Abstract
Objective:
The present study was designed to investigate the chemo preventive efficacy of bee propolis (BP) against diethylnitrosamine (DEN) initiated and ferric nitrilotriacetate (Fe-NTA) promoted renal carcinogenesis in Wistar rats. Chronic treatment of Fe-NTA induced oxidative stress, inflammation and cellular proliferation in Wistar rats. BP is a resinous material collected by bees from various plants which has been used from centuries in folk medicine.
Materials and Methods:
Renal cancer was initiated by single intraperitoneal injection of N-nitrosodiethylamine (DEN 200 mg/kg body weight) and promoted by twice weekly administration of Fe-NTA 9 mg Fe/kg body weight for 16 weeks. The chemo preventive efficacy of BP was studied in terms of lipid peroxidation (LPO), renal anti-oxidant armory such as catalase, superoxide dismustase, glutathione S-transferase, glutathione peroxidase, glutathione reductase and glutathione (GSH), serum toxicity markers, cell proliferation, tumor suppressor protein and inflammation markers.
Results:
Administration of Fe-NTA enhances renal LPO, with concomitant reduction in reduced GSH content and antioxidant enzymes. It induces serum toxicity markers, viz., blood urea nitrogen, creatinine and lactate dehydrogenase. Chemo preventive effects of BP were associated with upregulation of antioxidant armory and down regulation of serum toxicity markers. BP was also able to down regulate expression of proliferative cell nuclear antigen, cyclooxygenase-2, tumor necrosis factor-alpha and upregulated p53 along with induction of apoptosis. Histopathological changes further confirmed the biochemical and immunohistochemical results.
Conclusion:
These results provide a powerful evidence for the chemo preventive efficacy of BP against renal carcinogenesis possibly by modulation of multiple molecular pathways.
Keywords: Bee propolis, inflammation, oxidative stress, proliferation, renal carcinogenesis
INTRODUCTION
Renal cell carcinoma (RCC) is the most common type of kidney cancer accounting for around 90% of all kidney cancers. Each year about 273,000 new cases of kidney cancer are detected world-wide representing approximately 2% of all cancers.[1] Previously it has been reported that RCC is one of the most therapy resistant cancers. It responds to chemotherapy, hormonal therapy and radiation therapy either very feebly or not at all. However, the response rate is only 10-15% even for the immunotherapy, which is considered to be the best therapy for RCC.[2] It has also been evaluated that the standard survival following metastatic RCC is about 4 months with which merely 10% of patients survive for 1 year. Besides development of many novel chemotherapeutic agents over the past decade and the increase in the insights of molecular mechanisms involved in the development of RCC, yet it remains an incurable and lethal disease.[3] There are many risk factors involved in its development like environmental exposure to various toxicants.[4] Ferric nitrilotriacetate (Fe-NTA) is one such environmental toxicant, which is formed by the amalgamation of Fe-NTA acid, a known substitute for pyrophosphate used in various kinds of detergents. However, the detailed mechanism of its toxicity is not known. Previous reports show that Fe-NTA induces lipid peroxidation (LPO) which in turn generates oxidative stress and reactive oxygen species (ROS), also produces oxidative modifications in deoxyribonucleic acid (DNA).[5] Its prolonged exposure leads to widespread amplification in the levels of thiobarbituric acid reactive substances and 4-hydroxy-2-nonenal later found in renal proximal convoluted tubule (PCT). Henceforth, oxidative stress induced by Fe-NTA plays an essential and fundamental role in nephro toxicity and tumorigenesis.[6] It also causes DNA damage by stimulating the production of •OH radicals. In the kidney glutathione (GSH) cycle results in the production of cysteine reducing Fe3+-NTA to Fe2+-NTA, which is found in the lumen of PCT after glomerular filtration.[6] It has been reported that its extended exposure results in renal proximal tubular necrosis, which is consequently associated with the prominent incidence of renal adenocarcinoma in male mice and rats. Previous findings report that Fe-NTA enhances diethylnitrosamine (DEN)-induced renal tumorigenesis.[5] The correlation between oxidative stress and carcinogenesis is an established fact now. It has been found that ROS are involved in all stages of carcinogenesis. They can alter DNA and initiate carcinogenesis by reacting with it. The most abundant metal present in the human body is iron. Its catalytic effect on the generation of ROS has been found to be linked with carcinogenesis. Intraperitoneal (IP) administration of Fe-NTA (iron chelate) provides a captivating model of iron based oxidative stress induced carcinogenesis leading to renal proximal tubular damage that ultimately leads up to 90% incidence of RCC in rodents.[7] ROS can amend signal transduction pathways implicated in cellular repair, proliferation and inflammation such as p53, proliferative cell nuclear antigen (PCNA), tumor necrosis factor-alpha (TNF-α) and cyclooxygenase-2 (Cox-2).
