specific, since an unrelated protein, luciferase, did not coprecipitate with RID1 (Fig. 3, lane 3). The hTERT N terminus associated less efficiently with RID1 than did variants containing RT and C-terminal sequences, and N terminus-RID1 interactions appeared to be partly dependent on hTR (Fig. 3, lanes 4 and 5). We concluded that RID1 interacts with multiple sites in hTERT but associates predominantly with the C terminus and RT motifs. The putative hTERT C-terminal thumb, motif E and a telomerase-specific insertion in the fingers region of S. cerevisiae TERT, and a telomerase-specific residue in the RT region of Tetrahymena TERT have previously been shown to influence repeat addition processivity (12, 13, 31, 38). Interestingly, mutations in HIV-1 RT's p51 thumb, which interacts with the RNase H accessory domain of the p66 subunit, impair RNase H function (16). These observations suggest that RID1's association with hTERT's RT and putative C-terminal thumb sequences might be important for repeat addition processivity. Interactions between RID1 and the hTERT RT motifs and C terminus could also stimulate DNA synthesis efficiency as previously observed for the RNase H accessory domain of HIV-1 RT (30).
FIG. 3.
RID1 interacts with hTERT RT and C-terminal sequences. 35S-labeled protein fragments corresponding to different hTERT regions (N- and C-terminal regions and RT) (indicated by asterisks in the top gel) were coimmunoprecipitated with unlabeled Flag-tagged hTERT1-200 (bottom gel) in the presence (+) or absence (−) of wild-type (WT) hTR. Flag-hTERT1-200 (RID1) was detected by immunoblotting with anti-Flag antibody (αFlag). IP, immunoprecipitation.
RID1 sequences that mediate repeat addition processivity are required for in vitro association with the hTR template-pseudoknot domain.
Est2p RID1 sequences interact nonsequence specifically with DNA and RNA (56). Similarly, RID1 of hTERT can independently bind wild-type hTR, and RID1 mutations reduce hTR binding and abolished repeat addition processivity (Fig. 1D) (9, 44). The pseudoknot-template domain is an important mediator of vertebrate telomerase processivity (see below) (18).
We investigated the in vitro interaction of this hTR region with RID1. Though the pseudoknot-template domain is known to associate with hTERT, the hTERT sequences mediating this interaction have not been identified (6, 10). We found that the template-pseudoknot domain (nucleotides 1 to 209) appeared to interact more efficiently with RID1 than RID2 (Fig. 4B, lanes 9 and 10). On average, RID1 associated with hTR1-209 1.95 times more efficiently than with RID2 (P < 0.05) (five independent experiments were done).
Competition experiments performed with unlabeled competitor wild-type hTR and labeled hTR1-209 indicated that wild-type hTR inhibited binding of hTR1-209 to RID2 more efficiently than RID1 (Fig. 4D). This result suggests that hTR1-209 associates more strongly with RID1 than RID2. Interestingly, lower concentrations of competitor wild-type hTR frequently stimulated the association of hTR1-209 with both RID1 and RID2 (Fig. 4D). This stimulatory effect might be attributable to the ability of hTR 1-209 to dimerize with wild-type hTR (40). RID1 bound nucleotides 1 to 209 less efficiently than full-length hTERT, implying that the template-pseudoknot domain may associate with additional hTERT regions (Fig. 4B, lanes 8 and 10).
More precise mapping of the RID1 sequences that interact with nucleotides 1 to 209 revealed that RID1 residues implicated in complementation of the processivity and DNA synthesis defects of RID1 mutants were also required for pseudoknot-template interactions (compare Fig. 1D, lanes 13 to 15, and Fig. 4B, lanes 12 to 14). Interestingly, deleting amino acids 201 to 250 abolished hTR1-209 interactions, suggesting that the processivity- and DNA synthesis-regulating functions of these sequences may be mediated in part by hTERT-hTR interactions (Fig. 4B, lanes 11 and 12; Fig. 1D, lanes 12 and 13). Though fragment 1-200 was sufficient for complementation of the processivity defect of the Δ1-180 mutant, the presence of residues 200 to 250 in hTERT Δ1-180 might rescue the hTR interaction defect of fragment 1-200 in hybrid enzymes (Fig. 1D). Alternatively, the technical limitations of the RNA binding assay may preclude identification of sequences that interact weakly with hTR but are sufficient for minimal catalytic function. RID1 residues 1 to 24 were also essential for association with the pseudoknot-template domain (Fig. 4B, lane 14), implying that the RID1 domain in its entirety is required for interaction with this hTR region. Since neither fragment 1-200 nor 25-300 bound hTR1-209 efficiently, it is unlikely that amino acids 1 to 250 interacted nonspecifically with hTR. hTR1-209 did not interact with an unrelated protein, NKKβ (Fig. 4C, lane 3), and an unrelated RNA, DART-1, did not associate with RID1 (Fig. 4C, lane 9). Furthermore, deletion of residues 150 to 159, which abolished repeat addition processivity, reduces the interaction of hTERT with wild-type hTR (Fig. 1D) (44). These results suggested that RID1's regulation of processivity may depend partly on its association with the hTR template-pseudoknot domain, though RID1-mediated hTERT-hTERT and DNA primer interactions could also contribute to this catalytic function.
