Abstract
Bcl10 is a critical regulator of NF-κB activity in T and B cells, coupling antigen receptor signaling to NF-κB activation via protein kinase C (PKC). Here we show that PKC or T-cell receptor (TCR)/CD28 signaling results in downregulation of Bcl10 protein levels, thereby attenuating NF-κB transcriptional activity. Bcl10 degradation requires an intact caspase recruitment domain and is not observed after stimulation with tumor necrosis factor α or lipopolysaccharides. Bcl10 downregulation is not affected by proteasome inhibitors but is accompanied by transient localization to lysosomal vesicles, suggesting involvement of the lysosomal pathway rather than the proteasome. The HECT domain ubiquitin ligases NEDD4 and Itch promote ubiquitination and degradation of Bcl10, thus downmodulating NF-κB activation. Since CD3/CD28-induced activation of JNK is not affected by the decline of Bcl10, degradation of Bcl10 selectively terminates IKK/NF-κB signaling in response to TCR stimulation. Together, these results suggest a new mechanism of negative signaling in which TCR/PKC signaling initially activates Bcl10 but later promotes its degradation.
The NF-κB and Rel transcription factors regulate expression of genes involved in such diverse processes as inflammation, immune response, differentiation, proliferation, and apoptosis (23, 32). NF-κB plays a central role in innate immunity and is activated by signals initiated by tumor necrosis factor receptor, interleukin-1 receptor, and Toll-like receptors. NF-κB is also activated in T and B cells during the adaptive immune response (26, 37, 55). Activation of T lymphocytes requires interaction of the T-cell antigen receptor (TCR) with an antigen peptide presented by a major histocompatibility complex. While TCR engagement is essential for T-cell activation, productive T-cell activation requires additional costimulatory receptors, of which CD28 is the most prominent (2, 54). TCR/CD28 costimulation initiates a series of signal transduction events which modulate the activity of several nuclear transcription factors, including NF-κB, AP-1, and NF-AT, ultimately leading to the activation, differentiation, and proliferation of T lymphocytes (42).
In both B and T cells, signal transduction from antigen receptors to NF-κB requires protein kinase C (PKC) isoforms. In B lymphocytes, PKCβ appears to be the major isoform that transduces signals from the B-cell receptor to NF-κB (53, 59). A great body of biochemical, pharmacological, and genetic evidence supports a crucial role of PKCθ for activation of NF-κB and interleukin-2 expression in response to TCR stimulation in T cells (29). Nevertheless, conflicting results have been obtained regarding the extent of PKCθ contribution to NF-κB activation in peripheral T cells (45, 60). The protein Bcl10, originally cloned from the chromosomal translocation t(1;14) (p22;q32) found in mucosa-associated lymphoid tissue (MALT) B-cell lymphomas (69, 73), is also essential for antigen receptor-induced NF-κB signaling in B and T cells (51). Lack of Bcl10 selectively blocks antigen receptor-mediated NF-κB activation without affecting AP-1 activation, suggesting that Bcl10 acts downstream of PKCs in an NF-κB-specific pathway. Recently, it was demonstrated that Bcl10 controls the development and function of mature B cells (70).
Overexpression of Bcl10 activates NF-κB (33, 69), but the mechanism by which Bcl10 promotes NF-κB activation is not fully understood. Transfected Bcl10 is phosphorylated under certain conditions (20, 62), but the responsible protein kinase has not been identified, nor has the function of this phosphorylation been elucidated. Bcl10 contains an N-terminal caspase recruitment domain (CARD), which mediates self-oligomerization and is necessary and sufficient for NF-κB activation (33, 58). Bcl10 has been shown to associate with the paracaspase Malt1 (39), and target disruption in mice reveals that Malt1 operates downstream of the TCR, PKCθ, and Bcl10 in the IKK/NF-κB signaling cascade in T lymphocytes (50, 52). Furthermore, Bcl10 binds to proteins of the membrane-associated guanylate kinase (MAGUK) family, which include CARD9, CARD10 (Bimp1 or CARMA3), CARD11 (Bimp3 or CARMA1), and CARD14 (Bimp2 or CARMA2) via CARD-CARD interactions (9, 10, 20, 66).
Recent experiments have demonstrated that the Bcl10 binding protein CARMA1 is bridging antigen receptor proximal signaling in both B and T cells to JNK activation and Bcl10-mediated NF-κB induction (19, 25, 30, 47, 65). CARMA1 probably acts through coupling Bcl10 and PKCθ and other potential regulators to the membrane. Congruently, PKCθ, CARMA1, Bcl10, and IKKs are recruited to the membrane and into lipid raft microdomains in response to TCR ligation (13, 19, 67). In these lipid rafts, IKKs are most probably activated either through direct phosphorylation by an unknown IKK kinase or through the induction of structural changes in the IKK complex, which is subsequently activated through enhanced autophosphorylation of IKKβ/α (61).
Much genetic and biochemical evidence indicates that the RING finger-containing ubiquitin ligases c-Cbl and Cbl-b and the HECT (homology to the E6-AP carboxyl terminus) domain ubiquitin ligase Itch are critical negative regulators of T-cell activation (8, 18). Cbl-b-deficient mice show increased susceptibility to spontaneous or induced autoimmune disease (7, 11, 17), while mutation or lack of Itch results in aberrant activation of the immune response (16, 44). T cells from mice engineered to lack both c-Cbl and Cbl-b did not efficiently downmodulate surface TCR due to a defect in trafficking of the internalized TCR to the lysosomal compartment, resulting in sustained TCR signaling (41). Ubiquitination may target cellular proteins for destruction by the 26S proteasome (27) or direct trafficking of membrane-anchored proteins to lysosomal vesicles for degradation (31, 40). Even though it is evident that ubiquitin ligases play an important role in negatively regulating T-cell activation, considerably less is known about the signaling molecules that are substrates for degradation or the mechanism through which degradation occurs.
Here we demonstrate that, in T cells, Bcl10 is posttranslationally modified by phosphorylation and subsequently degraded in response to phorbol myristate acetate (PMA) stimulation or CD3/CD28 coligation. Degradation of Bcl10 involves activation of PKCs and is accompanied by ubiquitination and trafficking to lysosomal vesicles. The CARD of Bcl10 serves as a recognition motif for degradation. We show that Bcl10 is a substrate for ubiquitination by the HECT-type ubiquitin ligases NEDD4 and Itch, and loss of Bcl10 mediated by these E3 enzymes interferes with NF-κB activation. Depletion of Bcl10 correlates with cessation of TCR-induced NF-κB and IKK activation without affecting signaling to JNK. These results define a new negative-feedback mechanism in which downregulation of Bcl10 through degradation might selectively terminate induction of the NF-κB signaling pathway in response to TCR activation.
MATERIALS AND METHODS
Cell culture and treatment.
Jurkat T cells and 70Z/3 and 1.3E2 pre-B cells were grown in complete medium RPMI 1640 (including 10% fetal calf serum, 2 mM glutamine, and 100 U of penicillin-streptomycin per ml); 50 μM β-mercaptoethanol was added to the medium for 70Z/3 and 1.3E2 cells. Stable clones of 1.3E2 cells were obtained and grown as described previously (34). HEK 293 and COS7 cells were grown on culture dishes in complete Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 U of penicillin-streptomycin per ml, and 1 mM sodium pyruvate. CD4+ cells were isolated from spleen and lymph nodes of DO11.10 TCR transgenic mice by positive selection with anti-CD4 magnetic beads (Dynal) and differentiated into Th1 cells by standard protocols (1). The murine D5 (Ar-5) Th1 cell clone was grown as previously described (1).
Human Jurkat T cells were stimulated with 1 μg of CD3 (HIT3a) and 5 μg of CD28 (CD28.2) antibody per ml in the presence of rat anti-mouse immunoglobulin G2a and rat anti-mouse immunoglobulin G1 antibodies. Murine T cells were stimulated with 2.5 μg of CD3ɛ (145-2C11) and 2.5 μg of CD28 (37.51) antibodies per ml cross-linked with goat anti-hamster antibodies (all antibodies purchased from Pharmingen). Further treatments were as follows: 25 ng of TNF-α (Biomol) per ml, 200 ng of PMA (Calbiochem) per ml, 300 ng of ionomycin (Calbiochem) per ml, 10 μg of lipopolysaccharide (LPS) (Sigma) per ml, 25 μM MG132 (Calbiochem), and 50 μg of N-acetyl-Leu-Leu-norleucinal (ALLN; Calbiochem) per ml.
Plasmids and antibodies.
Bcl10 constructs (wild type, 1-140, 1-116, and L41Q) were cloned by standard PCR procedures. For expression of Xpress-tagged Bcl10, the constructs were cloned into pEF4His (Invitrogen). A pEFFlag vector was designed by replacing the cytomegalovirus promoter of Flag-tagged pcDNA3 (Invitrogen) with the EF promoter of pEF4His (Invitrogen). All epitope tags were fused to the amino terminus of Bcl10. All constructs were verified by sequencing. wild-type NEDD4 and HECT mutant NEDD4 (C-A) were cloned into pRK5 vector containing an amino-terminal Myc epitope and Cbl-b was hemagglutinin (HA) tagged at the carboxyl terminus and inserted into pcDNA3.
Antibodies directed against the following proteins were purchased from Santa Cruz Biotechnology: Bcl10 (C-17; Western blotting), Bcl10 (331.3; immunohistochemistry and Western blotting), IκBα (C-21), PKCδ (C-17), HA (Y-11), Myc (9E10 or A14), and LAMP1 (H-228). Antibodies against p65 (Biomol), PKCθ (Transduction Laboratories), ubiquitin (Babco), cathepsin B (Oncogene), FlagM2 (Sigma), Xpress (Invitrogen), IKKα (Pharmingen), and JNK1 (Pharmingen) were also obtained.