Nowadays research on herbal agents is upcoming fast because of their positive results in animal models as well as clinical trials. Hence, presently much investigation is being carried on assessment of safe and efficient plant based agents particularly against cancer because of the inadequate choice the modern system of medicine offers for the treatment. Thus, there is a huge urgency to extend alternate approach to handle it. It has been found that one of the most valuable options to prevent or delay the neoplastic commencement is the use of safe plant based compounds.[8]
Broadly, bee propolis (BP) is composed of 50% resin and vegetable balsam, 30% wax, 10% essential and aromatic oils, 5% pollen and 5% various other substances, including organic debris.[9] The principal components responsible for the biological activity of BP are flavonoids, aromatic acids, diterpenic acids and phenolic compounds. BP is assumed to demonstrate an extensive range of biological properties including antioxidant, anti-inflammatory, antihepatotoxic, anticancer, antiviral and antibacterial effects.[10] It is also being used in foods because of its wide range of biological activities to prevent diseases such as inflammation, cancer etc.[11] It has been reported that Crude (water, ethanol or lyophilized) BP extracts given orally to male Fischer 344 or Wistar rats and female Sprague Dawley rats inhibited the development of chemically induced colon and mammary carcinogenesis, respectively. Also, oral treatment with crude BP extracts reduced the development of pulmonary tumors in male ddY mice.[12]
In the present study, we examined its potential against Fe-NTA induced nephrotoxicity and its preclinical chemo preventive efficacy against two stage renal carcinogenesis induced by DEN initiation and Fe-NTA promotion for 16 weeks in Wistar rats by studying its possible potential molecular targets. To this effect, we studied the expression of a marker of DNA repair p53, proliferation marker PCNA and inflammatory marker Cox-2 and TNF-α which is known to be deregulated in cancer cells and hence might be one of the targets of the chemo preventive action of BP.
MATERIALS AND METHODS
Chemicals
Glutathione reductase (GR), oxidized glutathione disulfide and reduced GSH, 1,2-dithio-bis-nitrobenzoic acid, 1-chloro-2,4-dinitrobenzene, bovine serum albumin, oxidized and reduced nicotinamide adenine dinucleotide phosphate, flavine adenine dinucleotide, 2,6-dichlorophenolindophenol, thiobarbituric acid, tricholoracetic acid (TCA), Hydrogen peroxide, sulphosalicylic acid, perchloric acid, folin ciocalteau reagent, sodium potassium tartrate, di-sodium hydrogen phosphate, sodium dihydrogen phosphate, sodium hydroxide etc., were obtained from Sigma-Aldrich, USA. All other chemicals and reagents were of the highest purity grade and commercially available. Plant extract was purchased from Saiba Industries, Mumbai.
Animals
Male Wistar rats (150-200 g), 6-8 weeks old, were obtained from the Central Animal House Facility of Hamdard University. Animals received humane care in accordance with the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India, and prior permission was sought from the Institutional Animal Ethics Committee (IAEC No: 173/CPCSEA, 28 January 2000).
Preparation of Fe-NTA
The Fe-NTA solution was prepared by the method of Awai et al. briefly, ferric nitrate (0.16 mM/5.0 ml) solution was mixed with a fourfold molar excess or disodium salt of NTA (0.64 mM/5.0 ml) and the pH was adjusted to 7.4 with sodium bicarbonate. The solution was freshly prepared immediately before use and was injected on the basis of 10 ml/kg body weight.[13]
Experimental design
The treatment regimen for BP and the proposal of verifying its chemo preventive efficacy against renal carcinogenesis was based on the preliminary dose dependent pilot study which was carried out in our laboratory. To study the protective effects of BP on biochemical and serological changes induced by toxicity of Fe-NTA in rats, 30 male Wistar rats were randomly divided into five equal groups.
Group I animals received only distilled water for 14 consecutive days by oral gavage and thus served as untreated controls
Group II served as treated control and were administered distilled water by oral gavage for 14 consecutive days before a final dose of Fe-NTA on 15th day
Group III were pre-treated with oral gavage of BPD1 at a dose of 80 mg/kg body weight for 14 consecutive days followed by administration of Fe-NTA on 15th day
Group IV were pre-treated with oral gavage of BPD2 at a dose of 160 mg/kg body weight for 14 consecutive days followed by administration of Fe-NTA on 15th day
Group V received oral gavage of only BPD2 only, at a dose of 160 mg/kg body weight for 14 consecutive days.
Group V were used only to ensure that the higher dose does not produce any kind of nephrotoxicity itself. All animals were sacrificed within 1 h and exactly 12 h after Fe-NTA administration. Kidney tissues were processed for biochemical estimations. Blood was collected and serum separated out and processed for serological studies.
To study the effect of pre-treatment of BP on DEN initiated and Fe-NTA – promoted renal carcinogenesis. The complete treatment regimen followed in tumor study was as follows
Group I animals received only distilled water by oral gavage once daily for 16 weeks and served as controls
Group II also received only distilled water by oral gavage once daily for 16 weeks. In addition Group II were given IP injection of DEN in saline on the very 1st day of experiment and 10 days after the injection, the animals were promoted with IP injection of Fe-NTA, twice a week for 16 weeks
Group III were given same treatment as Group II and were also co-treated by oral gavages of BP once daily, at a dose of 80 mg/kg body weight, an hour prior to the treatment of Fe-NTA for a period of 16 weeks
Group IV were given same treatment as Group II and were also co-treated by oral gavage of BP once daily at a dose of 160 mg/kg body weight, an hour prior to the treatment of Fe-NTA for a period of 16 weeks.
