Abstract
Background:
Mild perifollicular inflammation is seen in both androgenetic alopecia (AGA) cases and normal controls, whereas moderate or dense inflammation with concentric layers of collagen, is seen in AGA cases but only in very few normal controls, and may lessen the response to topical minoxidil. Moderate or dense lymphocytic inflammation and perifollicular fibrosis have poor hair growth following transplantation.
Aim:
The purpose of the study is to evaluate the perifollicular lymphocytic inflammation and fibrosis in AGA patients during follicular unit hair transplantation (FUT) and its comparison in normal controls.
Materials and Methods:
A total of 21 male patients with AGA and 7 matched controls participated in the study. Histopathological analysis of biopsy specimens from donor strip of patients during the hair transplantation and two 4 mm punch biopsies on controls were performed. Morphometric analysis was performed and perifollicular fibrosis was scored based on the width of the condensed collagen at the lower infundibulum and isthmus from 0 to 3. Perifollicular infiltrate was also scored 0-3 and a total score of 3 or more out of 6 was considered significant.
Results:
Nearly 76% of AGA patients had perifollicular fibrosis more than 50 μm at ×200 magnification. Almost 33.33% patients had moderate/dense perifollicular lymphocytic infiltrate whereas none of the controls had it. Total score in AGA cases was significantly higher than controls (P = 0.012) using Chi-square test. Out of 21 patients, 13 had a score of 3 or more and were followed-up with monthly treatment with intralesional steroids using a dermaroller.
Conclusion:
Histopathological evaluation of the donor area is a must during hair transplantation to evaluate the extent of perifollicular inflammation and achieve better results by following it up with treatment directed to decrease the inflammation.
Keywords: Fibrosis, hair transplantation, inflammation
INTRODUCTION
Scalp biopsies are not always performed in androgenetic alopecia (AGA) patients[1] and in those who have poor growth following hair transplantation. The topic of poor growth is seldom a focus of discussion. Consequently, the incidence of suboptimal growth following hair transplant surgery and histopathological evaluation of possible etiologies remain to be explored.[2]
Previous studies on biopsy of AGA and normal patients have revealed that mild perifollicular fibrosis and infiltrates have also been found in normal patients.[3] Perifollicular inflammation was also present in the uninvolved occipital area, which serves as the donor area in follicular unit hair transplantation.[4] A quantitative measure of fibrosis and scoring of perifollicular inflammation has not been performed. We have tried to address these lacunae in our present study.
Aim and objectives
The aim is to evaluate the degree of perifollicular lymphocytic inflammation and fibrosis around the lower infundibula and isthmus in AGA and in normal controls. We also wanted to quantify the amount of perifollicular fibrosis and elucidate its significance in AGA in comparison with controls.
MATERIALS AND METHODS
Patients
A total of 21 patients with AGA undergoing follicular unit hair transplantation (FUT) and 7 normal subjects without AGA as controls participated in the study. Ages ranged from 21 to 30 years all were males. The normal controls taken were age and sex matched with the cases of AGA.
Ethical considerations
A written and signed informed explanatory consent was taken from all the patients before initiation of therapy. This clinical study was performed in accordance with the declaration of Helsinki after approval by the ethical committee of our hospital.
Biopsy
We performed histopathological analysis of biopsy specimens from the occiputs (donor strip) of the 21 patients during FUT. The triangular edges from both sides of the donor strip was cut and sent for histopathological examination [Figure 1]. Two 4 mm punch biopsies were performed on the occiput of the 7 controls and the wound was closed with 3-0 polyglactin 910.
Figure 1.
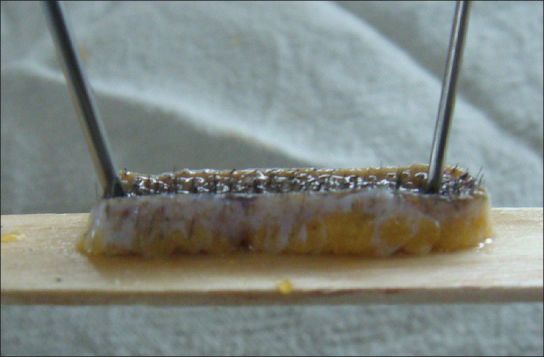
Hair transplant strip showing marked perifollicular fibrosis
Histopathologic analysis
For light microscopy, vertical and transverse sections were made and stained with hematoxylin and eosin. Van Gieson stain was used as a special stain to delineate perifollicular fibrosis clearly. Morphometric analysis was performed utilizing a microcomp image analysis system with a color camera mounted on an Olympus BH-2 research microscope. The quantity of inflammatory infiltrates and the width of perifollicular fibrosis in the lower infundibulum and isthmus were measured. An average of 5 readings at the lower isthmus under ×200 magnification was taken as the width of perifollicular fibrosis.
Scoring
Perifollicular fibrosis was scored from 0 to 3 based on the width of the condensed collagen at the lower infundibulum and isthmus into mild, significant, moderate and dense. Perifollicular infiltrate was also scored 0 to 3 into nil, mild, moderate and dense. Patients and controls were given a total score out of 6 after adding the values of fibrosis and inflammation. A total score of 3 or more out of 6 was considered to be significant.
Statistical analysis
The results were statistically analyzed using the statistical package for the social sciences version 17.0 (SPSS, Inc., Chicago, IL, USA). Differences were considered as statistically significant when P < 0.05. Chi-square test was performed to find if the perifollicular inflammation was significantly higher in cases when compared with controls. Quantitative measurement of width of fibrosis in the lower infundibulum and isthmus was plotted in receiver operating characteristic (ROC) curve and its significance between cases and controls was evaluated.
RESULTS
Nearly 76.19% (16 out of 21) of the patients had a thickness of perifollicular fibrosis in lower infundibulum and isthmus of more than 50 μm at ×200 magnifications [Table 1]. Perifollicular fibrosis [Figure 2] was significantly higher in AGA cases when compared to controls with Chi-square test (P = 0.006).
Table 1.
Width of perifollicular fibrosis in cases and controls at ×200 magnification