We found that the hTERT RID1 constitutes a single functional domain that is essential for repeat addition processivity. RID1 interacted with the processivity-regulating hTR pseudoknot-template domain, implying that RID1-hTR interactions might mediate processivity. In addition, RID2 sequences mediating high-affinity hTR interactions were required for DNA synthesis. Since hTR-hTERT interactions were important for both DNA synthesis and processivity, we examined the roles of the hTR CR4-CR5 and pseudoknot-template domains in the catalytic function of telomerase.
RID2-interacting sequences in the hTR CR4-CR5 domain are essential for DNA synthesis.
First, we examined the effects of CR4-CR5 domain mutations on telomerase activity (Fig. 5A and C). CR4-CR5-containing hTR sequences interact with hTERT RID2, and the stem structure of the mTR CR4-CR5 P6.1 helix is required for high-affinity interaction with wild-type mouse TERT (20, 35, 43). We found that disruption of base pairing in the hTR P6.1 stem abolished DNA synthesis (<1% wild-type first repeat DNA synthesis), and compensatory mutations that reconstitute the P6.1 stem structure and rescue TERT interactions restored wild-type levels of catalytic activity (Fig. 5C, lanes 7 to 9) (20). P6.1 base pairing mutants were defective in interactions with RID2 (Fig. 5E), indicating that the catalytic function of the P6.1 helix is likely mediated by binding to RID2. Mutation of other CR4-CR5 sequences had more modest effects on DNA synthesis (Fig. 5C, lanes 1 to 5). Interestingly, an hTR variant containing altered sequences immediately 3′ of the P6.1 stem-loop also exhibited a 75% reduction in repeat addition processivity (Fig. 5C, lane 5). We concluded that the RID2-interacting CR4-CR5 P6.1 stem-loop is essential for DNA synthesis. Since hTR binding-defective RID2 mutants exhibited comparable DNA synthesis defects (Fig. 1B), we concluded that high-affinity interactions between the P6.1 stem-loop and RID2 are likely required for the basic DNA synthesis function of telomerase.
Nucleotides in the hTR pseudoknot-template domain are essential for repeat addition processivity.
Next, we investigated the effects of pseudoknot-template domain mutations on DNA synthesis and repeat addition processivity. We found that nucleotides outside the template were not essential for DNA synthesis, though deleting these sequences reduced the amount of DNA synthesized (Fig. 6A, lanes 9 to 11 and 15). In contrast, most mutations inhibited processivity, consistent with recent reports that the pseudoknot-template domain is important for this catalytic function (Fig. 5D, lanes 2 to 4; Fig. 6A, lanes 9 to 10) (also substitution 36 to 45 and substitution 122 to 129 [data not shown]) (18, 36, 41). Interestingly, four hTR mutants, Δ65-144, Δ145-208, hTR170, and hTR180, exhibited elongation defects consistent with a complete loss of processivity (<2%) (Fig. 5D, lanes 3 and 4; Fig. 6A, lanes 9 and 10).
hTR170 and hTR180 contain complementary substitutions at positions 170 to 179 (hTR170) and 180 to 189 (hTR180) in the P3 helix (Fig. 5B). Substitution of hTR residues in this region with mTR sequences has been demonstrated to affect telomerase activity levels when chimeric TRs are assembled with hTERT, though a role in repeat addition processivity was not identified (18). We found that the hTR170 and hTR180 P3 mutants synthesized DNA less efficiently than wild-type enzyme but were catalytically active and generated only one repeat of elongation products with a pattern similar to the wild-type profile. Another P3 helix mutant containing a two-base substitution found in a family with autosomal dominant dyskeratosis congenita (DKC) exhibited activity levels similar to hTR170 but was fully processive (Fig. 5D, lane 5) (52). Substitution of nucleotides 190 to 199 in the adjacent P1 helix reduced processivity but did not affect the efficiency of DNA synthesis (Fig. 5D, lane 2). We recently found that the hTR190 and hTR180 mutants contain an additional small deletion of hTR nucleotides 200 and 201 (see Materials and Methods). The distinct catalytic phenotypes of the hTR180 and hTR190 mutants (Fig. 5D) suggest that the deletion of nucleotides 200 and 201 is not a major determinant of the catalytic properties of these mutants. Since nucleotides 170 to 189 were essential for repeat addition processivity, we investigated the mechanism underlying the severe processivity defects of the hTR170 and hTR180 mutants.
Pseudoknot-mediated hTR dimerization is necessary but not sufficient for repeat addition processivity.