At the end of 24 weeks, all the animals were euthanized by light ether anesthesia and exsanguinations. Their kidneys were quickly removed and processed for various molecular, histopathological and immunohistochemical studies.
Biochemical estimation
Assay for catalase (CAT), LPO, superoxide dismustase (SOD), reduced GSH, glutathione peroxidase (GPx), GR, blood urea nitrogen (BUN), creatinine, lactate dehydrogenase (LDH) and protein estimation was done by the method as described by Rashid et al.[14]
Assay for TNF-α
The level of TNF-α was quantitated using an enzyme-linked immunosorbent assay based kit (eBioscience, Inc., San Diego., USA) following instructions of the manufacturer.
Immunohistochemistry
Kidney sections on polylysine coated slides obtained were fixed in neutral buffered formalin, and embedded in paraffin and was treated for p53, PCNA and Cox-2 antibody for immunohistochemical analysis. The procedure was processed according to the manufacturer's protocol with slight modifications.
Histopathological examination
The kidneys were quickly removed after sacrifice and preserved in 10% neutral buffered formalin for histopathological processing. The kidneys were embedded in paraffin wax and longitudinally sectioned with a microtome. Hematoxylin and eosin staining of the sections was observed under an Olympus microscope.
Statistical analysis
Differences between groups were analyzed using analysis of variance followed by Dunnet's multiple comparisons test. All data points are presented as the treatment group's mean ± standard error.
RESULTS
Effect of BP on Fe-NTA-induced oxidative stress
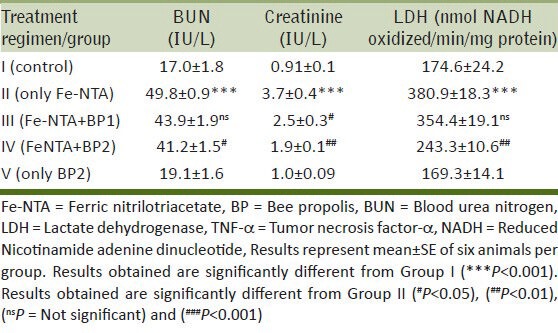
The protective effects of BP on Fe-NTA induced depletion of antioxidant armory, elevation in serum kidney toxicity markers are shown in Tables 1 and 2. Fe-NTA induced LPO substantially (P < 0.001) in Group II compared to Group I, but the administration of BP at both doses attenuated LPO levels significantly (P < 0.05 and P < 0.001). Renal reduced GSH was depleted in Group II (P < 0.001) due to Fe-NTA administration in comparison to Group I i.e., untreated control. Lower dose didn’t replenish GSH significantly (ns), but was alleviated significantly by higher dose (P < 0.01) of BP. There was a concomitant and significant decrease in the activity of glutathione dependent antioxidant enzymes (Reduced glutathione, GPx, GR, and SOD) compared to the control group (**P < 0.01 and ***P < 0.001). However, pre-treatment with a lower dose of BP restored the activity (ns and #P < 0.05) and higher dose of BP ameliorated considerably (P < 0.05 and P < 0.01). There was also marked decrease (P < 0.001) found in CAT activity due to Fe-NTA administration in Group II. Its activity however was not maintained significantly at both doses of BP. Serological chemistry revealed that Fe-NTA induced acute nephropathy as evident by the significant increase in kidney toxicity markers viz. BUN and creatinine in the serum compared to Group I (P < 0.001). Prophylactic treatment of BP at a higher dose prevented the Fe-NTA induced elevation in the serum levels of BUN (P < 0.001) and creatinine (P < 0.01) respectively. The cytotoxicity marker LDH, also showed marked elevation in serum (P < 0.001) due to Fe-NTA induced cytotoxicity, but its release in serum was suppressed by the treatment of BP at a higher dose significantly (P < 0.05). However, the treatment of BP at a higher dose in Group V did not produce any kind of adverse effects itself on the kidney of rats. It was found that there was no significant difference in the activity of these enzymic and non-enzymic antioxidants and serum toxicity markers between the control group and Group V (only BP group) [Tables 1 and 2].
Table 1.
Results of BP on CAT, SOD, LPO, GSH, GR and GPx on Fe-NTA administration in kidney of Wistar rats

Table 2.
Results of modulatory effect of BP on BUN, creatinine, LDH and TNF-α on Fe-NTA induced renal toxicity
Effect of BP on TNF-α production in rat kidney
Level of proinflammatory cytokine TNF-α was found to be elevated significantly in DEN initiated and Fe-NTA treated group for 16 weeks in comparison to the control (***P < 0.001). Pre-treatment with BP in Groups III and IV significantly (P < 0.05, P < 0.01) alleviated the TNF-α level. There is no significant difference in the level of TNF-α between control and higher dose of BP treated group (Group V) [Figure 1].