Figure 2.

Marked perifollicular fibrosis (>90 μ) in lower infundibulum and isthmus in longitudinal section with with Van Gieson, ×200 magnification
33.33% patients had moderate or dense perifollicular lymphocytic infiltrate [Figure 3] while none of the controls had it [Figure 4]. Only 9.52% patients had no inflammation in contrast to 57.14% of controls [Table 2]. Perifollicular inflammation was also significantly higher in cases as compared with controls using Chi-square test (P = 0.04).
Figure 3.

Perifollicular lymphocytic infiltrate-moderate in longitudinal section
Figure 4.

Normal control-mild fibrosis and no inflammation in longitudinal section
Table 2.
Perifollicular lymphocytic infiltrate in cases and controls

Total score in AGA cases was significantly higher than controls (P = 0.012) using Chi-square test [Table 3]. 13 out of 21 patients (61.9%) had a score of 3 or more and were followed-up with monthly treatment with intralesional steroids using a dermaroller [Figure 5].
Table 3.
Total scores of cases and controls

Figure 5.

Post-transplant intralesional treatment with dermaroller and steroids
ROC curve was plotted using quantitative measurement of width of fibrosis in μm at the level of lower infundibulum and isthmus and its significance between AGA cases and controls was computed. A width of 44.5 μm at lower infundibulum and isthmus had a sensitivity of 81% and specificity of 71.4% for significant fibrosis identification. The 47.5 μm width had a sensitivity of 81% and specificity of 100%. Hence, we postulate any fibrosis over 50 μm width in lower isthmus and infundibulum must be considered significant for AGA.
DISCUSSION
Significant perifollicular fibrosis and inflammation is a cause of sub-optimal hair growth post-transplant. As seen in our study 13 out of 21 cases had significant perifollicular inflammation and fibrosis when compared with controls. Identifying moderate or dense perifollicular infiltrate and fibrosis in the donor area during hair transplant would necessitate treatment with intralesional steroids at monthly intervals post hair transplant for optimal results. Treatment with immunosupressives is also recommended for AGA cases with a score of 3 or above. In other words, AGA cases with a perifollicular fibrosis more than 50 μm at the level of lower infundibulum and isthmus with moderate to dense perifollicular inflammation warrants topical immunosuppressive therapy with dermaroller[5] in AGA.
Observations in AGA patients supports the idea that the inflammatory process around lower infundibulum and isthmus may be responsible for the progression of AGA.[6] Lichen planuspilaris (LPP) like changes has been reported to occur after hair transplantation.[7,8] LPP is basically a histopathologic diagnosis characterized by perifollicular lymphocytic inflammation and fibrosis around the infundibular and isthmus.[9,10] These changes were presumed to be due to factors including koebnerization, loss of hair follicle immunoprivilege, autoimmunity, coincidence or misdiagnosis.[7]