Consistent with previous reports that the P3 helix is not essential for hTERT binding (6, 40), we found that the hTR170 and hTR180 substitutions did not prevent hTR from binding to full-length hTERT, RID1, or RID2 (data not shown). Since hTR interaction with the hTERT RID1 was not sufficient for repeat addition processivity, we investigated whether altered hTR-hTR interactions could account for the processivity defects of the hTR170 and hTR180 mutants. Substitution of nucleotides 170 to 189 with complementary sequences is predicted to disrupt the pseudoknot P3 helix, which is formed by long-range base pairing between nucleotides in the phylogenetically conserved CR2 and CR3 regions (Fig. 5B) (17). Interestingly, disruption of long-range base pairing potential in the Tetrahymena and Kluyveromyces lactis TR pseudoknots inhibits repeat addition processivity and affects template usage, respectively (36, 51). hTR dimerization is mediated by trans base pairing of the CR2 and CR3 sequences from separate hTR molecules (Fig. 5B) (40).
To investigate the hypothesis that P3-mediated hTR-hTR interactions might regulate processivity, we analyzed the in vitro dimerization potential of the hTR170 and hTR180 mutants. We found that hTR170 exhibited dimerization defects as extensive as those observed for an hTR mutant lacking all of CR2 (Fig. 5F to G, hTR170 and Δ65-144). However, hTR180 dimerized as efficiently as wild-type hTR, implying that the impaired processivity of this mutant was not attributable to dimerization defects detectable by this assay (Fig. 5F to G). We also found that the fully processive DKC mutant dimerized more efficiently than wild-type hTR (Fig. 5F to G). These data imply that trans P3 base pairing between full-length hTR molecules may not require the 3′ end of the P3 helix (nucleotides 107 and 108 and nucleotides 180 to 183) and may be regulated differently than the P3 interactions reconstituted in oligonucleotide model systems (21, 40, 50). A recent study found that not all compensatory base pairing substitutions in the K. lactis pseudoknot restored wild-type telomerase activity (51). Though it is not known whether the K. lactis TR dimerizes, these observations suggest that the pseudoknot base pairing structure alone is not sufficient for catalytic function. Similarly, we concluded that P3 helix-mediated hTR dimerization is not sufficient for repeat addition processivity.
Though the processivity impairment of the hTR180 mutant could not be attributed to a loss of hTR-hTR interactions, we found that reductions in dimerization efficiency coincided with processivity defects in other mutants. The processivity-impaired hTR190 mutant dimerized as inefficiently as hTR170, indicating that P1 helix residues are important for hTR-hTR interactions (Fig. 5F to G). In addition, deletion of CR2- or CR3-containing sequences inhibited dimerization and abolished processivity (Δ65-144 and Δ145-208 mutants) (Fig. 5F to G and Fig. 6A). Interestingly, the Δ145-208 mutant, which lacks both CR3 and P1 helix sequences, dimerized less efficiently than the Δ65-144 mutant, supporting the conclusion that the P1 helix participates in hTR-hTR interactions (Fig. 5F to G). Since all of the dimerization-impaired hTR mutants we tested were also defective in processivity (Fig. 5 and 6), we concluded that hTR dimerization may be necessary though not sufficient for wild-type levels of repeat addition processivity.
trans-acting hTRs restore dimerization and repeat addition processivity to a P3 helix mutant.
To test the hypothesis that hTR dimerization is important for repeat addition processivity, we asked whether trans-acting hTR fragments could restore processivity and dimerization to the hTR170 mutant. First, we mixed hTR170 with nucleotides 1 to 209 or 207 to 451 and found that only the pseudoknot-template domain could complement the processivity defects of the hTR170 mutant (Fig. 6A, lanes 4 to 6). An inactive CR4-CR5 mutant also reconstituted processivity when mixed with hTR170, confirming that trans complementation of processivity defects can occur in the context of overlapping hTR molecules and is not dependent on the presence of a functional CR4-CR5 domain in the complementing molecule (Fig. 6A, lane 7).
Next, we asked which nucleotides in the pseudoknot-template domain were responsible for trans complementation of the processivity defects of the hTR170 mutant. We deleted nucleotides 1 to 64, 65 to 144, or 145 to 208 from hTRs containing an intact 3′ region (nucleotides 209 to 451). Nucleotides 1 to 64 contain the template, upstream sequences involved in P1 helix formation, and a downstream processivity-determining element (Fig. 4A) (17, 18). As expected, deletion of the hTR template region abolished telomerase activity (Fig. 6A, lane 11). Nucleotides 65 to 144 include all of the sequences required to form the P2a.1, P2a, and P2b helices, in addition to the CR2 residues involved in P3 base pairing. Deletion of nucleotides 145 to 208 removes the entire upper strand of the pseudoknot region, including the CR3 sequences required for P3 base pairing with CR2 (Fig. 4A) (17). Deletion of nucleotides 145 to 208 or 65 to 144 disrupted hTR dimerization and abolished processivity (Fig. 5F to G; Fig. 6A, lanes 9 and 10).