Figure 1.
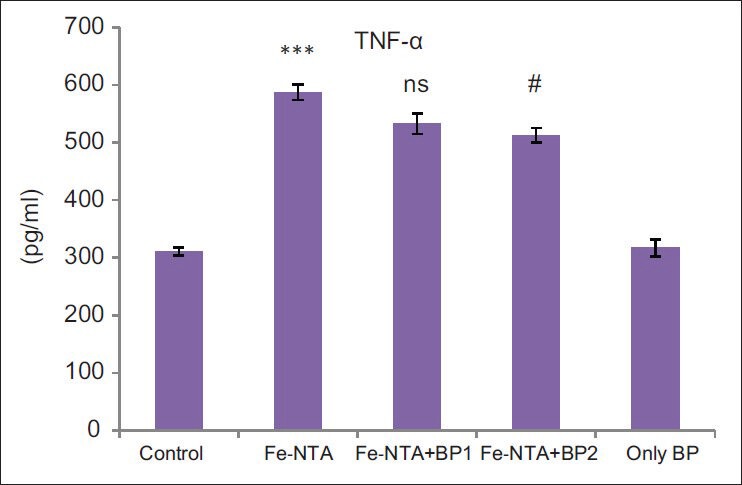
Results represent mean ± standard error of six animals per group. Results obtained were significantly increased in Group II (***P < 0.001). Prophylactic treatment prevented diethylnitrosamine initiated and ferric nitrilotriacetate promoted renal up regulation in tumor necrosis factor-alpha levels significantly at higher dose as compared to control (#P < 0.05)
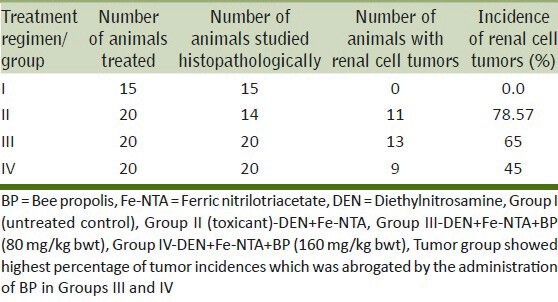
Effect of BP on tumor incidence and histopathological observations in tumor study
The data given in Table 3 gives a summary of the percentage incidence of renal tumors (RTs) in different treatment groups. It is evident that Group I did not show any incidences of kidney tumors. However, treatment with DEN-initiated and Fe-NTA treated animals for 16 weeks enhanced the development of RTs in 78.57% of the animals studied. In comparison, tumor incidence in the Group III i.e., group that was co-treated with BP (80 mg/kg body weight) was 65% whereas in the group receiving the higher dose of BP (160 mg/kg body weight), the tumor incidence was found reduced by 45%.
Table 3.
Modulatory effect of BP on tumor data in DEN-initiated and Fe-NTA-promoted renal tumors
The histology of the rat kidney tissues showed normal histo architecture in the control group. Kidneys of rats in the Group II that were DEN initiated and chronically promoted with Fe-NTA for 16 weeks were abnormal resulted in disruption of the normal renal architecture which was well evident by blood sinusoids, interstitial hemorrhages, glomerular congestion, tubular congestion and atrophy [Figure 2]. Furthermore, BP treatment significantly showed protective changes in the glomeruli and tubules in a dose dependent manner. Group III treated with BP (80 mg/kg body weight) shows mild inflammatory cell invasion, glomerular and tubular congestion and very few hyperchromatic deposits, whereas, high dose of BP (160 mg/kg body weight) attenuated renal histological features. The glomerular and tubular structures were more intact with no signs of glomerular atrophy and hyperchromatism.
Figure 2a-e.
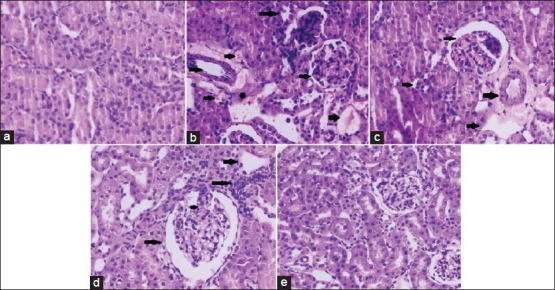
Histopathological examination of rat kidney ×40 (a) Normal histology of kidney (b) Disruption of the normal renal architecture by diethylnitrosamine + ferric nitrilotriacetate administration was observed as shown by arrows (c and d) treatment with bee propolis showed protective changes in the glomeruli and tubules and the morphology of tubular epithelial cells was restored on higher dose of BP
Expression of proliferative protein in tumor study
Detection of PCNA, a well-known cell proliferation marker by immunohistochemistry (IHC) has been used to characterize the proliferation of cells in many fields, such as in tumor studies. The immunohistochemical evaluation showed intense expression of PCNA in DEN initiated and Fe-NTA treated rats for 16 weeks compared to untreated controls [Figure 3]. Moderate expression of PCNA was found in the low dose modulator (80 mg/kg body weight) treated group compared to Group II. However, higher dose of BP (160 mg/kg body weight) treated rats showed considerably lesser expression of PCNA which means that BP has attenuated renal cell proliferation in kidneys compared to Group II. Hence confirmed the anti-proliferative potential of BP.