Transfection, reporter assay, extraction, Western blotting, EMSA, and kinase assay.
Jurkat cells were transfected by electroporation with a Bio-Rad gene pulser set at 200 V and 950 μF with 30 μg of DNA (pEF4His). Selection was started 2 days after transfection with 1.5 μg of zeocin per ml. After about 3 to 4 weeks, stable pools of Jurkat cells were taken for further analysis.
Transfection of HEK 293 cells by calcium phosphate precipitation and COS7 cells with Lipofectamine (Invitrogen) was done according to standard protocols. Stimulations were carried out for the last 5 h of transfection. After 24 h, cells were lysed in passive lysis buffer and analyzed by the dual luciferase reporter assay (Promega); 6×NF-κBluc was used for the determination of NF-κB activity (34). The pTK-luciferase plasmid (Clontech) was transfected as an internal standard.
Whole-cell extracts, Western blotting, and electrophoretic mobility shift assay (EMSA) were performed essentially as described previously (reference 34 and references therein). For kinase assays, the cells were extracted in lysis buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1% Triton X-100, 10% glycerol, 0.4 mM sodium orthovanadate, 10 mM sodium fluoride, 8 mM β-glycerophosphate [three phosphatase inhibitors], and complete protease inhibitor cocktail [Roche]). After immunoprecipitation with either IKKα, JNK, or PKCθ antibodies, the pellets were washed and incubated in kinase assay buffer (20 mM HEPES [pH 7.5], 10 mM MgCl2, 20 μM ATP, 20 mM β-glycerophosphate, 50 μM sodium orthovanadate, 1 mM dithiothreitol) in the presence of the specific substrate (glutathione S-transferase [Gst]-IκBα 1-53, Gst-Jun 1-79, or myelin basic protein [MBP], respectively) for 20 min at 37°C. After boiling in loading buffer, the kinase reactions were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and analyzed by autoradiography.
Indirect immunofluorescence and confocal microscopy.
For immunohistochemistry, Jurkat cells were seeded and attached to chamber slides with BD cell tag (BD-Bioscience). PMA stimulation was performed after attachment of the cells. Cells were fixed for 15 min in 4% paraformaldehyde and permeabilized for 5 min with 0.1% Triton X-100. Endogenous Bcl10 was detected with monoclonal Bcl10 antibody 331.3 as the primary antibody, and indocarbocyanine-labeled donkey anti-mouse immunoglobulin antibody (red; Jackson Laboratories) was used as secondary antibody. Polyclonal cathepsin B or LAMP1 antibodies were stained with a secondary Alexa 488-conjugated goat anti-rabbit immunoglobulin antibody (green; Alexa). Chamber slides were analyzed with an Axioplan 2 microscope (Zeiss) with the software AxioVision (for indirect immunofluorescence microscopy) or Pascal (for confocal microscopy).
Ubiquitination assay.
Jurkat cells or transfected 293 cells were lysed in ubiquitin lysis buffer containing 50 mM Tris (pH 7.5), 150 mM NaCl, 0.5% Triton X-100, 30 mM N-ethylmaleimide (NEM), 1 mM dithiothreitol, 0.4 mM sodium orthovanadate, 10 mM sodium fluoride, 8 mM β-glycerophosphate (three phosphatase inhibitors), and complete protease inhibitor cocktail (Roche). Immunoprecipitation with Bcl10 antibody C-17 or FlagM5 antibody was performed for 14 h at 4°C, and precipitates were collected with protein G-Sepharose (Amersham). Beads were washed three times with ubiquitin lysis buffer, boiled in SDS loading buffer, and analyzed by SDS-PAGE and subsequent Western blotting.
RESULTS
Bcl10 is degraded in activated T cells.
Bcl10 is an essential regulator of antigen receptor- and PMA-induced NF-κB signaling in B and T lymphocytes (51). We examined the protein levels and modification status of Bcl10 in activated Jurkat T cells. PMA stimulation induced a strong reduction of Bcl10 protein amounts within 30 to 60 min following stimulation (Fig. 1A, top panel). This result was seen with different Bcl10 antibodies and did not result from the movement of Bcl10 into a detergent-insoluble fraction (data not shown), indicating that it was due to a true loss of Bcl10 protein as a result of proteolysis rather than an apparent loss caused by protein modification or cellular translocation. Indirect immunofluorescence analysis confirmed that stimulation of Jurkat T cells with PMA for 2 h resulted in the disappearance of endogenous Bcl10 protein (Fig. 1B). PMA signaling also promoted a decrease in the novel PKC isoform PKCθ, but the decline was visible only after several hours, when Bcl10 levels were already partially restored (Fig. 1A, middle panel). As expected, proteasomal degradation of the NF-κB inhibitor IκBα occurred much more rapidly, already maximal by 15 min of PMA treatment (Fig. 1A, bottom panel). Thus, all three essential regulators of NF-κB signaling in T cells, IκBα, PKCθ, and Bcl10, are prone to signal-induced degradation, but their proteolytic removal is apparently controlled by very distinct mechanisms.
FIG. 1.
Bcl10 is degraded in response to PMA or CD3/CD28 costimulation in T cells. (A) Kinetics of Bcl10 degradation in response to PMA. Jurkat cells were treated with PMA as indicated, and Bcl10, PKCθ, and IκBα protein levels were determined by Western blotting. (B) Immunofluorescence analysis of Bcl10 degradation. Jurkat cells were untreated (upper panel) or treated (lower panel) with PMA for 120 min, and Bcl10 (red) was visualized by indirect immunofluorescence. Nuclei were stained with 4′,6′-diamidino-2-phenylindole (DAPI) (right panel). (C) CD3/CD28 clustering induces degradation of Bcl10 in Jurkat cells. Cells were treated with CD3 antibody or CD3/CD28 antibodies for 3 h, and Bcl10 and PKCθ protein was detected by Western blotting. (D) T-cell activation but not TNF-α signaling induces Bcl10 degradation. Jurkat cells were treated with CD3/CD28 antibodies, PMA, or TNF-α, and NF-κB DNA binding and Bcl10 and IκBα protein levels were determined by EMSA and Western blotting, respectively. (E) Bcl10 phosphorylation and degradation coincide with enhanced PKCθ kinase activity. The murine D5 (Ar.5) Th1 cell clone was incubated with CD3 and CD28 antibodies. Western blotting of Bcl10 was performed, and PKCθ kinase activity was determined after immunoprecipitation with myelin basic protein (MBP) as the substrate. (F) Primary murine Th1 cells were stimulated with CD3 or CD3 and CD28 antibodies for 1 or 2 h, and extracts were analyzed for Bcl10 and p65 protein.
Since PMA activates PKCθ directly, bypassing TCR signaling (29), we determined whether Bcl10 protein levels could also be downregulated in response to TCR stimulation. Activation of Jurkat cells by combined stimulation with CD3 and CD28 resulted in a strong decrease in Bcl10 protein levels within 3 h after stimulation (Fig. 1C and D, top panels). CD3 ligation in the absence of the costimulus had only a minor effect (Fig. 1C). Tumor necrosis factor alpha (TNF-α) stimulation, which initiates NF-κB signaling in Jurkat cells independent of PKCθ (38), did not reduce the steady-state amounts of Bcl10 (Fig. 1D). Loss of Bcl10 in response to CD3-CD28 clustering correlated with transient NF-κB activation, while lack of Bcl10 degradation in response to TNF-α stimulation correlated with more sustained NF-κB activation (Fig. 1D, bottom panel). Strong degradation of Bcl10 was also observed in primary murine Th1 cells in response to CD3/CD28 coligation (Fig. 1F) and in the untransformed, interleukin-2-dependent murine T-cell clone D5 (Ar-5) (Fig. 1E, top panel). Bcl10 was shown to be phosphorylated in HEK 293 cells under conditions of PKC overexpression (65). Interestingly, murine D5 T cells also showed, in parallel with Bcl10 degradation, the appearance of a phosphorylated Bcl10 form, indicated by slower migration (Fig. 1E, top panel). Bcl10 phosphorylation and degradation coincided with enhanced PKCθ kinase activity, as judged by in vitro kinase reactions with myelin basic protein as a substrate (bottom panel).
Bcl10 degradation involves PKC signaling and is mediated by the CARD.
To analyze the upstream requirements for Bcl10 degradation, we used the mouse pre-B-cell line 70Z/3 and its derivative 1.3E2. Due to the absence of IKKγ expression, 1.3E2 cells are defective in NF-κB activation in response to LPS, interleukin-1β, or PMA (71). In addition, 1.3E2 cells carry a defect in PKC signaling that can be rescued by stable introduction of novel PKCθ or PKCδ (34). In 70Z/3 pre-B cells, Bcl10 was degraded in response to PMA but not LPS stimulation (Fig. 2A, top panel); we have shown that the former but not the latter response involves activation of novel PKCs (34). Like CD3/CD28-stimulated D5 T cells (Fig. 1E), PMA-stimulated 70Z/3 cells showed a slower-migrating form of Bcl10 (Fig. 2A), which regained its original mobility after phosphatase treatment (data not shown), demonstrating that Bcl10 is indeed transiently phosphorylated prior to degradation. It is plausible that Bcl10 is a substrate for phosphorylation by PKCs and that this modification targets the protein for subsequent degradation in activated T and B cells.
FIG. 2.