Mixing hTR170 with Δ65-144 or Δ1-64, which confers P3 base pairing potential in trans, stimulated processivity by approximately 10-fold and restored dimerization to wild-type levels (Fig. 6A, lanes 13 and 14; Fig. 6B and C). Similarly, mixing hTR180 with Δ65-144 or Δ1-64 reconstituted processivity though less efficiently than hTR170, suggesting that trans-acting CR3 sequences also contribute to processivity (data not shown). However, mixing Δ145-208 with hTR170 restored neither dimerization nor processivity (Fig. 6A, lane 12; Fig. 6B and C). We concluded that reconstitution of trans P3 base pairing potential and dimerization can confer processivity to the hTR170 mutant. Recent studies have identified a role for trans-acting Tetrahymena pseudoknot elements in repeat addition processivity (36, 41). Though the mechanistic contribution of pseudoknot sequences and structures to repeat addition processivity remains unclear, our results establish a role for hTR-hTR interactions in the repeat addition processivity of human telomerase.
DISCUSSION
Functional organization of the human telomerase complex.
We found that processivity-mediating RID1 residues interact with the processivity-regulating hTR pseudoknot-template domain and that hTR-interacting residues in RID2 and a RID2-interacting helix in CR4-CR5 are essential for DNA synthesis. These results imply that hTERT-hTR interactions contribute to both the DNA synthesis and processivity functions of human telomerase. In addition, we found that trans-acting hTERTs or hTRs confer repeat addition processivity to processivity-defective hTERT or hTR mutants, respectively, suggesting a role for telomerase multimerization in repeat addition processivity. RID1 interacted with processivity-determining hTERT C-terminal sequences, and hTR dimerization also contributed to repeat addition processivity.
A schematic summary of the hTERT-hTR, hTERT-hTERT, and hTR-hTR interactions reported in this study and other studies (10, 20, 35, 40) is presented in Fig. 7. The role of hTERT multimerization in repeat addition processivity and DNA synthesis may be analogous to the interdependent functioning of the polymerase of HIV-1 RT and RNase H accessory domains (see below). hTR-hTR interactions at the P3 helix interface could stabilize the hTR dimer structure, permitting one TR to allosterically influence the function or conformation of the other TR (40). hTR interactions with RID1 and RID2 hTERT sequences essential for processivity and DNA synthesis, respectively, could facilitate the dynamic hTR rearrangements that are likely involved in telomerase catalysis. Such rearrangements could include the alternating use of the two potential active sites of telomerase (46, 55), though it is unknown whether the active site or template switching is an obligatory feature of the catalytic function of telomerase. An alternative model for the function of telomerase dimerization is that dimerization facilitates the simultaneous extension of two DNA substrates (55). Such a hypothesis might account for the observation that the abundant Tetrahymena telomerase is active and processive as a monomer (15).
FIG. 7.
Proposed functional domain organization of the human telomerase multimer. Schematic of hTERT-hTR, hTERT-hTERT, and hTR-hTR interactions in the human telomerase complex. RID1 is connected to the hTERT core polymerase domain (RID2, RT, and C terminus [C]) by a flexible, catalytically inessential linker. The RID2-P6.1 interaction site is likely essential for DNA synthesis. RID1-pseudoknot-template and RID1-C-terminus interactions may regulate repeat addition processivity. RID1 and the C terminus can functionally interact in trans (see text), implying that RID1 and the polymerase domain of a single hTERT may interact with different hTR molecules. P3 helix-mediated hTR dimerization also contributes to repeat addition processivity and may facilitate the allosteric regulation of one hTR monomer by the other (see text). This schematic is based on the recently proposed hTR dimer structure (40). Contacts between RID2 and the C terminus or RT sequences (2) are not depicted in this model but could form during the dynamic rearrangements likely entailed by telomerase catalysis.
RID1 as the major accessory domain of hTERT.
Nucleic acid polymerases contain unique, structurally unrelated major accessory domains that confer the specialized activities of each enzyme and interact with nucleic acids upstream of the polymerase active site but are not required for basic polymerase function (reviewed in reference 48). Previous work indicates that RID1 interacts with full-length hTR and is physically separable from the remainder of hTERT (9). Also, deletion of RID1 reduces but does not abolish telomerase activity and affects the ability of human telomerase to extend nontelomeric primers (9). We have identified the processivity-regulating hTR pseudoknot-template domain as a site in hTR that interacts with RID1. Furthermore, using a direct primer extension telomerase assay, we found that RID1 is not essential for DNA synthesis and is physically and functionally separable from, though functionally interdependent with, the core polymerase domain of hTERT (RID2, RT, and C terminus). These data suggest that RID1 may be the major accessory domain of hTERT and that the unique catalytic property it confers is repeat addition processivity.