Figure 3a-d.
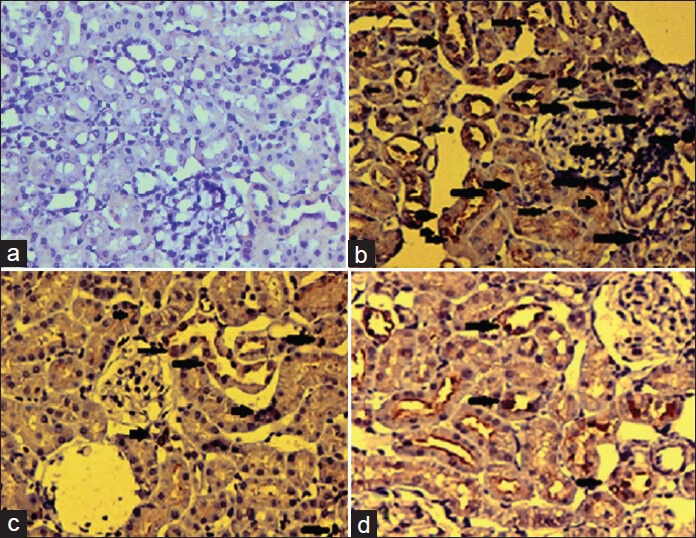
Photomicrographs of proliferative cell nuclear antigen determined by immunohistochemistry (a) no expression of bax was observed in case of control rats (b) Diethylnitrosamine initiated and ferric nitrilotriacetate promotion increased the number of PCNA positive cells in glomerular and tubular region of renal sections of animals represented by arrows in the figure (c) DEN + Fe-NTA + bee propolis (BPD1) treated animals showed very slightly lesser number of PCNA positive cells as compared to Group II as is evident from the figure (d) DEN + Fe-NTA + BPD2 treated animals showed slightly lesser number of PCNA positive cells as compared to Group II
Effect of BP on p53 expression in DEN initiated and Fe-NTA treated rat kidneys
The immunohistochemical evaluation showed very low positive staining of p53 protein in rats treated with only DEN and Fe-NTA for 16 weeks compared to control [Figure 4]. There was considerably very less p53 expression in the low dose modulator (80 mg/kg body weight) treated group compared to Group II. However, higher dose of BP (160 mg/kg body weight) treated rats showed considerably positive staining of p53 protein in kidneys compared to Group II which means that the higher dose of BP upregulated the expression of p53, which in necessary for regulation of normal cell cycle process.
Figure 4a-d.
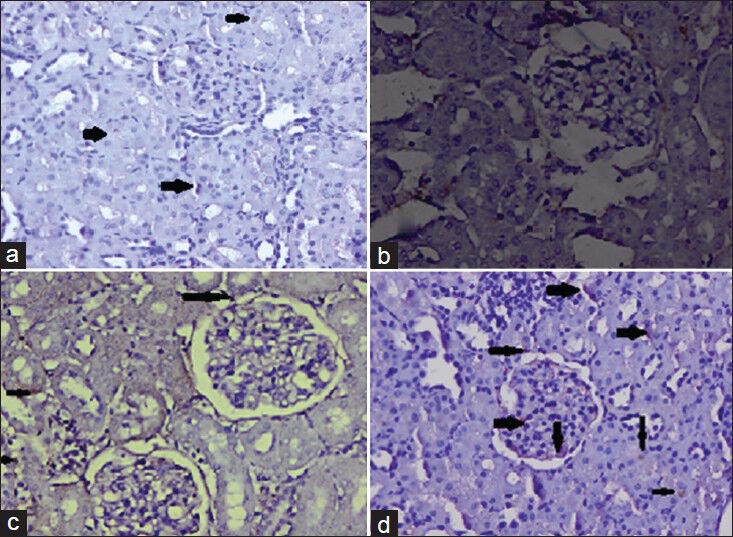
Photomicrographs of p53 determined by immunohistochemistry (a) There is almost negligible expression of p53 in the renal sections of control group (b) Diethylnitrosamine initiation and ferric nitrilotriacetate administration decreased strongly p53 expression in renal sections (c) There was partial expression of p53 as evidenced by weak immunostaining in the rat kidneys treated with lower dose of bee propolis (d) The expression of p53 was increased markedly on administration of higher dose of BP which was well evident by the positive staining
Effect of BP on Cox-2 expression in DEN initiated and Fe-NTA treated rat kidneys
The expression pattern of Cox-2 in rat kidney tissue was highly induced by repeated Fe-NTA treatment for 16 weeks in Group II compared to control group, but its expression was down regulated significantly by BPD1 (80 mg/kg body weight) and BPD2 (160 mg/kg body weight) substantially and dose dependently [Figure 5].