We also postulate that poor hair growth seen in previous studies post hair transplant revealing LPP-like lesions[7,8] may be due to transplantation of hair follicles with moderate to significant perifollicular inflammation in the donor area and recommend histopathological evaluation of donor strip in all cases of hair transplant. Follow-up with topical immunosupressives is required in cases with significant inflammation.
CONCLUSION
Histopathological evaluation of the donor area is a must during hair transplantation to evaluate the extent of perifollicular inflammation and achieve better results by following it up with treatment directed to decrease the inflammation.
Future directions
Further studies can be undertaken to find the efficacy of immunosuppressive therapies in AGA cases with significant perifollicular inflammation and computing it before and after the therapeutic intervention.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Kligman AM. The comparative histopathology of male-pattern baldness and senescent baldness. Clin Dermatol. 1988;6:108–18. doi: 10.1016/0738-081x(88)90074-0. [DOI] [PubMed] [Google Scholar]
- 2.Whiting DA. Diagnostic and predictive value of horizontal sections of scalp biopsy specimens in male pattern androgenetic alopecia. J Am Acad Dermatol. 1993;28:755–63. doi: 10.1016/0190-9622(93)70106-4. [DOI] [PubMed] [Google Scholar]
- 3.Whiting DA. Scalp biopsy as a diagnostic and prognostic tool in androgenetic alopecia. Dermatol Ther. 1998;8:24–33. [Google Scholar]
- 4.Lattanand A, Johnson WC. Male pattern alopecia a histopathologic and histochemical study. J Cutan Pathol. 1975;2:58–70. doi: 10.1111/j.1600-0560.1975.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 5.Dhurat R, Sukesh M, Avhad G, Dandale A, Pal A, Pund P. A randomized evaluator blinded study of effect of microneedling in androgenetic alopecia: A pilot study. Int J Trichology. 2013;5:6–11. doi: 10.4103/0974-7753.114700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sueki H, Stoudemayer T, Kligman AM, Murphy GF. Quantitative and ultrastructural analysis of inflammatory infiltrates in male pattern alopecia. Acta Derm Venereol. 1999;79:347–50. doi: 10.1080/000155599750010238. [DOI] [PubMed] [Google Scholar]
- 7.Chiang YZ, Tosti A, Chaudhry IH, Lyne L, Farjo B, Farjo N, et al. Lichen planopilaris following hair transplantation and face-lift surgery. Br J Dermatol. 2012;166:666–370. doi: 10.1111/j.1365-2133.2011.10692.x. [DOI] [PubMed] [Google Scholar]
- 8.Donovan J. Lichen planopilaris after hair transplantation: Report of 17 cases. Dermatol Surg. 2012;38:1998–2004. doi: 10.1111/dsu.12014. [DOI] [PubMed] [Google Scholar]
- 9.Jiménez F. Commentary: Lichen planopilaris after hair transplantation. Dermatol Surg. 2012;38:2005. doi: 10.1111/j.1524-4725.2012.02578.x. [DOI] [PubMed] [Google Scholar]
- 10.Mehregan DA, Van Hale HM, Muller SA. Lichen planopilaris: Clinical and pathologic study of forty-five patients. J Am Acad Dermatol. 1992;27:935–42. doi: 10.1016/0190-9622(92)70290-v. [DOI] [PubMed] [Google Scholar]