The HIV-1 RT is a well-characterized dimeric nucleic acid polymerase which has been used as a model for telomerase function in several previous structure-function studies (reviewed in reference 32). However, the similarities we observed between the domain organizations of HIV-1 RT of hTERT and the pattern of hTERT and RT accessory domain interactions with nucleic acids and polymerase sequences are also true of many other nucleic acid polymerases (references 28, 30, 48, 54, and references therein). Like the RNase H accessory domain of HIV-1 RT, the ability of RID1 to interact with several catalytically important hTERT regions could account for the effects of RID1 mutations on the DNA synthesis efficiency of telomerase (30). The association of the hTERT C terminus with RID1 could also influence repeat addition processivity by a mechanism analogous to p51 thumb's regulation of the RNase H function of the p66 subunit (16). Interestingly, we found that processivity-defective C-terminal mutants functionally complemented the processivity defects of a RID1 mutant, suggesting that RID1 and C-terminal processivity-determining sequences can be physically located on separate hTERT molecules (Fig. 7; Table 1; also data not shown). These observations imply that trans interactions in the TERT multimer can confer repeat addition processivity to human telomerase. Though protein fragments containing RID1 complemented overlapping RID1 mutants, our results do not indicate that RID1 always functions in trans in the human telomerase multimer.
Processivity-determining RID1 sequences also interacted with the hTR pseudoknot-template domain, which is a major determinant of repeat addition processivity (Fig. 7) (this study) (18). Recent work demonstrates that the vertebrate pseudoknot-template domain mediates mouse- and human-specific differences in telomerase activity in a TERT-dependent fashion (18). Though this study reported that the mTR pseudoknot interacts more efficiently with hTERT than the hTR pseudoknot, TR-TERT interactions were evaluated using wild-type hTERT and an hTR pseudoknot-template domain missing the first 43 nucleotides of hTR (18). These hTR sequences are important for human telomerase activity and have been recently implicated in 5′ template boundary definition and repeat addition processivity (10, 19, 39). Since hTR residues 1 to 39, which are absent in mTR, may be implicated in hTERT interactions (6, 10) and since these nucleotides are protected in vivo, but not in vitro (1), it is possible that hTERT-hTR1-209 interactions could partially account for species-specific differences in repeat addition processivity. Interestingly, substitution of nucleotides 36 to 45 or 190 to 199, which alters P1 helix sequences, reduced processivity, suggesting that this region might be a candidate for RID1 interactions (Fig. 5D; also data not shown). The accessory domains of a number of polymerases, such as the HIV-1 RT, regulate the movement of nucleic acids during catalysis (7), and repeat addition processivity is likely to entail dynamic rearrangements of the template RNA and/or DNA substrate within the catalytic active site. Our observations suggest a similar function for RID1.
The HIV-1 RNase H accessory domain also contributes to the stability of RT-primer-template complexes (reviewed in reference 24). Repeat addition processivity may be partly regulated by telomerase-DNA interactions at a protein-dependent anchor site that is distinct from the catalytic core (references 25 and 53 and references therein). Interestingly, in vitro-reconstituted human telomerase lacking RID1 cannot elongate nontelomeric primers, but this defect is rescued by coexpressing a second, inactive hTERT containing an intact RID1 (9). Together with our data demonstrating that trans complementation restores processivity to RID1 mutants, these observations suggest that RID1 may interact with DNA substrates and could constitute the TERT anchor site (9, 56).
RNA-dependent strand transfers as a model for telomerase repeat addition processivity.
Our data indicate that the hTERT RID1 and dimerization-mediating hTR sequences are essential for repeat addition processivity. These observations, and the similarities we have found between RID1 and the HIV-1 RNase H accessory domain, suggest that repeat addition processivity may resemble the strand transfer events mediated by HIV-1 and other RTs (reviewed in reference 24). During replication of the double-stranded RNA genome of HIV-1, RNase H-dependent strand transfers at the 5′ end of RNA templates facilitate the translocation of newly synthesized minus-strand DNA to the 3′ end of the RNA acceptor strand. By a mechanism that recalls the processivity-determining features of the 3′ template-3′ DNA substrate interactions of telomerase (18), the 3′ end of translocated donor strand DNA then hybridizes to complementary sequences at the 3′ end of either one of the homologous copies of genomic RNA, permitting a second round of DNA synthesis to begin. RNA-dependent strand transfers require the cleavage activity of the RNase H domain, suggesting that RID1 could mediate a similar function in hTERT. Endonuclease cleavage activity has been reported for yeast and ciliate telomerases (reviewed in reference 27). However, the roles of specific TERT sequences in endonucleolytic cleavage have not been characterized, and the similarities shared by the RNase H and RID1 accessory domains may not include cleavage function.