PMA-induced degradation of Bcl10 depends on an intact PKC signaling pathway and is mediated by the CARD. (A) PMA but not LPS induces Bcl10 degradation in 70Z/3 cells. Bcl10 and p65 protein amounts were analyzed in extracts from LPS- or PMA-stimulated 70Z/3 cells by Western blotting. (B) PKC signaling is required for Bcl10 degradation. 70Z/3 cells as well as clones of 1.3E2 cells expressing IKKγ or PKCθ, -δ, or -βII alone or in combination, as indicated (panels 1 to 6), were treated with PMA for 10, 30, or 180 min, and NF-κB binding activity as well as Bcl10 and p65 protein amounts were determined. The migration of a nonspecific band in the EMSA is indicated by a star. (C) Schematic representation of Bcl10 and Bcl10 mutants. The amino-terminal CARD including an essential leucine at amino acid position 41 is depicted. (D) The CARD of Bcl10 mediates degradation. Stable pools of Jurkat cells transfected with pEF4 expression vectors containing wild-type XBcl10, XBcl10 L41Q, and XBcl10 1-116 were stimulated with PMA. XBcl10 amounts were analyzed by Western blotting, and the migration of the proteins is indicated by an arrow. As a control for equal loading and stimulation, p65 and IκBα levels were determined in parallel. Nonspecific bands are marked ns.
We analyzed Bcl10 degradation in 1.3E2 cells expressing IKKγ and/or selected PKCs (Fig. 2B) (34). Unlike the parental 70Z/3 cells (Fig. 2B, panel 1), wild-type 1.3E2 cells (data not shown) and 1.3E2 cells expressing IKKγ alone (panel 2) showed no loss of Bcl10 protein following PMA stimulation. In contrast, introduction of either novel PKCθ or PKCδ (alone or in combination with IKKγ) restored Bcl10 degradation in response to PMA (panels 3 to 5). The PKCβII isoform, which does not restore NF-κB signaling in this cellular system (34), also did not restore Bcl10 degradation (panel 6). In summary, both phosphorylation and degradation of Bcl10 are independent of IKK activation but require an intact PKC signaling pathway.
Bcl10 is a bipartite protein with an amino-terminal CARD and a carboxyl terminus with no apparent structural motif (Fig. 2C). To determine the region of Bcl10 that is required for degradation, we generated deletion mutants that were truncated from the carboxyl terminus (Bcl10 1-140 and 1-116) and a point mutation (Bcl10 L41Q) containing a leucine-to-glutamic acid substitution which prevents CARD-CARD interactions (reference 24 and data not shown). We stably introduced Xpress-tagged Bcl10 (XBcl10) constructs into Jurkat T cells and stimulated the cells with PMA (Fig. 2D). Just like endogenous Bcl10, the protein levels of wild-type XBcl10 were considerably reduced after 3 h of stimulation. The CARD mutant Bcl10 L41Q was completely stable. In contrast, a construct that consisted almost exclusively of the CARD (Bcl10 1-116), created by a deletion that removed the entire carboxyl terminus of Bcl10, was still efficiently degraded in response to PMA stimulation. Thus, the CARD is critical for mediating PKC-dependent degradation of Bcl10.
Bcl10 localizes to lysosomal vesicles and is ubiquitinated prior to degradation.
We tested the effect of the proteasome inhibitors MG132 and ALLN on Bcl10 degradation (Fig. 3A). Despite their ability to inhibit IκBα degradation and NF-κB activation (top and third panels, respectively), we did not observe any stabilization of Bcl10 (bottom panel). Similar results were obtained with the proteasomal inhibitors lactacystin and epoxomicin as well as ALLN in 70Z/3 cells (data not shown). These data indicate that Bcl10 degradation occurs independently of the proteasomal degradation machinery. To test whether degradation might involve the alternative pathway of lysosomal trafficking, we used confocal microscopy to determine the subcellular distribution of Bcl10 before and after stimulation (Fig. 3B). In resting Jurkat cells, Bcl10 showed a slightly granular staining that was detected predominantly in the cytoplasm (left panel); after 30 min of PMA stimulation, it localized almost completely to vesicle-like structures within the cell (right panel) prior to substantial degradation at 2 h (Fig. 1B). Costaining with LAMP1 (lysosome-associated membrane protein 1) and cathepsin B antibodies, both markers for late endosomal and lysosomal vesicles, revealed that after PMA induction, Bcl10 at least partially colocalized with cathepsin B-positive or LAMP1-positive compartments (Fig. 3C and D, respectively). Thus, before degradation, Bcl10 translocates to lysosomal vesicles, suggesting that degradation might be executed by lysosomal proteases instead of the 26S proteasome.
FIG. 3.
Bcl10 degradation is insensitive to proteasomal inhibition and coincides with localization to lysosomal vesicles. (A) Proteasomal inhibitors fail to impede Bcl10 degradation. Jurkat cells were treated with MG132 or ALLN 1 h prior to stimulation as indicated. After whole-cell extraction, steady-state amounts of Bcl10, IκBα, and p65 were determined by Western blotting and NF-κB activity was determined by EMSA. The migration of nonspecific bands is indicated by a star. (B) Bcl10 localizes to vesicles after PMA induction. Jurkat cells were control treated or stimulated with PMA for 20 min, stained for Bcl10, and analyzed by confocal microscopy. (C and D) Costaining of Bcl10 and lysosomal marker proteins cathepsin B and LAMP1. Jurkat cells were treated with PMA for 20 min and costained for Bcl10 (red) and cathepsin B or LAMP1 (green; C and D, respectively). Arrows indicate vesicles stained for Bcl10 and cathepsin B or LAMP1.
Ubiquitination has been shown to target proteins for proteasomal destruction as well as regulation of trafficking to lysosomes (27, 40). We examined whether Bcl10 is inducibly ubiquitinated prior to degradation in Jurkat T cells (Fig. 4). Jurkat cells were stimulated with PMA and extracted in the presence of NEM, an inhibitor of deubiquitination, and extracts were immunoprecipitated with Bcl10 antibodies (Fig. 4A). An enhancement of Bcl10 ubiquitination was already detectable after 5 min of PMA stimulation and was strongest after 30 min (bottom panel); at this time point, strong Bcl10 ubiquitination was observed, ranging from short-chain conjugates to polyubiquitination in the high-molecular-weight range. At later time points (1 h and 3 h), when most of the Bcl10 had been depleted, almost no ubiquitination could be detected. The presence of proteasome inhibitors did not lead to an accumulation of ubiquitinated Bcl10 (Fig. 4B, left panels), indicating again that Bcl10 degradation is not carried out by the proteasome. In contrast, detection of polyubiquitinated IκBα after PMA stimulation was completely dependent on blockade of the proteasomal degradation pathway (Fig. 4B, right panels).
FIG. 4.
Bcl10 is ubiquitinated prior to degradation. (A) Kinetics of Bcl10 ubiquitination. Jurkat cells were treated with PMA as indicated, and extracts were immunoprecipitated (IP) with Bcl10 antibody. The input levels of Bcl10 (upper panel) as well as the amounts of immunoprecipitated Bcl10 (middle panel) and ubiquitin conjugation with ubiquitin antibody (lower panel) were determined after Bcl10 immunoprecipitation. (B) Proteasomal inhibition fails to stabilize Bcl10 ubiquitin conjugates in Jurkat cells. Jurkat cells were untreated or incubated with ALLN for 1 h and afterwards stimulated with PMA for 20 min, as indicated. Extracts were immunoprecipitated with Bcl10 (left panel) or IκBα (right panel) antibodies, and the input as well as the immunoprecipitates were analyzed by Western blotting (WB) for Bcl10, IκBα, and ubiquitin conjugates. (C) Ubiquitin is specifically conjugated to Bcl10. Jurkat cells were treated with PMA for 20 min, and extracts were prepared in ubiquitin lysis buffer containing 1% SDS. Extracts were diluted by a factor of 10 in ubiquitin lysis buffer before immunoprecipitation with Bcl10 antibody and analyzed as indicated. (D) CD3/CD28 ligation induces Bcl10 ubiquitination. Cells were incubated for 20 min with PMA or CD3 and CD28 antibodies. Extraction and immunoprecipitation were performed as for panel A. Migration of immunoglobulin heavy and light chains is indicated by an asterisk.
To rule out the possibility that ubiquitin is conjugated not to Bcl10 but to an associated protein, we prepared extracts in the presence of 1% SDS to disrupt protein interactions prior to immunoprecipitation; under these stringent conditions, ubiquitination was still detectable, proving that ubiquitin is indeed directly conjugated to Bcl10 (Fig. 4C). Furthermore, Bcl10 ubiquitination was also enhanced in response to CD3-CD28 ligation in Jurkat T cells (Fig. 4D), demonstrating that antigen receptor-induced Bcl10 degradation is also preceded by ubiquitination. These results suggest that ubiquitination is involved in induced Bcl10 degradation, probably by targeting the protein for lysosomal trafficking.
Ubiquitination and degradation of Bcl10 are promoted by NEDD4 and Itch.