Figure 5a-d.
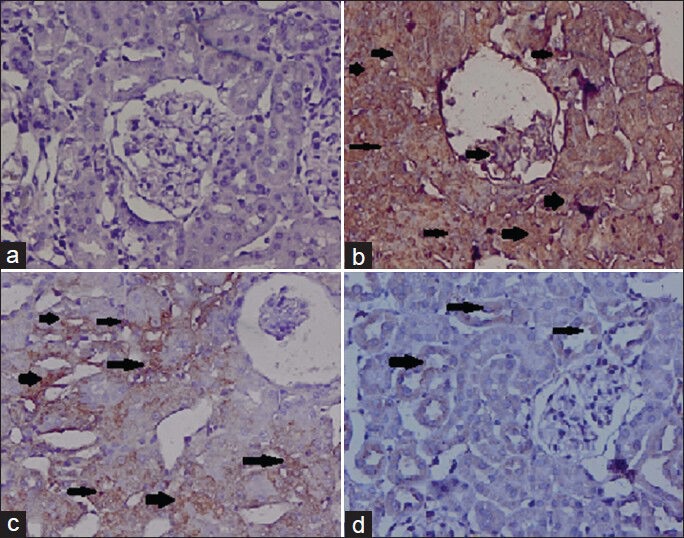
Photomicrographs of cyclooxygenase-2 (Cox-2) determined by immunohistochemistry (a) there is almost negligible expression of Cox-2 in the renal sections of control group (b) diethylnitrosamine + ferric nitrilotriacetate administration strongly induced Cox-2 expression in renal sections (c) there is partial inhibition of Cox-2 expression as evidenced by weak immunostaining in the rat kidneys treated with lower dose of bee propolis (d) higher dose of BP treatment showed lesser expression which implies that Cox-2 has been inhibited
DISCUSSION
Kidney cancer is widespread around the world as well as in the Indian population.[15] The occurrence of RCC is growing annually due to its resistance to various kinds of therapies and deficit of early warning signs.[16] Modern therapeutic interventions for the treatment of renal cancer have shown inadequate efficacy which has stimulated the need for the development of alternative strategies. Phytochemicals have been found to be the most helpful alternative to delay or thwart carcinogenesis. Henceforth there is a rise in the investigation of safe and effective phytochemicals for the management of renal cancer. BP is a resinous material collected by bees from various plants which has been used from centuries in folk medicine.[17] It is used as a disinfectant and acts as glue in beehive. It consists of a variety of polyphenols, flavonoids, terpenes, sterols, vitamins, amino acids, etc. Its chemopreventive potential have been proved against a variety of tumors like mammary cancers, colon cancers in vivo in animal models and in cancer cells in vitro models also.[11,12] It has been reported that until early promotion stage carcinogenesis is reversible, and involves a recurring and extended exposure to promoter. Henceforth, its mitigation at promotional stage is possible and suitable.
In the present study, we have demonstrated BP to inhibit several aspects of DEN initiated and Fe-NTA promoted tumor induction and promotion response in kidney. It has been reported that oxidative stress and inflammation plays an essential role in tumor promotion. Oxidative stress plays an imperative role in the pathogenesis of nephrotoxicity caused by Fe-NTA which plays an elementary role not only in nephrotoxicity but also in tumorigenesis. This has been reported previously from our lab.[18] Administration of Fe-NTA results in iron deposition within kidney tissues which is found to be the main generator of ROS, leading to oxidative damage which further causes LPO. In the present study, it was found that there was an increase in LPO production in Fe-NTA administered group as compared to control group. But prophylactic treatment of BP at both the doses alleviated LPO levels significantly. Further it was found that there was depletion of renal GSH content and antioxidant armory of kidney including GR, GPx, CAT, SOD in Fe-NTA treated group as compared to control which was in concurrence with earlier reports.[19] However, it is evident from the present results that prophylactic treatment of BP abrogated GSH and antioxidant armory which was earlier exhausted in scavenging the ROS produced by Fe-NTA administration. There was concomitant increase in serum toxicity markers like BUN, creatinine and LDH in Fe-NTA treated group as compared to control. Our results are in good agreement with those previously reported.[19] On the contrary, lower BUN, creatinine and LDH levels were found in the rats administered with BP as compared to Fe-NTA treated group. Hence the decrease in the levels of these enzymes and attenuation of antioxidant armory may be possibly by nephroprotective efficacy of BP.[20]
Histopathological examination of the kidneys of animals treated for 16 weeks with Fe-NTA revealed tubular necrosis, glomerular congestion, massive inflammatory response and renal cell tumors. All these pathological alterations were down regulated by BP treatment. Our data was further supported by the immunohistochemical results of proliferation and inflammation marker proteins. Carcinogenesis is featured by unlimited cell proliferation is a fact now. In this context we studied the expression of PCNA protein, which is a marker of hyper proliferation. It has been reported by Howard et al.