RNA-dependent strand transfers also require dimerization-initiating sequences in the double-stranded RNA template, though the function of template dimerization in strand transfers is not fully understood (8). The primary RNase H-mediated invasion step of strand transfer can occur at sites that are physically distant from the dimerization-initiating sequences, implying that dimerization is not required for the initiation of this process (8). RNA-dependent strand transfers can proceed in either an intra- or intermolecular fashion, though template switching is not obligatory. A strand transfer model of repeat addition processivity could therefore explain the ability of telomerase dimers to use both their templates (46, 55). Though RNA-dependent strand transfers and telomerase repeat addition processivity share some interesting features, it is unlikely that the mechanisms mediating these two processes are identical. The extent of their potential similarities and the roles of RID1 and the TR pseudoknot in repeat addition processivity remain to be defined.
In this study, we identified elements in hTR and hTERT that regulate DNA synthesis and repeat addition processivity. Our data implicate telomerase multimerization in repeat addition processivity and suggest that the mechanism mediating this telomerase-specific catalytic function may represent a variation on a catalytic mechanism shared by other RTs.
Acknowledgments
We thank L. Harrington for donating the pCR3.1hTERT plasmid used to generate Flag-hTERT constructs, R. Lin for the Flag-tagged NKKβ construct, and M.-C. Boulanger and S. Richard for donating the DART-1 construct. We thank M. A. Cerone, M. Götte, S. Huard, M. Taboski, and R. J. Ward for comments on the manuscript; J. Demers, S. Dupuis, S. Huard, Y. Sun, and M. Taboski for providing technical assistance or constructs; and C. Lalonde for help in preparing the figures.
This work was supported by CIHR grant MOP-14026 to C.A. T.J.M. is the recipient of a CIHR doctoral research scholarship. C.A. was supported by Boehringer Ingelheim (Canada) Young Investigator and FRSQ Chercheur-Boursier awards.
REFERENCES
- 1.Antal, M., E. Boros, F. Solymosy, and T. Kiss. 2002. Analysis of the structure of human telomerase RNA in vivo. Nucleic Acids Res. 30:912-920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai, K., K. Masutomi, S. Khurts, S. Kaneko, K. Kobayashi, and S. Murakami. 2002. Two independent regions of human telomerase reverse transcriptase are important for its oligomerization and telomerase activity. J. Biol. Chem. 277:8538-8544. [DOI] [PubMed] [Google Scholar]
- 3.Armbruster, B., S. Banik, C. Guo, A. Smith, and C. Counter. 2001. N-terminal domains of the human telomerase catalytic subunit required for enzyme activity in vivo. Mol. Cell. Biol. 21:7775-7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Autexier, C., R. Pruzan, W. D. Funk, and C. W. Greider. 1996. Reconstitution of human telomerase activity and identification of a minimal functional region of the human telomerase RNA. EMBO J. 15:5928-5935. [PMC free article] [PubMed] [Google Scholar]
- 5.Bachand, F., and C. Autexier. 1999. Functional reconstitution of human telomerase expressed in Saccharomyces cerevisiae. J. Biol. Chem. 274:38027-38031. [DOI] [PubMed] [Google Scholar]
- 6.Bachand, F., and C. Autexier. 2001. Functional regions of human telomerase reverse transcriptase and human telomerase RNA required for telomerase activity and RNA-protein interactions. Mol. Cell. Biol. 21:1888-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bahar, I., B. Erman, R. L. Jernigan, A. R. Atilgan, and D. G. Covell. 1999. Collective motions in HIV-1 reverse transcriptase: examination of flexibility and enzyme function. J. Mol. Biol. 285:1023-1037. [DOI] [PubMed] [Google Scholar]
- 8.Balakrishnan, M., B. P. Roques, P. J. Fay, and R. A. Bambara. 2003. Template dimerization promotes an acceptor invasion-induced transfer mechanism during human immunodeficiency virus type 1 minus-strand synthesis. J. Virol. 77:4710-4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beattie, T., W. Zhou, M. Robinson, and L. Harrington. 2001. Functional multimerization of the human telomerase reverse transcriptase. Mol. Cell. Biol. 21:6151-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beattie, T., W. Zhou, M. Robinson, and L. Harrington. 2000. Polymerization defects within human telomerase are distinct from telomerase RNA and TEP1 binding. Mol. Biol. Cell 11:3329-3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bibi, E., and H. R. Kaback. 1990. In vivo expression of the lacY gene in two segments leads to functional lac permease. Proc. Natl. Acad. USA 87:4325-4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosoy, D., and N. F. Lue. 2004. Yeast telomerase is capable of limited repeat addition processivity. Nucleic Acids Res. 32:93-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryan, T., K. Goodrich, and T. Cech. 2000. A mutant of Tetrahymena telomerase reverse transcriptase with increased processivity. J. Biol. Chem. 275:24199-24207. [DOI] [PubMed] [Google Scholar]