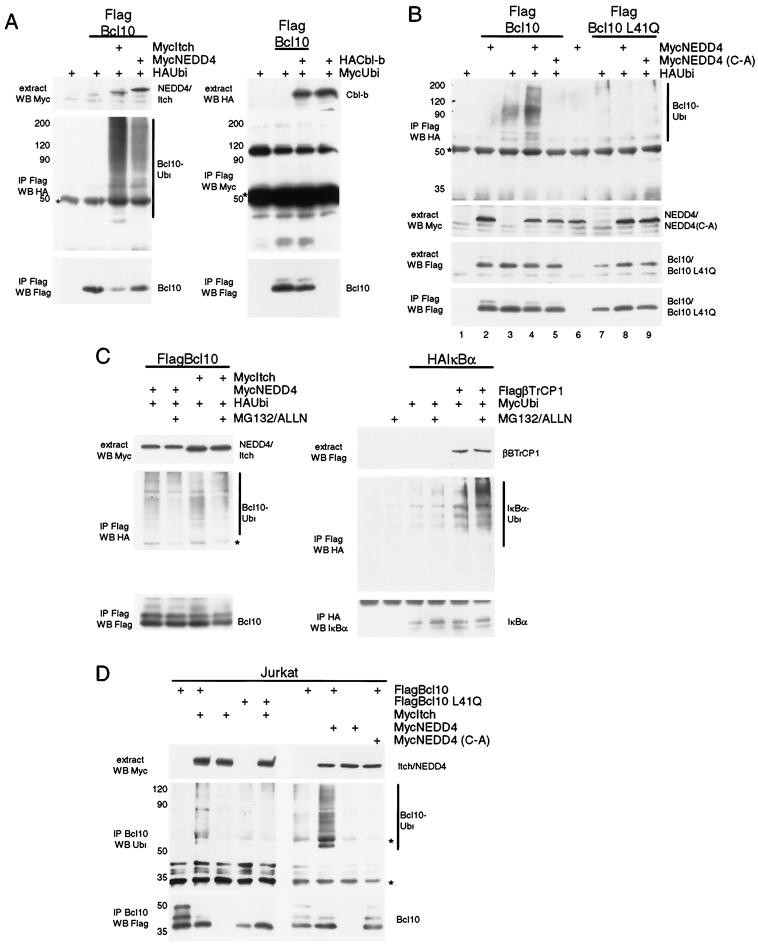
To identify E3 ligases that might mediate Bcl10 ubiquitination and degradation, we coexpressed different E3 ubiquitin ligases with epitope-tagged ubiquitin and Flag-tagged Bcl10 in HEK 293 cells (Fig. 5). We were especially interested in Itch and Cbl-b, E3 ligases whose mutation or deletion in mice leads to aberrant activation of the immune response (7, 11, 17, 44). We also tested NEDD4, a close relative of Itch that is ubiquitously expressed in T cells and other cell types (3). Ectopic expression of the HECT-type (catalytic) E3 ligases Itch and NEDD4 promoted conjugation of ubiquitin to Bcl10 (Fig. 5A, left panel); in contrast, expression of the RING finger (adapter) E3 ligase Cbl-b did not enhance ubiquitin ligation to Bcl10 (right panel).
FIG.5.
Expression of the HECT domain ubiquitin ligases NEDD4 and Itch mediates ubiquitination and degradation of Bcl10. (A) NEDD4 and Itch induce ubiquitination of Bcl10. HEK 293 cells were transfected with Flag-Bcl10, HA-ubiquitin, and Myc-NEDD4 or Myc-Itch (left panel) or Flag-Bcl10, Myc-ubiquitin, and HA-Cbl-b (right panel) expression constructs as indicated and subjected to immunoprecipitation with Flag antibody (Ab). Protein input levels, HA/Myc-ubiquitin conjugates, and Flag-Bcl10 amounts after immunoprecipitation were analyzed by Western blotting. (B) Ligase activity of NEDD4 is required for CARD-dependent ubiquitination of Bcl10. After transfection of Flag-Bcl10, HA-ubiquitin, and Myc-NEDD4, or Myc-NEDD4 (C-A), immunoprecipitation and analysis were done as for panel A. (C) NEDD4- and Itch-induced ubiquitination of Bcl10 is not augmented by proteasomal inhibitors. COS7 cells were transfected with Flag-Bcl10, Myc-NEDD4, Myc-Itch, and HA-ubiquitin (left panel) or HA-IκBα, Flag-β-transducin repeat-containing protein 1 (FlagβTrCP1), and Myc-ubiquitin (right panel) and where indicated, MG132 and ALLN were added 2 h before lysis. After immunoprecipitation with Flag or HA antibody, ubiquitination of Flag-Bcl10 and HA-IκBα was analyzed as indicated. (D) Itch and NEDD4 promote Bcl10 ubiquitination in Jurkat cells. Jurkat cells were transfected with Myc-Itch, Myc-NEDD4, Myc-NEDD4 (C-A), Flag-Bcl10 or Flag-Bcl10 L41Q and subjected to immunoprecipitation with Bcl10 antibody. Proteins were analyzed as indicated. The migration of immunoglobulin heavy chains is indicated by asterisks.
To test whether the ligase activity is required for ubiquitin conjugation, we used a NEDD4 mutant in which the cysteine in the active site was mutated to alanine (NEDD4 [C-A]) (Fig. 5B). Expression of this ligase-inactive mutant was unable to promote ubiquitin attachment to Flag-tagged Bcl10 (Fig. 5B, top panel, compare lanes 4 and 5) and was even found to inhibit ubiquitination by an endogenous E3 ligase (compare lanes 3 and 5). Furthermore, functional impairment of the CARD in Bcl10 L41Q also prevented ubiquitin attachment (lanes 6 to 9). While enhanced ubiquitination of IκBα mediated by the F-box E3 ligase β-transducin repeat-containing protein could be visualized only in the presence of proteasomal inhibitors (Fig. 5C, right panel), MG132 and ALLN did not alter the amount of NEDD4- or Itch-catalyzed ubiquitin conjugation to Bcl10 (Fig. 5C, left panel). Similarly, in Jurkat T cells, expression of Itch or NEDD4 promoted ubiquitin conjugation to Bcl10 (Fig. 5D). Again, ubiquitination was dependent on the integrity of the CARD in Bcl10 and required the catalytic activity of NEDD4. Ubiquitin conjugates ranged from mono- or short-chain ubiquitination to multiubiquitination.
We tested whether expression of ubiquitin ligases would affect Bcl10 protein levels in 293 cells (Fig. 6). Coexpression of either NEDD4 or Itch caused a strong decline in Bcl10 protein levels (Fig. 6A, lanes 2 and 3); in contrast, Cbl-b only marginally affected Bcl10 expression (lane 4). Again, NEDD4-mediated downregulation of Bcl10 was not seen with the HECT domain mutant NEDD4 (C-A) (Fig. 6B, left panel, lanes 4 to 6), indicating that the ubiquitin ligase activity is strictly required. Furthermore, the CARD sufficed for NEDD4-induced degradation of Bcl10 (Fig. 6B, right panels), and point mutation of the CARD (Bcl10 L41Q) led to Bcl10 stabilization (Fig. 6B, left panel, lanes 7 to 9). Thus, expression of the HECT domain ubiquitin ligases NEDD4 and Itch enhances ubiquitination and degradation of Bcl10. Analogous to the degradation induced in T cells, the CARD of Bcl10 is necessary and sufficient to mediate this proteasome-independent degradation process.
FIG. 6.
NEDD4 and Itch promote degradation of Bcl10. (A) HEK 293 cells were transfected with Flag-Bcl10 alone or in the presence of either Myc-NEDD4, Myc-Itch, or HA-Cbl-b, and protein amounts were determined by Western blotting. (B) Degradation of Bcl10 depends on NEDD4 ligase activity and is mediated by the CARD. Flag-Bcl10 and different Flag-Bcl10 mutants (L41Q, 1-140, and 1-116) were transfected with increasing amounts of Myc-NEDD4 or Myc-NEDD4 (C-A), and protein amounts were determined as for Fig. 5.
NEDD4 and Itch interfere with Bcl10-mediated NF-κB activation.
We asked if induced reduction in Bcl10 protein amounts would negatively influence NF-κB signaling (Fig. 7). We used an ectopic expression system in which expression of Bcl10 in 293 cells or PMA stimulation of 293 cells individually resulted in moderate activation of an NF-κB reporter construct but in combination (i.e., PMA stimulation of Bcl10-expressing cells) led to strong synergistic enhancement of NF-κB activity (Fig. 7A, bars 1 to 4). This synergy was specific for PKC signaling, since Bcl10 expression did not synergize with TNF-α stimulation to upregulate NF-κB activity (bars 5 and 6). Synergy was also observed after cotransfection of Bcl10 and constitutively active PKCθ (data not shown), consistent with previous findings that B- and T-cell receptor signaling as well as PMA signaling in lymphocytes critically depends on the presence of PKCs (34, 53, 59, 60).
FIG. 7.
Ubiquitin ligases NEDD4 and Itch inhibit synergistic NF-κB activation by Bcl10 and PMA. (A) Bcl10 and PMA synergize in NF-κB activation. HEK 293 cells were transfected with 2 μg of Flag-Bcl10, and cells were stimulated for the last 5 h before extraction with PMA or TNF-α. (B) The CARD of Bcl10 is necessary and sufficient to augment PMA induction of NF-κB. We transfected 1 μg of expression plasmids for XBcl10, XBcl10 L41Q, XBcl10 1-140, or XBcl10 1-116, and cells were stimulated for the last 5 h with PMA. (C and D) NEDD4 and Itch interfere with Bcl10-mediated NF-κB activation. HEK 293 cells were transfected with 2 μg of Flag-Bcl10 and the indicated amounts of Myc-NEDD4, Myc-Itch, or HA-Cbl-b expression constructs, and cells were stimulated with PMA or TNF-α as for panel A. (F) The CARD of Bcl10 is necessary for NEDD4-mediated inhibition of NF-κB. We transfected 2 μg of Flag-Bcl10 constructs in HEK 293 cells in the absence or presence of Myc-NEDD4, and cells were treated with PMA as indicated. Duplicates and standard deviations of a representative experiment are shown.