[21] that PCNA expression via IHC can be used as well marker of proliferation in tumor studies. Its expression is highest during the synthesis phase of cell cycle. In the present study, we observed the abrupt up regulation of PCNA after 16 weeks of Fe-NTA treatment in DEN initiated and Fe-NTA treated group as compared to the control group. Further we also observed that prophylactic treatment of BP alleviated the expression of PCNA positive nuclei at both the doses which is well evident from IHC slides signifying its anti-tumor and anti-proliferative potential. We also studied the expression of p53 protein which is said to be a tumor suppressor protein, a well-marked check point in regulation of cell cycle. We found that there was no expression of p53 in DEN initiated and Fe-NTA promoted Group for 16 weeks as compared to control which signifies that there is faulty machinery which leads to non-regulation of cell cycle by p53 protein. Nonetheless prophylactic treatment of BP mitigated p53 levels in BP treated group's dose dependently. Since p53 is a tumor suppressor gene implicated in regulation of gene transcription, DNA repair, and apoptosis. Thus, we can suggest that BP scavenged the ROS and triggered apoptosis, hence limiting the oxidative stress which ultimately activates p53 respectively. Cox-2 over expression is interrelated with renal inflammation and tumor promotion. Now it is well-known that Cox-2 activation is implicated in various features of oncogenic process such as cellular inflammation, proliferation, deregulation of apoptosis, contributing to the metastatic and angiogenic ability of tumor cells etc. Thus, inhibition of Cox-2 is now recognized as a valuable tool to thwart the carcinogenic development. Fe-NTA exposure was found to activate Cox-2 in renal tissues in our study which is consistent with earlier reports.[8,22] Thus suppressive effect of BP on Cox-2 production may be implicated in its antitumor and anti-inflammatory activities. Thus, inhibition of Fe-NTA induced Cox-2 expression by BP may also plausibly be implicated in protection against DEN initiated and Fe-NTA promoted renal carcinogenesis. Another proinflammatory marker cytokine of inflammation is TNF-α. It plays an essential role in various diseases including cancer because of its mutagenicity and proliferative capacity. Therefore, TNF-α plays an essential role in transformation and later survival of transformed cells which results into tumor development by promoting the accumulation of mutations. It also stimulates the production of other inflammatory mediators such as ROS via oxidative stress-responsive genes which enhances and extends inflammation.[23,24] Chronic exposure of Fe-NTA for 16 weeks in tumor group in the present study induced the expression of TNF-α as compared to control group. Prophylactic treatment of BP down regulated the expression dose dependently further supports its anti-inflammatory action.
CONCLUSION
Our data demonstrates that prophylactic treatment with BP strongly restrains against DEN initiated and Fe-NTA induced up-regulation of PCNA, Cox-2, TNF-α and down regulation of p53 via mitigation of renal oxidative stress, proliferative index, apoptosis, inflammatory response and cytokine release. Thus, inhibition in their secretion by BP seems to play an important role in its protective effect against renal tumorigenesis. Hence it could also be inferred that BP possess potent antioxidant, anti-inflammatory, anti-proliferative, pro apoptotic, nephroprotective activities. The present data together with reports available in literature, suggest BP may be a promising candidate for chemoprevention of renal carcinogenesis after further confirmatory studies both at pre-clinical and clinical levels.
Footnotes
Source of Support: UGC-SAP BSR-II.
Conflict of Interest: None declared.
REFERENCES
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Lyon, France: International Agency for Research on Cancer; 2010. GLOBOCAN. Cancer Incidence and Mortality Worldwide: IARC; p. 1. [Google Scholar]
- 2.Shi T, Liou LS, Sadhukhan P, Duan ZH, Novick AC, Hissong JG, et al. Effects of resveratrol on gene expression in renal cell carcinoma. Cancer Biol Ther. 2004;3:882–8. doi: 10.4161/cbt.3.9.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bullock A, McDermott DF, Atkins MB. Management of metastatic renal cell carcinoma in patients with poor prognosis. Cancer Manag Res. 2010;2:123–32. [PMC free article] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Iqbal M, Giri U, Giri DK, Alam MS, Athar M. Age-dependent renal accumulation of 4-hydroxy-2-nonenal (HNE)-modified proteins following parenteral administration of ferric nitrilotriacetate commensurate with its differential toxicity: Implications for the involvement of HNE-protein adducts in oxidative stress and carcinogenesis. Arch Biochem Biophys. 1999;365:101–12. doi: 10.1006/abbi.1999.1135. [DOI] [PubMed] [Google Scholar]