- 14.Bryan, T., K. Goodrich, and T. Cech. 2000. Telomerase RNA bound by protein motifs specific to telomerase reverse transcriptase. Mol. Cell 6:493-499. [DOI] [PubMed] [Google Scholar]
- 15.Bryan, T. M., K. J. Goodrich, and T. R. Cech. 2003. Tetrahymena telomerase is active as a monomer. Mol. Biol. Cell 14:4794-4804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cameron, C. E., M. Ghosh, S. F. J. LeGrice, and S. J. Benkovic. 1997. Mutations in HIV reverse transcriptase which alter RNase H activity and decrease strand transfer efficiency are suppressed by HIV nucleocapsid protein. Proc. Natl. Acad. Sci. USA 94:6700-6705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen, J.-L., M. A. Blasco, and C. W. Greider. 2000. Secondary structure of vertebrate telomerase RNA. Cell 100:503-514. [DOI] [PubMed] [Google Scholar]
- 18.Chen, J.-L., and C. W. Greider. 2003. Determinants in mammalian telomerase RNA that mediate enzyme processivity and cross-species incompatibility. EMBO J. 22:304-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen, J.-L., and C. W. Greider. 2003. Template boundary definition in mammalian telomerase. Genes Dev. 17:2747-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen, J.-L., K. Keyer Opperman, and C. W. Greider. 2002. A critical stem-loop structure in the CR4-CR5 domain of mammalian telomerase RNA. Nucleic Acids Res. 30:592-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comolli, L. R., I. Smirnov, L. Xu, E. H. Blackburn, and T. L. James. 2002. A molecular switch underlies a human telomerase disease. Proc. Natl. Acad. Sci. USA 99:16998-17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman, K. L., and T. R. Cech. 1999. Essential functions of amino-terminal domains in the yeast telomerase catalytic subunit revealed by selection for viable mutants. Genes Dev. 13:2863-2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gavory, G., M. Farrow, and S. Balasubramanian. 2002. Minimum length requirement of the alignment domain of human telomerase RNA to sustain catalytic activity in vitro. Nucleic Acids Res. 30:4470-4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gotte, M., X. Li, and M. A. Wainberg. 1999. HIV-1 reverse transcription: a brief overview focused on structure-function relationships among molecules involved in initiation of the reaction. Arch. Biochem. Biophys. 365:199-210. [DOI] [PubMed] [Google Scholar]
- 25.Hammond, P. W., T. N. Lively, and T. R. Cech. 1997. The anchor site of telomerase from Euplotes aediculatus revealed by photo-cross-linking to single- and double-stranded DNA primers. Mol. Cell. Biol. 17:296-308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy, C. D., C. S. Schultz, and K. Collins. 2001. Requirements for the dGTP-dependent repeat addition processivity of recombinant Tetrahymena telomerase. J. Biol. Chem. 276:4863-4871. [DOI] [PubMed] [Google Scholar]
- 27.Harrington, L. 2003. Biochemical aspects of telomerase function. Cancer Lett. 194:139-154. [DOI] [PubMed] [Google Scholar]
- 28.Hopfner, K. P., A. Eichinger, R. A. Engh, F. Laue, W. Ankenbauer, R. Huber, and B. Angerer. 1999. Crystal structure of a thermostable type B DNA polymerase from Thermococcus gorgonarius. Proc. Natl. Acad. Sci. USA 96:3600-3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hossain, S., S. M. Singh, and N. F. Lue. 2002. Functional analysis of the C-terminal extension of telomerase reverse transcriptase: a ′putative' thumb domain. J. Biol. Chem. 277:36174-36180. [DOI] [PubMed] [Google Scholar]
- 30.Hostomsky, Z., Z. Hostomska, G. O. Hudson, E. W. Moomaw, and B. R. Nodes. 1991. Reconstitution in vitro of RNase H activity by using purified N-terminal and C-terminal domains of human immunodeficiency virus type 1 reverse transcriptase. Proc. Natl. Acad. Sci. USA 88:1148-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huard, S., T. J. Moriarty, and C. Autexier. 2003. The C terminus of the human telomerase reverse transcriptase is a determinant of enzyme processivity. Nucleic Acids Res. 31:4059-4070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelleher, C., M. T. Teixeira, K. Forstemann, and J. Lingner. 2002. Telomerase: biochemical considerations for enzyme and substrate. Trends Biochem. Sci. 27:572-579. [DOI] [PubMed] [Google Scholar]
- 33.Kim, N. W., M. A. Piatyszek, K. R. Prowse, C. B. Harley, M. D. West, P. L. C. Ho, G. M. Coviello, W. E. Wright, S. L. Weinrich, and J. W. Shay. 1994. Specific association of human telomerase activity with immortal cells and cancer. Science 266:2011-2015. [DOI] [PubMed] [Google Scholar]