We tested whether the CARD was important for synergism between Bcl10 and PKC (Fig. 7B). Mutants Bcl10 1-140 and 1-116, in which the carboxyl terminus of Bcl10 was removed but the CARD was left intact, resembled wild-type Bcl10 in their ability to activate NF-κB and synergize with PMA. In contrast, destruction of the CARD in Bcl10 L41Q not only prevented NF-κB activation but also led to loss of synergism between Bcl10 and PMA, proving that the CARD is necessary and sufficient to augment PMA-induced NF-κB activation. Furthermore, NEDD4 and Itch, which promoted Bcl10 degradation (Fig. 6), also interfered with NF-κB activation induced by overexpression of Bcl10 (Fig. 7C to E). Increasing amounts of NEDD4 inhibited synergistic activation of the NF-κB reporter by Bcl10 and PMA in 293 cells but had much less effect on TNF-α-induced NF-κB activation (Fig. 7C). As expected from the ubiquitination-degradation analysis (compare Fig. 5 and 6), expression of Itch also led to a reduction of NF-κB activation mediated by Bcl10 and PMA, while expression of Cbl-b did not (Fig. 7D). An intact CARD of Bcl10 was necessary and sufficient for NEDD4-induced inhibition of Bcl10-mediated NF-κB activation (Fig. 7E). Thus, expression of the HECT ubiquitin ligases NEDD4 and Itch promote ubiquitination and degradation of Bcl10, thereby inhibiting its ability to function as a positive regulator of NF-κB signaling in conjunction with PMA.
Degradation of Bcl10 selectively terminates TCR-induced NF-κB signaling.
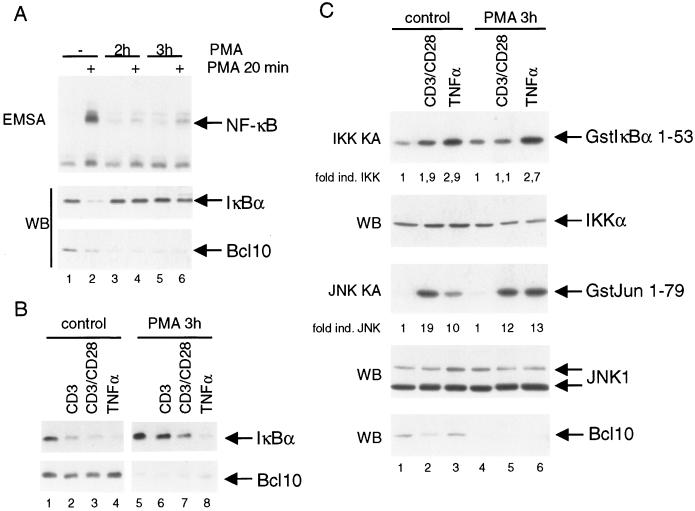
Since mitogen-activated protein kinase and AP-1 activation are normal in Bcl10-deficient lymphocytes (51), we postulated that Bcl10 degradation might selectively terminate NF-κB activation in B and T cells. To test this hypothesis, we stimulated Jurkat cells for 2 h or 3 h with PMA and subsequently restimulated the cells with PMA for 20 min (Fig. 8A). Strikingly, after prior PMA stimulation, neither NF-κB DNA binding activity (top panel) nor IκBα degradation (middle panel) was observed in response to a secondary PMA treatment, correlating with the almost complete loss of Bcl10 protein in response to the prior stimulation (bottom panel; compare lanes 1 and 2 with lanes 3 and 4 and lanes 5 and 6). Similarly, 3 h of preincubation with PMA strongly inhibited IκBα degradation in response to CD3 or CD3/CD28 ligation (Fig. 8B, top panel, compare lanes 2 and 3 with lanes 6 and 7), again correlating with loss of Bcl10 (bottom panel). PKCθ levels did not decrease during 3 h of PMA treatment (compare Fig. 1A). Loss of Bcl10 did not affect IκBα degradation in response to 15 min of TNF-α stimulation (Fig. 8B, lane 8), confirming that TNF-α-mediated NF-κB activation is independent of Bcl10.
FIG. 8.
Degradation of Bcl10 in Jurkat T cells leads to a selective termination of NF-κB signaling in response to PMA and TCR stimulation. (A) PMA induces unresponsiveness towards secondary stimulation. Jurkat cells were left untreated or stimulated with PMA for 2 or 3 h before a secondary round of stimulation with PMA for 20 min. Extracts were analyzed by EMSA for NF-κB DNA binding and for IκBα and Bcl10 protein amounts by Western blotting. (B) PMA pretreatment selectively inhibits TCR-induced IκBα degradation in Jurkat cells. Cells were left untreated or initially treated with PMA for 3 h, followed by stimulation with CD3 antibody, CD3 and CD28 antibodies, or TNF-α for 15 min. Extracts were analyzed for IκBα and Bcl10 protein amounts by Western blotting as indicated. (C) Bcl10 degradation selectively prevents IKK activation in Jurkat cells. Cells were incubated with PMA for 3 h. Secondary stimulation was performed for 15 min with CD3 and CD28 antibodies or 5 min with TNF-α. Extracts were immunoprecipitated with either IKKα or JNK1 antibodies. IKK and JNK1 kinase assays were performed after immunoprecipitation with Gst-IκBα1-53 or Gst-Jun 1-79 as the substrate. The values represent the induction of kinase activity after stimulation as determined by densitometric analysis. Western blotting was performed to control input levels of IKKα, JNK1, and Bcl10.
To determine whether Bcl10 degradation selectively downmodulates NF-κB signaling, we examined IKK and JNK kinase activity (Fig. 8C). Jurkat T cells were subjected to prolonged PMA stimulation (3 h) and restimulated with CD3/CD28 for 15 min or TNF-α for 5 min. The cells were then lysed, and IKKα or JNK1 immunoprecipitates were analyzed for kinase activity against their specific substrates in vitro. PMA pretreatment inhibited activation of the IKK complex in response to subsequent CD3/CD28 ligation (top panel, compare lanes 1 and 2 with 4 and 5) but barely affected upregulation of JNK1 kinase activity (third panel, compare lanes 1 and 2 with 4 and 5). As expected, PMA pretreatment also had no effect on IKK and JNK1 activation in response to TNF-α stimulation (compare lanes 3 and 6). Thus, PKC activation, which induces a sustained loss of Bcl10 protein, also results in long-lasting impairment of IKK and NF-κB activation in response to subsequent stimulation with PMA or CD3/CD28. Importantly, TCR-induced JNK activation and TNF-α-induced IKK activation were not impaired through the prolonged PMA treatment, ruling out that loss of NF-κB activation results from the inactivation of other signaling mediators either upstream (e.g., PKCθ and CARMA1) or downstream (e.g., IKKs) of Bcl10.
In conclusion, we propose that Bcl10 degradation is carried out through a proteolytic pathway that is independent of the proteasome and most likely involves lysosomal trafficking. Removal of Bcl10 contributes to the termination of NF-κB signaling, hence modulating the duration of NF-κB-dependent gene transcription initiated by T-cell activation.
DISCUSSION
In this study, we show that PKC or TCR/CD28 stimulation induces degradation of the CARD-containing protein Bcl10, an essential mediator of NF-κB activation in lymphocytes. Removal of Bcl10 is triggered by CD3/CD28 cross-linking in primary T cells and T-cell lines but not by TNF-α or LPS stimulation. The CARD is both necessary and sufficient for Bcl10 degradation. Bcl10 downregulation is mediated through novel PKCs as well as the HECT-type E3 ligases NEDD4 and Itch in a mechanism that appears to involve lysosomal rather than proteasomal degradation. Thus, PKC activation not only activates NF-κB signaling via Bcl10; it also promotes Bcl10 degradation and concomitant downregulation of NF-κB transcriptional activity. Our data define a novel negative-feedback pathway that downregulates and terminates NF-κB signal transduction in activated T cells.
Bcl10 degradation involves ubiquitination and lysosome trafficking.
Although we cannot completely exclude a role of the proteasomal destruction pathway, our data suggest that Bcl10 degradation involves ubiquitin conjugation and subsequent sorting to endocytic vesicles. Proteasomal inhibition did not significantly affect the stability of Bcl10; furthermore, immunohistochemistry revealed that Bcl10 degradation is preceded by its appearance in vesicular compartments, some of which were identified as late endosomes or lysosomes by the presence of the markers cathepsin B and LAMP1. Through binding to ubiquitin-interacting proteins such as Hrs or Tsg101 localized on endosomes, ubiquitinated proteins, which are targeted for endocytosis, are directed to the invaginating multivesicular body and hence the lysosome compartment (6, 48). The requirements for this sorting event are not completely defined, but in general, mono- or short-chain ubiquitination is sufficient (28), although polyubiquitination (long branched-chain ubiquitination on a single lysine) or multiubiquitination (multiple short branches on a few lysines) was shown to serve the same purpose in the case of macrophage colony-stimulating factor (36).
Why is ubiquitinated Bcl10 not prone to proteasomal destruction? Several scenarios are possible. (i) The high-molecular-weight smear of ubiquitinated Bcl10 could arise from a population of molecules bearing different numbers of mono-, di-, or short-chain ubiquitins conjugated to multiple lysine residues instead of one or more polyubiquitin chains conjugated to a single lysine. (ii) Bcl10 rapidly translocates to vesicular structures, where it is already visible 10 min after PMA treatment (data not shown), suggesting that internalization into endosomes, possibly directed via mono- or short-chain ubiquitination, is a very efficient process. Endosomal localization might protect polyubiquitinated Bcl10 from the proteasomal degradation machinery. (iii) Ubiquitin addition to Bcl10 could involve an atypical linkage, e.g., via conjugation of lysine 63 instead of 48, so that the conjugates are not recognized by the 26S proteasome. For several permeases in Saccharomyces cerevisiae, diubiquitin chains linked through Lys63 enhanced the internalization rates (21, 57).