- 6.Kaur G, Lone IA, Athar M, Alam MS. Protective effect of Didymocarpus pedicellata on ferric nitrilotriacetate (Fe-NTA) induced renal oxidative stress and hyperproliferative response. Chem Biol Interact. 2007;165:33–44. doi: 10.1016/j.cbi.2006.10.012. [DOI] [PubMed] [Google Scholar]
- 7.Dutta KK, Nishinaka Y, Masutani H, Akatsuka S, Aung TT, Shirase T, et al. Two distinct mechanisms for loss of thioredoxin-binding protein-2 in oxidative stress-induced renal carcinogenesis. Lab Invest. 2005;85:798–807. doi: 10.1038/labinvest.3700280. [DOI] [PubMed] [Google Scholar]
- 8.Kaur G, Athar M, Alam MS. Dietary supplementation of silymarin protects against chemically induced nephrotoxicity, inflammation and renal tumor promotion response. Invest New Drugs. 2010;28:703–13. doi: 10.1007/s10637-009-9289-6. [DOI] [PubMed] [Google Scholar]
- 9.Kalogeropoulos N, Konteles SJ, Troullidou E, Mourtzinos I, Karathanos VT. Chemical composition, antioxidant activity and antimicrobial properties of propolis extracts from Greece and Cyprus. Food Chem. 2009;116:452–61. [Google Scholar]
- 10.Lahouel M, Boutabet K, Wided K, Alyane M. Polyphenolic fractions of Algerian propolis reverses doxorubicin induced acute renal oxidative stress. Afr J Pharm Pharmacol. 2010;4:712–20. [Google Scholar]
- 11.Ozkul Y, Silici S, Eroğlu E. The anticarcinogenic effect of propolis in human lymphocytes culture. Phytomedicine. 2005;12:742–7. doi: 10.1016/j.phymed.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 12.Said RA, Grassi TF, Scolastici C, Alves de Lima RO, Darros BR, Barbisan LF, et al. Absence of chemopreventive influence of propolis on the rat liver altered foci development. Exp Toxicol Pathol. 2010;62:405–12. doi: 10.1016/j.etp.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 13.Awai M, Narasaki M, Yamanoi Y, Seno S. Induction of diabetes in animals by parenteral administration of ferric nitrilotriacetate. A model of experimental hemochromatosis. Am J Pathol. 1979;95:663–73. [PMC free article] [PubMed] [Google Scholar]
- 14.Rashid S, Ali N, Nafees S, Ahmad ST, Hasan SK, Sultana S. Abrogation of 5-flourouracil induced renal toxicity by bee propolis via targeting oxidative stress and inflammation in Wistar rats. J Pharm Res. 2013;7:189–94. [Google Scholar]
- 15.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 16.Koul H, Huh JS, Rove KO, Crompton L, Koul S, Meacham RB, et al. Molecular aspects of renal cell carcinoma: A review. Am J Cancer Res. 2011;1:240–54. [PMC free article] [PubMed] [Google Scholar]
- 17.Lu LC, Chen YW, Chou CC. Antibacterial and DPPH free radical-scavenging activities of the ethanol extract of propolis collected in Taiwan. J Food Drug Anal. 2003;11:277–82. [Google Scholar]
- 18.Ahmad ST, Arjumand W, Seth A, Nafees S, Rashid S, Ali N, et al. Preclinical renal cancer chemopreventive efficacy of geraniol by modulation of multiple molecular pathways. Toxicology. 2011;290:69–81. doi: 10.1016/j.tox.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 19.U Rehman M, Sultana S. Attenuation of oxidative stress, inflammation and early markers of tumor promotion by caffeic acid in Fe-NTA exposed kidneys of Wistar rats. Mol Cell Biochem. 2011;357:115–24. doi: 10.1007/s11010-011-0881-7. [DOI] [PubMed] [Google Scholar]
- 20.Newairy AS, Salama AF, Hussien HM, Yousef MI. Propolis alleviates aluminium-induced lipid peroxidation and biochemical parameters in male rats. Food Chem Toxicol. 2009;47:1093–8. doi: 10.1016/j.fct.2009.01.032. [DOI] [PubMed] [Google Scholar]
- 21.Howard PC, Warbritton A, Voss KA, Lorentzen RJ, Thurman JD, Kovach RM, et al. Compensatory regeneration as a mechanism for renal tubule carcinogenesis of fumonisin B1 in the F344/N/Nctr BR rat. Environ Health Perspect. 2001;109(Suppl 2):309–14. doi: 10.1289/ehp.01109s2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Surh YJ, Chun KS, Cha HH, Han SS, Keum YS, Park KK, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflammatory phytochemicals: Down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480-481:243–68. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 23.Zhou YH, Yu JP, Liu YF, Teng XJ, Ming M, Lv P, et al. Effects of Ginkgo biloba extract on inflammatory mediators (SOD, MDA, TNF-alpha, NF-kappaBp65, IL-6) in TNBS-induce. colitis in rats. Mediators Inflamm 2006. 2006 doi: 10.1155/MI/2006/92642. 92642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khan AQ, Khan R, Qamar W, Lateef A, Ali F, Tahir M, et al. Caffeic acid attenuates 12-O-tetradecanoyl-phorbol-13-acetate(TPA)-induced NF-κB and COX-2 expression in mouse skin: Abrogation of oxidative stress, inflammatory responses and proinflammatory cytokine production. Food Chem Toxicol. 2012;50:175–83. doi: 10.1016/j.fct.2011.10.043. [DOI] [PubMed] [Google Scholar]