- 34.Kim, N. W., and F. Wu. 1997. Advances in quantification and characterization of telomerase activity by the telomeric repeat amplification protocol (TRAP). Nucleic Acids Res. 25:2595-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai, C., J. Mitchell, and K. Collins. 2001. RNA binding domain of telomerase reverse transcriptase. Mol. Cell. Biol. 21:990-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai, C. K., M. C. Miller, and K. Collins. 2003. Roles for RNA in telomerase nucleotide and repeat addition processivity. Mol. Cell 11:1673-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, S. R., M. Y. Wong, and K. Collins. 2003. Human telomerase reverse transcriptase motifs required for elongation of a telomeric substrate. J. Biol. Chem. 278:52531-52536. [DOI] [PubMed] [Google Scholar]
- 38.Lue, N. F., Y. C. Lin, and I. S. Mian. 2003. A conserved telomerase motif within the catalytic domain of telomerase reverse transcriptase is specifically required for repeat addition processivity. Mol. Cell. Biol. 23:8440-8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ly, H., E. H. Blackburn, and T. G. Parslow. 2003. Comprehensive structure-function analysis of the core domain of human telomerase RNA. Mol. Cell. Biol. 23:6849-6856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ly, H., L. Xu, M. A. Rivera, T. G. Parslow, and E. H. Blackburn. 2003. A role for a novel ′trans-pseudoknot' RNA-RNA interaction in the functional dimerization of human telomerase. Genes Dev. 17:1078-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason, D. X., E. Goneska, and C. W. Greider. 2003. Stem-loop IV of Tetrahymena telomerase RNA stimulates processivity in trans. Mol. Cell. Biol. 23:5606-5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGuffin, L. J., K. Bryson, and D. T. Jones. 2000. The PSIPRED protein structure prediction server. Bioinformatics 16:404-405. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell, J., and K. Collins. 2000. Human telomerase activation requires two independent interactions between telomerase RNA and telomerase reverse transcriptase. Mol. Cell 6:361-371. [DOI] [PubMed] [Google Scholar]
- 44.Moriarty, T. J., S. Huard, S. Dupuis, and C. Autexier. 2002. Functional multimerization of human telomerase requires an RNA interaction domain in the N terminus of the catalytic subunit. Mol. Cell. Biol. 22:1253-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peng, Y., I. Mian, and N. Lue. 2001. Analysis of telomerase processivity: mechanistic similarity to HIV-1 reverse transcriptase and role in telomere maintenance. Mol. Cell 7:1201-1211. [DOI] [PubMed] [Google Scholar]
- 46.Prescott, J., and E. H. Blackburn. 1997. Functionally interacting telomerase RNAs in the yeast telomerase complex. Genes Dev. 11:2790-2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Romero, D. P., and E. H. Blackburn. 1991. A conserved secondary structure for telomerase RNA. Cell 67:343-353. [DOI] [PubMed] [Google Scholar]
- 48.Sousa, R. 1996. Structural and mechanistic relationships between nucleic acid polymerases. Trends Biochem. Sci. 21:186-190. [PubMed] [Google Scholar]
- 49.Szatmari, I., and J. Aradi. 15. January 2001. Telomeric repeat amplification, without shortening or lengthening of the telomerase products: a method to analyze the processivity of telomerase enzyme. Nucleic Acids Res. 29:E3. [Online.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Theimer, C. A., L. D. Finger, L. Trantirek, and J. Feigon. 2003. Mutations linked to dyskeratosis congenita cause changes in the structural equilibrium in telomerase RNA. Proc. Natl. Acad. Sci. USA 100:449-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tzfati, Y., Z. Knight, J. Roy, and E. H. Blackburn. 2003. A novel pseudoknot element is essential for the action of a yeast telomerase. Genes Dev. 17:1779-1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vulliamy, T., A. Marrone, F. Goldman, A. Dearlove, M. Bessler, P. Mason, and I. Dokal. 2001. The RNA component of telomerase is mutated in autosomal dominant dyskeratosis congenita. Nature 413:432-435. [DOI] [PubMed] [Google Scholar]
- 53.Wallweber, G., S. Gryaznov, K. Pongracz, and R. Pruzan. 2003. Interaction of human telomerase with its primer substrate. Biochemistry 42:589-600. [DOI] [PubMed] [Google Scholar]
- 54.Wang, J., A. K. M. A. Sattar, C. C. Wang, J. D. Karam, W. H. Konigsberg, and T. A. Steitz. 1997. Crystal structure of a pol α family replication DNA polymerase from bacteriophage RB69. Cell 89:1087-1099. [DOI] [PubMed] [Google Scholar]
- 55.Wenz, C., B. Enenkel, M. Amacker, C. Kelleher, K. Damm, and J. Lingner. 2001. Human telomerase contains two cooperating telomerase RNA molecules. EMBO J. 20:3526-3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xia, J., Y. Peng, I. Mian, and N. Lue. 2000. Identification of functionally important domains in the N-terminal region of telomerase reverse transcriptase. Mol. Cell. Biol. 20:5196-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zen, K. H., E. McKenna, E. Bibi, D. Hardy, and H. R. Kaback. 1994. Expression of lactose permease in contiguous fragments as a probe for membrane-spanning domains. Biochemistry 33:8198-8206. [DOI] [PubMed] [Google Scholar]