Our data implicate a role for the HECT domain ubiquitin ligases NEDD4 and Itch in Bcl10 downmodulation. Overexpression of these E3 ligases induced ubiquitination and degradation of Bcl10 in 293 cells and Jurkat T cells, whereas the RING-type E3 ligase Cbl-b did not. The possible involvement of Itch in Bcl10 degradation in vivo is particularly interesting because Itch-deficient T cells display an activated phenotype that could at least partially be explained by an increased stability of Bcl10 (16, 44). NEDD4 can be recruited to lipid rafts in different cell types (35, 46) and has been shown to play a role in membrane targeting, intracellular localization, trafficking. and endocytosis (reviewed in reference 49); the closely related Itch protein may have similar functions in T cells. Mutational analysis of Bcl10 revealed that the CARD was both necessary and sufficient for Bcl10 degradation: destruction of the CARD, as in the case of Bcl10 L41Q, abolished induced Bcl10 ubiquitination and degradation. The CARD mediates self-oligomerization of Bcl10 (33); it is also implicated in the interaction of Bcl10 with MAGUKs, which could be involved in membrane association (19), as well as recruitment of Bcl10 to cytoplasmic filaments (24). Thus, the CARD may function directly as an interaction motif for recognition by the E3 ligases; alternatively, CARD-mediated oligomerization, protein-protein interaction, or correct intracellular localization may be essential for the ubiquitination and degradation of Bcl10.
Bcl10 degradation terminates PKC- and TCR-mediated NF-κB activation.
To define the consequences of Bcl10 downregulation for NF-κB signaling, we took advantage of an ectopic expression system in which Bcl10 in conjunction with PMA stimulation or expression of active PKCθ enhances NF-κB reporter activity, a situation that mimics PKCθ-dependent NF-κB signaling in T cells. Reduction of Bcl10 levels by coexpression of NEDD4 and Itch severely interfered with NF-κB activation. Similarly, in T cells, induced degradation of Bcl10 may contribute at least partially to the transient duration of NF-κB activity in response to PKC and TCR/CD28 signaling. In addition to NF-κB activation, TCR clustering initiates JNK signaling via PKCθ in T cells (5, 22, 68). However, Bcl10 downregulation did not impede JNK activation (Fig. 8), suggesting that proteolytic downmodulation of Bcl10 might selectively impair NF-κB signal transduction in activated T cells.
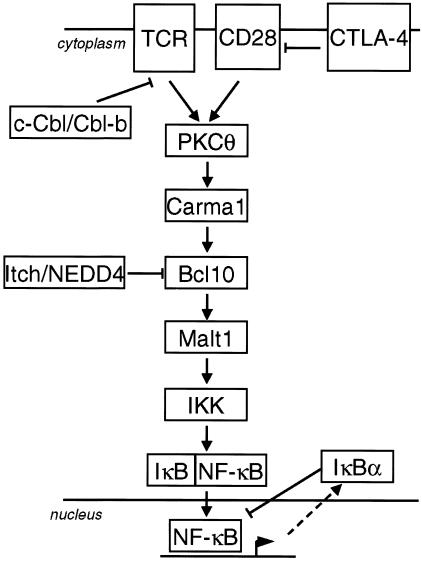
Negative regulation of T-cell signaling is essential to maintain T-cell homeostasis, prevent abnormal lymphocyte activation, and regulate the duration of signaling (56). A number of negative-feedback mechanisms have been proposed to regulate the duration of TCR signaling and subsequent NF-κB activation (Fig. 9). Autoregulatory upregulation of IκBα represents a negative-feedback mechanism that is required for postinductive dissociation of NF-κB from the DNA and reshuttling to the cytoplasm. Even though resynthesis of IκBα is essential for inactivation of NF-κB, it mostly does not suffice to downregulate NF-κB in the presence of continuous upstream signaling (4).
FIG. 9.
Model for positive- and negative-acting regulators in response to TCR-initiated NF-κB signal transduction. See text for details.
Several mechanisms have been suggested to downregulate TCR-proximal signaling. Expression of the inhibitory coreceptor CTLA-4 on activated T cells may either compete for ligand binding with CD28 (64) or directly interfere with the TCR or CD28 signaling pathway (15). Accumulation of CTLA-4 in the immunological synapse is proportional to the strength of the TCR-peptide/major histocompatibility complex interaction, suggesting that T cells receiving stronger stimuli are more susceptible to CTLA-4-mediated inhibition (14). Furthermore, the RING finger ubiquitin ligases c-Cbl and Cbl-b attenuate T-cell responsiveness, and as one possible way to terminate TCR signals, these ligases cooperate in the regulation of ligand-induced trafficking of the TCR to the lysosome compartment (41).
We propose that downstream of PKCθ and CARMA1, Bcl10 is prone to proteolytic removal in CD3/CD28-activated T cells, a process which might be specifically required for the downmodulation of NF-κB signaling in response to very strong costimulatory T-cell activation. Bcl10 is a substrate for Itch and NEDD4 or closely related HECT domain ligases. Congruently, Itch-deficient T cells have an activated phenotype, suggesting that this E3 ligase is necessary for negative regulation of T-cell activation (16, 44). Removal of Bcl10 abolishes NF-κB activation and protects the T cells from unopposed signaling. Bcl10 deficiency has profound effects on the immune system, and Bcl10 plays a role in lymphocyte differentiation and function (51, 70). Many defects associated with bcl10 deficiency are likely to result from defective NF-κB activation, which has been shown to control lymphocyte proliferation and survival and cytokine production (37). Given the severe consequences of bcl10 deficiency in the immune system, proteolytic downregulation of Bcl10 is certainly expected to influence lymphocyte function. Thus, enhancement of apoptosis induced through loss of Bcl10 and subsequent NF-κB inactivation could be involved in the elimination of self-reactive lymphocytes. Furthermore, coreceptors, such as CD28 and CTLA-4, have been implicated in the induction of peripheral T-cell tolerance (43, 63), and induced degradation of Bcl10 may contribute to the development of T-cell unresponsiveness to subsequent stimulation (anergy) or T-cell tolerance in vivo.
Our data indicate that TCR/CD28 stimulation induces downregulation of Bcl10 and thereby terminates NF-κB signaling. The regulation of Bcl10 protein amounts contributes to the strength and the duration of NF-κB in response to T-cell activation, and processes that interfere with induced Bcl10 downregulation may be associated with the development of immunological disorders.
Acknowledgments
We thank Claus Scheidereit for discussion and constant support. We thank P. Vito, J. Huibregtse, D. Birnbaum, S. Lipkowitz, G. Baier, M. Karin, and D. Bohmann for kindly providing expression plasmids for Bcl10, NEDD4, Itch/AIP4, Cbl-b, PKCθ constructs, IKKβ, and ubiquitin, respectively. We also thank Sabine Jungmann for excellent technical assistance and Thomas Sommer for valuable discussion.
This work was supported by an EMBO long-term fellowship to V.H.
REFERENCES
- 1.Agarwal, S., and A. Rao. 1998. Modulation of chromatin structure regulates cytokine gene expression during T cell differentiation. Immunity 9:765-775. [DOI] [PubMed] [Google Scholar]
- 2.Alegre, M. L., K. A. Frauwirth, and C. B. Thompson. 2001. T-cell regulation by CD28 and CTLA-4. Nat. Rev. Immunol. 1:220-228. [DOI] [PubMed] [Google Scholar]
- 3.Anan, T., Y. Nagata, H. Koga, Y. Honda, N. Yabuki, C. Miyamoto, A. Kuwano, I. Matsuda, F. Endo, H. Saya, and M. Nakao. 1998. Human ubiquitin-protein ligase Nedd4: expression, subcellular localization and selective interaction with ubiquitin-conjugating enzymes. Genes Cells 3:751-763. [DOI] [PubMed] [Google Scholar]
- 4.Arenzana-Seisdedos, F., J. Thompson, M. S. Rodriguez, F. Bachelerie, D. Thomas, and R. T. Hay. 1995. Inducible nuclear expression of newly synthesized IκB alpha negatively regulates DNA-binding and transcriptional activities of NF-κB. Mol. Cell. Biol. 15:2689-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avraham, A., S. Jung, Y. Samuels, R. Seger, and Y. Ben-Neriah. 1998. Costimulation-dependent activation of a JNK-kinase in T lymphocytes. Eur. J. Immunol. 28:2320-2330. [DOI] [PubMed] [Google Scholar]
- 6.Babst, M., G. Odorizzi, E. J. Estepa, and S. D. Emr. 2000. Mammalian tumor susceptibility gene 101 (TSG101) and the yeast homologue, Vps23p, both function in late endosomal trafficking. Traffic 1:248-258. [DOI] [PubMed] [Google Scholar]
- 7.Bachmaier, K., C. Krawczyk, I. Kozieradzki, Y. Y. Kong, T. Sasaki, A. Oliveira-dos-Santos, S. Mariathasan, D. Bouchard, A. Wakeham, A. Itie, J. Le, P. S. Ohashi, I. Sarosi, H. Nishina, S. Lipkowitz, and J. M. Penninger. 2000. Negative regulation of lymphocyte activation and autoimmunity by the molecular adaptor Cbl-b. Nature 403:211-216. [DOI] [PubMed] [Google Scholar]
- 8.Ben-Neriah, Y. 2002. Regulatory functions of ubiquitination in the immune system. Nat. Immunol. 3:20-26. [DOI] [PubMed] [Google Scholar]
- 9.Bertin, J., Y. Guo, L. Wang, S. M. Srinivasula, M. D. Jacobson, J. L. Poyet, S. Merriam, M. Q. Du, M. J. Dyer, K. E. Robison, P. S. DiStefano, and E. S. Alnemri. 2000. CARD9 is a novel caspase recruitment domain-containing protein that interacts with BCL10/CLAP and activates NF-κB. J. Biol. Chem. 275:41082-41086. [DOI] [PubMed] [Google Scholar]
- 10.Bertin, J., L. Wang, Y. Guo, M. D. Jacobson, J. L. Poyet, S. M. Srinivasula, S. Merriam, P. S. DiStefano, and E. S. Alnemri. 2001. CARD11 and CARD14 are novel caspase recruitment domain (CARD)/membrane-associated guanylate kinase (MAGUK) family members that interact with BCL10 and activate NF-κB. J. Biol. Chem. 276:11877-11882. [DOI] [PubMed] [Google Scholar]
- 11.Chiang, Y. J., H. K. Kole, K. Brown, M. Naramura, S. Fukuhara, R. J. Hu, I. K. Jang, J. S. Gutkind, E. Shevach, and H. Gu. 2000. Cbl-b regulates the CD28 dependence of T-cell activation. Nature 403:216-220. [DOI] [PubMed] [Google Scholar]
- 12.Costanzo, A., C. Guiet, and P. Vito. 1999. c-E10 is a caspase-recruiting domain-containing protein that interacts with components of death receptors signaling pathway and activates nuclear factor-κB. J. Biol. Chem. 274:20127-20132. [DOI] [PubMed] [Google Scholar]
- 13.Dienz, O., A. Moller, A. Strecker, N. Stephan, P. H. Krammer, W. Droge, and M. L. Schmitz. 2003. Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa and phospholipase Cgamma1 are required for NF-κB activation and lipid raft recruitment of protein kinase Ctheta induced by T cell costimulation. J. Immunol. 170:365-372. [DOI] [PubMed] [Google Scholar]
- 14.Egen, J. G., and J. P. Allison. 2002. Cytotoxic T lymphocyte antigen-4 accumulation in the immunological synapse is regulated by TCR signal strength. Immunity 16:23-35. [DOI] [PubMed] [Google Scholar]
- 15.Fallarino, F., P. E. Fields, and T. F. Gajewski. 1998. B7-1 engagement of cytotoxic T lymphocyte antigen 4 inhibits T cell activation in the absence of CD28. J. Exp. Med. 188:205-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fang, D., C. Elly, B. Gao, N. Fang, Y. Altman, C. Joazeiro, T. Hunter, N. Copeland, N. Jenkins, and Y. C. Liu. 2002. Dysregulation of T lymphocyte function in itchy mice: a role for Itch in TH2 differentiation. Nat. Immunol. 3:281-287. [DOI] [PubMed] [Google Scholar]
- 17.Fang, D., and Y. C. Liu. 2001. Proteolysis-independent regulation of PI3K by Cbl-b-mediated ubiquitination in T cells. Nat. Immunol. 2:870-875. [DOI] [PubMed] [Google Scholar]
- 18.Fang, N., D. Fang, H. Y. Wang, A. Altman, and Y. C. Liu. 2002. Regulation of immune responses by E3 ubiquitin-protein ligases Curr. Dir. Autoimmun. 5:161-175. [DOI] [PubMed] [Google Scholar]
- 19.Gaide, O., B. Favier, D. F. Legler, D. Bonnet, B. Brissoni, S. Valitutti, C. Bron, J. Tschopp, and M. Thome. 2002. CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-κB activation. Nat. Immunol. 3:836-843. [DOI] [PubMed] [Google Scholar]
- 20.Gaide, O., F. Martinon, O. Micheau, D. Bonnet, M. Thome, and J. Tschopp. 2001. Carma1, a CARD-containing binding partner of Bcl10, induces Bcl10 phosphorylation and NF-κB activation. FEBS Lett. 496:121-127. [DOI] [PubMed] [Google Scholar]
- 21.Galan, J. M., and R. Haguenauer-Tsapis. 1997. Ubiquitin lys63 is involved in ubiquitination of a yeast plasma membrane protein. EMBO J. 16:5847-5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ghaffari-Tabrizi, N., B. Bauer, A. Villunger, G. Baier-Bitterlich, A. Altman, G. Utermann, F. Uberall, and G. Baier. 1999. Protein kinase Ctheta, a selective upstream regulator of JNK/SAPK and IL-2 promoter activation in Jurkat T cells. Eur. J. Immunol. 29:132-142. [DOI] [PubMed] [Google Scholar]
- 23.Ghosh, S., and M. Karin. 2002. Missing pieces in the NF-κB puzzle. Cell 109:S81-S96. [DOI] [PubMed] [Google Scholar]
- 24.Guiet, C., and P. Vito. 2000. Caspase recruitment domain (CARD)-dependent cytoplasmic filaments mediate bcl10-induced NF-κB activation. J. Cell Biol. 148:1131-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hara, H., T. Wada, C. Bakal, I. Kozieradzki, S. Suzuki, N. Suzuki, M. Nghiem, E. K. Griffiths, C. Krawczyk, B. Bauer, F. D'Acquisto, S. Ghosh, W. C. Yeh, G. Baier, R. Rottapel, and J. M. Penninger. 2003. The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity 18:763-775. [DOI] [PubMed] [Google Scholar]
- 26.Hatada, E. N., D. Krappmann, and C. Scheidereit. 2000. NF-κB and the innate immune response. Curr. Opin. Immunol. 12:52-58. [DOI] [PubMed] [Google Scholar]
- 27.Hershko, A., A. Ciechanover, and A. Varshavsky. 2000. Basic Medical Research Award. The ubiquitin system. Nat. Med. 6:1073-1081. [DOI] [PubMed] [Google Scholar]
- 28.Hicke, L. 2001. Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell. Biol. 2:195-201. [DOI] [PubMed] [Google Scholar]
- 29.Isakov, N., and A. Altman. 2002. Protein kinase C(theta) in T cell activation. Annu. Rev.. Immunol. 20:761-794. [DOI] [PubMed] [Google Scholar]
- 30.Jun, J. E., L. E. Wilson, C. G. Vinuesa, S. Lesage, M. Blery, L. A. Miosge, M. C. Cook, E. M. Kucharska, H. Hara, J. M. Penninger, H. Domashenz, N. A. Hong, R. J. Glynne, K. A. Nelms, and C. C. Goodnow. 2003. Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity 18:751-762. [DOI] [PubMed] [Google Scholar]
- 31.Kane, L. P., J. Lin, and A. Weiss. 2000. Signal transduction by the TCR for antigen. Curr. Opin. Immunol. 12:242-249. [DOI] [PubMed] [Google Scholar]
- 32.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-[κ]B activity. Annu. Rev.. Immunol. 18:621-663. [DOI] [PubMed] [Google Scholar]
- 33.Koseki, T., N. Inohara, S. Chen, R. Carrio, J. Merino, M. O. Hottiger, G. J. Nabel, and G. Nunez. 1999. CIPER, a novel NF κB-activating protein containing a caspase recruitment domain with homology to herpesvirus-2 protein E10. J. Biol. Chem. 274:9955-9961. [DOI] [PubMed] [Google Scholar]
- 34.Krappmann, D., A. Patke, V. Heissmeyer, and C. Scheidereit. 2001. B-cell receptor- and phorbol ester-induced NF-κB and c-Jun N-terminal kinase activation in B cells requires novel protein kinase C's. Mol. Cell. Biol. 21:6640-6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lafont, F., and K. Simons. 2001. Raft-partitioning of the ubiquitin ligases Cbl and Nedd4 upon IgE-triggered cell signaling. Proc. Natl. Acad. Sci. USA 98:3180-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee, P. S., Y. Wang, M. G. Dominguez, Y. G. Yeung, M. A. Murphy, D. D. Bowtell, and E. R. Stanley. 1999. The Cbl protooncoprotein stimulates CSF-1 receptor multiubiquitination and endocytosis, and attenuates macrophage proliferation. EMBO J. 18:3616-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 38.Lin, X., A. O'Mahony, Y. Mu, R. Geleziunas, and W. C. Greene. 2000. Protein kinase C-theta participates in NF-κB activation induced by CD3-CD28 costimulation through selective activation of IκB kinase beta. Mol. Cell. Biol. 20:2933-2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lucas, P. C., M. Yonezumi, N. Inohara, L. M. McAllister-Lucas, M. E. Abazeed, F. F. Chen, S. Yamaoka, M. Seto, and G. Nunez. 2001. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-κ B signaling pathway. J. Biol. Chem. 276:19012-19019. [DOI] [PubMed] [Google Scholar]
- 40.Marx, J. 2002. Cell biology. Ubiquitin lives up to its name. Science 297:1792-1794. [DOI] [PubMed] [Google Scholar]
- 41.Naramura, M., I. K. Jang, H. Kole, F. Huang, D. Haines, and H. Gu. 2002. c-Cbl and Cbl-b regulate T cell responsiveness by promoting ligand-induced TCR down-modulation. Nat. Immunol. 3:1192-1199. [DOI] [PubMed] [Google Scholar]
- 42.Okamura, H., and A. Rao. 2001. Transcriptional regulation in lymphocytes. Curr. Opin. Cell Biol. 13:239-243. [DOI] [PubMed] [Google Scholar]
- 43.Perez, V. L., L. Van Parijs, A. Biuckians, X. X. Zheng, T. B. Strom, and A. K. Abbas. 1997. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity 6:411-417. [DOI] [PubMed] [Google Scholar]
- 44.Perry, W. L., C. M. Hustad, D. A. Swing, T. N. O'Sullivan, N. A. Jenkins, and N. G. Copeland. 1998. The itchy locus encodes a novel ubiquitin protein ligase that is disrupted in a18H mice. Nat. Genet. 18:143-146. [DOI] [PubMed] [Google Scholar]
- 45.Pfeifhofer, C., K. Kofler, T. Gruber, N. G. Tabrizi, C. Lutz, K. Maly, M. Leitges, and G. Baier. 2003. Protein kinase C theta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 197:1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Plant, P. J., F. Lafont, S. Lecat, P. Verkade, K. Simons, and D. Rotin. 2000. Apical membrane targeting of Nedd4 is mediated by an association of its C2 domain with annexin XIIIb. J. Cell Biol. 149:1473-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pomerantz, J. L., E. M. Denny, and D. Baltimore. 2002. CARD11 mediates factor-specific activation of NF-κB by the T cell receptor complex. EMBO J. 21:5184-5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Raiborg, C., K. G. Bache, D. J. Gillooly, I. H. Madshus, E. Stang, and H. Stenmark. 2002. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 4:394-398. [DOI] [PubMed] [Google Scholar]
- 49.Rotin, D., O. Staub, and R. Haguenauer-Tsapis. 2000. Ubiquitination and endocytosis of plasma membrane proteins: role of Nedd4/Rsp5p family of ubiquitin-protein ligases. J. Membr. Biol. 176:1-17. [DOI] [PubMed] [Google Scholar]
- 50.Ruefli-Brasse, A. A., D. M. French, and V. M. Dixit. 2003. Regulation of NF-κB-dependent lymphocyte activation and development by paracaspase. Science 302:1581-1584. [DOI] [PubMed] [Google Scholar]
- 51.Ruland, J., G. S. Duncan, A. Elia, I. del Barco Barrantes, L. Nguyen, S. Plyte, D. G. Millar, D. Bouchard, A. Wakeham, P. S. Ohashi, and T. W. Mak. 2001. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-κB and neural tube closure. Cell 104:33-42. [DOI] [PubMed] [Google Scholar]
- 52.Ruland, J., G. S. Duncan, A. Wakeham, T. W. Mak, A. A. Ruefli-Brasse, D. M. French, and V. M. Dixit. 2003. Differential requirement for Malt1 in T and B cell antigen receptor signaling Regulation of NF-κB-dependent lymphocyte activation and development by paracaspase. Immunity 19:749-758. [DOI] [PubMed] [Google Scholar]
- 53.Saijo, K., I. Mecklenbrauker, A. Santana, M. Leitger, C. Schmedt, and A. Tarakhovsky. 2002. Protein kinase C beta controls nuclear factor κB activation in B cells through selective regulation of the IκB kinase alpha. J. Exp. Med. 195:1647-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharpe, A. H., and G. J. Freeman. 2002. The B7-CD28 superfamily. Nat. Rev. Immunol. 2:116-126. [DOI] [PubMed] [Google Scholar]
- 55.Silverman, N., and T. Maniatis. 2001. NF-κB signaling pathways in mammalian and insect innate immunity. Genes Dev. 15:2321-2342. [DOI] [PubMed]
- 56.Singer, A. L., and G. A. Koretzky. 2002. Control of T cell function by positive and negative regulators. Science 296:1639-1640. [DOI] [PubMed] [Google Scholar]
- 57.Springael, J. Y., J. M. Galan, R. Haguenauer-Tsapis, and B. Andre. 1999. NH4+-induced down-regulation of the Saccharomyces cerevisiae Gap1p permease involves its ubiquitination with lysine-63-linked chains. J. Cell Sci. 112:1375-1383. [DOI] [PubMed] [Google Scholar]
- 58.Srinivasula, S. M., M. Ahmad, J. H. Lin, J. L. Poyet, T. Fernandes-Alnemri, P. N. Tsichlis, and E. S. Alnemri. 1999. CLAP, a novel caspase recruitment domain-containing protein in the tumor necrosis factor receptor pathway, regulates NF-κB activation and apoptosis. J. Biol. Chem. 274:17946-17954. [DOI] [PubMed] [Google Scholar]
- 59.Su, T. T., B. Guo, Y. Kawakami, K. Sommer, K. Chae, L. A. Humphries, R. M. Kato, S. Kang, L. Patrone, R. Wall, M. Teitell, M. Leitges, T. Kawakami, and D. J. Rawlings. 2002. PKC-beta controls IκB kinase lipid raft recruitment and activation in response to BCR signaling. Nat. Immunol. 3:780-786. [DOI] [PubMed] [Google Scholar]
- 60.Sun, Z., C. W. Arendt, W. Ellmeier, E. M. Schaeffer, M. J. Sunshine, L. Gandhi, J. Annes, D. Petrzilka, A. Kupfer, P. L. Schwartzberg, and D. R. Littman. 2000. PKC-theta is required for TCR-induced NF-κB activation in mature but not immature T lymphocytes. Nature 404:402-407. [DOI] [PubMed] [Google Scholar]
- 61.Tegethoff, S., J. Behlke, and C. Scheidereit. 2003. Tetrameric oligomerization of IκB kinase gamma (IKKγ) is obligatory for IKK complex activity and NF-κB activation. Mol. Cell. Biol. 23:2029-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thome, M., O. Gaide, O. Micheau, F. Martinon, D. Bonnet, M. Gonzalez, and J. Tschopp. 2001. Equine herpesvirus protein E10 induces membrane recruitment and phosphorylation of its cellular homologue, bcl-10. J. Cell Biol. 152:1115-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vacchio, M. S., and R. J. Hodes. 2003. CD28 costimulation is required for in vivo induction of peripheral tolerance in CD8 T cells. J. Exp. Med. 197:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van der Merwe, P. A., D. L. Bodian, S. Daenke, P. Linsley, and S. J. Davis. 1997. CD80 (B7-1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J. Exp. Med. 185:393-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang, D., Y. You, S. M. Case, L. M. McAllister-Lucas, L. Wang, P. S. DiStefano, G. Nunez, J. Bertin, and X. Lin. 2002. A requirement for CARMA1 in TCR-induced NF-κB activation. Nat. Immunol. 3:830-835. [DOI] [PubMed] [Google Scholar]
- 66.Wang, L., Y. Guo, W. J. Huang, X. Ke, J. L. Poyet, G. A. Manji, S. Merriam, M. A. Glucksmann, P. S. DiStefano, E. S. Alnemri, and J. Bertin. 2001. Card10 is a novel caspase recruitment domain/membrane-associated guanylate kinase family member that interacts with BCL10 and activates NF-κB. J. Biol. Chem. 276:21405-21409. [DOI] [PubMed] [Google Scholar]
- 67.Weil, R., K. Schwamborn, A. Alcover, C. Bessia, V. Di Bartolo, and A. Israel. 2003. Induction of the NF-κB cascade by recruitment of the scaffold molecule NEMO to the T cell receptor. Immunity 18:13-26. [DOI] [PubMed] [Google Scholar]
- 68.Werlen, G., E. Jacinto, Y. Xia, and M. Karin. 1998. Calcineurin preferentially synergizes with PKC-theta to activate JNK and IL-2 promoter in T lymphocytes. EMBO J. 17:3101-3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Willis, T. G., D. M. Jadayel, M. Q. Du, H. Peng, A. R. Perry, M. Abdul-Rauf, H. Price, L. Karran, O. Majekodunmi, I. Wlodarska, L. Pan, T. Crook, R. Hamoudi, P. G. Isaacson, and M. J. Dyer. 1999. Bcl10 is involved in t(1;14) (p22;q32) of MALT B cell lymphoma and mutated in multiple tumor types. Cell 96:35-45. [DOI] [PubMed] [Google Scholar]
- 70.Xue, L., S. W. Morris, C. Orihuela, E. Tuomanen, X. Cui, R. Wen, and D. Wang. 2003. Defective development and function of Bcl10-deficient follicular, marginal zone and B1 B cells. Nat. Immunol. 4:857-865. [DOI] [PubMed] [Google Scholar]
- 71.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 72.Yoneda, T., K. Imaizumi, M. Maeda, D. Yui, T. Manabe, T. Katayama, N. Sato, F. Gomi, T. Morihara, Y. Mori, K. Miyoshi, J. Hitomi, S. Ugawa, S. Yamada, M. Okabe, and M. Tohyama. 2000. Regulatory mechanisms of TRAF2-mediated signal transduction by Bcl10, a MALT lymphoma-associated protein. J. Biol. Chem. 275:11114-11120. [DOI] [PubMed] [Google Scholar]
- 73.Zhang, Q., R. Siebert, M. Yan, B. Hinzmann, X. Cui, L. Xue, K. M. Rakestraw, C. W. Naeve, G. Beckmann, D. D. Weisenburger, W. G. Sanger, H. Nowotny, M. Vesely, E. Callet-Bauchu, G. Salles, V. M. Dixit, A. Rosenthal, B. Schlegelberger, and S. W. Morris. 1999. Inactivating mutations and overexpression of BCL10, a caspase recruitment domain-containing gene, in MALT lymphoma with t(1;14) (p22;q32). Nat. Genet. 22:63-68. [DOI] [PubMed] [Google Scholar]